Abstract
The incorporation of [1-13C]- and [2,3,4,5-13C4]1-deoxy-d-xylulose into β-carotene, lutein, phytol, and sitosterol in a cell culture of Catharanthus roseus was analyzed by NMR spectroscopy. The labeling patterns of the isoprene precursors, isopentenyl pyrophosphate and dimethylallyl pyrophosphate, were obtained from the terpenes by a retrobiosynthetic approach. 13C Enrichment and 13C13C coupling patterns showed conclusively that 1-deoxy-d-xylulose and not mevalonate is the predominant isoprenoid precursor of phytol, β-carotene, and lutein. Label from 1-deoxyxylulose was also diverted to phytosterols to a minor extent (6% relative to carotene and phytol formation). The data demonstrate that the formation of isopentenyl pyrophosphate from pentulose occurs strictly by an intramolecular rearrangement process.
Keywords: carotenoids, phytol, sitosterol, NMR spectroscopy, Catharanthus
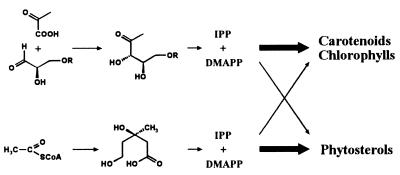
Whereas the mevalonate pathway to terpenoids has been studied in considerable detail (for reviews see refs. 1 and 2), it has been observed on several occasions that angiosperms incorporate little, if any, [14C]mevalonate into β-carotene and the phytol side chain of chlorophyll as well as into some other terpenoids, and the possibility that different pathways of terpenoid biosynthesis might be operative even in different compartments of plant cells has been considered (3–5).
Further evidence for an alternative, nonmevalonoid pathway of terpenoid biosynthesis was obtained by Arigoni and coworkers (6), who observed that Menyanthes trifoliata incorporates [2-14C]mevalonate into triterpenes and sterols, but not into the monoterpene glycoside, loganin. Additional observations that could not be accommodated within the framework of the mevalonate pathway have been reported by several research groups. Various interpretations, such as compartmentalization of nonexchanging acetate pools as well as terpene biosynthesis pathways via valine or leucine as precursors, have been proposed (7–11).
More recent studies by Arigoni and his research group established 1-deoxy-d-xylulose (hereafter designated deoxyxylulose) as the committed precursor of the alternative pathway in plants and bacteria (12, 13, §). The carbohydrate was incorporated into menaquinone with high efficacy by Escherichia coli and was also shown to serve as precursor for ginkgolides in Ginkgo biloba, whereas steroids were biosynthesized in the same plant via the mevalonate pathway. Deoxyxylulose had been isolated earlier from Streptomyces hygroscopicus (14, 15) and had been shown to serve as a biosynthetic precursor for thiamin (16, 17) and pyridoxal (18, 19).
Labeling data of the ginkgolides and of the E. coli terpenoids obtained in vivo from labeled glucose could be explained by formation of the deoxypentulose (or its 5-phosphate) from glyceraldehyde and active acetaldehyde generated in the thiamin pyrophosphate catalyzed decarboxylation of pyruvate (12, 13, §). The labeling patterns were identical with those detected independently by Rohmer, Sahm, and coworkers for the formation of hopanoids and other terpenes in various bacteria (20, 21).
Meanwhile, it has been shown that the deoxyxylulose pathway is also involved in higher plants in the biosynthesis of diterpenes in Salvia miltiorrhiza (A. Cartayrade, M. K. Schwarz, and D.A., unpublished data), of taxoids in Taxus chinensis (22), of essential oils in Mentha piperita (23), of chlorophyll and carotenoids in Lemna gibba, Hordeum vulgare, Daucus carota (24), and Scenedesmus obliquus (25), and of isoprene in higher plants (26).
This paper describes studies with single and multiple 13C-labeled deoxyxylulose using a photosynthetically active culture of Catharanthus roseus. The data show that the pentulose is efficiently incorporated into carotenoids and phytol, and that the pathway involves a skeletal rearrangement rather than a fragmentation/reassociation process.
EXPERIMENTAL PROCEDURES
Plant Material.
Photomixotrophic suspension cultures of C. roseus were maintained for more than 10 years on Linsmaier and Skoog medium (27) containing 3% glucose instead of sucrose. The cells were grown under continuous light conditions (2,400 lx) on a rotary shaker (100 rpm) at 28°C. Ten 1-liter flasks, each containing 250 ml of medium, 11 mg of labeled deoxyxylulose, and 2.5 g (dry weight) of C. roseus cells were incubated for 7 days.
Preparation of Labeled Precursors.
