Abstract
Inorganic polyphosphate [poly(P)] levels in Escherichia coli were reduced to barely detectable concentrations by expression of the plasmid-borne gene for a potent yeast exopolyphosphatase [poly(P)ase]. As a consequence, resistance to H2O2 was greatly diminished, particularly in katG (catalase HPI) mutants, implying a major role for the other catalase, the stationary-phase KatE (HPII), which is rpoS dependent. Resistance was restored to wild-type levels by complementation with plasmids expressing ppk, the gene for PPK [the polyphosphate kinase that generates poly(P)]. Induction of expression of both katE and rpoS (the stationary-phase σ factor) was prevented in cells in which the poly(P)ase was overproduced. Inasmuch as this inhibition by poly(P)ase did not affect the levels of the stringent-response guanosine nucleotides (pppGpp and ppGpp) and in view of the capacity of additional rpoS expression to suppress the poly(P)ase inhibition of katE expression, a role is proposed for poly(P) in inducing the expression of rpoS.
Inorganic polyphosphate [poly(P)] is a polymer of tens or hundreds of orthophosphate (Pi) residues linked by high-energy phosphoanhydride bonds (1). Poly(P) accumulates in massive amounts in many bacteria and fungi and in smaller amounts in every microbe, plant, and animal examined (1–3). Potential functions of poly(P) include the following: (i) a substitute for ATP for sugar and adenylate kinases (4–6), (ii) a phosphate reservoir (1), (iii) a chelator for divalent cations (7, 8), (iv) a buffer for alkaline stress (9), (v) a component in competence for DNA entry and transformation (10, 11), and (vi) a factor in regulatory responses to stresses and nutritional deficiencies (12).
An Escherichia coli mutant (ppk−) that lacks polyphosphate kinase (PPK), the enzyme that makes poly(P), is deficient in functions expressed in the stationary phase and fails to survive (13). The mutant shows a lack of resistance to an oxidant (H2O2), to heat, and to an osmotic challenge (12). The heat sensitivity of the ppk mutant is suppressed by extra copies of rpoS, the gene encoding the stationary-phase-specific RNA polymerase σ factor (12). Expression of rpoS is a central element in a regulatory network that governs the expression of many stationary-phase-induced and osmotically regulated genes, including katE (14–16). These lines of evidence indicate that poly(P) relates to rpoS in its transcription or translation or in stabilization of the RpoS (σ38) protein, inasmuch as the cellular content of σ38 is regulated at both the transcriptional and post-transcriptional levels (16).
To elucidate the functions of poly(P) in vivo, we have examined how poly(P) might be involved in H2O2 resistance by removing poly(P) with a plasmid-borne exopolyphosphatase [poly(P)ase, PPX1] (17). By this approach to achieve a poly(P)-less state, the emergence of resistant variants (12) in the ppk mutant can be avoided as well as the supplying of poly(P) by an alternative pathway (11). We report here that the high sensitivity to H2O2 was dependent mainly on the katE gene product, HPII catalase (18), and that poly(P) is necessary for induction of the transcription not only of katE but also of rpoS.
MATERIALS AND METHODS
Bacterial Strains and Plasmids.
The E. coli strains used are as follows: CSH7 (lacY, rpsL, thi-1) (18); UM178 (as CSH7 but katE1, his, lac+) (18); UM196 (as UM178 but katG17::Tn10) (18); NY001 (P1 transduction of katG17::Tn10 from UM196 to CSH7); KT1008 (F−, Δ(arg–lac)U169, araD139, rpsL150, ptsF25, flbB5301, rbsR, deoC1) (19); KT1008EL (as KT1008 but λRS45: katE–lacZ) (19); KT1008SL (as KT1008 but λRS45: rpoS–lacZ); JM101 {Δ(lac–proAB), supE, thi-1/F′ [traD36, proAB+, lacIq, lacZΔMI5]}; CA10 (as JM101 but ppk, ppx) (12).
λ lysogens carrying katE–lacZ (KT1008EL) were constructed as described (19), and rpoS–lacZ (KT1008SL) was constructed as follows. A DNA fragment corresponding to the 1.4-kb ClaI–DraI fragment of the rpoS promoter region was inserted into the operon fusion plasmid pRS551 (20), and the lacZ fusion construct was subsequently cloned in λRS45 (21). XL1-Blue (Stratagene) was the host strain for plasmid preparations.
