Abstract
Sympathetically mediated tachycardia is a characteristic feature of the physiological response to emotional or psychological stress in mammals. Activation of neurons in the region of the dorsomedial hypothalamus appears to play a key role in the integration of this response. Tachycardia evoked by chemical stimulation of the dorsomedial hypothalamus can be suppressed by microinjection of the GABAA receptor agonist and neuronal inhibitor muscimol into the raphe pallidus (RP). Therefore, we tested the hypothesis that neuronal excitation in the RP mediates tachycardia seen in experimental air stress in rats. Microinjection of the GABAA receptor antagonist bicuculline methiodide (BMI) into the RP evoked increases in heart rate. At the same sites, microinjection of muscimol (80 pmol (100 nl)−1) had no effect on heart rate under baseline conditions but virtually abolished air stress-induced tachycardia, while microinjection of lower doses (10 or 20 pmol) produced transient but clear suppression. Microinjection of muscimol at sites outside the RP had no effect on stress-induced tachycardia, although modest suppression was apparent after injection at two sites within 500 μm of the RP. In another series of experiments, microinjection of muscimol (80 pmol (100 nl)−1) into the RP failed to influence the changes in heart rate produced by baroreceptor loading or unloading. These findings indicate that activity of neurons in the RP plays a previously unrecognized role in the generation of stress-induced tachycardia.
Sympathetically mediated tachycardia is a common feature of the response to stress in many mammals, including man. Historically, the hypothalamus has been thought to play a key role in integrating and generating the physiological changes typically seen in stress in mammals. Recent evidence points to such a role for neurons specifically in the region of the dorsomedial hypothalamus (DMH; for review see DiMicco et al. 2002). Chemical stimulation of this region in rats provokes a pattern of behavioural and physiological changes resembling those seen in experimental stress, including marked increases in heart rate. Conversely, microinjection of the GABAA receptor agonist muscimol into this region can virtually abolish the increases in heart rate seen in air stress in rats (Stotz-Potter et al. 1996a,b). These findings have led to the suggestion that activation of neurons in the region of the DMH generates various components of the response to stress, including tachycardia.
If neurons in the DMH do play such a role, then the pathway through which they might influence the activity of cardiac-related sympathetic preganglionic neurons in the spinal cord under conditions of experimental stress is unclear. Few if any neurons in this region project directly to the spinal cord, pointing to the existence of at least one intermediate relay in the pathway. However, no specific brainstem region has been strongly implicated in the generation of stress-induced increases in heart rate. Recently, we found that microinjection of muscimol into the medullary raphe pallidus (RP) suppressed the tachycardia induced by chemical stimulation of the DMH in rats (Samuels et al. 2002). Neurons in the region of the RP are known to both project to spinal sympathetic centres (Jansen et al. 1995) and receive projections from neurons in the region of the DMH (Hosoya et al. 1987; Hermann et al. 1997). Disinhibition of neurons in the RP evokes a pattern of cardiovascular changes resembling that produced by chemical stimulation of the DMH, the salient feature of which is marked tachycardia (Morrison et al. 1999; Samuels et al. 2002). Thus, neurons in the RP may provide the critical relay mediating hypothalamic influences on cardiac sympathetic nerve activity under conditions of psychological or emotional stress.
To test this hypothesis, we examined changes in heart rate seen under baseline conditions and in experimental air stress after microinjection of muscimol or saline vehicle into the RP in conscious rats. In order to establish the specificity of any effect noted, we performed parallel studies to determine the effect of identical treatment with muscimol on changes in heart rate evoked through the baroreceptor reflex.
Methods
Animal preparation
Male Sprague-Dawley rats (300 ± 10 g) from Harlan (Indianapolis, IN, USA) were maintained under standard animal housing conditions with food and water ad libitum. All procedures conformed to guidelines set forth by the NIH and were approved by the Institutional Animal Care and Use Committee. Surgeries were performed under ketamine-xylazine anaesthesia (80 mg kg−1 ketamine and 11.5 mg kg−1 xylazine, i.p., supplemented (4-8 mg kg−1 ketamine and 0.6-1.15 mg kg−1 xylazine, i.p.) as required).
Experimental protocol: effect of microinjection of muscimol into the RP on cardiovascular responses to stress
The role of neuronal activity in the RP in the response to stress was examined in a total of 12 rats. A Dataquest telemetry system (Data Sciences, MN, USA) was used for measurement of arterial blood pressure, heart rate and locomotor activity. (Locomotor activity is calculated from changes in strength of the transmitter signal over time, where an activity unit is approximately equivalent to movement of 1 cm s−1.) Rats were anaesthetized and the flexible catheter of the transmitter (Model TA11PA-C40) was inserted into the abdominal aorta through the right femoral artery. The body of the transmitter was placed into the abdominal cavity and sutured to the abdominal wall.