[U-13C6]Glucose, [2-13C]pyruvate, and [3-13C]pyruvate (99% 13C abundance) were purchased from Isotec. [U-13C3]Glyceraldehyde was prepared from [U-13C6]glucose (28). [1-13C]1-Deoxy-d-xylulose was prepared by enzymatic condensation of [3-13C]pyruvate with unlabeled d-glyceraldehyde (29, 30). [2,3,4,5-13C4]Deoxyxylulose was prepared similarly from [2-13C]pyruvate and [U-13C3]d-glyceraldehyde. The following 13C NMR data were obtained for [2,3,4,5-13C4]deoxyxylulose in dimethyl sulfoxide-d6: C-1, 26.7 ppm; C-2, 211.6 ppm (13C coupling to C-3, 42.3 Hz; 13C coupling to C-5, 2.4 Hz); C-3, 77.0 ppm (13C coupling to C-4, 39.8 Hz); C-4, 72.5 ppm (13C coupling to C-5, 42.5 Hz); C-5, 61.9 ppm.
Isolation of Terpenoids.
C. roseus cells (1 kg wet weight) were harvested, frozen, thawed, and extracted exhaustively with acetone and a mixture of acetone/hexane (1:1 vol/vol). The extract was reduced to a volume of 200 ml, and the resulting two-phase mixture was extracted with hexane and chloroform. This final organic phase was then evaporated to a volume of 5 ml under reduced pressure. The solution was applied to a column of silica gel 60 (220–440 mesh; column size, 40 × 6 cm), which was subsequently developed with a mixture of hexane/ethyl acetate (1:1 vol/vol). Fractions containing β-carotene, lutein, chlorophyll, and phytosterols, respectively, were combined and taken to dryness.
The crude carotene fraction was dissolved in a mixture of hexane/chloroform (5:1 vol/vol) and chromatographed on a column of silica gel 60 (column size, 30 × 3 cm) using the same solvent mixture as eluent. The fraction containing β-carotene was collected and taken to dryness (yield, 3 mg).
The crude lutein fraction was chromatographed on a column of silica gel 60 (column size, 30 × 3 cm) using ligroin/acetone/chloroform (5:5:4 vol/vol) as eluent. The lutein fractions were combined and taken to dryness (yield, 7 mg).
The fractions containing chlorophyll a, chlorophyll b, and phytosterols were combined. The solution was taken to incipient dryness. The residue was boiled under reflux in methanol containing 3% KOH for 30 min. The reaction mixture was extracted with petrol ether (40–60°C). The petrol ether phase was washed to neutrality with water, dried over sodium sulfate, taken to dryness, and applied to a column of silica gel 60 (column size, 24 × 3 cm). The column was developed with a mixture of CH2Cl2/ethyl acetate (9:1 vol/vol). Fractions containing phytosterols were combined and taken to dryness (yield, 95 mg). The phytol fraction was further resolved on a second column of silica gel 60 (column size, 15 × 2.5 cm) using hexane/ethyl acetate (85:15 vol/vol) as eluent. The phytol fractions were combined and taken to dryness (yield, 38 mg).
NMR Spectroscopy.
1H and 13C NMR spectra of phytol, β-carotene, lutein, and sitosterol were recorded in CDCl3 using a Bruker DRX 500 spectrometer equipped with an ASPECT station. 13C NMR spectra were measured as follows: 45° pulse (3 μs); repetition time, 3.2 s; spectral width, 29 kHz; data set, 64 kilo-words; temperature, 17°C; zero-filling to 128 kilo-words before to Fourier transformation; Gaussian apodization; 1H decoupling by WALTZ 16 during acquisition and relaxation. Two-dimensional experiments and data processing routines were performed according to standard Bruker software (xwinnmr 1.1).
13C signal assignments were confirmed by two-dimensional INADEQUATE experiments performed with metabolites obtained from the incorporation experiment with [2,3,4,5-13C4]deoxyxylulose. 13C abundance in terpenoids was analyzed by quantitative NMR spectroscopy. 1H-decoupled 13C NMR spectra of samples from incorporation experiments and of samples with natural 13C abundance (1.1% 13C) were recorded under identical conditions. Relative 13C abundance of individual carbon atoms was then calculated by comparison of 13C signal integrals between 13C-labeled and unlabeled material. To determine absolute 13C abundance, 13C-coupled satellite signals in 1H NMR spectra were analyzed. The absolute 13C enrichment was then used to standardize the relative 13C abundance of all carbon atoms. The fraction of multiply labeled isotopomers was calculated from 1H-decoupled 13C NMR spectra as the fraction of 13C13C-coupled satellites in the global 13C signal of the respective carbon atom.
RESULTS
A cell culture of C. roseus was grown in presence of 0.3 mM [1-13C]- or [2,3,4,5-13C4]deoxyxylulose. The growth rate and the biosynthesis of terpenoids were not affected by the deoxyxylulose supplement. β-Carotene, lutein, and sitosterol were isolated as described and were analyzed by one- and two-dimensional NMR spectroscopy. Chlorophyll a and b were hydrolyzed to yield trans-phytol for NMR analysis.
