Abstract
We determined whether mitogen-activated protein kinase (MAPK) and 5′-AMP-activated protein kinase (AMPK) signalling cascades are activated in response to intense exercise in skeletal muscle from six highly trained cyclists (peak O2 uptake (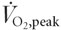 ) 5.14 ± 0.1 l min−1) and four control subjects (
) 5.14 ± 0.1 l min−1) and four control subjects (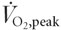 3.8 ± 0.1 l min−1) matched for age and body mass. Trained subjects completed eight 5 min bouts of cycling at ≈85% of
3.8 ± 0.1 l min−1) matched for age and body mass. Trained subjects completed eight 5 min bouts of cycling at ≈85% of 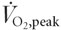 with 60 s recovery between work bouts. Control subjects performed four 5 min work bouts commencing at the same relative, but a lower absolute intensity, with a comparable rest interval. Vastus lateralis muscle biopsies were taken at rest and immediately after exercise. Extracellular regulated kinase (ERK1/2), p38 MAPK, histone H3, AMPK and acetyl CoA-carboxylase (ACC) phosphorylation was determined by immunoblot analysis using phosphospecific antibodies. Activity of mitogen and stress-activated kinase 1 (MSK1; a substrate of ERK1/2 and p38 MAPK) and α1 and α2 subunits of AMPK were determined by immune complex assay. ERK1/2 and p38 MAPK phosphorylation and MSK1 activity increased (P < 0.05) after exercise 2.6-, 2.1- and 2.0-fold, respectively, in control subjects and 1.5-, 1.6- and 1.4-fold, respectively, in trained subjects. Phosphorylation of histone H3, a substrate of MSK1, increased (P < 0.05) ≈1.8-fold in both control and trained subject. AMPKα2 activity increased (P < 0.05) after exercise 4.2- and 2.3-fold in control and trained subjects, respectively, whereas AMPKα1 activity was not altered. Exercise increased ACC phosphorylation (P < 0.05) 1.9- and 2.8-fold in control and trained subjects. In conclusion, intense cycling exercise in subjects with a prolonged history of endurance training increases MAPK signalling to the downstream targets MSK1 and histone H3 and isoform-specific AMPK signalling to ACC. Importantly, exercise-induced signalling responses were greater in untrained men, even at the same relative exercise intensity, suggesting muscle from previously well-trained individuals requires a greater stimulus to activate signal transduction via these pathways.
with 60 s recovery between work bouts. Control subjects performed four 5 min work bouts commencing at the same relative, but a lower absolute intensity, with a comparable rest interval. Vastus lateralis muscle biopsies were taken at rest and immediately after exercise. Extracellular regulated kinase (ERK1/2), p38 MAPK, histone H3, AMPK and acetyl CoA-carboxylase (ACC) phosphorylation was determined by immunoblot analysis using phosphospecific antibodies. Activity of mitogen and stress-activated kinase 1 (MSK1; a substrate of ERK1/2 and p38 MAPK) and α1 and α2 subunits of AMPK were determined by immune complex assay. ERK1/2 and p38 MAPK phosphorylation and MSK1 activity increased (P < 0.05) after exercise 2.6-, 2.1- and 2.0-fold, respectively, in control subjects and 1.5-, 1.6- and 1.4-fold, respectively, in trained subjects. Phosphorylation of histone H3, a substrate of MSK1, increased (P < 0.05) ≈1.8-fold in both control and trained subject. AMPKα2 activity increased (P < 0.05) after exercise 4.2- and 2.3-fold in control and trained subjects, respectively, whereas AMPKα1 activity was not altered. Exercise increased ACC phosphorylation (P < 0.05) 1.9- and 2.8-fold in control and trained subjects. In conclusion, intense cycling exercise in subjects with a prolonged history of endurance training increases MAPK signalling to the downstream targets MSK1 and histone H3 and isoform-specific AMPK signalling to ACC. Importantly, exercise-induced signalling responses were greater in untrained men, even at the same relative exercise intensity, suggesting muscle from previously well-trained individuals requires a greater stimulus to activate signal transduction via these pathways.
Members of the mitogen-activated protein kinase (MAPK) signalling cascades integrate intracellular signals from diverse extracellular stimuli, including growth factors and/or cellular stress, to regulate gene transcription and protein synthesis in various cell culture systems (Cohen, 1997). At least three parallel MAPK signalling cascades, including extracellular regulated kinase (ERK1/2; p42/p44 MAPK), p38 MAPK and c-jun NH2-terminal kinase (JNK), are activated in skeletal muscle in response to exercise, a physiological form of stress, in both moderately trained (Boppart et al. 2000; Yu et al. 2001) and untrained subjects (Aronson et al. 1997b; Widegren et al. 1998; Boppart et al. 1999). Exercise also increases activity of MAPK substrates, including mitogen- and stress-activated protein kinase (MSK) 1, MSK2, p90 ribosomal S6 kinase (p90rsk, also known as MAPKAPK1) and MAPK-activated protein kinase 2 (MAPKAPK2) (Krook et al. 2000). These enzymes are directly activated by ERK1/2 and p38 MAPK in rat skeletal muscle in response to contraction (Ryder et al. 2000) and in cultured cells in response to growth factors or stress (Cuenda et al. 1995; Beyaert et al. 1996; Zhao et al. 1996; Deak et al. 1998). Collectively, these findings offer support for the hypothesis that contraction-induced MAPK signalling increases transcriptional activity, as these kinases have been directly implicated in the phosphorylation of transcription factors (Cohen, 1997; Deak et al. 1998).
MAPK signalling pathways act directly on chromatin components to regulate DNA-template processes (Cheung et al. 2000). Activated MSK1 phosphorylates histone H3 (Thomson et al. 1999a), a member of a highly conserved family of proteins that assemble with DNA to form nucleosomes. This finding is intriguing since the timing of histone H3 phosphorylation closely corresponds to the transient expression of activated immediate-early genes (Thomson et al. 1999a), suggesting histone modification is linked to transcription activation. However, histone H3 phosphorylation in response to a physiological stimulus such as exercise has yet to be determined.
In addition to MAPK cascades, AMP-activated protein kinase (AMPK) has recently been identified as a candidate signal transducer involved in transcriptional regulation by repressing genes involved in the glucose-signalling system in hepatocytes (Salt et al. 1998; Woods et al. 2000) and up-regulating genes involved in glucose uptake and substrate metabolism in skeletal muscle (Holmes et al. 1999; Ojuka et al. 2000; Winder et al. 2000), even in severely diabetic rodents (Song et al. 2002). Recent evidence suggests AMPK may activate other downstream effectors such as p38 MAPK and mitogen-activated protein kinase kinase 3 (MKK3) (Xi et al. 2001), providing a link between MAPK and AMPK pathways. AMPK is activated by cellular stress associated with ATP depletion (Hardie & Carling, 1997) and is likely to be one of several critical regulators of mitogenic and metabolic events in response to exercise in skeletal muscle (Mu et al. 2001). Low-to-moderate intensity aerobic exercise (< 70 % of 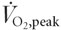 ) induces an isoform-specific and intensity-dependent increase in AMPKα2, but not AMPKα1 activity in moderately trained subjects (Fujii et al. 2000; Stephens et al. 2002; Wojtaszewski et al. 2000). This may be related to the finding that AMPK complexes containing the α2 rather than the α1 isoform have a greater dependence on AMP (Salt et al. 1998; Chen et al. 2000). Conversely, activity of AMPKα1 and α2 are increased in response to a 30 s ‘all-out’ sprint requiring power outputs 2- to 3-fold greater than attained during maximal aerobic exercise (Chen et al. 2000), consistent with the observation that rapid fuel depletion increases the activity of both α1 and α2 isoforms of the AMPK catalytic subunit in skeletal muscle (Hayashi et al. 2000). The in vivo regulation of AMPK and the downstream substrate, acetyl CoA-carboxylase (ACC), has not been determined in skeletal muscle from highly trained athletes capable of sustaining both high relative and absolute power outputs. Accordingly, trained athletes may have a greater capacity to avoid a negative energy balance in skeletal muscle during intense exercise and may have a differentiated AMPK response compared with less well-trained individuals.
