Abstract
In acute experiments, we demonstrated previously that nitric oxide (NO) donors exogenously applied to the nucleus tractus solitarii (NTS) depressed the baroreceptor cardiac reflex. In this study, we determined a role for endogenous endothelial nitric oxide synthase (eNOS) activity in the NTS for chronically regulating baroreceptor reflex function in conscious rats. A recombinant adenoviral vector directing expression of a truncated form of eNOS was microinjected bilaterally into the NTS to inhibit endogenous eNOS activity. Arterial pressure was monitored continuously using radio-telemetry in freely moving animals and spontaneous baroreceptor reflex gain (sBRG) determined by a time-series method. sBRG showed a gradual increase from day 7 to 21 after gene transfer and the value at day 21 (1.68 ± 0.20 ms mmHg−1, n = 6) was significantly higher than that before gene transfer (1.13 ± 0.09 ms mmHg−1, P < 0.001). This value was also significantly higher than that in rats in which enhanced green fluorescent protein (eGFP) was expressed in the NTS (1.04 ± 0.21 ms mmHg−1; n = 6, P < 0.01) and saline-treated groups (1.12 ± 0.15 ms mmHg−1; n = 4, P < 0.05), which did not change from control levels. In addition, heart rate decreased from 336 ± 6 to 318 ± 8 b.p.m. (P < 0.05) 21 days after gene transfer. This value was also significantly lower than that in control groups (eGFP: 348 ± 9 b.p.m., n = 6, P < 0.01; saline: 347 ± 5 b.p.m., n = 4, P < 0.05). Gene transfer did not affect arterial pressure. These findings suggest that in the conscious rat eNOS is constitutively active within the NTS and is a factor regulating baroreceptor reflex gain and heart rate.
The nucleus tractus solitarii (NTS) is the central termination site of arterial baroreceptor afferents (reviewed by Blessing, 1997) and provides a powerful site for modulation of reflex function. One potential modulator is nitric oxide (NO). In recent studies, nitric oxide synthase (NOS) has been found in the NTS (Ruggiero et al. 1996; Lawrence et al. 1998; Batten et al. 2000; Paton et al. 2001b) and several groups, including ourselves, have revealed a role for NO in the NTS for controlling the circulation. For example, we recently found that NTS microinjection of either l-arginine (a NO precursor) or NO donors (sodium nitroprusside or diethylamine nonoate) reduced the cardiac vagal component of the baroreceptor reflex gain in an unanaesthetized decerebrate rat model, the working heart-brainstem preparation (Paton et al. 2001b). This is consistent with the observation that NOS inhibitors in the NTS increased baroreflex gain in both anaesthetized (Li et al. 2002) and conscious rats (Pontieri et al. 1998). There are, however, other reports that failed to find such a role for NO in regulating baroreflex gain (see Harada et al. 1993; Zanzinger et al. 1995).
Some forms of arterial hypertension, which have been related to heightened activity of angiotensin II (ANGII), are associated with a depressed baroreceptor reflex function (e.g. Miyajima et al. 1999). Whether a depressed baroreceptor reflex contributes to hypertension is not clear (see Dickinson & Sleight, 2001, for debate) although the recent data from Thrasher (2002) would support this. Interestingly, similar to the effect of NO, ANGII acting within the NTS also depresses the baroreceptor reflex (e.g. Casto & Phillips, 1986; Michelini & Bonagamba, 1990; Luoh & Chan, 1998; Paton & Kasparov, 1999). Using adenoviral-mediated gene transfer of a dominant negative protein to genetically disable endothelial NOS (eNOS) within the NTS, we found that the effect of exogenously applied ANGII on the baroreceptor reflex was blocked, suggesting its action was mediated by activation of this NOS isoform (Paton et al. 2001b). Based on this acute study, we hypothesized that endogenous eNOS activity in the NTS plays a role in long-term sensitivity of baroreceptor reflex gain.
There were two requirements for the present study: (i) to chronically and specifically block eNOS activity within a circumscribed region of the NTS; and (ii) to monitor cardiovascular variables in unrestrained, freely moving animals to provide a definitive measure of arterial pressure and cardiac baroreceptor reflex function. Thus, we applied a recombinant adenoviral vector directing expression of a truncated mutant form of eNOS (‘TeNOS’; see Lee et al. 1995) into the NTS to inhibit endogenous eNOS activity chronically (see Paton et al. 2001b). This approach allowed acquisition of control data in the same animal (i.e. before gene manipulation). Furthermore, we adopted radio-telemetry to monitor multiple cardiovascular variables chronically. This allowed a continuous time-dependent assessment of changes in the cardiovascular system that was void of the complexities of restraint, anaesthesia and decerebration.
Our novel findings indicate that chronic blockade of eNOS activity in the NTS produces changes in cardiovascular variables and baroreceptor reflex gain using both a modified time-series technique (Bertinieri et al. 1988; Oosting et al. 1997b) and a conventional pharmacological method (i.e. injection of vasoactive agents). We demonstrate for the first time that endogenous eNOS activity in the NTS exists and that this influences baroreceptor reflex function in conscious rats.
Methods
Experimental animals and animal care
Male Wistar rats weighing between 275 and 325 g were used. The procedures were carried out according to the Animals (Scientific Procedures) Act 1986. The animals were housed individually, allowed normal rat chow and drinking water ad libitum, and kept on a 12 h light : 12 h dark cycle.
Chronic measurement of arterial pressure
(i) Recording system
We used a telemetry system (Data Sciences International, St Paul, MN, USA) for recording arterial pressure. The system consists of three basic elements: (1) a transmitter for monitoring arterial pressure (TA11PA-C40); (2) a receiver (RPC-1); and (3) an adapter (R11CPA) with an ambient pressure monitor (APR-1) to output analogue signals of arterial pressure. The system is calibrated relative to atmospheric pressure. A computer-based data acquisition system (Maclab/8s, AD Instruments and PowerBook 3400c, Apple Computer Inc.) was used to acquire, display, store and analyse the telemetered data.
(ii) Implantation of transmitters
The transmitter was implanted at least 7 days before recordings began. The rat was anaesthetized with an intramuscular injection of ketamine (60 mg kg−1) and medetomidine (250 μg kg−1). The level of anaesthesia was checked frequently by assessing limb withdrawal reflexes to noxious pinching. A midline incision of the abdominal wall was made in the supine position and the intestines were moved aside to allow good visualization of the abdominal aorta. The tip of the catheter (ca. 0.7 mm, thin-walled thermoplastic membrane) of the transmitter (TA11PA-C40; ca. 15 × 20 mm) was inserted into the abdominal aorta caudal to the root of the left renal artery. The transmitter was sutured to the ventral wall of the abdominal cavity. Penicillin (1000 U) was injected intramuscularly. The medetomidine anaesthesia was reversed with a subcutaneous injection of atipamezole (1 mg kg−1) after the surgery and the animal returned to its home cage for recovery.