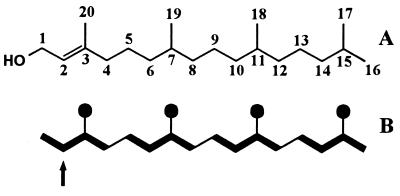
The 13C abundance of individual carbon atoms in the terpenoid metabolites was determined as described. In the experiment with [1-13C]deoxyxylulose, the methyl carbons C-17, C-18, C-19, and C-20 of trans-phytol showed high 13C enrichment (Table 1; average 13C abundance, 17.7 ± 1.2%; labeled positions shown as solid circles in Fig. 1), whereas the other atoms were virtually not labeled (average 13C abundance, 1.2 ± 0.2%).
Table 1.
13C NMR analysis of trans-phytol from a cell culture of C. roseus supplied with [1-13C]deoxyxylulose or [2,3,4,5-13C4]deoxyxylulose
| Position* | Chemical shift, ppm | Proffered deoxyxylulose
|
|||
|---|---|---|---|---|---|
| [1-13C] | [2,3,4,5-13C4]
|
||||
| % 13C | % 13C | % 13C13C | JCC, Hz† | ||
| 3 | 140.23 | 1.11 | 9.83 | 90.2 | 72.7 (2), 41.8 (4), 1.3 (1) |
| 2 | 123.09 | 1.11 | 8.85 | 88.8 | 72.7 (3), 47.3 (1), 2.4 (4) |
| 1 | 59.39 | 1.07 | 8.58 | 88.7 | 47.3 (1), 1.3 (3), 4.7 (4) |
| 4 | 39.85 | 1.63 | 8.31 | 87.2 | 41.8 (3), 2.4 (2), 4.7 (1) |
| 14 | 39.35 | 1.13 | 8.79 | 82.2 | 34.7 (13, 15) |
| 10 | 37.41 | 1.14 | 8.56‡ | ND | 34.5 (9, 11) |
| 8 | 37.35 | ND | 8.56‡ | ND | 34.7 (7), 2.8 (5) |
| 12 | 37.28 | 1.54 | 8.56‡ | ND | 34.7 (11), 2.9 (9) |
| 6 | 36.65 | 1.13 | 8.94 | 88.0 | 34.8 (5, 7) |
| 11 | 32.77 | 1.10 | 8.67‡ | 86.8 | 34.7 (10, 12) |
| 7 | 32.67 | 1.12 | 8.67‡ | 87.4 | 34.7 (6, 8) |
| 15 | 27.95 | 1.32 | 9.14 | 87.1 | 34.7 (14, 16) |
| 5 | 25.12 | 1.09 | 8.82 | ND | 34.7 (6), 2.6 |
| 13 | 24.78 | 1.04 | 8.54 | ND | 34.7 (14), 2.8 (16) |
| 9 | 24.45 | 1.09 | 8.26 | ND | 34.5 (10), 2.8 (12) |
| 17 | 22.69 | 16.44 | ND | ND | |
| 16 | 22.60 | 1.73 | ND | 86.3 | 34.9 (15), 2.6 (13) |
| 19§ | 19.72 | 17.94 | 1.39‡ | ND | |
| 18§ | 19.69 | 17.25 | 1.39‡ | 26.9 | |
| 20 | 16.14 | 19.29 | 0.91 | ND | |
ND, not determined due to signal overlapping.
Assignments are based on ref. 31 and were confirmed by INADEQUATE experiments.
13C13C coupling constants obtained from one-dimensional proton-decoupled 13C NMR spectra. Carbon atoms coupled to the respective index carbon are in parentheses and were determined by INADEQUATE spectroscopy.
Averaged intensities due to signal overlapping.
Assignments may be interchanged.
Figure 1.
Structure (A) and 13C labeling pattern (B) of trans-phytol obtained by saponification of chlorophyll. Solid circles represent carbon atoms that acquired label from [1-13C]deoxyxylulose. Bold lines connect carbon atoms that were transferred from the same molecule of [2,3,4,5-13C4]deoxyxylulose. Arrow indicates the carbon atom documented by the NMR pattern in Fig. 3A.
It is well established that phytol is formed from isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) via geranylgeranyl pyrophosphate. Each of the labeled atom positions C-17, C-18, C-19, and C-20 is biosynthetically equivalent to C-5 of IPP or DMAPP. It follows that C-1 of deoxyxylulose is specifically incorporated into C-5 of IPP and DMAPP, respectively. It should be noted that the carbon atoms C-4, C-12, and C-16 show an average 13C abundance of 1.6 ± 0.1%, well above the average abundance of the other “unlabeled” carbon atoms (1.1 ± 0.1%). For the corresponding C-8, the signal could not be evaluated. The three carbon atoms with slightly elevated 13C abundance are biosynthetically equivalent to C-4 of IPP/DMAPP. The increased 13C abundance of these carbon atoms reflects the somewhat imperfect stereocontrol of the enzyme, IPP/DMAPP isomerase.