) induces an isoform-specific and intensity-dependent increase in AMPKα2, but not AMPKα1 activity in moderately trained subjects (Fujii et al. 2000; Stephens et al. 2002; Wojtaszewski et al. 2000). This may be related to the finding that AMPK complexes containing the α2 rather than the α1 isoform have a greater dependence on AMP (Salt et al. 1998; Chen et al. 2000). Conversely, activity of AMPKα1 and α2 are increased in response to a 30 s ‘all-out’ sprint requiring power outputs 2- to 3-fold greater than attained during maximal aerobic exercise (Chen et al. 2000), consistent with the observation that rapid fuel depletion increases the activity of both α1 and α2 isoforms of the AMPK catalytic subunit in skeletal muscle (Hayashi et al. 2000). The in vivo regulation of AMPK and the downstream substrate, acetyl CoA-carboxylase (ACC), has not been determined in skeletal muscle from highly trained athletes capable of sustaining both high relative and absolute power outputs. Accordingly, trained athletes may have a greater capacity to avoid a negative energy balance in skeletal muscle during intense exercise and may have a differentiated AMPK response compared with less well-trained individuals.
The present study was undertaken to test the hypothesis that intense exercise in highly trained individuals activates kinase cascades involving parallel MAPKs (ERK1/2 and p38) and the downstream substrate MSK1. Furthermore, we assessed signal transduction at the level of AMPK (α2 and α1 isoform-specific activity) and the downstream substrate ACC. Finally, we determined whether histone H3 is phosphorylated in response to exercise. Exercise effects on signal transduction were compared between highly trained athletes and untrained control subjects. Analysis of exercise-induced responses on MAPK and AMPK cascades in skeletal muscle from athletes with a prolonged history of endurance training versus untrained control subjects will provide additional support for a physiological role of these cascades in the regulation of mitogenic and metabolic events.
Methods
Subjects
Six highly trained endurance cyclists (aged 27 ± 2 years, body mass 76.3 ± 1.2 kg, peak O2 uptake (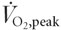 ) 5.14 ± 0.1 l min−1; mean ± s.e.m.), who were riding 368 ± 141 km week−1 and who had not undertaken any high-intensity interval training during the 4 weeks prior to investigation, were recruited to participate in this study. The trained subjects had participated in previous studies (Stepto et al. 2001) and were familiar with testing procedures. Four untrained control subjects (aged 25 ± 3 years, body mass 76.1 ± 7.3 kg,
) 5.14 ± 0.1 l min−1; mean ± s.e.m.), who were riding 368 ± 141 km week−1 and who had not undertaken any high-intensity interval training during the 4 weeks prior to investigation, were recruited to participate in this study. The trained subjects had participated in previous studies (Stepto et al. 2001) and were familiar with testing procedures. Four untrained control subjects (aged 25 ± 3 years, body mass 76.1 ± 7.3 kg, 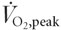 3.8 ± 0.1 l min−1, mean ± s.e.m.), who were not participating in any formal exercise programme, were also studied. All subjects were fully informed of the potential risks involved in the study before providing their written consent. The Human Research Ethics Committee of RMIT University and the Ethics Committee of the Australian Institute of Sport approved this study. All experiments were carried out in accordance with the Declaration of Helsinki.
3.8 ± 0.1 l min−1, mean ± s.e.m.), who were not participating in any formal exercise programme, were also studied. All subjects were fully informed of the potential risks involved in the study before providing their written consent. The Human Research Ethics Committee of RMIT University and the Ethics Committee of the Australian Institute of Sport approved this study. All experiments were carried out in accordance with the Declaration of Helsinki.
Preliminary exercise trials
On the first visit to the laboratory, subjects participated in a 10 min self-paced warm-up, followed by a maximal, incremental cycle test to exhaustion on an electro-magnetically braked ergometer (Lode, Gronigen, the Netherlands). The test protocol has been described in detail previously (Hawley & Noakes, 1992). A first-principles calibration rig was used to evaluate the accuracy and reliability of the cycle ergometer. Expected power output (W) was within ±2 % of actual output from 200 to 800 W when the pedal frequency was between 90 and 140 r.p.m. Throughout the maximal exercise test and for parts of the subsequently described intense cycling exercise protocol, subjects inspired air through a two-way Hans Rudolf valve attached to a custom-built automated Douglas bag gas analysis system (Australian Institute of Sport, ACT, Australia), which incorporated O2 and CO2 analysers (Ametek N-22 electrochemical O2 sensor, model S3A, and Ametek P-61B infrared CO2 sensor, Applied Electrochemistry, Ametek Instruments, Pittsburgh, PA, USA) and two Tissot gasometers (Warren E. Collins Inc., Braintree, MA, USA) interfaced to an IBM personal computer by Optical Rotary Encoders (RS 341-597, Berne, Switzerland) that calculated the rate of O2 consumption (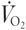 ), CO2 production (
), CO2 production (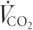 ), minute ventilation (
), minute ventilation (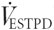 ) and the respiratory exchange ratio (RER) every 30 s from conventional equations. Before each maximal test and all subsequent experimental trials, the analysers were calibrated with commercially available gases of known O2 and CO2 content. Before and after the study an automated high-capacity calibrator for open-circuit indirect calorimetry was used to simultaneously check the gas analysers, volume device and software of the custom-built system (Gore et al. 1997). This device can calibrate to the high ventilation volumes (≈100 l min−1) measured when well-trained athletes work for sustained periods at high (80-90 % of
) and the respiratory exchange ratio (RER) every 30 s from conventional equations. Before each maximal test and all subsequent experimental trials, the analysers were calibrated with commercially available gases of known O2 and CO2 content. Before and after the study an automated high-capacity calibrator for open-circuit indirect calorimetry was used to simultaneously check the gas analysers, volume device and software of the custom-built system (Gore et al. 1997). This device can calibrate to the high ventilation volumes (≈100 l min−1) measured when well-trained athletes work for sustained periods at high (80-90 % of 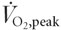 ) exercise intensities.
) exercise intensities. 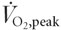 was defined as the highest O2 uptake a subject attained during two consecutive 30 s sampling periods. The results of the maximal test were used to determine the power output that corresponded to ≈85 % of each subject's
was defined as the highest O2 uptake a subject attained during two consecutive 30 s sampling periods. The results of the maximal test were used to determine the power output that corresponded to ≈85 % of each subject's 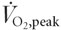 for the subsequently described intense cycling exercise protocol.
for the subsequently described intense cycling exercise protocol.
Subject training status and dietary control
Training and nutritional status of the subjects was controlled 24 h prior to an experimental trial to standardise muscle and liver glycogen stores. All subjects reported to the laboratory between 07.00 and 08.00 h the day before an experiment and completed a 60 min ride at ≈60 % of individual 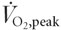 . Subjects were then provided with a standard diet of 50 kcal (kg body mass)−1 (BM), composed of 63 % carbohydrate (8 g (kg BM)−1), 20 % fat and 17 % protein, to be consumed over the next 24 h. During this time the trained subjects refrained from any further training activity, while the control subjects were instructed to avoid participation in all strenuous physical activity.
