Abstract
Polymorphisms in the gene encoding the β2-adrenoceptor have been associated with interindividual differences in blood pressure and the diagnosis of hypertension. A common polymorphism resulting in a change from arginine to glycine at amino acid 16 (Arg16 → Gly) enhances agonist-promoted downregulation of receptor expression in vitro. It is unknown whether genotype-dependent differences in nitric oxide generation contribute to differences in vasodilator responses to β2-agonists in vivo. To address this question, venous occlusion plethysmography was used to measure forearm blood flow responses to graded brachial artery infusions of the β-agonist isoproterenol in 41 healthy normotensive Caucasian adults (mean age (± s.d.) = 29 ± 6 years), who were either Arg16 (n = 18) or Gly16 (n = 23) homozygotes. Compared to Arg16 homozygotes, Gly16 homozygotes demonstrated significantly greater blood flow responses to isoproterenol (P = 0.02). After inhibition of nitric oxide synthase by Nγ-monomethyl-l-arginine, blood flow responses did not differ significantly between genotype groups (P = 0.27). Consequently, effects of the Arg16 → Gly polymorphism on forearm blood flow responses to isoproterenol appear to be dependent on differences in endothelial generation of nitric oxide. In contrast to previous reports based on systemic infusions of β2-agonists, our findings indicate that regional blood flow responses to locally infused isoproterenol are significantly greater in Gly16 than in Arg16 homozygotes.
Previous studies have reported altered function of β2-adrenoceptors in subjects with essential hypertension (Feldman et al. 1984; Feldman, 1987) and impaired vasodilator responses to β2-agonists in normotensive persons at increased risk of developing hypertension (Lang et al. 1995; Stein et al. 1995). Moreover, vasodilator responses to β2-agonists appear to be partially dependent on endothelial generation of nitric oxide (Dawes et al. 1997), which is impaired in persons with hypertension (Panza et al. 1993). Several polymorphisms in the gene encoding the β2-receptor (Reihsaus et al. 1993) have been reported to alter receptor function in vitro (Green et al. 1993, 1994). Studies exploring associations of these polymorphisms with essential hypertension and other cardiovascular diseases have mainly focused on the change from adenine to guanine at nucleotide 46, which results in glycine instead of arginine at amino acid 16 (Svetkey et al. 1996; Kotanko et al. 1997; Bray et al. 2000) (Arg16 → Gly) and was associated with greater agonist-promoted receptor down regulation in vitro (Green et al. 1994).
Relatively few studies have explored relationships between the Arg16 → Gly polymorphism of the β2-adrenoceptor and intermediate physiological characteristics that may contribute to differences in blood pressure. Systemic infusions of selective β2-agonists appeared to evoke greater vasodilation in Arg16 than Gly16 homozygotes (Gratze et al. 1999; Hoit et al. 2000), whereas local infusions of the β-agonist isoproterenol demonstrated that regional vasodilator responses were greater in Gly16 than Arg16 homozygotes (Cockcroft et al. 2000; Dishy et al. 2001). However, none of these studies assessed whether the effects of the Arg16 → Gly polymorphism were dependent on nitric oxide-dependent or -independent pathways (Dawes et al. 1997). Therefore, the present study addressed whether the Arg16 → Gly polymorphism is associated with differences in forearm blood flow responses to brachial artery administration of the β-agonist isoproterenol, and whether such differences are associated with differences in the endothelial nitric oxide pathway.
Methods
Study group
Between February and November of the year 2000, 41 healthy normotensive unrelated non-Hispanic white individuals (23 women and 18 men), 18-44 years of age, volunteered for this study in Rochester, MN, USA. Candidates were considered ineligible if they were men over 40 years of age, women over 50 years of age (or post-menopausal), used tobacco products, or had any acute or chronic disorders associated with alterations in cardiovascular structure or function (such as hypertension or diabetes). Based on the measured Arg16 → Gly genotype (see below), subjects were recruited into two groups: one homozygous for the Arg16 variant (n = 18) and the other homozygous for the Gly16 variant (n = 23).
