Abstract
The sperm-activating peptide speract and fucose-sulphate glycoconjugate (FSG) are sea urchin egg-envelope components that modulate sperm ion permeability. They influence motility and induce acrosomal reaction (AR), respectively. A fluorescent Na+-sensitive dye (Na+-binding benzofuran isophthalate, SBFI) was used to determine how these egg envelope components influence sperm Na+ permeability. [Ca2+]i and pHi were also measured to correlate their changes in response to speract and FSG with those observed in [Na+]i. SBFI determinations indicate that the resting [Na+]i is 20 ± 8 mm in sea urchin sperm. Saturating levels of speract increased [Na+]i by ≈15 mm, while similar levels of FSG caused a further elevation of ≈30 mm. The kinetics of the [Na+]i, [Ca2+]i and pHi changes induced by saturating levels of speract were faster than those induced by FSG. Both egg ligands appeared to activate more than one Na+ transport system. Nifedipine, Ni2+ and TEA+ inhibited the ionic changes and the AR induced by FSG but, importantly, did not alter those caused by speract. Thus, there are differences in some of the ionic transport mechanisms that operate in the speract and FSG responses. ZD2788, a blocker of hyperpolarization and cyclic-nucleotide-gated (HCN) channels such as SpHCN present in sea urchin sperm, did not decrease the speract-induced [Na+]i increase, but slowed its kinetics. Therefore, SpHCN does not play a major role in the uptake of Na+ triggered by this decapeptide. KB-R7943, an inhibitor of Na+/Ca2+ exchangers, decreased the resting [Na+]i and did not change significantly the speract-induced [Ca2+]i increase, but slowed its recovery.
Ion fluxes play an essential role in the dialogue between gametes (Wassarman et al. 2001). Diffusible egg components prepare and guide sperm towards the egg (Morisawa, 1994; Miller & Vogt, 1996). The sperm-activating peptide speract, a decapeptide that is present in the egg jelly coat of Hemicentrotus pulcherrimus (Suzuki et al. 1981) and Strongylocentrotus purpuratus (Hansbrough & Garbers, 1981a), elicits Na+ and Ca2+ influx, K+ and H+ efflux and augments the levels of cGMP and cAMP (Garbers, 1989; Ward & Kopf, 1993). These ionic changes lead to increases in [Na+]i, [Ca2+]i and pHi (Darszon et al. 2001).
The egg-jelly component that induces the acrosome reaction (AR), a crucial event for fertilization, is a fucose sulphated glycoconjugate (FSG; Vacquier, 1998). Notably, this egg-jelly component also induces Ca2+ and Na+ uptake and release of K+ and H+, which results in an elevation of [Ca2+]i and pHi, and a change in the membrane potential (Shackmann, 1989; Darszon et al. 2001). FSG also activates a kinase and a phosphatase and elevates the cAMP and InsP3 levels (Garbers, 1989).
Regulation of [Na+]i is of vital importance; changes in [Na+]i modulate cellular processes such as H+ and Ca2+ homeostasis and nutrient and neurotransmitter uptake (Limbird, 1984; Watson et al. 1989). As mentioned earlier, speract elicits Na+ influx, a finding that was initially demonstrated isotopically (Hansbrough & Garbers, 1981b; Repaske & Garbers, 1983). The increase in [Na+]i was postulated to occur through a peculiar voltage-dependent, amiloride-insensitive Na+/H+ 1:1 exchanger that regulates pHi (Lee, 1984a,b; Schackmann & Chock, 1986). This stoichiometry was estimated from 22Na+ uptake measurements and pH determinations with a pH electrode. Since [Ca2+]i and pHi increase over a period of milliseconds during the speract response (Nishigaki et al. 2001), it seemed important to perform fast (less than 1 s) [Na+]i determinations, a difficult quest when measuring 22Na+ influx. Assaying [Na+]i changes with a better time resolution would yield valuable information about the mechanisms involved in Na+ transport in sperm. To date, the molecular identity of the transporters responsible for Na+/H+ exchange and the Na+ uptake activated by speract is unknown.
The introduction of the fluorescent indicator Na+-binding benzofuran isophthalate (SBFI; Minta & Tsien, 1989) has helped us to understand Na+ homeostasis in many cell types (Harootunian et al. 1989; Minta & Tsien, 1989; Negulescu et al. 1990; Sato et al. 1991; Levi et al. 1994; Rose & Ransam, 1997). SBFI was used previously to qualitatively assay Na+ uptake during the speract response in swollen sea urchin sperm (Cook & Babcock, 1993). We have found appropriate loading conditions for SBFI in sea urchin sperm to measure the changes in [Na+]i induced by speract and those that occur during the AR. Our results show that the resting [Na+]i is 20 ± 8 mm and that the changes in [Na+]i, pHi and [Ca2+]i induced by the speract and FSG are different. AR blockers inhibit the FSG-induced ionic permeability changes but, importantly, do not influence those triggered by speract. These results indicate that there are differences in at least some of the ion transport mechanisms that operate in the speract and FSG responses. Other inhibitors were used to explore the contribution of a Na+-permeable channel belonging to the HCN family present in sea urchin sperm and the contribution of the Na+/Ca2+ exchanger to the influx of Na+ triggered by speract.
Methods
Gametes and reagents
S. purpuratus sea urchins were obtained from Marinus (Long Beach, CA, USA) and from Pamanes (Ensenada, Baja, CA, USA). Spawning was induced by an intracelomic injection of 0.5 m KCl. Dry sperm were collected and kept on ice until used. SBFI AM, fura-2 AM, fluo-3 AM, 2′7’-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein, acetoxymethylester (BCECF AM), the fluorescent dyes used to monitor [Na+]i, [Ca2+]i and pHi, respectively, were obtained from Molecular Probes (Eugene, OR, USA). 4-Ethylphenylamino-1,2-dimethyl-6-methylaminopyrimidinium chloride (ZD7288) was purchased from Tocris (Bristol, UK) and dissolved in Hepes (10 mm) pH 7.0 to obtain a 100 mm stock solution. 2-(2-(4-nitrobenzyloxy) phenyl) isothiourea methanosulphonate (KB-R7943) was a gift from Dr Vacquier (University of California, San Diego, CA, USA); it was dissolved in DMSO. The rest of the reagents used were of the highest quality available.
FSG was purified from egg jelly according to Garbers et al. (1983). FSG was quantified according to its fucose concentration (μg ml−1), which was determined by the cysteine-sulphuric method (Guerrero & Darszon, 1989b). FSG concentrations that induce a maximum response were used. The added volume never exceeded 1 % of the recording volume.
Artificial sea water (ASW) was made with (mm): 486 NaCl, 26 MgCl2, 10 KCl, 30 MgSO4, 10 CaCl2, 2.5 NaHCO3 and 0.1 EDTA; the pH was adjusted to 8.0 with NaOH. Ca2+-free or 1 mm Ca2+ ASWs were the same with the exception of the ion indicated. The two solutions used to obtain various external Na+ concentrations to calibrate the SBFI [Na+]i signal were (mm): (1) 135 NaCl, 364 Na+ methanesulphonate, 10 CaCl2, 26 MgCl2, 30 MgSO4, 10 Hepes and 0.1 EDTA at pH 7.2; (2) 135 KCl, 364 K+ methanesulphonate, 10 CaCl2, 26 MgCl2, 30 MgSO4, 10 Hepes and 0.1 EDTA at pH 7.2. Intracellular medium (IM) was composed of (mm): 750 mannitol, 175 KCl, 30 NaCl, 5 MgCl2 and 50 Hepes, pH 7.2 (Castellano et al. 1995).
