Abstract
The M2 protein of influenza A viruses forms a proton channel involved in modifying virion and trans Golgi pH during infection. Previous studies of the proton current using whole-cell patch clamp of mouse erythroleukaemia (MEL) cells expressing the M2 protein of the ‘Weybridge’ strain provided evidence for two protonation sites, one involved in permeation, the other in activation by acid pH. The present report compares the M2 channels of two different strains of influenza virus, ‘Weybridge’ (WM2) and ‘Rostock’ (RM2). Whereas with external acid pH the current-voltage relations showed similar small degrees of inward rectification, a similar apparent Kd of approximately 10 μm for proton permeation and a high selectivity for protons over Na+, the two M2 proteins differed in whole-cell conductance at low and high pH. The proton conductance of unit membrane area was on average 7-fold greater in RM2- than WM2-expressing MEL cells. At high external pH WM2 was shown previously to have small conductance for outward current at positive driving potential. In contrast, RM2 shows high conductance for outward current with high external pH, but shows small conductance for inward current with high internal pH, conditions in which WM2 shows high conductance for inward current. The different properties of the conductances due to the two channels at high pH were determined by three amino acids in their transmembrane domains. All intermediate mutants possessed one or other property and transformation of the WM2 phenotype into that of RM2 required substitution in all three residues V27I, F38L and D44N; single substitutions in RM2 effected the opposite phenotypic change. The significance of this difference for virus replication is not clear and it may be that the higher proton flux associated with RM2 is the main factor determining its increased ability to dissipate pH gradients. It is apparent, however, from the specific differences in the sidedness of the pH-induced changes in voltage dependence of the whole-cell current that this is an intrinsic property of the virus proton channel which may have parallels with regulation of other proton channels.
The M2 proton channel of influenza A viruses has roles in two stages of virus replication (reviewed in Hay, 1992). On the one hand, in virus entry into cells it is considered to promote acidification of the virion interior during endocytosis, dissociating the internal ribonucleoprotein- matrix protein structure and facilitating release of the active RNA transcription complex following haemagglutinin (HA)-mediated membrane fusion. On the other hand, during infection by pathogenic avian viruses, co-transport of functional M2 with HA is required to elevate the pH of the trans Golgi Network, to allow passage of the native, acid-sensitive, cleaved HA1/HA2 to the plasma membrane. Both functions are blocked by the anti-influenza drugs, amantadine and rimantadine; in the latter case the resulting low pH of the trans Golgi causes an irreversible conformational change and consequent inactivation of the HA (Sugrue et al. 1990; Steinhauer et al. 1991; Ciampor et al. 1992). The M2 proteins of different viruses have been shown to vary in their ability to alter trans Golgi pH (Grambas et al. 1992). In particular, of the M2 proteins of two closely related strains of avian influenza A H7 viruses, ‘Rostock’ and ‘Weybridge’, the greater activity of the former Rostock M2 (RM2) in reducing acidity of the trans Golgi correlates with the higher pH of fusion (lower acid stability) of Rostock HA, 5.9 compared with 5.3 for the HA of the Weybridge virus (Grambas & Hay, 1992).
The M2 channel is a homotetramer of subunits comprising a 24 residue N-terminal external domain, a 19 amino acid (hydrophobic) membrane-spanning domain and a 54 residue internal/cytoplasmic domain (Sugrue & Hay, 1991; Holsinger & Lamb, 1991; Sakagachi et al. 1997). Structural studies, including solid-state NMR (Kovacs & Cross, 1997; Wang et al. 2001) and site-directed infrared dichroism (Kukol et al. 1999) of a transmembrane peptide (residues 22-46) have indicated that the pore of the channel is formed by a symmetric left-handed bundle of four (nearly ideal) α-helices, tilted approximately 30-38 deg with respect to the bilayer normal.
The channel is highly selective for H+ and has a relatively low permeability for other physiological ions (Chizhmakov et al. 1996a; Mould et al. 2000). Histidine 37 has been implicated both in ion selectivity and in the mechanism of proton conductance (Wang et al. 1995; Pinto et al. 1997; Gandhi et al. 1999). It has been proposed that the residue may act as a proton shuttle and that protonation of the histidine(s) may activate the channel. In this respect, UV resonance Raman spectroscopy gave a pKa of 5.7 for His 37 (Okada et al. 2001), close to the apparent dissociation constant for proton permeation (Wang et al. 1995; Chizhmakov et al. 1996a). Salom et al. (2000) used proton NMR to show that protonation of the peptide monomer, corresponding to that of the first histidine of the tetramer, occurs near neutral pH, which may correspond to protonation of the ‘activation’ site (Chizhmakov et al. 1996a).
In this paper we report a comparison of the properties of the M2 channels of the Rostock (RM2) and Weybridge (WM2) viruses which shows that the changes in proton conductance in response to high internal or external pH (>7), respectively, are mainly determined by pH-induced alterations in voltage dependence. Furthermore, mutational analyses showed that three amino acid differences, at residues 27, 38 and 44 within the transmembrane domains of the two channels, all contribute to the different sidedness of the responses by the two channels and that single substitutions at any one of these positions, I27V, L37F or N44D, can switch between the two distinct properties of the R and W (double mutant) channels.
Methods
Cloning, mutagenesis and transfection of MEL cells
cDNAs of the M2 proteins of influenza viruses A/Chicken/ Germany/27 (H7N7, Weybridge strain) and A/Chicken/ Germany/34 (H7N1, Rostock strain) were reverse transcribed from mRNA prepared from virus-infected cells and cloned into the Bgl II site of plasmid PEV3, as described previously (Chizhmakov et al. 1996a; Ogden et al. 1998). Mutations (S20N, S18R S20N, V27I, F38L, D44N) were introduced into the Weybridge M2 sequence cloned into pTZ19U (USB Biochemicals, Cleveland, OH, USA) by the Kunkel template method (Kunkel, 1985); primer sequences are available on request.