In vivo gene transfer in the NTS
(i) TeNOS adenoviral vector
Ad-CMV-TeNOS (4 × 1010 pfu ml−1) is a replication-deficient recombinant adenoviral vector that directs expression of ‘TeNOS’ under the constitutive control of the cytomegalovirus (CMV) promoter-enhancer (Kantor et al. 1996). TeNOS lacks catalytic activity yet retains the NH2-terminal sequences required for cotranslational NH2-terminal glycine myristoylation (Liu & Sessa, 1994) and membrane localization (Busconi & Michel, 1993). TeNOS acts as a dominant negative inhibitor of wild-type eNOS activity through heterodimerization with the native protein (Lee et al. 1995). As a viral control, Ad-CMV-eGFP (4.9 × 1010 pfu ml−1) expressing enhanced green fluorescent protein (eGFP) was used.
(ii) Surgical procedure for in vivo gene transfer in the NTS
Animals with pre-implanted transmitters were re-anaesthetized with an intramuscular injection of ketamine (60 mg kg−1) and medetomidine (250 μg kg−1). They were placed in a stereotaxic head holder and, through a midline incision in the dorsal neck, the caudal dorsal medulla was exposed. Bilateral microinjections (five 100 nl injections per side) were made at separate sites spanning 500 μm rostral-caudal to the calamus scriptorius, 350-700 μm from the midline and 500-600 μm below the dorsal surface of the medulla. This region of the NTS was studied since it mediated a depression of the baroreceptor reflex gain following microinjection of l-arginine, NO donors and ANGII, which we showed mediates its effect by releasing NO (see Paton et al. 2001b). Three groups of animals were injected. The first viral group received NTS microinjections of Ad-CMV-TeNOS (n = 6). A second group acted as a control for viral transfection, in which animals received bilateral microinjections of Ad-CMV-eGFP (n = 6). A third group of animals received bilateral microinjections of saline as a control (n = 4). The wound was sutured, cleaned and treated with neomycin powder, and medetomidine anaesthesia reversed with a subcutaneous injection of atipamezole (1 mg kg−1). Animals were returned to their home cages for recovery.
Timing of telemetry measurements
On the day before NTS microinjection of either virus or saline and on the 7th, 14th, 21st and 28th day after microinjections, six 5 min epochs of arterial pressure data were collected randomly during the light phase. Heart rate (HR) was derived from the interpulse interval. Averaged mean blood pressure (MBP) and HR were obtained on each day and the baroreceptor reflex gain (BRG) calculated (see below).
Evaluation of baroreceptor reflex function
(i) Spontaneous baroreceptor reflex gain (sBRG)
To evaluate time-dependent changes in baroreceptor reflex function, the sBRG was determined from spontaneous changes in systolic blood pressure (SBP) and pulse interval (PI) using a modified time-series method established by Oosting et al. (1997b). First, moving averages of the SBP and the PI over ten consecutive beats were calculated to filter out respiration-induced fluctuations. Second, from the moving average data, spontaneously occurring ramps of either decreasing or increasing SBP of four beats or more were used to calculate baroreceptor reflex gain. Third, for each pair of SBP and PI ramps, measurements were made at delays of three, four and five beats; this was based on the finding that baroreceptor reflex-related changes in PI become apparent after a delay of between three and five beats following a pressure change (Oosting et al. 1997b). Fourth, from these ramps plots were made of the changes in PI vs. SBP to form slopes for each of the delays (i.e. 3 plots). The spontaneous baroreceptor reflex gain (sBRG) values quoted represent the mean value of the three slopes. Unlike Oosting et al. (1997b), we only used positive slope values, thereby avoiding contamination of our baroreceptor reflex data with non-baroreceptor-mediated changes in PI. We used systolic pressure and not the mean pressure as used by Oosting et al. (1997b) for measurement of sBRG since clear systolic signals were collected successfully by using radio-telemetry over the entire experimental period.
(ii) Baroreceptor reflex gain from manipulating blood pressure with vasoactive agents
In the 4th-5th week after viral injection the BRG was determined pharmacologically in conscious rats. Before this test, a polyethylene catheter (SP-31, Natsme) was inserted into the right jugular vein under ketamine (60 mg kg−1; i.m.) and medetomidine (250 μg kg−1; i.m.) anaesthesia. Forty-eight hours after installation of this venous line either phenylephrine (10-20 μg kg−1, total volume 2.75-6.50 μl; Sigma-Aldrich) or nitroprusside (10-20 μg kg−1, total volume 2.75-6.50 μl; Sigma-Aldrich) was injected using a syringe pump (Harvard Apparatus) to raise or lower, respectively, arterial pressure by about 25-50 mmHg. Both pressor and depressor challenges were performed at least twice. In order to assess the baroreceptor reflex gain, the slope of the linear regression function between the beat-to-beat changes in SBP and the related beat-to-beat changes in PI at a delay that scored the highest correlation coefficient was calculated (Struyker-Boudier et al. 1982; Oosting et al. 1997b).
(iii) Effect of NTS microinjections of ANGII in Ad-CMV-TeNOS-transfected rats
To test whether TeNOS was still present within the NTS 5-6 weeks after viral injection we microinjected ANGII bilaterally into the NTS to assess whether it depressed the baroreceptor reflex. This was a physiological assessment for the presence of the transgene based on our previous finding that the depressant effect of ANGII in the NTS depends on the integrity of eNOS (Paton et al. 2001b). Rats were terminally anaesthetized with urethane (1.4 g kg−1, i.p.). They were placed in a stereotaxic head holder and, through a midline incision in the dorsal neck, the caudal dorsal medulla was exposed. A single bilateral microinjection of ANGII (500 fmol, Sigma-Aldrich) was made from a multi-barrelled micropipette (tip diameter 30-40 μm), which was held in a micromanipulator and driven into the medulla to a depth of 600 μm ventral to the dorsal surface, ± 400 μm rostrocaudal relative to calamus scriptorius and between 350 and 500 μm from the midline (see Paton & Kasparov, 1999; Paton et al. 2001b). The injected volume (50 nl) was measured by observing the movement of the meniscus through a binocular microscope fitted with a calibrated eye-piece graticule. Arterial pressure was monitored via radio-telemetry and phenylephrine (10≈20 μg kg−1, Sigma-Aldrich) was injected thorough the jugular vein using a syringe pump (Harvard Apparatus) to raise arterial pressure by about 30-50 mmHg. Nitroprusside was not tested. In order to assess the baroreceptor reflex gain, the peak changes in SBP (ΔSBP) and corresponding peak reflex changes in PI (ΔPI) were measured and the ΔPI : ΔSBP ratio calculated. The baroreceptor reflex tests were performed before and after either ANGII or saline microinjection into the NTS. Saline microinjections were without effect on baseline cardiovascular variables or the baroreceptor reflex. At the end of experiment the position of the micropipette was marked by injection of 50 nl of 2 % Pontamine Sky Blue. The animals were killed by an overdose of urethane (3 g kg−1i.v.), the brainstem removed, fixed with 4 % paraformaldehyde for at least 24 h, and transferred to phosphate-buffered saline (PBS) containing 30 % sucrose before sectioning (40-80 μm) using a freezing microtome. Sections were mounted, stained with Neutral Red, dehydrated and cleared for viewing.