Fluorescence measurements
The experiments were performed in a SLM 8000 Aminco spectrofluorimeter with a temperature-controlled cell holder equipped with a magnetic stirrer, at 16 °C. Stock solutions (1 mm) of SBFI AM, fura-2 AM, fluo-3 AM and BCECF AM were made in DMSO. Sperm were loaded by diluting dry sperm (≈10-20 μl) 1:5 in 1 mm Ca2+ ASW, pH 7.0 containing 25 μm SBFI AM/0.6 % Pluronic F-127, 20 μm fura-2 AM/0.6 % Pluronic F-127, 10 μm fluo-3 AM/0.6 % Pluronic F-127 or 20 μm BCECF AM for 3 h (or more) at 14 °C in the dark (Guerrero & Darszon, 1989a; Harootunian et al. 1989; Sage et al. 1991). After loading, 10 ml of 1 mm Ca2+ ASW pH 7.0 was added and celomocyte cells and spines were removed from the sperm suspension by centrifugation at 121 × g for 7 min at 4 °C. The pellet was discarded and the dye remaining in the media removed by centrifugation (1000 × g for 8 min at 4 °C). The sperm pellet was resuspended in the original volume (50-100 μl) of 1 mm Ca2+ ASW, pH 7.0 and kept on ice until used. Of this suspension, 10 μl was added to a round cuvette containing 1.6 ml ASW, pH 8 at 14 °C. The suspension was stirred constantly and left to equilibrate for 2 min. SBFI fluorescence was monitored at 500 nm using dual excitation at 340 and 380 nm. Fluo-3, BCECF and fura-2 fluorescence was measured at 525, 540 and 500 nm, respectively, following excitation at 505, 500 and 340 nm, respectively. Sperm autofluorescence was less than 10 % of the total fluorescence, therefore it was not taken into account.
The concentration of intracellular SBFI was calculated according to Levi et al. (1994). Briefly, a cell suspension was divided into two identical samples, one of which was loaded with SBFI as described previously, and the other was treated equally but without dye (same amount of Pluronic-F 127 acid and DMSO). After a 3 h incubation, cells were washed and external dye removed as indicated above, and the pellet was resuspended in the original volume. Sperm (10 μl of suspension) were solubilized in the cuvette by adding 5 % Triton X-100 (TX-100). The sample was then centrifuged to eliminate cellular debris, and the emission spectrum of the loaded and not-loaded cell supernatants was recorded following excitation at 340 nm. The difference between the spectra of both samples gave the SBFI-dependent fluorescence. The signal at 500 nm was then compared to the standard curve of the free acid SBFI to estimate its concentration in the supernatant. The intracellular SBFI concentration calculated in our experiments was 80 ± 15 μm (n = 6).
Time course of incorporation of SBFI-AM into sea urchin sperm
To optimize the dye-loading conditions, sperm were incubated with SBFI AM for various times at 14 °C. Speract, which is easier to obtain than FSG and was shown to induce an increase in [Na+]i (Cook & Babcock, 1993), was used as a test response. The basal sperm fluorescence at 380 nm increased for up to 6 h (Fig. 1A); however, the fluorescence changes induced by speract were similar between 3 and 9 h (Fig. 1B). These results show that a loading time of 3 h at 14 °C is enough to record adequate speract responses, thus allowing the use of fresher cells.
Figure 1. Optimization of Na+-binding benzofuran isophthalate (SBFI) loading, cell distribution and pHi independence in sea urchin sperm.
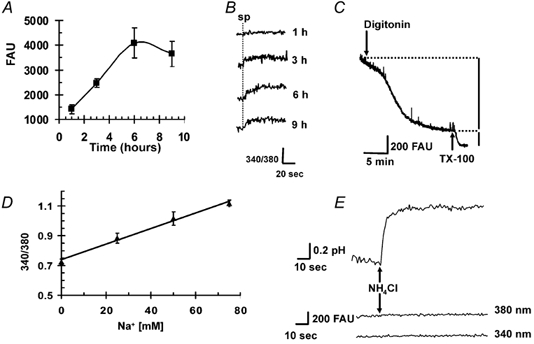
A, sea urchin sperm were exposed to SBFI AM/Pluronic F-127 for various periods of time at 14 °C. After washing away external dye, as described in Methods, basal sperm fluorescence was recorded at 500 nm after excitation at 380 nm for each incubation time. The results are presented as the mean ± s.e.m. of at least three experiments. B, the spermactivating peptide speract (sp, 100 nm) induced increases in [Na+]i for each incubation time. Measurements were made as in A, but also following excitation at 340 nm and plotting the emission ratio 340/380 at 500 nm. The dotted vertical line indicates when speract was applied. Incubation times are shown on the right of the traces. C, sperm suspended in intracellular medium (IM, see Methods) were loaded with SBFI for 3 h, the optimal time, to allow estimation of the percentage of dye in the cytosol. The fluorescence measured at 500 nm following excitation at ≈368 nm, the isosbestic point insensitive to Na+ decreased when digitonin (3 μm) was added, indicating that > 75 % of the dye was present in cytoplasm (see text). The subsequent decrease in fluorescence caused by applying 2 % Triton X-100 (TX-100) reflects the SBFI present in internal organelles. This experiment is representative of three with different sperm batches. D, relationship between Na+ and the SBFI fluorescence ratio (340/380). Sperm loaded for 3 h with SBFI were suspended in buffer containing different Na+ concentrations and then exposed to gramicidin (20 μm). The points represent the mean ± s.e.m. of 15 experiments made with at least six different sperm batches. E, sperm loaded with 2′7’-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein, acetoxymethylester (BCECF AM) and SBFI were exposed to 5 mm NH4Cl, which increased pHi from ≈7.2 to 7.7, but did not change SBFI fluorescence following excitation at either 340 or 380 nm. The traces are representative of three experiments with different batches of sperm. FAU = fluorescence arbitrary units.
Permeabilization
Since SBFI has been observed to accumulate in intracellular organelles (Harootunian et al. 1989), such fluorescence may contribute, in addition to the cytoplasmic dye, to the total signal measured from cells. SBFI distribution was determined using digitonin (3 μm) and TX-100 (2 %) to release dye from the cytoplasm and organelles, respectively (Levi et al. 1994). Digitonin, a non-ionic detergent, has been used to permeabilize the sea urchin sperm plasma membrane (Castellano et al. 1995). To ensure that fluorescence signals were not due to changes in [Na+]i caused by the addition of digitonin, measurements were carried out at the SBFI isosbestic point (368 nm) with sperm suspended in IM at pH 7.2. Figure 1C shows that although sperm permeabilization was made in a medium that mimics the cytoplasm, we observed a decrease in fluorescence when digitonin was added. It is known that SBFI in the cytoplasm of intact cells exhibits altered spectral properties compared with those it displays in vitro. Comparison of the emission spectra of loaded sperm in IM before and after digitonin addition shows a decrease in SBFI fluorescence at 500 nm upon dye release. A similar decrease was reported using digitonin in rat ventricular myocytes and cultured rat hippocampal neurons (Donoso et al. 1992; Rose & Ransam, 1997). The fluorescence decrease provoked by digitonin reflects the percentage of dye present in the cytoplasm. The subsequent fluorescence decrease caused by the addition of TX-100 is due to the release of the dye localized in intracellular organelles. Our results indicate that after 3 h of loading at 14 °C, more than 75 % of the dye is in the cytoplasm. As anticipated, fluorescence microscopy revealed that the SBFI fluorescence was mainly in the cytoplasm, and the remainder mostly in the mitochondria and acrosome (data not shown).
SBFI calibration
The relationship between [Na+]i and SBFI fluorescence was determined adding gramicidin D (20 μm) or palytoxin (20 nm) to equilibrate extracellular Na+ ([Na+]e) and [Na+]i in a medium where [Na+]e was varied from 0 to 75 mm (Negulescu et al. 1990; Donoso et al. 1992; Levi et al. 1994). Gramicidin and palytoxin induced similar changes in sea urchin sperm [Na+]i, so both were used. Two calibration solutions were prepared and mixed to obtain the desired [Na+]e (see gametes and reagents; solutions 1 and 2). The Cl− concentration in the calibration solutions was chosen to be approximately equal to the [Cl−]i in sperm to minimize Cl− fluxes and the consequent changes in cell volume that may occur when the transmembrane monovalent cation gradients are collapsed by gramicidin or palytoxin (Robertson & Foskett, 1995). The relationship between the fluorescence ratio and [Na+]i was then plotted and used as a calibration curve (Fig. 1D). The results are given as [Na+]i obtained by direct comparison with the calibration curve. The fluorescence of unloaded cells was negligible compared to that of loaded cells. The calibration may include a small error (maximum 10 %) due to the dye that is not in the cytoplasm (< 25 %). The magnitude of this error was estimated from the fluorescence change induced by gramicidin in sperm loaded with SBFI and permeabilized with digitonin in cytoplasmic media without Na+ or with 30 mmNaCl.
