Abstract
The vestibular system was activated by galvanic electrical stimulation in 19 normal subjects. With the head turned to one side so that the stimulating anode was on the posterior mastoid process, stimulation caused standing subjects to sway backwards in the sagittal plane. Electromyography showed bilateral activation of erector spinae, gluteus maximus, biceps femoris, soleus and intrinsic foot (toe flexor) muscles. When head direction or electrode polarity was reversed so that the anode was anterior, all those muscles became less active and the subjects swayed forwards. With the head facing forward, stimulation caused sideways sway in the coronal plane, towards the anode, with excitation of the erector spinae on the anode side and reduced activity on the cathode side. The limb muscles were activated on the side opposite the anode and showed complex responses on the anode side. Responses were detectable in the erectores spinae muscles in sitting subjects. No responses in limb muscles were detected in the sitting posture. Subject responses in erector spinae recorded at L3/L4 had latencies from 59 to 110 ms, using a 2 mA stimulus. Latencies in lower limb muscles were longer. The results suggest a role for the vestibular system and descending brain stem motor pathways to the erectores spinae muscles in the control of postural orientation of the back when sitting and standing. The conduction velocity in the motor pathway was estimated to be 13 ± 10 m s−1 (mean ± s.d., n = 12 subjects).
Maintenance of the characteristic upright bipedal posture in man requires that the centre of mass be kept over the support surface provided by the feet. Small amounts of sway are instantaneously resisted by the stiffness of the active postural muscles (Winter et al. 2001). Muscle activity and thus the body orientation (Hlavacka et al. 1996) or postural orientation (Horak & MacPherson, 1996; Horak & Hlavacka, 2001, 2002) is determined by a combination of vestibular and proprioceptive reflexes (Inglis et al. 1995) and voluntary control. Vestibular, proprioceptive and visual reflexes also contribute to stabilisation at low frequencies of sway (Dichgans & Diener, 1989). The responses to rapid perturbations of standing posture consist of combinations of triggered reactions (Runge et al. 1999).
Most published work has been directed to the role of lower limb muscles in the control of sway and orientation in the sagittal (pitch) plane of standing subjects. Rather little attention has been paid to the muscles of the back, despite the fact that the upper body constitutes some two-thirds of total body mass. Positioning of the torso on the pelvis in both sagittal and coronal/frontal (roll) planes is an essential element of postural control in both standing and sitting positions.
The present work has investigated vestibular actions on back muscles in the context of the control of body orientation, using the method of galvanic vestibular stimulation to activate vestibular afferent nerve fibres (Day, 1999). Comparisons are made with lower limb muscles. Observations on lower limb muscles have been extended to include the intrinsic (toe flexor) muscles of the feet. Both sagittal and coronal movement planes have been studied.
Methods
Subjects
Nineteen healthy subjects (7 male, 12 female) between the ages of 18 and 59 years participated (median age 24 years; height 1.67 ± 0.10 m, mean ± s.d.). Subjects gave informed, written consent. Procedures conformed to the Declaration of Helsinki and were approved by the Central Oxfordshire (study number: C01.057) and Aylesbury Vale Local (study NC1005) Research Ethics Committees.
Task
Subjects were studied in both standing and sitting postures. Standing was performed in a relaxed manner on a firm surface wearing shoes. Subjects were permitted to choose a comfortable position for the feet (typically with the inner borders separated by around 150 mm, slightly divergent at the toes) and with equal loading. Sitting took place on a firm chair or stool with the feet on the floor. In both postures the subjects were asked to flex the back (by around 7 deg measured between T11 and L5) thereby inducing tonic electromyographic activity in the erectores spinae muscles that are extensors of the back. This postural activity serves to resist any further flexion under the influence of gravity (Floyd & Silver, 1955; Andersson et al. 1974).
During standing, the forward lean also induced activity in gluteus maximus, biceps femoris and the intrinsic foot (toe flexor) muscles, and enhanced the normal postural activity in soleus. Particular attention was directed to ensuring that the arms were fully relaxed and pendent, with the hands not touching anything other than the body; in sitting subjects the arms rested against the lateral aspects of the thighs. This precaution was taken for two reasons. First, to avoid activity in the superficial back muscles (trapezius and latissimus dorsi) and second because hand contact with a fixed object is known to reduce the amplitude of responses evoked in limb muscles by vestibular stimulation (Britton et al. 1993).
Subjects kept their eyes closed. The head was placed in the forward facing position or turned to the maximal comfortable position to the right or left.
Monitoring movement
In some experiments, movement was recorded at the ankle joints and in the lower back using twin axis electronic goniometers (Biometrics Ltd, Cwmfelinfach, UK).
Vestibular stimulation
Bipolar binaural galvanic electrical stimulation was applied using an isolated constant-current stimulator connected to electrodes placed on the mastoid processes. The procedure was identical to that used previously (Iles & Pisini, 1992), except that stronger currents (up to 4 mA) were employed. Muscle activity was recorded for 0.1 s before the stimulus was applied and for 0.4 s after stimulus onset. The stimulus was maintained until the end of the recording period in order to avoid off-effects (Fitzpatrick et al. 1994). The repetition rate was around 0.25 Hz.
Motor cortex stimulation
In some experiments, the responses of the erector spinae muscle to vestibular system and motor cortex stimulation were compared. The corticospinal tract was activated by transcranial magnetic stimulation of the motor cortex with a Novametrix Magstim 200 stimulator. A small, flat figure of eight coil was used to stimulate the cortex at a point around 40 mm lateral to the vertex and contralateral to the erector spinae muscle from which recordings were made. The induced stimulating current direction was posterior to anterior.