Analysis of HR variability
HR variability before and 3 weeks after gene transfer was analysed using the maximum-entropy method with high resolution (MemCalc, Suwa Trust, Sapporo, Japan). According to Murasato et al. (1998), the magnitude of power was integrated in both the low-frequency (LF) band between 0.27 and 0.75 Hz and the high-frequency (HF) band (0.75-3.3 Hz).
Immunohistochemistry
To visualize expression of Ad-CMV-TeNOS within the NTS, immunohistochemical detection of eNOS was performed in the 5th-6th week after viral injection. Serial sections (40 μm each) of the medulla were obtained for immunohistochemistry as described above. The sections were rinsed in PBS three times each for 5-10 min, transferred to 1 % BSA with 0.1 % Triton-X for 30 min at room temperature, followed by incubation in antibody to NOS3 (Santa Cruz Biotechnology) diluted with 1 % BSA (1 : 200). After overnight incubation at 4 °C, the sections were rinsed three times in PBS for 5-10 min each and then incubated in 1 : 100 diluted anti-rabbit IgG (TRITC conjugate, Sigma-Aldrich) at room temperature in the dark for 2 h. Finally, sections were again washed in PBS before mounting in Vectashield (Vector Laboratories). The stained sections were photographed using a fluorescence microscope (Leica) and the immunoreactivity in TeNOS-transfected rats compared with that in both the eGFP-transfected and saline-treated rats. In eGFP-transfected rats, NTS regions expressing eGFP were also identified with a fluorescence microscope.
Data analysis
Data for TeNOS, eGFP and saline controls are expressed as means ± s.e.m. To evaluate time-dependent changes of cardiovascular variables by expressing TeNOS within NTS we used repeated-measures ANOVA and the Bonferroni test for multiple comparisons of cardiovascular variables across time and between different groups. Data were taken as significant at a probability of 0.05. In acute experiments, the differences between the values before and after viral or saline injections were compared using Student's paired t test, while differences between the three different groups were compared by one-way ANOVA followed by the Bonferroni test for multiple comparisons.
Results
Effects of chronic inhibition of eNOS activity in the NTS on arterial pressure, HR and sBRG
Before viral injection, baseline MBP, HR and sBRG in the TeNOS-transfected group were 102 ± 2 mmHg, 336 ± 6 b.p.m. and 1.13 ± 0.09 ms mmHg−1, respectively (Fig. 1). These values were not different from those of either the eGFP-transfected rats (MBP: 100 ± 4 mmHg; HR: 340 ± 8 b.p.m.; sBRG: 1.03 ± 0.08 ms mmHg−1) or the saline-treated group (MBP: 98 ± 2 mmHg; HR: 346 ± 3 b.p.m.; sBRG: 1.08 ± 0.07 ms mmHg−1).
Figure 1. Effects of chronic inhibition of eNOS activity in the NTS on mean blood pressure (MBP), heart rate (HR) and spontaneous baroreceptor reflex gain (sBRG) in conscious rats.
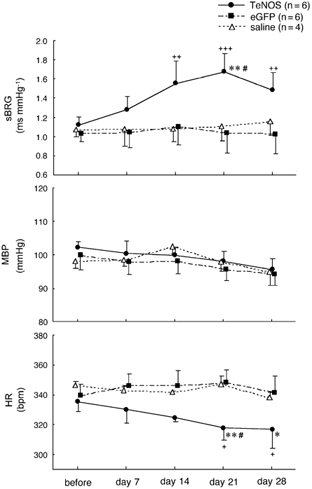
A significant increase in sBRG was observed 14, 21 and 28 days after the Ad-CMV-TeNOS transfection. In contrast, sBRG did not change in eGFP-transfected and saline-treated groups. In the TeNOS-transfected group, significant decreases in HR were also observed 21 and 28 days post-adenoviral injection. +P < 0.05, ++P < 0.01 and +++P < 0.001 values compared before and after gene transfer. *P < 0.05 and **P < 0.01 values compared to eGFP-transfected group. #P < 0.05 values compared to saline-treated group.
After viral transfection, the TeNOS-transfected group, sBRG and HR showed a time-dependent change between the 7th and 21st day after transfection but MBP was unaffected. Over this period sBRG increased such that the value at day 21 (1.68 ± 0.20 ms mmHg−1) was significantly higher than before gene transfer (1.13 ± 0.09 ms mmHg−1; n = 6, P < 0.001; Fig. 1). This value was also significantly higher than those in both the eGFP-transfected (1.04 ± 0.21 ms mmHg−1; n = 6, P < 0.01) and saline-treated groups (1.12 ± 0.15 ms mmHg−1; n = 4, P < 0.05). In addition, HR showed a time-related decrease from the 7th to the 21st day after gene transfer in the TeNOS-transfected group (Fig. 1); the value at day 21 (318 ± 8 b.p.m.) was significantly lower than before gene transfer (336 ± 6 b.p.m.; P < 0.05) or in control groups (eGFP: 348 ± 9 b.p.m., n = 6, P < 0.01; saline: 347 ± 5 b.p.m., n = 4, P < 0.05). At day 28 after TeNOS microinjection, sBRG and HR did not return to pre-transfection values (sBRG: 1.13 ± 0.09 vs. 1.49 ± 0.18 ms mmHg−1, P < 0.01; HR: 336 ± 6 vs. 317 ± 13 b.p.m., P < 0.05; Fig. 1). In contrast, baseline MBP, HR and sBRG in both eGFP-transfected and saline-treated rats did not change significantly over the entire observation period (Fig. 1).