Five days after transmitter implantation, the rats were again anaesthetized and placed in a stereotaxic apparatus with the incisor bar set 3.3 mm below the interaural line for placement of a microinjection guide cannula into the RP. The skin overlying the dorsal surface of the skull was cut and retracted, and soft tissue was removed from the exposed surface. In eight rats, the guide cannula (26 gauge, Plastics One Inc., Roanoke, VA, USA) was then positioned to target the RP (coordinates: AP −2.8 mm; LR 0.0 mm; HD −1.1 mm; interaural line as reference point) and secured by three stainless-steel screws, Vetbond glue and cranioplastic cement. Dummy wire cannulae were inserted in the guides, and rats were returned to their home cages for recovery. In experiments intended to serve as controls for neuroanatomical specificity, guide cannulae were intentionally placed lateral or posterior to the RP. Three to 4 days later, the rats were re-anaesthetized and femoral arterial and venous catheters were implanted for blood sampling (see below).
Three days later, appropriate placement of the guide cannula was assessed in each rat by noting the cardiovascular response to microinjection of the GABAA receptor antagonist bicuculline methiodide (BMI, 40 pmol (100 nl)−1) in the conscious freely moving subject under baseline conditions. These and all other microinjections were performed as follows. On the day of the experiment, rats were placed in their home cage on the telemetry receiver plate. The dummy cannula was then removed, the microinjector (33 gauge, Plastics One Inc.) connected to a 10 μl Hamilton syringe with Teflon FEP tubing (i.d. = 0.12 mm; o.d. = 0.65 mm; BAS, USA) was inserted into the guide cannula, and the rat was left undisturbed for an additional 30-40 min. The Hamilton syringe was mounted in an infusion pump (Sage, Boston, MA, USA) that was used to deliver a volume of 100 nl of injectate over 30 s. After the injection of BMI, the injector was left in place for at least 20 min while the animal remained undisturbed in its cage. The microinjection was considered successful if, immediately after removal of the microinjector, flow appeared within 5 s after the pump was reactivated, indicating that the injector was not clogged.
All rats were then subjected to four different trials at 2 to 3 day intervals in random order. In all rats, either saline (100 nl) or muscimol (80 pmol) was microinjected, followed 5 min later by a 10 min period of air stress, as described previously (Stotz-Potter et al. 1996a,b). The injector was removed 2-4 min after microinjections in these experiments, and rats were placed in a Plexiglas restraining tube that was sufficiently confining as to prevent the animal from turning around completely. An air jet (40 l min−1) was then directed at the rat's head for the duration of the trial, i.e. from +5 to +15 min after the microinjections. At the end of the 10 min period, the rat was released from the tube into its home cage. Three of the six rats in which guide cannulae had been placed to target the RP were subjected to two additional air-stress trials after microinjection of muscimol at lower doses (20 and 10 pmol). The remaining nine rats were subjected to trials in which treatment with saline vehicle or muscimol (80 pmol) was followed by continued observation under baseline conditions in trials designed to assess the effect of microinjection of muscimol in the relative absence of stress. For consistency, the injector in these trials was removed aproximately 2-4 min after the microinjection, and this evidently constituted a mild stress in all trials (see below). In order to avoid inclusion of cardiovascular data representing either the artifact associated with removal of the injection cannula or the subsequent physical manipulation resulting from placing the rat in the restraining tube, cardiovascular data were summarized for analysis by averaging heart rate and blood pressure for the period representing the last 5 min of the 10 min stress period in air-stress trials (i.e. from 10 to 15 min after microinjection). In all these studies, blood samples (0.35 ml) were taken 5 min before microinjection and at the tenth minute of stress in air-stress trials or at the corresponding time point in unstressed trials for measurement of plasma ACTH (data to be reported elsewhere).
Experimental protocol: effect of microinjection of muscimol into the RP on baroreflex responses
The effect of similar microinjections of muscimol on changes in heart rate evoked through the baroreflex was assessed in a separate group of four rats. These animals were instrumented with guide cannulae for microinjection into the RP as described above and with arterial and venous lines. At least 3 days later, the arterial line was connected to a blood pressure transducer via PE-50 tubing. A microinjector was connected to a syringe filled with a solution of muscimol as described above and fixed in the guide cannula. After allowing at least 30-40 min for attainment of a stable baseline, the baroreflex was tested by assessing the response to bolus intravenous injections of phenylephrine (4 μg kg−1) and, after return to baseline (8-10 min later), sodium nitroprusside (4 μg kg−1). After recovery of baseline arterial pressure and heart rate, muscimol (80 pmol (100 nl)−1) was microinjected into the RP, and the baroreflex was tested once again with phenylephrine and nitroprusside in identical fashion.