Electromyography
Records were taken bilaterally from the erectores spinae muscles of the back at up to four vertebral levels (defined by the spinous processes) and from muscles in the lower limbs. The paired recording electrodes were placed on the skin along the length of the chosen muscle with a separation of 30 mm and an earth electrode was placed around 35 mm lateral to the recording electrodes. The electrodes were placed 45 mm lateral to the mid-sagittal line when recording from erector spinae. Electrodes were placed along the midline of the foot sole in order to record from the intrinsic flexor muscles. The EMG signals were usually amplified, band-pass filtered (3 Hz to 3 kHz), full-wave rectified, integrated with a 10 ms time constant, and digitised at 4 kHz before averaging the muscle responses during forty or more stimuli to the vestibular system. The recording system has been described in a previous publication (Iles et al. 2000). Because the vestibular stimulus consisted of a large maintained current it was necessary to take measures to reduce the stimulus artefact, particularly when recording from the upper thoracic region. An earth electrode was placed around the neck and the recording amplifiers were muted for 1 ms at the stimulus onset.
EMG responses to motor cortex stimulation were neither rectified nor integrated before digitisation and averaging.
Results
The effects of experimental conditions on muscle responses to vestibular stimulation
Galvanic vestibular stimulation induced excitatory responses in the soleus muscles of subjects standing with the head turned to the right and the stimulating anode on the right mastoid process. Responses were not detectable in the raw EMG, even after averaging. Responses were clearer when the signals were rectified before averaging. A combination of rectification and integration, followed by digitisation and averaging produced the best records for measuring amplitude and latency (Fig. 1A).
Figure 1. EMG responses in soleus and erector spinae muscles to galvanic vestibular stimulation: signal processing.
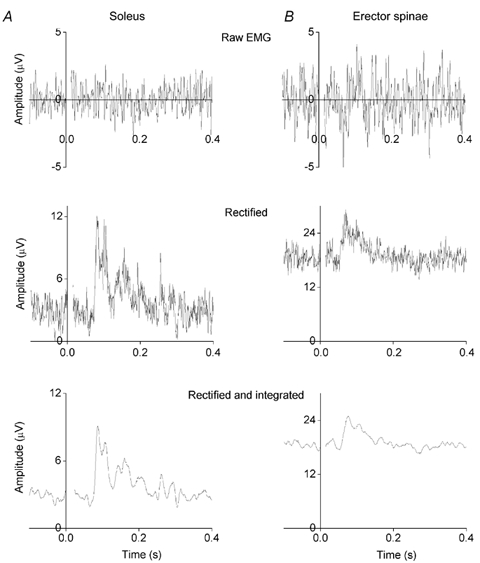
The subject was standing with the head turned around 60 deg to the right and the stimulating anode was placed on the right (posterior) mastoid process. A, EMG responses recorded from the right soleus muscle. Each record is the average of 120 responses. The upper record is an average of the raw EMG responses; the middle record consists of the same responses full-wave rectified and then averaged. The lower record consists of the rectified responses integrated with a 10 ms time constant and then averaged. The vestibular system was stimulated (2 mA) from time 0 to 0.4 s. A period of 0.1 s was recorded before stimulus onset in order to provide a measure of background EMG level (which is large because the subject was leaning forward). A ML response is present at a latency of 85 ms. B, for comparison, EMG responses from the right erector spinae muscle recorded at L3/L4. Each record is the average of 160 responses. A ML response is present at a latency of 60 ms.
The main excitatory response was sometimes preceded by a smaller response of opposite polarity. These will be referred to as ML (medium latency) and SL (short latency) responses respectively. The SL response became more prominent when the vestibular stimulus intensity was increased beyond the 2-2.5 mA routinely used in the current experiments (cf. Fitzpatrick et al. 1994; Watson & Colebatch, 1997). The earliest movement measured at the ankle correlated in sign and amplitude with the ML responses. Later responses were not studied.
The ML response latency was measured from stimulus onset to response onset in selected records where the onset was not obscured by background activity. Latency values were agreed between two of the authors. In some cases when a SL response was present, it clearly finished before ML onset, which was measured in the usual way. If a SL response was not clearly separated then latency was measured to the transition from SL to ML responses (the time of maximum positive slope).
The relationship between ML response latency and galvanic stimulus strength was explored in two subjects. The ML response latency was reduced when vestibular stimulus intensity was increased up to 2 mA and then remained constant if the stimulus was increased further up to 4 mA (the maximum used, Fig. 2). To avoid this stimulus dependence of ML latency and also to reduce SL responses, a standardised stimulus intensity of 2-2.5 mA was used in most experiments.
Figure 2. Soleus ML response latency: dependence on galvanic stimulus strength.
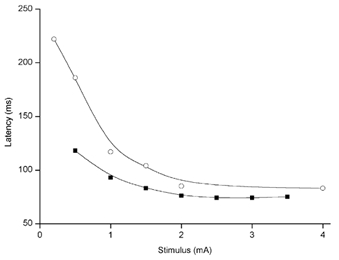
The subject was standing with the head turned around 60 deg to the left and the stimulating anode was placed on the left (posterior) mastoid process. The ML response latencies were measured from averaged rectified and integrated EMG records from the right soleus muscle. Data from two experiments performed 1 year apart on the same subject are plotted. ML latency is shortest for stimuli of 2 mA or larger. Weaker stimuli evoke responses at longer latencies.
Responses similar to those in soleus, but of smaller amplitude and shorter latency were recorded from the erectores spinae muscles at L3 (Fig. 1B). The latency of excitatory ML responses in erector spinae in the 18 different subjects varied from 59 to 110 ms (in the nineteenth subject only limb muscles were studied). Latency was significantly and positively related to subject height (linear regression: P = 0.002, R2 = 45 %).
Responses in erectores spinae muscles compared to responses in lower limb muscles
In subjects with the head turned to one side and the anode on the posterior mastoid process, vestibular stimulation induced a backward sway in the sagittal plane. This resulted from bilateral activation of soleus and erector spinae muscles, as described in the previous section. Gluteus maximus, biceps femoris and the intrinsic foot (toe flexor) muscles were also activated bilaterally (Fig. 3A). The excitatory ML response latencies in the subject illustrated in Fig. 3 (height 1.64 m) increased with the distance between brainstem and muscle (averaged values: erector spinae 61 ms; gluteus 69 ms; biceps femoris 68 ms; soleus 85 ms; foot flexors 95 ms).
