Abstract
The acid-sensitive ion channel ASIC1 is a proton-gated ion channel from the mammalian nervous system. Its expression in sensory neurons and activation by low extracellular pH suggest that ASIC is involved in transmitting nociceptive impulses produced by the acidification caused by injury or inflammation. However, ASIC1 expression is not restricted to sensory neurons. To understand the functional role of ASIC1 in the CNS we investigated its expression and subcellular distribution therein. In particular, we examined the presence of ASIC1 in domains where the local pH may drop sufficiently to activate ASIC1 under physiological conditions. Immunostaining with specific antibodies revealed broad expression of ASIC1 in many areas of the adult rat brain including the cerebral cortex, hippocampus and cerebellum. Within cells, ASIC1 was found predominantly throughout the soma and along the branches of axons and dendrites. ASIC1 was not enriched in the microdomains where pH may reach low values, such as in synaptic vesicles or synaptic membranes. Pre- or postsynaptic ASIC1 was not gated by synaptic activity in cultured hippocampal neurons. Blockage or desensitization of ASIC1 with amiloride or pH 6.7, respectively, did not modify postsynaptic currents. Finally, the ontogeny of ASIC1 in mouse brain revealed constant levels of expression of ASIC1 protein from embryonic day 12 to the postnatal period, indicating an early and almost constant level of expression of ASIC1 during brain development.
Acid-sensitive ion channel (ASIC)1α, also known as brain Na+ channel 2 (BNaC2), is one of six acid-sensitive ion channels so far cloned from the mammalian nervous system (Garcia-Añoveros et al. 1997; Waldmann et al. 1997). Expression of ASIC1 in neurons from the dorsal root ganglia (DRG) and gating of the channel by external protons imply that ASIC1 may constitute a receptor that is able to detect acidification (Waldmann et al. 1997; Sutherland et al. 2001). Indeed, many noxious stimuli are associated with extracellular acidification, such as that caused by injury, inflammation or ischaemia. Expression of ASIC1 in certain populations of DRG neurons overlaps with the expression of the vallinoid receptor, which is another molecule that is gated directly by low pHo (Tominaga et al. 1998), suggesting that activation of ASIC1 may contribute to the response to low pHo.
In the CNS, ASIC1 is the most abundantly expressed channel of the ASIC family (Garcia-Añoveros et al. 1997; Waldmann et al. 1997); however, the function of ASIC1 in the CNS has not been established. Many endogenous substances and drugs modulate noxious responses by acting on neurons from the CNS. However, current data on the distribution of ASIC1 is not consistent with a link to the relay function for nociception. With the aid of in situ hybridization, ASIC1 mRNA has been found in the olfactory bulb, cerebral cortex, hippocampus, basolateral amydgaloid nuclei, subthalamic nuclei and cerebellum (Garcia-Añoveros et al. 1997; Waldmann et al. 1997). Recently, a mouse model with inactivation of the ASIC1 gene has been generated. Defects in nociception or other sensory modalities were not reported in the study, but the animals exhibited mild deficits in spatial learning and impaired eyeblinking conditioning, indicating the importance of ASIC1 in the CNS (Wemmie et al. 2002).
Effective activation of ASIC1 requires rapid and large decreases in pHo of approximately one pH unit (Waldmann et al. 1997; Alvarez de la Rosa et al. 2002; Baron et al. 2002; Benson et al. 2002). Protons also induce desensitization, thus, reactivation of channels requires the pHo to return to values greater than 7.3. Recovery from desensitization is slow, with less than 50 % recovery after 4 s of exposure to neutral pHo (Benson et al. 2002). When all of these properties are considered, it is concluded that efficient activation of ASIC1 in the CNS may occur in locations where the pHo can change rapidly, profoundly and reversibly. Those conditions may be met in a few microenvironments such as the lumen of intracellular vesicles or the synaptic cleft. The latter is an attractive possibility because there, synaptic vesicles repeatedly release acidic content (≈pH 5.6; Miesenbock et al. 1998) in a small and delimited space. In response to repetitive high-frequency stimulation, the release of the acidic content of synaptic vesicles could temporarily overwhelm the mechanisms for proton buffering, diffusion and extrusion in the synaptic cleft, and the pHo could decrease sufficiently to gate ASIC1. It should be noticed, however, that the kinetics and extent of pHo changes in the synaptic cleft have not been determined experimentally.
To further the understanding of the functional roles of ASIC1 in the CNS, we sought to establish the distribution of ASIC1 protein in the adult brain. In particular, we investigated whether ASIC1 was enriched in domains where the pH may undergo rapid and transient changes, such as synaptic vesicles and synapses, and whether synaptic transmission could be a stimulus for activation of ASIC1 in CNS neurons. We also examined the ontogeny of ASIC1 in the mouse to correlate expression of this channel with synaptogenesis in the developing brain.
Methods
Primary antibodies
A new anti-ASIC antibody directed against the extracellular domain of the rat ASIC1 was developed in rabbits. A glutathione-S-transferase fusion protein was generated in the plasmid pGEX-2T containing the sequence N119-F157. The peptide was produced in Escherichia coli, isolated with agarose-glutathione beads and used as the antigen for immunization. This serum is referred to as anti-ASIC1-EL to distinguish it from a previously developed antibody against the carboxyterminus: anti-ASIC1-CT (Alvarez de la Rosa et al. 2002). A monoclonal antibody against microtubule-associated protein (MAP)2 was a kind gift from Dr Pietro de Camilli. Monoclonal anti-postsynaptic density protein (PSD)-95 was obtained from Affinity Bioreagents (Golden, CO, USA). Monoclonal anti-β-tubulin and monoclonal anti-synaptophysin (clone SVP-38) were from Sigma (St Louis, MO, USA). Monoclonal antibody anti-NMDA receptor 1 (NMDAR1) was from PharMingen (San Diego, CA, USA).
Western blots and digestion of proteins with peptide: N-glycosidase F (PNGase-F)
Protein samples from Xenopus oocytes injected with ASIC1α cRNA and from rat brain were prepared by homogenization with a Potter-Elvehjem tissue grinder in lysis buffer (mm): 50 Tris-HCl pH 7.5, 5 EDTA, 150 NaCl, 1 % Triton X-100, protease inhibitor cocktail (Roche Biochemicals, Indianapolis, IN, USA). Homogenates were spun at 10 000 g and the supernatants were used as a source of protein. Protein concentration was measured with the BCA protein assay kit (Pierce, Rockford, IL, USA). Equal amounts of protein were resolved in 10 % SDS-polyacrylamide gels and transferred to Immobilon-P membranes (Millipore, Bedford, MA, USA) for Western blot analysis. To examine the distribution of ASIC1 in rat brain we used a commercial ReadyBlot Adult Brain Protein Explorer from Alpha Diagnostic (San Antonio, TX, USA), which contains ≈100 μg of brain tissue proteins per lane. After blocking the membranes with 5 % dry-milk in Tris buffer saline Tween 20 (TBST: 20 mm Tris, pH 7.6, 120 mmNaCl, 0.1 % Tween), they were incubated with a 1:2000 dilution of the specific purified ASIC1 (Alvarez de la Rosa et al. 2002) or anti-β-tubulin (Sigma) antibodies at room temperature for 1-2 h, followed by extensive washes with TBST. A 1:10 000 dilution of HRP-conjugated anti-rabbit IgG antibody was added and membranes were incubated for 1 h (Sigma). Blots were developed with enhanced chemiluminescence plus (Amersham Pharmacia Biotech) and exposed to BioMax MR film (Eastman Kodak, New Haven, CT, USA). Where indicated, proteins extracted from oocytes or brain were deglycosylated with PNGase-F (Roche Biochemicals) following the manufacturer's instructions, prior to loading onto the SDS-PAGE system.
