Abstract
This study investigated the effects of the oxidants hydrogen peroxide (H2O2) and 2,2′-dithiodipyridine (DTDP), and reductants, glutathione (GSH) and dithiothreitol (DTT), on the properties of the contractile apparatus of rat fast- and slow-twitch skeletal muscle fibres, in order to assess how oxidation affects muscle function. Skinned muscle fibres were activated in heavily-buffered Ca2+ solutions. The force-[Ca2+] relationship before and after various treatments was fitted by a Hill curve described by the maximum Ca2+-activated force, pCa50 (-log10[Ca2+] giving half-maximum force) and nH (the Hill coefficient). Exposing freshly skinned fibres to strong reducing conditions (i.e. 10 mm DTT or 5 mm GSH) had little if any effect on Ca2+ sensitivity (pCa50 or nH). The effect of oxidants H2O2 and DTDP depended on whether the fibre was relaxed (in pCa > 9) or activated during the exposure. In both fast- and slow-twitch fibres a 5 min exposure to 10 mm H2O2 at pCa > 9 had no effect on pCa50, causing only a reduction in nH. In contrast, when fast-twitch fibres were activated in the presence of 10 mm H2O2 (or 100 μm DTDP) there was a substantial increase in pCa50 (by ≈0.06 and 0.1, respectively), as well as larger decreases in nH than occurred in relaxed fibres, with all effects being reversed by DTT (10 mm, 10 min). In slow-twitch soleus fibres, the activation-dependent effect of DTDP was even greater (pCa50 increased by ≈0.35), and it was found that the rate of reversal in DTT was also increased by activation. A separate important phenomenon was that fast-twitch fibres that had been oxidised with H2O2 or DTDP (while either relaxed or activated) showed a paradoxical increase in Ca2+ sensitivity (≈0.04 and 0.25 increase in pCa50, respectively) when briefly exposed to the endogenous reductant GSH (5 mm, 2 min). This effect was reversed by DTT or longer (> 20 min) exposure to GSH, did not occur in slow-twitch soleus fibres, and may contribute to post-tetanic potentiation in fast-twitch muscle. Maximum force was not affected by any of the above treatments, whereas exposure to a high concentration of DTDP (1 mm) did greatly reduce force production. These findings reveal a number of novel and probably important effects of oxidation on the contractile apparatus in skeletal muscle fibres.
Reactive oxygen species (ROS) are a group of often short lived, but highly reactive molecules that are generated within most cells including skeletal muscle (Reid, 2001). The targets of ROS modification are numerous and include protein thiol (R-SH) residues and lipids, and it has been suggested that ROS could play a role in muscle fatigue (Barclay & Hansel, 1991). Exposure of intact skeletal muscle fibres to millimolar concentrations of hydrogen peroxide (H2O2) initially causes potentiation of twitch size within minutes, but with longer exposure there is a decline and even abolition of force production (Reid et al. 1993; Oba et al. 1996). Oba et al. (1996) suggested that the initial potentiation of the twitch was due to an oxidation effect on the dihydropyridine receptor, which acts as voltage sensor in the transverse-tubular (T-) system (Melzer et al. 1995), resulting in an increase in Ca2+ release. Reid et al. (1993) also postulated that the potentiation was due to increased Ca2+ release but that this was caused by oxidation of the ryanodine receptor (RyR)-Ca2+ release channel in the sarcoplasmic reticulum (SR). This was based on the earlier finding that under certain circumstances oxidation activates the RyR and triggers Ca2+ release from the SR (see Salama et al. 1992). In contrast to the suggestions of both Oba et al. (1996) and Reid et al. (1993), it was subsequently found that in isolated fast-twitch mouse fibres exposure to H2O2 (100-300 μm) caused potentiation of submaximal tetanic force responses without any increase in cytoplasmic [Ca2+], with the effect instead being due to an apparent increase in the Ca2+ sensitivity of the contractile apparatus (≈0.08 pCa unit increase; Andrade et al. 1998a). It is therefore somewhat puzzling that to date no study examining the properties of the contractile apparatus in skinned muscle fibres has observed any increase in pCa50 at any concentration of H2O2 (Brotto & Nosek, 1996; Plant et al. 2000; Callahan et al. 2001; Darnley et al. 2001), with the only changes reported in trunk-limb skeletal muscle being a reduction in the Hill coefficient (nH; i.e. steepness) of the force-[Ca2+] relationship and a small decrease in maximum force (Plant et al. 2000).
Thus, at present there is a major unexplained disparity between intact and skinned fibres as to the effect of H2O2 oxidation on the contractile apparatus. Further, it seems that in skinned fibres other oxidants have markedly different effects from H2O2, and that these too are not in close accord with those occurring in intact fibres. For example, oxidation with the nitric oxide donor, nitroprusside, in skinned fibres caused a decrease in pCa50 and also a decrease in maximum force that could be reversed by exposure to dithiothreitol (DTT; Perkins et al. 1997); in intact fibres it caused a decrease in pCa50 with no change in maximum force (Andrade et al. 1998b). Exposure of skinned fibres to the sulphydryl-specific reagent, 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB; applied at 10 mm in pCa >9 at pH 8.6) caused a large reduction in maximum force, which was accompanied by a substantial reduction in nH and a small decrease in pCa50, and a brief exposure to DTT partially reversed the reduction in maximum force and caused a very large increase in pCa50 (≈0.3) such that the Ca2+ sensitivity of the contractile apparatus was much greater than before DTNB treatment (Wilson et al. 1991). Finally, we have shown that another oxidant, the reactive disulphide 2,2′-dithiodipyridine (DTDP), at 100 μm, caused an increase in Ca2+ sensitivity of the contractile apparatus in skinned fibres, without any appreciable change in maximum force (Posterino & Lamb, 1996). Thus, this latter investigation using DTDP is the only skinned fibre study to date to observe oxidative effects on the contractile apparatus that were at all like the initial changes occurring in intact fibres with H2O2 treatment (Andrade et al. 1998a). The effects of DTDP in the skinned fibres however were complex and not fully characterised, and it is by no means clear how to relate all the various findings in intact and skinned fibres.
Here, we comprehensively examine a number of redox agents and treatment parameters, revealing a number of phenomena which help relate previous findings. We show that both H2O2 and DTDP cause an increase in Ca2+ sensitivity in skinned fibres if present during fibre activation but not if the fibre is kept relaxed during the exposure as was the case in all previous skinned fibre studies with H2O2. Furthermore, we also document and characterise a quite separate phenomenon in which brief exposure of oxidised fibres to glutathione (GSH), a reducing agent present endogenously in intact fibres, paradoxically causes a large increase in Ca2+ sensitivity that can be subsequently reversed by exposure to DTT or by prolonging the exposure to GSH. Interestingly, the effect of GSH occurs only in fast-twitch EDL fibres and not in soleus fibres, whereas the use-dependent effect of DTDP is much larger in slow-twitch fibres than in fast-twitch fibres. In showing the above two mechanisms in which exposure to oxidants can cause an increased Ca2+ sensitivity of the contractile apparatus, the present study gives considerable further insight into the physiological effects of oxidation in muscle and enables the observations in intact and skinned fibres to be reconciled. It seems likely that these oxidation effects are important in vivo, and possibly contribute to the phenomenon of post-tetanic potentiation. Finally, we document how the overall effect of oxidants is altered by prolonging the exposure or by increasing the concentration, producing reductions in maximum force and a decrease in Ca2+ sensitivity, which helps explain other outstanding differences between previous experiments.
Methods
Solutions
All chemicals were obtained from Sigma (St Louis, USA). A potassium ‘relaxing’ solution with 50 mm EGTA (pCa = -log10[Ca2+] > 9) and 1 mm free Mg2+ was made as described previously (Stephenson & Williams, 1981) and contained (mm): 126 K+, 37 Na+, 50 EGTA, 8 total ATP, 10.3 total Mg2+, 10 creatine phosphate, 90 Hepes, 1 , pH 7.10 ± 0.01). A matching Ca-EGTA solution was made similarly but with 49.5 mm total Ca2+ (pCa ≈4.5) and with 8.1 mm total Mg2+ to maintain the free [Mg2+] at 1 mm. Both solutions had an osmolality of ≈290 mosmol (kg solvent)−1. Solutions with intermediate [Ca2+] were made by appropriate mixture of these two solutions and the free [Ca2+] was verified with a Ca2+-sensitive electrode (Orion Research, Boston, MA, USA). A similar solution made with Sr2+ instead of Ca2+, with pSr (= -log10[Sr2+]) of 5.2, was used to distinguish between fast- and slow-twitch fibres in soleus muscles (Bortolotto et al. 2000) (see below).
DTT was made as a 1 m stock in double distilled water and diluted 100- (or 1000-)fold in the final solution. Stocks of glutathione in its reduced form (GSH) and oxidised form (GSSG) were made at 100 mm and 50 mm respectively in a potassium solution with 50 mm 1,6-diaminohexane-N,N,N’,N‘-tetraacetic acid (HDTA) that otherwise matched the 50 mm EGTA solution, with the pH re-adjusted to 7.10 with KOH. The GSH and GSSG stocks (mixed together where appropriate) were diluted 20-fold in the final solution, with the same amount of potassium HDTA solution added to matching control solutions; solutions were made with 5 mm GSH, or with 2.5 mm GSSG, or with the ratio of GSG:GSSG either 30:1 or 3:1. Hydrogen peroxide was added directly from a 30 % aqueous stock solution to the experimental solutions at a final concentration of 10 mm or diluted to give 200 μm. A 100 mm stock solution of 2, 2′-dithiodipyridine (DTDP) was made in absolute ethanol and in most experiments was diluted 1000-fold in the final solutions to 100 μm; matching control solutions with the same amount of ethanol (0.1 %) had no noticeably different effect than controls without ethanol. In experiments examining the effect of exposure to 1 mm DTDP, the stock was diluted 100-fold in pCa > 9 solution, and exposure to a matching control solution with the same amount of ethanol was found to have no obvious effect on the subsequent force responses (see protocol below).