Figure 3. Responses in erector spinae and lower limb muscles to vestibular stimulation inducing sway in the sagittal plane.
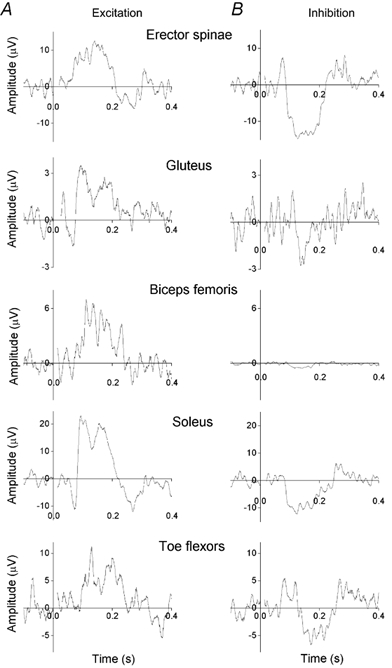
The subject was standing with the head turned about 60 deg to the side and leaning forward. A, excitatory EMG responses recorded from erector spinae at L3/L4 and lower limb muscles on the side to which the subject was facing and with the stimulating anode facing to the posterior. B, inhibitory EMG responses recorded from erector spinae and lower limb muscles with the stimulating anode facing to the anterior. Each record is the average of 160 or more responses. All responses have had the background EMG level (recorded from – 0.1 to 0 s) subtracted (the levels were 62, 20, 43, 84 and 33 μV, from the top to the bottom records).
In subjects with the head turned and the anode on the anterior mastoid process, vestibular stimulation caused a reduction in EMG activity in all the five muscles investigated (Fig. 3B). The subjects swayed forward in the sagittal plane.
When subjects faced forwards and the anode was placed on the right mastoid process, vestibular stimulation induced a sway to the right in the coronal plane. This resulted from excitation of erector spinae on the right (anode) side and inhibition on the left (cathode) side. In the lower limbs, gluteus maximus, biceps femoris, soleus and foot muscles were excited on the cathode side at latencies corresponding to the ML responses seen during sway in the sagittal plane. In the most distal muscles (soleus and foot flexors) these excitatory ML responses were preceded by large inhibitory SL responses. On the anode side the limb muscles were inhibited during the ML excitatory responses of the contralateral limb and large excitatory SL responses were seen in the two most distal muscles (Fig. 4). When the anode was placed on the left mastoid process all the responses reversed in sign and the subject swayed to the left.
Figure 4. Responses in erector spinae and lower limb muscles to vestibular stimulation inducing sway in the coronal plane.
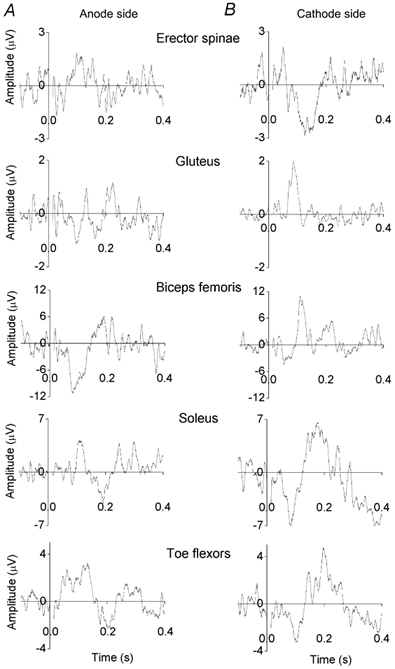
The subject (the same as in Fig. 3) was standing facing forward and leaning forward. A, EMG responses recorded from muscles on the same side as the stimulating anode. B, EMG responses recorded from muscles on the side of the stimulating cathode. Each record is the average of 120 or more responses. Background EMG level has been subtracted (the levels were 13, 13, 67, 39 and 23 μV, from the top to the bottom records). In the soleus and foot flexor muscles the ML responses are preceded by large SL responses.
ML responses in the erectores spinae were observed in both sitting and standing subjects (Fig. 5). Responses in limb muscles were absent in sitting subjects, even if they performed a voluntary contraction of soleus of a magnitude matched to that measured in standing.
Figure 5. Responses in erectores spinae muscles in a sitting subject.
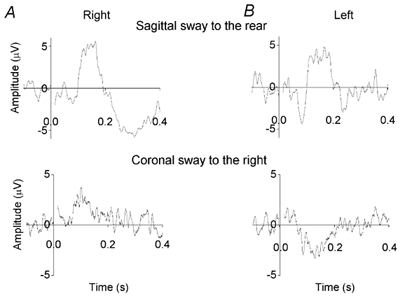
The subject was sitting and leaning forward. A, EMG responses recorded from the right erector spinae at L3/L4. B, EMG responses recorded from the left erector spinae at L3/L4. Upper records were obtained during sway in the sagittal plane (anode on the right mastoid process, head turned to the right). Lower records were obtained during sway in the coronal plane (anode on the right, head facing forward). Each record is the average of at least 120 responses. Background EMG level (10, 22 μV) has been subtracted.
Excitatory responses in erectores spinae muscles during induced movements in both sagittal and coronal planes were observed in all 18 subjects examined. Inhibitory responses were observed in all the subjects tested (four during movement in the sagittal plane and ten during movement in the coronal plane). ML response amplitude in erector spinae muscles varied with posture. Responses were larger when standing than sitting (ratio for subject of Fig. 3 and Fig. 4: 2.2 ± 0.5, mean ± s.e.m., n = 10). Responses were larger when the head was turned (movement in the sagittal plane) than with the head facing forward (movement in the coronal plane; ratio 3.0 ± 0.7, mean ± s.e.m., n = 10). Responses were larger on the side towards which the head was turned (ratio 2.3 ± 0.8, mean ± s.e.m., n = 13).