Immunohistochemistry of rat brain
Adult male Sprague-Dawley rats were anaesthetized with 0.4 ml Nembutal, administered intraperitoneally. Animals were perfused through the heart first with Ca2+-free Tyrode solution, followed by fixative (4 % paraformaldehyde and 0.2 % picric acid in 0.1 m phosphate buffer). The brain was dissected and transferred into 10 % sucrose buffer solution overnight prior to cutting. Frozen sections (10 or 30 μm) were processed for immunofluorescence histochemistry. Sections were incubated for 16-18 h at 4 °C with primary antibody diluted 1:1000 in PBS/0.3 % TritonX-100/0.1 % bovine serum albumin (BSA)/10 % goat serum. After several washes with the same buffer, sections were incubated with anti-rabbit IgG goat antibody (1:200) labelled with Alexafluor (Molecular Probes). As a control for the specificity of the antibody, we pre-adsorbed the primary antibody with a 10-fold excess of the cognate peptide in incubation buffer for 3 h at room temperature before adding to tissue sections.
Primary hippocampal and cortical cultures
Cultures of rat hippocampus or mouse cortex were prepared essentially as described by Wilcox et al. (1994). Briefly, mouse embryos at embryonic days (E)16-17 or rat embryos at E18-E19 were removed from the uterine horn and placed in Hanks’ balanced salts solution (HBS-S). Hippocampi or cortices were isolated, the meninges removed, and the tissue cut into small pieces. Tissue pieces were incubated with 0.03 % w/v trypsin in HBS-S (hippocampi) or with papain 0.3 U ml−1, dispase 0.1 % w/v, DNase I 0.01 % w/v in HBS-S (cortices) for 15 min at 37 °C. Cells were then dispersed with fire-polished Pasteur pipettes of progressively smaller diameter. Cells were plated in neurobasal medium supplemented with B-27 (Invitrogen Life Technologies, Carlsbad, CA, USA) on glass coverslips coated with poly-l-lysine (Peptide-International, Louisville, KY, USA) for hippocampal neurons or with poly-l-lysine and laminin (Invitrogen Life Technologies) for cortical neurons. The Yale University Committee on Animal Research approved all of the animal procedures used in this study.
Immunostaining of neurons
Cultures of cells at 10-14 days old were fixed with 4 % paraformaldehyde and 4 % sucrose in PBS (PBSS) for 15 min at room temperature. Cells were washed three times in PBSS and permeabilized with 0.1 % Triton X-100 in PBSS. Non-specific protein binding sites were blocked with 10 % goat serum and 1 % BSA in PBSS for 1 h at room temperature. Primary antibodies were diluted in blocking solution and incubated overnight at 4 °C. Dilutions were as follows: ASIC1-CT and ASIC1-EL, 1:500; MAP2 1:500; PSD-95 1:80. After washing three times with PBSS, cells were blocked again for 30 min in blocking solution. Secondary goat anti-mouse Texas Red-conjugated antibody and goat anti-rabbit Cy2-conjugated antibody (Jackson Immunoresearch Laboratories) were diluted 1:200 in blocking solution and the cells were incubated for 1 h at room temperature. Cells were washed three times with PBSS, once with water, and then coverslips were mounted with Aquamount medium (BDH Chemicals, Poole, Dorset, UK). Digital images were obtained on a Nikon Eclipse TE200 microscope equipped with a CCD camera. Images were compiled with Adobe Photoshop 5.0.
Isolation of fetal brain tissue and analysis of ASIC1 expression during development
Pregnant mice were obtained from Charles River Laboratories (Wilmington, MA, USA) and brains from animals at E12-E16 and postnatal day (P)0-P10 were collected. At each stage, material from several animals was pooled and transferred to ice cold lysis buffer (10 mm Hepes pH 7.4, 1.7 % sucrose). The buffer was supplemented with a protease inhibitor cocktail (Roche Biochemicals). Tissues were homogenized by 10-20 strokes with a Potter-Elvehjem tissue grinder operating at 2000 r.p.m. The sucrose concentration was adjusted to 8 % and the homogenate was centrifuged at 1000 g for 10 min. The postnuclear supernatant was then centrifuged at 100 000 g for 1 h to obtain a crude microsomal fraction, which was then resuspended in lysis buffer. Protein concentration was measured with the bicinchoninic acid procedure (BCA kit, Pierce) and equal amounts of protein were loaded on a 10 % SDS-PAGE gel. Gel replicas were stained with Coomassie blue as a control for protein loading or transferred to Immobilon-P membranes (Millipore). ASIC1 protein abundance was examined by Western blot using anti-ASIC1-CT purified antibody.
Isolation of synaptic vesicles from rat brain
Synaptic vesicles were prepared by differential centrifugation, as described previously (Huttner et al. 1983). Adult rat brain tissue was homogenized in 320 mm sucrose, 4 mm Hepes-NaOH, pH 7.4. The homogenate (H) was centrifuged at 800 g and the pellet (P1) was saved. The supernatant (S1) was centrifuged at 9200 g, yielding a pellet (P2) and supernatant (S2); this procedure was repeated by centrifugation of the resuspended pellet P2 at 10 200 g, yielding a washed synaptosomal pellet (P2′). The supernatants from both centrifugations (S2 and S2′) were combined and centrifuged at 184 000 g, yielding pellet P3 and supernatant S3, which corresponds to the cytosolic fraction. The washed synaptosomes (P2′) were resuspended, lysed hypotonically and centrifuged at 25 000 g. The pellet (LP1) was saved and the supernatant (LS1) was centrifuged at 184 000 g. The resulting pellet (LP2), was a fraction containing crude synaptic vesicles. Equal amounts of protein from the different fractions were analysed by Western blot with anti-ASIC1 antibody or with an anti-synaptophysin monoclonal antibody, a marker for synaptic vesicles.
