Abstract
Convincing evidence has accumulated to identify the Frizzled proteins as receptors for the Wnt growth factors. In parallel, a number of secreted frizzled-like proteins with a conserved N-terminal frizzled motif have been identified. One of these proteins, Frzb-1, binds Wnt-1 and Xwnt-8 proteins and antagonizes Xwnt-8 signaling in Xenopus embryos. Here we report that Frzb-1 blocks Wnt-1 induced cytosolic accumulation of β-catenin, a key component of the Wnt signaling pathway, in human embryonic kidney cells. Structure/function analysis reveals that complete removal of the frizzled domain of Frzb-1 abolishes the Wnt-1/Frzb-1 protein interaction and the inhibition of Wnt-1 mediated axis duplication in Xenopus embryos. In contrast, removal of the C-terminal portion of the molecule preserves both Frzb-Wnt binding and functional inhibition of Wnt signaling. Partial deletions of the Frzb-1 cysteine-rich domain maintain Wnt-1 interaction, but functional inhibition is lost. Taken together, these findings support the conclusion that the frizzled domain is necessary and sufficient for both activities. Interestingly, Frzb-1 does not block Wnt-5A signaling in a Xenopus functional assay, even though Wnt-5A coimmunoprecipitates with Frzb-1, suggesting that coimmunoprecipitation does not necessarily imply inhibition of Wnt function.
The Wnt family of signaling molecules is of great interest because of its roles in developmental processes and in oncogenesis (1, 2). The recent implication of β-catenin, one effector protein in the Wnt signaling pathway, in colon cancers and melanomas has underscored the importance of this pathway in human cancers (3–5). A major advance in the understanding of Wnt signaling was provided by the discovery that Dfz2, a member of the class of Frizzled proteins, functions as a receptor for Drosophila Wingless (Wg), the homologue of vertebrate Wnt-1 (6). In addition, overexpression of rat fz-1 in Xenopus embryos recruits Dishevelled, another component of the Wnt signaling pathway (7), to the plasma membrane. These findings led to the proposal that the frizzled group within the G-protein receptor superfamily comprises receptors for the Wnt proteins (6).
The Wnts have been divided into two operationally defined classes. Class I Wnts (e.g., Wnt-1) induce both transformation of cultured mammalian cells and axis duplication in Xenopus embryos; class II Wnts (e.g., Wnt-5A) do not. The observation that several, but not all, mammalian frizzled proteins are able to confer Wg binding in cell biological assays, suggests that some Frizzled proteins may be selective for particular members of the Wnt family (6). This is supported by the demonstration that rat Fz-1 is involved in Wnt signaling in a manner that discriminates between the functionally distinct Xwnt-8 (class I) and Xwnt-5A (class II) (7).
Frzb-1, originally discovered by primary protein sequencing of highly purified cartilage-derived protein preparations, contains an N-terminal domain with ≈50% identity to the cysteine-rich domain (CRD) of Drosophila Frizzled (8). Because this domain has been proposed to be the ligand binding domain of the Frizzled proteins (6), we explored the possibility of structural and functional interactions between Frzb-1 and members of the Wnt family. The observation of complementary expression patterns of Xfrzb-1 and Xwnt-8 in developing embryos and the demonstration of their direct interaction led to the finding that Frzb-1 is a Wnt-8 antagonist (9–11).
In this paper, we demonstrate that Frzb-1 prevents Wnt-1 induced cytosolic accumulation of β-catenin in human embryonic kidney cells. Structure/function studies indicate that the N-terminal CRD of Frzb-1 is critical for the inhibition of Wnt-1 signaling, and suggest a supportive role for the C terminus. We further show that Frzb-1 also binds to murine Wnt-5A in immunoprecipitation experiments. However, this interaction does not necessarily predict inhibition of Wnt function, as our findings indicate that Frzb-1 does not block Wnt-5A signaling.
MATERIALS AND METHODS
Constructs and Plasmids.