Mechanically skinned fibre preparation
Long Evans hooded rats (Rattus norvegicus) aged 14 to 24 weeks were killed by halothane overdose in accordance with ethical guidelines of the Animal Ethics Committee of La Trobe University. The extensor digitorum longus (EDL) and soleus muscles were removed and immediately placed in paraffin oil and kept cool on ice. Single muscle fibres were dissected free at one end from the muscle and mechanically skinned with fine jeweller's forceps as described previously (Lamb & Stephenson, 1994; Posterino & Lamb, 1996). The skinned fibre segment was mounted onto a force transducer (AME801, SensoNor, Horten, Norway), and stretched to 120 % of its resting length. Fibre diameter of the skinned segment was then measured (range 25 to 50 μm). The fibre was then placed into a 2 ml Perspex bath containing the 50 mm EGTA solution (pCa > 9) to equilibrate for 2 min, before being activated in solutions with higher [Ca2+]. All experiments were performed at room temperature (23 ± 2 °C).
Ca2+ activation of the contractile apparatus
The force-[Ca2+] relationship in each fibre was determined by exposing the fibre to a sequence of solutions at progressively higher free [Ca2+], allowing the fibre to reach close to a steady force level in each solution before moving to the next. The last in each sequence was the solution at pCa ≈4.5, and the force reached in that solution was defined as the maximum Ca2+-activated force. The fibre was then fully relaxed in the pCa > 9 solution for at least 1 min before repeating the activation sequence. Successive control sequences were normally highly reproducible, apart from a small progressive reduction in maximum force (see Results).
The same fibre was then subjected to one or more experimental protocols; these are specifically described in the relevant sections of Results and fall into two broad types. In one, the fibre was exposed to some reagent (e.g. 100 μm DTDP) in the relaxing solution (pCa > 9) for a set time, washed for 1 min in the relaxing solution and then tested again (usually twice in succession) with the control activating solution sequence (e.g. Fig. 2). Where appropriate, the fibre was then subjected to another reagent (e.g. 10 mm DTT to reverse the effect of DTDP) as above and tested again. In the other type of protocol (e.g. Fig. 3), a fibre was activated in a solution sequence with the given reagent present in all solutions; these solutions were otherwise identical to those of the control sequence, having been made together and then split before adding the reagent to each of those in the test sequence. In most instances the fibre was subjected to three successive activation sequences in the presence of the reagent (over ≈5-6 min) before being washed in the standard relaxing solution and tested again (usually twice) with the control sequence.
Figure 2. After pre-exposure to the oxidant DTDP, GSH causes a large increase Ca2+ sensitivity.
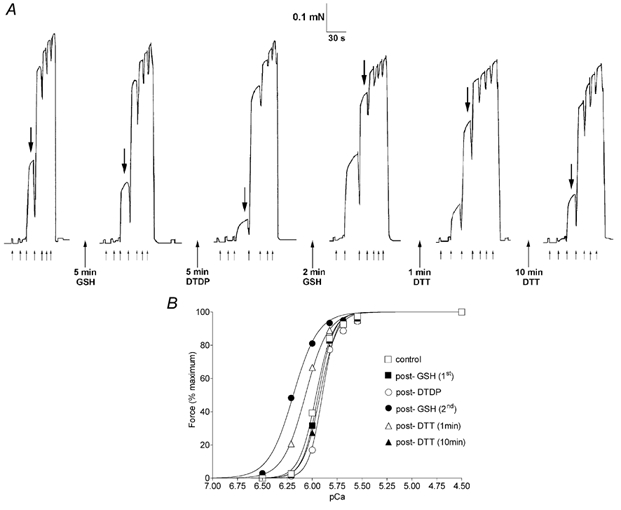
A, force produced in an EDL fibre repeatedly exposed to a sequence of solutions of progressively higher [Ca2+]. The first activation sequence shows the third elicited under the control conditions (no oxidants or reductants) after skinning. The fibre was then exposed to a particular reagent (in the standard relaxing solution, pCa > 9) for the indicated time and tested twice with the control solution sequence; sequence pairs were virtually identical in every case (except for a small decline in maximum force) and only one of each pair is shown. After a 5 min exposure to 5 mm GSH the force response indicated a slight decrease in Ca2+ sensitivity. (The large downward arrows mark the same solution (pCa 6.00) in each sequence.) Exposure to 100 μm DTDP for 5 min caused a further reduction in sensitivity and re-exposure to 5 mm GSH then caused a very large increase in sensitivity, which was reversed ≈50 % and then fully by exposure to 10 mm DTT for 1 and 10 min, respectively. The fibre was initially bathed in a solution at pCa > 9 and then at pCa (applied at each small arrow) 6.50, 6.21, 6.00, 5.83, 5.69, 5.55, 4.5, with the force declining upon return to pCa > 9. B, Hill curve fits to the data in A. The pCa50 values following treatments in the order (control, 5 mm GSH 1st, 100 μm DTDP, 5 mm GSH 2nd, 1 min DTT, 10 min DTT) were: 5.961, 5.945, 5.892, 6.186, 6.069, 5.912 and the corresponding Hill coefficients were: 5.1, 5.4, 6.3, 3.5, 3.8, 5.1.
Figure 3. Activation in the presence of 100 μm DTDP causes a large increase in Ca2+ sensitivity in a soleus fibre.
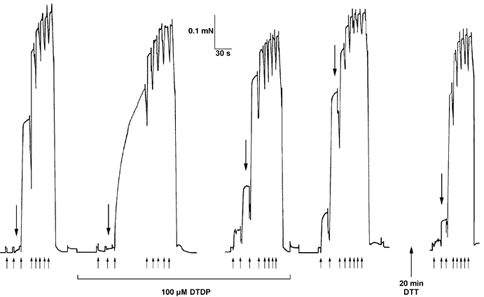
In the first activation sequence in the presence of 100 μm DTDP (second sequence from left), the force produced at a submaximal [Ca2+] (pCa 6.00) had still not reached steady state after ≈1 min (between third and fourth upward arrows) due to a progressive increase in Ca2+ sensitivity over the course of the activation. The next two sequences in DTDP (only last shown) were similar to each other, displaying increased Ca2+ sensitivity. The full extent of the use-dependent increase in Ca2+ sensitivity was apparent upon washout of the DTDP (compare fourth to first sequence). This large increase in Ca2+ sensitivity was almost fully reversed after a total of 20 min exposure to 10 mm DTT (intervening sequences not shown). In each sequence the large downward arrow indicates the force produced at pCa 6.30. Upward arrows indicate the start of exposure to solution at pCa 6.60, 6.30, 6.00, 5.80, 5.63, 5.49, 5.35, 4.5, with the fibre in pCa > 9 at other times. This soleus fibre produced near-maximal force in pSr 5.2 (not shown) indicating that it was a slow-twitch fibre.
In the case of soleus muscle fibres, after testing as above, each fibre was exposed to a Sr2+-based solution at pSr 5.2 in order to distinguish fast-twitch fibres (≈15 %) from slow-twitch fibres (Bortolotto et al. 2000); fast-twitch fibres gave < 20 % of maximal force in such a solution whereas slow-twitch fibres gave near maximal force.
Analysis
The data for each activation sequence in a fibre were analysed individually. The maximum force achieved in a sequence was measured and the force produced at each pCa expressed relative to that maximum. This force-pCa data was then fitted with a Hill curve (GraphPad Software Inc., San Diego, CA, USA) to obtain the pCa50 (the pCa giving 50 % maximum force) and Hill coefficient (nH). The effect of a treatment was assessed by measuring the change in maximum Ca2+-activated force, pCa50 and nH relative to the values before that treatment in the same fibre. Results are given as means ± s.e.m. for a sample of n fibres. Statistical significance was examined using a one-way analysis of variance with Dunnett's multiple comparison test, or where appropriate with Student's two- or one-tailed t test, with a significance level of P < 0.05.
Results
This paper describes a number of treatments that cause substantial changes in the Ca2+ sensitivity and/or maximum force production of the contractile apparatus. To put these in perspective, it is first necessary to consider a number of control experiments and other treatments that had little if any effect. Proper description of Ca2+ sensitivity requires both the pCa50 (the pCa at half-maximum force) and nH (Hill coefficient) values; when nH is little changed, increases and decreases in pCa50 straightforwardly indicate increases and decreases in Ca2+ sensitivity, respectively. In considering the pCa50 changes here, it is important to distinguish between positive and negative values, and to note that the values are logarithmic and that for example a change of ≈0.08 units (Andrade et al. 1998a) means that a [Ca2+] that initially produces a force response of ≈40 % of maximum Ca2+-activated force gives ≈60 % of maximum force after the change (see also Fig. 2B).
