Abstract
Continuous positive airway pressure (CPAP) is the main treatment of the obstructive sleep apnoea syndrome (OSAS). We assessed its effects on the upper airway (UA) dynamics in response to bilateral anterior magnetic phrenic nerve stimulation (BAMPS) in 17 awake untreated OSAS patients (15 males; 52 ± 7 years) whose effective CPAP (Peff) had been determined beforehand by a conventional titration sleep study. All twitch-related inspirations were flow-limited, flow first rising to a maximum (V̇Imax), then decreasing to a minimum (V̇Imin), and then increasing again (M-shaped pattern). Up to V̇Imin, the relationship between driving pressure (Pd) and flow (V̇) could adequately be fitted to a polynomial regression model (V̇ = k1Pd + k2Pd2; r2 = 0.71-0.98, P < 0.0001). At atmospheric pressure V̇Imax was 700 ± 377 ml s−1, V̇Imin was 458 ± 306 ml s−1, k1 was 154.5 ± 63.9 ml s−1 (cmH2O)−1, and k2 was 10.7 ± 7.3 ml s−1 (cmH2O)−1. CPAP significantly increased V̇Imax and V̇Imin (peak values 1007 ± 332 ml and 837 ± 264 ml s−1, respectively) as well as k1 and k2 (peak values 300.9 ± 178.2 ml s−1 (cmH2O)−1 and 55.2 ± 65.3 ml s−1 (cmH2O)−1, respectively). With increasing CPAP, k1/k2 increased up to a peak value before decreasing. We defined as Peff,stim the CPAP value corresponding to the highest k1/k2 value. Peff,stim was correlated with Peff (Peff = 7.0 ± 2.0; Peff,stim = 6.4 ± 2.6 cmH2O; r = 0.886; 95 % CI 0.696-0.960, P < 0.001). We conclude that CPAP improves UA dynamics in OSAS and that the therapeutic CPAP to apply can be predicted during wakefulness using BAMPS.
The obstructive sleep apnoea syndrome is characterised by recurrent sleep-related episodes of complete or partial upper airway closure (Guilleminault et al. 1976; Remmers et al. 1978) that result from upper airway (UA) instability and lead to intermittent hypoxia and hypercarbia. Together with the perception of the increasing intensity of inspiratory effort that is conveyed by rib cage afferents (Hudgel & Harasick, 1990), the increase in pharyngeal pressure leads to arousal or awakening, which in turn increases UA muscle dilator activity and breaks the apnoea. This sequence of events recurs hundreds of times in a night. The disruption of sleep architecture leads to daytime sleepiness with its own consequences (Olson et al. 1995; Lloberes et al. 2000). The repeated hypoxic stimulations and their neurovegetative counterparts may account for the increased frequency of cardiovascular events (Peppard et al. 2000), in part through a hypercoagulable state (Eisenher & Noachtar, 2001; Arnulf et al. 2002). Diagnosing and treating the obstructive sleep apnoea syndrome is thus of the utmost importance. Currently, the gold standard treatment consists of ‘splinting’ the UA by applying a positive pressure (continuous positive airway pressure, CPAP) (Sullivan et al. 1981) that preserves its patency throughout the night. This dramatically improves sleep architecture and the stability of gas exchanges (Sullivan et al. 1981; Lamphere et al. 1989) with positive effects on daytime vigilance and cognitive performance (Derderian et al. 1988), and a reversal of cardiovascular (Akashiba et al. 1999) and coagulation abnormalities (Chin et al. 1996; Chin & Ohi, 1998).
One of the main mechanisms of sleep-related UA closure is an asynchrony between UA dilator muscles and inspiratory muscles (Hudgel & Harasick, 1990). Indeed, UA dilator muscles normally contract before the inspiratory muscles, to open and stabilise the UA during inspiration. During sleep, the decrease in the neural drive to the UA dilator muscles delays their action and decreases their efficiency. The UA is therefore functionally passive or quasi so when the contraction of the diaphragm begins. The loss of the counterforce that normally opposes the negative inspiratory pressure explains why the airways tend to close at their most collapsible locus, namely the pharynx (Series et al. 1999, 2000; Verin et al. 2002a). It is currently possible to reproduce this sequence of events in conscious patients by the use of phrenic nerve stimulation. Indeed, with this technique, the inspiratory driving pressure is generated by a diaphragm contraction that is not preceded by any pre-activation of the UA dilator muscles. Studying the relationship of inspiratory flow to driving pressure in response to phrenic nerve stimulation is thus an accurate method of characterising UA mechanics in a state very close to sleep (namely, not ‘passive’ because of the preserved tonic muscular activity, but not phasically active (Series et al. 1999, 2000; Verin et al. 2002a)). It has been previously shown that diaphragm twitches in response to phrenic nerve stimulation induce flow-limited inspirations in normal subjects and in patients with the obstructive sleep apnoea syndrome (Series et al. 1999, 2000; Verin et al. 2002a), and that they can be used to study the effects of external factors (Series et al. 2000; Verin et al. 2002a). The effects of varying CPAP levels on the behaviour of the UA confronted with the inspiratory-related drop in UA transpharyngeal pressure gradient remain unknown. This is in part due to the difficulties inherent in studies conducted during sleep, where numerous factors interfering with UA properties are impossible to control for (e.g. sleep stability and sleep stages, body position, nasal resistance, hypoxaemia/ hypercarbia- induced ventilatory responses…). Phrenic nerve stimulation in conscious patients, being free of such influences should eliminate these difficulties.