The bovine (B)Frzb-1 plasmid was described previously (8). Δ C-term construct (see Fig. 2A) was made by deletion of amino acids 160–316; Δ CRD, by deletion of amino acids 39–145; Δ 7C, by deletion in the CRD of amino acids 79–149; Δ 2C, by deletion of amino acids 124–149; and Δ 57–95, by deletion of amino acids 57–95, which contains the hydrophobic domain (see Fig. 2A). The pFrzb-1-FLAG was made by replacement of the last seven residues of Frzb-1 by the Flag-tag (DYKDDDDK). All constructs were subcloned into pcDNA3 (Invitrogen). The plasmids pLNCW1-hemagglutinin (HA) and pLNCW5A-HA, carrying the respective Wnt gene family members, have been reported (12).
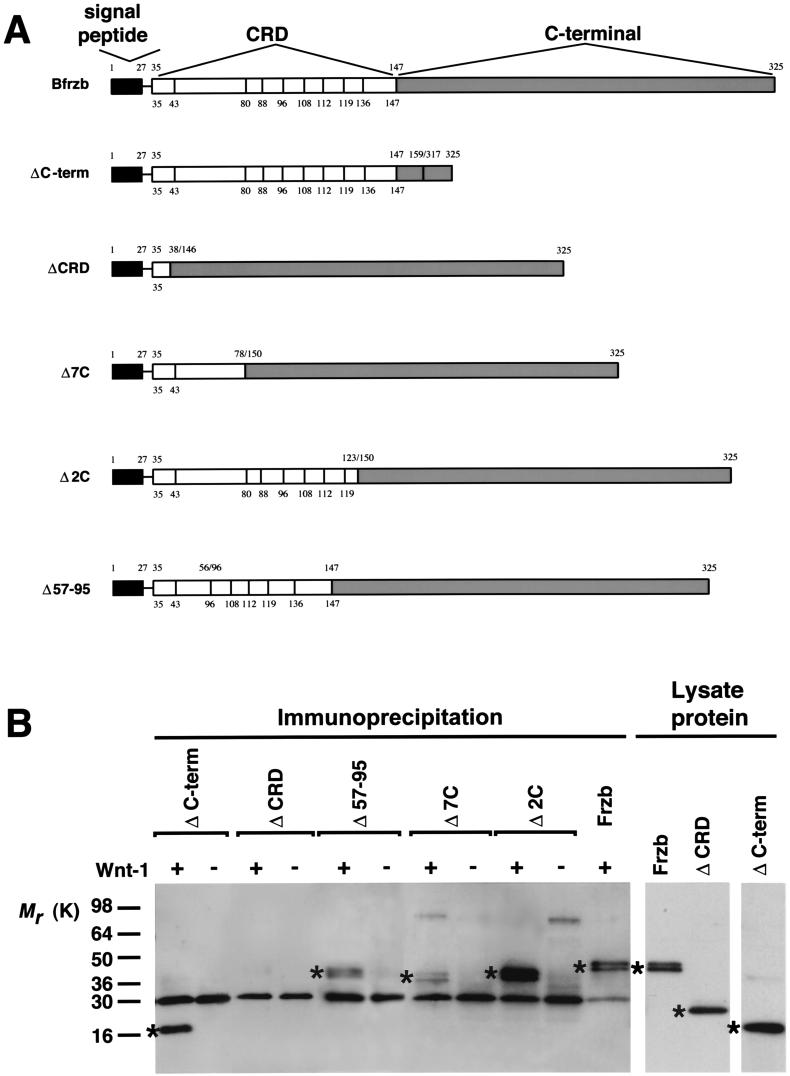
Figure 2.
Coimmunoprecipitation of Wnt-1 with Frzb-1 deletion constructs. (A) Schematic drawing of the BFrzb-1 deletion constructs (Δ). The numbers above the bars indicate the amino acid residue numbers and the junction sites (zigzag lines), the numbers below and vertical bars in the CRD indicate the position of the conserved cysteines. (B) COS7 cells were (co-)transfected with HA-tagged Wnt-1 and BFrzb-1 deletion constructs. Cell lysates were analyzed directly for protein expression by Western analysis or were immunoprecipitated with anti-HA antibody and probed with N374 PEP antiserum. Asterisks indicate detected Frzb-1 protein.
Transient Transfections.