Prior to the study protocol, each subject was evaluated by one of the investigators who reviewed the subject's medical history, including use of medications, and performed a physical examination including measurement of the subject's height (by wall stadiometer), weight (by electronic balance) and blood pressure (by random zero sphygmomanometer). Three blood pressure readings taken 2 min apart were recorded after the subject had been seated quietly for at least 5 min. The second and third readings were averaged and used in the analyses. The results of the screening examination (including chemistry, lipid and haematology profiles) established that all subjects were free of acute or chronic medical problems. Any medications that could alter blood pressure or plasma lipids were discontinued at least 4 weeks before forearm blood flow measurements. No pregnant or lactating women were recruited or enrolled, and women of childbearing potential were required to have a negative pregnancy test before participation. The study protocol was approved by the Institutional Review Board of the Mayo Clinic and carried out in accordance with institutional guidelines. Each study participant signed an informed consent document.
Genotype determination
The Arg16 → Gly polymorphism was genotyped by amplifying a surrounding 107 bp fragment from genomic DNA by polymerase chain reaction using the forward primer:
and the reverse primer:
The reaction included 30 ng of DNA, 1.5 mm magnesium chloride, 0.5 U Taq polymerase (Invitrogen, Carlsbad, CA, USA), 8.5 % DMSO and standard concentrations of nucleotides and buffer in a 20 μl reaction volume. After initial denaturation at 94 °C for 4 min, the fragments were amplified by 35 cycles of 1 min at 94 °C, 1 min at 61 °C, 1 min at 72 °C, followed by 5 min at 72 °C and 5 min at 98 °C. The amplicons were then digested by exposure to 5 U of the restriction enzyme KpnI, followed by electrophoretic separation on 3 % agarose gels, staining with ethidium bromide and visualization using UV light. The Arg16/Arg16 genotype is represented by a single 107 bp band, the Arg16/Gly16 genotype by 25, 82 and 107 bp bands, and the Gly16/Gly16 genotype by 82 and 25 bp bands.
Study protocol
One week before measurements of forearm blood flow, subjects were placed on a diet containing 150 mmol of sodium daily. Food items remained the same throughout the week to provide constant daily amounts of protein (1.4 g (kg body weight)−1 day−1), potassium (100 mmol day−1) and calcium (1100 mg day−1). The caloric content of the diet was adjusted using the Harris Benedict equation to maintain constant body weight, and no more than 35 % of calories were provided by fat. All meals were prepared in the diet kitchen of the General Clinical Research Center (GCRC) where subjects ate two of their three meals each day. On day 6, urine was collected for 24 h for measurement of sodium, potassium and creatinine excretion; that night, subjects slept in the GCRC. The next morning, they remained fasting until the measurements of forearm blood flow were completed in the integrative physiology core of the GCRC.
The morning of the forearm blood flow measurements, the volume of the non-dominant arm was measured by water displacement. The arm was then instrumented with a 5 cm long, 20 gauge brachial artery catheter placed using aseptic technique after local anaesthesia. The catheter and transducer system were configured to permit simultaneous measurements of arterial pressure and infusion of study drugs (Dietz et al. 1994). The total dead space in the catheter/transducer system was 0.5 ml. Forearm blood flow was measured four times each minute using venous occlusion plethysmography with mercury-in-silastic strain gauges (Greenfield et al. 1963). During the measurements, the hand was excluded by inflation of a wrist cuff to 250 mmHg.