Double mutants V27I F38L, V27I D44N and F38L D44N and the triple mutant V27I F38L D44N were made by sequential mutagenesis. The mutant sequences were amplified by PCR using primers M2 5′E and M2 3′B, which introduced 5′EcoRI and 3′Bgl II sites, respectively, and were cloned into the EcoRI and Bgl II sites of pEV3. The N44D mutant of Rostock M2 was made by PCR mutagenesis using the primers SN44D and AN44D. The two first round PCR reactions with pEV3-RM2, containing the Rostock M2 sequence, used primer pairs M2 5′E and AN44D and M2 3′B and SN44D. PCR products were gel purified and used as templates in a second PCR reaction with the primers M2 5′E and M2 3′B. Full length mutant M2 was cloned into pEV3. Sequences were confirmed by automated DNA sequencing. pEV3-M2 plasmids were electroporated into mouse erythroleukaemia (MEL C88) cells as described by Needham et al. (1992) and clones expressing high levels of M2 after induction with 2 % DMSO were selected for study. MEL cell clones expressing the various wild-type and mutant M2 proteins which were used in the present study are listed in Table 1. The amount of M2 protein expressed in MEL cells was determined by Western blot analysis, as described by Grambas et al. (1992), and reached a maximum 3-4 days after induction.
Table 1.
Influence of amino acid substitutions on the properties of mutant Weybridge and Rostock M2 proteins
| Cell clone | Amino acid substitutions* | Phenotypet† | ||||
|---|---|---|---|---|---|---|
| 18 | 20 | 27 | 38 | 44 | ||
| M2-39 (wt) | S | S | V | F | D | W |
| Wm15 (clone 24) | R | N | V | F | D | W |
| Wm16 (clone 23) | S | S | I | F | D | W |
| Wm17 (clone 5) | S | S | V | L | D | W |
| Wm19 (clone 14B) | S | S | I | L | D | W |
| Wm 20 (clone 22B) | S | S | I | F | N | W |
| Wm21 (clone 43) | S | S | V | L | N | W |
| Wm22 (clone 31) | S | S | I | L | N | R |
| Rm10 (clone 61) | R | N | I | L | D | W |
| M2-R4B (wt) | R | N | I | L | N | R |
The mutant proteins expressed by MEL cell clones Wm15, 16, 17, 19, 20, 21 and 22 were derived by mutagenesis of the Weybridge sequence; the mutant M2 of Rm10 was derived by mutagenesis of the Rostock sequence.
Substitutions of amino acid residues 18, 20, 27, 38 and 44 (single letter code) are indicated in bold.
W and R indicate characteristics similar to the Weybridge and Rostock wt M2 proteins, respectively.
Electrophysiological recording
M2-transformed MEL cells were used 3 or 4 days after induction with 2 % DMSO. Cells were voltage clamped with the whole-cell patch clamp configuration (Hamill et al. 1981). To optimize pH control, high concentrations of pH buffer were used as impermeant ions. The patch pipette contained 90 mmN-methyl-d-glucamine (NMDG), 10 mm EGTA and either 180 mmN(2-hydroxyethyl)piperazine-N‘(2-ethanesulphonic acid) (Hepes) pH 7.2-7.4, or 180 mm 2-(N-morpholino) ethanesulphonic acid (Mes) pH 6.0 or 6.5. The bath contained a similar solution (280 mosmol l−1) with 2 mm CaCl2 replacing EGTA. In ion selectivity experiments NaCl and other salts were substituted iso-osmotically for NMDG-Hepes. The ‘NaCl/KCl solutions’ used in a number of experiments contained 100 mm NaCl (external solution) and 100 mm KCl (internal solution). The presence of CaCl2 facilitated seal formation; experiments performed using CaCl2-free extracellular solutions showed a similar pH dependence. Membrane current was recorded at room temperature, digitized at 0.1 kHz and stored and analysed in a PC AT computer. Current was measured at 10 mV increments of membrane potential over the range −100 to +100 mV. Internal pH (pHi) was constant. External pH (pHo) was changed at each potential by fast perfusion close to the cell with the U-tube method (Krishtal & Pidoplichko, 1980) and washout by slow perfusion for up to 15 s. The rate of change in proton concentration on application of low or high pH solutions was estimated from the speed of change in proton current. The rate did not depend on pH, but was variable, within 50-70 ms. Successive pHo pulses were applied within 20-30 s after recovery of the initial current. Rimantadine hydrochloride was applied by slow perfusion. Chemicals were from BDH or Sigma.
M2 current was determined by subtracting rimantadine-resistant (leak) current from total current at each potential and external pH. All current traces are presented without correction. Each I-V dependence was plotted after subtraction of rimantadine-resistant current. The curvature of I-V plots was characterized by the ratio of conductances at −60 and +60 mV. At each pH, the chord conductance was calculated by division of current by (E – EH), where EH is the equilibrium proton potential. Reversal potentials (Erev) were estimated by linear interpolation of least squares fits to two or three data points of the I-V relation on each side of zero current. The zero current intercept and its statistical error were calculated from the best-fit parameters. The data recorded between different solutions were corrected for the presence of liquid junction potentials of up to 6 mV (Chizhmakov et al. 1996a).
Membrane capacitance was determined by exponential fit to the current transients (digitized at 60 kHz) evoked by 5 mV voltage pulses applied to the pipette. The average capacitance was 9.5 ± 5.6 pF (mean ± s.d.) and series resistance (Rs) 55 ± 8.5 MΩ. In voltage step experiments Rs was minimized to give values of τ ≤ 200 μs.
Results
Similarities between RM2 and WM2 conductance
Current due to M2 expression was studied in response to changes in pHo at membrane potentials between −100 and +100 mV. The pH and voltage dependence of current due to Rostock M2 (RM2) at pH ≤ 7 were similar to those previously described for Weybridge M2 (WM2) (Chizhmakov et al. 1996a) but differed in showing current densities (pA pF−1) which were about 5-8 times greater for RM2-expressing cells than for WM2-expressing cells (see below). The current traces shown in Fig. 1A, obtained from a RM2-expressing cell at −60 mV in NaCl/KCl solutions, show an increase in inward proton current (initial current ≈15 pA) on reduction of pHo from 7.0 to values between 6.1 and 4.1, due to the increase in H+ gradient. The current reached a steady-state within 50-100 ms, remained constant during application of low pHo solution and returned to the initial level following termination of the pHo pulse. RM2 current was inhibited by 50 μm rimantadine, as previously described (Chizhmakov et al. 1996b). In generating I-V curves and normalized plots, the rimantadine-resistant current was subtracted from total current to obtain a precise measure of M2 current uncontaminated by leak current.