COS7 cells (1.6 × 106 initial seeding density) were transfected either with 5 μg of plasmid DNA, or cotransfected with 4 μg for each plasmid DNA per 100-mm dish by using 30 μl LipofectAMINE reagent (Life Technologies, Gaithersburg, MD). Transfections were carried out for 6 hr in serum-free Opti-MEM I (Life Technologies). Subsequently, equal amounts of 10% fetal bovine serum in Opti-MEM I were added to the transfections and the cultures were continued for 18 hr. The cells were further incubated at 37°C for 24 hr in serum-free Opti-MEM I. The cultures were then extracted for 30 min on ice with RIPA buffer (50 mM Tris/150 mM NaCl/1.0% Nonidet P-40/0.5% deoxycholic acid/0.1% SDS). Varying amounts of plasmid were used to transfect 293 cells (ref. 13; as shown in the legend of Fig. 1). After two days, cells were lysed in an hypotonic buffer and insoluble (membrane-associated) and soluble (cytosol-associated) material was recovered by preparative ultracentrifugation at 100,000 × g. Protein contents were determined using a protein assay kit (Bio-Rad). Samples were adjusted to 1× Laemmli sample buffer before denaturation by boiling (14).
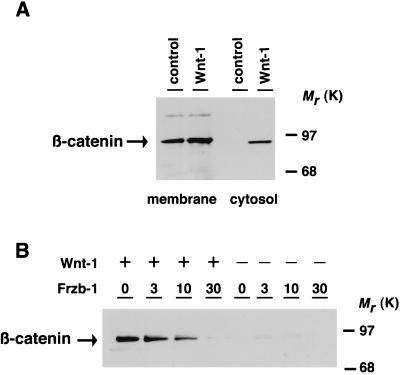
Figure 1.
Frzb-1 expression blocks wnt-1 mediated accumulation of cytosolic β-catenin. 293 cells were transiently transfected with Frzb-1 or Wnt-1 expressing plasmids. After transfection, equal amounts of denatured pellet (membrane) and supernatant (cytosol)-derived protein were electrophoresed, blotted onto nitrocellulose membranes and exposed to anti-β-catenin antibody. (A) Distribution of β-catenin in membrane and cytosolic fractions transfected by a control cytomegalovirus promoter-containing vector (control) or vector containing Wnt-1 cDNA (0.5 μg). (B) The accumulation of cytosolic β-catenin by Wnt-1 is blocked by Frzb-1 expression. Cells were transfected with variable amounts of a cytomegalovirus promoter-containing plasmid carrying the frzb-1 gene in the presence of a control expression vector containing a lac z gene (Right) or Wnt-1 gene (Left, 0.5 μg). The total amount of DNA (30 μg) transfected was kept constant by supplementation with vector DNA.
Coimmunoprecipitation.
Immunoprecipitations with HA Antiserum. Protein A-Agarose (50 μl; Boehringer Mannheim) were incubated with 100 μl of hybridoma supernatant containing anti-HA antibody 12CA5 in 450 μl of 50 mM Tris⋅HCl (pH 7.4) 150 mM NaCl by rotating overnight. Cell extracts (100–400 μl) and RIPA buffer (0–300 μl) were added to a final volume of 1 ml and the incubation was continued for another hour. The agarose beads were washed twice in 50 mM Tris⋅HCl (pH 7.4), 150 mM NaCl, twice in 50 mM Tris⋅HCl (pH 7.4), 500 mM NaCl, and once in 50 mM Tris⋅HCl (pH 7.4). After the last wash, the beads were suspended in 30–50 μl of 2× Laemmli sample buffer with 4% 2-mercaptoethanol, boiled, and separated on denaturing 4–20% gradient Tris⋅glycine gels (NOVEX, San Diego). Samples were blotted onto Immobilon-P membrane (Millipore) by using a GENIE electrophoretic blotter (Idea Scientific, Corvallis, OR). Membranes were blocked 30 min in blocking buffer consisting of 10 mM Tris (pH 7.5), 0.9% NaCl, 0.05% Tween 20, and 4% BSA. The primary antiserum (N374-PEP) (8) was incubated with the membranes in 1/10 blocking buffer and 9/10 TBST buffer (10 mM Tris, pH 7.5/0.1% Tween/150 mM NaCl) at a dilution of 1:2,500. The membranes then were washed four times for 5 min in TBST. The membranes were subsequently incubated with the secondary antibody at a dilution of 1:10,000 for 60 min. Blots were developed by using the SuperSignalCL Substrate system (Pierce) and exposed to Kodak XAR-5 film for 1–10 min.
Immunoprecipitation with Anti-FLAG M2 Antiserum.