Isolation of postsynaptic densities (PSDs) from rat brain
PSDs were isolated from rat brain as described by Carlin et al. (1980). Briefly, the whole brain (≈1.7 g wet weight) removed from an adult rat was homogenized by 12 strokes with a motor-operated Teflon-glass homogenizer in solution A (mm: 320 sucrose, 1 NaHCO3, 1 MgCl2, 0.5 CaCl2) and diluted to 10 % (w/v) in solution A. A low-speed pellet (1400 g) was obtained and washed by resuspension in a 10 % volume of solution A. A second centrifugation was performed at 710 g for 10 min. The supernatant was taken and centrifuged at 13 800 g for 10 min. The resulting pellet, containing synaptosomes and mitochondria, was resuspended in solution B (mm: 320 sucrose, 1 NaHCO3) using 2.4 ml 1 g−1 starting tissue. Of the resulting material, 8 ml was placed onto a sucrose step density (gradient no. 1) containing 10 ml each of 0.85, 1.0 and 1.2 m sucrose solutions in 1 mm NaHCO3. Gradient no. 1 was run in a SW28 rotor and Beckman L8-70M ultracentrifuge at 82 500 g for 2 h. The band between 1.0 and 1.2 m sucrose was removed by aspiration with a 21-gauge needle and diluted with solution B to a volume of 6 ml 1 g−1 of initial material and an equal volume 1 % (v/v) of Triton X-100 in 0.32 m sucrose and 12 mm Tris-HCl (pH 8.1). The suspension was stirred at 4 °C for 15 min and then centrifuged at 32 800 g. The pellet was resuspended in 0.25 ml of solution B per 1 g original wet weight brain, and 0.5 ml of this material was layered on a gradient composed of 4 ml of 2.0 m sucrose, 3 ml of 1.5 m sucrose-1 mm NaHCO3, and 3 ml of 1.0 m sucrose-1 mm NaHCO3, and centrifuged at 201 800 g for 2 h. The band between 1.5 and 2.0 m sucrose containing PSDs was removed by needle aspiration and diluted to a volume of 2.4 ml with solution B and an equal volume of 0.1 % Triton-150 mm KCl. This suspension was centrifuged for 20 min at 201 800 g. The resulting pellet was resuspended by homogenization with a Teflon-glass homogenizer. The amounts of protein in the PSD, synaptosome and total membrane fractions were measured with BCA protein assay kit (Pierce). Equal amounts of protein were loaded onto a 10 % SDS-PAGE system and transferred to Immobilon-P membranes (Millipore) for Western blot analysis. Anti-NMDAR1 at 1:1000 and anti-ASIC1antibody at 1:2000 were used for Western blot analysis.
Electrophysiology
Whole-cell perforated-patch recordings were performed from monosynaptically connected pairs of cultured hippocampal neurons 8-12 days after plating. Micropipettes with a resistance of 2-4 MΩ were filled with intracellular solution (pH 7.4) containing (mm) potassium gluconate 120, NaCl 8, MgCl2 1, EGTA 0.5, Hepes 10 and 150 μg ml−1 amphotericin B for perforation (Rae et al. 1991). The bath solution contained (mm) NaCl 124, KCl 3, CaCl2 2, MgCl2 1, Hepes 3 and d-glucose 5 (pH 7.4 or 6.7). Amiloride (50 μm) from Sigma, [d, l]-2-amino-5-phosphonovalerate (APV, 50 μm) and 6,7-dinitroquinoxaline-2,3-dione (DNQX, 10 μm) from Tocris (Ellisville, MO, USA), were added to the bath solution where indicated in the figures. The recording chamber had a total volume of 150 μl and was perfused with bath solution at room temperature at a rate of 1.5 ml min−1. The presence of proton-gated ion channels was assessed by applying a solution containing (mm) NaCl 124, KCl 3, CaCl2 2, MgCl2 1, Mes 10 and d-glucose 5 (pH 5.5) by gravity flow through a glass capillary held in close proximity (50-100 μm) to the recorded neuron. Neurons were visualized by phase-contrast microscopy using a Nikon TE300 inverted microscope. Voltage-clamp recordings were performed using Axopatch 200B amplifiers (Axon Instruments, Union City, CA, USA). Recorded neurons were held at −80 mV, signals were filtered at 5 kHz, sampled at a rate of 10 kHz, and analysed using pClamp8.0 software (Axon Instruments). Recordings were rejected for analysis if series resistance (15-40 MΩ), whole-cell capacitance and input resistance varied by greater than 20 % in the course of the experiments. For stimulation of presynaptic neurons, cells were injected with 0.3-0.8 nA, 1 ms depolarizing current steps and induced action potentials were monitored in current-clamp mode. Trains of stimuli at 100 Hz, 3 s or 30 Hz, 1 s were given at 30 s intervals to allow recovery from short-term depression. Recordings were accepted for analysis only if postsynaptic currents had a latency of onset of < 3 ms relative to the peak of the presynaptic action potential and if postsynaptic currents before application of drugs or low-pH bath solution did not change by more than 10 % (s.e.m.).
Results
Expression of ASIC1 in the CNS
Expression of ASIC1 has been demonstrated previously at the mRNA level by in situ hybridization and Northern blot analysis (Garcia-Añoveros et al. 1997; Waldmann et al. 1997). However, in situ hybridization is limited by the relatively low spatial and quantitative resolution, but most importantly because it does not provide information on the subcellular distribution of the protein. In the study presented here, we have investigated the distribution of ASIC1 protein in the CNS using a specific antibody that recognizes rat ASIC1, both the ASIC1α and ASIC1β isoforms (Alvarez de la Rosa et al. 2002). We first estimated the distribution and abundance of ASIC1 in various regions of rat brain and in spinal cord using Western blot analysis (Fig. 1). The membrane was probed first with anti-ASIC1 antibody and subsequently with a commercial anti-β-tubulin antibody that served as control for the amount of protein loading. The results show that ASIC1 is expressed in almost all areas of the rat CNS, including the forebrain, midbrain, brainstem, pons, cerebellum, hippocampus and spinal cord. Densitometric analysis of ASIC1 relative to β-tubulin indicates that ASIC1 is expressed at similar levels in many areas of the brain.
Figure 1. Western blot analysis of acid-sensitive ion channel (ASIC)1 expression in the rat CNS.
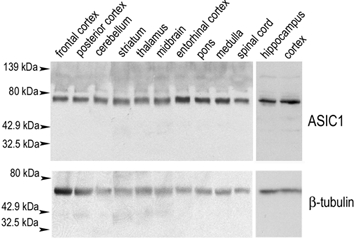
Protein extracts from the indicated areas of rat CNS (100 μg) were analysed by Western blot using consecutively anti-ASIC1-CT (upper panel) and anti-β-tubulin (lower panel) antibodies. ASIC1 immunoreactivity was present in all areas examined. Molecular weight markers are shown on the left.