Effects of chronic inhibition of eNOS activity in the NTS on BRG as determined using vasoactive agents
Before drug injections, baseline MBP in the TeNOS-transfected group was 101 ± 14 mmHg, and the value was not different from eGFP-transfected (103 ± 7 mmHg) and saline-treated groups (103 ± 12 mmHg). As seen in Fig. 2, the value of BRG determined by injection of phenylephrine in the 4th-5th week after NTS transfection with TeNOS was 2.21 ± 0.37 ms mmHg−1 (n = 5), which was higher than that in both eGFP-transfected (1.09 ± 0.34 ms mmHg−1; n = 5, P < 0.05) and saline-treated groups (1.13 ± 0.10 ms mmHg−1; n = 4, P < 0.05). When BRG was determined by injections of nitroprusside, however, there were no differences between the groups (TeNOS: 1.54 ± 0.31 ms mmHg−1; eGFP: 1.36 ± 0.31 ms mmHg−1; saline: 1.57 ± 0.09 ms mmHg−1).
Figure 2. Effects of chronic inhibition of eNOS activity in the NTS on the baroreceptor reflex gain (BRG) in conscious rats.
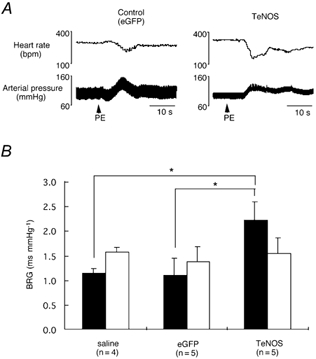
A, original recordings of arterial pressure and HR from 2 rats in which the NTS was transfected with either eGFP (left) or TeNOS (right). Arterial pressure was increased by bolus injection of phenylephrine (PE; 10 μg kg−1). The baroreceptor reflex was potentiated significantly in the TeNOS-transfected rat compared to the eGFP-transfected rat. B, BRG was determined pharmacologically by i.v. injections of phenylephrine (▪; 10-20 μg kg−1) and nitroprusside (□; 10-20 μg kg−1) in conscious animals in the 4th and 5th week post-adenoviral injection. BRG in response to phenylephrine in TeNOS-transfected rats is significantly larger than that in eGFP-transfected and saline-treated groups. *P < 0.05 between different groups.
Effects of chronic inhibition of eNOS activity in the NTS on ANGII-induced depression of the baroreceptor reflex
All groups (TeNOS, eGFP and saline) were anaesthetized with urethane and bilateral NTS microinjections of 500 fmol ANGII caused transient effects (≈60 s) on arterial pressure (hypotension) and HR (bradycardia). These effects were not quantified further in the present study. Baseline MBP and HR in the TeNOS-transfected group were 77 ± 5 mmHg and 354 ± 10 b.p.m., respectively. These values were not different from those of either the eGFP-transfected rats (MBP: 76 ± 2 mmHg; HR: 376 ± 28 b.p.m.) or the saline-treated group (MBP: 73 ± 5 mmHg; HR: 381 ± 34 b.p.m.). BRG was determined by injection of phenylephrine (Fig. 3 and Fig. 4). In eGFP-transfected and saline-treated groups, BRG was strongly attenuated by ANGII microinjections (eGFP: 0.52 ± 0.13 vs. 0.28 ± 0.07 ms mmHg−1; n = 5, P < 0.05; saline: 0.73 ± 0.14 vs. 0.29 ± 0.08 ms mmHg−1; n = 4, P < 0.01). In contrast, AII microinjected into the NTS of TeNOS-transfected rats failed to affect BRG (0.83 ± 0.25 vs. 0.82 ± 0.28 ms mmHg−1; n = 6; Fig. 3 and Fig. 4).
Figure 3. Effects of chronic inhibition of eNOS activity in the NTS on ANGII-induced depression of the baroreceptor reflex in anaesthetized rats.
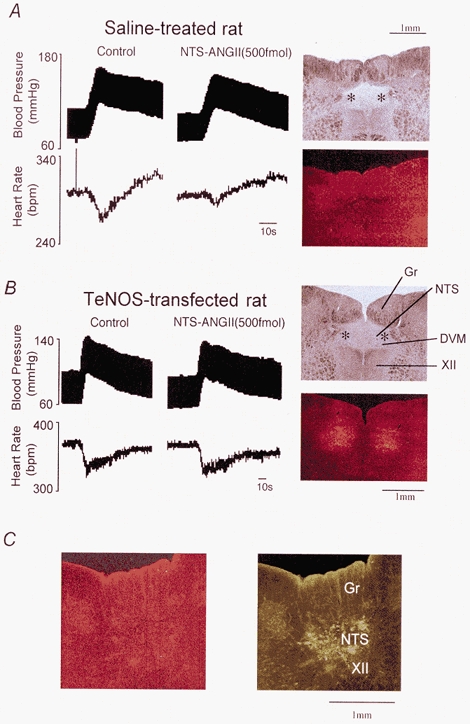
A, original recordings of arterial pressure and HR from a rat in which the NTS had received microinjections of saline. Arterial pressure was increased by bolus injection of phenylephrine (10 μg kg−1). A bilateral NTS microinjection of ANGII (500 fmol) attenuated the reflex cardiac response. The photomicrographs on the right show the microinjection sites of ANGII (top, indicated by *) and the immunoreactivity for eNOS (bottom) on the same section. B, original recordings of arterial pressure and HR in a rat in which the NTS was transfected with TeNOS. A bilateral NTS microinjection of ANGII (500 fmol) failed to affect the baroreceptor reflex. The photomicrographs on the right show the microinjection sites of ANGII (top, indicated by *) and the enhanced immunoreactivity for eNOS (bottom) on the same section. In this rat the immunoreactivity for eNOS evaluated through the fluorescence microscope was positive, indicating that TeNOS protein was expressed site-specifically within cells contained in the NTS and the dorsal vagal motor nucleus (DVM). This eNOS immunoreactivity was obviously greater than that in the control rat which received saline injections (see above in A). C, the photomicrographs show representative transverse sections (the caudal part of the microinjected site) where eGFP was expressed in the NTS (right) but eNOS immunoreactivity was obviously less than in TeNOS-transfected rats (left). Abbreviations: DVM, dorsal vagal motonucleus; Gr, gracile nucleus; XII, hypoglossal motor nucleus.
Figure 4. Comparison of the effect of bilateral NTS microinjections of ANGII on the baroreceptor reflex gain (BRG) in TeNOS-transfected, eGFP-transfected and saline-treated rats.
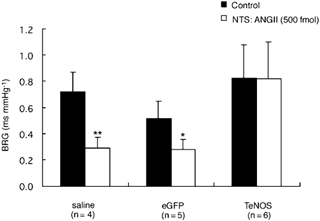
Bilateral NTS microinjection of ANGII (500 fmol) depressed the BRG in eGFP-transfected and saline-treated rats. In contrast, ANGII microinjected into the NTS of rats chronically transfected with TeNOS failed to affect BRG. *P < 0.05 and **P < 0.01 compared to control values.