The aim of the present work was thus to further elucidate the effects of CPAP on upper airway inspiratory flow dynamics in patients with the obstructive sleep apnoea syndrome. In addition, a mathematical model of the pressure-flow relationship during diaphragm twitches was used to predict the effective level of external pressure needed to prevent flow limitation with reference to the conventionally determined value (CPAP titration over an all-night polysomnographic recording).
Methods
Patients
Seventeen patients (Table 1) with a polysomnographically established diagnosis of obstructive sleep apnoea syndrome (American Academy of Sleep Medicine Task Force report, 1999) participated in this study. In all cases, nocturnal CPAP was retained as the treatment of choice. The protocol was designed and conducted in accordance with the Declaration of Helsinki, and had been approved by the ethical review board of the institution where the experiments were performed (Université Laval, Québec, Canada). Written consent was obtained from each participant after giving them full information on the aim of the study and the methods used.
Table 1.
Anthropomorphic and polysomnographic characteristics of the 17 patients included in the study
| Patient | Age (years) | Sex | BMI (kg m−2) | AHI (n h−1) | AI (n h−1) | Peff (cmH2O) |
|---|---|---|---|---|---|---|
| 1 | 39 | F | 48.4 | 26.5 | 19.3 | 12 |
| 2 | 60 | M | 33.2 | 51.1 | 31.3 | 9 |
| 3 | 52 | M | 30.5 | 9.6 | 6.2 | 4 |
| 4 | 50 | M | 24 | 9.4 | 5.4 | 5 |
| 5 | 53 | M | 31.3 | 33.9 | 16.9 | 6 |
| 6 | 54 | M | 35.2 | 25 | 11.6 | 10 |
| 7 | 50 | M | 27.3 | 28.9 | 8.5 | 5 |
| 8 | 54 | M | 32.3 | 76.1 | 28.5 | 8 |
| 9 | 63 | M | 24.9 | 35.6 | 27.5 | 5 |
| 10 | 65 | M | 25.3 | 22.2 | 3.4 | 5 |
| 11 | 57 | M | 30.8 | 46.7 | 19.8 | 7 |
| 12 | 46 | M | 27.8 | 37.4 | 11.2 | 8 |
| 13 | 39 | M | 30.1 | 33.5 | 9.8 | 7 |
| 14 | 49 | M | 27.3 | 62.8 | 56.3 | 5 |
| 15 | 46 | M | 31.3 | 23 | 13.7 | 6 |
| 16 | 45 | M | 32.6 | 58.6 | 35 | 7 |
| 17 | 60 | M | 30.6 | 15.4 | 3.9 | 5 |
| Mean | 52 | 30.6 | 35.0 | 18.1 | 7 | |
| s.d. | 7 | 5.4 | 18.2 | 13.5 | 2 | |
BMI: body mass index; AHI: apnoea hypopnoea index number of obstructive events per hour; AI: apnoea index (number of apoea per sleep hour); Peff. effective continuous positive airway pressure.
Sleep recordings
The polysomnographic recordings consisted of in-lab continuous acquisition of data from electroencephalograms, electroocculograms, submental electromyograms, transcutaneous pulsed oxymetry (SpO2), naso-oral airflow measured with thermistors, nasal pressure measured with nasal cannula (Series & Marc, 1999), chest and abdominal movements measured by impedance plethysmography (Respitrace, Ambulatory Monitoring Inc., Ardsley, NY, USA), electrocardiograms, and breath sounds measured by means of two microphones connected to a calibrated sound analyser (Series et al. 1993). Sleep position was continuously assessed by the attending technician, on the monitor of an infrared camera. Two sleep studies were performed in each patient, the first to establish the diagnosis of OSA and the second to determine the optimal CPAP pressure level (Peff) (American Academy of Sleep Medicine Task Force report, 1999). During this last study, nasal flow was recorded using a pneumotachograph connected to the nasal CPAP mask, in place of the thermistors and nasal cannula. Peff was defined as the pressure level that abolished snoring and obstructive events in all sleep stages and body positions (Bureau & Series, 2000). All variables were digitally recorded (Sandman Elite system, Mallinckrotd, Kenilworth, NJ, USA).
Phrenic nerve stimulation
Measurements
Surface recordings of the right and left costal diaphragmatic electromyographic activity were obtained using silver cup electrodes placed in the lowest accessible intercostal space, close to the chondro-costal junction, in the mid-clavicular line (Verin et al. 2002b), and connected to an electromyograph (Biopac system, Biopac, Santa Barbara, CA, USA). Oesophageal pressure (Poes) was measured using a balloon-catheter system (Jaeger, Witzburg, Germany) passed through a nostril after topical anaesthesia (xylocaine 1 %), positioned into the lower third of the oesophagus (Baydur et al. 1982), and connected using a 1.4 mm internal diameter polyethylene catheter to a differential pressure transducer linear over the ± 100 cmH2O range (Validyne, Northridge, CA, USA). A nasal stent was placed in the anterior nostrils (Nozovent; WPM international AB; Göteborg, Sweden) to prevent nasal collapse. An airtight nasal mask was then placed over the nose (Profile Light Nasal Mask, Respironics, Pittsburg, PA, USA). Mask pressure (Pmask) was measured from a side port in the mask connected to a second pressure transducer of identical type. Poes was referenced to Pmask to provide driving pressure (Pd). A pneumotachograph (Hans Rudolph, model 112467-3850A, Kansas City, MO, USA) was connected to the mask, that was either open to the atmosphere or connected to a CPAP apparatus (Healthdyne Marietta, GA, USA), via a non-rebreathing valve (Respironics, Pittsburg, PA, USA). Pressures and flow were digitally recorded at a 300 Hz sampling rate (Digidata 1320, Axon Instrument, Union City, CA, USA) whereas EMG signals were digitised at a 10 000 kHz sample rate.