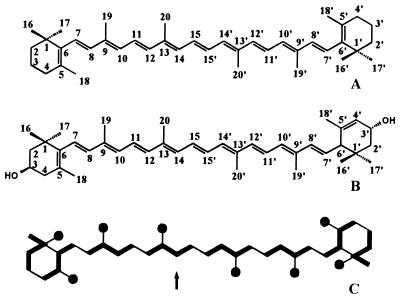
Label from [1-13C]deoxyxylulose was also specifically incorporated into all but two methyl positions of β-carotene (average 13C abundance of labeled positions, 16.3 ± 0.3%) and lutein (average 13C abundance of labeled positions, 17.2 ± 1.8%). The labeled methyl groups are shown in Fig. 2C as solid circles. The labeled positions were again biosynthetically equivalent to C-5 of IPP and DMAPP. In light of the relatively low concentration of deoxyxylulose in the culture medium (0.3 mM), the high 13C enrichment values in trans-phytol and carotenoids suggest that deoxyxylulose is a precursor with close metabolic proximity to IPP and DMAPP.
Figure 2.
Structure of β-carotene (A) and lutein (B). The same labeling pattern (C) was obtained for both compounds. Arrow indicates the carbon atom documented by the NMR patterns in Fig. 3 B and C. For additional details see the legend to Fig. 1.
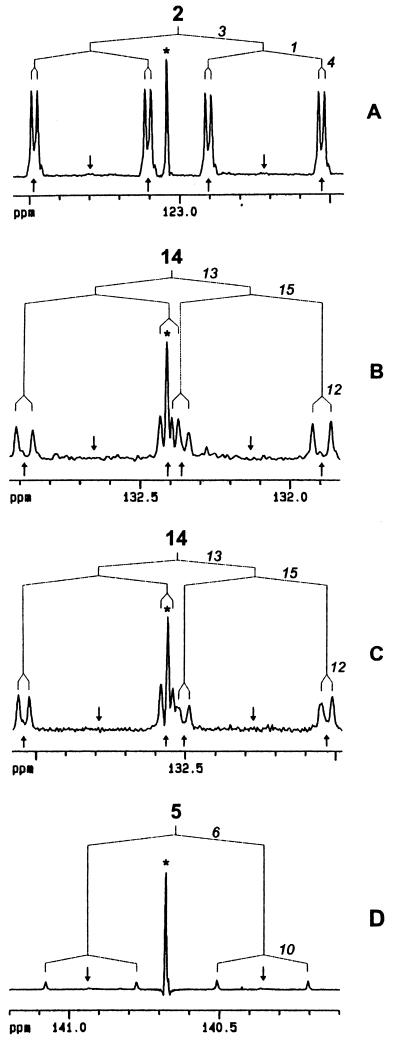
In the experiment with [2,3,4,5-13C4]deoxyxylulose, 13C label was diverted to all positions of phytol (Table 1) and carotenoids (data not shown), with the exception of those that were labeled in the experiment with [1-13C]deoxyxylulose (Figs. 1 and 2). The average 13C enrichment of the labeled positions was 8.7 ± 0.4% in trans-phytol, 7.1 ± 0.7% 13C in β-carotene, and 7.0 ± 0.7% 13C in lutein. As shown in Fig. 3, 13C signals of trans-phytol, β-carotene, and lutein were characterized by intense 13C-coupled satellites (approximately 85% of signal intensity relative to the overall 13C signal intensity). As shown below, these satellite signals arise by 13C13C coupling resulting from joint incorporation of 13C atoms from [2,3,4,5-13C4]deoxyxylulose into C-1, C-2, C-3, and C-4 of IPP and DMAPP. The uncoupled central signals marked by asterisks in Fig. 3 represent isotopomers with a single 13C atom, which were formed from the natural 13C abundance glucose in the culture medium.
Figure 3.
NMR patterns of metabolites derived from [2,3,4,5-13C4]deoxyxylulose. (A) C-2 of phytol. (B) C-14 of β-carotene. (C) C-14 of lutein. (D) C-5 of sitosterol. Signals of natural 13C abundance isotopomers biosynthesized from proffered glucose are marked by asterisks. 13C coupling patterns are indicated. Coupled carbon atoms are shown in italics. Arrows indicate the predicted signal position of isotopomers with fewer than four contiguous 13C atoms per biosynthetic isoprenoid intermediate.