Measurements of pHi and [Ca2+]i
BCECF calibration was performed after each experiment using 0.01 % TX-100 to permeabilize the cells, then adding Hepes pH 7.0 (1 m) to achieve 20 mm and 1 m HCl twice (5 μl; Guerrero & Darszon, 1989b). Fura-2 fluorescence was calibrated as reported previously (Guerrero et al. 1998). Fluo-3 signals were normalized using the ratio between the fluorescence reached after a speract or inhibitor addition (F) and the basal fluorescence (before addition; F0): F/F0.
The intracellular buffering power (β) was estimated according to Wu et al. (1994) using gradual addition of NH4Cl to increase pHi. As recommended, overestimation of β was avoided by estimating it under conditions that inhibit known membrane pHi regulators. The value of β obtained in sea urchin sperm was 7 ± 1.7 mm pH unit −1(n = 6), which is within the range of those that have been reported previously (Wu et al. 1994).
Intracellular pH independence of SBFI-loaded sperm fluorescence
The reported pKa (-log of the dissociation constant) of SBFI in cytoplasmic-like solutions is 6.1 (Minta & Tsien, 1989), suggesting that this dye is relatively unaffected by pHi changes under physiological conditions. However, it was important to show that the sperm pHi changes occurring in response to speract and FSG do not influence SBFI fluorescence. Exposing sperm to 5 mm NH4Cl increases pHi by ≈0.6 pH units (BCECF measurements, Fig. 1E), as NH3 enters the cell and combines with H+. Although this pHi change is even larger than that observed in response to speract or FSG (Schackmann & Chock, 1986; Guerrero & Darszon, 1989b), it did not alter SBFI fluorescence (Fig. 1E).
Results
It is not known whether different Na+ transport systems operate in sea urchin sperm during the response to speract and FSG, the factor that triggers the AR. On the other hand, the time course of the increase in [Na+]i induced by FSG had not been documented. Figure 2A shows that it is possible to record the fluorescence changes induced by this egg ligand in SBFI-loaded sea urchin sperm. We also measured the [Ca2+]i and pHi changes triggered by FSG (Fig. 2B and C) to correlate them with those determined for [Na+]i.
Figure 2. Fucose-sulphate glycoconjugate (FSG)-induced changes in [Na+]i, [Ca2+]i and pHi in the absence (A, B, C) and presence of saturating concentrations of speract (D, E, F).
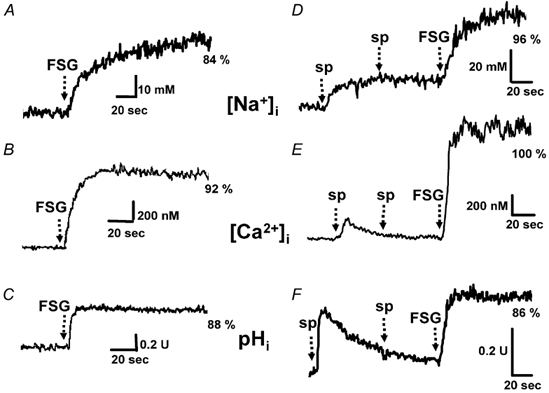
Sperm loaded with SBFI AM, fura-2 AM and BCECF AM, as indicated in Methods, were suspended in artificial sea water (ASW) for 2 min before the recordings were started. At the time indicated by the arrows, saturating levels of FSG (5-20 μl), the acrosome reaction (AR) inducer, were added. In D, E and F, two 100 nm speract (sp) additions were made before exposing the sperm suspension to saturating levels of FSG. The percentage of AR (%) is shown at the end of the traces. The [Na+]i, [Ca2+]i and pHi records shown are representative of at least nine different batches of sea urchin sperm, with the exception of the [Na+]i increase induced by FSG alone, where n = 3.
The [Ca2+]i and pHi changes induced in sperm by speract and FSG have been measured independently using fura-2 and BCECF, respectively (Schackmann & Chock, 1986; Trimmer et al. 1986; Babcock et al. 1992; González-Martínez et al. 2001). Using the sequential addition of a sperm-activating peptide and the AR-inducing factor, Hoshino et al. (1992) suggested that the [Ca2+]i and pHi changes induced by the two egg components involve distinct transport systems. This experimental design mimics sperm physiology at fertilization, but may complicate the interpretation, as speract will have activated mechanisms that may alter the response to FSG alone. Figure 2D illustrates that even if enough speract is added to saturate its response, FSG is still able to increase [Na+]i and trigger the AR. These findings suggest that either different Na+ transport systems are involved in the responses to these two egg-jelly components or that FSG changes the ionic gradients in a way that speract-activated Na+ uptake is further stimulated. Figure 2 and Table 1 show that the magnitude of the changes in [Na+]i, [Ca2+]i and pHi induced by saturating levels of FSG in the absence or presence of saturating speract are similar.
Table 1.
Increase (Δ) in [Na+]i, [Ca2+]i and pHi induced by fucose-sulphate glycoconjugate (FSG) in the presence and absence of the sperm-activating peptide, speract
| Ion | ΔFSG | ΔSperact + FSG |
|---|---|---|
| [Na+]i | 31 ± 9 | 30 ± 6 |
| (mm) | (n = 3) | (n = 9) |
| [Ca2+]i | 1700 ± 157 | 1857 ± 220 |
| (nm) | (n = 10) | (n = 9) |
| pHi | 0.45 ± 0.06 | 0.42 ± 0.10 |
| (pH units) | (n = 10) | (n = 9) |
Sperm were loaded with Na+-binding benzofuran isophthalate (SBFI AM), fura-2 AM and 2′7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein, acetoxymethylester (BCECF AM), as indicated in Methods. the values are the mean ± s.e.m. of the maximum increase (Δ) of at least three experiments.
Under optimized SBFI-loading conditions, basal sperm [Na+]i is 20 ± 8 mm (n = 12). The resting [Ca2+]i and pHi found (63 ± 9 nm; n = 45 and 7.1 ± 0.1; n = 9, respectively) were in the range of those that have been reported previously (Christen et al. 1982; Trimmer et al. 1986; Guerrero & Darszon, 1989b; Guerrero et al. 1998). When sperm are exposed to a saturating concentration of speract (100 nm), [Na+]i is elevated to 35 ± 1 mm (n = 6) with a time constant (τ1/2) of 2.4 ± 0.7 s (n = 6), [Ca2+]i increases to 310 ± 83 nm (n = 9) with a τ1/2 of 0.50 ± 0.01 s (n = 9) and decays with τ1/2 of 12.0 ± 0.5 s (n = 9) and pHi is elevated to 7.4 ± 0.1 (n = 9) with a τ1/2 of 0.35 ± 0.04, decaying with a τ1/2 25.0 ± 4.7 s (n = 6; see Table 2 and Fig. 3A). The τ1/2 values for the pHi and [Ca2+]i changes were statistically different (t test, P < 0.05). The speract concentration required to achieve a half-maximal increase in [Na+]i, [Ca2+]i and pHi, assuming one speract-binding site, was: 0.6 ± 0.1 (n = 9), 5.0 ± 1.6 (n = 9) and 0.30 ± 0.07 nm (n = 9), respectively. It is worth noting that around a 10-fold higher concentration of speract is required to achieve the half-maximal [Ca2+]i elevation than that required for pHi (Schakmann & Chock, 1986) and [Na+]i.
Table 2.