Highly reproducible force responses were obtained when exposing EDL and soleus skinned fibres to successive activation sequences under control conditions (i.e. to successive series of incremental steps in pCa from 9 to 4.5, in the absence of any oxidising or reducing agent), with there being only a small decline (≈2-5 %) in maximum Ca2+-activated force and no significant change in pCa50 or nH (see Tables 1 and 3). The small progressive decline in maximum force, which is typically seen in skinned fibre studies, depended on the size and duration of the preceding activation sequence and not on the time between sequences, with a similar decline being observed even if the sequences were ten or more minutes apart (see later). The change in maximum force following any of the treatments in this study was not noticeably different from that occurring with successive control sequences (except for treatment with a very high concentration (1 mm) of DTDP as described later). Note that unless it is specifically mentioned that a fibre was activated in the presence of a reagent, treatment with a reagent means that the fibre was bathed in relaxing solution (pCa > 9) in the presence of that reagent for the indicated time, and tested with a sequence of activating solutions only after washout of the reagent.
Table 1.
Effect of H2O2 and other treatments on pCa50 and Hill coefficient in EDL fibres
| Treatment | ΔpCa50 | ΔnH | n |
|---|---|---|---|
| (1) Repeated control sequences | −0.002 ± 0.002 | 0.0 ± 0.1 | 57 |
| (2) 5 mm GSH at pCa > 9 (5 min) | −0.013 ± 0.005 | 0.0 ± 0.3 | 6 |
| (3) 10mm DTT at pCa > 9 (10 min) | −0.011 ± 0.005 | 0.5 ± 0.3 | 5 |
| (4) 200 μm H2O2 at pCa > 9(5 min) | −0.010 ± 0.001 | 0.2 ± 0.3 | 3 |
| (5) 10mm H2O2 at pCa > 9 (5 min), subsequent 10mm DTT (10 min) | −0.009 ± 0.007 | −1.6 ± 0.3* | 9 |
| −0.018 ± 0.006† | +1.5 ± 0.4 † | 5 | |
| (6) 10mm H2O2 during activation, | +0.060 ± 0.006* | −3.6 ± 0.7* | 5 |
| subsequent 10mm DTT (10 min) | −0.052 ± 0.003† | +2.0 ± 0.2† | 5 |
| (7) 5 mm GSH after 10mm H2O2 at pCa > 9, | +0.026 ± 0.006* | +0.1 ± 0.4 | 4 |
| subsequent 10mm DTT (10 min) | −0.050 ± 0.006† | +0.5 ± 0.1† | 4 |
The force–pCa relationship in a fibre was examined before and after various treatments and each case fitted with a Hill curve described by the parameters pCa50 and Hill coefficient (nH) (e.g. Fig.2 B). The mean (± s.e.m.) values of pCa50 and nH in EDL fibres in the whole study (n = 82) were 5.89 ± 0.02 and 5.7 ± 0.1, respectively. The table shows the mean change (± s.e.m.) in pCa50 and nH resulting from each of the indicated treatments ((1) to (7)), with n being the number of EDL fibres. Each applied reagent was washed out before activating the fibre, except in treatment 6. Where indicated some or all of the same fibres were subjected to subsequent treatment with DTT, and the values show the resulting mean change. In treatment 6,H2O2 was present during three activation sequences and washed out (∼5 min total exposure to H2O2) and the values are for the change between control sequences before and after.
Mean is significantly different from repeated control sequence case (treatment 1; one way ANOVA).
Mean change after DTT treatment is significantly different from zero (Student's two-tailed t test).
Table 3.
Effect of H2O2 and other treatments on pCa50 and Hill coefficient in soleus fibres
| Treatment | ΔpCa50 | ΔnH | n |
|---|---|---|---|
| (1) Repeeted control sequence | −0.002 ± 0.002 | −0.1 ± 0.1 | 28 |
| (2) 5 mm GSH (5 min) at pCa > 9 | −0.004 ± 0.001 | −0.1 ± 0.1 | 3 |
| (3) 10 mm H2O2 at pCa > 9 (5 min) | −0.011 ± 0.008 | −2.1 ± 0.8* | 3 |
| (4) 10 mm H2O2 during activation, | −0.003 ± 0.008 | −1.2 ± 0.2* | 10 |
| subsequent 10 mm DTT (10 min) | −0.003 ± 0.007 | +0.2 ± 0.2 | 7 |
| (5) 10 mm H2O2 during activation, | +0.012 ± 0.004 | −0.9 ± 0.2* | 3 |
| subsequent 5 mm GSH (2 min) | −0.010 ± 0.008 | +0.1 ± 0.1 | 3 |
| (6) 100 μm DTDP (5 min) at pCa > 9, | −0.059 ± 0.027‡ | −1.1 ± 0.5* | 5 |
| subsequent 5 mm GSH (2 min), | +0.001 ± 0.002 | −0.1 ± 0.1 | 5 |
| subsequent 10 mm DTT (10 min) | +0.023 ± 0.011 | +0.3 ± 0.2 | 4 |
| (7) 100 μm DTDP during activation, | +0.353 ± 0.081* | −1.6 ± 0.3* | 5 |
| subsequent 5 mm GSH (2 min), | +0.000 ± 0.002 | 0.0 ± 0.1 | 3 |
| subsequent 10 mm DTT (20 min) | −0.351 ± 0.069† | +1.9 ± 0.2† | 3 |
Mean change (± s.e.m.) in pCa50 and nH resulting from each of the indicated treatments, with n being the number of slow-twitch soleus fibres. Mean (± s.e.m.) values of pCa50 and nH in soleus fibres in the whole study (n = 28) were 6.09 ± 0.02 end 4.3 ± 0.2, respectively.
Mean is significantly different from repeated control sequence case (treatment 1; one way ANOVA).
Mean change after subsequent treatment (DTT or GSH) is significantly different from zero (Students two-tailed t test).
For treatment 6 indicates significance at P < 0.05 using Student's one-tailed t test. Fibres for treatment 5 are a subset of those used for the initial part of treatment 4.
Effects of DTT and GSH
Exposure of skinned EDL fibres to the reducing agent DTT (10 mm) for 10 min had no significant effect on pCa50 or nH (Table 1, treatment 3), and the decline in maximum force (2.6 ± 0.8 %, n = 5) was not noticeably different from control repetitions; these findings are consistent with previous results (Posterino & Lamb, 1996; Plant et al. 2000). Similarly, exposure of EDL fibres to 5 mm glutathione (GSH) for 5 min caused little if any change in pCa50 or nH (Table 1, treatment 2). Furthermore, when the force-[Ca2+] relationship was measured in the presence of 5 mm GSH in four other EDL fibres it was not significantly different from that in the absence of GSH (change in pCa50 and nH: −0.007 ± 0.004 and −0.2 ± 0.3, respectively).
The cytoplasm is thought to be a strongly reducing environment normally with the ratio of reduced glutathione (GSH) to oxidised GSH (GSSG) being ≈30:1 (total ≈5 mm), whereas in the SR lumen the ratio is ≈3:1 (see Feng et al. 2000). The force-[Ca2+] relationship in EDL fibres that were placed into a solution with a GSH:GSSG ratio of 30:1 (5 mm total) immediately after skinning was not noticeably different from that of fibres placed initially in control solution (no GSH or GSSG). Activation sequences were performed after 5 and 15 min with GSH and GSSG present in the ratio 30:1, then again in the ratio 3:1, and finally once more in the ratio 30:1. There was no significant difference in pCa50 or nH either 5 or 15 min after changing from the 30:1 condition to the 3:1 condition or after changing back again (mean change in 3:1 after 15 min: −0.008 ± 0.006 and −0.3 ± 0.2, respectively, and in 30:1 after 15 min: −0.010 ± 0.004 and −0.4 ± 0.2, respectively, n = 4), and the maximum force only showed the small progressive decline expected for repeated sequences. Similarly, there was no significant difference when changing from the presence of 5 mm GSH to 2.5 mm GSSG for 5-10 min (change in pCa50 −0.007 ± 0.006 and nH −0.2 ± 0.1, n = 3). Thus, the Ca2+ sensitivity of the contractile apparatus does not appear to be appreciably affected by even large changes in the ratio of GSH:GSSG in the cytoplasm.
Activation-dependent effects of oxidants
In agreement with previous findings (Brotto & Nosek, 1996; Plant et al. 2000), if H2O2 (200 μm or 10 mm) was applied to an EDL fibre for 5 min whilst it was relaxed in a low [Ca2+] solution (pCa > 9) there was little or no change in pCa50 when the fibre was tested back in control conditions (i.e. in the absence of H2O2; see Table 1, treatments 4 and 5). The only substantial change was a ≈25 % decrease in nH after exposure to a high concentration (10 mm) H2O2. In contrast, if H2O2 was present whilst the fibre was activated (three activation sequences with a total time of ≈5 min in H2O2), there was a substantial increase in pCa50 (+0.060) and a large decrease in nH, with these changes persisting unchanged after washout of H2O2 (Table 1, treatment 6). This increase in Ca2+ sensitivity could be observed during its onset by examining the response to repeated applications of a submaximal [Ca2+] with H2O2 present (e.g. Fig. 1); in such a situation the peak force increased progressively upon successive activations, reaching a stable level after a total of ≈1 min of activation in the presence of H2O2 (and a total of ≈2-3 min exposure to H2O2). Importantly, the increase in pCa50 could be fully reversed by exposing the fibre to 10 mm DTT for 10 min, with the Hill coefficient also recovering substantially (Table 1, treatment 6); this clearly shows that the activation-dependent effects of H2O2 involved oxidation reactions.
Figure 1. H2O2 increases Ca2+ sensitivity in an activation-dependent manner.