Stimulations
Supramaximal bilateral anterior magnetic phrenic nerve stimulation (BAMPS) was performed with two Magstim 200 stimulators (Magstim Ltd, Whitland, Dyfed, UK) equipped with double 43 mm figure-of-eight coils of the ‘branding iron’ type, according to the technique described by Mills et al. (1996). The application of this technique to upper airway studies has been described in detail elsewhere (Series et al. 1999, 2000). In brief, each stimulating coil was positioned antero-laterally over the anatomical landmark of the phrenic nerve in the neck, at the posterior border of the sternomastoid muscle at the level of the cricoid cartilage, the handle of the coil making a 45 ° axis with both the mid-sagittal plane of the body and the horizontal plane. The intensity of stimulation was set at the maximum possible output of the stimulators. A simplified recruitment curve (motor response to stimulation against stimulation intensity) was performed to verify the supramaximal nature of the stimulation. The patients were studied seated in a comfortable armchair with a 60 ° inclination and with their head maintained in a natural, ‘neutral’, position by a moulded pillow. Special attention was paid to avoiding any change in body, neck or head position during the experiments. All stimulations were delivered at the end of a relaxed expiration according to the monitoring of the flow and Poes traces, to control as precisely as possible for the confounding effects of lung volume and abdominal configuration on the output of phrenic stimulation (Chen et al. 2000).
Protocol
The phrenic nerve stimulation studies were performed within the week following the CPAP titration studies. BAMPS was applied with the patient breathing room air at the atmospheric pressure and under CPAP (3 cmH2O with 1 cmH2O stepwise increases in pressure up to Peff + 3 cmH2O). After 5 min of quiet breathing at each CPAP level, five stimuli were delivered at four to five breath intervals, and thereafter averaged.
Data analysis
Flow limitation
The twitch-induced breaths were considered flow-limited when, beyond a maximal value (V̇Imax), flow plateaued or decreased in spite of a persistent increase in driving pressure. The driving pressure value corresponding to V̇Imax is henceforth termed limiting pressure (Pd,lim). Beyond V̇Imax, flow decreased to a minimal value (V̇Imin) whereas Pd continued to increase, up to a peak value, Pd,peak (Fig. 1).
Figure 1. Example of the changes in inspiratory flow (A) and driving pressure (B) in response to a phrenic nerve stimulation-induced diaphragm twitch, in a representative subject.
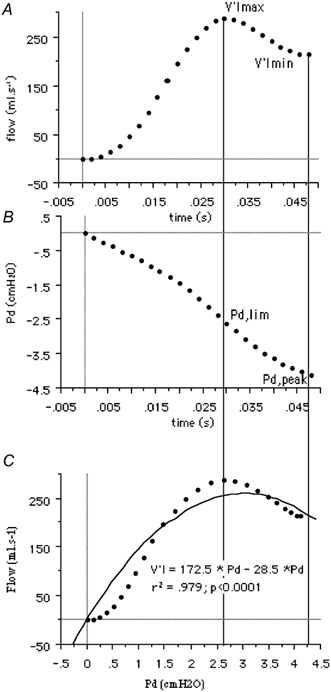
V̇Imax and V̇Imin stand respectively for the maximal and minimal flow values reached during the twitch. Pd,lim indicates the driving pressure corresponding to V̇Imax. Pd,peak indicates the driving pressure corresponding to V̇Imin. C, flow-pressure relationship up to V̇Imin. The dotted line corresponds to the actual data points. The solid line corresponds to the pressure-flow fit by a polynomial of order 2.
The resistance of the respiratory system was calculated at V̇Imax and V̇Imin (RV̇Imax or RV̇Imin) as the ratio of the Pd,lim to V̇Imax and of Pd,peak to V̇Imin, respectively (Verin et al. 2002a). No correction was applied to take into account the portion of the measured pressure required to overcome the elastic properties of the lung, because the volume change during the analysed portion of the twitch has been considered as minimal in this regard (below 100 ml).
Driving pressure-flow relationship
Because of its hyperbolic shape, the twitch-related pressure-flow relationship from zero flow to V̇Imin was fitted to a polynomial equation of the form V̇I = k1Pd + k2Pd2 using the least squares method.
Statistical Analysis
Statistical analysis was performed using the SuperAnova 4.5 software (Abacus Concept, Berkeley, CA, USA) running on an Apple Macintosh computer. Values are expressed as means ± standard deviation (s.d.). Statistical associations between the characteristics of the twitch-related inspiratory flow and the severity of sleep-related respiratory disturbances were studied using the z test for correlation. The conventionally determined Peff and the phrenic stimulation-derived estimate of Peff (Peff,stim see Results) were tested for agreement using the regression procedure described by Passing & Bablok (1983) and a graphical Bland and Altman representation (Bland & Altman, 1986). Statistical results were considered significant when the probability P of a type I error was 0.05 or less.