Responses in erector spinae at different medio-lateral recording positions
In one subject, pairs of recording electrodes (each orientated rostro-caudal) were placed on the mid-sagittal line and at three positions more lateral on the right side up to 98 mm from the midline). The amplitudes of the ML responses to vestibular stimulation at each electrode pair were recorded during eight separate averages. One experimental session recorded responses at the T8/T9 level and a second at L3/L4.
The largest response was recorded from the midline in one set of averaged responses; from the first position lateral to the midline in nine sets and from the next lateral position in six sets (out of a total of 16). There were no consistent differences in the position of the largest responses recorded under different conditions (e.g. sitting and standing, coronal and sagittal sway). In order to interpolate between the four recording positions, the eight plots of response amplitude versus recording position in each experimental session were fitted with quadratic functions and the peak of the function was taken to represent the optimal recording position. The optimal position for the eight sets of averaged responses at the T8/T9 level was 40 ± 7 mm and for the L3/L4 level was 48 ± 2 mm (mean ± s.e.m.) lateral to the mid-sagittal line.
Response amplitude in erector spinae at different rostro-caudal recording positions
In all subjects there was a trend for the ML responses in erector spinae to become smaller as the recording position was moved more rostral. This was investigated in two subjects by making simultaneous recordings with the standard electrode configuration, 45 mm lateral to the midline, at different rostro-caudal levels. The amplitudes of both excitatory and inhibitory responses recorded at rostral levels were normalised to the amplitude recorded at L3/L4. Responses recorded at T3 were only 22 % and at T7 were 34 % of the response at L3/L4, irrespective of whether the vestibular stimulus was inducing movement in the coronal or sagittal plane.
Response latency in erector spinae at different rostro-caudal recording positions
ML response latency in the erector spinae muscle increased with the distance from the brain stem to the recording site. This latency progression was used to estimate the conduction velocity (CV) in the spinal motor pathway conveying signals about vestibular stimulation from brain stem to segmental levels.
The time course of the response recorded at L3/L4 tended to be slower than that recorded at thoracic levels. This complication was ignored and latency was simply measured to ML response onset. Linear regression of latency of excitatory ML responses on vertical distance from inion to vertebral recording level, in each of twelve subjects, gave estimates of the conduction velocity in the spinal pathway of 6 to 33 m s−1. However, the relations were slightly convex upwards rather than linear. In particular, for eight subjects the latencies measured to the onsets of the responses recorded at T11/T12 and L3/L4 were not different. This suggested that a simple regression of latency on distance could overestimate the conduction velocity.
If the spinal pathway conduction velocity is lower than that in motor axons then some non-linearity will result from the fact that the spinal cord terminates at the L1 vertebra and much of the vertical conduction distance in the lumbar region consists of faster conducting motor axons. We attempted to reanalyse the data taking this in to account, by partitioning the conduction distance from inion to most proximal recording electrode, into CNS and motor axon components using the proportions from a published anatomical description (Haymaker & Woodhall, 1945).
The latency measured from the start of vestibular stimulation to the start of the response recorded from erector spinae at some vertebral level is comprised of three components:
(1) time taken for the signal to travel within the CNS from brain stem to the spinal segment (CNS distance/CNS CV),
(2) time taken for the signal to travel from spinal cord to muscle at the recording site (motor distance/motor axon CV),
(3) fixed central delays, including conduction of the signal in the vestibular nerves and processing in the brain stem.
These components give the following linear relation:
latency – (motor distance/motor axon CV)
= (1/CNS CV)(CNS distance) + central delay.
Using an estimated 50 m s−1 for the conduction velocity in motor axons, the data from twelve subjects were analysed by linear regression to give CNS conduction velocity and central delay (P values ranged from 0.001 to 0.13, R2 values ranged from 76 to 99 %). The conduction velocity in the pathway descending from the brain stem for the population of twelve subjects was 13.1 ± 9.6 m s−1 (mean ± s.d.). The central delay was 26.3 ± 20.2 ms (mean ± s.d.). In one subject with an estimated brain stem descending pathway conduction velocity of 26 m s−1, transcranial magnetic stimulation gave a conduction velocity of 55 m s−1 for corticospinal tract actions on erector spinae, a value close to that presented in other recent studies (Taniguchi & Tani, 1999; Hashimoto et al. 2000).
Because of the uncertainties inherent in estimating conduction velocity and central delay from recordings at different levels in single subjects, particularly the difficulty of measuring the latencies of small ML responses recorded at upper thoracic levels, we also made estimates using data from all 18 subjects in whom we had recorded from the lumbar erector spinae. A regression of ML response latency recorded at L3/L4 on the distance from inion to recording site, in the 18 subjects, showed a significant positive relationship (P = 0.02, R2 = 28 %). The slope of the relation corresponded to a spinal pathway conduction velocity of 10.4 m s−1 and the intercept to a central delay of 26.1 ms.
Discussion
Muscle responses to vestibular stimulation
The responses observed in the limb muscles are comparable with those reported earlier (Iles & Pisini, 1992) but have larger amplitudes and smaller latencies. The enhanced amplitude results from the fact that more intense vestibular stimulation was used in the present experiments: medium latency (ML) response amplitude scales with vestibular stimulus strength (Iles & Pisini, 1992). The present work has shown that ML response latency shortens as the galvanic stimulus intensity is increased (Fig. 2). This relationship is consist with published values: stimuli of 0.35 mA were associated with latencies of around 200 ms (Iles & Pisini, 1992) and stimuli of 3 mA give an average ML latency in soleus of 104 ms (Rosengren & Colebatch, 2002). Response latencies in different muscles, subjects or conditions can only be compared if the same stimulus amplitudes are used or the stimuli are strong enough to ensure that latency is at its minimum value. This dependence of latency on stimulus strength is probably also responsible for the prolonged ML response latencies reported using slowly rising current ramp stimuli (Rosengren & Colebatch, 2002).