The plasmids used are listed in Table 1. Plasmid pTrcPPX1 contains the entire coding region of the yeast PPX1 gene (1,484-bp BamHl fragment) and overproduces histidine-tagged PPX1 under control of the trc promoter (17); pTrcHisB (Invitrogen) is the original expression vector of pTrcPPX1; pLGPPX1 and pSUPPX1 were constructed by inserting a 3.0-kb SphI–BglII fragment that contains the lacIq gene, trc promoter, and PPX1 gene of pTrcPPX1 into SphI–BamHI double-digested pLG339 (22) and pSU2719 (23), respectively; pLGHisB and pSUHisB are control vectors of pLGPPX1 and pSUPPX1, respectively, that lack the BamHI fragment containing the PPX1 gene but have the lacIq gene and trc promoter; pLGPPX1 and pLGHisB are low-copy-number plasmids that are derivatives of pSC105; pSUPPX1 and pSUHisB are medium-copy-number plasmids that are derivatives of pACYC184; pBC29 is a derivative of pUC18 that has the whole region of the ppk gene (24); pBS-rpoS, which carries the entire coding region of the rpoS gene under the lac promoter in pBluescript II SK (+) (Stratagene), was provided by K. Makino (Osaka University, Japan).
Reagents.
Restriction and DNA modification enzymes were obtained from Takara Shuzo (Kyoto) or New England Biolabs. [32P]Orthophosphate ([32P]Pi) was supplied by Amersham. Polyethyleneimine-cellulose thin-layer chromatography (PEl-TLC) plastic plates were from Merck. Anti-RpoS antibody was prepared as described (15). Purified histidine-tagged PPX1 enzyme used in poly(P) estimation was prepared as described (17).
Measurement of H2O2 Sensitivity.
To cells grown overnight in Luria–Bertani (LB) medium, isopropyl β-d-thiogalactopyranoside (IPTG) was added to a final concentration of 1 mM with a further incubation for 1 hr. The stationary-phase cells were washed and resuspended in 150 mM NaCl to an OD600 of 5.0. H2O2 was added to a final concentration of 42 mM. At the times indicated, samples were diluted immediately in 150 mM NaCl and spread on LB plates to determine viable cell numbers.
Starvation Conditions for β-Galactosidase Assay and Western Blotting.
Exponentially growing cells (OD600 ≈ 0.5) in LB, collected and washed with M9 minimal medium (25) without NH4Cl and supplemented with glucose (16 mM), 0.2% Casamino acids, and 1 mg/ml thiamin, were concentrated to 1/10 the original culture volume in M9 medium lacking NH4Cl and starved for several hours at 37°C with shaking. At the times indicated, samples were collected for β-galactosidase assay and Western blotting.
Estimation of Cellular Poly(P).
Exponentially growing KT1008 cells harboring the plasmid containing PPX1 (pTrcPPX1) or the control vector (pTrcHisB) were labeled with [32P]Pi for at least 3 hr in LB until growth reached OD600 ≈ 0.5. The Pi concentration in LB, determined by the method of Chen et al. (26), is near 2.4 mM. Cells were washed once, concentrated to 1/10 the original culture volume, and starved in MOPS medium (27) containing glucose (16 mM), K2HPO4 (2.4 mM), and [32P]Pi at the same specific activity as that of LB. From cells collected by centrifugation at the times indicated, poly(P) was extracted and estimated by the DEAE filter method (N.N.R., S. Liu, and A.K., unpublished results).
Visualization of Catalases (Hydroperoxidases HPI and HPII) on Acrylamide Gels.
Typical HPI and HPII bands were visualized by electrophoresis of whole-cell extracts on an 8% polyacrylamide gel and then stained with a 50:50 solution of 2% K3Fe(CN)6 and 2% FeCl3. Bovine serum catalase (Sigma) was used as a standard.
Other Procedures.
The β-galactosidase assay was performed as described (28). Western blotting and all DNA manipulations were done by the methods described by Sambrook et al. (25). Cellular (p)ppGpp was estimated as described by Manoil and Kaiser (29). Concentrations of poly(P) are given in terms of phosphate residues.
RESULTS
Overproduction of Polyphosphatase Decreases Poly(P) to Barely Detectable Levels, and the Reduced Poly(P) Levels Can Be Restored by the ppk Gene.