Resting [Na+]i, [Ca2+]i and pHi levels in sea urchin sperm and the time constants (τ1/2) of the changes induced by speract and FSG
| Ion | Resting | Saturated speract | τ1/2 rise (s) | τ1/2 decay (s) | Saturated FSG | τ1/2 rise (s) |
|---|---|---|---|---|---|---|
| [Na+]i | 20 ± 8 | 35 ± 1 | 2.4 ± 0.7 | > 1 min | 65 ± 6 | 11 ± 2 |
| (mm) | (n = 12) | (n = 6) | (n = 6) | (n = 9) | (n = 6) | |
| [Ca2+]i | 63 ± 9 | 310 ± 83 | 0.50 ± 0.01 | 12.0 ± 0.5 | 1920 ± 220 | 1.4 ± 0.1 |
| (nm) | (n = 45) | (n = 9) | (n = 9) | (n = 9) | (n = 9) | (n = 9) |
| pHi | 7.1 ± 0.1 | 7.4 ± 0.1 | 0.35 ± 0.04 | 25.0 ± 4.7 | 7.60 ± 0.09 | 3.8 ± 1.3 |
| (pH units) | (n = 9) | (n = 9) | (n = 6) | (n = 6) | (n = 9) | (n = 6) |
Sperm were loaded with Fura-2, BCECF and SBFI, as indicated in Methods. The values are presented in the form mean ± s.e.m. (n = number of experiments) and the τ1/2 rise and decay of the speract and FSG- induced changes are given.
Figure 3. Kinetic differences in the increases in [Na+]i, [Ca2+]i and pHi induced by speract (A) and FSG (B).
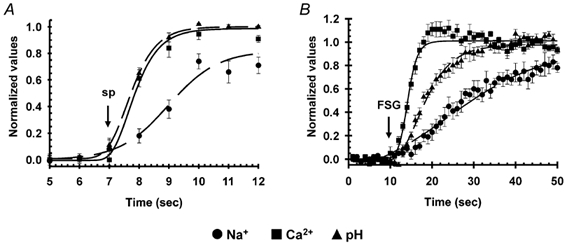
The results shown are averages of at least six experiments (± s.e.m.) performed as indicated in Fig. 2. The difference between the time constant (τ1/2) values of the pHi and [Ca2+]i changes induced by speract are statistically different (*P < 0.05; see Table 2).
Saturating concentrations of FSG increased [Na+]i to 65 ± 6 mm (n = 6), [Ca2+]i to 1920 ± 220 nm (n = 9) and pHi to 7.60 ± 0.09 (n = 9). These values were significantly higher than those achieved by speract, except for the pHi change, whose magnitude was similar in both responses (Table 1). The FSG-induced increases in [Na+]i, [Ca2+]i and pHi occurred with a τ1/2 of 11 ± 2 s (n = 6), 1.4 ± 0.1 s (n = 9) and 3.8 ± 1.3 s (n = 6), respectively. [Na+]i and [Ca2+]i did not decrease for at least 120 s and pHi slowly returned to its resting level. These results are summarized in Table 2 and Fig. 3B. Because FSG has a very high molecular weight (106) and is quite viscous (Vacquier & Moy, 1997), the τ1/2 values for the changes it induces may be largely overestimated. However, our findings suggest that speract first changes pHi and then [Ca2+]i, while FSG first increases [Ca2+]i and then pHi. The [Na+]i elevations appeared to be slower, and more so for FSG, but because of the lower sensitivity of SBFI to detect small rises in this cation, their kinetic analysis is more difficult. The overall findings indicate that the sequence of ionic changes induced by speract and FSG are different, implying the involvement of at least some distinct transport mechanisms in the two responses (see Discussion).
To further substantiate that there are differences in the ionic transport systems that participate in the sperm responses to these two egg-jelly components, the following inhibitory conditions of the AR were tested: nifedipine (20 μm, only in [Ca2+]i) and Ni2+ (300 μm), both of which are Ca2+ channel blockers (Fox et al. 1987; González-Martínez et al. 2001), TEA+ (10 mm), a blocker of many types of K+ channels (Hille, 1992), and Ca2+-free ASW, which inhibits the AR but does not significantly alter the pHi and membrane potential changes induced by speract (Darszon et al. 2001). Under these conditions, the changes in [Na+]i, [Ca2+]i and pHi induced by FSG were inhibited but, importantly, those triggered by speract were not influenced (Fig. 4). A summary of these results is shown in Fig. 5. Taken together, our observations indicate that the [Na+]i, [Ca2+]i and pHi changes induced by speract and by FSG occur in a distinct sequence. The Ca2+ uptake mechanisms activated by the two egg ligands differ in their pharmacological profile and are thus probably different; however, whether these two signalling pathways use the same or distinct Na+ and H+ transporters remains to be seen (see Discussion).
Figure 4. AR inhibitors do not have major effects on the ionic changes induced by speract.
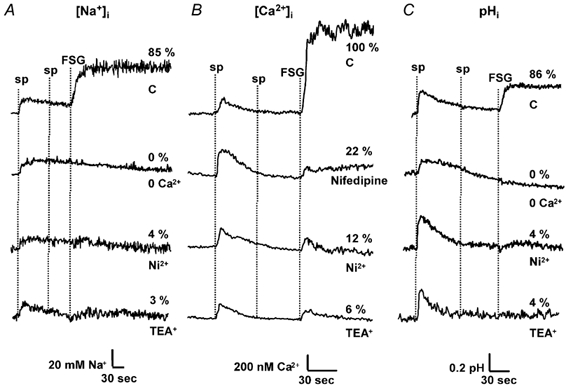
Sperm were loaded with (A) SBFI AM, (B) fura-2 AM and (C) BCECF AM, as described previously. Following 2 min of incubation in ASW containing either nifedipine (20 μm; only for [Ca2+]i), Ni2+ (300 μm), TEA+ (10 mm) or in Ca2+-free ASW (0 Ca2+), 100 nm speract (sp) and saturating levels of FSG were added. The percentage (%) of AR is shown at the end of the traces.
Figure 5. Summary of the influence of AR inhibitors on the speract and FSG responses.
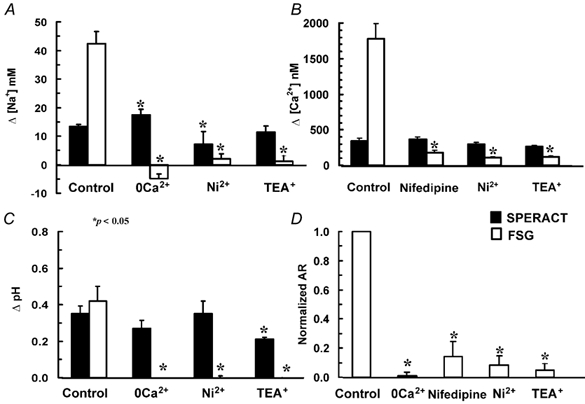
Experiments were performed as in Fig. 4. Bars represent the means ± s.e.m. (n > 3) of the increase in [Na+]i (A), [Ca2+]i (B), pHi (C) and normalized AR (D) induced by speract (sp; black bars) and FSG (white bars). Statistically significant differences (*P < 0.05) between inhibitory conditions and their control in the [Na+]i, [Ca2+]i and pHi changes induced by speract and FSG are indicated by an asterisk (*).
During the speract response, cAMP levels increase (Garbers, 1989) and cAMP and hyperpolarization-gated, nonselective cation channels are present in sea urchin sperm. These channels have a permeability (P) ratio (PK/PNa), of ≈5; their opening in sea water would allow Na+ influx and would depolarize the sperm (Labarca et al. 1995; Gauss et al. 1998). One of these channels, named SpHCN, has been cloned and is a member of the HCN channel family (Gauss et al. 1998; Kaupp & Seifert, 2001). ZD7288, a blocker of this channel (Shin et al. 2001), was used to explore the possible participation of SpHCN in the speract response. Incubation of sperm for 10 min with 300 μm ZD7288 did not reduce the speract-induced increase in [Na+]i and [Ca2+]i, but significantly slowed the rate of Na+ uptake (Fig. 6 and Table 3).
Figure 6. ZD7288 does not inhibit the increases in [Na+]i and [Ca2+]i induced by speract, but significantly slows the rate of [Na+]i uptake.