The complex satellites were analyzed by deconvolution of the multiplets using 13C13C coupling constants that had been unequivocally established by two-dimensional INADEQUATE spectroscopy using the metabolites from the [2,3,4,5-13C4]deoxyxylulose experiment. As shown in Fig. 3A, the NMR signature of C-2 of phytol (arrow in Fig. 1B) consists of nine lines. This carbon atom is biosynthetically equivalent to C-2 of the terpenoid precursors. The satellite signals arise by one bond coupling of the observed carbon to two adjacent carbon atoms (coupling constants of 72.7 and 47.3 Hz, respectively, Table 1) and by long-range 13C13C coupling via two bonds (2.4 Hz). The two-dimensional INADEQUATE (not shown) reveals unequivocally that the long-range coupling is to carbon 4. All four contiguous carbon atoms are part of one isoprenoid moiety (Fig. 1). The coupled signals are not arranged symmetrically with respect to the signal from the natural abundance material marked by an asterisk in Fig. 3A. The substantial upfield shift (4.6 Hz) of the coupled signals is caused by heavy isotope shifts of the neighboring 13C atoms (α shifts by carbons 3 and 1 and β shift by carbon 4). It should be noted that only two isotopomers are observed in Fig. 3A for the segment under investigation, namely [2-13C1]phytol (natural abundance metabolite from unlabeled glucose) and [1,2,3,4-13C4]phytol. Other isotopomers, if present, would have yielded additional signals at the positions marked by arrows in Fig. 3A.
The arguments used for interpretation of Fig. 3A can likewise be applied for the interpretation of Figs. 3 B and C. The carbon atoms under specific investigation in carotene and lutein correspond to the one marked by an arrow in Fig. 2C. Each of these carbon atoms is biosynthetically equivalent to C-2 of IPP/DMAPP. The observed carotenoid carbons in Figs. 3 B and C show coupling to three other 13C atoms, which are all biosynthetically derived from the same isoprenoid precursor molecule. In close agreement with the observations on phytol, the data confirm that only the [1,2,3,4-13C4]isotopomers of DMAPP and IPP have been formed biosynthetically from the proffered [2,3,4,5-13C4]deoxypentulose.
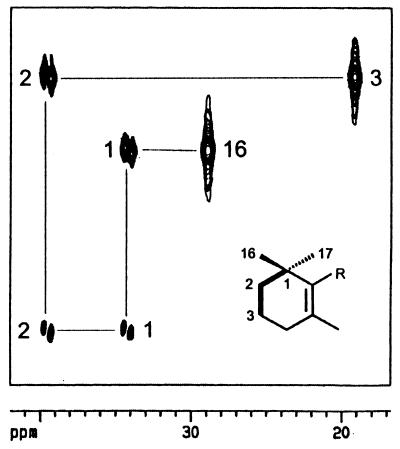
An isoprenoid unit of β-carotene is also shown in the two-dimensional INADEQUATE spectrum in Fig. 4. Carbon atoms 16, 1, 2, and 3 are shown to be connected via single bond 13C13C coupling. The signals for C-1 and C-2 appear as doublets as a consequence of their simultaneous coupling to two adjacent 13C atoms via single bonds (coupling constants, approximately 35 Hz).
Figure 4.
Section of INADEQUATE spectrum of β-carotene biosynthesized from [2,3,4,5-13C4]deoxyxylulose. (Inset) The relevant part of the β-carotene structure. Contiguous 13C atoms are connected by bold lines.
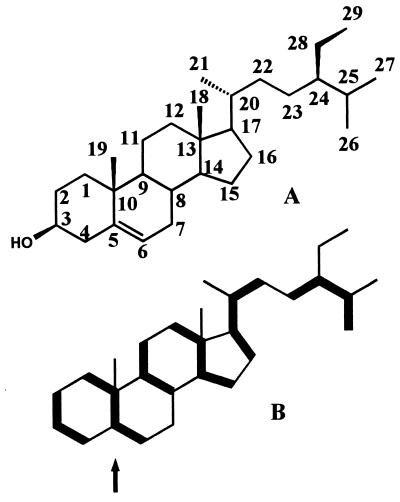
According to the data in Fig. 3D and Table 2, [2,3,4,5-13C4]deoxyxylulose was also incorporated into sitosterol as indicated in Fig. 5. The 13C signal of C-5 of sitosterol, which is biosynthetically equivalent to C-2 of IPP/DMAPP, comprises four satellite signals, whereas the carbon atoms in phytol (Fig. 3A) and carotenoids (Fig. 3 B and C), which are biosynthetically equivalent to C-2 of IPP/DMAPP, comprise eight satellite lines. The long-range couplings observed in Fig. 3 A–C are transferred via sp2 carbon atoms conducing to relatively large two-bond coupling constants. On the other hand, the C-5 of sitosterol is connected to C-1 via single bonds resulting in long-range coupling constants of less than 0.5 Hz, which cannot be resolved experimentally. However, it is obvious from the analysis of the other signals that each IPP/DMAPP unit contributed from [2,3,4,5-13C4]deoxyxylulose carried four contiguous 13C atoms (Table 2).