Localization of injection sites
After the last session, rats were injected with pentobarbital (100 mg kg−1i.p.), and microinjection sites were marked with 100 nl of 2.5 % Alcian Blue dye. Brains were immediately perfused in situ with 60 ml of cold saline (4 °C) followed by 60 ml of 4 % paraformaldehyde. Brains were then removed and postfixed in 4 % paraformaldehyde at least overnight, and then saturated with 30 % sucrose and cut in 30 μm coronal sections on a freezing microtome. Mounted sections were stained with 1 % Neutral Red. Injection sites were determined according to the atlas of Paxinos & Watson (1998) by a ‘blind’ observer.
Statistics
Results are expressed as means ± s.e.m. Comparisons were made between groups with repeated measures ANOVA where appropriate. Fisher's LSD was used for post hoc analysis. Limits of probability considered significant were 5 % or less.
Results
Effect of microinjection of muscimol into the RP on cardiovascular responses to stress
Of the rats tested in stress protocols, injection sites were highly restricted to the RP in six rats in which this region was specifically targeted (Fig. 1). In another six rats, injection sites were judged to be at other sites. In three of these rats, injection sites were located dorsally or dorsolaterally within approximately 500 μm of the targeted zone of the RP (one filled and two half-filled circles in Fig. 1A). Injection sites in the remaining three rats in the latter group were located more than 500 μm lateral and/or posterior to the RP (filled circles in Fig. 1A and B). For convenience, these sites are referred to below as adjacent to the RP (n = 3) and outside the RP (n = 3), respectively, and collectively as other injection sites (n = 6).
Figure 1.
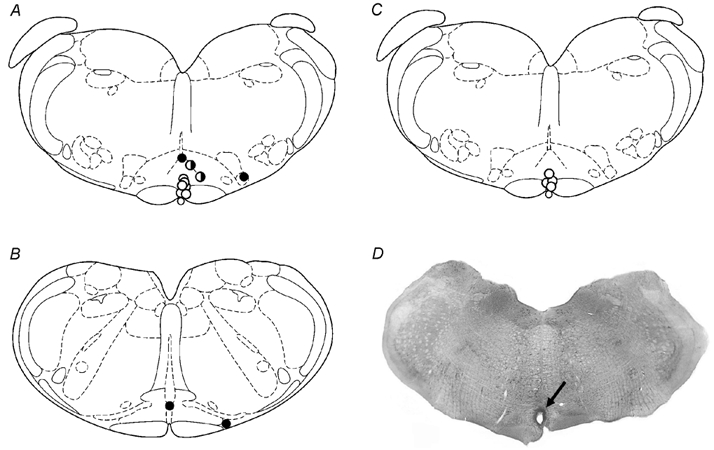
A-C, approximate locations of all injection sites depicted on schematic coronal sections through the brainstem of the rat, representing, relative to bregma, −11.60 (A and C) and −12.30 (B; adapted from the atlas of Paxinos & Watson, 1998). All microinjections involved volumes of 100 nl as described in the text. A and B, injection sites in all rats included in stress studies (n = 12); open circles, sites in active region of the RP; filled or half-filled circles, other sites (see text for details). C, injection sites in rats examined in baroreflex experiments (n = 4). D, fixed and stained coronal section through brainstem of a rat depicting injection site in raphe pallidus (arrow).
Injection of 40 pmol BMI into the RP caused marked increases in heart rate in all six rats (mean maximal change = +75 ± 12 beats min−1) accompanied by slight increases in arterial pressure while locomotor activity was unaffected (not shown). Heart rate returned to baseline 15-20 min after injection of BMI. In contrast to the consistent increases in heart rate seen after injection into the RP, heart rate responses were highly variable after microinjection of BMI at six other sites (mean maximal change = +39 ± 27 beats min−1). However, closer examination revealed that injection of BMI at sites adjacent to the RP elicited clear increases in heart rate (mean maximal change = +89 ± 19 beats min−1; n = 3), while similar injections outside the region did not (mean maximal change = −12 ± 27 beats min−1; n = 3).