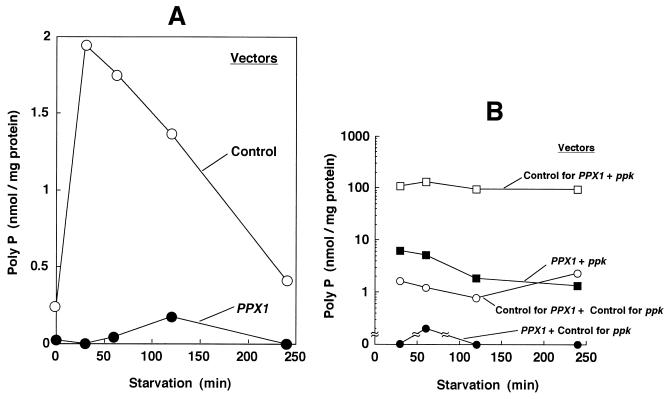
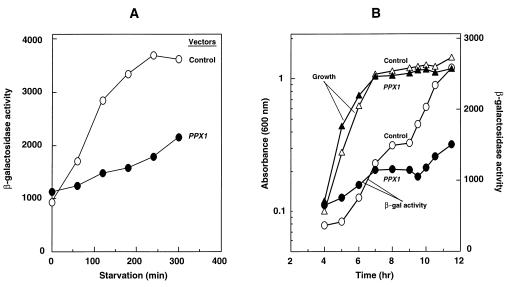
Reduction of poly(P) levels was achieved with a plasmid (pTrcPPX1) that overproduces the yeast exopoly(P)ase (PPX1). After the cells (KT1008) were transformed by either pTrcPPX1 or the corresponding vector plasmid lacking PPX1 (pTrcHisB), they were cultured to mid-logarithmic phase (OD600 = 0.4) and starved for 4 hr as described in Materials and Methods. Poly(P) accumulated within the first 30 min of starvation of vector-only-bearing cells and then decreased gradually (Fig. 1A). In cells transformed with plasmids containing PPX1, poly(P) accumulation was below detectable levels (0.1 nmol/mg of protein).
Figure 1.
(A) Starvation-induced poly(P) accumulation. KT1008 cells, harboring either the plasmid with the PPX1 gene (pTrcPPX1) (see text) or the control vector (pTrcHisB) were starved in nitrogen-free MOPS minimal medium (27). (B) Poly(P) levels reduced by poly(P)ase overproduction are restored by extra copies of the ppk gene. KT1008 cells harbored pairs of plasmids as follows: PPX1 gene (pLGPPX1) and ppk gene (pBC29) (▪); control vector for PPX1 (pLGHisB) and control vector for ppk (pUC18) (○); and control vector for PPX1 (pLGHisB) and ppk gene (pBC29) (□). Poly(P) values for cells harboring both the PPX1 gene (pLG PPX1) and the control vector for ppk (pUC18) (•) were below detectable levels (0.1 nmol/mg of protein).
The reduced poly(P) levels due to poly(P)ase overproduction could be overcome by complementation of the strain with extra copies of the ppk gene (Fig. 1B). The concentrations of poly(P) were brought down below the detectable limit (0.1 nmol/mg of protein) in a strain that carried a plasmid with PPX1 and a control vector for ppk. When the ppk control vector was replaced by the same vector bearing the ppk gene in the poly(P)ase-overproducing strain, the levels of poly(P) rose above 1 nmol/mg of protein and fluctuated between 1 and 8 nmol/mg of protein (Fig. 1B). A strain that contained control vectors for both ppk and PPX1 accumulated about 1–2 nmol/mg of protein of poly(P), a value similar to the one observed with the control strain in Fig. 1A. A strain containing multiple copies of ppk and the control vector for PPX1 accumulated a very high, but constant, amount (about 100 nmol/mg of protein) of poly(P), as expected (Fig. 1B).
Poly(P)ase Overproduction Results in Sensitivity to H2O2.