We used 50 μl of protein G-agarose (Boehringer Mannheim) and 5 μg anti-FLAG M2 antibody (Eastman Kodak) for immunoprecipitation, 1:1,000 dilution of anti-HA-peroxidase antibody (horseradish peroxidase-HA antibody, Boehringer Mannheim) for Western blot analysis, and SuperSignal ULTRA Substrate (Pierce) for the chemiluminescent detection.
Detection of β-Catenin in Transiently Transfected 293 Cells.
Membrane or cytosol preparations were generated as described above with some modifications (M. Giarré and A.M.C. Brown, personal communication). Fifty micrograms protein from cell extracts were separated on denaturing SDS/8% polyacrylamide gels. Proteins were transferred from gels onto nitrocellulose filters by electroblotting and blocked overnight at 4°C in TBST containing 1% BSA (fraction V). Blots then were incubated in anti-mouse β-catenin mAb (Transduction Laboratories, Lexington, KY) diluted 1:4,000 in TBST, at room temperature for 2 hr. The primary antibody was then removed by washing in TBST at room temperature three times for 5 min each. Blots were exposed to a 1:5,000 dilution of horseradish peroxidase-conjugated sheep anti-mouse Ig G (Amersham). Blots were then incubated 5 min in horseradish peroxidase substrate and exposed to Kodak XAR-5 films.
Xenopus Embryo Manipulations.
Frogs and their embryos were maintained and manipulated using standard methods (15). mRNA injection experiments were performed by standard procedures as described (16). Immunoblotting experiments were done with total embryo lysates prepared by sonication (9). The Xwnt-5A assay was scored as reported previously (17).
RESULTS
Frzb-1 Blocks Wnt-1 Signaling in Mammalian Cells.
We determined whether the inhibitory effect of Frzb-1 on Wnt-1 signaling, observed initially in Xenopus embryos, also could be demonstrated in mammalian cells. It has been shown that the Wingless/Wnt-1 pathway involves posttranslational stabilization of armadillo/β-catenin, leading to its accumulation in the cytoplasm and the nucleus (18, 19). Ectopic Wnt-1 expression in human embryonic kidney cells induced the accumulation of β-catenin within the cytosol, whereas membrane-associated levels of β-catenin remained virtually unchanged (Fig. 1A). Fig. 1B shows that the induction of cytosolic β-catenin by Wnt-1 is attenuated in the presence of increasing amounts of plasmid encoding Frzb-1. Partial inhibition was observed when 3 μg Frzb-1 plasmid were transfected and complete inhibition was achieved with 30 μg. Frzb-1 expression, in the absence of Wnt-1, had no effect on the accumulation of β-catenin in the cytosol.
The CRD of Frzb-1 Is Required for Wnt-1 Binding and Inhibition of Wnt-1 Function.
The observation that the extracellular domain of Dfz2 containing the CRD confers Wg binding (6) suggested that the CRD of Frzb-1 may be sufficient for Wnt binding. We confirmed this by performing immunoprecipitation experiments with lysates of COS7 cells cotransfected with wnt-1 cDNA and several Frzb-1 deletion constructs retaining the signal peptide (Fig. 2A). Interestingly, whereas removal of the entire CRD indeed resulted in loss of coimmunoprecipitation with the Wnts (Fig. 2B), the other CRD deletions had little or no effect on the outcome of the immunoprecipitations (Fig. 2B). Analysis of the protein lysates by immunoblotting confirmed similar overall expression of the constructs (data not shown, Fig. 2B).
We further evaluated the structural requirements for inhibition of Wnt-1 signaling in vivo. Injection of Wnt-1 mRNA into Xenopus embryos results in duplication of the dorsal axis (20, 21) and can be scored easily by direct inspection (Fig. 3 A and B). Coinjection of Wnt-1 with Frzb-1 results in the complete inhibition of secondary axis formation (10). Previous reports suggested that this effect was due to blockade of Wnt signaling (9, 10). After confirming comparable protein expression levels for the constructs tested in total embryo lysates (data not shown, Fig. 3C), we compared the ability of these constructs to block Wnt signaling with that of wild-type Frzb-1. Substantial inhibition of Wnt-1 mediated axis duplication also was observed when the CRD of Frzb-1 only was coinjected with Wnt-1 (Fig. 3D). Conversely, removal of the entire CRD abolished the inhibitory activity of Frzb-1 (Fig. 3D). It is noteworthy that the C-terminal domain may play some functional role with regard to Frzb-1 activity, as inhibition appeared to be more efficient in the presence of this domain (Fig. 3D). In contrast to the coimmunoprecipitation data however, inhibition of Wnt signaling was not observed in the in vivo assay by any of the deletion constructs affecting the CRD domain (Fig. 3D). Even a limited deletion of a small domain (Δ 2C, 27 amino acids, see Materials and Methods, Fig. 3D) containing the last two of the 10 conserved cysteines, resulted in an almost complete loss of Wnt-1 inhibition. These data support the critical role of a preserved cysteine core of the CRD for inhibition of Wnt-1 activity.