Results
Of the 491 twitches recorded, 95.4 % could be analysed. In patient number 7, the CPAP-induced changes in inspiratory flow dynamics could not be studied, because of a leak around the nasal mask that produced major artefacts.
Flow limitation
Flow-limitation was consistently absent during tidal breathing. Conversely, twitch-induced breaths were flow-limited in all subjects and at all CPAP levels.
Driving pressure-flow relationship
A typical example is shown in Fig. 1. The V̇I = k1Pd + k2Pd2 model adequately described the relationship between driving pressure and the flow in all twitches (r2 = 0.71 to 0.98, P < 0.0001) in all cases. The mean values at atmospheric pressure were as follows: V̇Imax = 700 ± 377 ml s−1; V̇Imin = 458 ± 306 ml s−1; Pd,lim = −7.7 ± 4.7 cmH2O; Pd,peak = −12.6 ± 5.1 cmH2O; k1 = 154.5 ± 63.9 ml s−1 (cmH2O)−1, k2 = 10.7 ± 7.3 ml s−1 (cmH2O)−1. CPAP significantly increased V̇Imax and V̇Imin (1007 ± 332 ml s−1 and 837 ± 264 ml s−1, respectively), at the highest CPAP value tested in each patient, corresponding to mean increases of 43 and 85 % as compared to baseline (P < 0.0001 and P < 0.05). The k1 and k2 coefficients also increased (300.9 ± 178.2 ml s−1 (cmH2O)−1 and 55.2 ± 65.3 ml s−1 (cmH2O)−1, respectively), corresponding to 94 and 414 % increases compared to baseline (P < 0.0001). Conversely, Pd,lim and Pd,peak did not significantly change with CPAP. As a result, RV̇Imax or RV̇Imin both decreased with increasing CPAP (P < 0.001; Fig. 2).
Figure 2. Effects of an increasing level of continuous positive airway pressure (CPAP) on the measured variables.
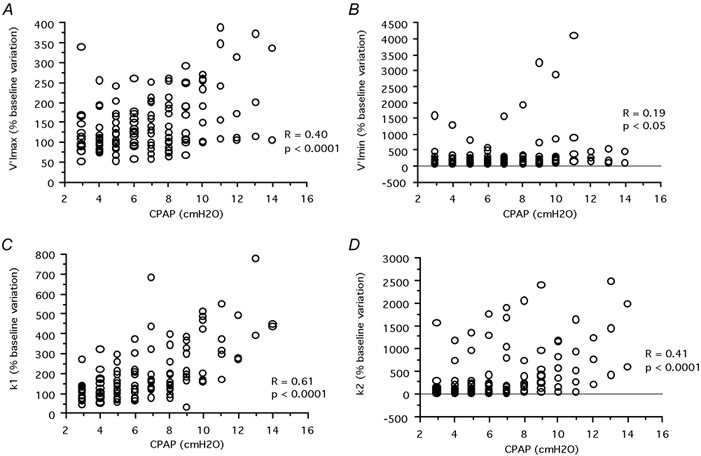
A, effect on the maximal inspiratory flow observed in response to the diaphragm twitch (V̇Imax,), B, effect on the minimal inspiratory flow (V̇Imin,), and C and D, the effects on coefficients k1 (C) and k2 (D) of the V̇I = k1Pd + k2Pd2 equation describing the flow-pressure relationship up to maximal flow, see text for details. Each data point represents the averaged results of all stimulations performed at a given CPAP level in a given subject.
Solving V̇I = k1Pd + k2Pd2 for V̇ = 0 with Pd different from zero gives Pd = k1/k2, which is the Pd value that should correspond to full airway collapse. The k1/k2 ratio is thus a descriptor of UA stability (the higher the value of k1/k2, the better the upper airway opposes the inspiration-related collapsing force). In all but two cases, increasing CPAP resulted in a progressive increase of k1/k2 up to a maximum that was followed by a subsequent decrease. (Fig. 3). k1/k2 as function of CPAP level was accurately described by a polynomial regression of order 2 (r2 from 0.69 to 0.99, P < 0.05 except in patient number 3 where P = 0.06). The k1/k2 peak was assumed to correspond to the CPAP level ensuring maximal UA stability and thus termed Peff,stim (Fig. 4).
Figure 3. Illustration, in one patient, of the effects of increasing the level of positive pressure applied at the airway (Pmask).
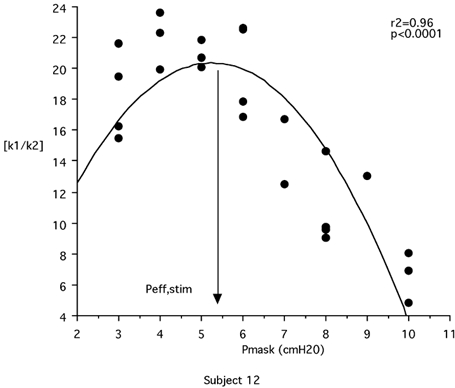
Effects on the absolute value of the k1/k2 ratio (k1 and k2, coefficients of the V̇I = k1Pd + k2Pd2 equation describing the flow-pressure relationship up to maximal flow, see text for details) are shown. The peak of the k1/k2 curve corresponds to optimal upper airway stability. From the pressure-flow response to phrenic nerve stimulation established at various CPAP levels, it is thus possible to calculate what would theoretically be the efficient pressure Peff (Peff,stim) in a given individual. In this patient, Peff,stim was 5.3 cmH2O, whereas the Peff conventionally determined during a titration sleep recording was 7 cmH2O.