Distribution of ASIC1 examined by immunohistochemistry of rat brain sections
In order to define the cellular distribution of ASIC1 in the CNS, sections from adult rat brain were stained with the anti-ASIC1-CT antibody. Immunoreactivity was observed in neurons but not in glia. Figure 2A shows a low-magnification view of a section of brain cortex to demonstrate ASIC1-CT immunoreactivity in neurons from all layers of the cerebral cortex. At a higher magnification we can appreciate the immunolabelling of the soma and dendrites of pyramidal cells (Fig. 2B). All regions of the hippocampus were positive for ASIC1 (Fig. 2C), the signal being more intense over the soma of pyramidal cells (Fig. 2D). In the cerebellum, the soma and dendritic trees of Purkinje cells were strongly labelled (Fig. 2E). Preabsorption of the primary antibody with the cognate peptide completely abolished the fluorescent signal in all areas of the brain (Fig. 2F).
Figure 2. Immunohistochemical staining of endogenous ASIC1 in the rat brain and spinal cord.
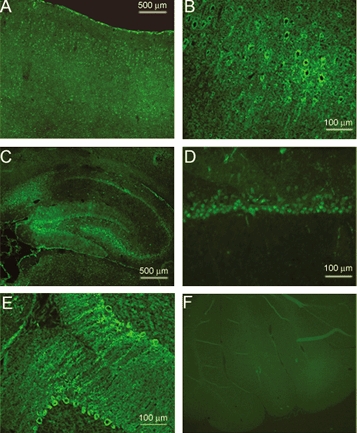
Brain sections (10 μm thick) were immunostained with anti-ASIC1-CT antibody. A, area of brain cortex exhibiting staining of most pyramidal cells. B, same region as in A but at a higher magnification, showing labelling of axons and dendrites. C, low-magnification sagittal view of the whole hippocampus showing immunoreactivity in all hippocampal regions. D, hippocampal neurons from the CA1 region at higher magnification. E, view of the cerebellar cortex showing Purkinje cells stained over the soma and dendritic tree. F, section of cerebellum stained with anti-ASIC-CT antibody pre-incubated with an excess of the cognate fusion protein.
Expression of ASIC1 during development of the mouse nervous system
To gain insight into the functional role of ASIC1 during development of the nervous system, and in particular to determine whether the expression of ASIC1 coincides with the stages of rapid synapse formation in the brain, we examined the expression of ASIC1 in the developing mouse brain. The presence and relative amounts of ASIC1 protein were assessed by Western blot analysis using anti-ASIC1-CT antibody. Equal amounts of protein from mouse CNS that were collected at different embryonic and postnatal days were resolved using SDS-PAGE. Figure 3 shows that ASIC1 is already expressed at the E12 stage and remains at approximately the same level throughout development until adulthood. These findings are consistent with a previous report in which the levels of ASIC1α mRNA were examined in mouse embryos by Northern blot analysis, showing that it is already abundant by E11 and remains at the same level at E15 and E17 (Garcia-Añoveros et al. 1997).
Figure 3. ASIC1 expression during mouse CNS development.
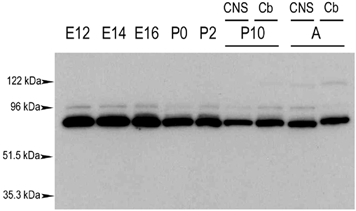
Protein extracts from mouse CNS at different embryonic (E) or postnatal (P) days were collected and analysed for ASIC1 expression by Western blot. A sample from adult animals was included (A). P10 and adult samples were obtained either from cerebellum (Cb) or from total CNS. Molecular weight markers are show on the left.
Subcellular distribution of ASIC1 in neurons examined by membrane fractionation
The previous experiments indicate that ASIC1 is distributed broadly in many types of neurons in the CNS. To investigate the presence of ASIC1 in specialized domains, we separated whole brain membranes and isolated the fractions enriched with synaptic vesicles or PSDs, compartments where pHo may fall rapidly and reversibly. First, we prepared crude synaptic vesicles (Huttner et al. 1983) and examined the fractions by Western blot analysis using anti-ASIC-CT and anti-synaptophysin antibodies (Fig. 4). As expected, all fractions containing membranes were positive for ASIC1 and synaptophysin, whereas S3, which corresponds to the cytosolic fraction, was devoid of these proteins. The crude synaptic vesicle fraction (LP2) was enriched fivefold in synaptophysin, contrary to ASIC1, whose abundance decreased in this fraction (Fig. 4A).
Figure 4. Subcellular distribution of ASIC1 examined by differential fractionation of membranes from rat brain.
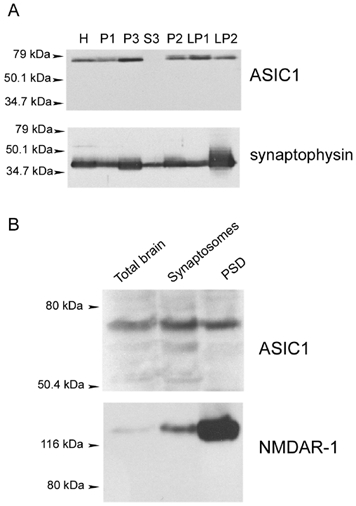
A, synaptic vesicle preparation. Equal amounts of proteins from each fraction of synaptic vesicle preparation were loaded onto an SDS-PAGE system and analysed by Western blot with anti-ASIC1-CT and with anti-synaptophysin antibodies. H = whole homogenate, P2 = second pellet from a 9200 g spin, P3 = pellet from 184 000 g spin, S3 = supernatant from 184 000 g spin, LP1 = pellet from hypotonically lysed synaptosomes centrifuged at 25 000 g, LP2 = crude synaptic vesicle fraction. B, PSD preparation. Equal amounts of proteins from rat CNS (total brain), synaptosomes or PSD fractions were analysed by Western blot using consecutively anti-ASIC1-CT and anti-NMDA-receptor type I (NMDAR-1) antibodies.
Next we examined ASIC1 localization in the PSDs by isolating these structures with the protocol established by Carlin et al. (1980). Figure 4B shows a representative Western blot of total brain membranes and fractions enriched with synaptosomes and PSDs probed with anti-ASIC1-CT antibody and a monoclonal antibody for glutamate receptor-1 (NMDAR-1). The PSD fraction was highly enriched with NMDAR-1 (≈20-fold), a finding that confirms the quality of our preparation. In contrast, PSDs were not enriched with ASIC1, although the channel was also detected in the PSD fraction by Western blotting.