After baseline measurements, a reactive hyperaemia trial was conducted. An arm cuff was inflated to 250 mmHg for 5 min, and blood flow was measured for 3 min following release of the cuff. Reactive hyperaemia provides a measure of maximal forearm blood flow response to a standard physiological stimulus that is not dependent on β2-receptors or nitric oxide generation (Engelke et al. 1996). Ten minutes after the reactive hyperaemia trial, a dose-response curve to sodium nitroprusside, a nitric oxide donor whose vasodilatory action is endothelium-independent (Oates & Brown, 2001), was measured during graded infusions at doses of 0.25, 0.50, 1.0 and 2.0 mg (100 ml limb volume)−1 min−1. Each dose was given for 2-3 min and the total duration of the trial lasted ≈10 min. Ten minutes later, a dose-response curve to the nonselective β-agonist isoproterenol was measured during graded infusions at doses of 1.0, 3.0, 6.0 and 12.0 ng (100 ml limb volume)−1 min−1. After an additional 10 min, endothelium-dependent vasodilator response was tested by administering acetylcholine at doses of 1.0, 2.0, 4.0 and 8.0 mg (100 ml limb volume)−1 min−1. As before, doses of the latter drugs were each infused over 2-3 min and the total duration of each trial lasted ≈10 min. For each drug dose, the final four measurements of forearm blood flow were averaged and used in the analyses.
Following the initial dose-response curves, the inhibitor of nitric oxide synthase Nγ-monomethyl-l-arginine (l-NMMA; Clinalfa, Laufelfingen, Switzerland) was infused into the forearm as a 50 mg loading dose given over 10 min followed by a maintenance dose of 1 mg min−1 given over ≈50 min. The efficacy of l-NMMA to inhibit nitric oxide synthesis was verified by re-administering acetylcholine as described above. Dose-response curves to nitroprusside and isoproterenol were then repeated as before, and a second reactive hyperaemia trial was conducted. The investigators who performed the forearm blood flow measurements and processed the raw data (M.J.J. and N.M.D.) were blinded to genotypes of the subjects.
Statistical analysis
Analyses were conducted with SAS version 8.0 (SAS Institute, Cary, NC, USA). Test statistics were considered statistically significant when the associated probability was ≤ 0.05. Measured characteristics were summarized by calculating means and standard deviations for quantitative variables and proportions for categorical variables. The repeated measures analysis of variance was used to assess statistical significance of differences between or among genotype groups for the within-subject responses to reactive hyperaemia and to increasing doses of acetylcholine, nitroprusside and isoproterenol, both before and after l-NMMA. For each drug dose (or reactive hyperaemia trial), unpaired and paired t-tests were used to contrast mean response values between groups and post- vs. pre-l-NMMA, respectively. Since the baseline measurements of mean arterial pressure and heart rate did not differ significantly between genotype groups, and the local drug infusions were not associated with statistically significant changes in these measurements (analyses not shown), the subsequent presentation focuses on forearm blood flow rather than calculated vascular resistance or conductance. Moreover, because linear regression adjustment of the forearm blood flow responses to isoproterenol for differences in the responses to nitroprusside or to acetylcholine did not alter inferences, only the raw unadjusted forearm blood flows are presented.
Results
The descriptive characteristics of the subjects did not differ significantly between the Arg16 and Gly16 homozygote groups at the time of study enrolment or after the controlled sodium diet (Table 1). Prior to administration of l-NMMA, increases in forearm blood flow in response to reactive hyperaemia and to acetylcholine were statistically significant (for each response, P < 0.0001), but neither differed significantly between the Arg16 and Gly16 homozygote groups (Table 2), indicating no underlying differences in vasodilator capacity or endothelial function. In contrast, Gly16 homozygotes demonstrated significantly greater forearm blood flow responses to isoproterenol than Arg16 homozygotes (P = 0.02) (Fig. 1, upper panel). There was also a trend toward greater responses to nitroprusside in Gly16 than Arg16 homozygotes, although this difference did not achieve statistical significance (P = 0.07; Table 2). Moreover, after adjustments by linear regression for differences in responses to nitroprusside or to acetylcholine, residual differences between the homozygote groups in the adjusted forearm blood flow responses to isoproterenol remained statistically significant (at the highest doses, P < 0.05 and P < 0.007, respectively).
Table 1.
