The idea that the thalamus is merely a relay station for incoming signals in their route to the cerebral cortex is no longer tenable since accumulating data indicate that the responses of thalamic neurons to sensory stimuli display higher selectivity and larger coefficients of variation than responses recorded from prethalamic input sources. The higher complexity of thalamic responses suggests that inhibitory neurons play an important role in discriminative and integrative processes (Steriade, 2001a). During the 1960s and 1970s, some investigators postulated that thalamocortical neurons are disinhibited upon natural awakening or forebrain activation induced by brainstem reticular stimulation, through inhibition of inhibitory thalamic neurons. This was an embarrassing conclusion since brain operations during wakefulness are associated with the blockade of prolonged inhibitory potentials, which generate long-range oscillations during slow-wave sleep, but require preservation of short inhibitory postsynaptic potentials (IPSPs), which may underlie the accurate analysis of rapidly recurring signals during an adaptive behavioural state.
One of the two GABAergic cell classes, thalamic reticular and local-circuit neurons, which may assist thalamocortical neurons with discriminatory functions, is the topic of the article by Perreault et al. (2003) in this issue of The Journal of Physiology. The authors address the development of dendritic branching of local interneurons in the rat lateral geniculate nucleus, and report that the development of GABAA- and GABAB-mediated IPSPs in thalamocortical neurons is associated with the dendritic maturation of presynaptic interneurons. This is an important topic since it has previously been shown that the inhibitory surround in the receptive field of kittens’ lateral geniculate neurons is weaker than in adult cats.
The complexity of thalamic IPSPs transcends the biphasic GABAA-B sequence of IPSPs, first described in thalamic slices (Hirsch & Burnod, 1987; Crunelli et al. 1988). The dendritic appendages of interneurons are equipped with presynaptic vesicles and they contact, within thalamic glomeruli, the dendrites of thalamocortical neurons and other local-circuit cells. The importance of this intraglomerular circuitry resides in the discrete localization of inhibition. As direct recordings from presynaptic dendrites are not yet feasible, their possible role has been determined by analysing in somatic impalements the responses of thalamocortical neurons in anterior nuclei of the cat (Paré et al. 1991), a species in which these nuclei do not receive afferents from the other source of GABAergic input, thalamic reticular neurons (Steriade et al. 1984). Our study revealed that stimulation of mammillothalamic neurons gives rise in anterior thalamic neurons to an early, short-lasting, Cl−-dependent IPSP (termed the a-IPSP), ascribed to intraglomerular operations of presynaptic dendrites, and distinct from the following biphasic sequence of IPSPs with longer durations, produced by the axonal firing of interneurons and mediated by GABAA and GABAB receptors. The top trace in Fig. 1 (modified from the study of Paré et al. 1991) shows the miniature GABAa IPSP in isolation, using minimal intensity of mammillary nucleus stimulation, while the bottom trace shows all three IPSPs (GABAa followed by GABAA-B) evoked by increasing the stimulation intensity. Subsequently, the ‘miniature’ (GABAa) IPSP was also detected in thalamocortical neurons from the lateral geniculate nucleus, in response to optic tract stimulation (Soltesz & Crunelli, 1992).
Figure 1.
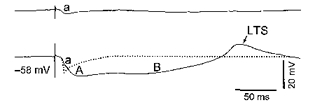
LTS, low-threshold spike.
What is the evidence that the earliest IPSP is crucially important for inhibitory sculpting, an operation that basically takes place during waking and attentive states? During wakefulness, mesopontine cholinergic neurons with thalamic projections discharge at high rates (Steriade et al. 1990). The enhanced release of acetylcholine in the thalamus (Williams et al. 1994) hyperpolarizes local interneurons and suppresses their bursting firing through muscarinic action (Zhu & Heggelund, 2001). However, the presynaptic dendrites of local interneurons may continue to participate in the regulation of afferent excitatory inputs. Indeed, stimulation of mesopontine cholinergic nuclei, the experimental model of brain awakening, leads to the reduction up to suppression of prolonged GABAA-B IPSPs, but does not affect and even enhances the ‘miniature’ GABAa IPSP (see Fig. 1, dotted line in the bottom trace). This dissociation between various GABA-mediated IPSPs upon forebrain activation (Curró Dossi et al. 1992) suggests that the earliest IPSP may be involved in the high-fidelity transfer of information because it prevents summation of EPSPs and its very short duration (less than 10 ms) ensures a rapid return of the membrane potential to resting level.
Further investigations, specifically directed to the developmental study of thalamic reticular neurons, are necessary to assess the contribution of the latter elements to the process of feedback inhibition in the visual thalamus. There has been a lot of speculation implicating thalamic reticular neurons in selective attention but, whereas intensive research has pointed to the crucial role played by these neurons in long-range sleep oscillations, research into their role in attentive and discriminatory functions at the neuronal level is still lacking. The problem is complicated by the projections of thalamic reticular neurons to local-circuit inhibitory cells, with yet unknown consequences (including disinhibition) at their ultimate targets, thalamocortical neurons. This circuit has generated a hypothesis of focusing attention (see Steriade, 2001b) that awaits experimental testing.
References
- Crunelli V, Haby M, Jassik-Gerschenfeld D, Leresche N, Pirchio M. J Physiol. 1988;399:153–176. doi: 10.1113/jphysiol.1988.sp017073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curró Dossi R, Pare D, Steriade M. Neurosci. 1992;47:279–289. doi: 10.1016/0306-4522(92)90244-v. [DOI] [PubMed] [Google Scholar]
- Hirsch JC, Burnod Y. Neurosci. 1987;23:457–468. doi: 10.1016/0306-4522(87)90069-8. [DOI] [PubMed] [Google Scholar]
- Paré D, Curro Dossi R, Steriade M. J Neurophysiol. 1991;66:190–1204. [Google Scholar]
- Perreault M-C, Qin Y, Heggelund P, Zhu JJ. J Physiol. 2003;546:137–148. doi: 10.1113/jphysiol.2002.030643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltesz I, Crunelli V. In: Progress in Brain Research. Mize RR, Marc RE, Sillito AM, editors. Vol. 90. Amsterdam: Elsevier; 1992. pp. 151–169. [DOI] [PubMed] [Google Scholar]
- Steriade M. The Intact and Sliced Brain. Cambridge, MA, USA: The MIT Press; 2001a. [Google Scholar]
- Steriade M. J Neurophysiol. 2001b;86:1–39. doi: 10.1152/jn.2001.86.1.1. [DOI] [PubMed] [Google Scholar]
- Steriade M, Datta S, Paré D, Oakson G, Curró Dossi R. J Neurosci. 1990;10:2541–2559. doi: 10.1523/JNEUROSCI.10-08-02541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Parent A, Hada J. J Comp Neurol. 1984;229:531–547. doi: 10.1002/cne.902290407. [DOI] [PubMed] [Google Scholar]
- Williams JA, Comisarow J, Day J, Fibiger HC, Reiner PB. J Neurosci. 1994;14:5236–5242. doi: 10.1523/JNEUROSCI.14-09-05236.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JJ, Heggelund P. J Neurosci. 2001;21:1148–1159. doi: 10.1523/JNEUROSCI.21-04-01148.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]