Short latency (SL) responses of opposite polarity were sometimes seen, but only became prominent when the stimulus amplitude was increased to around 4 mA (excepting distal limb muscles during sway in the coronal plane when SL responses were large). The responses recorded from erector spinae had smaller amplitudes and shorter latencies, but were otherwise similar to the responses in limb muscles.
Task dependence of responses to vestibular stimulation
Binaural vestibular stimulation probably produces an asymmetry in the activity of the left and right vestibular nerves and induces sway towards the anode in standing subjects (Severac Cauquil et al. 1997). For example with the anode on the right mastoid process and the head turned to the right (or anode left and head turned left) vestibular stimulation causes backwards sway. This can be considered to be the response to a false vestibular signal indicating forward sway (Lund & Broberg, 1983). The movement results from bilateral activation of posterior lower limb muscles (gluteus, biceps femoris and soleus). The present work shows that there is bilateral activation of the erectores spinae muscles and of intrinsic foot (toe flexor) muscles as part of this synergy. Coactivation of erector spinae, gluteus and hamstrings has previously been noted in spontaneous and voluntary trunk movements (Oddsson & Thorstensson, 1990). Standing subjects experiencing posterior platform translation activate the same group of muscles (Keshner et al. 1988). Activation of the intrinsic flexor muscles of the feet is not surprising because they are known to be active in the stance phase of locomotion (Mann & Inman, 1964) and flexor digitorum brevis is activated by imposed dorsiflexion of the foot during standing (Schieppati et al. 1995), both observations indicating a postural role. When the anode was positioned to produce forwards sway then the same group of muscles showed a reduction in activity. Previous work has suggested that this involves inhibition of motoneurones (and primary afferents) in addition to withdrawal of excitation (Iles & Pisini, 1992). In the sitting posture, responses in the limb muscles were abolished. Responses in the back muscles persisted, but with their amplitude approximately halved. The strong dependence of muscle response on head position results from an integration of vestibular and head position signals, probably in the brain stem (Fransson et al. 2000; Andersson & Magnusson, 2002). The vestibular system can only control posture through limb and trunk muscles if the head position relative to the trunk is taken into account.
When subjects stand facing forwards, vestibular stimulation induces sway towards the anode in the coronal plane (Coats, 1973; Tokita et al. 1989; Day et al. 1997; Pavlik et al. 1999). Sway to the right results from activation of gluteus maximus, biceps femoris and soleus in the left limb. More complex responses occur in the same muscles of the right limb; the opposite is true for sway to the left. These results in limb muscles are broadly similar to those described by Day et al. (1997). The present work shows that sway to the right is also accompanied by activation of the intrinsic flexors of the left foot. The erector spinae is active on the right side and shows reduced activity on the left during sway to the right. This pattern, consisting of activation of the erector spinae on the anode side and limb muscles in the opposite limb has been illustrated by Ardic et al. (2000) for erectores spinae and gastrocnemii muscles, and termed a ‘low back strategy’. Responses in the erectores spinae during movement in the coronal plane were around one-third the amplitude of those seen during movements in the sagittal plane. The responses would presumably have been larger if the subjects had been instructed to adopt a narrow stance width (Day et al. 1997).
In the sitting posture, responses in the limb muscles are not seen (unless very strong stimuli are applied, when facilitated H reflexes have been reported: Kennedy & Inglis, 2001). Responses in the erectores spinae persist when sitting during both sagittal and coronal sway, but with amplitude more than halved. The torso is tilted relative to the pelvis, with the tilt amplitude approximately halved during sitting compared to standing with a comfortable stance width, as has been noted before (Day et al. 1997). The reductions in back muscle activation when changing from the sagittal to the coronal plane and from standing to sitting were approximately multiplicative. Similar multiplicative attenuation has been noted for sensory actions on galvanic stimulation-induced responses in soleus (Welgampola & Colebatch, 2001).
These results suggest that there are two entirely different synergies employed according to whether the vestibular stimulus signals movement in the sagittal or coronal plane. In the sagittal plane, muscle responses are bilaterally synergist and the erectores spinae muscles are co-active with gluteus maximus, biceps femoris, soleus and intrinsic flexors. In the coronal plane, responses are bilaterally antagonistic and the erectores spinae muscles are co-active with the lower limb muscles on the opposite side. Corresponding synergies have been demonstrated in sitting subjects in response to applied movement in sagittal and coronal planes (Zedka et al. 1998).
Some asymmetry was evident in the muscle responses to stimuli producing sway in the sagittal plane, as noted earlier for limb muscles (Britton et al. 1993; Watson & Colebatch, 1997). For example, with the anode on the right mastoid process and the head turned to the right, responses were larger in the erector spinae muscle on the right side compared to the left. In the limb muscles, larger responses were recorded on the left side. However, subjects turned their heads by less than 90 deg, suggesting that in these circumstances there is a component of sway in the coronal plane to the right in addition to the posterior sway in the sagittal plane, which could produce just such asymmetries. This is supported by the observation that a comfortable head rotation of 60 deg to the right leads to a vestibular-induced sway direction of around 150 deg, not 180 deg (Lund & Broberg, 1983). Direction-specific responses to stance perturbation reported for the erector spinae (Carpenter et al. 1999) are maximal around 135 deg. Small changes in posture can alter the degree of activation of postural muscles (Houtz & Fischer, 1961) and might also modify the responses to vestibular stimulation.