. Subjects were then provided with a standard diet of 50 kcal (kg body mass)−1 (BM), composed of 63 % carbohydrate (8 g (kg BM)−1), 20 % fat and 17 % protein, to be consumed over the next 24 h. During this time the trained subjects refrained from any further training activity, while the control subjects were instructed to avoid participation in all strenuous physical activity.
Experimental cycling trial and muscle biopsy procedure
Subjects reported to the laboratory between 07.00 and 08.00 h after an overnight fast. The subjects agreed to undergo two muscle biopsies, one immediately before and one after exercise. Accordingly, the demands of the entire intense cycling exercise protocol (described subsequently), rather than the metabolic response to a single 5 min work bout, were assessed. A Teflon cannula was inserted in the forearm antecubital vein for rapid continuous blood sampling via a sterile stop-cock. The cannula was regularly flushed with 0.9 % sterile saline to keep the vein patent. Blood samples (≈5 ml) were taken immediately after the warm-up and at the end of the final work bout and immediately analysed for blood lactate concentration. After local anaesthesia had been administered to the skin, subcutaneous tissue and fascia of the vastus lateralis, a resting biopsy (≈150 mg) was taken using a 6 mm Bergström needle (Bergström, 1962) with suction applied (Evans et al. 1982). Muscle was frozen (17 ± 3 s) in liquid nitrogen and stored at −80 °C until subsequent analysis. At this time, a separate site on the same leg (≈5 cm distal) was prepared for the second biopsy.
Subjects then mounted the cycle ergometer and commenced a standardised warm-up, consisting of 2 min at 30 %, 55 % and 65 % of 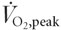 . After a 2 min rest, during which the subjects remained seated on the ergometer, the intense cycling exercise protocol was initiated. The protocol for the trained subjects consisted of eight 5 min work bouts at ≈85%
. After a 2 min rest, during which the subjects remained seated on the ergometer, the intense cycling exercise protocol was initiated. The protocol for the trained subjects consisted of eight 5 min work bouts at ≈85% 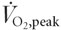 , with 60 s active recovery (a work:rest ratio of 5:1). Because control subjects were unable to exercise at the same absolute power output as the well-trained athletes, they commenced the intense cycling protocol at the same relative intensity, with an identical work:rest interval for four 5 min work bouts. The vast majority of cross-sectional investigations that have compared physiological responses with exercise between untrained and trained subjects have employed exercise protocols performed at the same percentage of
, with 60 s active recovery (a work:rest ratio of 5:1). Because control subjects were unable to exercise at the same absolute power output as the well-trained athletes, they commenced the intense cycling protocol at the same relative intensity, with an identical work:rest interval for four 5 min work bouts. The vast majority of cross-sectional investigations that have compared physiological responses with exercise between untrained and trained subjects have employed exercise protocols performed at the same percentage of 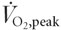 (for review see Coggan & Williams, 1995). During the 60 s recovery between work bouts, trained subjects cycled at a workload of ≈100 W, while control subjects cycled at a power output of ≈50 W. Subjects were allowed to ingest water ad libitum throughout exercise.
(for review see Coggan & Williams, 1995). During the 60 s recovery between work bouts, trained subjects cycled at a workload of ≈100 W, while control subjects cycled at a power output of ≈50 W. Subjects were allowed to ingest water ad libitum throughout exercise.
Tissue processing
Skeletal muscle biopsies (60-70 mg) were homogenised in ice-cold buffer (50 mm Tris HCl, 0.1 % Trition X-100, 1 mm EDTA, 1 mm EGTA, 50 mm NaF, 5 mm Na2P2O7, 10 mm glycerophosphate, 1 mm Na3VO4, 1 μm microcystin, 0.1 % β-mercaptoethanol, 1 mm benzimidine and 0.1 mm PMSF). All reagents were analytical grade (Sigma, St Louis, MI, USA), unless otherwise specified. Homogenates were rotated for 60 min at 4 °C, centrifuged at 12 000 g for 10 min at 4 °C, and the protein concentration of the resulting supernatant determined using a commercial kit (Bio-Rad, Richmond, CA, USA). For preparation of nuclear proteins for the histone H3 analysis, a separate piece of muscle biopsy was homogenised in ice-cold buffer. Homogenates were subjected to sonification (twice for 10 s at medium power). Muscle lysate (1 mg protein in 700 μl buffer) was digested by addition of 18 μl of stock DNAse (final concentration 80 000 kU ml−1). Samples were rotated for 30 min at 4 °C.
Immunoblot analysis
Aliquots of muscle lysate (40 μg for ERK1/2, p38, AMPK and ACC) or nuclear extracts (100 μg for histone H3) were mixed with Laemmli sample buffer, and proteins were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE; 6 % for ACC, 10 % for ERK1/2, p38 and AMPK, and 12 % for histone H3). Following electrophoresis, proteins were transferred to polyvinylidenedifluoride (PVDF) membranes (Millipore, Bedford, MA, USA). Membranes were blocked in TBST (10 mm Tris, 100 mm NaCl, 0.02 % Tween-20) containing 7.5 % non-fat milk for 2 h at room temperature, washed with TBST for 10 min, and then incubated with appropriate primary antibody overnight at 4 °C. To determine ERK1/2 phosphorylation, membranes were immunoblotted with a phosphospecific antibody that recognises ERK1/2 (p42/44 MAPK) kinase when phosphorylated at Thr202 and Tyr204 (New England Biolabs, Beverly, MA, USA). To determine p38 MAPK phosphorylation, membranes were immunoblotted with a phosphospecific p38 MAPK antibody that recognises p38 MAPK phosphorylated at Thr180 and Tyr182 (New England Biolabs). For histone H3 phosphorylation, membranes were immunoblotted with a phosphospecific antibody that recognises histone H3 when phosphorylated at Ser10 (Upstate Biotechnology, Lake Placid, NY, USA). To determine AMPK phosphorylation, membranes were immunoblotted with a phosphospecific antibody that recognises the AMPK pan-α subunit phosphorylated at Thr172 (Cell Signaling Technology Inc., Beverly, MA, USA). For ACC phosphorylation, membranes were immunoblotted with a phosphospecific antibody raised against a peptide corresponding to the sequence in rat ACCα containing the Ser79 phosphorylation site that also recognises human ACCβ phosphorylated at Ser221 (Upstate Biotechnology). Following incubation with primary antibodies, membranes were washed with TBST and incubated with appropriate secondary antibody for 1 h at room temperature, followed by washing in TBST. Immunoreactive proteins were detected using enhanced chemiluminescence (ECL) reagents (Amersham, Arlington Heights, IL, USA) and quantified by densitometric scanning. Horseradish peroxidase-conjugated goat anti-rabbit and anti-mouse immunoglobulin G was from Bio-Rad Laboratories (Richmond, CA, USA).
MSK1 activity
Muscles were homogenised in an ice-cold buffer as described above for ‘Immunoblot analysis’. Aliquots of the supernatant (500 μg) were immunoprecipitated with an anti-MSK1 antibody coupled to protein G sepharose for 1 h at 4 °C. Characteristics of the MSK1 antibody have been described previously (Alessi et al. 1995; Clifton et al. 1996; Deak et al. 1998). Immunoprecipitates were then washed three times in ice-cold buffer A (0.5 m NaCl, 50 mm Tris HCl, pH 7.5, 0.1 % Triton X-100, 1 mm EDTA, 1 mm EGTA, 50 mm NaF, 5 mm Na2P2O7, 10 mm β-glycerophosphate, 1 mm Na3VO4, 1 μm microcystin, 0.1 % β-mercaptoethanol), twice in ice-cold buffer B (50 mm Tris HCl, pH 7.5, 0.03 % Brij 35, 0.1 mm EGTA, 0.1 % β-mercaptoethanol), and resuspended in 30 μl of buffer C (50 mm Tris HCl, pH 7.5, 16.7 mm Mg(C2H3O2)24H2O, 50 μm peptide substrate (Crosstide), 0.1 mm EGTA, 0.1 % β-mercaptoethanol, 17 μm PKI, 20 μCi [γ32P]ATP). After incubation (10 min at 30 °C), the reaction was terminated by incubation on ice. Incorporation of 32P into the peptide substrate was determined by resolving the products on a 40 % acrylamide gel. The gel was visualised on a PhosphorImager (Bio-Rad, Richmond CA, USA), and the band corresponding to the peptide substrate was quantified.