Characterization of a newly developed ASIC1 antibody
Techniques of membrane separation are designed to identify cellular fractions that are enriched in certain proteins, but they do not provide details about subcellular localization. That type of information can be obtained by immunofluorescence of cultured neurons with specific antibodies. To assure the validity of the results, we decided to compare the data from two ASIC1 antibodies directed against different domains of the protein. For that purpose we raised another antibody that recognizes an epitope located in the extracellular domain of ASIC1 (ASIC1-EL). This epitope is also common to the two ASIC1 isoforms. Figure 5A shows the results of Western blotting experiments that demonstrate the specificity of the ASIC1-EL antibody. Lane 1 from Fig. 5A corresponds to non-injected oocytes, and the other lanes to oocytes expressing ASIC1. The sample in lane 3 was treated with the endoglycosidase PNGase-F prior to loading on the gel. Lanes 2 and 3 reveal single immunoreactive bands with molecular weights of 70 and 66 kDa, respectively. The ≈4 kDa decrease in molecular weight indicates the removal of two asparagine-linked core oligosaccharides by PNGase-F. The molecular weight predicted from the rat ASIC1 sequence is 59.6 kDa. However, we have consistently observed slightly slower migration after deglycosylation or after in vitro translation of the protein (data not shown). Lane 4 demonstrates the absence of immunoreactivity after competition with the cognate peptide. Protein extracts from whole rat brain yielded bands identical to those from injected oocytes, indicating that endogenous ASIC1 protein is a glycoprotein and, most importantly, that the antibody ASIC-EL recognizes the native protein with high specificity (Fig. 5B).
Figure 5. Characterization of the anti-ASIC1-EL antibody.
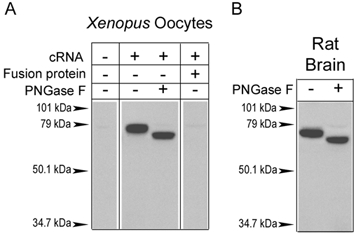
Western blot analysis of proteins extracted from ASIC1 cRNA-injected oocytes (A) or rat brain (B) probed with anti-ASIC1-EL antibody. Proteins in lanes 3 (A) and 2 (B) were pretreated with PNGase-F. No signal was detected in water-injected oocytes or after competition of the antibody with the cognate peptide. Single bands of ≈70 kDa were detected in ASIC1-injected oocytes and in adult rat brain. Treatment with PNGase-F reduced the molecular weight by ≈4 kDa, which is consistent with the removal of two core N-linked oligosaccharides. The migration of molecular weight markers is indicated by arrowheads.
Localization of ASIC1 and synaptic markers by immunocytochemistry of brain cortical neurons in primary culture
To gain more information about the distribution of ASIC1 within cells we used primary cultures of mouse cortical neurons. We examined colocalization of ASIC1 with markers for dendrites (MAP2) and synapses (PSD-95), respectively. After 10-14 days in culture, cells were labelled with two different ASIC1 antibodies: anti-ASIC1-CT and anti-ASIC1-EL. Figure 6A and D shows staining of cultured cortical neurons with each of the ASIC1 antibodies. The signal is similar in both cases; slightly punctate and of higher intensity over the soma than on dendrites or axons. Overlays of anti-ASIC1 antibodies and MAP2 (Fig. 6C and F) show colocalization of ASIC1 with MAP2 in dendrites but not in axons (indicated by the arrows). Co-staining of neurons with anti-ASIC1-CT and the postsynaptic density marker anti-PSD-95 shows the signal of PSD-95 in dendritic spines, while ASIC1 does not cluster in this area. As indicated in the enlarged view of a dendrite (inset from Fig. 6I), colocalization of ASIC1 and PSD-95 is minimal in dendrites. A similar pattern of staining was obtained using rat hippocampal neurons in culture (Fig. 6J). Hippocampal neurons were co-stained with anti-PSD-95 and ASIC1 antibodies (Fig. 6K), and overlay of the signal showed very little colocalization of these markers (Fig. 6L).
Figure 6. Distribution of ASIC and colocalization with synaptic markers in cultured embryonic cortical and hippocampal neurons.
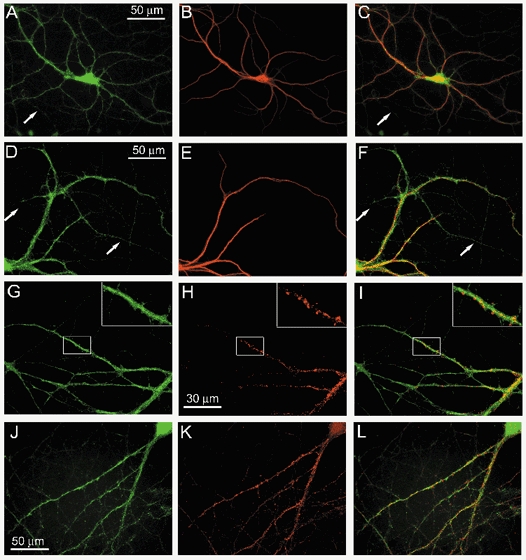
A and B, co-staining of cortical neurons with anti-ASIC1-CT (A, green) and MAP2 (B, red) antibodies. C, overlay of A and B indicates that ASIC1 is expressed on the soma, dendrites and axons, whereas MAP2 is present predominantly in the dendrites and in the soma. Arrows show axons stained exclusively with ASIC1. D-F, co-staining of cortical neurons with anti-ASIC1-EL (D, green) and MAP2 (E, red), showing that both ASIC1 antibodies yield an identical pattern of staining (F, overlay). G and H, co-staining of cortical neurons with anti-ASIC1-CT (G, green) and anti-PSD-95 (H, red). I, overlay of G and H. Insets show a dendrite at high magnification showing synapses intensely labelled by PSD-95 and little overlap with ASIC1. Co-staining of hippocampal neurons with anti-ASIC-CT (J, green) and anti-PSD-95 (K, red). L, overlay of signals shown in J and K.
Synaptic transmission does not activate ASIC1 from cultured hippocampal neurons
So far, our results have not demonstrated enrichment of ASIC in synapses. However, since an ASIC1 signal has been found in the PSD preparation, and in a recent report by Wemmie et al. (2002), ASIC1 was implicated in synaptic transmission, we pursued the search for ASIC1 in synapses. We rationalized that if ASIC1 is expressed in pre- or postsynaptic membranes at low levels, perhaps not readily detectable by immunocytochemical techniques, we could use instead a functional assay to confirm the presence of ASIC1 in synapses. For these studies we preferred to use hippocampal cells in culture because this was the preparation used by Wemmie et al. (2002) and because expression of ASIC1 currents in these cells has been documented by several groups. We first performed whole-cell patch-clamp recordings of hippocampal neurons in dissociated cultures. In all neurons tested, rapid application of an acidic solution (pH 5.5) induced an inward current of average (± s.e.m.) amplitude 535 ± 52 pA (n = 9) that was largely attenuated by either the addition of the ASIC inhibitor amiloride (50 μm) (KD = 10 μm; Waldmann et al. 1997) or by lowering the pH of the bath solution to 6.7, a proton concentration known to inactivate ASIC1 (Alvarez de la Rosa et al. 2002; Fig. 7A). It is well known that AMPA- and NMDA-type glutamate receptor currents constitute the overwhelming majority of the postsynaptic current elicited by single presynaptic stimuli (Forsythe & Westbrook, 1988; Andreasen et al. 1989), making it unlikely that ASIC1 currents contribute to the postsynaptic responses under conditions of basal, low-frequency stimulation. It is possible, however, that the release of multiple synaptic vesicles, such as in response to a high-frequency train of presynaptic stimuli, the proton concentration at a site in the synaptic cleft becomes high enough to activate otherwise ‘silent’ ASIC1 receptors, thus increasing postsynaptic charge transfer. To test this hypothesis, we recorded whole-cell synaptic currents from monosynaptically connected pairs of neurons and delivered a high-frequency (100 Hz) stimulus train to the presynaptic neuron in the presence of glutamate receptor antagonists. Whereas in the absence of any antagonists, large AMPA-receptor- and NMDA-receptor-mediated synaptic currents were observed, inhibition of AMPA receptors and NMDA receptors with DNQX (10 μm) and APV (50 μm), respectively, totally abolished all postsynaptic currents (Fig. 7B, top trace) in all excitatory synaptic connections examined (n = 7). Likewise, we did not detect a residual postsynaptic current at inhibitory synaptic connections stimulated with a 100 Hz train after inhibition of GABAA receptors with picrotoxin (10 μm, data not shown).