Back muscle origin of responses to vestibular stimulation
Up to this point the back extensor muscles have been referred to as the erectores spinae, a collective term for iliocostalis, longissimus and spinalis dorsi (Winckler, 1937; Macintosh & Bogduk, 1987; Kalimo et al. 1989). Earlier work has shown that surface electrodes can localise muscle responses in the lower back (Andersson et al. 1974) and so it is valid to consider whether the EMG changes in response to vestibular stimulation are localised to the erector spinae, as opposed to deeper and more medially located muscles such as the multifidi, which have previously been regarded as occupying a discrete transversospinalis compartment of the erector spinae (Jonsson, 1969, 1970a).
The data obtained by recording from different medio-lateral positions at the T8/T9 and L3/L4 levels show that maximum responses were obtained at positions 40 and 48 mm lateral to the midline respectively. This is lateral to the lateral border of the multifidi and located centrally over the main mass of both the thoracic and lumbar parts of iliocostalis lumborum and longissimus (Jonsson, 1970b; Macintosh & Bogduk, 1987). Whilst this does not exclude a contribution from the multifidi, the simplest conclusion is that responses to vestibular stimulation are predominantly localised to iliocostalis and longissimus. These muscles have been shown to be co-active in other movements (Masselli et al. 1994).
The same data suggest that the extensive superficial muscles, latissimus dorsi and trapezius do not contribute very much to the recorded responses. We attempted to reduce involvement of these muscles by keeping the upper limbs relaxed and pendent. Latissimus dorsi is not very active in unloaded standing (Lavender et al. 1994). The longitudinal arrangement of the recording electrode pairs will also have favoured recording from the longitudinally orientated erector spinae muscle fibres and will have minimised recording from the superficial muscles with more laterally directed muscle fibres.
Response latencies and conduction velocity in the descending motor pathway
Estimating the conduction velocity in the descending spinal motor pathway activated by vestibular stimulation was complicated by the slightly non-linear relationship between response latency and the vertical distance from the inion to the erector spinae recording electrodes on the back. A contribution to the non-linearity probably results from the gross anatomy of the spinal cord and the fact that much of the vertical conduction path to lower lumbar muscles consists of relatively fast-conducting motor axons in the cauda equina. This will lead to overestimates of the brain stem pathway motor conduction velocity (estimation of corticospinal tract conduction velocity will be relatively unaffected because it is, fortuitously, similar to the conduction velocity of motor axons). The non-linearity was most pronounced between the lowest thoracic and the lumbar recording levels where there are the largest differences in anatomy (Kalimo et al. 1989).
Another complication was the slower time course of responses recorded at L3/L4. This may have resulted from increased collateralisation at lumbar levels (Gough & Kopke, 1968; Bogduk et al. 1982) or to differences in the anatomy of the muscles themselves. Erector spinae responses to cortical stimulation have also been shown to have a different time course at lower lumbar levels (Taniguchi & Tani, 1999; Hashimoto et al. 2000).
Linear regression of ML latency on conduction distance, corrected for the motor axon component, was performed for 12 subjects. Both conduction velocity and central delay showed considerable inter-subject variation. Some of the variation may result from inaccuracies in the experimental method, but repeated estimates in one of the authors showed a conduction velocity consistently one standard deviation above the group mean, indicating some real variation between subjects. The group mean calculated for conduction velocity in the pathway descending from the brain stem producing the ML responses in erector spinae (13±10 m s−1, mean ± s.d., n = 12) is much lower than the value of 60-80 m s−1 estimated for the SL response in limb muscles (Britton et al. 1993) and lower than published values for the corticospinal tract. The estimate of the time taken for processing in the brain stem (26±20 ms, mean ± s.d., n = 12) is similar to the 30 ms calculated for SL responses by Britton et al. (1993). SL responses had higher thresholds than ML responses and in distal limb muscles SL responses were larger for movement in the coronal plane than in the sagittal plane. It has recently been shown that SL responses decline with age, whereas ML responses are preserved (Welgampola & Colebatch, 2002). SL response amplitude (but not ML) is reduced when ramped galvanic stimuli have slow rates of increase (Rosengren & Colebatch, 2002). These results all suggest that different descending motor pathways are responsible for SL and ML responses, possibly the lateral vestibulospinal tract and reticulospinal tracts respectively.
However, a very different model could be proposed. Responses in triceps brachii have a longer latency than those in soleus (Britton et al. 1993). This has been suggested to result from a greater central delay for the upper limb ML responses. If central delay can vary from muscle to muscle then it would be possible to explain the relationship between latency of ML response and inion to recording point distance in terms of progressively increasing central delay for signals travelling to more caudal components of erector spinae, combined with a fast conducting descending motor pathway. When recording latencies from individual subjects, this model cannot be distinguished from the one with single values for central delay and a slow conduction velocity, proposed above. A distinction can be made by utilising data from several subjects. If it is assumed that the variation in inion to recording site distance in subjects of different height reflects variation in the length of the spinal motor pathway (and neglecting scaling elsewhere in the circuitry), then a regression of ML response latency measured at L3 on distance will provide an unambiguous measure of conduction velocity in the motor pathway. The value for conduction velocity obtained this way was low (10.4 m s−1) and similar to that obtained by measuring latency at four recording positions in single subjects (13.1 m s−1). This suggests that the conduction velocity really is low and that the first model, with a single central delay is correct.
Acknowledgments
This work was supported by an equipment grant from the Wellcome Trust. A.S.A. was supported by a Nathalie Rose Barr studentship from the International Spinal Research Trust.