Baseline values for heart rate and arterial pressure prior to any of the treatments in rats subjected to stress protocols were not different from one another. After injection of saline into the RP, air-jet stress evoked marked tachycardia and variable pressor responses (Fig. 2), as reported previously (Stotz-Potter et al. 1996a,b). Heart rate rose sharply at the onset of air stress to a plateau that was maintained between 470 and 490 beats min−1 for the duration of the stress period and returned gradually to baseline over 15-25 min after its termination. After microinjection of 80 pmol muscimol into the RP, tachycardia was greatly reduced throughout the 10 min stress trial compared to that seen under similar conditions after injection of saline (Fig. 2). Thus, stress-associated increases from baseline heart rate observed after injection of saline were suppressed after microinjection of 80 pmol muscimol into the RP in all six rats (mean suppression for last 5 min of air-stress period = 76 ± 8 %; range, 100-60 %; n = 6). After treatment with this dose of muscimol, the heart rate during much of the post-stress observation period was also lower than that seen after treatment with saline (Fig. 2). Similar injection of 80 pmol muscimol at all other sites produced no significant effect when considered as a group (mean suppression = 13 ± 8 %; n = 6; Fig. 3). However, examination of data from individual experiments revealed that injection of 80 pmol muscimol at two of the three injection sites adjacent to the RP appeared to produce moderate suppression of the increases (i.e. by 34 and 42 %; half-filled circles in Fig. 1A). No suppression was evident after injection of muscimol at any of the four other sites tested (filled circles, Fig. 1A and B).
Figure 2.
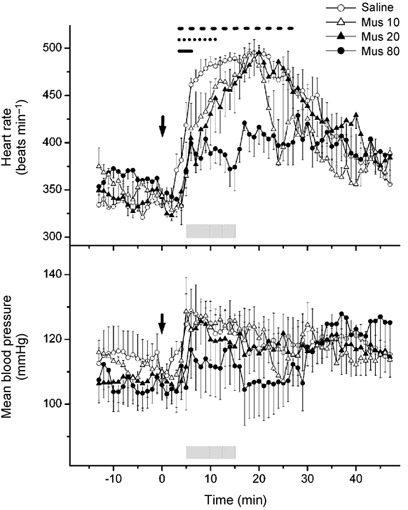
Mean heart rate (top) and blood pressure (bottom) over time before, during and after air-jet stress following microinjection of each of three doses of muscimol into RP. Mus 80, 80 pmol (100 nl)−1 (n = 6); Mus 20, 20 pmol (100 nl)−1 (n = 3); Mus 10, 10 pmol (100 nl)−1 (n = 3); Saline, saline vehicle (100 nl; n = 6). Microinjections were performed at t = 0 min (arrows), and rats were subjected to air-jet stress during stress trial between t = +5 min and t = +15 min (shaded bars). Horizontal lines (top) indicate significant differences from corresponding values after treatment with saline: dashes, 80 pmol; dots, 20 pmol; continuous, 10 pmol.
Figure 3.
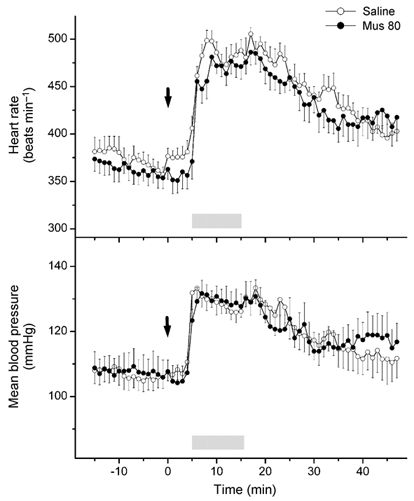
Mean heart rate (top) and blood pressure (bottom) over time before, during and after air-jet stress following microinjection of muscimol (Mus 80, 80 pmol (100 nl)−1; n = 6) or saline vehicle (100 nl; n = 6) into other sites in the brainstem, i.e. outside or adjacent to the RP (see filled and half-filled circles in Fig. 1A and B). Microinjections were performed at t = 0 min (arrows), and rats were subjected to air-jet stress during stress trial between t = +5 min and t = +15 min (shaded bars). No significant differences were found at any time point for corresponding values after injection of saline and of muscimol.
In three animals, microinjection of 10 and 20 pmol muscimol produced suppression of stress-induced tachycardia that appeared to be most marked early in the trial and to wane progressively over time (Fig. 2). Thus, 1 min after the beginning of air stress, heart rate was elevated above baseline by 112 ± 24 beats min−1 after injection of saline (n = 6), but only by 42 ± 6 beats min−1 after microinjection of 20 pmol muscimol (n = 3) and 70 ± 31 beats min−1 after microinjection of 10 pmol muscimol (n = 3). However, by the end of the period of air stress, the heart rate in animals treated with each of the two lower doses of muscimol had attained levels that were no different from those seen after injection of saline vehicle (Fig. 2). Increases in heart rate averaged over either the first or second 5 min period of air stress were highly inversely correlated with the dose of muscimol injected (linear regression analysis; first 5 min, r = −0.66, P = 0.02; second 5 min, r = −0.83, P = 0.001).