Analysis of HR variability
The HF power in TeNOS-transfected rats was 7.2 ± 1.3 ms2 3 weeks after gene transfer, which was significantly higher than before gene transfection (i.e. 3.8 ± 0.7 ms2; Fig. 5A, n = 6, P < 0.05). The HF power in eGFP-transfected and saline-treated rats did not change over the same observation period (Fig. 5A). In all groups, there were no significant differences in the ratio of LF power to HF power (LF/HF) before and after gene transfer (Fig. 5B).
Figure 5. Chronic inhibition of eNOS activity in the NTS increases high-frequency (HF) power of HR variability in rats.
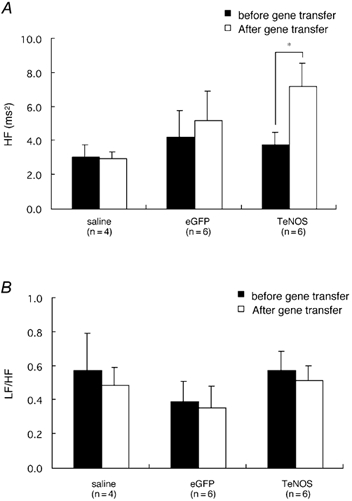
HF power was increased 3 weeks after TeNOS transfection while HF power in eGFP-transfected and saline-treated rats did not change (A). In all groups, there were no significant differences in LF/HF before and after gene transfer (B). This suggests that blockade of endogenous eNOS activity in the NTS enhances cardiac vagal tone.
Histological verification of transfection and microinjection sites of ANGII
Despite the microinjection of relatively large volumes of virus into the NTS, we saw no evidence of tissue damage. In eGFP-transfected rats, eGFP was expressed in the NTS and, in some cases, in restricted regions of the gracile nucleus and dorsal vagal motor nucleus (DVM; Fig. 3C). Figure 3A and B depicts the transfected regions in a representative animal from a TeNOS-transfected and saline-treated groups together with microinjection sites of ANGII. In TeNOS-transfected rats, numerous NTS neurons and blood vessels were immunopositive to eNOS, while saline-treated rats and eGFP-transfected rats showed much less immunoreactivity, indicating that TeNOS protein was highly expressed in the NTS 5-6 weeks after transfection. In all rats, transfection and ANGII microinjection sites overlapped and were located caudal to the obex within the NTS.
Discussion
The new finding of this study is that eNOS activity within the caudal NTS is constitutively active in the conscious normotensive rat and acts to attenuate the cardiac component of the baroreceptor reflex. This was revealed by a significant increase in reflex gain, which persisted for several weeks, following its chronic blockade by adenoviral-mediated expression of a dominant negative protein, TeNOS. Our results are consistent with the acute study by Pontieri et al. (1998), in which NTS microinjections of a nonselective NOS inhibitor increased baroreceptor reflex gain in conscious normotensive rats. Our study reveals both the NOS isoform involved and that it exerts a chronic effect. Although we found immunohistochemical evidence of TeNOS protein in dorsal regions of the DVM in some rats, we believe that our findings are mainly confined to the endogenous eNOS activity in the NTS. Clearly, future studies need to specifically assess a role for NO in the DVM before we can completely rule out its contribution to the present data.
Technical considerations of the sBRG measurement
Time-series analysis of sBRG has been shown to detect differences between normo- and hypertensive rats and to be sensitive to both baroreceptor afferent denervation and lesions of the NTS (see Oosting et al. 1997a,b, for details). We found qualitatively similar results of disabling eNOS in the NTS on baroreceptor reflex gain using either time-series analysis or a pharmacological approach. However, there were quantitative differences (i.e. an increase in gain of +49 vs. +96 % after viral transfection of NTS using sequence and pharmacological testing, respectively). There may be a number of reasons to explain this. First, as discussed by Oosting et al. (1997b), the sBRG does not fully consider the sympathetic component of the baroreceptor cardiac reflex because the HR responses measured are too rapid and transient and mediated predominantly by the vagus. This is likely to be relevant if NO decreases the baroreceptor cardiac reflex through both sympathetic and parasympathetic components as found previously (Liu et al. 1996). Second, the sBRG is based on measures of both bradycardia and tachycardia reflexly evoked by increases and decreases, respectively, in arterial pressure. If NO only modulates reflex bradycardia to pressor responses (as argued below) then any change in the sBRG will be compromised. This may be the case for NO, since ANGII releases NO in the NTS and the depressant effect of ANGII on the baroreceptor reflex is dependent on NO and is asymmetrical (i.e. depresses the reflex bradycardia to a rise in pressure only). With both these considerations the sBRG measurements may underestimate changes in baroreceptor reflex gain compared to data following injection of vasoactive drugs. Nevertheless, the sBRG measurements are not invalid. Combined with radio-telemetry, we believe that the time-series method is very powerful for chronic, time-dependent assessment of changes in baroreceptor reflex gain. The method is advantaged by allowing a continuous assessment of the reflex during physiological changes in arterial pressure and void of artificial stimulation with drug injections that can cause stress to the animal (Frankel et al. 1993; Iellamo, 2001).
Assessing transgene expression in the NTS
In the present study we showed that exogenous ANGII microinjected into the NTS was ineffective in attenuating the baroreceptor reflex in TeNOS-NTS transfected rats but not in controls (NTS expressing eGFP or NTS microinjection of saline). The absence of effect of ANGII in the NTS of transfected rats is physiological confirmation of the existence of the transgene 5 weeks after viral injection. These data also confirm our previous finding that ANGII attenuation of the baroreceptor reflex was via activation of eNOS (Paton et al. 2001b). Furthermore, we also showed the presence of TeNOS immunocytochemically using an antibody against eNOS. TeNOS protein expression was greater in the NTS of transfected rats than in controls. Both the physiological and immunocytochemical data support a long lasting expression that persisted for several weeks using a titre of 4 × 1010 pfu ml−1 in this strain of rat.