References
- Andersson BJ, Jonsson B, Ortengren R. Myoelectric activity in individual lumbar erector spinae muscles in sitting. A study with surface and wire electrodes. Scand J Rehabil Med Suppl. 1974;3:91–108. [PubMed] [Google Scholar]
- Andersson GB, Magnusson M. Neck vibration causes short-latency electromyographic activation of lower leg muscles in postural reactions of the standing human. Acta Otolaryngol. 2002;122:284–288. doi: 10.1080/000164802753648169. [DOI] [PubMed] [Google Scholar]
- Ardic FN, Latt LD, Redfern MS. Paraspinal muscle response to electrical vestibular stimulation. Acta Otolaryngol. 2000;120:39–46. doi: 10.1080/000164800760370819. [DOI] [PubMed] [Google Scholar]
- Bogduk N, Wilson AS, Tynan NW. The human lumbar dorsal rami. J Anat. 1982;134:383–397. [PMC free article] [PubMed] [Google Scholar]
- Britton T, Day B, Brown P, Rothwell J, Thompson P, Marsden C. Postural electromyographic responses in the arm and leg following galvanic vestibular stimulation in man. Exp Brain Res. 1993;94:143–151. doi: 10.1007/BF00230477. [DOI] [PubMed] [Google Scholar]
- Carpenter MG, Allum JH, Honegger F. Directional sensitivity of stretch reflexes and balance corrections for normal subjects in the roll and pitch planes. Exp Brain Res. 1999;129:93–113. doi: 10.1007/s002210050940. [DOI] [PubMed] [Google Scholar]
- Coats A. Effect of varying stimulus parameters on the galvanic body-sway response. Ann Otol Rhinol Laryngol. 1973;82:96–102. doi: 10.1177/000348947308200119. [DOI] [PubMed] [Google Scholar]
- Day BL. Galvanic vestibular stimulation: new uses for an old tool. J Physiol. 1999;517:631. doi: 10.1111/j.1469-7793.1999.0631s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BL, Severac Cauquil A, Bartolomei L, Pastor MA, Lyon IN. Human body-segment tilts induced by galvanic stimulation: a vestibularly driven balance protection mechanism. J Physiol. 1997;500:661–672. doi: 10.1113/jphysiol.1997.sp022051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichgans J, Diener H-C. The contribution of vestibulospinal mechanisms to the maintenance of human upright posture. Acta Otolaryngol. 1989;107:338–345. doi: 10.3109/00016488909127518. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick R, Burke D, Gandevia S. Task-dependent reflex responses and movement illusions evoked by galvanic vestibular stimulation in standing humans. J Physiol. 1994;478:363–372. doi: 10.1113/jphysiol.1994.sp020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd WF, Silver PHS. The function of the erectores spinae muscles in certain movements and postures in man. J Physiol. 1955;129:184–203. doi: 10.1113/jphysiol.1955.sp005347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson PA, Karlberg M, Sterner T, Magnusson M. Direction of galvanically-induced vestibulo-postural responses during active and passive neck torsion. Acta Otolaryngol. 2000;120:500–503. doi: 10.1080/000164800750045992. [DOI] [PubMed] [Google Scholar]
- Gough JG, Kopke GH. Electromyographic determination of motor root levels in erector spinae muscles. Arch Phys Med Rehabil. 1968;47:9–11. [PubMed] [Google Scholar]
- Hashimoto T, Uozumi T, Tsuji S. Paraspinal motor evoked potentials by magnetic stimulation of the motor cortex. Neurology. 2000;55:885–858. doi: 10.1212/wnl.55.6.885. [DOI] [PubMed] [Google Scholar]
- Haymaker W, Woodhall B. Peripheral Nerve Injuries: Principles of Diagnosis. 1. Philadelphia and London: WB Saunders Company; 1945. p. 227. [Google Scholar]
- Hlavacka F, Mergner T, Krizkova M. Control of the body vertical by vestibular and proprioceptive inputs. Brain Res Bull. 1996;40:431–434. doi: 10.1016/0361-9230(96)00138-4. [DOI] [PubMed] [Google Scholar]
- Horak FB, Hlavacka F. Somatosensory loss increases vestibulospinal sensitivity. J Neurophysiol. 2001;86:575–585. doi: 10.1152/jn.2001.86.2.575. [DOI] [PubMed] [Google Scholar]
- Horak FB, Hlavacka F. Vestibular stimulation affects medium latency postural muscle responses. Exp Brain Res. 2002;144:95–102. doi: 10.1007/s00221-002-1041-9. [DOI] [PubMed] [Google Scholar]
- Horak FB, Macpherson JM. Postural orientation and equilibrium. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. New York: American Physiological Society; 1996. [Google Scholar]
- Houtz SJ, Fischer FJ. Function of leg muscles acting on foot as modified by body movements. J Appl Physiol. 1961;16:597–605. doi: 10.1152/jappl.1961.16.4.597. [DOI] [PubMed] [Google Scholar]
- Iles JF, Ali A, Pardoe J. Task-related changes of transmission in the pathway of heteronymous spinal recurrent inhibition from soleus to quadriceps motor neurones in man. Brain. 2000;123:2264–2272. doi: 10.1093/brain/123.11.2264. [DOI] [PubMed] [Google Scholar]
- Iles JF, Pisini JV. Vestibular evoked postural reactions in man and modulation of transmission in spinal reflex pathways. J Physiol. 1992;455:407–424. doi: 10.1113/jphysiol.1992.sp019308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis JT, Shupert CL, Hlavacka F, Horak FB. Effect of galvanic vestibular stimulation on human postural responses during support surface translations. J Neurophysiol. 1995;73:896–901. doi: 10.1152/jn.1995.73.2.896. [DOI] [PubMed] [Google Scholar]
- Jonsson B. Morphology, innervation, and electromyographic study of the erector spinae. Arch Phys Med Rehabil. 1969;50:638–641. [PubMed] [Google Scholar]