Table 2.
13C NMR analysis of sitosterol from a cell culture of C. roseus supplied with [1-13C] deoxyxylulose or [2,3,4,5-13C]deoxyxylulose
| Position* | Chemical shift, ppm | Proffered deoxyxylulose
|
|||
|---|---|---|---|---|---|
| [1-13C] | [2,3,4,5-13C4]
|
||||
| % 13C | % 13C | % 13C13C | JCC, Hz† | ||
| 5 | 140.69 | 1.14 | 1.74 | 29.0 | 71.6 (6), 38.3 (10) |
| 6 | 121.72 | 1.22 | 1.74 | 34.7 | 71.8 (5), 1.5 (6) |
| 3 | 71.77 | 1.24 | 1.87 | 38.6 | 36.2 (2, 4) |
| 14 | 56.70 | 1.10 | 1.26 | 16.3 | 32.3 (15, 13) |
| 17 | 55.95 | ND | 1.97 | ND | ND |
| 9 | 50.03 | 1.22 | 1.26 | ND | ND |
| 24 | 45.73 | ND | 1.41 | 37.3 | 34.8 (23, 25) |
| 13 | 42.26 | 1.19‡ | 1.69‡ | ND | ND |
| 4 | 42.23 | 1.19‡ | 1.69‡ | ND | ND |
| 12 | 39.70 | 1.16 | 1.28 | 14.1 | 35.6 (13) |
| 1 | 37.18 | 1.39 | 1.69 | ND | 34.1 (10), 1.8 (6) |
| 10 | 36.45 | 1.11 | 2.08 | ND | ND |
| 20 | 36.11 | ND | 0.88 | ND | ND |
| 22 | 33.85 | ND | 0.60 | 29.8 | 34.1 (20) |
| 7 | 31.87 | 1.42 | 1.38‡ | ND | ND |
| 8 | 31.83 | 1.32 | 1.38‡ | ND | ND |
| 2 | 31.60 | 1.28 | 1.27 | ND | ND |
| 25 | 29.01 | ND | 0.73 | ND | ND |
| 16 | 28.23 | ND | 1.41 | ND | 33.6 (17) |
| 23 | 25.90 | ND | 0.67 | 34.9 | 34.9 (24), 1.3 (26) |
| 15 | 24.27 | 1.22 | 1.35 | 16.8 | 33.3 (14) |
| 28 | 22.98 | ND | 0.45 | ND | ND |
| 11 | 21.03 | 1.29 | 1.60 | 16.0 | 34.7 (9) |
| 26 | 19.82 | ND | 0.67 | 38.5 | 35.4 (25), 1.4 (23) |
| 19 | 19.38 | ND | 1.10 | 4.9 | 35.2 (10) |
| 27 | 18.97 | 1.38 | ND | ND | ND |
| 21 | 18.74 | 1.49 | ND | ND | ND |
| 29 | 11.95 | ND | ND | ND | ND |
| 18 | 11.83 | 1.49 | 1.10 | ND | ND |
ND, not determined due to signal overlapping.
13C13C coupling constants obtained from one-dimensional proton-decoupled 13C NMR spectra. Carbon atoms coupled to the respective index carbon are in parentheses and were determined by INADEQUATE spectroscopy.
Averaged intensities due to signal overlapping.
Figure 5.
Structure (A) and 13C labeling pattern (B) of sitosterol. For details see Fig. 1. Arrow indicates the position documented by the NMR spectrum in Fig. 3C.
As determined from the intensities of the satellite lines relative to the uncoupled natural abundance signal (marked by asterisks in Fig. 3D), the diversion of deoxyxylulose to sitosterol was approximately 15 times less efficient than in the case of phytol and carotenoids. A similar dilution of the isotopic label was also detected in the experiment with [1-13C]deoxyxylulose where the maximum 13C abundance observed was at best 1.5%.
In summary, a total of 22 spectroscopically nonequivalent isoprenoid units could be analyzed by the detailed study of four metabolites yielding highly redundant information on the labeling patterns of the isoprenoid intermediates, IPP and DMAPP. These data show unequivocally that only one respective isotopomer of IPP and DMAPP had been formed from [2,3,4,5-13C4]deoxyxylulose, namely [1,2,3,4-13C4]DMAPP and [1,2,3,4-13C4]IPP. These labeled precursors were diverted with high and closely similar efficacy to carotene, lutein, and phytol. They were also diverted to sitosterol, but with 15 times lower efficacy.