Figure 1. pH dependence of RM2 proton current.
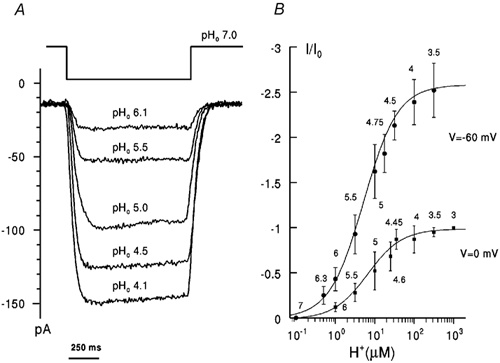
A, superimposed current traces obtained in a single cell in NaCl/KCl solutions (see Methods) in response to shifts in pHo (as indicated; pHi 7.0) at −60 mV. Each pHo pulse was applied within 20-30 s after recovery of the initial current. B, dependence of the inward proton current on [H+]o at different voltages (as indicated) in NaCl-free solutions (pHi 7.0). Rimantadine-sensitive current was normalized with respect to current, Io, at 0 mV, pHo 3.0 (negative values to indicate inward current) and plotted against [H+]o (pHo indicated). Lines were fitted by least-squares regression with a single site binding function. Points represent the means ± s.e.m., n = 6-8 cells. The apparent dissociation constant Kd was 7.5 μm at −60 mV and 8.3 μm at 0 mV.
Dependence on [H+]o
When plotted against external [H+]o current increased on a saturating curve and reached saturation at pHo approximately 3.5 at membrane potentials between −60 and 0 mV in NaCl-containing (data not shown) and NaCl-free solutions (Fig. 1B). The dependence of RM2 current on external H+ concentration was fitted well by a single binding site model which allowed estimates of an apparent dissociation constant, Kd = 7-10 μm, in both NaCl-free and physiological saline solutions. These are similar to the apparent Kd for WM2 of ≈10 μm (Chizhmakov et al. 1996a).
Current-voltage relations
With pHo< 7.0 the I-V relationships for RM2 current were similar to those of WM2 for both inward (Fig. 2) and outward pH gradients (data not shown). For example, with pHi 7.0, pHo 5.0, I-V curves for RM2 and WM2 exhibited a similar small degree of inward rectification (Fig. 2B). The ratio of conductances at −60 and +60 mV (g (-60)/g (+60)) was 2.9 and 2.5 for RM2 and WM2, respectively. The current density at zero voltage was 15.5 ± 8.2 pA pF−1 (s.d., n = 5) and 2.2 ± 1.8 pA pF−1 (s.d., n = 6) for RM2 and WM2, respectively.
Figure 2. Voltage dependence of RM2 and WM2 inward proton currents.
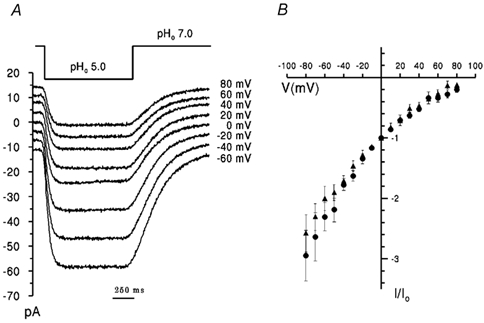
A, superimposed data records of membrane current for RM2 obtained with the same cell in NaCl-free solutions evoked by changing pHo from 7.0 to 5.0 (indicated above traces) at membrane potentials (indicated) between −60 mV and +80 mV. B, I-V plots of RM2(•) and WM2 (▴) rimantadine-sensitive current under the same solution conditions. Current, I, was normalized to current, Io, at 0 mV. Data points represent mean values for 4-6 cells ± s.e.m.
Reversal potentials
Zero current potentials (Erev) for RM2, determined as described in Chizhmakov et al. (1996a), were close to the equilibrium potential for H+ (EH) calculated from the pH gradient with the Nernst equation, and were not influenced significantly by the presence of inwardly or outwardly directed NaCl concentration gradients (Fig. 3). Linear regression of Erev with respect to pH gradient gave a slope of 62.4 ± 2.7 mV in the presence of NaCl and 59.1 ± 3.8 mV in NMDG- Hepes or NMDG-Mes alone, both slopes close to Nernst value of 58 mV at room temperature. Estimates of the permeability to Na+ relative to H+ at the different H+ and Na+ gradients presented in Fig. 3 (see Fig. 3 legend) gave a mean value for RM2 of 8 × 10−7(n = 10) demonstrating a high selectivity for H+ over Na+, similar to that found for WM2 (PNa/PH = 6 × 10−7, Chizhmakov et al. 1996a).
Figure 3. Reversal potentials of RM2 current.
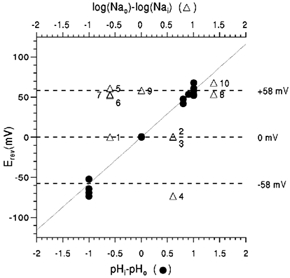
Reversal potentials were determined from I-V curves, as described by Chizhmakov et al. (1996a), and plotted against the pH gradient (•, lower abscissa) and log of [Na+] gradient (▵, upper abscissa). •, values obtained in NaCl-free NMDG-Hepes (Mes). The numbered points (▵) were obtained with pH gradients of 0 (1-3), −1 (4) and +1 (5-10) and internal/external NaCl concentrations of (mm): 40/10 (1, 5, 6 and 7), 10/10 (9), 10/40 (2, 3 and 4) and 5/120 (8 and 10). The continuous line has a Nernst slope of 58.2 mV per decade; the upper, middle and lower dotted lines indicate +58, 0 and −58 mV, respectively. Least-squares regression of Erev on pHi-pHo was calculated to be 59.1 ± 3.8 mV for NaCl-free solutions and 62.4 ± 2.7 mV for NaCl-containing solutions.
Thus at pHo < 7 and pHi between 6.0 and 7.0, the M2 channels of the Rostock and Weybridge viruses exhibited similar properties as regards high proton selectivity, sensitivity to rimantadine, saturation of current at low pH and a small degree of inward rectification of I-V relations. However, differences in the characteristics of the two channels were observed, as described below.