Mechanism by which NO from eNOS depresses the baroreflex in NTS
Since the depressant effects of ANGII in the NTS on the baroreceptor reflex are dependent on the release of NO from eNOS (Paton et al. 2001b), we suggest that the mechanism of reflex attenuation by NO may be comparable to that of ANGII. Although we have limited data on downstream actions of NO in the NTS, we have shown that blocking GABAA receptors (Paton et al. 2001a) antagonizes the depressant effect of ANGII on the reflex. Similarly, both ANGII and NO enhance GABA transmission in the paraventricular and supraoptic nucleus of the hypothalamus (Bains & Ferguson, 1997; Ferguson & Latchford, 2000; Ozaki et al. 2000). Thus, we propose that ANGII-released NO acts presynaptically to baroreceptive neurones to stimulate release of GABA from GABAergic NTS interneurones thereby shunting baroreceptor inputs to NTS neurones. This is consistent with an absence of a change in both membrane potential and input resistance of baroreceptive neurones exposed to ANGII (Paton & Kasparov, 2000) and the enhancement of synaptically evoked IPSPs recorded from some NTS neurones in the presence of ANGII in vitro (Kasparov & Paton, 1999). Thus, inhibiting eNOS activity in the NTS may increase baroreceptor reflex gain via disinhibition.
Reasons for endogenous eNOS activity in the NTS
Although many factors regulate eNOS activity (see Forstermann et al. 1998), we believe that circulating hormones, such as ANGII, may contribute to the production of NO in the NTS of conscious rats. It is established that circulating ANGII is a potent stimulant of eNOS located in endothelial cells (Millatt et al. 1999). In support of circulating ANGII driving eNOS activity in the NTS, it is of interest that in an arterially perfused rat preparation devoid of circulating ANGII, NTS microinjection of either l-NAME or l-NMMA had no effect on baroreceptor reflex function. However, the depressant effect of exogenously applied ANGII in the NTS was prevented by NOS inhibitors, indicating that ANGII releases NO (Paton et al. 2001a). In addition, centrally derived ANGII may also play a role. All the biochemical machinery to produce ANGII resides within the NTS (Fuxe et al. 1994) and external sources such as the hypothalamus, which contains a high number of ANGII-containing neurones (Lind et al. 1985), may project to the NTS and release ANGII from their terminals.
Role of eNOS in the NTS for arterial pressure control
Recently, Sakai et al. (2000) reported that over-expression of eNOS in NTS using an adenovirus produced a bradycardia and hypotension indicative of a general excitatory effect. No data on baroreceptor reflex gain were reported. Based on our data with TeNOS, we would predict that excess eNOS activity would depress the NTS and produce an opposite pattern of response (i.e. tachycardia/hypertension). One possible reason for this inconsistency is the differences in NTS microinjection sites. The microinjection sites in the study of Sakai et al. (2000) included NTS regions rostral to the obex. Our microinjection sites were caudal to the obex. We selected this localized region based on the effective NTS sites that mediated the ANGII-induced depression of the baroreceptor reflex (Paton & Kasparov, 1999; Paton et al. 2001b). Thus, regarding the inconsistency in the role of NO in the NTS for regulating HR, there may be functional differences between the anatomical sites studied in the NTS in the present study vs. those studied by Sakai et al. (2000).
Sakai et al. (2000) attributed the bradycardia/depressor response induced by over-expression of eNOS activity in the NTS to NO enhancing release of glutamate. However, our results are consistent with the idea that NO produced by eNOS primarily influenced release of GABA, which is supported by others (Kano et al. 1998). Indeed, NO preferentially enhancing GABA transmission was also found in the paraventricular and supraoptic nucleus of the hypothalamus (Bains & Ferguson, 1997; Ferguson & Latchford, 2000; Ozaki et al. 2000). It should also be noted that expression of a dominant negative eNOS protein, as described in this study, would only affect NTS cells that normally express eNOS naturally. In contrast, an over-expression of exogenous eNOS using an unspecific promoter, such as CMV, will result in ‘ectopic’ eNOS expression in virtually all cells at the site of transfection. Based on this fact, we believe that the dominant negative approach gives a more realistic account of the physiological role of endogenous eNOS in the NTS of conscious rats.
Plausible mechanism of decreased HR by chronic TeNOS expression in the NTS
Although we did not detect a change in arterial pressure following chronic blockade of eNOS activity in the NTS of our normotensive rats, heart rate was lowered significantly. In contrast, Lewis et al. (1991) showed that microinjections of S-nitrosocysteine, a NO donor, into the NTS decreased both heart rate and arterial pressure in anaesthetized rats. These data may not be comparable to the present findings because of differences in the animal model used (conscious vs. anaesthetized) and because chronic vs. acute effects may be mediated by entirely separate processes. Consistent with our study, however, was that inhibition of NOS in the NTS did not affect the baseline level of arterial pressure in conscious animals (Pontieri et al. 1998). The absence of any change in arterial pressure after chronic blockade of eNOS in the present study may reflect compensatory processes outside the NTS, such as within the ventrolateral medulla. Alternatively, neuronal circuitry regulating the heart rate in the NTS may be more sensitive to NO. Consistent with this notion was the finding that NOS inhibitors (albeit given intravenously) enhanced the heart rate but not the renal sympathetic nerve activity response following electrical stimulation of the aortic depressor nerve (Liu et al. 1996). Whilst we fully accept that the fall in heart rate recorded after chronically disabling eNOS activity in the NTS is small, and likely to be functionally insignificant, it was a significant response. Although we did not assess the relative changes in sympathetic vs. parasympathetic drives to the heart pharmacologically after eNOS blockade in the NTS, we did analyse heart rate variability. It is generally accepted that the high-frequency component of heart rate variability is mediated by cardiac parasympathetic tone whereas the ratio of low : high frequency of heart rate variability is an index of cardiac sympathetic tone (Murasato et al. 1998). Because high-frequency power was significantly increased following TeNOS expression in the NTS but low : high frequency did not change, the decreased basal HR may be mediated by an increased level of cardiac vagal tone.
Absence of effect of disabling eNOS in the NTS on baroreflex tachycardia
In comparison to the enhanced reflex bradycardia evoked by phenylephrine-induced pressor responses in TeNOS-NTS transfected rats, the reflex tachycardia in response to a depressor response (evoked by nitroprusside) remained unaltered. This result is reminiscent of a previous report in conscious rats where oral intake of a NOS inhibitor enhanced baroreceptor reflex gain due to a potentiation of reflex bradycardia only (Vasquez et al. 1994). Furthermore, ANGII microinjections into the NTS affect baroreceptor reflex bradycardia but not tachycardia (e.g. Michelini & Bonagamba, 1990). We cannot provide a definitive reason for this asymmetrical effect of NO (or ANGII). A possibility is that raising arterial pressure enhances GABAergic tone within the NTS that is sensitive to modulation by NO, whereas lowering arterial pressure does not affect GABA release to the same extent.
In conclusion, we have shown that in the conscious rat endogenous eNOS is constitutively active within the NTS and is important for setting the baroreceptor reflex gain and resting level of HR. This eNOS activity may provide a mechanism to dampen excessive vagally mediated bradycardic reflex responses during transient rises in arterial pressure. This is particularly important given the fast time course and potent nature of the cardiac vagus. Whether excessive eNOS activity in the NTS contributes to the blunted baroreceptor reflex gain in pathological conditions of hypertension remains to be elucidated.