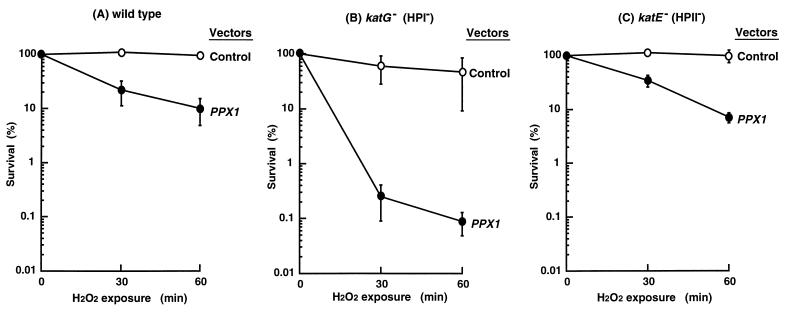
Just as the ppk mutant with low poly(P) levels showed sensitivity to H2O2 (12, 13), so does the cell in which poly(P)ase is overproduced by PPX1 contained in a plasmid (Fig. 2). The 10-fold increase in H2O2 sensitivity (Fig. 2A) became 1000-fold in a katG mutant (lacking the HPI catalase). Both the katE mutant (lacking HPII, the stationary-phase-induced catalase) and the wild type show the same levels of sensitivity (Fig. 2 A and C). Thus, the catalase most dependent on poly(P) in stationary-phase cells is HPII, the only catalase present in katG mutant cells (Fig. 2B). At the same time, the sharply decreased resistance to H2O2 of these cells when deprived of poly(P) is evidence for the contribution by HPII to this resistance.
Figure 2.
Effects of poly(P)ase overproduction on H2O2 sensitivity in catalase-deficient strains. The wild-type strain (CSH7) in A, or the katG mutant (NY001) in B, or the katE mutant (UM178) in C, harbored either the plasmid with the PPX1 gene (pTrcPPX1) (•) or the control vector (pTrcHisB) (○). Cells were exposed to 42 mM H2O2 at 25°C; viable cell numbers were determined by plating onto LB agar.
Although the basal expression of katG also depends on the RpoS level in stationary phase (30), the katG expression dependent on the OxyR activator may be sufficient to remove H2O2, thus accounting for the greater H2O2 sensitivity of the katG mutant at low poly(P) levels (Fig. 2).
Furthermore, resistance to H2O2 was also restored in poly(P)ase-overproducing cells to almost the same level as in transformants that harbored both PPX1- and ppk-bearing plasmids (Table 2). Thus, the heightened sensitivity to H2O2 caused by poly(P)ase overproduction can be ascribed to a decreased level of poly(P).
Transcription of katE Is Not Induced in Cells Lacking Poly(P).
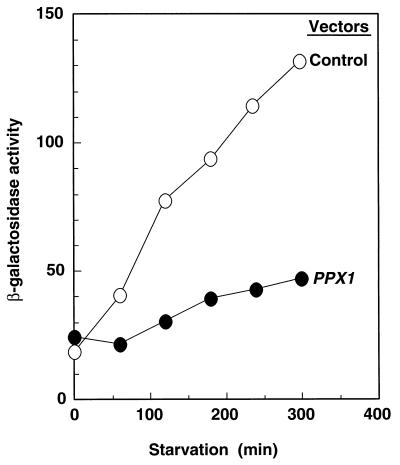
The level of katE expression as monitored by β-galactosidase activity with a katE–lacZ transcriptional fusion was induced by starvation and reached a maximum value after 5 hr (Fig. 3). However, no significant induction of expression was observed when poly(P)ase was overproduced. Thus, the high resistance to H2O2 (Fig. 2, Table 2) is likely due to the dependence of katE gene expression on adequate levels of poly(P).
Figure 3.
Expression of the katE–lacZ operon fusion monitored by β-galactosidase activity. KT1008EL cells harboring either plasmids with the PPX1 gene (pTrcPPX1) or the control vector (pTrcHisB) were starved in a M9 minimal medium (25). β-Galactosidase activity was measured in Miller units (28).
Production of HPII Depends on Poly(P) and rpoS.
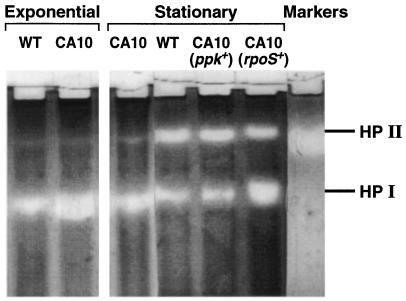
Direct assays of the levels of HPII, the katE gene product, demonstrate this catalase to be a stationary-phase-induced enzyme, the induction of which is not observed in ppk mutant cells (Fig. 4). This failure can be overcome by supplying the ppk gene or, remarkably, by extra copies of rpoS.
Figure 4.
Visualization of catalase activities (HPI and HPII). Electrophoresis of extracts of wild-type (WT) and CA10 (ppk−) cells grown to exponential or stationary phase was performed on an 8.5% acrylamide nondenaturing gel; extracts of stationary-phase CA10 cells complemented with plasmids bearing ppk (pBC29) or rpoS (pMMkatF3) were also tested.