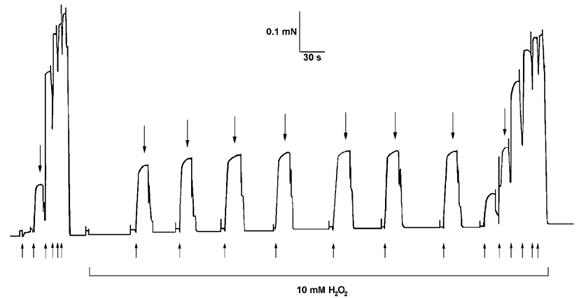
The contractile apparatus in a mechanically skinned EDL fibre was activated by exposure to heavily buffered Ca2+ solutions (50 mm total EGTA). The peak force produced at a submaximal [Ca2+] (pCa 6.00, large downward arrows) increased progressively with repeated activation in the presence of 10 mm H2O2, due to an increase in Ca2+ sensitivity of the contractile apparatus (cf. final and initial full sequences). No such change occurred if a fibre was kept relaxed during exposure to H2O2 (see Table 1). Upward arrows below initial and final staircases indicate the time at which the fibre was moved sequentially into solutions with pCa at 6.21, 6.00, 5.83, 5.69, 5.55, 4.5, and with pCa at 6.00 for intervening responses. The fibre was kept in pCa > 9 between activation sequences.
A comparable activation-dependent increase in Ca2+ sensitivity was also seen with another oxidant, the specific sulphydryl reagent DTDP. If an EDL fibre was kept in a relaxed state in pCa > 9 solution during exposure to 100 μm DTDP for 5 min, there was a decrease in pCa50 of ≈0.06 units with no change in nH (Table 2, treatment 1; Fig. 2, third record). If instead, the fibre was activated in the presence of 100 μm DTDP (three activation sequences over ≈5 min), the pCa50 measured back under control conditions (i.e. in the absence of DTDP) was increased ≈0.05 relative to the original control, and nH was also decreased substantially (by ≈30 %) (Table 2, treatment 4; also see Fig. 3 showing an analogous, though larger, effect in a soleus fibre). The activation-dependent changes were maximal after two or three sequences in DTDP (each ≈1 min in duration), with no further effect being found with more repetitions. Taking into account the above decrease in pCa50 occurring after simply applying and washing out DTDP, the overall increase indicates that activation in the presence of DTDP actually caused ≈0.11 unit increase in pCa50 (i.e. 0.05 + 0.06); changes of this order could indeed be seen by comparing the first and third sequences in the presence of DTDP (not shown). Both the exposure-dependent decrease in pCa50, and the activation-dependent increase in pCa50 and reduction in nH were fully reversed by 10 min exposure to 10 mm DTT (e.g. Table 2, treatment 3). This shows that all these effects of DTDP were due to oxidation reactions.
Table 2.
Effect of DTDP and other treatments on pCa50 and Hill coefficient in EDL fibres
| Treatment | ΔpCa50 | ΔnH | n |
|---|---|---|---|
| (1) 100 mm DTDP (5 min) at pCa > 9, | −0.065 ± 0.008* | −0.5 ± 0.4 | 12 |
| subsequent 5 mm GSH (2 min), | +0.276 ± 0.015† | −1.2 ± 0.4† | 12 |
| subsequent 10 mm DTT (10 min) | −0.227 ± 0.012† | +1.4 ± 0.3† | 10 |
| (2) 100 mm DTDP (15 s) at pCa > 9, | −0.056 ± 0.009* | −0.3 ± 0.4 | 6 |
| subsequent 5 mm GSH (2 min), | +0.200, +0.220 | −1.2, −1.1 | 2 |
| subsequent 10 mm DTT (10 min) | −0.167, −0.151 | +0.7, +0.7 | 2 |
| (3) 100 mm DTDP (5 min) at pCa > 9, | −0.041 ± 0.011* | −0.3 ± 0.6 | 3 |
| subsequent 10 mm DTT (10 min), | +0.051 ± 0.010† | +0.6 ± 0.3 | 3 |
| subsequent 5 mm GSH (2 min) | −0.004 ± 0.002 | −0.2 ± 0.1 | 3 |
| (4) 100 mm DTDP during ectivetion, | +0.048 ± 0.017* | −1.9 ± 0.4* | 5 |
| subsequent 5 mm GSH (2 min), | +0.299 ± 0.021† | −1.0 ± 0.1† | 3 |
| subsequent 10 mm DTT (10 min) | −0.258, −0.392 | +1.5, +1.4 | 2 |
Mean change (± s.e.m.) in pCa50 and nH (or individual velues where n = 2) resulting from each of the indicated treatments (1 to 4), with n being the number of EDL fibres (see Table 1). Eech epplied reagent was washed out before activating the fibre, except in treatment 4. In treatment 4, each fibre was subjected to three activation sequences in the presence of DTDP, and the pCa and n velues after washout of DTDP were compared to those before DTDP exposure (e.g. like comparing fourth to first sequence in Fig. 3).
Mean chenge is significantly different from that for repeated control sequence case (treatment 1 in Teble 1) (one way ANOVA). Where fibres were subjected to a subsequent treatment (GSH or DTT exposure)
indicates the resulting mean chenge is significantly different from zero (Student's two-tailed t test).
It was also evident that the presence of DTDP (100 μm) had a direct, inhibitory effect on force production. As this effect was reversible upon washout (without any treatment with a reductant), it evidently did not involve an oxidation reaction and was not studied further. Note that this direct effect of DTDP does not bear on the main findings which all relate to measurements made under control conditions (i.e. in the absence of DTDP).
Paradoxical effect of GSH on DTDP-oxidised EDL fibres
Experiments aimed at using the endogenous reductant GSH to reverse the oxidising effects of H2O2 and DTDP revealed a major novel effect. After an EDL fibre had been ‘oxidised’ by a 5 min exposure to 100 μm DTDP (at pCa > 9, with the DTDP washed out afterwards), a 2 min exposure to 5 mm GSH (at pCa > 9) caused an extremely large increase in pCa50 (≈0.28) and a decrease (≈25 %) in nH (Table 2 second part of treatment 1). An example of this is shown in Fig. 2A, with the Hill equation fitted to the force-[Ca2+] relationship shown in Fig. 2B; note that the initial GSH exposure (before oxidation with DTDP) had no such effect, causing if anything a small decrease in pCa50. Maximum force was not noticeably affected by the post-DTDP exposure to GSH on average in the fibres examined. The effect of GSH was fully reversed by 10 min exposure to 10 mm DTT, with pCa50 and nH returning to levels indistinguishable from those before the DTDP treatment. This could be seen in each fibre individually as well as in the mean data (see Table 2, treatment 1: change with DTT is approximately the sum of the preceding changes; and in Fig. 2A compare last trace with second trace). This whole procedure could then be repeated with virtually identical results (not shown), showing that the oxidation-reduction reactions involved in the phenomenon were highly reproducible.
If an EDL fibre was treated with DTDP and exposed to DTT for 10 min, GSH had no effect (Table 2, treatment 3). This is consistent with the GSH effect occurring only when a fibre is in an oxidised state. The effect of GSH application after oxidation with DTDP was similar, irrespective of whether the fibre was activated in the presence of DTDP or simply exposed to DTDP (compare second parts of treatments 1 and 4 in Table 2). This shows that the effect of GSH on a DTDP-oxidised fibre did not necessitate the fibre being activated during the exposure to DTDP or the exposure to GSH. Application of GSH:GSSG in the ratio 30:1 had an effect indistinguishable from that of GSH alone (not shown). In contrast, exposure to GSSG (2.5 mm) had no detectable effect at all on DTDP-oxidised fibres (change in pCa50: 0.000 and 0.008, and in nH: 0.0 and 0.0, in two fibres examined), even though GSH treatment immediately afterwards had the expected effect (e.g. as in treatment 1, Table 2). This shows that the sensitivity change involves a redox reaction that depends on glutathione being in its reduced form (see Discussion).
As mentioned above, exposure to 10 mm DTT for 10 min completely reversed the effects on both pCa50 and nH occurring with a 2 min exposure to GSH in DTDP-oxidised fibres. It was further found that a 1 min exposure to 10 mm DTT caused a 50 ± 1 % reversal of the pCa50 shift (n = 3; Fig. 2A), though only an 18 ± 9 % reversal of the change in nH. Importantly, it was also found that prolonged exposure to 5 mm GSH also reversed (at least partially) the changes produced by the initial brief (2 min) exposure to GSH. In the two fibres examined, a 20 and 25 min exposure to GSH, respectively, caused a 56 and 61 % reversal of the pCa50 increase and a 35 and 30 % reversal of the decrease in nH, with subsequent exposure to 10 mm DTT completing the full reversal of these parameters in each case. Thus, the initial effect of GSH seemed somewhat paradoxical: when an EDL fibre had been oxidised with DTDP, prolonged exposure to GSH had the effect expected of a reducing agent, but initially the GSH exposure actually had exactly the opposite effect.
When fibres were exposed to the DTDP for only 15 s instead of 5 min before being subsequently exposed to GSH, the decrease in pCa50 with DTDP and increase in pCa50 with GSH were both approximately 80 % of that occurring with the 5 min exposure to DTDP (compare treatments 1 and 2 in Table 2). This probably indicates that the GSH is acting at the same site(s) as that responsible for the decrease in sensitivity with DTDP oxidation. It appeared that a 5 min exposure to 100 μm DTDP and a 2 min exposure to GSH gave close to maximal effect, and increasing the concentration of DTDP to 1 mm (5 min exposure) or increasing the GSH exposure time to 5 min gave a similar pCa50 shift (not shown). It was also found that after the full GSH effect had been induced, a further exposure to DTDP (again 100 μm for 5 min) had no significant effect. This indicates that the sensitivity change with GSH could not have been the result of the GSH restoring a proportion of the DTDP-oxidised sites to their original reduced state, as this would have been reversed by the re-application of DTDP. Interestingly, following such treatment (i.e. 5 min DTDP exposure, 2 min GSH exposure and then repeated 5 min DTDP exposure), if the fibre was activated in the presence of DTDP (100 μm, three activation sequences), it did display a further increase in pCa50 (+0.058 ± 0.024, n = 4, P < 0.05 Student's one-tailed t test) (change in nH −0.3 ± 0.2, not significant). Although this increase in pCa50 with activation in DTDP is not as large as that occurring in fibres not exposed to GSH (≈0.11, see ‘Activation-dependent effects of oxidants’), it still shows that at least a component of the activation-dependent increase in Ca2+ sensitivity occurs independently of the GSH-mediated increase.