AMPK activity
AMPK was immunoprecipitated from aliquots of muscle lysate (400 μg) by incubation with either anti-α1, or anti-α2 antibody bound to protein G-sepharose (St Louis, MO, USA) for 2 h at 4 °C. Sheep antibodies against the rat AMPKα1 and -α2 subunits were produced as described previously (Woods et al. 1996). Immune complexes were collected by brief centrifugation and washed extensively in buffer C (50 mm Tris HCl (pH 7.5), 50 mm NaF, 5 mm sodium pyrophosphate, 1 mm EDTA, 1 mm DTT, 10 % glycerol and 1 % Triton X-100). AMPK activity in the immune complex was determined by phosphorylation of the SAMS (full sequence: HMRSAMSGLHLVKRR) synthetic peptide substrate (Woods et al. 1996) as described previously (Fryer et al. 2000). Kinase reactions performed in reaction buffer (40 mmol l−1 Hepes buffer, pH 7.0, 0.2 mmol l−1 SAMS peptide, 0.2 mmol l−1 AMP, 80 mmol l−1 NaCl, 0.8 mmol l−1 DTT, 5 mmol l−1 MgCl2, 0.2 mmol l−1 ATP (containing 2 μCi [γ32P]ATP). Reactions were incubated on a vibrating platform for 60 min at 30 °C and terminated by centrifugation (9000 g, 30 s). Immediately thereafter, aliquots were spotted onto phosphocellulose units (Pierce, Rockford, IL, USA). Phosphocellulose P81 cation exchange paper was washed with 1 % phosphoric acid according to the manufacturer specifications. Incorporation of 32P into the peptide substrate was measured by liquid scintillation counting.
Statistics
All data are presented as means ± s.e.m. Differences were determined using non-parametric statistics (Sign test) for values obtained at rest versus those obtained after intense cycling exercise. Significance was accepted at P < 0.05.
Results
Responses to intense cycling exercise
All highly trained subjects completed eight 5 min bouts of intense cycling at a power output of 334 ± 18 W. This exercise protocol elicited an average relative intensity of 86 ± 2 % of 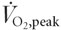 . At the end of the eighth work bout, RER was 0.92 ± 0.04 units, and blood lactate concentration was 4.7 ± 1.7 mmol l−1. Employing an identical exercise protocol and subjects of equivalent training status, we have previously reported a significant correlation between blood lactate concentration and the prevailing muscle lactate concentration (r = 0.91, P < 0.05) and a reduction in muscle glycogen content of ≈50 % (Stepto et al. 2001). Conversely, the control subjects were only able to complete four 5 min bouts of intense cycling at a power output of 241 ± 11 W. At the completion of the fourth work bout, the relative exercise intensity had increased from 84 ± 1 % (bout 1) to 91 ± 1 % of
. At the end of the eighth work bout, RER was 0.92 ± 0.04 units, and blood lactate concentration was 4.7 ± 1.7 mmol l−1. Employing an identical exercise protocol and subjects of equivalent training status, we have previously reported a significant correlation between blood lactate concentration and the prevailing muscle lactate concentration (r = 0.91, P < 0.05) and a reduction in muscle glycogen content of ≈50 % (Stepto et al. 2001). Conversely, the control subjects were only able to complete four 5 min bouts of intense cycling at a power output of 241 ± 11 W. At the completion of the fourth work bout, the relative exercise intensity had increased from 84 ± 1 % (bout 1) to 91 ± 1 % of 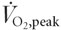 . RER values at this time were 1.03 ± 0.01 while blood lactate concentration had increased to 8.0 ± 0.4 mmol l−1.
. RER values at this time were 1.03 ± 0.01 while blood lactate concentration had increased to 8.0 ± 0.4 mmol l−1.
ERK1/2 MAPK phosphorylation
In control subjects, intense cycling exercise led to a 2.6-fold increase in ERK1/2 MAPK phosphorylation (P < 0.05, range 1.6- to 4.3-fold over resting levels, Fig. 1). In trained subjects, intense cycling exercise led to a 1.5-fold increase in ERK1/2 MAPK phosphorylation (P < 0.05, range 1.1- to 2.8-fold over resting levels, Fig. 1).
Figure 1. ERK1/2 MAPK phosphorylation.
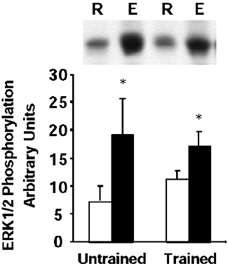
Representative immunoblot and mean ± s.e.m. arbitrary densitometric units for ERK1/2 MAPK phosphorylation in skeletal muscle obtained at rest (R, open boxes) and after exercise (E, filled boxes). *P < 0.05 rest vs. exercise.
p38 MAPK phosphorylation
In control subjects, exercise was associated with a 2.1-fold increased in p38 MAPK phosphorylation (P < 0.05, range 1.3- to 4.9-fold over resting levels, Fig. 2), whereas in trained subjects there was a 1.6-fold increase in p38 MAPK phosphorylation (P < 0.05, 1.1- to 3.2-fold over resting levels, Fig. 2).
Figure 2. p38 MAPK phosphorylation.
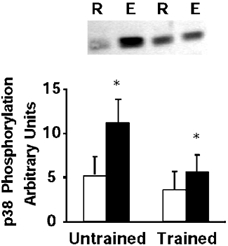
Representative immunoblot and mean ± s.e.m. arbitrary densitometric units, for p38 MAPK phosphorylation in skeletal muscle obtained at rest (R, open boxes) and after exercise (E, filled boxes). *P < 0.05 rest vs. exercise.
MSK1 activity
MSK1 is believed to function as a downstream substrate of both ERK1/2 and p38 MAPK (Deak et al. 1998). In control subjects, exercise was associated with a 2-fold increased in MSK1 activity (P < 0.05, range 1.3- to 2.9-fold, Fig. 3). In trained subjects, exercise led to a 1.4-fold increase in MSK1 activity (P < 0.05, range 1.1- to 2.3-fold over resting levels, Fig. 3).
Figure 3. MSK1 activity.
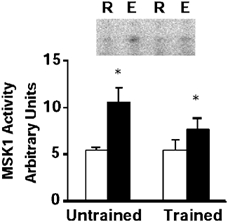
Representative phosphoimage of reaction products (phosphorylated Crosstide peptide) and mean ± s.e.m. arbitrary densitometric units for MSK1 activity in skeletal muscle obtained at rest (R, open boxes) and after exercise (E, filled boxes). *P < 0.05 rest vs. exercise.
Nuclear protein histone H3 phosphorylation
Previous studies have provided evidence that the nuclear protein histone H3/HMG14 is a physiological substrate of MSK1 in 293 cells (Thomson et al. 1999a). In control subjects, exercise increased histone H3 phosphorylation 1.9-fold (P < 0.05, range 1.2- to 3.7-fold, Fig. 4). In trained subjects, exercise led to a 1.8-fold increase in histone H3 phosphorylation (P < 0.05, 1.4- to 2.5-fold over resting levels, Fig. 4). While the fold-increase in histone H3 phosphorylation was similar between trained and control subjects, resting histone H3 phosphorylation was slightly greater (1.6-fold) in trained subjects.