Cellular Content of RpoS Fails to Increase in Cells Lacking Poly(P).
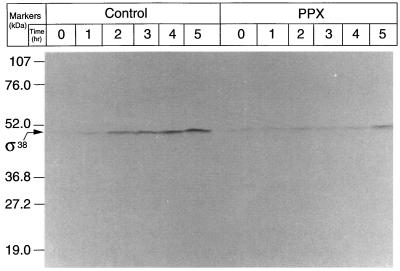
Because katE expression is dependent on RpoS, the cellular content of this σ factor (σ38) in a cell overproducing poly(P)ase was determined. As estimated by Western blotting (Fig. 5), the σ38 level increased with starvation time in control cells but no significant increase was observed in cells that overproduced poly(P)ase, even after 4 hr of starvation. Because the apparent molecular mass of σ38 in this gel is higher than the reported value, we used extracts of both an rpoS knockout mutant and an RpoS overproducer as controls, and confirmed that the product is really σ38. Once again, these results suggest that the high sensitivity to H2O2 is caused by lack of rpoS expression.
Figure 5.
Levels of RpoS (σ38) in KT1008EL cells harboring plasmids with either the PPX1 gene or the control vector during starvation. The plasmids used were pTrcPPX1 or pTrcHisB. Proteins were subjected to Western blotting with anti-σ38 antiserum and visualized with an alkaline phosphatase-conjugated second antibody.
Transcription of the rpoS Gene in Cells Lacking Poly(P).
In a λ lysogen of E. coli carrying the rpoS–lacZ transcriptional fusion gene, rpoS transcription was monitored by β-galactosidase activity (Fig. 6). Cells bearing the poly(P)ase gene or the control vector were grown to mid-logarithmic phase and then starved in a minimal medium. In the vector-only transformants, rpoS expression increased with starvation time, reaching a maximum level after 4 hr (Fig. 6A) (31). On the other hand, rpoS expression was induced only 2-fold after 5 hr of starvation in the transformants lacking poly(P) (Fig. 6A).
Figure 6.
Expression by the rpoS–lacZ operon fusion monitored by β-galactosidase activity (Miller units). KT1008SL cells harbored either plasmids with the PPX1 gene (pTrcPPX1) or the control vector (pTrcHisB). (A) Induction of rpoS transcription was monitored after starvation in an M9 minimal medium (25). (B) Expression of rpoS-lacZ fusion was tested during growth in LB.
Expression of rpoS was also monitored by β-galactosidase activity in cultures grown in LB (Fig. 6B). A vector-only control showed induction of rpoS during entry into stationary phase, with expression increasing nearly 5-fold after 12 hr. In the poly(P)ase-overproducing transformants, the increase in expression was only 2-fold. Inasmuch as growth rates of control and PPX1 transformants were very much the same, implying no gross differences in metabolism, these results suggest that stationary-phase induction of rpoS expression is modulated by poly(P) levels.
Poly(P)ase Overproduction Does Not Affect Accumulation of (p)ppGpp.
To see whether the overproduction of poly(P)ase affected the levels of pppGpp and ppGpp, the positive signals for accumulation of σ38 (32, 33), the levels of (p)ppGpp were monitored after starvation. Both ppGpp and pppGpp accumulated after 30 min of starvation, and they remained at the same level for 2 hr in the presence of the poly(P)ase overproducer compared with cells that harbored the control vector for PPX1 (data not shown).
DISCUSSION
Mutants that fail to express ppk, the gene that encodes the poly(P) kinase (PPK) responsible for the synthesis of poly(P) in E. coli, fail to develop the resistance to heat, oxidants, and osmotic stresses characteristic of stationary-phase cells and lose their viability within a few days (12). A relationship of poly(P) level to the expression of rpoS, the σ38 factor that controls the induction of some 50 genes in the stationary phase, was observed in the capacity of extra copies of rpoS to overcome the deficiencies in the ppk mutant (12). However, detailed genetic and physiologic studies of the ppk mutant and the regulatory role of poly(P) have been hampered by the genetic instability of the mutant and residual levels of poly(P) presumably synthesized by another route.