Baseline characteristics of study subjects according to Arg16 → Gly genotype
| Homozygote group | |||
|---|---|---|---|
| Characteristic | Argl6 (n = 18) | Gly16 (n = 23) | P |
| Gender (females/males) | 11/7 | 12/11 | 0.57 |
| Age (years) | 27.6 ± 1.6 | 28.7 ± 1.2 | 0.59 |
| Systolic BP (mmHg) | 105.9 ± 2.4 | 106.1 ± 1.6 | 0.93 |
| Diastolic BP (mmHg) | 68.6 ± 2.0 | 71.8 ± 1.4 | 0.17 |
| Heart rate (beats min−1) | 74.7 ± 1.3 | 70.7 ± 1.5 | 0.06 |
| Body mass index (kg m−2) | 24.1 ± 0.7 | 24.1 ± 0.5 | 0.97 |
| Waist-to-hip ratio | 0.8 ± 0.02 | 0.8 ± 0.02 | 0.89 |
| 24 h Na+ excretion (mmol) | 133.5 ± 7.0 | 128.3 ± 6.6 | 0.60 |
Table entries are proportions for gender or means ± standard deviations for other characteristics. P value is for contrast of means between homozygote groups. BP, blood pressure; Na+, sodium.
Table 2.
Forearm blood flow (ml (100 ml limb volume)−1 min−1) before and after administration of Nγ-monomethyl-l-arginine (l-NMMA)
| Before l-NMMA | After l-NMMA | |||||
|---|---|---|---|---|---|---|
| Homozygote group | Homozygote group | |||||
| Argl6 (n = 18) | Glyl6 (n = 23) | Argl6 (n = 18) | Glyl6 (n = 23) | |||
| Baseline | 1.91 ± 0.58 | 1.79 ± 0.60 | 2.04 ± 0.65 | 2.21 ± 0.88 | ||
| Reactive | ||||||
| hyperaemia | 22.1 ± 5.57 | 20.3 ± 9.48 | 24.7 ± 6.42 | 26.8 ± 8.59 | ||
| Pgenotype | 0.48 | 0.21 | ||||
| Pinteraction | 0.53 | 0.25 | ||||
| Nitroprusside | ||||||
| 0.0 | 1.87 ± 0.47 | 1.89 ± 0.84 | 2.11 ± 0.96 | 2.16 ± 0.89 | ||
| 0.25 | 8.21 ± 2.98 | 10.1 ± 4.39 | 10.9 ± 5.18‡ | 11.2 ± 438 | ||
| 0.50 | 9.99 ± 4.21 | 11.7 ± 4.40 | 12.0 ± 5.97† | 13.6 ± 5.72 | ||
| 1.0 | 12.2 ± 5.79 | 15.8 ± 5.64 | 14.0 ± 7.61† | 17.9 ± 8.31 | ||
| 2.0 | 15.3 ± 7.74 | 19.7 ± 6.79 | 17.3 ± 9.48 | 23.4 ± 10.8† | ||
| Pgenotype | 0.07 | 0.15 | ||||
| Pinteraction | 0.10 | < 0.01 | ||||
| Isoproterenol | ||||||
| 0.0 | 2.54 ± 1.04 | 2.64 ± 1.63 | 2.06 ± 0.71 | 1.76 ± 0.79‡ | ||
| 1.0 | 6.48 ± 3.47 | 6.76 ± 2.40 | 4.43 ± 2.13† | 3.53 ± 1.10§ | ||
| 3.0 | 7.67 ± 3.41 | 9.18 ± 4.41 | 5.13 ± 2.33‡ | 4.76 ± 1.86§ | ||
| 6.0 | 8.52 ± 3.40 | 12.0 ± 4.88* | 6.39 ± 3.02† | 7.30 ± 3.38§ | ||
| 12.0 | 10.3 ± 4.99 | 15.2 ± 6.25* | 7.85 ± 3.67† | 10.1 ± 5.41§ | ||
| Pgenotype | 0.02 | 0.27 | ||||
| Pinteraction | < 0.001 | < 0.01 | ||||
| Acetylcholine | ||||||
| 0.0 | 3.20 ± 1.25 | 3.73 ± 1.70 | 1.58 ± 0.42§ | 1.66 ± 0.64§ | ||
| 1.0 | 13.2 ± 10.2 | 17.5 ± 9.5 | 8.06 ± 6.00 | 10.2 ± 6.73‡ | ||
| 2.0 | 14.7 ± 11.8 | 17.4 ± 10.3 | 8.39 ± 6.82† | 8.95 ± 8.54§ | ||
| 4.0 | 15.9 ± 11.5 | 19.3 ± 11.3 | 10.0 ± 6.80 | 11.7 ± 8.70† | ||