Differences between RM2 and WM2 conductance
The current density, normalized to whole-cell capacitance (pA pF−1), was severalfold greater for cells expressing RM2 than for cells expressing WM2 under comparable conditions of pH and potential. The amounts of M2 expressed in RM2 and WM2 expressing cells, estimated by Western blot analyses, differed by less than 2-fold. Densities calculated at external pH 4.5 at −60 mV for a number of cells in cultures showing similar levels of protein expression were −5.5 ± 4.7 pA pF−1 (s.d., n = 12) for WM2 and −40.9 ± 29.4 pA pF−1 (s.d., n = 7) for RM2 (P << 0.01, Student's t test). Thus, larger current densities in MEL cells expressing RM2 was a clear and consistent difference between the data obtained for the two wild-type M2 channels at similar levels of expression.
Current-voltage relations at external pH > 7.0
To increase the outward current the internal pH was reduced to 6.0, thus increasing the internal proton concentration. We reported previously (Chizhmakov et al. 1996a) that, contrary to expectation from the increase in electrochemical gradient, outward WM2 current was substantially reduced when pHo was raised from 6 to 7 or higher at positive membrane potentials. This is shown for WM2 in Fig. 4A. At positive potential, going from pHo 6.0 to pHo 6.5 the outward current increased as expected in response to the increase in outward electrochemical gradient. However, at higher pHo, the initial increase was followed by subsequent decrease to a steady level, which at +60 mV and pHo > 7 was less than the initial current (Fig. 4A, top panel). The transient increase of current can be explained by the slow change of pH through intermediate values from 6 to 8 as the solution changed. At 0 mV, a similar pH dependence was observed (Fig. 4A, lower panel). Similar experiments with RM2 gave a different result. In the records shown in Fig. 4B, on increasing pHo to 6.5 or 7.0 the outward current increased in response to the increase in electrochemical gradient with no initial peak. At higher pHo, an initial increase was seen, followed by a decline. Unlike WM2, the peak was greater than the steady-state current at pH 7, indicating that the subsequent decline is due to loss of H+ permeability in RM2 at high pH. The stability of the steady-state current during the pHo pulse indicates that pHi is not changing as a result of H+ flux and that the initial decline of current is probably not due to a decrease of the electrochemical potential by H+ accumulation or depletion. Thus, unlike WM2, RM2 showed a steady-state outward current at high external pH which increased with the increase in proton gradient, with only a small component of inactivation at high pH.
Figure 4. Outward proton currents evoked by increasing pHo.
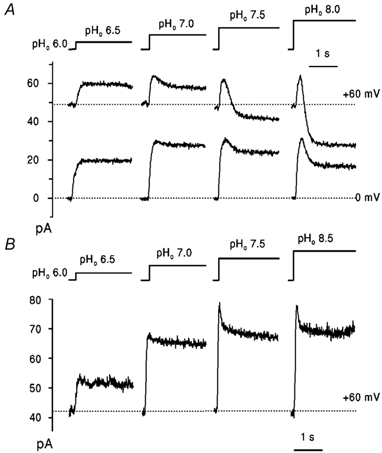
Current traces obtained with a WM2 expressing cell (A) and a RM2 expressing cell (B), in NaCl-free solutions (pHi 6.0; pHo and voltage indicated). Dotted lines indicate the levels of initial current recorded under symmetrical pH conditions (pHo = pHi 6.0). Each pHo pulse was applied within 20-30 s after recovery (not shown) of initial current.
The voltage dependence of RM2 and WM2 currents, with pHo 8.0 and pHi 6.0, are shown in Fig. 5A and C. Looking first at negative membrane potentials, the initial current at pHo = pHi 6.0 was inward and for both RM2 and WM2 decreased on raising pHo to 8, as expected from the decrease in external proton concentration (Fig. 5A and C). At positive potentials the currents through RM2 and WM2 differ. The RM2 outward current increased (Fig. 5A) as the electrochemical gradient increased, in the direction expected from the pH gradient. However, in similar conditions the WM2 outward current increased (for 50-70 ms) to a maximum and then declined towards zero, the size of the decline increasing at more positive potentials (Fig. 5C). Thus the anomalous reduction in outward current of WM2 described above (Fig. 4A) exhibited a strong voltage dependence. Comparison of the voltage dependence for RM2 and WM2 current at the same pH conditions (Fig. 5B) shows that steady-state RM2 current increased substantially at positive voltages while current due to WM2 depended only slightly on voltage. The ratio of chord conductance at +60 mV to that at −60 mV was 0.54 for WM2 and 1.85 for the RM2 channel. The current density at zero voltage was 3.1 ± 1.8 pA pF−1 (s.d., n = 6) and 0.9 ± 0.4 pA pF−1 (s.d., n = 4) for RM2 and WM2, respectively.
Figure 5. Voltage dependence of RM2 and WM2 proton currents at high pHo.

A and C, superimposed current traces obtained with RM2 (A) and WM2 (C) in NaCl-free solutions (pHi 6.0) when pHo was shifted from 6.0 to 8.0 at different membrane potentials (as indicated). B, I-V plots of rimantadine-sensitive RM2 (•) and WM2 (▴) current, (pHi 6.0, pHo 8.0) normalized to the value at 0 mV. Data points represent mean values ± s.e.m. for 5-7 cells.
Thus, at high pHo the RM2 conductance shows strong outward rectification whereas the conductance of WM2 shows an opposite voltage dependence.
Current-voltage relations at internal pH > 7.0
WM2 and RM2 conductance differed also at high intracellular pH. Figure 6A and B shows current traces obtained at different membrane potentials from single WM2- (Fig. 6A) and RM2- (Fig. 6B) expressing cells when pHo was reduced from 8.0 to 6.0 with pHi 8.0. At pH 8 the initial current (indicated by the dotted lines) was close to the rimantadine-resistant leak and the amplitude of current generated at pHo 6.0 is due to M2 current. In both cells shown, the increase in [H+]o evoked an inward current at membrane potentials between −80 and +100 mV. However, the voltage dependence in WM2 showed inward rectification whereas in RM2 current showed little change with potential. The I-V relation for WM2 current (squares, Fig. 6C) was similar to that for the same transmembrane pH gradient of 2.0 at the lower pHi of 7.0 (i.e. pHo step from 7.0 to 5.0, Fig. 2B). The ratio of chord conductance at −60 mV to that at +60 mV was 1.4. In contrast, the RM2 current was influenced much less by membrane potential (Fig. 6C) and the curvature of the I-V curve over the range −80 to +100 mV was opposite to that for WM2. The ratio of conductance at −60 mV to that at +60 mV was 0.45 (i.e. < 1). The current density at zero voltage was 2.8 ± 1.9 pA pF−1 (s.d., n = 6) and 1.5 ± 0.8 pA pF−1 (s.d., n = 4) for RM2 and WM2, respectively.