To circumvent these difficulties, another approach to deplete the cell of poly(P) was taken in the present studies. Poly(P) levels in cells transformed with a high-copy-number plasmid bearing the gene for the potent yeast exopolyphosphatase [poly(P)ase, PPX1], are reduced to virtually undetectable levels, thus enabling an evaluation of the influence of various genetic backgrounds, transcription efficiencies, and physiologic consequences. The large accumulations of poly(P) that result from overproduction of PPK by a multicopy plasmid bearing ppk are markedly reduced by overexpression of poly(P)ase (PPX1) (Fig. 1B); plasmid vectors that lacked the ppk and PPX1 genes served as controls.
Sensitivity to H2O2 evinced by transformants that overproduce poly(P)ase can be attributed to depressed levels of katE, the stationary-phase catalase (HPII) (Fig. 2). The dependence of katE on poly(P), as measured by peroxide sensitivity, was 100-fold greater than that of the exponential-phase catalase (HPI), product of the katG gene. The relative contributions of these catalases to resistance to peroxide is dependent on cell density, among other factors, and is thus difficult to assess. Under our conditions, the susceptibilities of the katE and katG mutants to peroxide were no greater than that of the wild type (Fig. 2).
The dependence on poly(P) of the expression of rpoS was observed in four ways: (i) β-galactosidase activity produced from an rpoS–lacZ operon fusion was sharply reduced in cells with overproduced PPX1 (Fig. 6); (ii) a similar result was obtained with an rpoS-activated gene, as in the katE–lacZ operon fusion (Fig. 3); (iii) HPII, the catalase product of katE expression, was absent from a ppk mutant, a defect that could be complemented with either ppk or rpoS (Fig. 4); and (iv) the cellular content of RpoS (σ38) failed to increase upon starvation in cells lacking poly(P) (Fig. 5).
That the low level of induction of katE transcription in a poly(P)ase overproducer is due to a decrease in the induction of rpoS expression was also demonstrated by the introduction of extra copies of rpoS gene on a multicopy plasmid (pBS-rpoS) into a poly(P)ase overproducer. katE was induced as fully when cells harbored plasmids for overproduction of both poly(P)ase and rpoS as with cells that harbored a plasmid for rpoS and a control vector (data not shown). However, no induction was observed in the strain bearing the poly(P)ase gene and a control vector. These results are consistent with observations (12) that the introduction of the rpoS gene in a multicopy plasmid into a ppk mutant increased its resistance to heat to the wild-type level.
The principal signals for stress responses in E. coli are the guanosine tetra- and pentaphosphates [(p)ppGpp], which accumulate in response to deficiencies in phosphate, nitrogen, amino acids, and other nutrients, as well as to undefined factors that operate in starvation and initiation of the stationary phase (32, 33). In the present study, we observed no effect on the levels of (p)ppGpp upon the virtually complete removal of poly(P). Instead, it has been demonstrated that pppGpp, in particular, profoundly inhibits the E. coli poly(P)ase, but not the PPK, thus leading to 100- to 1,000-fold accumulations of poly(P) (34). It has also been shown that (p)ppGpp is essential for the induction of rpoS expression (32).
On the basis of available evidence, it is clear that the generation of (p)ppGpp is often the primary response to stresses and nutritional deficiencies; accumulations of poly(P) and rpoS follow (32). Furthermore, poly(P) appears to figure in the expression of rpoS with the resultant activation of the many stationary-phase genes under rpoS control. What remains to be determined are the molecular mechanisms by which poly(P) exercises its regulatory role over the induction, maintenance, and actions of rpoS. To date, no activating role for poly(P) with reconstituted RNA polymerase systems has been observed.
Although poly(P) complexes of stationary-phase RNA polymerase have been reported, the nature of the holoenzyme, the specificity of gene activation, and details of the reactants are all unclear (35). E. coli RNA polymerase holoenzymes reconstituted from the core polymerase and a σ factor (e.g., RpoD, RpoS) showed no specificity in activating a stationary-phase gene (e.g., katE) (H. Wurst and A.K., unpublished results). In fact, the RpoS holoenzyme was uniquely and profoundly inhibited by substoichiometric amounts of a long-chain poly(P) (about 700 residues). Many reasons can be imagined for failures under in vitro conditions, among them, the involvement of other factors (e.g., cAMP), DNA topology, and especially the lack of a putative poly(P)-binding protein, which could have been stripped from the core polymerase early in the course of its isolation from the crude cell extracts.
Table 1.