Figure 4. Comparison of the values of efficient pressure determined conventionally during a titration sleep recording (Peff) with the value of efficient pressure derived from phrenic nerve stimulation performed at various levels of positive pressure applied to the airway (Peff,stim).
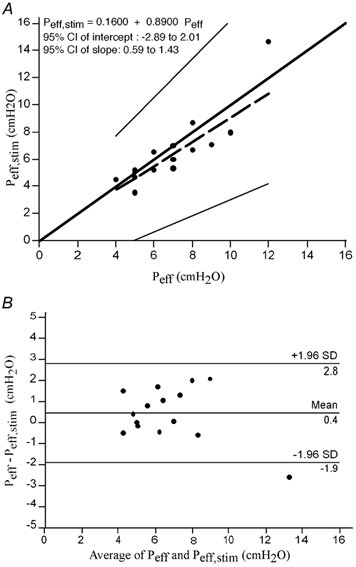
A, results of the Passing & Bablok regression (1983) of Peff,stim against Peff. The thick solid line is the line of identity; the dashed line is the regression line; the thin solid lines mark the 95 % confidence interval of the regression. There was no significant deviation from linearity (P > 0.10). The 95 % confidence interval for the intercept contained zero and the 95 % confidence interval for the slope contained 1, indicating the absence of systematic difference between the two methods. B, scatter diagram of the differences plotted against the averages of the two measurements, according to the method devised by Bland & Altman (1986).
Comparison of nocturnal and diurnal effective pressure values
Peff,stim was strongly correlated with the conventional Peff (Peff = 7.0 ± 2.0; Peff,stim = 6.4 ± 2.6 cmH2O; r = 0.886; 95 % confidence interval 0.696-0.960, P < 0.001). A regression analysis according to the method of Passing & Bablok (1983) showed that there was no significant systematic difference between the two methods. The Bland and Altman graphic representation (Bland & Altman, 1986; Fig. 4A) set the lower limit of agreement between the two methods at −1.9 cmH2O and the upper limit at 2.8 cmH2O and showed that only one data point fell outside this interval.
Discussion
This study confirms that the dynamics of inspiratory flow in sleep apnoea patients is dramatically influenced by a positive pressure applied to the upper airway and provides, seemingly for the first time, a quantitative description of this phenomenon. It also suggests that the adequate positive pressure level to treat a given patient could be predicted from phrenic nerve stimulation performed during consciousness.
Study population
Three patients (patients 3, 4, 17) had an upper airway high resistance syndrome, defined as repetitive episodes of partial upper airway closure with flow limitation, leading to arousal but not causing hypopnoea. (Guilleminault et al. 1993). They were included in the study because its primary aim was to evaluate the influence of CPAP on UA dynamics in patients requiring this therapeutic intervention. As expected, Peff values were low in these three cases (4.0, 5.0 and 5.0 cmH2O, respectively), but they were well matched with the Peff,stim values (4.5, 4.6 and 5.1 cmH2O). The inclusion of these patients, could be viewed as a problem because it made the study population heterogeneous, but was, in fact, an advantage because it broadened the range of the comparison of Peff with Peff,stim (Fig. 4).
Of note, the severity of the obstructive sleep apnoea syndrome, assessed in terms of the number of obstructive events per unit of time (apnoea-hypopnoea index, AHI), varied greatly in our patients (Table 1). The relationship of the AHI with Peff in the OSAS is not a simple one, as illustrated by the lack of statistical association between the two parameters in this study and in others (Sforza et al. 1995). Peff,stim was not correlated with the AHI, and, more importantly, nor was the Peff - Peff,stim difference (R = 0.08, P = 0.31). Figure 4 shows that there was no systematic deviation of Peff,stim from Peff as a function of the Peff level, suggesting that phrenic nerve stimulation could be useful regardless of the severity of upper airway abnormalities.
Driving pressure-flow relationship
Because we used oesophageal pressure instead of supralaryngeal pressure to measure driving pressure, the calculated resistance was that of the respiratory system as a whole and not solely that of the upper airway. However, UA resistance accounts for most of the total respiratory resistance during nasal breathing (Skatrud & Dempsey, 1985; Hudgel, 1986), and it is likely that the differences in flow dynamics that we observed during continuous positive pressure trials were mainly due to changes induced at the UA level and not below.