| 8.0 | 19.1 ± 13.9 | 24.5 ± 13.3 | 13.2 ± 8.81† | 15.8 ± 9.48‡ | ||
| Pgenotype | 0.25 | 0.41 | ||||
| Pinteraction | 0.65 | 0.84 | ||||
Table entries are means ± standard deviations. Drug infusions were mg (100 ml limb volume)−1 min−1 for nitroprusside and acetylcholine and ng (100 ml limb volume)−1 min−1 for isoproterenol. Pgenotype., statistical significance of genotype effect on profile of response to reactive hyperaemia or drug administration. Pinteraction, statistical significance of interaction effect on profile of response (i.e. genotype-by-reactive hyperaemia or genotype-by-drug). Statistical significance of unpaired t-tests contrasting means between genotypes is denoted by:
P < 0.01.
Statistical significance of paired t-test contrasts of means pre- vs. post-l-NMMA are denoted by:
P < 0.05
P < 0.01
P < 0.001.
Figure 1. Forearm blood flow responses to graded infusion of isoproterenol in subjects homozygous for the β2-adrenoceptor Arg16 variant (n = 18) or the Gly16 variant (n = 23).
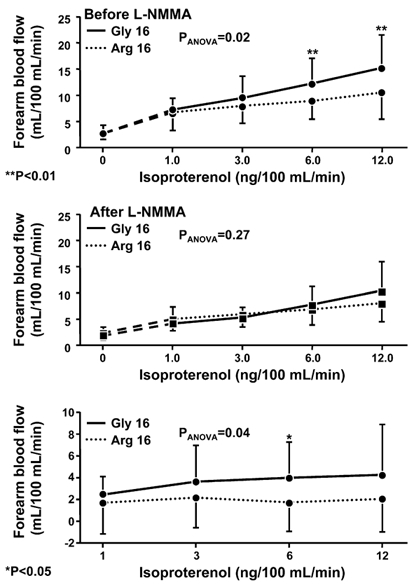
Upper panel, responses before administration of Nγ-monomethyl-l-arginine (l-NMMA); middle panel, responses after administration of l-NMMA; and lower panel, difference in response pre-l-NMMA vs. post-l-NMMA. PANOVA is the P value for the contrast between genotypes from the repeated measures ANOVA; at each dose of isoproterenol, statistically significant differences between genotype groups are denoted by: *P < 0.05; **P < 0.01.
To determine the contributions of nitric oxide-dependent and -independent pathways to these observed differences, we repeated the same set of experiments after administration of l-NMMA (Table 2). The efficacy of l-NMMA to inhibit endothelial nitric oxide synthase was documented by significant decreases in the forearm blood flow responses to acetylcholine in both homozygote groups (for each decrease, P < 0.02), the magnitudes of which did not differ significantly between the groups (P = 0.24). After l-NMMA, the forearm blood flow responses to isoproterenol were significantly reduced in both the Arg16 and Gly16 homozygotes (P < 0.01 and P < 0.001, respectively), and they no longer differed significantly between the genotype groups (P = 0.27) (Fig. 1, middle panel). Consequently, the nitric oxide-dependent component of the vasodilator response to isoproterenol, calculated by subtracting the post-l-NMMA responses from the pre-l-NMMA responses, was significantly greater in Gly16 than Arg16 homozygotes (P = 0.04)(Fig. 1, bottom panel).