Figure 4. Histone H3 phosphorylation.
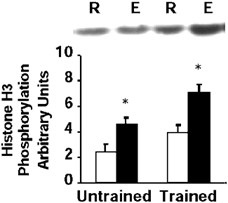
Representative immunoblot and mean ± s.e.m. arbitrary densitometric units for histone H3 phosphorylation in skeletal muscle obtained at rest (R, open boxes) and after exercise (E, filled boxes). *P < 0.05 rest vs. exercise.
AMP kinase activity and phosphorylation
AMPKα1 activity was not altered by exercise in either trained or control subjects (Fig. 5A). Exercise increased AMPKα2 activity 4.2-fold over resting levels in control subjects (P < 0.05, range 3.7- to 4.5-fold, Fig. 5B). In trained subjects, AMPKα2 activity was increased 2.3-fold after exercise (P < 0.05, 1.2- to 2.9-fold over resting levels, Fig. 5B). Essentially similar profiles were observed for pan-AMPKα phosphorylation (Fig. 5C). Exercise led to a 2.2- and 1.4-fold increase in AMPKα phosphorylation in control and trained subjects, respectively (P < 0.05, Fig. 5C).
Figure 5. AMPK activity and phosphorylation.
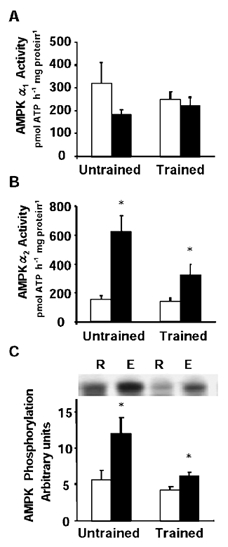
Graphs are means ± s.e.m. for AMPKα1 activity (A), AMPKα2 activity (B) and AMPKα phosphorylation (C) in skeletal muscle obtained at rest (R, open boxes) and after exercise (E, filled boxes). *P < 0.05 rest vs. exercise.
ACC phosphorylation
A direct correlation between phosphorylation of AMPK and ACC in rodent skeletal muscle has been recently observed in response to either electrical stimulation or treadmill running (Park et al. 2002). ACC phosphorylation was increased in control subjects 1.9-fold after exercise (P < 0.05, 1.6- to 2.2-fold over resting levels, Fig. 6). In trained subjects, exercise led to a 2.8-fold increase in ACC phosphorylation after exercise (P < 0.05, range 1.2- to 4-fold over resting levels, Fig. 6).
Figure 6. ACC phosphorylation.
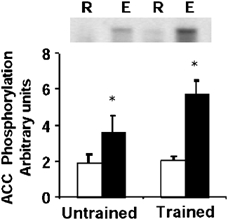
Representative immunoblot and mean ± s.e.m. arbitrary densitometric units for ACC phosphorylation in skeletal muscle obtained at rest (R, open boxes) and after exercise (E, filled boxes). *P < 0.05 rest vs. exercise.
Discussion
The ability of skeletal muscle to respond to physical exercise by executing the appropriate metabolic and transcriptional response is dependent upon cellular signal transduction through phosphorylation cascades (Widegren et al. 2001). One area of emerging interest is the identification of putative phosphorylation kinase cascades controlling transcriptional regulation in skeletal muscle in response to exercise. MAPK and AMPK cascades constitute molecular targets involved in the regulation of exercise-induced responses on gene expression (Zierath, 2002). We hypothesised that intense exercise in highly trained athletes would activate parallel MAPK kinase cascades and AMPK signal transduction. Specific responses of these signalling cascades in skeletal muscle from highly trained endurance athletes versus less-trained control subjects were determined. Here we provide evidence that acute exercise in already well-trained individuals increases MAPK signalling via ERK1/2, p38 and the downstream substrate MSK1. A novel finding was that these responses were coupled to phosphorylation of the nuclear protein histone H3. Furthermore, an increase in AMPKα2 isoform-specific activity was observed, concomitant with an increase in phosphorylation of the downstream substrate ACC. However these parameters were not statistically correlated, supporting the observation that human adaptation to exercise is variable. Importantly, we provide evidence to suggest that even during exercise performed at the same relative intensity, the activation of these signalling intermediates was generally greater in skeletal muscle from less-trained control subjects. This suggests that muscle from highly trained athletes with a prolonged history of endurance training requires a greater stimulus to activate signal transduction through MAPK and AMPK pathways.
Exercise (Aronson et al. 1997b; Widegren et al. 1998; Yu et al. 2001) and muscle contraction (Martineau & Gardiner, 2001) increase ERK1/2 and p38 MAPK phosphorylation. In untrained humans, exercise-induced MAPK signalling in skeletal muscle has been proposed to be intensity dependent, as ERK1/2 phosphorylation is greater in high (75 % of one-leg 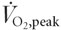 ) versus low (40 % of one-leg
) versus low (40 % of one-leg 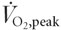 ) intensity cycling exercise (Widegren et al. 2000). In the present study, ERK1/2 phosphorylation was increased to a similar extent in trained versus less-trained subjects. However, the p38 response was greater in untrained subjects. Since both groups of subjects were exercising at approximately the same relative intensity (≈85-90 % of
) intensity cycling exercise (Widegren et al. 2000). In the present study, ERK1/2 phosphorylation was increased to a similar extent in trained versus less-trained subjects. However, the p38 response was greater in untrained subjects. Since both groups of subjects were exercising at approximately the same relative intensity (≈85-90 % of 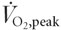 ), our findings provide evidence to suggest that highly trained athletes may undergo a partial adaptation of p38 MAPK signalling in skeletal muscle. In support of this contention, after an acute bout of treadmill running, total p38 MAP kinase expression in muscle from chronically trained rats was reported to be downregulated compared with that of previously untrained animals, suggesting that prior exercise training leads to a selective post-exercise activation of intracellular signalling pathways (Lee et al. 2002). Future studies of MAPK signalling in untrained subjects before and after an intensive training period may reveal the full extent of adaptation along these pathways.
), our findings provide evidence to suggest that highly trained athletes may undergo a partial adaptation of p38 MAPK signalling in skeletal muscle. In support of this contention, after an acute bout of treadmill running, total p38 MAP kinase expression in muscle from chronically trained rats was reported to be downregulated compared with that of previously untrained animals, suggesting that prior exercise training leads to a selective post-exercise activation of intracellular signalling pathways (Lee et al. 2002). Future studies of MAPK signalling in untrained subjects before and after an intensive training period may reveal the full extent of adaptation along these pathways.
MSK1, a common downstream substrate of ERK1/2 and p38 (Deak et al. 1998), is activated in skeletal muscle in response to electrical stimulation (Ryder et al. 2000) and exercise (Krook et al. 2000). In highly trained endurance athletes and less-trained control subjects, MSK1 activity increased in parallel with phosphorylation of ERK1/2 and p38 MAPK in skeletal muscle after intense exercise. However, the reduction in MSK1 activity in highly trained versus less-trained subjects mirrored results for p38 rather than ERK1/2 phosphorylation. In 293 cells, activated MSK1 is localised in the nucleus and phosphorylates the transcription factor CREB at Ser133 (Deak et al. 1998). However, exercise is not associated with increased CREB phosphorylation, rather CREB phosphorylation is either unchanged (Widegren et al. 2000) or repressed (Widegren et al. 1998). While MSK1 is a highly efficient CREB kinase in vitro (Deak et al. 1998), it does not appear to be linked with CREB phosphorylation in skeletal muscle after exercise (Widegren et al. 2000). Interestingly, activation of JNK and ERK1/2 in response to skeletal muscle contraction is associated with a rapid induction of the early response genes c-jun and c-fos (Aronson et al. 1997a). Thus, other mechanisms by which exercise regulates intracellular signal transduction from MSK1 to transcriptional machinery in the nucleus to modulate gene expression need to be considered.