Acknowledgments
The authors wish to thank Dr Kiyoaki Katahira (Experimental Animal Center, Fukushima Medical University School of Medicine) for his advice on the telemetry system. This study was carried out as a part of the ‘Ground Research Announcement for Space Utilization’ promoted by the Japan Space Forum. The study was also financially supported by the British Heart Foundation (BS/93003).
References
- Bains JS, Ferguson AV. Nitric oxide regulates NMDA-driven GABAergic inputs to type I neurones of the rat paraventricular nucleus. J Physiol. 1997;499:733–746. doi: 10.1113/jphysiol.1997.sp021965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batten TFC, Atkinson L, Deuchars J. Nitric oxide systems in the medulla oblongata and their involvement in autonomic control. In: Steinbusch HWM, De Vente J, editors. Handbook of Chemical Neuroanatomy, Functional Neuroanatomy of the Nitric Oxide System. XIV. Amsterdam: Elsevier; 2000. pp. 177–213. [Google Scholar]
- Bertinieri G, Di Rienzo M, Cavallazzi A, Ferrari AU, Pedotti A, Mancia G. Evaluation of baroreceptor reflex by blood pressure monitoring in unanesthetized cats. Am J Physiol. 1988;254:H377–383. doi: 10.1152/ajpheart.1988.254.2.H377. [DOI] [PubMed] [Google Scholar]
- Blessing WW. The Lower Brainstem and Bodily Homeostasis. New York: Oxford University Press; 1997. Anatomy of the lower brainstem; pp. 29–99. [Google Scholar]
- Busconi L, Michel T. Endothelial nitric oxide synthase. N-terminal myristoylation determines subcellular localization. J Biol Chem. 1993;268:8410–8413. [PubMed] [Google Scholar]
- Casto R, Phillips MI. Angiotensin II attenuates baroreflexes at nucleus tractus solitarius of rats. Am J Physiol. 1986;250:R193–198. doi: 10.1152/ajpregu.1986.250.2.R193. [DOI] [PubMed] [Google Scholar]
- Dickinson CJ, Sleight P. The baroreflex bandwagon: time to get off? J Hypertens. 2001;19:157–161. doi: 10.1097/00004872-200101000-00021. [DOI] [PubMed] [Google Scholar]
- Ferguson AV, Latchford KJ. Local circuitry regulates the excitability of rat neurohypophysial neurones. Exp Physiol. 2000;85S:153S–161S. doi: 10.1111/j.1469-445x.2000.tb00019.x. [DOI] [PubMed] [Google Scholar]
- Forstermann U, Boissel JP, Kleinert H. Expressional control of the ‘constitutive’ isoforms of nitric oxide synthase (NOS I and NOS III) FASEB J. 1998;12:773–790. [PubMed] [Google Scholar]
- Frankel RA, Metting PJ, Britton SL. Evaluation of spontaneous baroreflex sensitivity in conscious dogs. J Physiol. 1993;462:31–45. doi: 10.1113/jphysiol.1993.sp019541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K, Aganti LF, Covenas R, Norvaez JA, Bunnemann B, Bjelke B. Volume transmission in transmitter peptide co-storing neurons in the medulla oblongata. In: Barraco IRA, editor. Nucleus of the Solitary Tract. London: CRC Press; 1994. pp. 75–89. [Google Scholar]
- Harada S, Tokunaga S, Momohara M, Masaki H, Tagawa T, Imaizumi T, Takeshita A. Inhibition of nitric oxide formation in the nucleus tractus solitarius increases renal sympathetic nerve activity in rabbits. Circulation Res. 1993;72:511–516. doi: 10.1161/01.res.72.3.511. [DOI] [PubMed] [Google Scholar]
- Iellamo F. Neural mechanisms of cardiovascular regulation during exercise. Auton Neurosci. 2001;90:66–75. doi: 10.1016/S1566-0702(01)00269-7. [DOI] [PubMed] [Google Scholar]
- Kano T, Shimizu-Sasamata M, Huang PL, Moskowitz MA, Lo EH. Effects of nitric oxide synthase gene knockout on neurotransmitter release in vivo. Neuroscience. 1998;86:695–699. doi: 10.1016/s0306-4522(98)00179-1. [DOI] [PubMed] [Google Scholar]
- Kantor DB, Lanzrein M, Stary SJ, Sandoval GM, Smith WB, Sullivan BM, Davidson N, Schuman EM. A role for endothelial NO synthase in LTP revealed by adenovirus-mediated inhibition and rescue. Science. 1996;274:1744–1748. doi: 10.1126/science.274.5293.1744. [DOI] [PubMed] [Google Scholar]
- Kasparov S, Paton JFR. Differential effects of angiotensin II in the nucleus tractus solitarii of the rat - plausible neuronal mechanisms. J Physiol. 1999;521:227–238. doi: 10.1111/j.1469-7793.1999.00227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AJ, Castillo-Melendez M, McLean K, Jarrott B. The distribution of nitric oxide synthase-, adenosine deaminase- and neuropeptide Y-immunoreactivity through the entire rat nucleus tractus solitarius. Effects of unilateral nodose ganglionectomy. J Chem Neuroanat. 1998;15:27–40. doi: 10.1016/s0891-0618(98)00020-9. [DOI] [PubMed] [Google Scholar]
- Lee CM, Robinson LJ, Michel T. Oligomerization of endothelial nitric oxide synthase. Evidence for a dominant negative effect of truncation mutants. J Biol Chem. 1995;270:27 403–27 406. doi: 10.1074/jbc.270.46.27403. [DOI] [PubMed] [Google Scholar]
- Lewis SJ, Ohta H, Machado B, Bates JN, Talman WT. Microinjection of S-nitrosocysteine into the nucleus tractus solitarii decreases arterial pressure and heart rate via activation of soluble guanylate cyclase. Eur J Pharmacol. 1991;202:135–136. doi: 10.1016/0014-2999(91)90269-v. [DOI] [PubMed] [Google Scholar]
- Li J, Smith SA, Mitchell JH. The arterial baroreflex attenuates the pressor response to exercise by increasing nitric oxide formation within the nucleus tractus solitarius. Proceedings of the Experimental Biology 2002 Meeting. 2002;131:4. [Google Scholar]
- Lind RW, Swanson LW, Ganten D. Organization of angiotensin II immunoreactive cells and fibers in the rat central nervous system. An immunohistochemical study. Neuroendocrinology. 1985;40:2–24. doi: 10.1159/000124046. [DOI] [PubMed] [Google Scholar]