Figure 6. Voltage dependence of WM2 and RM2 inward proton currents at high pHi.
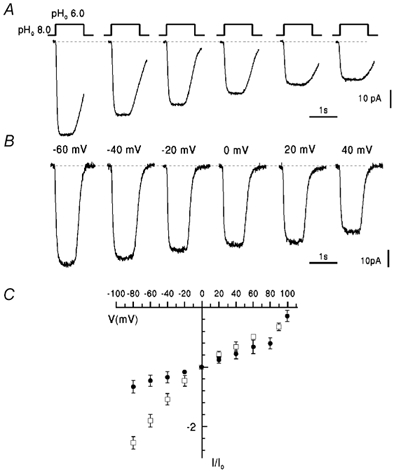
A and B, currents evoked by lowering pHo to 6.0 (pHi 8.0) in NaCl-free solutions for WM2 (A) and RM2 (B) at different voltage, as indicated. C, I-V plot of rimantadine-sensitive WM2 (□) and RM2 (•) currents at pHi 8.0, pHo 6.0 normalized to the value in each cell at 0 mV, Io (negative values indicate inward current). Data points represent mean values ± s.e.m. for 5-7 cells.
Time course of conductance induced by voltage change
The time course of conductance changes in WM2 and RM2 were investigated with voltage jumps from 0 to −60 mV with internal pH 8.0, (using the small initial current in M2 at external pH 8.0 to provide a control for subtraction of leak and capacity currents) and external pH 6.0. Series resistance compensation was not applied and the time constant of the membrane potential change through the series resistance was estimated as 200 μs. Both WM2 and RM2 currents were established immediately on this time scale, in the case of WM2 showing activation in < 1 ms of the inward rectification seen in the steady-state current and for RM2 no slow inactivation to account for the small slope conductance in this potential range. Thus, there appear to be no slow gating reactions requiring times approaching 1 ms, and the conductance change is related to fast permeation or submillisecond ‘gating’ conformation changes.
Mutant M2 proteins
The M2 proteins of Rostock and Weybridge viruses differ in five amino acids, at positions 18, 20, 27, 38 and 44 of the 97 amino acid polypeptide (Hay et al. 1985). To investigate which of these amino acid substitutions contribute to the differences in RM2 and WM2 conductance at high pH, we constructed a series of mutant proteins with amino acid sequences intermediate between those of the two wild-type virus proteins, as listed in Table 1, and analysed the pH and voltage dependence of rimantadine-sensitive currents in cells expressing the mutant M2s.
Substitution of two serine residues, 18 and 20, in the external domain of WM2 by arginine and asparagine, respectively, mutant Wm15, as present in the Rostock protein, had no effect on the pH and voltage dependence relative to those of WM2. Similarly, mutant proteins containing single substitutions (Wm16 and Wm17) or combinations of two substitutions (Wm19, Wm20 and Wm21) of the three amino acids at positions 27, 38 and 44 within the transmembrane domain (comprising amino acids 24-44) of the Weybridge protein also possessed the characteristics of the WM2 channel. Thus, as shown for the double mutant Wm20 (V27I, D44N) in Fig. 7A, high external pH (pHo 8.0, pHi 6.0) at positive membrane potentials caused inactivation of the current similar to that seen with wild-type WM2. I-V dependencies (Fig. 7C) at this pH condition (g (+60)/g (-60) = 0.38) for outward current and for inward current with pHi 8.0, pHo 6.0 (g(-60)/g (+60) = 2.7; Fig. 7B) were similar to those for the wild-type WM2 channel. Only when all three substitutions were made in WM2, isoleucine for valine 27(V27I), leucine for phenylalanine 38 (F38L) and asparagine for aspartic acid 44 (D44N), did the triple mutant Wm22 (V27I, F38L, D44N) acquire properties corresponding to those of RM2. As illustrated in Fig. 8A and B, inward current with pHi 8.0, pHo 6.0 exhibited the low variation with voltage between −80 and +80 mV (g(+60)/g (-60) = 0.47) typical of the Rostock wild-type protein and the I-V dependence for outward current with pHi 6.0 and pHo 8.0 (Fig. 8B; g (+60)/g (-60) = 3.8) was similar to that for RM2 (Fig. 5B). The additional single amino acid changes at positions 27 (V27I), 38 (F38L) or 44 (D44N) were, therefore, necessary and sufficient to convert the WM2-like double mutants of the Weybridge protein into the RM2-like triple mutant. The converse was also true; for example, the single substitution of asparagine 44 in the Rostock protein by aspartic acid, present in the Weybridge M2, resulted in a mutant protein Rm10 (N44D) with properties corresponding to those of WM2, i.e. inactivation by high external pH (pHo 8.0, pHi 6.0) at positive membrane potentials (Fig. 8C and D; g(+60)/g(-60) = 0.97) and a I-V relation for inward current with pHi 8.0 (g (+60)/g (-60) =1.37, Fig. 8D) similar to that with pHi 7.0 (see Fig. 2B). None of the mutant proteins was observed to have properties differing significantly from either of the two wild-type proteins. With pHi 7.0 all mutant proteins exhibited saturation of inward current at low external pH and I-V relations with pHo 5.0 similar to those for the wild-type viruses shown in Fig. 1 and Fig. 2.
Figure 7. Voltage dependence of proton currents through the WM2 mutant V27I, D44N channel.