Plasmids
| Plasmid | Characteristics | Source or ref. |
|---|---|---|
| pTrcHisB | Expression vector of His-tagged protein with lacIq gene and trc promoter; Ap resistance | Invitrogen |
| PTrcPPX1 | pTrHisB derivative carrying PPX1 gene; Ap resistance | 17 |
| pLGHisB | Expression vector of His-tagged protein with lacIq gene and trc promoter derived from pSC105; Km resistance | This study |
| pLGPPX1 | pLGHisB derivative carrying PPX1 gene; Km resistance | This study |
| pSUHisB | Expression vector of His-tagged protein with lacIq gene and trc promoter derived from pACYC184; Cm resistance | This study |
| pSUPPX1 | pSUHisB derivative carrying PPX1 gene; Cm resistance | This study |
| pMMkatF3 | pBT153 (a pBR322 derivative) with rpoS gene; Ap resistance | 36 |
| pBC29 | pUC18 derivative carrying E. coli ppk gene; Ap resistance | 24 |
| pBS-rpoS | pBluescript II SK(+) derivative carrying rpoS gene; Ap resistance | K. Makino |
Ap, ampicillin; Km, kanamycin; Cm, chloramphenicol.
Table 2.
Resistance to H2O2 restored by extra copies of the ppk gene
| Vectors* | Genotype | Viability,† % |
|---|---|---|
| Control for PPX1 + control for ppk | Wild type | 100 |
| Control for PPX1 + ppk | ppk+++ | 31 |
| PPX1 + control for ppk | PPX1+++ | 0.5 |
| PPX1 + ppk | PPX1+++ + ppk+++ | 29 |
The plasmids were as follows: control for PPX1 = pLGHisB, control for ppk = pUC18, ppk+++ = pBC29, and PPX1+++ = pLGPPX1.
Stationary-phase cells were exposed to 42 mM H2O2 for 20 min. Viability with no H2O2 exposure is set at 100%.
Acknowledgments
We thank Drs. A. Ishihama and H. Wurst for kindly sharing unpublished data and Dr. S. Yonei for catalase mutant strains. The helpful advice and assistance of Drs. A. Kuroda and S. Liu are gratefully acknowledged. We thank Drs. E. Nakajima, N. Nakajima, and LeRoy Bertsch for assistance with manuscript preparation. Financial support was provided by the Akiyama Foundation (Sapporo, Japan), and by grants from the Ministry of Education, Science and Culture of Japan and the National Institutes of Health (U.S.).
ABBREVIATIONS
- poly(P)
inorganic polyphosphate
- poly(P)ase
exopolyphosphatase
- PPK
poly(P) kinase
- HP
hydroperoxidase (catalase)
- ppGpp
guanosine 5′-diphosphate 3′-diphosphate
- pppGpp
guanosine 5′-triphosphate 3′-diphosphate
- (p)ppGpp
ppGpp and pppGpp
References
- 1.Kulaev I S. The Biochemistry of Inorganic Polyphosphates. New York: Wiley; 1979. [DOI] [PubMed] [Google Scholar]
- 2.Kulaev I S, Vagabov V M. Adv Microbiol. 1983;15:731–738. doi: 10.1016/s0065-2911(08)60385-9. [DOI] [PubMed] [Google Scholar]
- 3.Wood H G, Clark J E. Annu Rev Biochem. 1988;57:235–260. doi: 10.1146/annurev.bi.57.070188.001315. [DOI] [PubMed] [Google Scholar]
- 4.Hsieh P C, Shenoy B C, Jentoft J E, Phillips N F B. Protein Expression Purif. 1993;4:76–84. doi: 10.1006/prep.1993.1012. [DOI] [PubMed] [Google Scholar]
- 5.Phillips N F, Horn P J, Wood H G. Arch Biochem Biophys. 1993;300:309–319. doi: 10.1006/abbi.1993.1043. [DOI] [PubMed] [Google Scholar]
- 6.Bonting C F C, Kortstee G J J, Zehnder A J B. J Bacteriol. 1991;173:6484–6488. doi: 10.1128/jb.173.20.6484-6488.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Veen H W, Abee T, Kortstee G J J, Koning W N, Zehnder A J B. J Biol Chem. 1993;268:19377–19383. [PubMed] [Google Scholar]
- 8.Archibald F S, Fridovich I. Arch Biochem Biophys. 1982;215:589–596. doi: 10.1016/0003-9861(82)90120-5. [DOI] [PubMed] [Google Scholar]