Since a relatively brief (2 min) exposure to GSH in DTDP-oxidised fibres had the opposite effect to prolonged exposure, we examined whether this was also the case with the other reducing agent DTT. In the five EDL fibres examined, DTDP exposure (100 μm for 5 min) caused a decrease in pCa50 of 0.041 ± 0.014 and a subsequent 15 s exposure to 10 mm DTT caused an increase of 0.059 ± 0.010 (with no significant changes in nH). Similarly, a 15 s exposure to a lower concentration of DTT (1 mm) did no more than reverse the decrease in sensitivity caused by DTDP exposure (decrease in pCa50 after DTDP: −0.062 ± 0.016; increase after DTT: +0.062 ± 0.005, in three fibres; with no further change after exposure to 10 mm DTT for 10 min). Thus, the effect of a relatively brief exposure to DTT was very different from that seen with GSH, with the DTT seeming to simply reverse the sensitivity decrease produced by DTDP exposure.
GSH effect following 5 min H2 O2 oxidation
Brief exposure to GSH also increased pCa50 in EDL fibres that had been exposed to H2O2. After a 5 min exposure to 10 mm H2O2 (at pCa > 9), which itself had no effect on pCa50 (see above), a 2 or 5 min exposure to 5 mm GSH caused a small (≈0.026) increase in pCa50 with no change in nH (Table 1, treatment 7). Subsequent exposure to DTT (10 mm, 10 min) reversed this effect on pCa50 and caused a significant increase in nH, the latter being consistent with partial reversal of the decrease in nH produced by the preceding exposure to H2O2 (see treatment 5 in Table 1). After such reversal with DTT, re-exposure to H2O2 and then GSH induced a similar effect to that seen before in the two cases examined (not shown). Thus, brief GSH treatment had a qualitatively similar effect in such H2O2-oxidised fibres to its effect in DTDP-oxidised fibres.
Effects in soleus slow-twitch fibres
In slow-twitch soleus fibres the effects of oxidants and GSH treatment showed many similarities, but also major differences, compared to those occurring in fast-twitch EDL fibres. When exposed to DTDP (100 μm, 5 min at pCa > 9) slow-twitch soleus fibres showed a similar decrease in pCa50 (≈0.06; Table 3, treatment 6), to that observed in EDL fibres. However, when activated in DTDP, the slow-twitch soleus fibres showed a very much larger increase in pCa50 (≈0.35) than occurred in EDL fibres (Table 3, treatment 7; thus, taking into account the decrease caused by exposure to DTDP (0.06), the actual increase was ≈0.41). An example of this is shown in Fig. 3. The progressive increase in Ca2+ sensitivity during activation in DTDP caused a marked prolongation of the rise in force in a submaximal [Ca2+] (see second trace in Fig. 3). The effect was evidently complete after the first activation sequence in DTDP (< 2 min) because the force responses of the second sequence (not shown) were virtually identical to those of the third sequence in DTDP. Slow-twitch fibres however did not show a significant increase in pCa50 during activation in the presence of 10 mm H2O2, even though there was a ≈25 % decrease in nH (Table 3 treatment 4), like that in EDL fibres.
The most striking difference in slow-twitch soleus fibres compared to fast-twitch EDL fibres was that a 2 min exposure to GSH had no effect whatsoever in fibres that had been exposed and/or activated in either DTDP or H2O2 (Table 3, treatments 5-7). Thus, the most prominent effect observed in EDL fibres, the marked increase in pCa50 when GSH was applied after an oxidant, was entirely absent in slow-twitch fibres. Interestingly, the two soleus fibres that were identified as fast-twitch fibres (by the extent of their activation in a pSr 5.2 solution, see Methods) were both found to show an increase in pCa50: (a) in an activation-dependent manner in 10 mm H2O2 (≈0.025) and (b) with GSH exposure after H2O2 exposure (≈0.028). Thus, these fast-twitch soleus fibres behaved quite similarly to the fast-twitch EDL fibres (see Table 1).
As mentioned, pCa50 increased in an activation-dependent manner in DTDP to a much greater extent in slow-twitch fibres than in fast-twitch EDL fibres (total increase of ≈0.41 versus ≈0.11). This shift was fully reversed by 20 min exposure to 10 mm DTT (Table 3, treatment 7) (e.g. Fig. 3), with a 10 min exposure giving noticeably less complete reversal. Thus, the reversal by DTT appeared to be somewhat slower in slow-twitch soleus fibres than in fast-twitch EDL fibres. It also appeared that the rate of reversal could be increased by activating the fibre in the presence of the DTT. In the two slow-twitch fibres examined, a 1 min period of partial activation (≈70 % maximum force) in the presence of 10 mm DTT caused a ≈3-fold greater decrease in pCa50 than did a 1 min period in DTT with the fibre relaxed (at pCa > 9). (Similar partial activation without DTT did not cause any reduction in pCa50.) Thus, activation of a fibre not only induced the oxidation effect, but also facilitated its reversal.
Effect of prolonged exposure to 10 mm H2O2
As described above, exposing a relaxed fibre to 10 mm H2O2 for 5 min caused no significant change in pCa50 in either EDL or soleus fibres (Table 1 treatment 5; Table 3 treatment 3); there was also no change with a briefer exposure (2 min, not shown). In contrast, following a 20 min exposure to 10 mm H2O2 (at pCa > 9), the pCa50 in EDL fibres (measured back in control conditions) decreased considerably (-0.079 ± 0.010, n = 7); nH decreased by 0.9 ± 0.1, which was comparable with the decrease for a 5 min exposure to H2O2, and the maximum force declined by 17 ± 3 %. These effects on pCa50 and maximum force were not noticeably reversed by a 10 min exposure to 10 mm DTT, although nH recovered ≈25 %. The changes after the H2O2 treatment could not be ascribed to effects of time alone, because a similar 20 min period in the absence of H2O2 had little effect on the parameters (mean changes in maximum force: 3 ± 1 %; pCa50 : −0.014 ± 0.005; and nH: 0.0 ± 0.1, measured in five of the EDL fibres preceding the 20 min H2O2 exposure). After the prolonged (20 min) exposure to H2O2, a 2 min exposure to GSH had no significant effect on Ca2+ sensitivity (or maximum force) (changes in pCa50: 0.003 ± 0.002; nH: 0.0 ± 0.0; maximum force: +1 ± 1 %, n = 6); thus, the Ca2+ sensitising effect of GSH evident after a briefer (5 min) exposure to H2O2 was apparently lost. Given all these observations, one can seemingly identify a sequence of oxidative changes that reconciles the findings in intact and skinned fibres (see Discussion).
Reduction of maximum force after exposure to 1 mm DTDP
Finally, the effect of a higher concentration of DTDP (1 mm) was also examined because it has been previously found that another sulphydryl specific reagent, 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB), applied at 10 mm (in pCa > 9 at pH 8.6), had effects on both maximum force and Ca2+ sensitivity in skinned fibres (Wilson et al. 1991). As described above, when 100 μm DTDP was applied to relaxed fibres (in pCa > 9) for 5 min and then washed out, there was no change in maximum force (other than the expected decline when repeating sequences), but there was a significant decrease in pCa50 of ≈0.06 units in both EDL and soleus fibres. Similar exposure to 1 mm DTDP resulted in a decrease in maximum force to ≈40 and ≈50 % of the original level in EDL and soleus (slow-twitch) fibres, respectively (Fig. 4). After a further 10 min exposure to 1 mm DTDP, force production was almost completely abolished in EDL fibres and maximum force in soleus fibres was reduced to only ≈10 % of the control level. Following a 10 min exposure to 10 mm DTT maximum force recovered to ≈45 and 80 % of the pre-DTDP level in EDL and soleus fibres, respectively, but it only increased slightly more with a further 10 min in DTT (Fig. 4B), with virtually no further increase occurring after an additional 60 min in DTT in the one soleus and two EDL fibres examined (e.g. Fig. 4A).
Figure 4. Prolonged exposure to a high concentration of DTDP abolishes force production in EDL and soleus fibres.
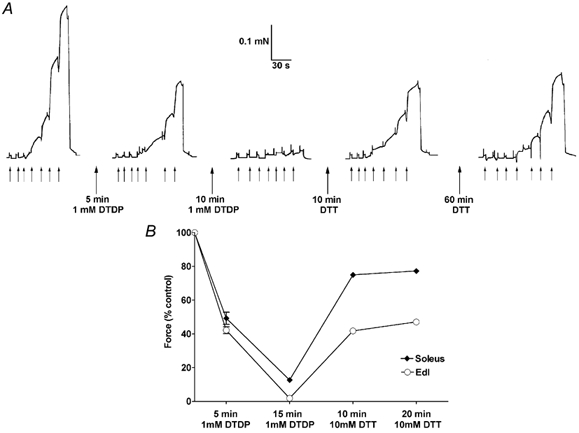
A, maximum force production in an EDL fibre was reduced to ≈40 % after a 5 min exposure to 1 mm DTDP (at pCa > 9), with the reduction in Ca2+ sensitivity being little different from that with brief (15 s) exposure to 100 μm DTDP (see text). Exposure to 1 mm DTDP for a further 10 min almost completely abolished force production, and force recovered to only ≈55 % of the original control level after a total of 70 min in DTT (pCa > 9). In each sequence the fibre was exposed successively to solutions at pCa 6.7, 6.41, 6.20, 6.00, 5.89, 5.75, 4.5, at times indicated by the small upward arrows. B, mean size (± s.e.m.) of maximum force in 3 soleus (♦) and 4 EDL (○) fibres after exposure to 1 mm DTDP for 5 min and then a further 10 min, and then to 10 mm DTT for two 10 min periods. Values are expressed relative to maximum force before exposure to DTDP. The s.e.m. is smaller than the symbol in most cases, showing that the effects are highly reproducible within each fibre type.