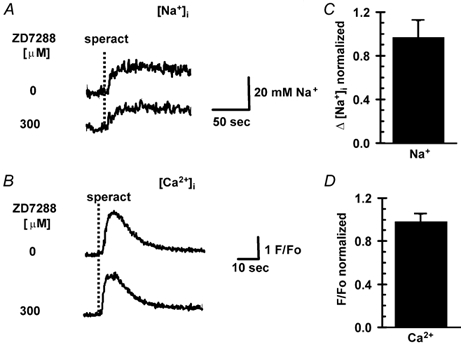
Sperm were loaded with SBFI AM (A) and fluo-3 AM (B), as indicated in Methods. Cells were incubated with ZD7288 (300 μm) for 10 min, after which speract (100 nm) was added (A and B). There was no difference in the normalized [Na+]i (C) and [Ca2+]i (D) changes (Δ) induced by speract between control and ZD7288-treated conditions. Each condition represents the mean ± s.e.m. (n > 6). The kinetic constants are given in Table 3.
Table 3.
Influence of ZD7288 and KB-R7943 on the kinetics of the speract-induced changes in sperm [Na+]i and [Ca2+]i
| Speract | Speract after ZD7288 | Speract after KB-R | |||||||
|---|---|---|---|---|---|---|---|---|---|
| τ1/2 rise (s) | τ1/2 decay (s) | n | τ1/2 rise (s) | τ1/2 decay (s) | n | τ1/2 rise (s) | τ1/2 decay (s) | n | |
| [Na+]i | 2.4 ± 0.4 | nd | 11 | 5.44 ± 0.04* | nd | 5 | 3.05 ± 0.09 | nd | 4 |
| [Ca2+]i | 0.58 ± 0.03 | 5.07 ± 0.45 | 13 | 0.64 ± 0.05 | 5.06 ± 0.14 | 4 | 0.92 ± 0.19 | 22.0 ± 0.7* | 4 |
Cells were loaded with SBFI and Fluo-3, as indicated in Methods. The values of the τ1/2 rise and decay of the speract responses are presented as mean ± s.e.m. (n = number of experiments). Statistically significant differences
(P < 0.05) between the kinetic constants of control and inhibitor-treated cells are indicated by an asterisk (*); nd, not done.
On the other hand, a K+-dependent Na+/Ca2+ exchanger was cloned from a sea urchin testis library and it has been shown to be present in the sperm flagella (Su & Vacquier 2002). A Na+/Ca2+ exchanger has been implicated in the [Na+]i and [Ca2+]i changes induced by speract (Schakmann & Chock, 1986). We used KB-R7943, a blocker of Na+/Ca2+ exchangers (Elias et al. 2001; Takano et al. 2001; Su & Vacquier, 2002). At 20 μm, this compound decreased [Na+]i and increased [Ca2+]i at rest, as expected if there were Na+/Ca2+ exchangers working in the forward-mode (Ca2+ efflux) (Fig. 7A and C). Taking the speract-induced changes (Δ) to be 100 %, the KB-R7943-induced Δ[Na+]i decrease was −65 %, while the Δ[Ca2+]i increase was 100 %. KB-R7943 did not significantly affect the increases in [Na+]i and [Ca2+]i induced by speract, but it slowed the [Ca2+]i recovery (Fig. 7C and D). The kinetics of the [Ca2+]i changes varied with different sperm batches, in particular the recovery to the resting level, which was never complete.
Figure 7. Effect of KB-R7943 on basal [Na+]i, basal [Ca2+]i, speract-induced increases in [Na+]i and [Ca2+]i, and recovery of [Ca2+]i.
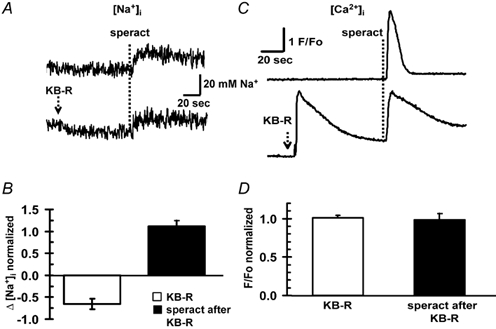
KB-R7943 (KB-R) decreased and increased basal [Na+]i (A) and [Ca2+]i (C), respectively; it did not affect the normalized speract-induced increases (Δ; n = 6) in [Na+]i (B) and [Ca2+]i (D), but it slowed the [Ca2+]i recovery. Changes in [Na+]i, and [Ca2+]i induced by speract (100 nm) were measured with SBFI AM(A) and fluo-3 AM (C), respectively, as indicated in Methods, with or without KB-7943 (20 μm).
Discussion
Diffusible components present in the jelly coat surrounding the egg modulate sperm ion permeability and play an essential role in gamete signalling. The two main egg-jelly components of S. purpuratus sea urchins are speract, a diffusible decapeptide that modulates sperm motility and respiration (Garbers, 1989) and FSG, which induces the AR (Vacquier, 1998).
During sperm activation, a Na+/H+ exchange present in flagella is turned on. In isolated flagella, a stoichiometry of one Na+ taken per H+ released was estimated using 22Na+ fluxes and a pH electrode (Lee, 1984b). Although electroneutral, this amiloride-insensitive exchanger is stimulated by cell hyperpolarization (Lee, 1984a,b). Speract transiently increases cGMP, causing a transitory hyperpolarization that is mediated by cGMP-modulated, K+-selective channels (Lee & Garbers, 1986; Babcock et al. 1992; Galindo et al. 2000) that activate this Na+/H+ exchanger and then stimulate other ion transporters (reviewed in Darszon et al. 2001). The changes in pHi, membrane potential and [Ca2+]i caused by speract occur within a few seconds (Galindo et al. 2000; Nishigaki et al. 2001), thus, the low temporal resolution of 22Na+ uptake measurements is not adequate to study the kinetics of uptake of this cation and its stoichiometric relationship to other ions flowing during the response. Considering this, it seemed appropriate to establish a faster assay for [Na+]i using the Na+-sensitive fluorescent dye SBFI. In addition, this would allow a better understanding of the transport and regulation of this cation during the sea urchin sperm AR. SBFI had been used to qualitatively assay Na+ uptake during the speract response without determining its cell distribution or detailed calibration (Cook & Babcock, 1993). More recently, this dye was utilized to study the Na+ uptake induced by progesterone in human sperm (Patrat et al. 2000) and Sodium Green in herring sperm (Vines et al. 2002).
In the present work we established an optimized loading protocol for SBFI into sea urchin sperm that considers its cellular distribution and responsiveness to egg ligands. This protocol allows the determination of the resting [Na+]i and its changes in response to speract and FSG in sperm suspensions. Intracellular SBFI, loaded as the AM-ester form, has been reported to localize in both the cytoplasm and intracellular organelles. Using digitonin to selectively permeabilize the plasma membrane to release the dye contained in the cytoplasm, we found more than 75 % of the dye had accumulated in the cytoplasm. This observation is in agreement with the distribution of SBFI described for other cells (Harootunian et al. 1989; Sato et al. 1991; Borin et al. 1993; Levi et al. 1994). Furthermore, fluorescence microscopy of loaded sperm after 3 h of incubation with SBFI revealed a homogeneous distribution of the dye in the cytoplasm (not shown). These results, together with those indicating that the speract-induced [Na+]i change measured with SBFI remains basically constant after between 3 and 9 h of incubation with the dye, indicate that the fluorescence signal reflects mainly an increase in cytoplasmic Na+.
It has been shown that SBFI senses [Na+]i independently of changes in pHi and [K+]i in fibroblasts and lymphocytes (Harootunian et al. 1989), smooth muscle (Moore et al. 1998), gastric glands (Negulescu et al. 1990) and other cells (Haigney et al. 1992). However, in isolated rabbit parietal cells, it has been demonstrated that raising pHi from 7.0 to 7.5 shifts the SBFI ratio by ≈4 mm Na+ (Negulescu et al. 1990). Using NH4Cl to alkalinize the sperm cytoplasm to the values reached during speract- and FSG-induced responses (7.4-7.6) did not affect SBFI fluorescence in these cells. Our results show that SBFI can be used to measure the [Na+]i changes triggered by speract or FSG, even though their responses also involve pHi changes.