Under baseline (unstressed) conditions, microinjection of vehicle (100 nl saline) into the RP in three rats was followed by moderate and transient increases in heart rate that returned to baseline within 5-6 min and were unaccompanied by significant changes in blood pressure (Fig. 4). Thus, at 10-15 min after injection, corresponding to the last 5 min of air stress in stress trials during which the analysis above was performed, heart rate and blood pressure were not different from baseline. The initial tachycardia seen after microinjection of saline occurred at the approximate time at which the injector was removed from the guide, but appeared to be absent after microinjection of 80 pmol muscimol into the RP in the same three animals (Fig. 4). In fact, injection of muscimol had no significant effect on baseline heart rate and blood pressure up to t = +15 min, the time corresponding to the end of the stress period in air-stress trials. Thereafter, modest but significant decreases in both heart rate and blood pressure were apparent at several time points between +15 min and +30 min. Microinjection of 80 pmol muscimol into other sites under baseline conditions likewise failed to alter heart rate and blood pressure (data not shown).
Figure 4.
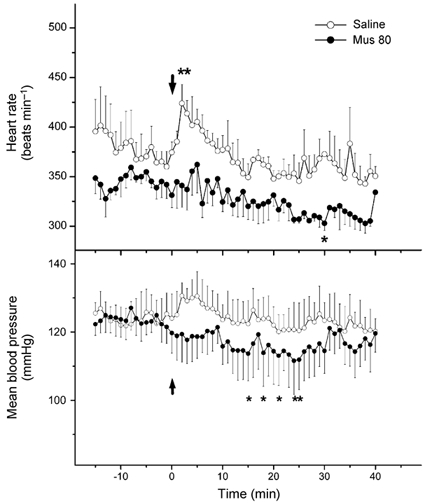
Mean heart rate (top) and blood pressure (bottom) over time before, during and after microinjection of muscimol (80 pmol (100 nl)−1) or saline vehicle (100 nl) into the RP under control (unstressed) conditions. Microinjections were performed at t = 0 min and rats were left undisturbed in their home cages except for removal of injector from guide cannula at approximately +2-4 min. Asterisks indicate time points at which the parameter was significantly different from original baseline (i.e. period 5 min prior to injection) by repeated measures ANOVA.
Effect of microinjection of muscimol into the RP on baroreflex responses
To assess the selectivity of the effect of muscimol on changes in heart rate seen in experimental stress, the ability of similar treatment to influence changes in heart rate evoked by baroreceptor loading and unloading was tested in four additional rats. Intravenous infusion of 4 μg kg−1 nitroprusside before and after microinjection of 80 pmol muscimol into the RP produced identical decreases in blood pressure (-18 ± 2 mmHg before injection of muscimol vs. −17 ± 1 mmHg after injection of muscimol) and increases in heart rate (+61 ± 9 beats min−1 before muscimol vs. +60 ± 9 beats min−1 after microinjection of muscimol). Similarly, injection of 4 μg kg−1 phenylephrine before and after injection of muscimol caused equivalent increases in blood pressure (+42 ± 2 mmHg before injection of muscimol vs. +41 ± 2 mmHg after injection of muscimol) and decreases in heart rate (-81 ± 12 beats min−1 before injection of muscimol vs. −79 ± 15 beats min−1). Thus, all calculated indices of cardiac baroreflex sensitivity remained unchanged after microinjection of 80 pmol muscimol into the RP.
Discussion
The results of this study indicate that activation of neurons in the RP is responsible for generating tachycardia seen in air stress in rats. Prior injection of muscimol into the RP produced dose-related suppression of the cardioacceleration associated with air stress and nearly abolished it at the highest dose tested, but had no discernible effect on heart rate under ‘unstressed’ conditions. Interestingly, even the small and transient increase in heart rate associated with removal of the injector from the guide cannula after saline injection was absent after injection of 80 pmol muscimol. Thus, the effect of injection of the highest dose of muscimol that was so striking with respect to air stress was even apparent with respect to a relatively modest stressor imposed as an artifact of our experimental design. Injection of lower doses of muscimol appeared to produce a trend toward suppression that was most marked early in the air-stress trial but was lost over time. Initial microinjection of the GABAA receptor antagonist BMI into the RP in this study increased heart rate under baseline conditions in conscious rats, a finding reported previously in anaesthetized preparations (Morrison et al. 1999; Samuels et al. 2002). Taken together, these data suggest that neurons in the RP with the potential to activate sympathetic pathways to the heart are subject to tonic GABAergic inhibition. These putative cardioacceleratory neurons appear to contribute negligibly to cardiac sympathetic nerve activity under baseline conditions because of this inhibitory mechanism. On the other hand, the tachycardia seen in experimental air stress is likely to rely on their recruitment, perhaps by means of withdrawal of GABA-mediated inhibition.