Different models have been used to describe the pressure- flow relationship in the upper airway, in conscious subjects (Rohrer, 1915) or during sleep (Hudgel et al. 1988). They could not be applied to our data, because of the particular shape of the pressure-flow relationship during diaphragm twitches. Indeed, in this setting, inspiratory flow rises in response to driving pressure up to a maximal value (V̇Imax) (Series et al. 1999)), then decreases to a minimal value (V̇Imin) corresponding to the peak driving pressure, and later increases again. This M-shape is markedly different from the plateau appearance typically observed in OSAS patients during spontaneous breathing. It is consistent with a ‘passive’, or more precisely ‘not phasically active’, behaviour of the UA when the diaphragm contraction occurs, the late second increase in flow probably being accounted for by a negative pressure-triggered reflex activation of UA dilator muscle (Series et al. 1999). The first part of the flow-pressure relationship was adequately described by a polynomial regression model V̇I = k1Pd + k2Pd2 with a negative k2 value. The k1 and k2 coefficients bore a strong relationship to the level of positive pressure applied to the UA, and are thus probably relevant descriptors of their mechanical characteristics. As a matter of fact, these terms relate to airway conductance, k1 being the counterpart of RuaV̇Imax and k2 that of RuaV̇Imin. Strong statistical associations were found between k1 and 1/RuaV̇Imax (R = 0.81, 95 % CI 0.78-0.84) and between k2 and 1/RuaV̇Imin (R = 0.71, 95 % CI 0.66-0.75). The k1/k2 ratio seems particularly interesting to examine. Indeed, it determines the driving pressure value that should lead to the complete collapse of the UA (see Results), and should therefore represent an index of UA stability. The fact that k1/k2 increased to a maximum with increasing levels of CPAP and then decreased is an important result. It indicates that positive pressure stabilises the UA up to a certain point beyond which it becomes less efficient. Together with the augmentation of critical pressure associated with excessive CPAP levels during sleep that has previously been reported by Schwartz et al. (1989), this gives a physiological basis to the therapeutic choice of the lowest efficient positive pressure in a given patient. This novel observation derives directly from the fact that phrenic stimulation, in response to which the inspiratory driving pressure builds up fully in the absence of UA dilator muscle activity (as opposed to what happens during spontaneous breathing even in OSAS patients), makes it possible to observe flow limitation in situations where it would not normally occur. This is the case in normal subjects (Series et al. 1999) and in OSAS patients receiving CPAP at values exceeding Peff (this study).
Comparison with previous data
The treatment of the obstructive sleep apnoea syndrome by continuous positive pressure was first described by Sullivan et al. (1981) who demonstrated that nasal CPAP dramatically reduced the frequency of apnoeas both during non-rapid eye movement sleep and rapid eye movement sleep. Several mechanisms are involved in the effectiveness of CPAP. They include relatively complex phenomena, such as an increased UA dilator muscle activity due to the stimulation of mechanoreceptors (Rapoport et al. 1983) or of superficial receptors in the nasal mucosa sensitive to airflow (Basner et al. 1989). The simplest mechanism at play is purely mechanical in nature and relates to the pneumatic splitting effect of the applied pressure (Series et al. 1992). In this respect, Schwartz et al. (1989) have shown that CPAP markedly decreased inspiratory resistance and increased UA collapsibility during sleep in six OSAS patients. Isono et al. (1993), by direct endoscopic examination performed in nine sleeping OSAS patients, have correlated this finding with a positive pressure-related increase in the cross-sectional area of the velopharynx. The observations that we made in our patients (effects of CPAP on UA dynamics, agreement between Peff and Peff,stim) are in line with these conclusions. This suggests that the effects of CPAP on UA dynamics are not radically different during sleep and wakefulness. It also suggests that the difference in upper airway dilator muscle tone that exists between sleep and wakefulness is not a major confounding factor relating to the behaviour of the upper airway during a diaphragm twitch when phrenic nerve stimulation is applied at end-expiration.
Prediction of Peff
Predicting Peff without resorting to all-night polysomnography with CPAP titration is a major clinical issue. It would save money, increase the availability of the sleep medicine resources, and shorten the time during which OSAS patients must wait for their treatment to begin. Several prediction models based on anthropomorphic data and the AHI have been devised (Miljeteig & Hoffstein, 1993; Hoffstein & Mateika, 1994). They are useful to shorten titration sleep studies (Hoffstein & Mateika, 1994) or to optimise the settings of automatic CPAP devices (Series, 2000). However, the information that they provide is thus only of a ‘statistical’ nature and they do not take pathophysiological data into account. The prediction method that we used in this study relies on the observation of actual pressure-flow relationships established from phrenic stimulation at various CPAP levels. Its ‘pathophysiological nature’ probably constitutes an important advantage in terms of clinical usefulness. For example, it can be anticipated that it would be useful in non-obese patients suffering from the OSAS. It must also be noted that phrenic stimulation allows an investigator to predict a Peff value corresponding not only to the suppression of apnoeas, hypopnoeas and snoring, but also to the suppression of flow-limited breaths inducing arousals (Meurice et al. 1998). The comparison of Peff and Peff,stim, shows an average difference between the two methods of 0.4 cmH2O with limits of agreement that appear to be clinically acceptable (Fig. 4). The clinical validity of our Peff prediction method must nevertheless be tested by prospective studies comparing the results of CPAP therapy adjusted from phrenic nerve stimulation data with in-laboratory conventional titration methods.
Meanwhile, we feel that the present study provides a potentially useful contribution to the understanding of the effects of CPAP on the behaviour of the UA in the sleep apnoea syndrome. The same approach could be used to characterise other therapeutic techniques such as mandibular advancement.
Acknowledgments
This study was supported by Medical Research Council of Canada, grant MT 13 768 and ADOREP (Association pour le Développement et l'Organisation de la Recherche en Pneumologie) Paris, France. The authors are indebted to Dr Alan Matthews for his help with the English language.