The resting [Na+]i in sea urchin sperm estimated using SBFI is 20 ± 8 mm (n = 12). This value is higher than that reported in other cells: 4.2 mm in fibroblasts, 9.4 mm in lymphocytes (Harootunian et al. 1989), 8-10 mm in gastric gland cells (Negulescu et al. 1990), 5.5 mm in human platelets (Sage et al. 1991) and 8.9 mm in cultured rat hippocampal neurons (Rose & Ransam, 1997). However, our result is not surprising considering the high concentration of Na+ present in sea water (488.5 mm).
We found that in response to speract (100 nm), pHi reaches its maximum level threefold faster than [Na+]i (Table 2). Considering the involvement of a Na+/H+ exchanger, this result most likely indicates that there is more than one pathway that allows Na+ influx during the speract response. Preliminary rough estimates suggest that during the first 5 s of the speract response, the Na+/H+ stoichiometry is ≈1.8, thereafter it increases to values up to 6. It is worth noting that the Na+/H+ stoichiometry of 1 was estimated in isolated S. purpuratus sperm flagella suspended in Na+ -free sea water after adding 10 mmNaCl (Lee, 1984a). Under these conditions the Na+ gradient and the membrane potential (Beltrán et al. 1996) are quite different than in whole sperm in sea water. The speract signalling system is not turned on, and so it is unlikely that cGMP increases inside the flagella. Thus, even over long periods of time (minutes) basically the Na+/H+ exchange is what is being measured. Therefore, it is not unexpected that the Na+/H+ stoichiometry during the speract response increases with time and is different from that measured in isolated flagella suspended in Na+-free sea water upon Na+ addition.
Stopped-flow experiments have revealed a delay in the pHi response to speract of ≈70 ms (Nishigaki et al. 2001) and the estimated τ1/2 for the change was 0.211 ± 0.029 s (n = 12), which is close to the τ1/2 of the pHi elevation induced by speract measured here (0.3 s). However, to avoid possible mixing-time limitations of the experimental set-up used in the present work, fast mixing techniques will be required to measure adequately the initial rates of the [Na+]i and pHi changes induced by speract and to determine the values and time course of changes in the Na+/H+ stoichiometry. Preliminary results along this line indicate that there is a fast initial change in [Na+]i, which would be consistent with the participation of a Na+/H+ exchanger in the early phase of the speract response. The measurements performed in the work presented here do indicate that saturating speract concentrations first change pHi and then [Ca2+]i, in agreement with previous fast kinetic measurements (Nishigaki et al. 2001). The change in [Na+]i reaches its plateau more slowly, but its initial increase could kinetically and quantitatively match that of pHi.
Besides the putative Na+/H+ exchanger, which sperm transport systems could allow Na+ influx during the speract response? In addition to an initial hyperpolarization and alkalinization, speract increases the cAMP levels. It has been proposed that cAMP elevation activates a Ca2+-permeable channel (Cook & Babcock 1993; Reynaud et al. 1993) and a hyperpolarization- and cAMP-gated, nonselective cation channel, possibly SpHCN (Labarca et al. 1995; Gauss et al. 1998; Darszon et al. 2001). The cAMP-gated cation channels have a PK/PNa of ≈5; their opening in sea water would allow Na+ influx and depolarize the sperm (Labarca et al. 1996; Gauss et al. 1998). Our experiments with ZD7288, a blocker of SpHCN, show that this compound does not inhibit the speract-induced increases in [Na+]i and [Ca2+]i, but it significantly slows the rate of [Na+]i increase. These findings suggest that SpHCN does not play a major role in the magnitude of the speract-induced increases in [Na+]i and [Ca2+]i observed in sperm populations. However, it is worth pointing out that ZD7288 is an open channel blocker (Shin et al. 2001). It is possible that even though we incubated for up to 10 min, as indicated in the literature, SpHCN is not open for enough time at rest to be thoroughly blocked.
A K+-dependent Na+/Ca2+ exchanger called suNCKX was recently cloned from sea urchin testis and found to be present in sperm. It was reported that suNCKX contributes to maintain low [Ca2+]i in a K+-dependent manner and that its function is important for sperm motility (Su & Vacquier, 2002). When membrane depolarization and Na+ uptake occur, this transporter can work in its reverse mode and could contribute to the speract-induced [Ca2+]i increase (Schakmann & Chock, 1986; Blaustein & Lederer, 1999). Here we show that KB-R7943, a blocker of Na+/Ca2+ (Elias et al. 2001) and K+-dependent Na+/Ca2+ exchangers (Takano et al. 2001), increases [Ca2+]i (Su & Vacquier, 2002) and decreases [Na+]i, as anticipated. The speract-induced increases in [Na+]i and [Ca2+]i were not significantly altered by KB-R7943. These results indicate that Na+/Ca2+ exchangers do not contribute greatly to the increases in [Na+]i and [Ca2+]i induced by speract in sperm populations.
The nature of the cAMP-modulated Ca2+ channel proposed to participate in the speract response is not known (Cook & Babcock, 1993). Since neither Ni2+ nor nifedipine greatly inhibit the speract-induced [Ca2+]i increase, it is unlikely that a typical voltage-dependent Ca2+ channel is responsible for this response (Fig. 5). On the other hand, FSG binding to the sperm induces Ca2+ and Na+ influx as well as K+ and H+ efflux, activation of adenylate cyclase and the AR (Darszon et al. 2001). Figure 4 illustrates that FSG induces [Na+]i, [Ca2+]i and pHi changes through mechanisms that, at least in part, differ from those involved in the speract response. This is evidenced by the different sequence of ionic changes triggered by FSG, and by their distinct pharmacology. The Na+/H+ exchanger that has been studied in sea urchin sperm operates during activation and the speract response (Bibring et al. 1984; Lee 1984a,b); however, the mechanisms that regulate pHi and [Na+]i in the AR have not been characterized. The pHi change induced by FSG is Ca2+ dependent (Guerrero et al. 1998; Fig. 5) and that induced by speract is not (Schackmann & Chock, 1986; Fig. 5). Thus, either different Na+/H+ exchange mechanisms are involved or, for instance, two different K+-selective channels: one regulated by cGMP and insensitive to TEA+ involved in the speract response and one regulated by Ca2+ and blocked by TEA+ that participates in the AR, could hyperpolarize sperm and stimulate the same voltage-dependent Na+/H+ exchanger. Both the speract- and the FSG-induced pHi changes are inhibited by high external K+ (Schackmann & Chock, 1986).
At saturating concentrations of FSG, the [Na+]i increase reaches a plateau more slowly (≈threefold) than does pHi. Therefore, although a Na+/H+ exchange system could participate in the early phase of the pHi changes during the AR, at least one other Na+ permeability pathway is activated in this process. Again, fast mixing experiments are required to dissect the kinetics of the different Na+ transport systems that participate in the AR.
The use of fluorescent Ca2+-sensitive dyes has revealed the participation of two different Ca2+ channels in the sea urchin sperm AR. The first one opens almost immediately (< 1 s after FSG binds to the receptor, it is highly Ca2+ selective and it is blocked by verapamil and nifedipine. Five seconds later, a second channel opens that does not inactivate, is pHi sensitive, permeable to Mn2+, poorly Ca2+ selective, blocked by Ni2+ and Co2+ and store operated (González-Martínez et al. 2001). The results presented here suggest that the second channel allows Na+ influx, since Ni2+ blocks almost completely the increase in [Na+]i triggered by FSG (Fig. 4). On the other hand, a comparison of the rise times estimated for the FSG-induced ionic increases indicates that Ca2+ uptake precedes the changes in pHi and [Na+]i.
In conclusion, SBFI is a suitable tool with which to determine Na+ influx and [Na+]i in sea urchin sperm suspensions. We found that the [Na+]i and [Ca2+]i increases were larger in the AR than in the speract response; however, the pHi changes were very similar. Our results also show that the changes in ion permeability elicited by speract and FSG are regulated independently and involve at least some different transport systems. Both of these egg-jelly components activate more than one Na+ transporter.