DISCUSSION
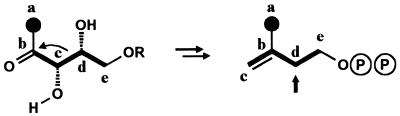
The combined labeling data from studies with 13C-labeled glucose, pyruvate, and deoxyxylulose (12, 13, 20–26, §) require that the biosynthesis of the isoprenoid intermediates proceeds by breaking of the C-3/C-4 bond of deoxypentulose and that a new bond is established between C-2 and C-4 of the original deoxypentulose intermediate (Fig. 6). This could occur by rearrangement or by fragmentation and reassociation of the fragments.
Figure 6.
The skeletal rearrangement conducive to the observed labeling patterns of IPP as reconstructed from the terpenoid compounds studied. 13C labels from [1-13C]deoxyxylulose are shown as solid circles. Labeling from [2,3,4,5-13C4]deoxyxylulose is shown by bold lines. Arrow indicates the carbon atom documented by the NMR patterns in Fig. 4. R = H or PO3H2.
We have now shown that [2,3,4,5-13C4]deoxyxylulose is transformed into quadruple-labeled isotopomers of IPP and DMAPP (i.e., [1,2,3,4-13C4]IPP and [1,2,3,4-13C4]DMAPP, respectively) in cells of C. roseus. The exclusive formation of quadruple-labeled isotopomers from [2,3,4,5-13C4]deoxyxylulose proves the strictly intramolecular nature of the process. A fragmentation/reassociation reaction would have resulted in the formation of isotopomers with fewer than four contiguous 13C atoms as a consequence of stochastic recombination between fragments arising from the isotope-labeled precursor and deoxyxylulose molecules formed in vivo from the natural abundance glucose that had been proffered in large excess.
Our interpretation is based on the analysis of more than a dozen isoprenoid moieties forming part of the metabolites under study. The close similarity of the enrichment values observed by biosynthetically equivalent carbon atoms in different isoprenoid units of each respective metabolite documents the high accuracy of the experimental method.
The relatively high isotope enrichments obtained in the presence of a low concentration of 13C-labeled deoxyxylulose show that the precursor is used with high efficiency for biosynthesis of phytol and carotenoids. It is an open question whether the free carbohydrate or a derivative such as a phosphoric acid ester acts as the committed precursor.
In our experimental system, the incorporation of deoxyxylulose into sitosterol is about 15-fold lower than in phytol and carotenoids. This is well in line with earlier studies by Arigoni and his coworkers in the plant, G. biloba, where ginkgolides were derived preferentially from deoxyxylulose, whereas steroids were derived preferentially from the acetate pool via mevalonate (13, §). In both cases, it seems that the mevalonate pathway is operative in the cytosol of the plant cells and the deoxyxylulose pathway is segregated in plastids, with some flux of metabolites between the different isoprenoid pools.
Preliminary data obtained with 13C-labeled acetate indicate that the mevalonate pathway is operative in the C. roseus cell line and that its metabolic products are preferentially channeled into the biosynthesis of sitosterol (Fig. 7). A more detailed analysis of metabolite partitioning of the two pathways in C. roseus is needed.
Figure 7.
Hypothetical partitioning of the mevalonate pathway and the alternative pathway in C. roseus. R = H or PO3H2.
Acknowledgments
We thank N. Hohenstein (Munich) for excellent technical assistance and F. Wendling, A. Werner, and H. Wagner (Garching) for help in the preparation of the manuscript. This work was supported by grants from the Deutsche Forschungsgemeinschaft, Bonn (SFB 369), and the Fonds der Chemischen Industrie.
ABBREVIATIONS
- IPP
isopentenyl pyrophosphate
- DMAPP
dimethylallyl pyrophosphate
- deoxyxylulose
1-deoxy-d-xylulose
Footnotes
Cartayrade, A., Schwarz, M. K., Jaun, B. & Arigoni, D., Second Symposium of the European Network on Plant Terpenoids, Strasbourg/Bischenberg, Jan. 23–27, 1994, abstr. P1.
References
- 1.Popják G, Cornforth J W. Adv Enzymol. 1960;22:281–335. doi: 10.1002/9780470122679.ch7. [DOI] [PubMed] [Google Scholar]
- 2.Bloch K. Steroids. 1992;57:378–383. doi: 10.1016/0039-128x(92)90081-j. [DOI] [PubMed] [Google Scholar]
- 3.Goodwin T W. In: Chemistry and Biochemistry of Plant Pigments. Goodwin T W, editor. London: Academic; 1965. pp. 143–154. [Google Scholar]
- 4.Loomis W D. In: Terpenoids in Plants. Pridham J B, editor. London: Academic; 1967. pp. 59–82. [Google Scholar]
- 5.Banthorpe D V, Charlwood B V, Francis M J O. Chem Rev. 1972;72:115–155. doi: 10.1021/cr60276a002. [DOI] [PubMed] [Google Scholar]
- 6.Brechbühler-Bader, S., Coscia, C. J., Loew, P., v. Sczepanski, C. & Arigoni, D. (1968) J. Chem. Soc. Chem. Commun. 136–137.