References
- Akashiba T, Minemura H, Yamamoto H, Itoh D, Kosaka N, Saitoh O, Horie T. Effects of nasal continuous positive airway pressure on pulmonary haemodynamics and tissue oxygenation in patients with obstructive sleep apnoea. Respirology. 1999;4:83–87. doi: 10.1046/j.1440-1843.1999.00159.x. [DOI] [PubMed] [Google Scholar]
- American Academy of Sleep Medicine Task Force report. Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- Arnulf I, Merino-Andreu M, Perrier A, Birolleau S, Similowski T, Derenne JP. Obstructive sleep apnea and venous thromboembolism. JAMA. 2002;287:2655–2656. doi: 10.1001/jama.287.20.2655. [DOI] [PubMed] [Google Scholar]
- Basner RC, Simon PM, Schwartzstein RM, Weinberger SE, Weiss JW. Breathing route influences upper airway muscle activity in awake normal adults. J Appl Physiol. 1989;66:1766–1771. doi: 10.1152/jappl.1989.66.4.1766. [DOI] [PubMed] [Google Scholar]
- Baydur A, Behrakis PK, Zin WA, Jaeger M, Milic-Emili J. A simple method for assessing the validity of the esophageal balloon technique. Am Rev Respir Dis. 1982;126:788–791. doi: 10.1164/arrd.1982.126.5.788. [DOI] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- Bureau MP, Series F. Comparison of two in-laboratory titration methods to determine effective pressure levels in patients with obstructive sleep apnoea. Thorax. 2000;55:741–745. doi: 10.1136/thorax.55.9.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Kayser B, Yan S, Macklem PT. Twitch transdiaphragmatic pressure depends critically on thoracoabdominal configuration. J Appl Physiol. 2000;88:54–60. doi: 10.1152/jappl.2000.88.1.54. [DOI] [PubMed] [Google Scholar]
- Chin K, Ohi M. New insights into the therapy and pathophysiology of patients with obstructive sleep apnoea syndrome. Respirology. 1998;3:139–143. doi: 10.1111/j.1440-1843.1998.tb00112.x. [DOI] [PubMed] [Google Scholar]
- Chin K, Ohi M, Kita H, Noguchi T, Otsuka N, Tsuboi T, Mishima M, Kuno K. Effects of NCPAP therapy on fibrinogen levels in obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 1996;153:1972–1976. doi: 10.1164/ajrccm.153.6.8665063. [DOI] [PubMed] [Google Scholar]
- Derderian SS, Bridenbaugh RH, Rajagopal KR. Neuropsychologic symptoms in obstructive sleep apnea improve after treatment with nasal continuous positive airway pressure. Chest. 1988;94:1023–1027. doi: 10.1378/chest.94.5.1023. [DOI] [PubMed] [Google Scholar]
- Eisenher I, Noachtar S. Haematological aspects of obstructive sleep apnoea. Sleep Med Rev. 2001;5:207–221. doi: 10.1053/smrv.2001.0158. [DOI] [PubMed] [Google Scholar]
- Guilleminault C, Stoohs R, Clerk A, Cetel M, Maistros P. A cause of excessive daytime sleepness: the upper airway resistance syndrome. Chest. 1993;104:781–787. doi: 10.1378/chest.104.3.781. [DOI] [PubMed] [Google Scholar]
- Guilleminault C, Tikian A, Dement W. The sleep apnea syndrome. Annu Rev Med. 1976;27:465–464. doi: 10.1146/annurev.me.27.020176.002341. [DOI] [PubMed] [Google Scholar]
- Hoffstein V, Mateika S. Predicting nasal continuous positive airway pressure. Am J Respir Crit Care Med. 1994;150:486–488. doi: 10.1164/ajrccm.150.2.8049834. [DOI] [PubMed] [Google Scholar]
- Hudgel D. Variable site of airway narrowing among obstructive sleep apnea patients. J Appl Physiol. 1986;61:1403–1409. doi: 10.1152/jappl.1986.61.4.1403. [DOI] [PubMed] [Google Scholar]
- Hudgel DW, Harasick T. Fluctuation in timing of upper airway and chest wall inspiratory muscle activity in obstructive sleep apnea. J Appl Physiol. 1990;69:443–450. doi: 10.1152/jappl.1990.69.2.443. [DOI] [PubMed] [Google Scholar]
- Hudgel DW, Hendricks C, Hamilton HB. Characteristics of the upper airway pressure-flow relationship during sleep. J Appl Physiol. 1988;64:1930–1935. doi: 10.1152/jappl.1988.64.5.1930. [DOI] [PubMed] [Google Scholar]
- Isono S, Morrison DL, Launois SH, Feroah TR, Whitelaw WA, Remmers JE. Static mechanics of the velopharynx of patients with obstructive sleep apnea. J Appl Physiol. 1993;75:148–154. doi: 10.1152/jappl.1993.75.1.148. [DOI] [PubMed] [Google Scholar]
- Lamphere J, Roehrs T, Wittig R, Zorick F, Conway WA, Roth T. Recovery of alertness after CPAP in apnea. Chest. 1989;96:1364–1367. doi: 10.1378/chest.96.6.1364. [DOI] [PubMed] [Google Scholar]
- Lloberes P, Levy G, Descals C, Sampol G, Roca A, Sagales T, de la Calzada MD. Self-reported sleepiness while driving as a risk factor for traffic accidents in patients with obstructive sleep apnoea syndrome and in non-apnoeic snorers. Respir Med. 2000;94:971–976. doi: 10.1053/rmed.2000.0869. [DOI] [PubMed] [Google Scholar]