Acknowledgments
This work was supported by grants from CONACyT and DGAPA-UNAM. E.S. was the recipient of a CONACyT graduate fellowship. The authors thank Claudia Treviño, Takuya Nishigaki and Pedro Labarca for critical reading of the manuscript.
References
- Babcock DF, Bosma MM, Battaglia DE, Darszon A. Early persistent activation of sperm K+ channels by the egg peptide speract. Proc Natl Acad Sci USA. 1992;89:6001–6005. doi: 10.1073/pnas.89.13.6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrán C, Zapata O, Darszon A. Membrane potential regulates sea urchin sperm adenylyl cyclase. Biochemistry. 1996;35:7592–7598. doi: 10.1021/bi952806v. [DOI] [PubMed] [Google Scholar]
- Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiol Rev. 1999;79:763–854. doi: 10.1152/physrev.1999.79.3.763. [DOI] [PubMed] [Google Scholar]
- Bibring T, Baxandall J, Harter CC. Sodium-dependent pH regulation in active sea urchin sperm. Dev Biol. 1984;101:425–435. doi: 10.1016/0012-1606(84)90157-x. [DOI] [PubMed] [Google Scholar]
- Borin ML, Goldman WF, Blaustein MP. Intracellular free Na+ in resting and activated A7r5 vascular smooth muscle cells. Am J Physiol. 1993;265:C1513–1524. doi: 10.1152/ajpcell.1993.264.6.C1513. [DOI] [PubMed] [Google Scholar]
- Castellano LE, López-Godinez J, Aldana G, Barrios-Rodiles M, Obregón A, García L, Darszon A, García-Soto J. The acrosome reaction in digitonin-permeabilized sea urchin sperm in the absence of the natural inducer. Eur J Cell Biol. 1995;67:23–31. [PubMed] [Google Scholar]
- Christen R, Schackmann RW, Shapiro BM. Metabolism of the sea urchin sperm. Interrelationship between intracellular pH, ATPase activity and mitochondrial respiration. J Biol Chem. 1982;258:5392–5399. [PubMed] [Google Scholar]
- Cook SP, Babcock DF. Activation of Ca2+ permeability by cAMP is coordinated through the pHi increase induced by speract. J Biol Chem. 1993;268:22408–22413. [PubMed] [Google Scholar]
- Darszon A, Beltrán C, Félix R, Nishigaki T, Treviño C. Ion transport in sperm signaling. Dev Biol. 2001;340:1–14. doi: 10.1006/dbio.2001.0387. [DOI] [PubMed] [Google Scholar]
- Donoso P, Mill JG, O'Neill SC, Eisner DA. Fluorescent measurements of cytoplasmic and mitochondrial sodium concentration in rat ventricular myocytes. J Physiol. 1992;448:493–509. doi: 10.1113/jphysiol.1992.sp019053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias CE, Lukas A, Shurraw S, Scott J, Omelchenco A, Gross GJ, Hnatowich M, Hryshko L. Inhibition of Na+/Ca2+ exchange by KB-R7943: transport mode selectivity and antiarrhythmic consequences. Am J Physiol Heart Circ Physiol. 2001;281:H1334–1345. doi: 10.1152/ajpheart.2001.281.3.H1334. [DOI] [PubMed] [Google Scholar]
- Fox A, Nowcky MC, Tsien RW. Kinetics and pharmacological properties distinguishing three types of calcium currents in chick sensory neurons. J Physiol. 1987;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo BE, Beltrán C, Cragoe EJJ, Darszon A. Participation of a K+ channel modulated directly by cGMP in the speract-induced signaling cascade of Strongylocentrotus purpuratus sea urchin sperm. Dev Biol. 2000;221:285–294. doi: 10.1006/dbio.2000.9678. [DOI] [PubMed] [Google Scholar]
- Garbers DL. Molecular basis of fertilization. Ann Rev Biochem. 1989;58:719–742. doi: 10.1146/annurev.bi.58.070189.003443. [DOI] [PubMed] [Google Scholar]
- Garbers DL, Kopf GS, Tubb DJ, Olson G. Elevation of sperm adenosine 3′5′-monophosphate concentration by fucose sulfate rich complex associated with egg: I structural characterization. Biol Reprod. 1983;29:1211–1220. doi: 10.1095/biolreprod29.5.1211. [DOI] [PubMed] [Google Scholar]
- Gauss R, Seifert R, Kaupp UB. Molecular identification of a hyperpolarization-activated (Ih) channel in sea urchin sperm. Nature. 1998;393:583–587. doi: 10.1038/31248. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Martínez MT, Galindo BE, De La Torre L, Zapata O, Rodriguez E, Florman HM, Darszon A. A sustained increase in intracellular Ca2+ is required for the acrosome reaction in sea urchin sperm. Dev Biol. 2001;236:220–229. doi: 10.1006/dbio.2001.0323. [DOI] [PubMed] [Google Scholar]
- Guerrero A, Darszon A. Egg jelly triggers a calcium influx which inactivates and is inhibited by calmodulin antagonists in the sea urchin sperm. Biochim Biophys Acta. 1989a;980:109–116. doi: 10.1016/0005-2736(89)90206-x. [DOI] [PubMed] [Google Scholar]
- Guerrero A, Darszon A. Evidence for the activation of two different Ca2+ channels during the egg jelly-induced acrosome reaction of sea urchin sperm. J Biol Chem. 1989b;264:19593–19599. [PubMed] [Google Scholar]
- Guerrero A, García L, Zapata O, Rodríguez E, Darszon A. Acrosome reaction inactivation in sea urchin sperm. Biochim Biophys Acta. 1998;1401:329–338. doi: 10.1016/s0167-4889(97)00127-4. [DOI] [PubMed] [Google Scholar]
- Haigney MC, Miyata H, Lakatta EG, Stern MD, Silverman HS. Dependence of hypoxic cellular calcium loading on Na(+)-Ca2+ exchange. Circ Res. 1992;71:547–557. doi: 10.1161/01.res.71.3.547. [DOI] [PubMed] [Google Scholar]
- Hansbrough JR, Garbers DL. Speract: Purification and characterization of a peptide associated with eggs that activate spermatozoa. J Biol Chem. 1981a;256:1447–1452. [PubMed] [Google Scholar]
- Hansbrough JR, Garbers DL. Sodium dependent activation of sea urchin spermatozoa by speract and monensin. J Biol Chem. 1981b;256:2235–2241. [PubMed] [Google Scholar]
- Harootunian AT, Kao JP, Eckert BK, Tsien RV. Fluorescence ratio imaging of cytosolic free Na+ in individual fibroblasts and lymphocytes. J Biol Chem. 1989;264:19458–19467. [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. 2. Sunderland, MA: Sinauer; 1992. p. 607. [Google Scholar]
- Hoshino K, Shimizu T, Sendai Y, Harumi T, Suzuki N. Differential effect of the egg jelly molecules FSG and SAP-1 on elevation of intracellular Ca2+ and pH in sea urchin sperm. Develop Growth Differ. 1992;34:403–311. doi: 10.1111/j.1440-169X.1992.00403.x. [DOI] [PubMed] [Google Scholar]
- Kaupp UB, Seifert R. Molecular diversity of pacemaker ion channels. Ann Rev Physiol. 2001;63:235–257. doi: 10.1146/annurev.physiol.63.1.235. [DOI] [PubMed] [Google Scholar]
- Labarca P, Santi C, Zapata O, Morales E, Beltrán C, Liévano A, Darszon A. A cAMP regulated K+ selective channel from the sea urchin sperm plasma membrane. Dev Biol. 1996;174:271–280. doi: 10.1006/dbio.1996.0072. [DOI] [PubMed] [Google Scholar]
- Labarca P, Zapata O, Beltrán C, Darszon A. Ion channels from mouse sperm plasma membrane in planar lipid bilayers. Zygote. 1995;3:199–206. doi: 10.1017/s0967199400002598. [DOI] [PubMed] [Google Scholar]
- Lee HC. Sodium and proton transport in flagella isolated from sea urchin spermatozoa. J Biol Chem. 1984a;259:4957–4963. [PubMed] [Google Scholar]