- Jonsson B. The functions of individual muscles in the lumbar part of the spinae muscle. Electromyography. 1970a;10:5–21. [PubMed] [Google Scholar]
- Jonsson B. Topography of the lumbar part of the erector spinae muscle. An analysis of the morphologic conditions precedent for insertion of EMG electrodes into individual muscles of the lumbar part of the erector spinae muscle. Zeit Anat Entwicklungs. 1970b;130:177–191. doi: 10.1007/BF00518805. [DOI] [PubMed] [Google Scholar]
- Kalimo H, Rantanen J, Viljanen T, Einola S. Lumbar muscles: structure and function. Ann Med. 1989;21:353–359. doi: 10.3109/07853898909149220. [DOI] [PubMed] [Google Scholar]
- Kennedy PM, Inglis JT. Modulation of the soleus H-reflex in prone human subjects using galvanic vestibular stimulation. Clin Neurophysiol. 2001;112:2159–2163. doi: 10.1016/s1388-2457(01)00665-4. [DOI] [PubMed] [Google Scholar]
- Keshner EA, Woollacott MH, Debu B. Neck, trunk and limb muscle responses during postural perturbations in humans. Exp Brain Res. 1988;71:455–466. doi: 10.1007/BF00248739. [DOI] [PubMed] [Google Scholar]
- Lavender S, Trafimow J, Andersson GB, Mayer RS, Chen IH. Trunk muscle activation. The effects of torso flexion, moment direction, and moment magnitude. Spine. 1994;19:771–778. [PubMed] [Google Scholar]
- Lund S, Broberg C. Effects of different head positions on postural sway in man induced by a reproducible vestibular error signal. Acta Physiol Scand. 1983;117:307–309. doi: 10.1111/j.1748-1716.1983.tb07212.x. [DOI] [PubMed] [Google Scholar]
- Macintosh JE, Bogduk N. 1987 Volvo award in basic science. The morphology of the lumbar erector spinae. Spine. 1987;12:658–668. doi: 10.1097/00007632-198709000-00004. [DOI] [PubMed] [Google Scholar]
- Mann R, Inman V. Phasic activity of intrinsic muscles of the foot. J Bone Joint Surg Am. 1964;46:469–481. [PubMed] [Google Scholar]
- Masselli MR, De Camargo AM, Berzin F. Electromyographic study of the longissimus dorsi and iliocostalis lumborum muscles during knee flexion and extension on a plain and on a tilt Roman table. Electromyogr Clin Neurophysiol. 1994;34:309–314. [PubMed] [Google Scholar]
- Oddsson L, Thorstensson A. Task specificity in the control of intrinsic trunk muscles in man. Acta Physiol Scand. 1990;139:123–131. doi: 10.1111/j.1748-1716.1990.tb08904.x. [DOI] [PubMed] [Google Scholar]
- Pavlik AE, Inglis JT, Lauk M, Oddsson L, Collins JJ. The effects of stochastic galvanic vestibular stimulation on human postural sway. Exp Brain Res. 1999;124:273–280. doi: 10.1007/s002210050623. [DOI] [PubMed] [Google Scholar]
- Rosengren SM, Colebatch JG. Differential effect of current rise time on short and medium latency vestibulospinal reflexes. Clin Neurophysiol. 2002;113:1265–1272. doi: 10.1016/s1388-2457(02)00121-9. [DOI] [PubMed] [Google Scholar]
- Runge CF, Shupert CL, Horak FB, Zajac FE. Ankle and hip postural strategies defined by joint torques. Gait and Posture. 1999;10:161–170. doi: 10.1016/s0966-6362(99)00032-6. [DOI] [PubMed] [Google Scholar]
- Schieppati M, Nardone A, Siliotto R, Grasso M. Early and late stretch reflex responses of human foot muscles induced by perturbation of stance. Exp Brain Res. 1995;105:411–422. doi: 10.1007/BF00233041. [DOI] [PubMed] [Google Scholar]
- Severac Cauquil A, Bousquet P, Costes Salon MC, Dupui P, Bessou P. Monaural and binaural galvanic vestibular stimulation in human dynamic balance function. Gait and Posture. 1997;6:210–217. [Google Scholar]
- Taniguchi S, Tani T. Motor-evoked potentials elicited from human erector spinae muscles by transcranial magnetic stimulation. Spine. 1999;24:154–156. doi: 10.1097/00007632-199901150-00014. [DOI] [PubMed] [Google Scholar]
- Tokita T, Ito Y, Takagi K. Modulation by head and trunk position of the vestibulospinal reflexes evoked by galvanic stimulation of the labyrinth. Observations by evoked EMG. Acta Otalaryngol. 1989;107:327–332. doi: 10.3109/00016488909127516. [DOI] [PubMed] [Google Scholar]
- Watson S R, Colebatch J G. EMG responses in the soleus muscles evoked by unipolar galvanic vestibular stimulation. Electroencephalogr Clin Neurophysiol. 1997;105:476–483. doi: 10.1016/s0924-980x(97)00044-1. [DOI] [PubMed] [Google Scholar]
- Welgampola M S, Colebatch J G. Vestibulospinal reflexes: quantitative effects of sensory feedback and postural task. Exp Brain Res. 2001;139:345–353. doi: 10.1007/s002210100754. [DOI] [PubMed] [Google Scholar]
- Welgampola M S, Colebatch J G. Selective effects of ageing on vestibular-dependent lower limb responses following galvanic stimulation. Clin Neurophysiol. 2002;113:528–534. doi: 10.1016/s1388-2457(02)00020-2. [DOI] [PubMed] [Google Scholar]
- Winckler G. La structure du muscle spinalis dorsi chez l'homme. Arch d'Anat d'Histol d'Embryol. 1937;23:183–206. [Google Scholar]
- Winter DA, Patla AE, Rietdyk S, Ishac MG. Ankle muscle stiffness in the control of balance during quiet standing. J Neurophysiol. 2001;85:2630–2633. doi: 10.1152/jn.2001.85.6.2630. [DOI] [PubMed] [Google Scholar]
- Zedka M, Kumar S, Narayan Y. Electromyographic response of the trunk muscles to postural perturbation in sitting subjects. J Electromyogr Kinesiol. 1998;8:3–10. doi: 10.1016/s1050-6411(96)00033-8. [DOI] [PubMed] [Google Scholar]