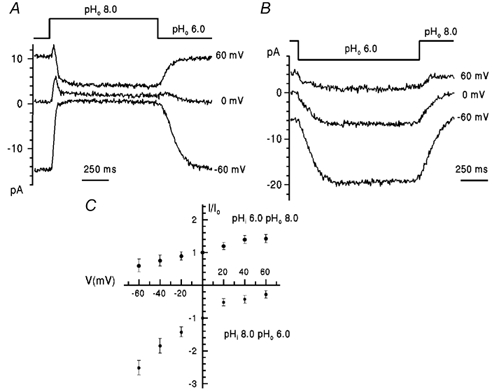
A and B, currents obtained with cells expressing the WM2 mutant V27I, D44N were evoked by shift of pHo at different voltage (pH and voltage indicated). C, I-V plots of rimantadine-sensitive current normalized to the values of current at 0 mV, Io, for different pH gradients (indicated). Data points represent mean values ± s.e.m. for 2-3 cells.
Figure 8. Voltage dependence of proton currents through the WM2 triple mutant, V27I, F38L, D44N channel and the RM2 single mutant N44D.

A and C, currents obtained in NaCl-free solutions at pHi 8.0 (A, WM2 triple mutant) and pHo 8.0 (C, RM2 single mutant) at different voltages (pH and voltage indicated). B and D, I-V plots of rimantadine-sensitive currents, normalized to the values of current at 0 mV, Io, (positive and negative values show outward and inward currents, respectively) for the WM2 triple mutant (B) and for the RM2 single mutant (D) (pHi and pHo indicated). Data points represent mean values ± s.e.m. for 2-3 cells.
Discussion
The M2 channels of the two closely related Weybridge and Rostock strains of influenza A viruses show several similarities in ion conductance when expressed in MEL cells. Both have a high selectivity for protons over Na+ (PNa/PH = 6-8 × 10−7), showing no detectable Na+ permeability, the currents are blocked irreversibly by low concentrations of rimantadine and amantadine and both can generate large inwardly directed proton fluxes at similar proton concentrations (apparent Kd = 10 μm) along the electrochemical gradient, corresponding in direction to proton uptake by the virus or proton efflux from the trans Golgi. Similar properties were shown in cells expressing M2 modified by point mutation to generate proteins intermediate in structure between WM2 and RM2. These properties are consistent with a role of M2 in acidifying the virion during infection and increasing the pH of the trans Golgi. The high proton selectivity is not inconsistent with the ability to modify the pH of the virion or trans Golgi compartments, the high surface to volume of both compartments permitting substantial changes in concentration as a result of moderate increase of charge stored on the membrane capacitance, and it is likely that other channels or transporters can provide counter-ion permeability.
One of the differences between cells expressing RM2 and WM2 is the much larger proton current in RM2-expressing cells. This was quantified as a 5- to 8-fold difference in current density for inward current in cells expressing similar levels of protein. It is consistent with observations made of the greater increase in trans Golgi pH by RM2 to maintain the native conformation of the more acid-sensitive Rostock virus HA during transport to the cell surface. The basis for the larger RM2 conductance is not clear from the present experiments, and may be either a greater single channel flux in RM2 or a greater proportion of conducting pores formed by RM2 than WM2 at a particular level of expression. The idea that a proportion of M2 present is non-conducting but in equilibrium with the pool of conducting pores is supported by the observations that the block by adamantanes develops with a rate much slower than expected for a diffusion-limited ligand binding to residues in the channel and is apparently irreversible (Wang et al. 1993; Chizhmakov et al. 1996a), indicating that slow conversion to a susceptible form determines the slow rate of block and unblock. In this regard, studies of amantadine binding to the transmembrane peptide suggest that the drug binds to the unprotonated, neutral state of the channel (Salom et al. 2000). The time course of change in conductance induced by change of voltage in both cases is within 1 ms, and suggests that a fast process within the permeation pathway is responsible for the non-linear characteristics seen. These characteristics are a strong dependence of WM2 on external pH, requiring external protons at > 0.1 μm concentration for outward H+ permeability, and dependence of RM2 on internal H+ at > 0.1 μm for inward permeability.
The mechanisms underlying the pH and voltage dependent differences between current-voltage relations of WM2- and RM2-expressing cells produce similar effects on conductance but are induced by pH changes on opposite sides of the membrane. Point mutations of the residues different between RM2 and WM2 showed only these two distinct phenotypes rather than a progressive transition between two extremes and no mutant exhibited both properties. Proteolysis of the external residues in M2 expressing cells shows that the orientation is N-terminus external for both WM2 and RM2 (A. Phillips & A. Hay, unpublished observations), so a simple reversal of the transmembrane orientation does not occur. Of the three amino acid differences between the transmembrane domains of the two proteins, substitution of any one, residue 27, 38 or 44, was sufficient to switch between susceptibility of double mutants of WM2 to external high pH and susceptibility of RM2 to internal high pH. The variation in both the location and nature of the amino acid changes suggests that the difference in direction of the responses may be due to differences in channel conformation rather than to differences in particular titratable residues.
Inward proton flux in both phenotypes at pH 7 internally shows a dependence on external pH described by a proton binding site of apparent Kd ≈ 10 μm and weakly dependent on potential and the external ionic environment. On the one hand, in WM2 the protonation of this site from the internal face may be slowed by a conformational change induced by deprotonation of an external site with apparent Kd ≈ 0.1 μm, interrupting proton efflux when the gradient is directed outwards. The Rostock phenotype, which is susceptible to internal pH, would require a corresponding conformational change on deprotonation of an internal site that restricts access of external protons to the permeation site. Evidence indicating that His37 is the site of proton binding during permeation suggests that it may be the accessibility of this residue to internal (WM2) or external (RM2) protons which is modified by conformational changes induced by, respectively, external (WM2) or internal (RM2) deprotonation. This simple interpretation could not be tested because in both cases it requires systematic changes of the internal pH during an experiment. In gramicidin, where proton permeation is explained by conduction along a hydrogen bonded chain of water molecules (a ‘water wire’), changes in I-V curves from supralinear to sublinear at low concentration of permeant ion (as observed in these studies of M2) have been interpreted as a shift in the rate-limiting step from the channel interior to the external channel mouth. Reorientation of the water molecules in the chain is considered to limit the rate of proton conductance and pH and voltage-dependent changes in channel properties can be accounted for in terms of changes in the dipole moment of channel constituents and membrane dipole potential which change the rate of water reorientation at the channel exit (Phillips et al. 1999).