- Lee HC. A membrane potential-sensitive Na+-H+ exchange system in flagella isolated from sea urchin spermatozoa. J Biol Chem. 1984b;259:15315–15319. [PubMed] [Google Scholar]
- Lee HC, Garbers DL. Modulation of the voltage-sensitive Na+/H+ exchanger in sea urchin spermatozoa through membrane potential changes induced by the egg peptide speract. J Biol Chem. 1986;261:16026–16032. [PubMed] [Google Scholar]
- Levi AJ, Lee CO, Brooksby P. Properties of fluorescent sodium indicator ‘SBFI’ in rat and rabbit cardiac myocytes. J Card Electrophysiol. 1994;5:241–257. doi: 10.1111/j.1540-8167.1994.tb01161.x. [DOI] [PubMed] [Google Scholar]
- Limbird LE. GTP and Na+ modulate receptor -adenyl cyclase coupling and receptor-mediated function. Am J Physiol. 1984;247:E59–68. doi: 10.1152/ajpendo.1984.247.1.E59. [DOI] [PubMed] [Google Scholar]
- Miller RL, Vogt R. An N-terminal partial sequence of the 13 kDa Pycnopodia helianthoides sperm chemoattractant ‘startrak’ possesses sperm-attracting activity. J Exp Biol. 1996;199:311–318. doi: 10.1242/jeb.199.2.311. [DOI] [PubMed] [Google Scholar]
- Minta A, Tsien RY. Fluorescent indicators for cytosolic sodium. J Biol Chem. 1989;264:19449–19457. [PubMed] [Google Scholar]
- Moore EDW, Tsien RY, Minta A, Fay FS. Measurement of the intracellular sodium with SBFI a newly developed fluorescent dye. FASEB J. 1998;2:A754–760. [Google Scholar]
- Morisawa M. Cell signaling mechanisms for sperm motility. Zoolog Sci. 1994;11:647–662. [PubMed] [Google Scholar]
- Negulescu PA, Harootunian A, Tsien RY, Machen TE. Fluorescence measurements of cytosolic free Na+ concentration, influx and efflux in gastric cells. Cell Regul. 1990;1:259–268. doi: 10.1091/mbc.1.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishigaki T, Zamudio FZ, Posanni LD, Darszon A. Time resolved sperm responses to an egg peptide measured by stopped-flow fluorimetry. Biochem Biophys Res Commun. 2001;284:531–535. doi: 10.1006/bbrc.2001.5000. [DOI] [PubMed] [Google Scholar]
- Patrat C, Serres C, Jouanne P. Induction of a sodium ion influx by progesterone in human spermatozoa. Biol Reprod. 2000;62:1380–1386. doi: 10.1095/biolreprod62.5.1380. [DOI] [PubMed] [Google Scholar]
- Repaske DR, Garbers DL. A hydrogen ion flux mediates stimulation of respiratory activity by speract in sea urchin spermatozoa. J Biol Chem. 1983;258:6025–6029. [PubMed] [Google Scholar]
- Reynaud E, De La Torre L, Zapata O, Liévano A, Darszon A. Ionic bases of membrane potential and intracellular pH changes induced by speract: possible implications for fertilization. FEBS Lett. 1993;329:54–69. doi: 10.1016/0014-5793(93)80223-h. [DOI] [PubMed] [Google Scholar]
- Robertson MA, Foskett K. Fluorescence measurements of cytosolic sodium concentration. Methods Neurosci. 1995;27:274–288. [Google Scholar]
- Rose CR, Ransam BR. Regulation of intracellular sodium in cultured rat hippocampal neurons. J Physiol. 1997;499:573–587. doi: 10.1113/jphysiol.1997.sp021951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage SO, Rink TJ, Mahaut-Smith MP. Resting and ADP-evoked changes in cytosolic free sodium concentration in human platelets loaded with the indicator SBFI. J Physiol. 1991;441:559–573. doi: 10.1113/jphysiol.1991.sp018767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Hayashi H, Noda N, Terada H, Kobayashi A, Yamashita Y, Kawai T, Hirano M, Yamazaki N. Quantification of intracellular free sodium ions by using a new fluorescent indicator, sodium-binding benzofuran isophthalate, in guinea pig myocytes. Biochem Biophy Res Commun. 1991;175:611–616. doi: 10.1016/0006-291x(91)91609-g. [DOI] [PubMed] [Google Scholar]
- Schackmann RW. Ionic regulation of the sea urchin sperm acrosome reaction and stimulation by egg-derived peptides. In: Schatte H, Schatten G, editors. The Cell Biology of Fertilization. San Diego, CA: Academic; 1989. pp. 3–29. [Google Scholar]
- Schackmann RW, Chock PB. Alteration of intracellular [Ca2+] in sea urchin sperm by the egg peptide speract: evidence that increased intracellular Ca2+ is coupled to Na+ entry and increased intracellular pH. J Biol Chem. 1986;261:8719–8728. [PubMed] [Google Scholar]
- Shin KS, Rothberg BS, Yellen G. Blocker state dependence and trapping in hyperpolarization-activated cation channels: evidence for an intracellular activation gate. J Gen Physiol. 2001;117:91–100. doi: 10.1085/jgp.117.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su YH, Vacquier VD. A flagellar K+-dependent Na+/H+ exchanger keeps Ca2+ low in sea urchin spermatozoa. Proc Natl Acad Sci U S A. 2002;99:6743–6748. doi: 10.1073/pnas.102186699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Nomura K, Othake H, Isaka S. Purification and the primary structure of sperm-activity peptides from the jelly coat of sea urchin eggs. Biochem Biophys Res Commun. 1981;99:1238–1244. doi: 10.1016/0006-291x(81)90752-x. [DOI] [PubMed] [Google Scholar]
- Takano S, Kimura J, Ono T. Inhibition of aggregation of rabbit and human platelets induced by adrenaline and 5-hydroxytryptamine by KB-R7943, a Na+/Ca2+ exchange inhibitor. Br J Pharm. 2001;132:1383–1388. doi: 10.1038/sj.bjp.0703928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimmer JS, Schackmann RW, Vacquier VD. Monoclonal antibodies increase intracellular Ca2+ in sea urchin spermatozoa. Proc Natl Acad Sci U S A. 1986;83:9055–9059. doi: 10.1073/pnas.83.23.9055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacquier VD. Evolution of gamete recognition proteins. Science. 1998;281:1995–1998. doi: 10.1126/science.281.5385.1995. [DOI] [PubMed] [Google Scholar]
- Vacquier VD, Moy GW. The fucose sulfate polymer of egg jelly binds to sperm REJ and is the inducer of the sea urchin sperm acrosome reaction. Dev Biol. 1997;192:125–135. doi: 10.1006/dbio.1997.8729. [DOI] [PubMed] [Google Scholar]
- Vines CA, Yoshida K, Griffin FG, Pillai MC, Morisawa M, Yanagimachi R, Cherr GN. Motility initiation in herring sperm is regulated by reverse sodium-calcium exchange. Proc Natl Acad Sci U S A. 2002;99:2026–2031. doi: 10.1073/pnas.042700899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward GR, Kopf G. Molecular events mediating sperm activation. Dev Biol. 1993;158:9–34. doi: 10.1006/dbio.1993.1165. [DOI] [PubMed] [Google Scholar]
- Wassarman PM, Jovine L, Litscher ES. A profile of fertilization in mammals. Nat Cell Biol. 2001;3:E59–64. doi: 10.1038/35055178. [DOI] [PubMed] [Google Scholar]
- Watson RL, Jacobson KL, Singh JC. Monovalent ion enhancement of beta-adrenergic stimulated adenylate cyclase activity in mouse parotid gland. Biochem Pharmacol. 1989;38:1069–1079. doi: 10.1016/0006-2952(89)90250-5. [DOI] [PubMed] [Google Scholar]
- Wu ML, Tsai ML, Tseng YZ. DIDS-sensitive pH regulation in single rat cardiac myocytes in nominally HCO3-free conditions. Circ Res. 1994;75:123–132. doi: 10.1161/01.res.75.1.123. [DOI] [PubMed] [Google Scholar]