- Liu J, Sessa WC. Identification of covalently bound amino-terminal myristic acid in endothelial nitric oxide synthase. J Biol Chem. 1994;269:11 691–11 694. [PubMed] [Google Scholar]
- Liu J-L, Murakami H, Zucker IH. Effects of NO on baroreflex control of heart rate and renal nerve activity in conscious rabbits. Am J Physiol. 1996;270:R1361–1370. doi: 10.1152/ajpregu.1996.270.6.R1361. [DOI] [PubMed] [Google Scholar]
- Luoh HF, Chan SHH. Participation of AT1 and AT2 receptor subtypes in the tonic inhibitory modulation of baroreceptor reflex response by endogenous angiotensins at the nucleus tractus solitarii in the rat. Brain Res. 1998;782:73–83. doi: 10.1016/s0006-8993(97)01198-0. [DOI] [PubMed] [Google Scholar]
- Michelini LC, Bonagamba LH. Angiotensin II as a modulator of baroreceptor reflexes in the brainstem of conscious rats. Hypertension. 1990;15:I45–I50. doi: 10.1161/01.hyp.15.2_suppl.i45. [DOI] [PubMed] [Google Scholar]
- Millatt LJ, Abdel-Rahman EM, Siragy HM. Angiotensin II and nitric oxide: a question of balance. Regul Pep. 1999;81:1–10. doi: 10.1016/s0167-0115(99)00027-0. [DOI] [PubMed] [Google Scholar]
- Miyajima E, Shigemasa T, Yamada Y, Tochikubo O, Ishii M. Angiotensin II blunts, while an angiotensin-converting enzyme inhibitor augments, reflex sympathetic inhibition in humans. Clin Exp Pharmacol Physiol. 1999;26:797–802. doi: 10.1046/j.1440-1681.1999.03122.x. [DOI] [PubMed] [Google Scholar]
- Murasato Y, Hirakawa H, Harada Y, Nakamura T, Hayashida Y. Effects of systemic hypoxia on R-R interval and blood pressure variabilities in conscious rats. Am J Physiol. 1998;275:H797–804. doi: 10.1152/ajpheart.1998.275.3.H797. [DOI] [PubMed] [Google Scholar]
- Oosting J, Janssen BJA, Struijker-Boudier HAJ. Autonomic control of ultradian and circadian rhythms of blood pressure, heart rate, and baroreflex sensitivity in SHR. J Hypertens. 1997a;15:401–410. doi: 10.1097/00004872-199715040-00011. [DOI] [PubMed] [Google Scholar]
- Oosting J, Struijker-Boudier HAJ, Janssen BJA. Validation of a continuous baroreceptor reflex sensitivity index calculated from spontaneous fluctuations of blood pressure and pulse interval in rats. J Hypertens. 1997b;15:391–399. doi: 10.1097/00004872-199715040-00010. [DOI] [PubMed] [Google Scholar]
- Ozaki M, Shibuya N, Kabashima T, Isse T, Noguchi J, Ueta Y, Inoue Y, Shigematsu A, Yamashita H. Preferential potentiation by nitric oxide of spontaneous inhibitory postsynaptic currents in rat supraoptic neurones. J Neuroendocrinol. 2000;12:273–281. doi: 10.1046/j.1365-2826.2000.00448.x. [DOI] [PubMed] [Google Scholar]
- Paton JFR, Boscan P, Murphy D, Kasparov S. Unravelling mechanisms of action of angiotensin II on cardiorespiratory function using in vivo gene transfer. Acta Physiol Scand. 2001a;173:127–137. doi: 10.1046/j.1365-201X.2001.00898.x. [DOI] [PubMed] [Google Scholar]
- Paton JFR, Deuchars J, Ahmad Z, Wong L-F, Murphy D, Kasparov S. Adenoviral vector demonstrates that angiotensin II-induced depression of the cardiac baroreflex is mediated by endothelial nitric oxide synthase in the nucleus tractus solitarii of the rat. J Physiol. 2001b;531:445–458. doi: 10.1111/j.1469-7793.2001.0445i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JFR, Kasparov S. Differential effects of angiotensin II on cardiorespiratory reflexes mediated by nucleus tractus solitarii - a microinjection study in the rat. J Physiol. 1999;521:213–225. doi: 10.1111/j.1469-7793.1999.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JFR, Kasparov S. Sensory channel specific modulation in the nucleus of the solitary tract. J Auton Nerv Syst. 2000;80:117–129. doi: 10.1016/s0165-1838(00)00077-1. [DOI] [PubMed] [Google Scholar]
- Pontieri V, Venezuela MK, Scavone C, Michelini LC. Role of endogenous nitric oxide in the nucleus tractus solitarii on baroreflex control of heart rate in spontaneously hypertensive rats. J Hypertens. 1998;16:1993–1999. doi: 10.1097/00004872-199816121-00021. [DOI] [PubMed] [Google Scholar]
- Ruggiero DA, Mtui EP, Otake K, Anwar M. Central and primary visceral afferents to nucleus tractus solitarii may generate nitric oxide as a membrane-permeant neuronal messenger. J Comp Neurol. 1996;361:51–67. doi: 10.1002/(SICI)1096-9861(19960101)364:1<51::AID-CNE5>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Sakai K, Hirooka Y, Matsuo I, Eshima K, Shigematsu H, Shimokawa H, Takeshita A. Overexpression of eNOS in NTS causes hypotension and bradycardia in vivo. Hypertension. 2000;36:1023–1028. doi: 10.1161/01.hyp.36.6.1023. [DOI] [PubMed] [Google Scholar]
- Struyker-Boudier HAJ, Evenwel RT, Smits JFM, Van Essen H. Baroreflex sensitivity during the development of spontaneous hypertension in rats. Clin Sci. 1982;56:163–167. doi: 10.1042/cs0620589. [DOI] [PubMed] [Google Scholar]
- Thrasher TN. Unloading arterial baroreceptors causes neurogenic hypertension. Am JP hysiol. 2002;282:R1044–1053. doi: 10.1152/ajpregu.00431.2001. [DOI] [PubMed] [Google Scholar]
- Vasquez EC, Cunha RS, Cabral AM. Baroreceptor reflex function in rats submitted to chronic inhibition of nitric oxide synthesis. Brazilian J Med Biol Res. 1994;27:767–774. [PubMed] [Google Scholar]
- Zanzinger J, Czachurski J, Seller H. Effects of nitric oxide on sympathetic baroreflex transmission in the nucleus tractus solitarii and caudal ventrolateral medulla in cats. Neurosci Lett. 1995;197:199–202. doi: 10.1016/0304-3940(95)11929-q. [DOI] [PubMed] [Google Scholar]