The change in pCa50 in the EDL fibres after a 5 min exposure to 1 mm DTDP (-0.075 ± 0.014, n = 4) was little, if at all, different from that occurring with only a 15 s exposure to 100 μm DTDP (Table 2), though the decrease in nH (-1.4 ± 0.4) was larger. The force-pCa relationship in EDL fibres could not be reliably measured after 15 min (total) in 1 mm DTDP, but following 10 min in DTT it was not significantly different from that after the initial 5 min in DTDP (pCa50 and nH change relative to data after 5 min in DTDP: +0.010 ± 0.015, −0.4 ± 0.2, respectively, n = 4), and after a further 10-70 min in DTT, pCa50 was little changed but nH recovered ≈50 % towards its original pre-DTDP level. If the total exposure time to 1 mm DTDP was only 5 min instead of 15 min, pCa50 and nH recovered to values close to their pre-DTDP levels. Thus, in contrast to the findings when DTT was applied following DTNB treatment (Wilson et al. 1991), DTT exposure of DTDP-treated fibres never increased the Ca2+ sensitivity (i.e. pCa50) above its original control level. GSH exposure (5 mm, 2 min) however did cause its usual large shift in pCa50 (+0.294 and 0.246) in the two fibres that had been exposed to 1 mm DTDP for 5 min, with maximum force remaining depressed at ≈50 % of the original control level. Thus, though the GSH effect was lost after prolonged (20 min) exposure to H2O2 (see previous section), it still occurred unchanged after stringent treatment with DTDP, and also occurred independently of any reduction in maximum force caused by the oxidation.
In the soleus fibres, the decrease in pCa50 after exposure to 1 mm DTDP for 5 min (-0.186 ± 0.015, n = 3) was much larger than that seen after exposure to 100 μm DTDP (Table 3; change in nH: −0.9 ± 0.4). After an additional 10 min in 1 mm DTDP, nH declined further (-0.9 ± 0.4) and was significantly smaller than the original level (2.5 ± 0.1 versus 4.3 ± 0.3, n = 3) but the pCa50 shifted back to close to its original (pre-DTDP) level in each fibre. Upon reduction for 10 min in 10 mm DTT the pCa50 actually increased substantially above its original level (+0.196 ± 0.025, n = 3) and nH started to increase towards its original level (change: +0.5 ± 0.2). With a further 10 min in DTT both the pCa50 and nH recovered ≈30-40 % more towards their original levels, and in the one fibre examined after a total of 80 min in DTT, both parameters had returned to close to their original (pre-DTDP) values. These effects, though seemingly complicated, were very reproducible across the three fibres and also were similar to those found when treating soleus fibres with DTNB and DTT (Wilson et al. 1991).
Discussion
This study has characterised the effects of the oxidants H2O2 and DTDP, and reductants GSH and DTT, on the properties of the contractile apparatus in mammalian muscle. It shows that both these oxidants can increase the Ca2+ sensitivity of contractile apparatus by two distinct mechanisms, with the relative potency of these two mechanisms differing greatly between fast-twitch and slow-twitch fibres. The specific characteristics of these mechanisms, namely dependence on activation or on the presence of the endogenous reductant GSH, helps explain the disparities in findings between previous experiments with intact fibres and skinned fibres. This study also illustrates how an oxidant can separately affect maximum force, pCa50 and nH, depending on the concentration of the oxidant and the exact treatment conditions, indicating the many and various means by which oxidation can modify contractile force.
Initial oxidative state and the effect of reductants
The finding that exposure to the strong reductant DTT readily reversed oxidative changes in the contractile apparatus and yet had little or no effect when applied to a freshly skinned fibre (Table 1), strongly suggests that the contractile apparatus: (a) is normally in a reduced state in rested muscle fibres, and (b) works well in such a state. In seeming contrast, treatment with DTT was found to cause a substantial reduction in the Ca2+ sensitivity of the contractile apparatus in intact murine fibres (Andrade et al. 1998a). This difference however could be due to the fact that those intact fibres were subject to some level of repeated activity whilst in a high O2 (95 %) environment, and as such may have been oxidised more than a rested fibre in situ.
The present study further found, in accord with the DTT results, that addition of 5 mm (potassium) GSH had little, if any, effect on the contractile apparatus in freshly skinned fibres, either by its presence or by its action as a reductant (Tables 1 and 3). (We previously reported that the addition of 10 mm sodium GSH caused an appreciable decrease in Ca2+ sensitivity (≈0.07 pCa50 decrease, Posterino & Lamb, 1996); this effect was probably not due simply to the higher concentration of GSH used in that study but instead to the accompanying increase in ionic strength and sodium concentration - compare the conclusion in Patterson et al. 2001.) There was also no apparent difference when GSH was present in a 30:1 ratio with GSSG, which is the situation normally prevailing in the cytoplasm in rested fibres (see Feng et al. 2000). Moreover, varying the redox potential of the cytoplasmic environment over a large range, by changing the GSH:GSSG ratio from 30:1 to 3:1, was also found to have no detectable effect over 15 min on maximum force, pCa50 or nH. This strongly suggests that when oxidative changes do occur in the contractile apparatus they are not caused by the oxidant(s) altering the overall redox potential of the cytoplasm, as is often thought, but instead are caused by direct action of the oxidant(s) on the contractile apparatus and events subsequent to that.
Physiological versus pharmacological effects of H2O2
The concentration of H2O2 required to cause oxidative changes in the skinned fibres in this study is several orders of magnitude higher than that occurring in intact muscle fibres in vivo. However, this is not unexpected and should not be taken to mean that the reported effects would not occur in vivo. Hydrogen peroxide is a relatively stable ‘reactive oxygen species’ (ROS), which probably exerts its effects by generating the hydroxyl radical, OH• a highly reactive free radical (Halliwell & Gutteridge, 1999). Homolytic fission of H2O2 into two OH• molecules normally occurs only to an extremely small extent in the solutions like those used in this study with 50 mm EGTA present to chelate heavy metal ions. However, in a muscle cell, H2O2 readily liberates Fe2+ from haem in myoglobin and rapidly generates OH• by the Fenton reaction, and can also react with GSH to generate OH• and GS• radicals (Halliwell & Gutteridge, 1999). Consequently, it is to be expected that H2O2 generated within, or applied to, an intact muscle cell would be far more efficacious than H2O2 applied to skinned fibres in this study, though the end effects could be expected to be quite similar (see also later).
Activation-dependent oxidative effects
Andrade et al. (1998a) showed that exposure of intact murine fast-twitch fibres to 100-300 μm H2O2 resulted in an increase in the Ca2+ sensitivity of the contractile apparatus within a few minutes (≈0.08 pCa increase), followed by a larger decrease in sensitivity upon longer exposure, particularly at a higher H2O2 concentration. The mechanisms involved in these changes were unclear, particularly because comparable changes were not observed in skinned fibres (see Introduction). Here, we first confirmed previous findings that exposing skinned fast-twitch fibres to H2O2 for 5 min whilst relaxed in a solution at pCa > 9 has little or no effect on pCa50 and reduces nH by ≈25 % (Table 1). Importantly, we further showed that the effects of H2O2 were quite different in other circumstances. If the skinned fibres were activated in the presence of 10 mm H2O2 (with the total exposure time maintained at ≈5 min) there was in fact an increase in pCa50 (≈0.06; e.g. Fig. 1), and a larger decrease in nH. Exposure to DTT (10 mm, 10 min) largely or fully reversed these effects, indicating that they were indeed due to oxidation reactions (Table 1 treatment 6). A comparable activation-dependent effect was also observed with the sulphydryl-specific oxidant, DTDP (100 μm) (net pCa50 increase ≈0.05; nH decrease ≈35 %; Table 2). The effect did not seem to depend on [Ca2+] per se as it only developed in an individual fibre if the [Ca2+] was sufficient to produce noticeable force. Thus, oxidants can directly increase the Ca2+ sensitivity of the contractile apparatus in skinned fibres but this effect is dependent on whether the fibre is activated or kept relaxed. This could be because the oxidants only have access to certain sites when the contractile apparatus is activated or because certain sites come sufficiently close together to be cross-linked under such circumstances.
It is possible that this activation-dependent oxidation mechanism was the predominant cause of the increase in pCa50 in the intact fibre study of Andrade et al. (1998a) because the fibres in that study were repetitively activated at 1 to 2 min intervals in the continued presence of H2O2. However, a reason to doubt this is that the periods of activation (submaximal tetani) only lasted ≈0.35 s, whereas the activation-dependent increase in Ca2+ sensitivity observed here (at a similar temperature) took ≈30 to 60 s of activation for the full effect to occur (Fig. 1 and also Fig. 3).