Owing to the ubiquity of GABAA receptors on central mammalian neurons (Johnston, 1996), local microinjection of muscimol has proven to be an effective way to inhibit neuronal activity acutely and reversibly in a discrete region of the brain. In this study, microinjection of the highest dose of muscimol tested at sites outside the RP, sites at which injection of BMI had no effect on baseline heart rate, failed to suppress air stress-induced tachycardia. In three experiments, injection sites were misplaced by 500 μm or less dorsal and/or lateral to the targeted area of the RP. At these sites, microinjection of BMI elicited variable increases in heart rate and injection of muscimol appeared to provoke modest suppression of air stress-induced tachycardia. These findings are consistent with spread or diffusion of the injected agents to sites of action in the RP for both effects. As shown in other studies, microinjections of this same dose and volume of muscimol into different hypothalamic regions also produced effects that appeared to be highly localized (Stotz-Potter et al. 1996a,b; Morin et al. 2001). Previously, identical treatment of the RP with 80 pmol muscimol reduced the tachycardia evoked by disinhibition of neurons in the DMH (Samuels et al. 2002) while injection of a much larger dose (1 nmol) into the rostral ventrolateral medulla (RVLM) did not (Fontes et al. 2001). Taken together, these results suggest that the observed effect of muscimol on air stress-induced tachycardia is likely to be mediated through inhibition of neurons in RP or its immediate vicinity.
The findings here are a logical extension of previous work indicating that (1) activation of neurons somewhere in the region of the DMH is responsible for air stress-induced tachycardia (Stotz-Potter et al. 1996a,b), and (2) the tachycardia evoked by chemical stimulation of the DMH in anaesthetized rats can be suppressed by microinjection of muscimol into the RP (Samuels et al. 2002). The simplest explanation for these findings is that the neural pathway responsible for the generation of stress-induced tachycardia involves excitation of neurons in the DMH acting through recruitment of latent cardioacceleratory neurons in the RP. Ample evidence exists for neural connections that are likely to comprise such a pathway. In a retrograde tracing study in cats, the RP contained the most labelled cells of any brain region after tracer was injected into the spinal cardioacceleratory sites at T3-T4 (Miura et al. 1983). Neurons in the RP are trans-synaptically labelled from the stellate ganglion, the origin of cardiac sympathetic innervation (Jansen et al. 1995). In the same study, labelled neurons were also noted in a specific subregion of the DMH, the dorsal hypothalamic area. Neurons in the dorsal hypothalamic area are known to project directly to the RP (Hosoya et al. 1987; Hermann et al. 1997; Nogueira et al. 2000). Thus, the tachycardia evoked by air stress and by chemical stimulation of the DMH is probably mediated, at least in part, through a direct projection from neurons in the dorsal hypothalamic area to the RP.
Although a role for neurons in the RP in stress-induced tachycardia has not been previously proposed, some evidence supports this notion. Neurons in the RP appear to be activated in diverse paradigms for emotional and physical stress in which heart rate is also elevated (Bonaz & Tache, 1994; Cullinan et al. 1995; Campeau & Watson, 1997), and at least some of these neurons send descending projections to the spinal cord (Senba et al. 1993). Activation of neurons in the RP elicits cutaneous vasoconstriction in the rabbit pinna (Blessing & Nalivaiko, 2000), a characteristic feature of the alerting response in this species (Yu & Blessing, 2001; Blessing & Nalivaiko, 2001). In the rat, activation of the RP provokes vasoconstriction in cutaneous vessels in the tail (Blessing & Nalivaiko, 2001) and excitation of sympathetic nerves innervating brown fat (Morrison et al. 1999), effects that, like cutaneous vasoconstriction in the rabbit ear (Pedersen & Blessing, 2001), serve to elevate core body temperature. Accordingly, the RP has been proposed as a key brainstem thermoregulatory centre (Tanaka et al. 2002). However, hyperthermia is also recognized as a facet of the physiological response to stress in rats and many other mammals (for review, see Oka et al. 2001). Interestingly, activation of the neurons in the DMH in rats also elicits sympathetically mediated thermogenesis in brown fat and increases in core body temperature that correlate with the accompanying increases in heart rate (Zaretskaia et al. 2002). Thus, thermogenesis evoked from the DMH, like the tachycardia that can be generated from the same region, may be mediated through neural projections from the dorsal hypothalamic area to the RP.