On the other hand, it may be protonation-deprotonation of His37 which is responsible for the changes in channel characteristics. Salom et al. (2000) showed that the pKa of His37 in monomers of a M2 transmembrane peptide was close to neutrality and estimated that protonation of the first histidine in the tetramer would occur with a pKa of ≈6.4. This may correspond to the pH dependence (pK ≈7.0) of the reduction in current at positive membrane potential for the intact WM2 channel protein, observed previously. In this case, structural differences between the RM2 and WM2 channels may affect the accessibility of this residue from inside or outside the cell, either by altering the orientation of His37 or the association between the subunits of the tetrameric channel. This would be consistent with His37 being located at the ‘narrowest’ point in the channel (Pinto et al. 1997; Shuck et al. 2000) where slight changes in channel structure may alter its internal/external accessibility and would readily account for the apparent symmetry in the responses of the two channels in terms of pH and I-V dependence. In this respect, His64 (pKa = 7.1), the catalytic proton shuttle of carbonic anhydrase II, undergoes conformational changes between pH 5.7 and 8.5, and, furthermore, mutations at other locations in the molecule can affect its orientation with respect to the efflux of protons from the catalytic site (Nair & Christianson, 1991; Krebs et al. 1991). The direct or indirect involvement of His37 in the modulation of M2 channel properties is supported by the absence of a similar change in voltage dependence of the H37A mutant of WM2 at external or internal pH 8.0 (N. Mulrine, D. Ogden & A. Hay, unpublished observations).
The latter considerations have parallels with models of the pH dependence of gating of voltage-activated proton channels, where changes in the pH gradient, due to increase in pHo or decrease in pHi, shift the voltage dependence of H+ extrusion to less positive potentials (Cherny et al. 1995). Key features of the model are that protonation sites are accessible to either the internal or the external side of the membrane at any one time and that deprotonation causes a conformational change which switches accessibility (see Fig. 10 in Cherny et al. 1995). In the case of the M2 channel, the analogy extends only to the conformational changes induced by deprotonation which alter the voltage dependence of conductance, the difference in conformation of WM2 and RM2 corresponding to the switch in accessibility.
As regards the significance of the different properties, the sequence of the WM2 channel is more representative, V27 and D44 being almost exclusively observed in the known sequences of natural virus isolates from various species, and the pH-dependent changes in WM2 are more typical of the M2s of other viruses, such as the human virus A/Port Chalmers/1/73 (Chizhmakov et al. 1996b) and A/Udorn/72 (Mould et al. 2000). In the case of these channels, the utility of a dependence on external H+ for inward flux along the electrochemical gradient into the virion or out of the trans Golgi is clear. On the one hand, the low permeability to outward flux at high external pH may favour H+ transfer into the virion and prevent elevation of pH within the virion when exposed to an alkaline environment, to preserve the integrity of the RNA genome. In contrast, the low permeability of RM2 for inward flux when the internal H+ concentration is low would not appear to facilitate internal acidification if internal pH is high. This peculiar characteristic of RM2 relies on the acquisition of two unusual amino acid substitutions. In the absence of any other phenotype the characteristics of RM2 may represent ‘switch off’ of the more general, but undesirable (for the Rostock virus), WM2 characteristics rather than selection of a specific advantageous alteration. In relation to the higher pH of fusion of the Rostock virus HA it may be that the overall higher proton flux associated with RM2, particularly at high ‘external’ pH, is the main factor in determining its increased ability to dissipate pH gradients during infection (Grambas et al. 1992). The relationship between the two properties of RM2, is, however, not known.
In conclusion, the two M2 channels, RM2 and WM2, have similar properties at pH below 7 in terms of high proton selectivity, saturation of inward current with respect to H+ and a small degree of rectification of inward current. They differ in a 5- to 8-fold greater current density for RM2 and in the direction of rectification induced by high (> 7) internal (RM2) or external (WM2) pH. The similarity in the pH-induced voltage-dependent change in proton conductance of the two channels suggests that it is an intrinsic property of the M2 channel and that amino acid changes at different locations within the transmembrane domain can determine whether internal or external pH affects the conductance.
Acknowledgments
We acknowledge the support of the Wellcome Trust in the form of a Collaborative Research Initiative Grant (056633/Z/99/Z) to I. Chizhmakov and a Research Travelling Fellowship (042871/2/94) to T. Betakova. We thank Seti Grambas for excellent assistance.
References
- Cherny VV, Markin VS, Decoursey TE. The voltage-activated hydrogen ion conductance in rat alveolar epithelial cells is determined by the pH gradient. J Gen Physiol. 1995;105:861–896. doi: 10.1085/jgp.105.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizhmakov IV, Geraghty FM, Ogden DC, Hayhurst A, Antoniou M, Hay AJ. Selective proton permeability and pH regulation of the influenza virus M2 channel expressed in mouse erythroleukaemia cells. J Physiol. 1996a;494:329–336. doi: 10.1113/jphysiol.1996.sp021495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizhmakov IV, Ogden DC, Geraghty FM, Betakova T, Skinner A, Hay AJ. Characteristics of the influenza A virus M2 proton channel. In: Brown LE, Hampson AW, Webster RG, editors. Options for the Control of Influenza III. Amsterdam: Elsevier Science BV; 1996b. pp. 343–350. [Google Scholar]
- Ciampor F, Bayley PM, Nermut MV, Hirst EMA, Sugrue RJ, Hay AJ. Evidence that the amantadine-induced, M2-mediated conversion of influenza A virus haemagglutinin to the low pH conformation occurs in an acidic trans Golgi compartment. Virology. 1992;188:14–24. doi: 10.1016/0042-6822(92)90730-d. [DOI] [PubMed] [Google Scholar]