- Meurice JC, Paquereau J, Denjean A, Patte F, Series F. Influence of correction of flow limitation on continuous positive airway pressure efficiency in sleep apnoea/hypopnoea syndrome. Eur Respir J. 1998;11:1121–1127. doi: 10.1183/09031936.98.11051121. [DOI] [PubMed] [Google Scholar]
- Miljeteig H, Hoffstein V. Determinants of continuous positive airway pressure level for treatment of obstructive sleep apnea. Am Rev Respir Dis. 1993;147:1526–1530. doi: 10.1164/ajrccm/147.6_Pt_1.1526. [DOI] [PubMed] [Google Scholar]
- Mills GH, Kyroussis D, Hamnegard CH, Polkey MI, Green M, Moxham J. Bilateral magnetic stimulation of the phrenic nerves from an anterolateral approach. Am J Respir Crit Care Med. 1996;154:1099–1105. doi: 10.1164/ajrccm.154.4.8887614. [DOI] [PubMed] [Google Scholar]
- Olson LG, King MT, Hensley MJ, Saunders NA. A community study of snoring and sleep-disordered breathing symptoms. Am J Respir Crit Care Med. 1995;152:707–710. doi: 10.1164/ajrccm.152.2.7633730. [DOI] [PubMed] [Google Scholar]
- Passing H, Bablok W. A new biometrical procedure for testing the equality of measurements from two different analytical methods. Application of linear regression procedures for method comparison studies in clinical chemistry, Part I. J Clin Chem Clin Biochem. 1983;21:709–720. doi: 10.1515/cclm.1983.21.11.709. [DOI] [PubMed] [Google Scholar]
- Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- Rapoport DM, Garay SM, Goldring RM. Nasal CPAP in obstructive sleep apnea: mechanisms of action. Bull Eur Physiopathol Respir. 1983;19:616–620. [PubMed] [Google Scholar]
- Remmers JE, Degroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol. 1978;44:931–938. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- Rohrer F. The resistance in the human airway and the influence of branching of bronchial systems on frequency of breathing at different lung volumes. Pflügers Arch. 1915;162:255–299. [Google Scholar]
- Schwartz AR, Smith PL, Wise RA, Bankman I, Permutt S. Effect of positive nasal pressure on upper airway pressure-flow relationships. J Appl Physiol. 1989;66:1626–1634. doi: 10.1152/jappl.1989.66.4.1626. [DOI] [PubMed] [Google Scholar]
- Series F. Accuracy of an unattended home CPAP titration in the treatment of obstructive sleep apnea. Am J Respir Crit Care Med. 2000;162:94–97. doi: 10.1164/ajrccm.162.1.9908023. [DOI] [PubMed] [Google Scholar]
- Series F, Cormier Y, La Forge J, Desmeules M. Mechanisms of the effectiveness of continuous positive airway pressure in obstructive sleep apnea. Sleep. 1992;15:S47–49. [PubMed] [Google Scholar]
- Series F, Demoule A, Marc I, Sanfacon C, Derenne JP, Similowski T. Inspiratory flow dynamics during phrenic nerve stimulation in awake normals during nasal breathing. Am J Respir Crit Care Med. 1999;160:614–620. doi: 10.1164/ajrccm.160.2.9812036. [DOI] [PubMed] [Google Scholar]
- Series F, Marc I. Nasal pressure recording in the diagnosis of sleep apnoea hypopnoea syndrome. Thorax. 1999;54:506–510. doi: 10.1136/thx.54.6.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Series F, Marc I, Atton L. Comparison of snoring measured at home and during polysomnographic studies. Chest. 1993;103:1769–1773. doi: 10.1378/chest.103.6.1769. [DOI] [PubMed] [Google Scholar]
- Series F, Straus C, Demoule A, Attali V, Arnulf I, Derenne JP, Similowski T. Assessment of upper airway dynamics in awake patients with sleep apnea using phrenic nerve stimulation. Am J Respir Crit Care Med. 2000;162:795–800. doi: 10.1164/ajrccm.162.3.9906135. [DOI] [PubMed] [Google Scholar]
- Sforza E, Krieger J, Bacon W, Petiau C, Zamagni M, Boudewijns A. Determinants of effective continuous positive airway pressure in obstructive sleep apnea. Role of respiratory effort. Am J Respir Crit Care Med. 1995;151:1852–1856. doi: 10.1164/ajrccm.151.6.7767530. [DOI] [PubMed] [Google Scholar]
- Skatrud JB, Dempsey JA. Airway resistance and respiratory muscle function in snorers during NREM sleep. J Appl Physiol. 1985;59:328–335. doi: 10.1152/jappl.1985.59.2.328. [DOI] [PubMed] [Google Scholar]
- Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1:862–865. doi: 10.1016/s0140-6736(81)92140-1. [DOI] [PubMed] [Google Scholar]
- Verin E, Series F, Locher C, Straus C, Zelter M, Derenne JP, Similowski T. Effects of neck flexion and mouth opening on inspiratory flow dynamics in awake humans. J Appl Physiol. 2002a;92:84–92. doi: 10.1152/jappl.2002.92.1.84. [DOI] [PubMed] [Google Scholar]
- Verin E, Straus C, Demoule A, Mialon P, Derenne JP, Similowski T. Validation of improved recording site to measure phrenic conduction from surface electrodes in humans. J Appl Physiol. 2002b;92:967–974. doi: 10.1152/japplphysiol.00652.2001. [DOI] [PubMed] [Google Scholar]