In contrast to the virtual abolition of stress-induced tachycardia resulting from microinjection of muscimol into the RP, changes in heart rate evoked by nitroprusside or phenylephrine were unchanged by the same treatment. Thus, neurons in the RP play a role in the tachycardia signalled from the hypothalamus in stress but not in that evoked through the baroreceptors. This conclusion is consistent with the exclusion of the RP from brainstem pathways involved in sympathetic nervous regulation of arterial pressure (Reis et al. 1994; Morrison, 2001). Instead, the RP appears to constitute a key brainstem centre responsible for integrating sympathetic adjustments signalled from the hypothalamus, including thermoregulatory responses (Nalivaiko & Blessing, 2001; Tanaka et al. 2002) and tachycardia (Samuels et al. 2002).
In summary, activation of neurons in the RP appears to play a previously unrecognized role in the generation of stress-induced tachycardia in rats. This response is likely to be generated through a direct projection from neurons in the dorsal hypothalamic area, a distinct subregion of the DMH.
Acknowledgments
This work was supported by NIH grant NS 19883.
References
- Blessing WW, Nalivaiko E. Regional blood flow and nociceptive stimuli in rabbits: patterning by medullary raphe, not ventrolateral medulla. J Physiol. 2000;524:279–292. doi: 10.1111/j.1469-7793.2000.t01-2-00279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blessing WW, Nalivaiko E. Raphe magnus/pallidus neurons regulate tail but not mesenteric arterial blood flow in rats. Neurosci. 2001;105:923–929. doi: 10.1016/s0306-4522(01)00251-2. [DOI] [PubMed] [Google Scholar]
- Bonaz B, Tache Y. Induction of Fos immunoreactivity in the rat brain after cold-restraint induced gastric lesions and fecal excretion. Brain Res. 1994;652:56–64. doi: 10.1016/0006-8993(94)90316-6. [DOI] [PubMed] [Google Scholar]
- Campeau S, Watson SJ. Neuroendocrine and behavioral responses and brain pattern of c-fos induction associated with audiogenic stress. J Neuroendocrinol. 1997;9:577–588. doi: 10.1046/j.1365-2826.1997.00593.x. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neurosci. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- DiMicco JA, Samuels BC, Zaretskaia MV, Zaretsky DV. The dorsomedial hypothalamus and the response to stress: Part renaissance, part revolution. Pharmacol Biochem Behav. 2002;71:469–480. doi: 10.1016/s0091-3057(01)00689-x. [DOI] [PubMed] [Google Scholar]
- Fontes MAP, Tagawa T, Polson JW, Cavanagh S-J, Dampney RAL. Descending pathways mediating cardiovascular response from dorsomedial hypothalamic nucleus. Am J Physiol. 2001;280:H2891–2901. doi: 10.1152/ajpheart.2001.280.6.H2891. [DOI] [PubMed] [Google Scholar]
- Hermann DM, Luppi PH, Peyron C, Hinckel P, Jouvet M. Afferent projections to the rat nuclei raphe magnus, raphe pallidus and reticularis gigantocellularis pars alpha demonstrated by iontophoretic application of choleratoxin (subunit b) J Chem Neuroanat. 1997;13:1–21. doi: 10.1016/s0891-0618(97)00019-7. [DOI] [PubMed] [Google Scholar]
- Hosoya Y, Ito R, Kohno K. The topographical organization of neurons in the dorsal hypothalamic area that project to the spinal cord or to the nucleus raphe pallidus in the rat. Exp Brain Res. 1987;66:500–506. doi: 10.1007/BF00270682. [DOI] [PubMed] [Google Scholar]
- Jansen AS, Nguyen XV, Karpitskiy V, Mettenleiter TC, Loewy AD. Central command neurons of the sympathetic nervous system: basis of the fight-or-flight response. Science. 1995;270:644–646. doi: 10.1126/science.270.5236.644. [DOI] [PubMed] [Google Scholar]
- Johnston GAR. GABAA receptor pharmacology. Pharmacol Therap. 1996;69:173–198. doi: 10.1016/0163-7258(95)02043-8. [DOI] [PubMed] [Google Scholar]
- Miura M, Onai T, Takayama K. Projections of upper structure to the spinal cardioacceleratory center in cats: an HRP study using a new microinjection method. J Auton Nerv Syst. 1983;7:119–139. doi: 10.1016/0165-1838(83)90041-3. [DOI] [PubMed] [Google Scholar]