Figure 3.
Inhibition of Wnt-1 induced axis duplication by Frzb-1 deletion constructs. (A and B) Induction of secondary axis by Wnt-1. Embryos injected with 150 pg preprolactin mRNA (A), or 15 pg Wnt-1 mRNA (B). (C) Immunoblot analysis of total embryo lysates coinjected with Wnt-1 (80 pg) and Frzb-1 deletion constructs (800 pg) and probed with N374-PEP antiserum; lane 1: Wnt-1; lane 2: Wnt-1+Frzb-1; lane 3: Wnt-1+FrzbΔCterm; lane 4: Wnt-1+FrzbΔCRD. The equivalent of one-half embryo was loaded per lane. Asterisks indicate immunodetected protein. (D) Embryos were coinjected with Wnt-1 (15 pg) and different constructs (150 pg each) as indicated. They were then scored for secondary axis formation. The number between brackets indicates the number of embryos injected.
Specifity of Frzb-1/Wnt Interactions.
To further investigate the specificity of Frzb-1/Wnt interactions, COS7 cells were cotransfected with Frzb-1 and Wnt-5A. Wnt-1 was used as a positive control (9). Frzb-1 coimmunoprecipitated with Wnt-5A (Fig. 4A), conversely, using a Flag-tagged Frzb-1 protein, Wnt-5A coimmunoprecipitated with Frzb-1 (Fig. 4B). These findings demonstrate that Frzb-1 has sufficient affinity to allow coimmunoprecipitation with both Wnt-1 and Wnt-5A. Lack of soluble Wnt proteins precluded classical binding studies. However, variation of the washing conditions after immunoprecipitation did not result in any noticable differences in the association between Frzb-1 and Wnt-1 or Wnt-5A (data not shown). We next analyzed whether Frzb-1 functioned as an antagonist to Wnt-5A signaling for which a Xenopus assay has been described (17). Overexpression of Wnt-5A in Xenopus embryos produces a characteristic phenotype with head and/or tail malformations (17). Coinjections of Wnt-5A (5 pg) with preprolactin (100 pg) resulted in ≈75% of the injected embryos, developing in an abnormal phenotype (two independent experiments, 40/53 and 27/36), and coinjection with Frzb-1 (100 pg) showed similar results (34/34 and 30/36). Therefore Frzb-1 does not appear to suppress the Wnt-5A induced phenotypic changes, and may even enhance the development of the head and/or tail abnormalities. This finding is consistent with recent reports that a dominant negative Xwnt-8 is also unable to block Wnt-5A activity in the same model (22).
Figure 4.
Frzb-1/Wnt coimmunoprecipitation experiments. COS7 cells were (co-)transfected with HA-tagged Wnt-1 and Wnt-5A and Frzb-1 (Flag-tagged) as indicated. The cell lysates were immunoprecipitated (see Materials and Methods) with anti-HA antibody (A) or anti-FLAG M2 antibody (B), and probed by immunoblot with N374-PEP antiserum (A) or horseradish peroxidase-HA antibody (B).
DISCUSSION
This report provides evidence that Frzb-1 can block the Wnt signaling cascade in a mammalian cell line, as measured by the inhibition of the Wnt-1 induced cytosolic accumulation of β-catenin. We and others demonstrated that Fzb-1 blocks Wnt-1 and Xwnt-8 signaling in Xenopus embryos, as assessed by its inhibition of Wnt-1/Xwnt-8 axis duplication or the induction of Xwnt-8 response genes Xnr3 and Siamois in animal cap assays (9, 10). These are relatively late read-out systems in the Wnt signaling pathway. β-catenin, an earlier component in the Wnt signaling cascade, has been implicated in the development of either colon cancer or melanomas (3–5). Therefore it is possible that Frzb-1 potentially could act as a tumor suppressor by inhibiting Wnt-mediated cell proliferation. Based on its mapping to chromosome 2q31–33, it previously was suggested that Frzb-1 may be a tumor suppressor gene (10). Loss of one copy of the 2q arm has indeed been associated with a high incidence of lung carcinomas, colorectal carcinomas, and neuroblastomas (23, 24).