GSH effect in oxidised fibres
It seems likely that a second, distinctly different, oxidative phenomenon occurring in fast-twitch fibres underlay, or substantially contributed to, the increase in pCa50 occurring upon H2O2 exposure in intact fibres. It was found that after a skinned EDL fibre had been exposed to an oxidant, either H2O2 or DTDP, a brief (2 min) subsequent exposure to GSH caused an increase in pCa50 (≈0.03 for H2O2 and ≈0.25-0.30 for DTDP; Tables 1 and 2). The effect was reversed not only by exposure to DTT (Fig. 2) but also by prolonged exposure (> 20 min) to GSH itself. The GSH effect occurred irrespective of whether or not the fibre was activated (Table 2), so given that GSH is normally present in intact fibres one would expect that the phenomenon would have occurred in the intact fibres in the study of Andrade et al. (1998a). The pCa50 increase with GSH exposure in H2O2-oxidised skinned fibres was comparatively small, less than half of the increase observed in intact fibres. This difference might be due simply to the generation of greater amounts of OH• (or related ROS, such as superoxide) in the intact fibres (see earlier). In skinned fibres that had been oxidised with DTDP, the GSH effect was extremely large (Fig. 2), almost 10-fold higher than with H2O2. Although DTDP is highly reactive, it should specifically react with free sulphydryl groups, and these same groups should also be targets for oxidation by OH• and other ROS, as well as by other species such as GS•. Reversal of these various oxidation events by GSH could be expected to generate similar intermediary molecules, and consequently differences in the extent of the pCa50 shifts may simply reflect differences in the extent of oxidation of certain free sulphydryl groups rather than a difference in the type of oxidation events at those sites (see Discussion, Possible basis of GSH effect in oxidised fibres).
Distinction between GSH-mediated and activation-dependent oxidation events
It appears that the sites involved in the ‘GSH effect’ are different from those involved in the ‘activation effect’. Firstly, the size of the GSH effect was similar irrespective of whether or not the activation effect had been induced (see pCa50 shifts with GSH in treatments 1 and 4 of Table 2). Secondly, some or all of the activation effect could still be induced after maximal induction of the GSH effect (see Results). Thirdly, in soleus slow-twitch fibres the activation effect with DTDP was very large but there was no GSH effect at all (Table 3). It seems likely that the GSH effect occurs when GSH acts on free sulphydryl sites that have been rapidly oxidised by DTDP (e.g. within ≈15 s exposure to 100 μm DTDP). In contrast, activating fibres after such DTDP oxidation did not give rise to the activation effect; this effect only occurred if DTDP was present in the bathing solution during the actual activation process. Thus, DTDP oxidation (or brief H2O2 oxidation) seems to ‘prime’ sites so that GSH exposure can subsequently induce its sensitising effect, but such oxidation does not enable activation to subsequently exert its effect.
Possible basis of GSH effect in oxidised fibres
The functional consequences of the ‘GSH effect’ are clear and pronounced. Furthermore, the conditions under which it occurs, and is reversed, are so specific that it seems possible to get some insight into its molecular basis even without knowing the particular proteins and sites involved, or indeed even the precise effect of the initial oxidation. DTDP (2,2′-dithiodipyridine) specifically reacts with free sulphydryls on cysteine residues in a target protein (R-SH) to create a mixed disulphide between the cysteine residue and one half of the DTDP molecule (i.e. creating RSSP) and liberating the other half of DTDP as thiopyridone (TP) (Brocklehurst, 1979; Zaidi et al. 1989). It may also be possible that, if two cysteine groups are appropriately positioned, DTDP could react to form a disulphide bridge between them (forming RSSR), and liberating two TP molecules (see analogous effect of DTNB; Wilson et al. 1991). These reactions would be effectively unidirectional because of the production of thiopyridone (Brocklehurst, 1979; Zaidi et al. 1989). Thus, the reduced Ca2+ sensitivity after DTDP exposure is most likely due to the attachment of pyridyl sulphide (one half of DTDP) to a cysteine in one of the contractile proteins, that is the formation of RSSP, or to the formation of a disulphide bond (RSSR) in the protein.
Now: (a) GSH initially caused the Ca2+-sensitising effect in DTDP-oxidised fibres and then subsequently itself reversed this effect; (b) after the initial GSH sensitising effect, reapplying DTDP had no further effect; (c) GSSG was entirely without effect in any situation; and (d) DTT reversed the DTDP-oxidation effect, and also the GSH effect, without itself having any Ca2+-sensitising action. These effects would seem to be well explained by the fact that GSH reduces disulphide bonds by a two-step reaction involving two GSH molecules, whereas a single DTT molecule can do so in effectively a one-step reaction (Cleland, 1964). Specifically, one GSH molecule reacts with a RSSR disulphide bridge to form a free R-SH group and the mixed disulphide RSSG, and the latter then has to react with another GSH molecule to produce the second free RSH group (and reduced glutathione, GSSG). Similarly, when GSH reduces RSSP, it might be expected to form thiopyridone and RSSG, before a second GSH molecule reacts with the latter to give a free RSH group and GSSG. Thus, all of the findings (a) to (d) above could be explained if the Ca2+ sensitivity of the contractile apparatus is greatly increased when RSSG is formed at the cysteine residue(s) where DTDP reacts. Specifically: (i) GSH exposure of DTDP-oxidised fibres would initially lead to the formation of RSSG, but longer exposure to GSH or DTT would reduce the RSSG back to RSH; (ii) DTDP would not react with either RSSG or RSSR; (iii) GSSG would be unable to replace GSH in its thiol-disulphide exchange reaction with RSSP and (iv) DTT would reduce RSSP or RSSR to free RSH groups without forming any intermediate (Cleland, 1964).
A similar situation can be expected when the fibre is oxidised by H2O2/OH•. Hydroxyl radicals will oxidise RSH groups to generate RS•, which readily reacts with GS− (the ionised form of GSH), with the end product being the same mixed disulphide as above, RSSG (Halliwell & Gutteridge, 1999). The same product would also be produced when OH• reacts with endogenous GSH to give GS•, which in turn would react readily with RS− groups. Thus, the same overall mechanism can account for increased sensitivity occurring when applying GSH to fibres oxidised with either DTDP or H2O2.
Oxidation effects and post-tetanic potentiation
Whatever its molecular basis, the ‘GSH effect’ (i.e. the increase in Ca2+ sensitivity occurring when GSH initially interacts with oxidised contractile apparatus) seems likely to be functionally important in muscle fibres. Muscle activity is known to generate reactive oxygen species (ROS; Reid, 2001). Consequently, one might expect some of these ROS to reach and interact with the contractile apparatus and then the GSH endogenously present would act on it, increasing the Ca2+ sensitivity, resulting in potentiation of twitches and submaximal tetani. This potentiation would then be spontaneously lost over many minutes, as the GSH present reversed its initial Ca2+ sensitising effect. In view of this, the GSH effect may contribute to the well-known phenomenon of post-tetanic potentiation, at least under some circumstances. Although post-tetanic potentiation is usually regarded as being due solely to phosphorylation of the myosin light chain (MLC; Sweeney et al. 1993), the poor correspondence between the levels of potentiation and MLC phosphorylation occurring in some cases (e.g. with prolonged stimulation and fatigue) has led to the conclusion that some other factor besides MLC phosphorylation contributes to the total level of post-tetanic potentiation (Tubman et al. 1996). It is also interesting to note that both the GSH effect and post-tetanic potentiation occur in only fast-twitch and not slow-twitch fibres (see Table 3; Sweeney et al. 1993).
Sequence of actions of H2O2 in an intact fibre
Brief (5 min) treatment with H2O2 had oxidative effects in EDL fibres that were qualitatively comparable with those found with DTDP treatment, in particular causing both GSH-dependent and activation-dependent increases in Ca2+ sensitivity, with these effects being readily reversed by DTT exposure. As DTDP specifically reacts with free sulphydryls, it seems likely that these effects of brief H2O2 treatment also involve free sulphydryl groups. In contrast, when the exposure to H2O2 was prolonged to 20 min, the GSH effect was seemingly lost and the Ca2+ sensitivity decreased and was not readily reversed by DTT exposure. No such difference was seen between brief and more prolonged treatments with DTDP (apart from a separate effect on maximum force discussed in the next section). Thus, it seems that prolonged treatment with H2O2 causes a different type of reaction; this is not surprising, as OH• (and related ROS products) will also react with many sites besides free sulphydryls, for example oxidising methionine to methionine sulphoxide, a reaction which is poorly reversible by DTT unless catalysed by an enzyme present in intact cells (Halliwell & Gutteridge, 1999).
Although the precise events are uncertain, it seems that the various findings described here in skinned fibres together seem to account for the changes observed with H2O2 treatment in intact fibres (Andrade et al. 1998a). The Ca2+ sensitivity initially increases with little or no change in maximum force, presumably due to the GSH effect and possibly also the activation-dependent effect, and then decreases substantially below the initial control level, presumably due to the effects of prolonged H2O2/OH• exposure directly on a different site on the contractile proteins. This type of situation, where the oxidant initially causes one type of effect and then more slowly causes additional effects, is broadly similar to that proposed by Andrade et al. (1998b) to explain the differences between intact and skinned fibre studies in regard to the effect of nitric oxide donors.