Figure 7. Effect of synaptic transmission on ASIC1 activation.
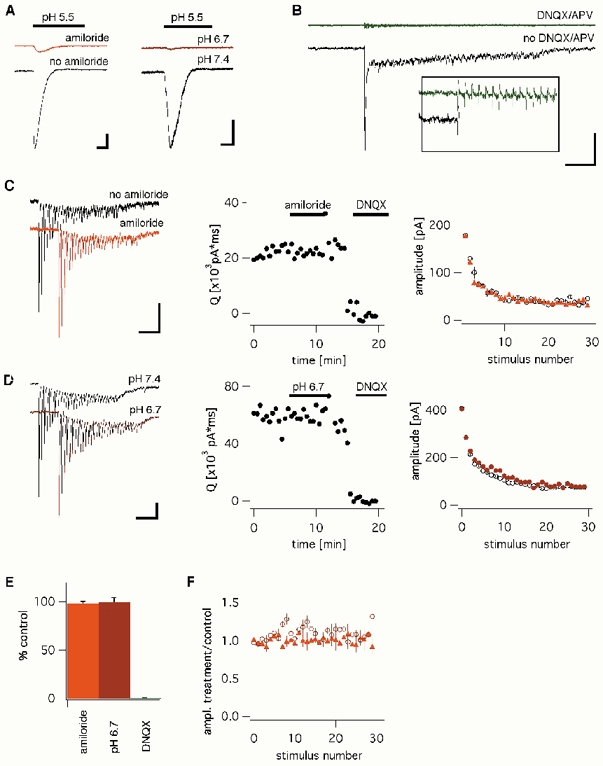
A, inward currents elicited in cultured hippocampal neurons by rapid application of acidic saline (pH 5.5) in the presence or absence of 50 μm amiloride (left panel) and at a bath pH of 6.7 or 7.4 (right panel). Scale bars 0.2 nA, 2 s. B, postsynaptic current elicited by high-frequency stimulation (3 s at 100 Hz) of a presynaptic glutamatergic neuron (bottom) is completely blocked by application of 10 μm 6,7-dinitroquinoxaline-2,3-dione (DNQX) and 50 μm [d, l]-2-amino-5-phosphonovalerate (APV, average of four traces, top). The inset shows a higher-magnification view of this trace during the first 15 stimuli of the train. Scale bar: 50 pA (inset 10 pA), 1 s (inset 100 ms). C, EPSCs produced in response to a 30 Hz, 1 s stimulus train in the presence or absence of amiloride (50 μm, left panel). Integrated postsynaptic currents elicited by the stimulus trains (middle panel) and average amplitudes of EPSCs to successive stimuli within the train (right panel) are shown. D, EPSCs in response to a 30 Hz stimulus in at a bath pH of 7.4 or 6.7. E, normalized integrated postsynaptic currents during amiloride application (98.2 ± 2.0 % of pre-application currents, n = 3), at a bath pH of 6.7 (99.5 ± 4.4 %; n = 3) or after application of DNQX and APV (0.4 ± 0.3 %, n = 3). F, postsynaptic currents recorded in response to successive stimuli within a train. Current amplitudes of EPSCs after application of amiloride or at pH 6.7 have been normalized to respective current amplitudes under control conditions.
This experimental protocol only examined the activation of ASIC1 localized in the postsynaptic membrane, but it did not exclude the possibility of activation of proton-gated channels in the presynaptic membrane. This possibility is technically difficult to address, since presynaptic membrane currents can only be studied in a few specialized preparations with very large presynaptic terminals that permit direct recordings (Forsythe, 1994). Instead, we examined whether activation of presynaptic ASIC1, induced by maximal exocytosis of synaptic vesicle contents, could alter presynaptic neurotransmitter release. If that is the case, inhibition of ASIC1 currents should modulate short-term synaptic plasticity, which is generally attributed to changes in presynaptic neurotransmitter release. However, we did not observe any differences in short-term plasticity upon blockade of ASIC1 with amiloride (Fig. 7C) or inactivation of ASIC1 by lowering the preconditioning pH to 6.7 (Fig. 7D). In summary, these results indicate that protons released during intense synaptic activity do not activate postsynaptic ASIC1 currents in excitatory synapses of cultured hippocampal neurons, and that any activation of presynaptic ASIC currents does not alter synaptic transmission.
Discussion
Functional implications of the localization of ASIC1 in neurons from the CNS
These studies are the first to examine the distribution of ASIC1 protein in the CNS and its subcellular localization in pyramidal cortical neurons. Our results indicate that ASIC1 is expressed broadly in many areas of the brain and thus confirm previous in situ hybridization studies (Garcia-Añoveros et al. 1997; Waldmann et al. 1997). The level of expression of ASIC1, examined on brain sections by immunohistochemistry techniques or by the more quantitative approach of Western blot analysis, is quite homogenous in most areas of the CNS. Numerous previous studies that examined proton-gated currents, prior to the cloning of ASIC1, also found that the magnitude of proton-gated whole-cell currents were very similar, irrespective of the area of the brain chosen for the patches. We found an average value of 540 pA in hippocampal neurons, and Baron et al. (2002) found ≈600 pA. In pyramidal cells from mouse brain cortex stimulated by pHo 6.3 the value was ≈400 nA (Varming, 1999); in rat hypothalamic neurons this value was ≈400 pA (Ueno et al. 1992); and in rat tectal neurons it was ≈500 pA (Grantyn & Lux, 1988). Together these data indicate that levels of expression of ASIC1 do not vary significantly among neurons with very diverse functional specialization or localization in the brain.