- 9.Pick U, Weiss M. Plant Physiol. 1991;97:1234–1240. doi: 10.1104/pp.97.3.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reusch R N, Sadoff H L. Proc Natl Acad Sci USA. 1988;85:4176–4180. doi: 10.1073/pnas.85.12.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castuma C E, Huang R, Kornberg A, Reusch R N. J Biol Chem. 1995;270:12980–12983. doi: 10.1074/jbc.270.22.12980. [DOI] [PubMed] [Google Scholar]
- 12.Rao N N, Kornberg A. J Bacteriol. 1996;173:1394–1400. doi: 10.1128/jb.178.5.1394-1400.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crooke E, Akiyama M, Rao N N, Kornberg A. J Biol Chem. 1994;289:6290–6295. [PubMed] [Google Scholar]
- 14.Hengge-Aronis R. Cell. 1993;72:165–168. doi: 10.1016/0092-8674(93)90655-a. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka K, Takayanagi Y, Fujita N, Ishihama A, Takahashi H. Proc Natl Acad Sci USA. 1993;90:3511–3515. doi: 10.1073/pnas.90.8.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loewen P C, Hengge-Aronis R. Annu Rev Microbiol. 1994;48:53–80. doi: 10.1146/annurev.mi.48.100194.000413. [DOI] [PubMed] [Google Scholar]
- 17.Wurst H, Shiba T, Kornberg A. J Bacteriol. 1995;177:898–906. doi: 10.1128/jb.177.4.898-906.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loewen P C, Switala J, Triggs-Raine B L. Arch Biochem Biophys. 1985;243:144–149. doi: 10.1016/0003-9861(85)90782-9. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka, K., Handel, K., Loewen, P. C. & Takahashi, H. (1997) Biochim. Biophys. Acta, in press. [DOI] [PubMed]
- 20.Takayanagi Y, Tanaka K, Takahashi H. Mol Gen Genet. 1994;243:525–531. doi: 10.1007/BF00284200. [DOI] [PubMed] [Google Scholar]
- 21.Simons R W, Houman F, Kleckner N. Gene. 1987;53:85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- 22.Stoker N G, Fairweather N F, Spratt B G. Gene. 1982;18:335–341. doi: 10.1016/0378-1119(82)90172-x. [DOI] [PubMed] [Google Scholar]
- 23.Chandler M S. Plasmid. 1991;25:221–224. doi: 10.1016/0147-619x(91)90016-p. [DOI] [PubMed] [Google Scholar]
- 24.Akiyama M, Crooke E, Kornberg A. J Biol Chem. 1993;268:633–639. [PubMed] [Google Scholar]
- 25.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 26.Chen P S, Toribara T, Warner H. Anal Chem. 1956;28:1756–1758. [Google Scholar]
- 27.Neidhardt F C, Bloch P L, Smith D F. J Bacteriol. 1974;119:736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller J H. Experiments in Molecular Genetics. Plainview, NY: Cold Spring Harbor Lab. Press; 1972. [Google Scholar]
- 29.Manoil C, Kaiser D. J Bacteriol. 1980;141:297–304. doi: 10.1128/jb.141.1.297-304.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ivanova A, Miller C, Glinsky G, Eisenstark A. Mol Microbiol. 1994;12:571–578. doi: 10.1111/j.1365-2958.1994.tb01043.x. [DOI] [PubMed] [Google Scholar]
- 31.Mulvey M R, Switala J, Borys A, Loewen P C. J Bacteriol. 1990;172:6713–6720. doi: 10.1128/jb.172.12.6713-6720.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gentry D R, Hernandez V J, Nguyen L H, Jensen D B, Cashel M. J Bacteriol. 1993;175:7982–7989. doi: 10.1128/jb.175.24.7982-7989.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lange R, Fischer D, Hengge-Aronis R. J Bacteriol. 1995;177:4676–4680. doi: 10.1128/jb.177.16.4676-4680.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuroda, A., Murphy, H., Cashel, M. & Kornberg, A. (1997) J. Biol. Chem. 272, in press. [DOI] [PubMed]
- 35.Ozaki M, Fujita N, Wada A, Ishihama A. Nucleic Acids Res. 1992;20:257–261. doi: 10.1093/nar/20.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mulvey M R, Sorby P A, Triggs-Raine G L, Loewen P C. Gene. 1988;73:337–345. doi: 10.1016/0378-1119(88)90498-2. [DOI] [PubMed] [Google Scholar]