Effects on maximum force
Finally, it was apparent that oxidation affected maximum force by a fundamentally different action from the actions (described above) involved in increasing pCa50 and decreasing nH. Maximum force was unaffected by the GSH and activation effect, and the GSH effect occurred seemingly unchanged even when maximum force had been substantially reduced by exposure to a high concentration (1 mm) of DTDP. Clearly, reduction in maximum force required exposure to the oxidant for a longer time, or at a higher concentration, than did the increase in pCa50 or decrease in nH. When the treatment was sufficiently stringent, both DTDP and H2O2 caused a reduction in maximum force, with the effect increasing with longer exposure time. As the reduction in maximum force with 1 mm DTDP is very similar to the effect observed previously with DTNB (10 mm, at pH 8; Wilson et al. 1991), it is very likely due to the same mechanism, that is, to oxidation of free sulphydryl groups SH1 and SH2 on the myosin heads.
We found that when maximum force in EDL fibres had been decreased by exposure to 1 mm DTDP, DTT exposure reversed or partially reversed the accompanying decrease in pCa50 without causing any ‘overshoot’. Thus, there was no increase in pCa50 above its original control level, as occurs when DTT is used to reverse the effects of DTNB treatment (Wilson et al. 1991). As mentioned, only GSH exposure produced such an overshoot effect in the EDL fibres here after treatment with 1 mm DTDP. In contrast to the EDL fibres, DTT exposure after 1 mm DTDP did produce a prominent overshoot effect in soleus fibres here. This seeming disparity might be explained by the simultaneous occurrence of independent (and opposing) oxidation effects. It was found here that the activation effect (i.e. the increase in pCa50 with activation in the presence of an oxidant) was extremely large in the soleus fibres with DTDP treatment, much larger than in the EDL fibres. It is possible that this effect, which at a relatively low oxidant concentration (i.e. 100 μm DTDP) occurs within minutes only if the fibre is activated, may also occur in a relaxed fibre given sufficient time and high enough concentration of oxidant. Thus, it is possible that the exposure to 1 mm DTDP, in addition to reducing maximum force: (a) decreases pCa50 by oxidation of one type of site, and (b) simultaneously increases pCa50 by oxidation of another site, possibly the site involved in the activation effect. When the fibre is exposed to DTT, the effect in (a) might be rapidly reversed, whereas the effect in (b) might be reversed more slowly. This would initially result in the pCa50 increasing above its original control level before declining to that level after longer DTT treatment, as observed by Wilson et al. (1991) and also here under some circumstances. Further, this type of scenario would fit well with our findings that the activation effect was far larger in soleus fibres than in EDL fibres and also was relatively slowly reversed by DTT exposure.
In conclusion, this study has identified and characterised a number of redox phenomena that contribute to the diverse effects of oxidation on the contractile apparatus in skeletal muscle. These results help reconcile and explain previous findings in intact and skinned fibres and give important insights into the physiological mechanisms by which oxidation modifies muscle performance.
Acknowledgments
We thank Maria Cellini and Aida Yousef for technical assistance, Professor George Stephenson and Dr Niels Ortenblad for helpful discussions and comments, and the National Health and Medical Research Council of Australia for financial support (Grant No. 991496).
References
- Andrade FH, Reid MB, Allen DG, Westerblad H. Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J Physiol. 1998a;509:565–575. doi: 10.1111/j.1469-7793.1998.565bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade FH, Reid MB, Allen DG, Westerblad H. Effect of nitric oxide on single skeletal muscle fibres from the mouse. J Physiol. 1998b;509:577–586. doi: 10.1111/j.1469-7793.1998.577bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay JK, Hansel M. Free radicals may contribute to oxidative skeletal muscle fatigue. Can J Physiol Pharmacol. 1991;69:279–284. doi: 10.1139/y91-043. [DOI] [PubMed] [Google Scholar]
- Bortolotto SK, Cellini M, Stephenson DG, Stephenson GM. MHC isoform composition and Ca2+ or Sr2+ -actiavtion properties of rat skeletal muscle fibers. Am J Physiol Heart Circ Physiol. 2000;267:H1010–1016. doi: 10.1152/ajpcell.2000.279.5.C1564. [DOI] [PubMed] [Google Scholar]
- Brocklehurst K. Specific covalent modification of thiols: applications in the study of enzymes and other biomolecules. Int J Biochem. 1979;10:259–274. doi: 10.1016/0020-711x(79)90088-0. [DOI] [PubMed] [Google Scholar]
- Brotto MA, Nosek TM. Hydrogen peroxide disrupts Ca2+ release from the sarcoplasmic reticulum of rat skeletal muscle fibers. J Appl Physiol. 1996;81:731–737. doi: 10.1152/jappl.1996.81.2.731. [DOI] [PubMed] [Google Scholar]
- Callahan LA, She ZW, Nosek TM. Superoxide, hydroxyl radical, and hydrogen peroxide effects on single-diaphragm fiber contractile apparatus. J Appl Physiol. 2001;90:45–54. doi: 10.1152/jappl.2001.90.1.45. [DOI] [PubMed] [Google Scholar]
- Cleland WW. Dithiothreitol, a new protective reagent for SH groups. Biochemistry. 1964;3:480–482. doi: 10.1021/bi00892a002. [DOI] [PubMed] [Google Scholar]
- Darnley GM, Duke AM, Steele DS, Macfarlane NG. Effects of reactive oxygen species on aspects of excitation-contraction coupling in chemically skinned rabbit diaphragm muscle fibres. Exp Physiol. 2001;86:161–168. doi: 10.1113/eph8602109. [DOI] [PubMed] [Google Scholar]
- Feng W, Liu GH, Allen PD, Pessah IN. Transmembrane redox sensor of ryanodine receptor complex. J Biol Chem. 2000;275:35902–35907. doi: 10.1074/jbc.C000523200. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. 3. Oxford: Oxford University Press; 1999. [Google Scholar]
- Lamb GD, Stephenson DG. Effects of intracellular pH and [Mg2+] on excitation-contraction coupling in skeletal muscle fibres of the rat. J Physiol. 1994;478:331–339. doi: 10.1113/jphysiol.1994.sp020253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer W, Herrmann-Frank A, Lüttgau HC. The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibres. Biochim Biophys Acta. 1995;1241:59–116. doi: 10.1016/0304-4157(94)00014-5. [DOI] [PubMed] [Google Scholar]
- Oba T, Koshita M, Yamaguchi M. H2O2 modulates twitch tension and increases Po of Ca2+ release channel in frog skeletal muscle. Am J Physiol. 1996;40:C810–818. doi: 10.1152/ajpcell.1996.271.3.C810. [DOI] [PubMed] [Google Scholar]
- Patterson MF, Stephenson GM, Stephenson DG. A study of the short-term effects of glucose 6-phosphate on the contractile activation properties of skinned single muscle fibres of the rat. Implications for solution design. Pflügers Arch. 2001;442:874–881. doi: 10.1007/s004240100605. [DOI] [PubMed] [Google Scholar]
- Perkins WJ, Han Y-S, Sieck GC. Skeletal muscle force and actomyosin ATPase activity reduced by nitric oxide donor. J Appl Physiol. 1997;83:1326–1332. doi: 10.1152/jappl.1997.83.4.1326. [DOI] [PubMed] [Google Scholar]
- Plant DR, Lynch GS, Williams DA. Hydrogen peroxide modulates Ca2+-activation of single permeabilized fibres from fast-and slow-twitch skeletal muscles of rats. J Muscle Res Cell Motil. 2000;21:747–752. doi: 10.1023/a:1010344008224. [DOI] [PubMed] [Google Scholar]
- Posterino GS, Lamb GD. Effects of reducing agents and oxidants on excitation-contraction coupling in skeletal muscle fibres of rat and toad. J Physiol. 1996;496:809–825. doi: 10.1113/jphysiol.1996.sp021729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MB. Redox modulation of skeletal muscle contraction: what we know and what we don't. J Appl Physiol. 2001;90:724–731. doi: 10.1152/jappl.2001.90.2.724. [DOI] [PubMed] [Google Scholar]
- Reid MB, Khawli FA, Moody MR. Reactive oxygen in skeletal muscle. III. Contractility of unfatigued muscle. J Appl Physiol. 1993;75:1081–1087. doi: 10.1152/jappl.1993.75.3.1081. [DOI] [PubMed] [Google Scholar]
- Salama G, Abramson JJ, Pike GG. Sulphydryl reagents trigger Ca2+ release from the sarcoplasmic reticulum of skinned rabbit psoas fibres. J Physiol. 1992;454:389–420. doi: 10.1113/jphysiol.1992.sp019270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson DG, Williams DA. Calcium-activated force responses in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures. J Physiol. 1981;317:281–302. doi: 10.1113/jphysiol.1981.sp013825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney HL, Bowman FB, Stull JT. Myosin light chain phosphorylation in vertebrate striated muscle: regulation and function. Am J Physiol. 1993;264:C1085–1095. doi: 10.1152/ajpcell.1993.264.5.C1085. [DOI] [PubMed] [Google Scholar]
- Tubman LA, Macintosh BR, Maki WA. Myosin light chain phosphorylation and post-tetanic potentiation in fatigued skeletal muscle. Pflügers Arch. 1996;431:882–887. doi: 10.1007/s004240050081. [DOI] [PubMed] [Google Scholar]
- Wilson GJ, dos Remedios CG, Stephenson DG, Williams DA. Effects of sulphydryl modification of skinned rat skeletal muscle fibres using 5,5′-dithiobis(2-nitrobenzoic acid) J Physiol. 1991;437:409–430. doi: 10.1113/jphysiol.1991.sp018603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi NF, Lagenaur CF, Abramson JJ, Pessah IN, Salama G. Reactive disulfides trigger Ca2+ release from sarcoplasmic reticulum via an oxidation reaction. J Biol Chem. 1989;264:21725–21736. [PubMed] [Google Scholar]