Our deletion studies showed that the CRD of Frzb-1 is required and sufficient for its interaction with the Wnt proteins. Functionally all the deletion constructs in the CRD resulted in the loss of the inhibitory activity of Fzb-1 using the Wnt-1-induced axis duplication assay in Xenopus embryos. Our coimmunoprecipitation experiments did not reveal a specific motif to be critical for the Frzb-1/Wnt interaction. Disruption of the cysteine core and/or removal of the hydrophobic domain within the CRD (57–95), potentially important for the Frzb-1/Wnt interaction, did not affect the outcome of the immunoprecipitation experiments significantly. Our functional data suggest that the affinity of the interactions between Frzb-1 and Wnts may be critical for modulation of Wnt signaling. However, immunoprecipitations are not quantitative and therefore do not provide information on binding affinity. Classical quantitative binding studies cannot be performed to address this point, because soluble Wnt proteins are not available.
Although not required for binding and inhibition of Wnt signaling, the C terminus may play a role in the stabilization of the tertiary structure of Frzb, in the binding affinity to the Wnts, in Frzb-1 turnover, or solubility of the protein.
Frzb-1 belongs to a novel family of secreted proteins containing a cysteine-rich N-terminal domain highly similar to the ligand binding domain of frizzled proteins. Indeed, other secreted proteins containing frizzled-like CRDs have been reported (25, 26, 27). It is of note that each of these has a lower degree of amino acid sequence identity to the frizzleds than does Frzb-1. Nevertheless, the data indicate that besides Frzb-1, another secreted frizzled-like protein, sFRP-2, also confers binding of Wg in transfected 293 cells (26). Because sequence alignment between the CRD of sFRP-2 and Frzb-1 reveals an identity of only about 25%, it is likely that several frizzled-like proteins will interact with the Wnts. However, as demonstrated by the lack of inhibition of Wnt-5A signaling by Frzb-1, it remains to be determined what the functional consequences of this interaction might be. In this regard it cannot be excluded that Frzb-1 and related proteins have additional yet undiscovered regulatory functions, independent of the Wnt signaling pathway.
Acknowledgments
We thank M. Kuehn, N. Ryba, J. T. Thomas, and R. Siraganian for their critical comments. This work was supported by grants to J.K. from the American Cancer Society (DB-81) and from the U.S. Army Medical Research and Material Command (DAMD17-94-J-4069).
Note Added in Proof
While this manuscript was being reviewed, another frizzled-related protein was reported to antagonize the actions of Wnt-1 and Wnt-8 in a Xenopus axis duplication assay (28).
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: CRD, cysteine-rich domain; HA, hemagglutinin.