- Morin SM, Stotz-Potter EH, DiMicco JA. Injection of muscimol into dorsomedial hypothalamus and stress-induced Fos expression in paraventricular nucleus. Am J Physiol. 2001;280:R1276–1284. doi: 10.1152/ajpregu.2001.280.5.R1276. [DOI] [PubMed] [Google Scholar]
- Morrison SF. Differential control of sympathetic outflow. Am J Physiol. 2001;281:R683–698. doi: 10.1152/ajpregu.2001.281.3.R683. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Sved AF, Passerin AM. GABA-mediated inhibition of raphe pallidus neurons regulates sympathetic outflow to brown adipose tissue. Am J Physiol. 1999;276:R290–297. doi: 10.1152/ajpregu.1999.276.2.R290. [DOI] [PubMed] [Google Scholar]
- Nalivaiko E, Blessing WW. Raphe region mediates changes in cutaneous vascular tone elicited by stimulation of amygdala and hypothalamus in rabbits. Brain Res. 2001;891:130–137. doi: 10.1016/s0006-8993(00)03210-8. [DOI] [PubMed] [Google Scholar]
- Nogueira MI, de Rezende BD, do Vale LE, Bittencourt JC. Afferent connections of the caudal raphe pallidus nucleus in rats: a study using the fluorescent retrograde tracers fluorogold and true-blue. Anat Anz. 2000;182:35–45. doi: 10.1016/s0940-9602(00)80118-1. [DOI] [PubMed] [Google Scholar]
- Oka T, Oka K, Hori T. Mechanisms and mediators of psychological stress-induced rise in core temperature. Psychosomat Med. 2001;63:476–486. doi: 10.1097/00006842-200105000-00018. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1998. [Google Scholar]
- Pedersen NP, Blessing WW. Cutaneous vasoconstriction contributes to hyperthermia induced by 3,4-methylenedioxymethamphetamine (ecstasy) in conscious rabbits. J Neurosci. 2001;21:8648–8654. doi: 10.1523/JNEUROSCI.21-21-08648.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis DJ, Golanov EV, Ruggiero DA, Sun MK. Sympatho-excitatory neurons of the rostral ventrolateral medulla are oxygen sensors and essential elements in the tonic and reflex control of the systemic and cerebral circulations. J Hypertens. 1994;12(suppl.):S159–S180. [PubMed] [Google Scholar]
- Samuels BC, Zaretsky DV, DiMicco JA. Tachycardia evoked by disinhibition of the dorsomedial hypothalamus is mediated through medullary raphe. J Physiol. 2002;538:941–946. doi: 10.1113/jphysiol.2001.013302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senba E, Matsunaga K, Tohyama M, Noguchi K. Stress-induced c-fos expression in the rat brain: activation mechanism of sympathetic pathway. Brain Res Bull. 1993;31:329–344. doi: 10.1016/0361-9230(93)90225-z. [DOI] [PubMed] [Google Scholar]
- Stotz-Potter EH, Morin SM, DiMicco JA. Effect of microinjection of muscimol into the dorsomedial or paraventricular hypothalamic nucleus on air stress-induced neuroendocrine and cardiovascular changes in rats. Brain Res. 1996a;742:219–224. doi: 10.1016/s0006-8993(96)01011-6. [DOI] [PubMed] [Google Scholar]
- Stotz-Potter EH, Willis LR, DiMicco JA. Muscimol acts in the dorsomedial hypothalamus but not paraventricular hypothalamic nucleus to suppress cardiovascular effects of stress. J Neurosci. 1996b;16:1173–1179. doi: 10.1523/JNEUROSCI.16-03-01173.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Nagashima K, McAllen RM, Kanosue K. Role of the medullary raphe in thermoregulatory vasomotor control in rats. J Physiol. 2002;540:657–664. doi: 10.1113/jphysiol.2001.012989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YH, Blessing WW. Neurons in amygdala mediate ear pinna vasoconstriction elicited by unconditioned salient stimuli in conscious rabbits. Auton Neurosci. 2001;87:236–242. doi: 10.1016/S1566-0702(00)00278-2. [DOI] [PubMed] [Google Scholar]
- Zaretskaia MV, Zaretsky DV, Shekhar A, DiMicco JA. Chemical stimulation of the dorsomedial hypothalamus evokes non-shivering thermogenesis in anesthetized rats. Brain Res. 2002;928:113–125. doi: 10.1016/s0006-8993(01)03369-8. [DOI] [PubMed] [Google Scholar]