In addition to phosphorylation of specific transcription factors, MAPK substrates can mediate alterations in the chromatin environment of specific genes by direct phosphorylation and/or acetylation of nucleosomal and chromatin proteins (Thomson et al. 1999b). Thus, one potential MSK1 substrate is the nuclear protein histone H3. Phosphorylation of nuclear protein histone H3 occurs at Ser10 of the N-terminal tail, and the time course of phosphorylation is closely coupled to the transient expression of activated immediate-early (IE) response genes (Thomson et al. 1999a). Histone H3 phosphorylation is associated with MAPK signalling to p90 rsk (Nebreda & Gavin, 1999; Sassone-Corsi et al. 1999) and MSK1 (Thomson et al. 1999a), thereby directly linking the MAPK pathway to induction of IE response gene expression. MSK1 is considered to be the likely kinase to mediate histone H3 phosphorylation since it is an extremely efficient kinase for histone H3 phosphorylation utilising physiologically relevant sites (Thomson et al. 1999a). In the present study, MSK1 activity and histone H3 phosphorylation were increased in skeletal muscle after intense exercise. While the experimental model employed in the present study cannot be used directly to link MSK1 activity with histone H3 phosphorylation, we provide the first demonstration of a physiological induction of histone H3 in mammalian tissue. Thus, activation of MSK1 in response to physical exercise provides a putative signalling mechanism to IE response genes and alterations in the nucleosome and chromatin structure though phosphorylation of histone H3.
AMPK pathways have been linked to metabolic and mitogenic events in skeletal muscle (Holmes et al. 1999; Ojuka et al. 2000; Winder et al. 2000; Song et al. 2002). Activation of AMPK mimics several classic exercise-mediated responses on gene expression, including increases in GLUT4 mRNA and protein content, hexokinase II mRNA and activity, UCP-3 mRNA, mitochondrial enzymes, and glycogen content in skeletal muscle (Holmes et al. 1999; Kraniou et al. 2000; Ojuka et al. 2000; Song et al. 2002; Winder et al. 2000; Zhou et al. 2000). In the present study, acute exercise was associated with an isoform-specific increase in AMPK activity: AMPKα2 activity was increased, whereas AMPKα1 activity was not altered. The increase in AMPKα2 was associated with an increase in ACC phosphorylation. Our results in highly trained endurance athletes are consistent with studies in untrained subjects showing exercise at 70-75 % 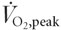 increases AMPKα2, but not AMPKα1 activity in skeletal muscle (Fujii et al. 2000; Wojtaszewski et al. 2000). Since AMPK complexes containing the α2 rather than the α1 isoform demonstrate preferential nuclear localisation (Salt et al. 1998), AMPKα2 may be an important molecular target mediating metabolic and mitogenic events in skeletal muscle in response to exercise. Indeed, a recent study provides evidence that MAPK and AMPK signalling pathways converge (Xi et al. 2001). In Clone 9 cells, AMPK activates downstream p38 MAPK and MKK3 (Xi et al. 2001). p38 MAPK was reported to be required for AICAR-stimulated glucose transport, since chemical inhibition of p38 MAPK (SB203580), or overexpression of dominant negative p38 MAPK mutant, was associated with a parallel inhibition of glucose transport (Xi et al. 2001). Further support for convergence of AMPK and MAPK pathways comes from the observation that the S. cerevisiae serine/threonine protein kinase sucrose non-fermenting 1 (SNF1) and the acetyltransferase Gcn5 function in an obligate sequence to enhance INOS transcription by modifying histone H3 phosphorylation (Lo et al. 2001). Since AMPK is closely related to SNF1 (Hardie et al. 1998), it is temping to speculate that activation of AMPK may also lead to histone H3 modification. Future work aimed at determining whether there is similar ‘cross-talk’ between AMPK and MAPK pathways in skeletal muscle will be important to understand the nature of signals that lead to changes in gene expression and metabolism in response to exercise.
increases AMPKα2, but not AMPKα1 activity in skeletal muscle (Fujii et al. 2000; Wojtaszewski et al. 2000). Since AMPK complexes containing the α2 rather than the α1 isoform demonstrate preferential nuclear localisation (Salt et al. 1998), AMPKα2 may be an important molecular target mediating metabolic and mitogenic events in skeletal muscle in response to exercise. Indeed, a recent study provides evidence that MAPK and AMPK signalling pathways converge (Xi et al. 2001). In Clone 9 cells, AMPK activates downstream p38 MAPK and MKK3 (Xi et al. 2001). p38 MAPK was reported to be required for AICAR-stimulated glucose transport, since chemical inhibition of p38 MAPK (SB203580), or overexpression of dominant negative p38 MAPK mutant, was associated with a parallel inhibition of glucose transport (Xi et al. 2001). Further support for convergence of AMPK and MAPK pathways comes from the observation that the S. cerevisiae serine/threonine protein kinase sucrose non-fermenting 1 (SNF1) and the acetyltransferase Gcn5 function in an obligate sequence to enhance INOS transcription by modifying histone H3 phosphorylation (Lo et al. 2001). Since AMPK is closely related to SNF1 (Hardie et al. 1998), it is temping to speculate that activation of AMPK may also lead to histone H3 modification. Future work aimed at determining whether there is similar ‘cross-talk’ between AMPK and MAPK pathways in skeletal muscle will be important to understand the nature of signals that lead to changes in gene expression and metabolism in response to exercise.
Our studies provide evidence that MAPK and AMPK signalling occur in parallel in response to intense exercise. In summary, MAPK signalling through ERK1/2 and p38 pathways is associated with activation of MSK1 and histone H3 phosphorylation in skeletal muscle from endurance-trained subjects in response to intense cycling exercise. These changes are observed in concert with increases in AMPKα2, but not AMPKα1 activity. Nevertheless, our data provide evidence to suggest that the activation of these signalling intermediates in response to cycling exercise undertaken at the same relative intensity was greater in the untrained control subjects than in well-trained individuals, even though the less-trained subjects completed 50 % of the exercise protocol performed by the athletes. Thus, even in skeletal muscle of highly trained athletes with a history of many years of endurance training, intense exercise still induces a physiological stimulus to mediate nucleosomal responses through activation of MSK1 and histone H3. Exercise-induced phosphorylation of histone H3 provides a potential cellular mechanism by which exercise facilitates gene transcription.
Acknowledgments
We thank Dr Louise Burke for dietary assistance for the subjects throughout the study and Dr Dario R. Alessi, Department of Biochemistry, University of Dundee, UK, for generously providing reagents. This study was supported by a RMIT Faculty grant (to J. A. H.) and grants from the Swedish Medical Research Council, the Swedish Diabetes Association, the Foundation for Scientific Studies of Diabetology, the Swedish National Centre for Research in Sports and from the following Research Foundations: Thurings, Wibergs, Magnus Bergwalls, Tore Nilsons, Novo-Nordisk, Harald and Greta Jeansson, Marcus and Amalia Wallenberg (to A. K., J. R. Z.) and from Diabetes UK (L. G. D. F. and D. C.) and Medical Research Council UK (D. C.).