References
- 1.Nusse R, Varmus H E. Cell. 1992;69:1073–1087. doi: 10.1016/0092-8674(92)90630-u. [DOI] [PubMed] [Google Scholar]
- 2.Parr B A, McMahon A P. Curr Biol. 1994;4:523–528. doi: 10.1016/0959-437x(94)90067-d. [DOI] [PubMed] [Google Scholar]
- 3.Korinek V, Barker N, Morin P J, van Wichen D, de Weger R, Kinzler K W, Vogelstein B, Clevers H. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 4.Morin P J, Sparks A B, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler K W. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 5.Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P. Science. 1997;275:1790–1792. doi: 10.1126/science.275.5307.1790. [DOI] [PubMed] [Google Scholar]
- 6.Bhanot P, Brink M, Samos C H, Hsieh J C, Wang Y, Macke J P, Andrew D, Nathans J, Nusse R. Nature (London) 1996;382:225–230. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- 7.Yang-Snyder J, Miller J R, Brown J D, Lai C J, Moon R T. Curr Biol. 1996;6:1302–1306. doi: 10.1016/s0960-9822(02)70716-1. [DOI] [PubMed] [Google Scholar]
- 8.Hoang B, Moos M, Jr, Vukicevic S, Luyten F P. J Biol Chem. 1996;271:26131–26137. doi: 10.1074/jbc.271.42.26131. [DOI] [PubMed] [Google Scholar]
- 9.Wang S, Krinks M, Lin K, Luyten F P, Moos M., Jr Cell. 1997;88:757–766. doi: 10.1016/s0092-8674(00)81922-4. [DOI] [PubMed] [Google Scholar]
- 10.Leyns L, Bouwmeester T, Kim S-H, Piccolo S, De Robertis E M. Cell. 1997;88:747–756. doi: 10.1016/s0092-8674(00)81921-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moon R T, Brown J D, Yang-Snyder J A, Miller J. Cell. 1997;88:725–728. doi: 10.1016/s0092-8674(00)81915-7. [DOI] [PubMed] [Google Scholar]
- 12.Munsterberg A E, Kitajewski J, Bumcrot D A, McMahon A P, Lassar A B. Genes Dev. 1995;9:2911–2922. doi: 10.1101/gad.9.23.2911. [DOI] [PubMed] [Google Scholar]
- 13.Pear W S, Nolan G P, Scott M L, Baltimore D. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Gurdon J B. Methods Cell Biol. 1977;16:125–139. doi: 10.1016/s0091-679x(08)60096-5. [DOI] [PubMed] [Google Scholar]
- 16.Moos M, Jr, Wang S, Krinks M. Development (Cambridge, UK) 1995;121:4293–4301. doi: 10.1242/dev.121.12.4293. [DOI] [PubMed] [Google Scholar]
- 17.Moon R T, Campbell R M, Christian J L, McGrew L L, Shih J, Fraser S. Development (Cambridge, UK) 1993;119:97–111. doi: 10.1242/dev.119.1.97. [DOI] [PubMed] [Google Scholar]
- 18.Peifer M, Sweeton D, Casey M, Wieschaus E. Development (Cambridge, UK) 1994;120:369–380. doi: 10.1242/dev.120.2.369. [DOI] [PubMed] [Google Scholar]
- 19.Yost C, Torres M, Miller J R, Huang E, Kimelman D, Moon R T. Genes Dev. 1996;10:1443–1454. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- 20.Smith W C, Harland R M. Cell. 1991;67:753–765. doi: 10.1016/0092-8674(91)90070-f. [DOI] [PubMed] [Google Scholar]
- 21.Sokol S, Christian J L, Moon R T, Melton D A. Cell. 1991;67:741–752. doi: 10.1016/0092-8674(91)90069-b. [DOI] [PubMed] [Google Scholar]
- 22.Hoppler S, Brown J D, Moon R T. Genes Dev. 1996;10:2805–2817. doi: 10.1101/gad.10.21.2805. [DOI] [PubMed] [Google Scholar]
- 23.Tsuchiya E, Nakamura Y, Weng S Y, Nakagawa K, Tsuchiya S, Sugano H, Kitagawa T. Cancer Res. 1992;52:2478–2481. [PubMed] [Google Scholar]
- 24.Kohno T, Morishita K, Takano H, Shapiro D N, Yokota J. Oncogene. 1994;9:103–108. [PubMed] [Google Scholar]
- 25.Shirozu M, Tada H, Tashiro K, Nakamura T, Lopez N D, Nazarea M, Hamada T, Sato T, Nakano T, Honjo T. Genomics. 1996;37:273–280. doi: 10.1006/geno.1996.0560. [DOI] [PubMed] [Google Scholar]
- 26.Rattner A, Hsieh J-H, Smallwood P M, Gilbert D J, Copeland N G, Jenkins N A, Nathans J. Proc Natl Acad Sci USA. 1997;94:2859–2863. doi: 10.1073/pnas.94.7.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rehn M, Pihlajaniemi T. J Biol Chem. 1995;270:4705–4711. doi: 10.1074/jbc.270.9.4705. [DOI] [PubMed] [Google Scholar]
- 28.Finch P W, He X, Kelley M J, Uren A, Schaudies R P, Popescu N C, Rudikoff S, Aaronson S A, Varmus H E, Rubin J. Proc Natl Acad Sci USA. 1997;94:6770–6775. doi: 10.1073/pnas.94.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]