Within neurons, ASIC1 was found predominantly on the plasma membrane of the soma and, to a lesser degree, over the dendrites and axon. Our two anti-ASIC antibodies yielded the same results. The ASIC1 signal was slightly punctate; this finding may reflect clustering of ASIC1 by protein interacting with C kinase (PICK1), a PDZ-domain-containing protein that binds the carboxyterminus of ASIC1 (Duggan et al. 2002; Hruska-Hageman et al. 2002). It is thought that interactions with PDZ-domain-containing proteins are important for targeting and determining the distribution of neurotransmitter transporters and ion channels into specialized areas of the cell (Xia et al. 1999; Deken et al. 2001). The functional significance of the interaction between ASIC1 and PICK1 has not been determined, but our finding suggests that it is involved in anchoring the channel within the plasma membrane.
In spite of our multiple attempts to demonstrate ASIC1 in synapses, in particular in dendritic spines, we did not find evidence of enrichment in this domain. Membrane fractionations as well as immunolocalization studies revealed very low levels of expression in synaptosomes and PSDs. Although these domains were not enriched in ASIC1, the presence of few channels could still be of functional significance. This rationale motivated us to perform functional studies designed to demonstrate proton-gated currents in synapses from hippocampal neurons in cultured. The notion that protons released by chemical synaptic transmission can gate ASIC1 is attractive, in particular because the synaptic cleft constitutes one of the few places where variations of pHo may meet the requirements for gating of ASIC1, thus providing a physiological function for these channels in the CNS. This hypothesis has gained support from recent reports suggesting that protons modulate synaptic activity by blocking the presynaptic Ca2+ channels that mediate vesicle release in retinal cones (De Vries, 2001). In addition, abnormalities in long-term potentiation in an ASIC1-knockout mouse were attributed to a lack of proton activation of ASIC1 in postsynaptic membranes (Wemmie et al. 2002).
In our experiments, low pHo applied to the soma elicited robust proton-gated currents, but no response was detected when the stimulus was synaptic transmission. In the presence of ionotropic receptor antagonists of AMPA and NMDA, an intense train of stimuli did not elicit detectable postsynaptic currents. DNQX and APV did not affect the expression of ASIC1 (data not shown), therefore, the absence of a residual postsynaptic current in the experiments shown in Fig. 7B indicates that repetitive release of synaptic vesicles did not activate ASIC1. In additional experiments designed to examine the effects of possible activation of ASIC1 in the presynaptic membrane (Fig. 7C-F), we did not observe any differences in short-term plasticity upon blockade of ASIC1 with amiloride or inactivation of ASIC1 by lowering the preconditioning pH to 6.7. In summary, these results indicate that protons released during intense synaptic activity do not activate either presynaptic or postsynaptic ASIC1 in cultured hippocampal neurons.
A potential caveat from these studies is that the conditions in culture do not mimic the environment of synapses in vivo. In particular, it can be argued that proton concentrations dissipate faster in cultured cells than in the densely packed synapses of the CNS. However, our experiments were performed with bath solutions (3 mm Hepes, pH 7.4) with a buffering capacity much lower than in the cerebrospinal fluid. Another argument against proton gating of ASIC1 in synapses comes from the experimental observations and calculations of pH changes in the synapse provided by De Vries (2001). The shift in the activation profile of presynaptic Ca2+ channels in cone photoreceptors induced by synaptic vesicle fusion was 7.5 mV, which indicates an underlying acidification of at most 0.2 pH units. Such a decrease in pH is insufficient to activate ASIC1; currents start to appear at values of pHo below 7.0 (Waldmann et al. 1997; Sutherland et al. 2001). On the contrary, a change of pHo to 7.1 induces desensitization of ASIC1 (Alvarez de la Rosa et al. 2002).
Developmental expression of ASIC1 in the mouse brain
The ASICs are excitatory channels in the nervous system, as shown by the fact that activation by protons induces transient membrane depolarization. It is known that other excitatory channels such as glutamate receptors are required for dendritic arbor development, synaptogenesis and formation of neuronal circuits in the fetal and perinatal brain (Cline, 2001). Glutamate receptor proteins, detected by 3H-ligand binding, are first identified in the rat at stage E14 and peak in the late embryonic/early postnatal period (Bahn et al. 1994). To investigate whether ASIC1 follows a similar developmental regulation, we used Western blot analysis to examine the expression of ASIC1 protein during pre- and postnatal periods in the mouse brain. ASIC1 was detected readily at E12, and the levels of expression remained constant until adulthood. In the mouse, E12 corresponds to E14 in the rat, where there is approximately a 2 day delay in the embryonic stages. Our results indicate that there is no increase in the expression of ASIC1 during the periods of most active synaptogenesis. ASIC1 appears early and persists at almost constant levels throughout the development of the mouse brain.
The very early expression of ASIC1 in the developing brain is consistent with previous studies that found proton-activated Na+ currents in rat embryonic neurons from stage E12. Grantyn & Lux (1988) documented large (≈500 pA) proton-activated Na+ currents from dissociated tectal primordium at E12 (Grantyn & Lux, 1988). Expression of proton-gated currents appeared earlier than voltage-gated, tetrodotoxin-sensitive Na+ currents or voltage-activated Ca2+ currents, which are expressed even earlier than GABA- and glutamate-activated currents. These authors concluded that in the period of neuronal differentiation, proton-activated Na+ currents are the largest and, in many cells, the only detectable cationic inward current. Our results demonstrate that the proton-activated Na+ current in early embryos is indeed mediated by ASIC1. Moreover, the level of expression of ASIC1 in individual early embryonic cells is similar to that in adult neurons, as indicated by the magnitude of whole-cell currents and quantification of ASIC1 protein by Western blotting during brain development and in adults (Fig. 3).
In summary, the data presented here indicate that ASIC1 is distributed widely throughout the CNS. Neurons with diverse function, regardless of being excitatory or inhibitory, express ASIC1. Within neurons, ASIC1 is localized predominantly at the plasma membrane over the soma and main branches of dendrites and axons. During development of the mammalian nervous system, ASIC1 appears early, prior to voltage- and ligand-gated channels, at a stage when synapses and neuronal circuits are poorly developed. Taken together, the evidence suggests that ASIC1 channels participate in functions that are ubiquitous to all types of neurons from very early stages of development. ASIC1 may generate membrane depolarizations to allow interneuronal communications in early development when cells are not able to generate action potentials. The degenerins, a family of ion channels closely related to the ASICs that are expressed in Caenorhabditis elegans, also operate in neurons that are devoid of action potentials (Goodman et al. 1998). In the adult CNS, external protons blunt Na+ channel conductance and cause a depolarizing shift in the voltage dependence of current activation (Hille, 1968). These changes are noticeable at pHo 6.5 and are robust bellow pHo 5.0, which correspond to the pHo range of ASIC1 activation (Hille, 1968). High-threshold Ca2+ channels localized in the dendrites and soma underlie the prolonged action potential in vertebrate neurons and they are especially sensitive to pHo (reviewed by Tombaugh & Somjen, 1998). Although our current results do not indicate the specific function of ASIC1, the data are consistent with a role for ASIC1 in the modulation of membrane excitability by counteracting the inhibitory effects of external H+ ions in voltage-gated Na+ and Ca2+ ion currents.