- Gandhi CS, Shuck K, Lear JD, Dieckmann GR, Degrado WF, Lamb RA, Pinto LH. Cu(II) inhibition of the proton translocation machinery of the influenza A virus M2 protein. J Biol Chem. 1999;274:5474–5482. doi: 10.1074/jbc.274.9.5474. [DOI] [PubMed] [Google Scholar]
- Grambas S, Bennett MS, Hay AJ. Influence of amantadine resistance mutations on the pH regulatory function of the M2 protein of influenza A viruses. Virology. 1992;191:541–549. doi: 10.1016/0042-6822(92)90229-i. [DOI] [PubMed] [Google Scholar]
- Grambas S, Hay AJ. Maturation of influenza A virus haemagglutinin-estimates of the pH encountered during transport and its regulation by the M2 protein. Virology. 1992;190:11–18. doi: 10.1016/0042-6822(92)91187-y. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hay AJ. The action of adamantanamines against influenza A viruses: inhibition of the M2 ion channel protein. Semin Virol. 1992;3:21–30. [Google Scholar]
- Hay AJ, Wolstenholme AJ, Skehel JJ, Smith MH. The molecular basis of the specific anti-influenza action of amantadine. EMBO J. 1985;4:3021–3024. doi: 10.1002/j.1460-2075.1985.tb04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsinger LJ, Lamb RA. Influenza virus M2 integral membrane protein is a homotetramer stabilized by formation of disulfide bonds. Virology. 1991;183:32–43. doi: 10.1016/0042-6822(91)90115-r. [DOI] [PubMed] [Google Scholar]
- Kovacs FA, Cross TA. Transmembrane four-helix bundle of influenza A M2 protein channel: structural implications from helix tilt and orientation. Biophys J. 1997;73:2511–2517. doi: 10.1016/S0006-3495(97)78279-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs JF, Fierke CA, Alexander RS, Christianson DW. Conformational mobility of His-64 in the Thr-200→ Ser mutant of human carbonic anhydrase II. Biochemistry. 1991;30:9153–9160. doi: 10.1021/bi00102a005. [DOI] [PubMed] [Google Scholar]
- Krishtal OA, Pidoplichko VI. A receptor for protons in the nerve cell membrane. Neuroscience. 1980;5:2325–2327. doi: 10.1016/0306-4522(80)90149-9. [DOI] [PubMed] [Google Scholar]
- Kukol A, Adams PD, Rice LM, Brunger AT, Arkin IT. Experimentally based orientational refinement of membrane protein models: a structure for the influenza A M2 H+ channel. J Mol Biol. 1999;286:951–962. doi: 10.1006/jmbi.1998.2512. [DOI] [PubMed] [Google Scholar]
- Kunkel TA. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985;82:488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mould JA, Drury JE, Frings SM, Kaupp UB, Pekosz A, Lamb RA, Pinto LH. Permeation and activation of the M2 ion channel of influenza A virus. J Biol Chem. 2000;275:31038–31050. doi: 10.1074/jbc.M003663200. [DOI] [PubMed] [Google Scholar]
- Nair SK, Christianson DW. Unexpected pH-dependent conformation of His-64, the proton shuttle of carbonic anhydrase II. J Am Chem Soc. 1991;113:9455–9458. [Google Scholar]
- Needham M, Gooding C, Hudson K, Antoniou M, Grosveld F, Hollis M. LCR/MEL: A versatile system for high-level expression of heterologous proteins in erythroid cells. Nucleic Acids Res. 1992;20:997–1003. doi: 10.1093/nar/20.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden DC, Chizhmakov IV, Geraghty FM, Hay AJ. Virus ion channels. Methods Enzymol. 1998;294:490–506. doi: 10.1016/s0076-6879(99)94029-6. [DOI] [PubMed] [Google Scholar]
- Okada A, Miura T, Takeuchi H. Protonation of histidine and histidine-tryptophan interaction in the activation of the M2 ion channel from influenza A virus. Biochemistry. 2001;40:6053–6060. doi: 10.1021/bi0028441. [DOI] [PubMed] [Google Scholar]
- Phillips LR, Cole CD, Hendershot RJ, Cotten M, Cross TA, Busath DD. Noncontact dipole effects on channel permeation. III. Anomalous proton conductance effects in gramicidin. Biophys J. 1999;77:2492–2501. doi: 10.1016/S0006-3495(99)77085-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto LH, Dieckmann GR, Gandhi CS, Papworth CG, Braman J, Shaughnessy MA, Lear JD, Lamb RA, Degrado WF. A functionally defined model for the M2 proton channel of influenza A virus suggests a mechanism for its ion selectivity. Proc Natl Acad Sci U S A. 1997;94:11301–11306. doi: 10.1073/pnas.94.21.11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi T, Tu Q, Pinto LH, Lamb RA. The active oligomeric state of the minimalistic influenza virus M2 ion channel is a tetramer. Proc Natl Acad Sci U S A. 1997;94:5000–5005. doi: 10.1073/pnas.94.10.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salom D, Hill BR, Lear JD, Degrado WF. pH-dependent tetramerization and amantadine binding of the transmembrane helix of M2 from the influenza A virus. Biochemistry. 2000;39:14160–14170. doi: 10.1021/bi001799u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuck K, Lamb RA, Pinto LH. Analysis of the pore structure of the influenza A virus M2 ion channel by the substituted-cysteine accessibility method. J Virol. 2000;74:7755–7761. doi: 10.1128/jvi.74.17.7755-7761.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhauer DA, Wharton SA, Skehel JJ, Wiley DC, Hay AJ. Amantadine selection of a mutant influenza virus containing an acid-stable hemagglutinin glycoprotein: evidence for virus-specific regulation of the pH of glycoprotein transport vesicles. Proc Natl Acad Sci U S A. 1991;88:11525–11529. doi: 10.1073/pnas.88.24.11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugrue RJ, Bahadur G, Zambon MC, Hall-Smith M, Douglas AR, Hay AJ. Specific structural alteration of the influenza haemagglutinin by amantadine. EMBO J. 1990;9:3469–3476. doi: 10.1002/j.1460-2075.1990.tb07555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugrue RJ, Hay AJ. Structural characteristics of the M2 protein of influenza A viruses: evidence that it forms a tetrameric channel. Virology. 1991;180:617–624. doi: 10.1016/0042-6822(91)90075-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Lamb RA, Pinto LH. Activation of the M2 ion channel of influenza virus: A role for the transmembrane domain histidine residue. Biophys J. 1995;69:1363–1371. doi: 10.1016/S0006-3495(95)80003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Takeuchi K, Pinto LH, Lamb RA. Ion channel activity of influenza A virus M2 protein: characterization of the amantadine block. J Virol. 1993;67:5585–5594. doi: 10.1128/jvi.67.9.5585-5594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Kim S, Kovacs F, Cross TA. Structure of the transmembrane region of the M2 protein H+ channel. Protein Sci. 2001;10:2241–2250. doi: 10.1110/ps.17901. [DOI] [PMC free article] [PubMed] [Google Scholar]