References
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase in vitro and in vivo. J Biol Chem. 1995;270:27 489–27 494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- Aronson D, Dufresne SD, Goodyear LJ. Contractile activity stimulates the c-Jun NH2-terminal kinase pathway in rat skeletal muscle. J Biol Chem. 1997a;272:25636–25640. doi: 10.1074/jbc.272.41.25636. [DOI] [PubMed] [Google Scholar]
- Aronson D, Violan MA, Dufresne SD, Zangen D, Fielding RA, Goodyear LJ. Exercise stimulates the mitogenic-activated protein kinase pathway in human skeletal muscle. J Clin Invest. 1997b;99:1251–1257. doi: 10.1172/JCI119282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström J. Muscle electrolytes in man: Determined by neutron activation analysis in needle biopsy specimens. A study on normal subjects, kidney patients, and patients with chronic diarrhea. Scand J Clin Lab Invest. 1962;14(suppl. 68):1–110. [Google Scholar]
- Beyaert R, Cuenda A, Vanden Berghe W, Plaisance S, Lee J, Haegeman G, Cohen P, Fiers W. The p38/RK mitogen-activated protein kinase pathway regulates interleukin-6 synthesis response to tumor necrosis factor. EMBO J. 1996;15:1914–1923. [PMC free article] [PubMed] [Google Scholar]
- Boppart MD, Aronson D, Gibson L, Roubenoff R, Abad LW, Bean J, Goodyear LJ, Fielding RA. Eccentric exercise markedly increases c-Jun NH2-terminal kinase activity in human skeletal muscle. J Appl Physiol. 1999;87:1668–1673. doi: 10.1152/jappl.1999.87.5.1668. [DOI] [PubMed] [Google Scholar]
- Boppart MD, Asp S, Wojtaszewski J FP, Fielding RA, Mohr T, Goodyear LJ. Marathon running transiently increases c-Jun NH2-terminal kinase and p38γ activities in human skeletal muscle. J Physiol. 2000;526:663–669. doi: 10.1111/j.1469-7793.2000.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z-P, McConell GK, Michell BJ, Snow RJ, Canny BJ, Kemp BE. AMPK signaling in contracting human skeletal muscle: acetyl-CoA carboxylase and NO synthase phosphorylation. Am J Physiol. 2000;279:E1202–1206. doi: 10.1152/ajpendo.2000.279.5.E1202. [DOI] [PubMed] [Google Scholar]
- Cheung P, Allis CD, Sassone-Corsi P. Signaling to chromatin through histone modifications. Cell. 2000;103:263–271. doi: 10.1016/s0092-8674(00)00118-5. [DOI] [PubMed] [Google Scholar]
- Clifton AD, Young PR, Cohen P. A comparision of the substrate specificity of MAPKAP kinase-2 and MAPKAP kinase-3 and their activation by cytokines and cellular stress. FEBS Lett. 1996;292:209–214. doi: 10.1016/0014-5793(96)00816-2. [DOI] [PubMed] [Google Scholar]
- Coggan AR, Williams BD. Metabolic adaptations to endurance training: Substrate metabolism during exercise. In: Hargreaves M, editor. Exercise Metabolism, Human Kinetics. Champaign, IL, USA: 1995. pp. 177–210. [Google Scholar]
- Cohen P. The search for physiological substrates of MAP and SAP kinases in mammalian cells. Trends Cell Biol. 1997;7:353–361. doi: 10.1016/S0962-8924(97)01105-7. [DOI] [PubMed] [Google Scholar]
- Cuenda A, Rouse J, Doza Y N, Meier R, Cohen P, Gallagher T-F, Young P R, Lee J C. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- Deak M, Clifton A, Lucocq L, Alessi D. Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J. 1998;15:6552–6563. doi: 10.1093/emboj/17.15.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans WJ, Phinney SK, Young VR. Suction applied to a biopsy maximizes sample size. Med Sci Sports Exerc. 1982;14:101–102. [PubMed] [Google Scholar]
- Fryer LGD, Hajduch E, Rencurel F, Salt IP, Hundal HS, Hardie DG, Carling D. Activation of glucose transport by AMP-activated protein kinase via stimulation of nitric oxide synthase. Diabetes. 2000;48:1978–1985. doi: 10.2337/diabetes.49.12.1978. [DOI] [PubMed] [Google Scholar]
- Fujii N, Hayashi T, Hirshman MF, Smith JT, Habinowski SA, Kaijser L, Mu J, Ljungqvust O, Birnbaum MJ, Witters LA, Thorell A, Goodyear LJ. Exercise induces isoform-specific increase in 5′-AMP-activated protein kinase activity in human skeletal muscle. Biochem Biophys Res Comm. 2000;273:1150–1155. doi: 10.1006/bbrc.2000.3073. [DOI] [PubMed] [Google Scholar]
- Gore CJ, Catcheside PG, French SN, Bennet JM, Laforgia J. Automated O2 max calibrator for open circuit indirect calorimetry systems. Med Sci Sports Exerc. 1997;29:1095–1103. doi: 10.1097/00005768-199708000-00016. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Carling D. The AMP-activated protein kinase. Fuel gauge of the mammalian cell? Eur J Biochem. 1997;246:259–273. doi: 10.1111/j.1432-1033.1997.00259.x. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Carling D, Carlson M. The AMP-activated/SNF-1 protein kinase subfamily: Metabolic sensors of the eukaryotic cell? Annu Rev Biochem. 1998;67:821–855. doi: 10.1146/annurev.biochem.67.1.821. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Hirshman MF, Fujii NSAH, Witters LA, Goodyear LJ. Metabolic stress and altered glucose transport: Activation of AMP-activated protein kinase as a unifying coupling mechanism. Diabetes. 2000;49:527–531. doi: 10.2337/diabetes.49.4.527. [DOI] [PubMed] [Google Scholar]
- Holmes BF, Kurth-Kraczek EJ, Winder WW. Chronic activation of 5′-AMP-activated protein kinase increases GLUT4, hexokinase and glycogen in muscle. J Appl Physiol. 1999;87:1990–1995. doi: 10.1152/jappl.1999.87.5.1990. [DOI] [PubMed] [Google Scholar]
- Kraniou Y, Cameron-Smith D, Misso M, Collier G, Hargreaves M. Effects of exercise on GLUT4 and glycogenin gene expression in human skeletal muscle. J Appl Physiol. 2000;88:794–796. doi: 10.1152/jappl.2000.88.2.794. [DOI] [PubMed] [Google Scholar]
- Krook A, Widegren U, Jiang XJ, Henriksson J, Wallberg-Henriksson H, Alessi D, Zierath J R. Effects of exercise on mitogen- and stress-activated kinase signal transduction in human skeletal muscle. Am J Physiol. 2000;279:R1716–1721. doi: 10.1152/ajpregu.2000.279.5.R1716. [DOI] [PubMed] [Google Scholar]
- Lee JS, Bruce CR, Spurell BE, Hawley JA. Effect of training on activation of extracellular signal-regulated kinase1/2 and p38 mitogen-activated protein kinase pathways in rat soleus muscle. Clin Exp Pharmacol Physiol. 2002;29:655–660. doi: 10.1046/j.1440-1681.2002.03713.x. [DOI] [PubMed] [Google Scholar]
- Lo W-S, Duggan L, Emre NCT, Belotserkovskya R, Lane WS, Shiekhattar R, Berger S L. Snf1-A histone kinase that works in concert with the histone acetyltransferase Gcn5 to regulate transcription. Science. 2001;293:1142–1146. doi: 10.1126/science.1062322. [DOI] [PubMed] [Google Scholar]