- 7.Pandian S, Saengchjan S, Raman T S. Biochem J. 1981;196:675–681. doi: 10.1042/bj1960675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cane D E, Rossi T, Tillman A M, Pachlatko J P. J Am Chem Soc. 1981;103:1838–1843. [Google Scholar]
- 9.Flesch G, Rohmer M. Eur J Biochem. 1988;175:405–411. doi: 10.1111/j.1432-1033.1988.tb14210.x. [DOI] [PubMed] [Google Scholar]
- 10.Kleinig H. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:39–59. [Google Scholar]
- 11.Rohmer, M., Sutter, B. & Sahm, H. (1989) J. Chem. Soc. Chem. Commun. 1471–1472.
- 12.Broers, S. T. J. (1994) Thesis (Eidgenössische Technische Hochschule, Zürich).
- 13.Schwarz, M. K. (1994) Thesis (Eidgenössische Technische Hochschule, Zürich).
- 14.Slechta L, Johnson L E. J Antibiot. 1976;29:685–687. doi: 10.7164/antibiotics.29.685. [DOI] [PubMed] [Google Scholar]
- 15.Hoeksema H, Baczynskyj L. J Antibiot. 1976;29:688–691. doi: 10.7164/antibiotics.29.688. [DOI] [PubMed] [Google Scholar]
- 16.White R H. Biochemistry. 1978;17:3833–3840. doi: 10.1021/bi00611a024. [DOI] [PubMed] [Google Scholar]
- 17.David S, Estramareix B, Fischer J-C, Thérisod M. J Am Chem Soc. 1981;103:7341–7342. [Google Scholar]
- 18.Hill R E, Sayer B G, Spenser I D. J Am Chem Soc. 1989;111:1916–1917. [Google Scholar]
- 19.Himmeldirk, K., Kennedy, I. A., Hill, R. E., Sayer, B. G. & Spenser, I. D. (1996) J. Chem. Soc. Chem. Commun. 1187–1188. [DOI] [PubMed]
- 20.Rohmer M, Knani M, Simonin P, Sutter B, Sahm H. Biochem J. 1993;295:517–524. doi: 10.1042/bj2950517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rohmer M, Seemann M, Horbach S, Bringer-Meyer S, Sahm H. J Am Chem Soc. 1996;118:2564–2566. [Google Scholar]
- 22.Eisenreich W, Menhard B, Hylands P J, Zenk M H, Bacher A. Proc Natl Acad Sci USA. 1996;93:6431–6436. doi: 10.1073/pnas.93.13.6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eisenreich W, Sagner S, Zenk M H, Bacher A. Tetrahedron Lett. 1997;38:3889–3892. [Google Scholar]
- 24.Lichtenthaler H K, Schwender J, Disch A, Rohmer M. FEBS Lett. 1997;400:271–274. doi: 10.1016/s0014-5793(96)01404-4. [DOI] [PubMed] [Google Scholar]
- 25.Schwender J, Seemann M, Lichtenthaler H K, Rohmer M. Biochem J. 1996;316:73–80. doi: 10.1042/bj3160073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeidler J G, Lichtenthaler H K, May H U, Lichtenthaler F W. Z Naturforsch C. 1997;52:15–23. [Google Scholar]
- 27.Linsmaier E M, Skoog F. Physiol Plant. 1965;18:100–127. [Google Scholar]
- 28.Schöpf C, Wild H. Chem Ber. 1954;87:1571–1575. [Google Scholar]
- 29.Yokota A, Sasajima K. Agric Biol Chem. 1984;48:149–158. [Google Scholar]
- 30.Yokota A, Sasajima K. Agric Biol Chem. 1986;50:2517–2524. [Google Scholar]
- 31.Shimizu M, Shogawa H, Matsuzawa T, Yonezawa S, Hayshi T, Arisawa M, Suzuki S, Yoshizaki M, Morita N, Ferro E, Basualdo J, Berganza L H. Chem Pharm Bull. 1990;38:2283–2284. doi: 10.1248/cpb.38.2283. [DOI] [PubMed] [Google Scholar]
- 32.Wright J L C, McInnes A G, Shimizu S, Smith D G, Walther J A, Idler D, Khalil W. Can J Chem. 1978;56:1898–1903. [Google Scholar]
- 33.Horibe, I., Nakai, H., Sato, T., Seo, S., Takeda, K. & Takatsuto, S. (1989) J. Chem. Soc. Perkin I 1957–1967.