Acknowledgments
We thank Dr Pietro de Camilli for the monoclonal MAP2 antibody and Tanya Stevens for primary cultures from brain cortex. The work was supported by NIH grant RO1 HL56163 to C. Canessa.
References
- Alvarez de la Rosa D, Zhang P, Shao D, White F, Canessa CM. Functional implications of the localization and activity of acid-sensitive channels in rat peripheral nervous system. Proc Natl Acad Sci U S A. 2002;99:2326–2331. doi: 10.1073/pnas.042688199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen M, Lambert JD, Jensen MS. Effects of new non-N-methyl-d-aspartate antagonists on synaptic transmission in the in vitro rat hippocampus. J Physiol. 1989;414:317–336. doi: 10.1113/jphysiol.1989.sp017690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahn S, Volk B, Wisden W. Kainate receptor gene expression in the developing rat brain. J Neurosci. 1994;14:5525–5547. doi: 10.1523/JNEUROSCI.14-09-05525.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron A, Waldmann R, Lazdunski M. ASIC-like, proton-activated currents in rat hippocampal neurons. J Physiol. 2002;539:485–494. doi: 10.1113/jphysiol.2001.014837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson CJ, Xie J, Wemmie JA, Price MP, Henss JM, Welsh MJ, Snyder PM. Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc Natl Acad Sci U S A. 2002;99:2338–2343. doi: 10.1073/pnas.032678399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin RK, Grab DJ, Cohen RS, Siekevitz P. Isolation and characterization of postsynaptic densities from various brain regions: enrichment of different types of postsynaptic densities. J Cell Biol. 1980;86:831–845. doi: 10.1083/jcb.86.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline HT. Dendritic arbor development and synaptogenesis. Curr Opin Neurobiol. 2001;11:118–126. doi: 10.1016/s0959-4388(00)00182-3. [DOI] [PubMed] [Google Scholar]
- Deken SL, Beckman ML, Quick MW. PICKing on transporters. Trends Neurosci. 2001;24:623–625. doi: 10.1016/s0166-2236(00)01933-0. [DOI] [PubMed] [Google Scholar]
- De Vries SH. Exocytosed protons feedback to suppress the Ca2+ current in mammalian cone photoreceptors. Neuron. 2001;32:1107–1117. doi: 10.1016/s0896-6273(01)00535-9. [DOI] [PubMed] [Google Scholar]
- Duggan A, Garcia-Añoveros J, Corey DP. The PDZ domain protein PICK1 and the sodium channel BNaC1 interact and localize at mechanosensory terminals of dorsal root ganglion neurons and dendrites of central neurons. J Biol Chem. 2002;277:5203–5208. doi: 10.1074/jbc.M104748200. [DOI] [PubMed] [Google Scholar]
- Forsythe ID. Direct patch recording from identified presynaptic terminals mediating glutamatergic EPSCs in the rat CNS, in vitro. J Physiol. 1994;479:381–387. doi: 10.1113/jphysiol.1994.sp020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe ID, Westbrook GL. Slow excitatory postsynaptic currents mediated by N-methyl-d-aspartate receptors on cultured mouse central neurones. J Physiol. 1988;396:515–533. doi: 10.1113/jphysiol.1988.sp016975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Añoveros J, Derfler B, Neville-Golden J, Hyman BT, Corey DP. BNaC1 and BNaC2 constitute a new family of human neuronal sodium channels related to degenerins and epithelial sodium channels. Proc Natl Acad Sci U S A. 1997;94:1459–1464. doi: 10.1073/pnas.94.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman MB, Hall DH, Avery L, Lockery SR. Active currents regulate sensitivity and dynamic range in C. elegans neurons. Neuron. 1998;20:763–772. doi: 10.1016/s0896-6273(00)81014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantyn R, Lux HD. Similarity and mutual exclusion of NMDA- and proton-activated transient Na+-currents in rat tectal neurons. Neurosci Lett. 1988;89:198–203. doi: 10.1016/0304-3940(88)90381-3. [DOI] [PubMed] [Google Scholar]
- Hille B. Charges and potentials at the nerve surface. Divalent ions and pH. J Gen Physiol. 1968;51:221–236. doi: 10.1085/jgp.51.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruska-Hageman AM, Wemmie JA, Price MP, Welsh MJ. Interaction of the synaptic protein PICK1 (protein interacting with C kinase 1) with the non-voltage gated sodium channels BNC1 (brain Na+ channel 1) and ASIC (acid-sensing ion channel) Biochem J. 2002;361:443–450. doi: 10.1042/0264-6021:3610443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner WB, Schiebler W, Greengard P, De Camilli P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J Cell Biol. 1983;96:1374–1388. doi: 10.1083/jcb.96.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesenbock G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- Rae J, Cooper K, Gates P, Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods. 1991;37:15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Sutherland P, Benson CJ, Adelman JP, McClenskey EW. Acid-sensing ion channel 3 matches the acid-gated current in cardiac ischemia-sensing neurons. Proc Natl Acad Sci U S A. 2001;98:711–716. doi: 10.1073/pnas.011404498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh GC, Somjen GG. pH modulation of voltage-gated ion channels. In: Kaila K, Ranson BR, editors. pH and Brain Function. New York: Wiley-Liss; 1998. pp. 373–416. [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Ueno S, Nakaye T, Akaike N. Proton induced sodium current in freshly dissociated hypothalamic neurons of the rat. J Physiol. 1992;447:309–327. doi: 10.1113/jphysiol.1992.sp019004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varming T. Proton-gated ion channels in cultured mouse cortical neurons. Neuropharmacology. 1999;38:1875–1881. doi: 10.1016/s0028-3908(99)00079-9. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- Wemmie JA, Chen J, Askwith CC, Hruska-Hageman AM, Price MP, Nolan BC, Yoder PG, Lamani E, Hoshi T, Freeman JH, Jr, Welsh MJ. The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron. 2002;34:463–477. doi: 10.1016/s0896-6273(02)00661-x. [DOI] [PubMed] [Google Scholar]
- Wilcox KS, Buchhalter J, Dichter MA. Properties of inhibitory and excitatory synapses between hippocampal neurons in very low density cultures. Synapse. 1994;18:128–151. doi: 10.1002/syn.890180206. [DOI] [PubMed] [Google Scholar]
- Xia J, Zhang X, Staudinger J, Huganir RL. Clustering of AMPA receptors by the synaptic PDZ domain-containing protein PICK1. Neuron. 1999;22:179–187. doi: 10.1016/s0896-6273(00)80689-3. [DOI] [PubMed] [Google Scholar]