- Martineau LC, Gardiner PF. Insight into skeletal muscle mechanotransduction: MAPK activation is quantitatively related to tension. J Appl Physiol. 2001;91:693–702. doi: 10.1152/jappl.2001.91.2.693. [DOI] [PubMed] [Google Scholar]
- Mu J, Brozinick JTJ, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell. 2001;7:1085–1094. doi: 10.1016/s1097-2765(01)00251-9. [DOI] [PubMed] [Google Scholar]
- Nebreda AR, Gavin A-C. Cell survival demands some Rsk. Science. 1999;286:1309–1310. doi: 10.1126/science.286.5443.1309. [DOI] [PubMed] [Google Scholar]
- Ojuka EO, Nolte LA, Holloszy JO. Increased expression of GLUT4 and hexokinase in rat epitrochlearis muscles exposed to AICAR in vitro. J Appl Physiol. 2000;88:1072–1075. doi: 10.1152/jappl.2000.88.3.1072. [DOI] [PubMed] [Google Scholar]
- Park SH, Gammon SR, Knippers JD, Paulsen SR, Rubink DS, Winder WW. Phosphorylation-activity relationships of AMPK and acetyl-CoA carboxylase in muscle. J Appl Physiol. 2002;92:2475–2482. doi: 10.1152/japplphysiol.00071.2002. [DOI] [PubMed] [Google Scholar]
- Ryder JW, Fahlman R, Wallberg-Henriksson H, Alessi DR, Krook A, Zierath JR. Effect of contraction on mitogen-activated protein kinase signal transduction in skeletal muscle: Involvement of the mitogen- and stress-activated protein kinase 1. J Biol Chem. 2000;275:1457–1462. doi: 10.1074/jbc.275.2.1457. [DOI] [PubMed] [Google Scholar]
- Salt I, Celler JW, Hawley SA, Prescott A, Woods A, Carling D, Hardie DG. AMP-activated protein kinase: Greater AMP dependence and preferential nuclear localization, of complexes containing the α2 isoform. Biochem J. 1998;334:177–187. doi: 10.1042/bj3340177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassone-Corsi P, Mizzen CA, Cheung P, Crosio C, Monaco L, Jacquot S, Hanauer A, Allis CD. Requirement of Rsk-2 for epidermal growth factor-activated phosphorylation of histone H3. Science. 1999;285:886–891. doi: 10.1126/science.285.5429.886. [DOI] [PubMed] [Google Scholar]
- Song XM, Fiedler M, Galuska D, Ryder JW, Fernström M, Chibalin AV, Wallberg-Henriksson H, Zierath JR. 5-Aminoimidazole-4-carboxamide ribonucleoside treatment improves glucose homeostasis in insulin-resistant diabetic (ob/ob) mice. Diabetologia. 2002;45:56–65. doi: 10.1007/s125-002-8245-8. [DOI] [PubMed] [Google Scholar]
- Stepto NK, Martin DT, Fallon KE, Hawley JA. Metabolic demands of intense aerobic interval training in competitive cyclists. Med Sci Sports Exerc. 2001;33:303–310. doi: 10.1097/00005768-200102000-00021. [DOI] [PubMed] [Google Scholar]
- Stephens TJ, Chen ZP, Canny BJ, Michell BJ, Kemp BE, McConell GK. Progressive increase in human skeletal muscle AMPKalpha2 activity and ACC phosphorylation during exercise. Am J Physiol. 2002;282:E688–694. doi: 10.1152/ajpendo.00101.2001. [DOI] [PubMed] [Google Scholar]
- Thomson S, Clayton AL, Hazzalin CA, Rose S, Barratt MJ, Mahadevan LC. The nucleosomal response associated with immediate-early gene induction is mediated via alternative MAP kinase cascades: MSK1 as a potential histone H3/HMG-14 kinase. EMBO J. 1999a;18:4779–4793. doi: 10.1093/emboj/18.17.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson S, Mahadevan LC, Clayton AL. MAP kinase-mediated signalling to nucleosomes and immediate-early gene induction. Semin Cell Dev Biol. 1999b;10:205–214. doi: 10.1006/scdb.1999.0302. [DOI] [PubMed] [Google Scholar]
- Widegren U, Jiang XJ, Krook A, Chibalin AV, Björnholm M, Tally M, Roth RA, Henriksson J, Wallberg-Henriksson H, Zierath JR. Divergent effects of exercise on metabolic and mitogenic signaling pathways in human skeletal muscle. FASEB J. 1998;12:1379–1389. doi: 10.1096/fasebj.12.13.1379. [DOI] [PubMed] [Google Scholar]
- Widegren U, Ryder JW, Zierath JR. Mitogen-activated protein kinase (MAPK) signal transduction in skeletal muscle: Effects of exercise and muscle contraction. Acta Physiol Scand. 2001;172:227–238. doi: 10.1046/j.1365-201x.2001.00855.x. [DOI] [PubMed] [Google Scholar]
- Widegren U, Wretman C, Lionikas A, Westerblad H, Henriksson J. Influence of exercise intensity on ERK/MAP kinase signalling in human skeletal muscle. Pflugers Arch. 2000;441:317–322. doi: 10.1007/s004240000417. [DOI] [PubMed] [Google Scholar]
- Winder WW, Holmes BF, Rubink DS, Jensen EB, Chen M, Holloszy JO. Activation of AMP-activated protein kinase increases mitochondrial enzymes in skeletal muscle. J Appl Physiol. 2000;88:2219–2226. doi: 10.1152/jappl.2000.88.6.2219. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JPF, Nielsen P, Hansen BF, Richter EA, Kiens B. Isoform-specific and exercise intensity-dependent activation of 5′-AMP-activated protein kinase in human skeletal muscle. J Physiol. 2000;528:221–226. doi: 10.1111/j.1469-7793.2000.t01-1-00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Azzout-Marniche D, Foretz M, Stein SC, Lemarchand P, Ferre P, Foufelle F, Carlin GD. Characterization of the role of AMP-activated protein kinase in the regulation of glucose-activated gene expression using constitutively active and dominant negative forms of the kinase. Mol Cell Biol. 2000;20:6704–6711. doi: 10.1128/mcb.20.18.6704-6711.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Salt I, Scott J, Hardie DG, Carling D. The α1 and α2 isoforms of the AMP-activated protein kinase have similar activities in rat liver but exhibit differences in substrate specificity in vitro. FEBS Lett. 1996;397:347–351. doi: 10.1016/s0014-5793(96)01209-4. [DOI] [PubMed] [Google Scholar]
- Xi X, Han J, Zhang J-Z. Stimulation of glucose transport by AMP-activated protein kinase via activation of p38 mitogen-activated protein kinase. J Biol Chem. 2001;276:41 029–41 034. doi: 10.1074/jbc.M102824200. [DOI] [PubMed] [Google Scholar]
- Yu M, Blomstrand E, Chiablin AV, Krook A, Zierath JR. Marathon running increases ERK1/2 and p38 MAP kinase signalling to downstream targets in human skeletal muscle. J Physiol. 2001;536:273–282. doi: 10.1111/j.1469-7793.2001.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Bjorbaek C, Moller DE. Regulation and interaction of pp90(RSK) isoforms with mitogen-activated protein kinase. J Biol Chem. 1996;271:29 773–29 779. doi: 10.1074/jbc.271.47.29773. [DOI] [PubMed] [Google Scholar]
- Zhou M, Lin B-Z, Coughlin S, Vallega G, Pilch PF. UCP-3 expression in skeletal muscle: Effects of exercise, hypoxia, and AMP-activated protein kinase. Am J Physiol. 2000;297:E622–629. doi: 10.1152/ajpendo.2000.279.3.E622. [DOI] [PubMed] [Google Scholar]
- Zierath JR. Invited Review: Exercise training-induced changes in insulin signaling in skeletal muscle. J Appl Physiol. 2002;93:773–781. doi: 10.1152/japplphysiol.00126.2002. [DOI] [PubMed] [Google Scholar]