After the administration of l-NMMA, the forearm blood flow responses to reactive hyperaemia and to nitroprusside also did not differ between genotype groups (Table 2). Moreover, baseline measurements of mean arterial blood pressure and heart rate did not differ significantly between genotype groups, either before or after l-NMMA, nor did these measurements change significantly in either genotype group during the tests of reactive hyperaemia or the administration of study drugs (analyses not shown).
Discussion
To our knowledge, this is the first study to provide evidence that the effects of polymorphic variation in the β2-adrenoceptor on forearm blood flow responses to the β-agonist isoproterenol are dependent on differences in the generation of nitric oxide. In Gly16 homozygotes, nearly 40 % of the forearm blood flow response to isoproterenol was inhibited by l-NMMA, suggesting a major role for the endothelial nitric oxide pathway. In contrast, the Arg16 homozygotes were much less sensitive to l-NMMA (Fig. 1), and after l-NMMA the responses to isoproterenol did not differ significantly between the genotype groups (Fig. 1). In so far as the results of l-NMMA administration are specific for the response of endothelial β2-adrenoceptors to isoproterenol, there do not appear to be significant genotype-dependent differences in the endothelium-independent component of vasodilator response due to stimulation of β2-adrenoceptors in vascular smooth muscle. The magnitude of differences between Gly16 and Arg16 homozygotes in the nitric oxide-sensitive component of forearm blood flow responses to isoproterenol is comparable to the impairment of endothelial function in subjects with hypercholesterolaemia (Creager et al. 1992) or that reported in young normotensive offspring of hypertensive parents (Taddei et al. 1996).
The other principal finding, that the forearm arterial vasodilator responses to isoproterenol are significantly greater in Gly16 than Arg16 homozygotes, extends a previous smaller study in which isoproterenol was infused intra-arterially and venous occlusion plethysmography was used to measure forearm blood flow response (Cockcroft et al. 2000). Cockcroft and coworkers also found that acute venodilator responses to isoproterenol were 3-fold greater in Gly16 than Arg16 homozygotes (Cockcroft et al. 2000). In a comparable experiment, Dishy et al. (2001) observed that the maximal venodilatory responses to isoproterenol were also greater in Gly16 than Arg16 homozygotes. However, the difference was attributed to effects of the Glu27 variant rather than the Gly16, because greater responses were present only in Gly16 + Glu27 homozygotes, not in Gly16 + Gln27 homozygotes, when compared to Arg16 + Gln27 homozygotes. In a post hoc analysis of the 41 (of 42) subjects in this study who were also genotyped for the Gln27 → Glu polymorphism, the largest difference in forearm blood flow responses to isoproterenol was observed between Arg16 + Gln27 and Gly16 + Gln27 homozygotes (peak responses: 10.3 ± 5.0 vs. 18.8 ± 90.7 ml (ml forearm volume)−1 min−1, respectively, P = 0.02). In contrast, among subjects carrying the Gly16 variant, the Gln27 → Glu polymorphism was not associated with significant differences in forearm blood flow responses to isoproterenol (P = 0.19). These observations further support the conclusion that the Arg16 → Gly polymorphism is associated with significant differences in forearm arterial vasodilator responses to isoproterenol.
In contrast to local infusion studies, when selective β2-agonists were infused systemically, the Arg16 variant of the β2-adrenoceptor, rather than the Gly16 variant, was associated with greater vasodilator responses. In particular, Hoit et al. (2000) reported greater increases in lower limb blood flow measured by plethysmography in response to terbutaline in Arg16 (n = 10) than Gly16 (n = 10) homozygotes. Gratze et al. (1999) made similar inferences based on greater decreases in total peripheral resistance in response to salbutamol in Arg16 homozygotes (n = 11) than in Gly16 carriers (n = 42). However, in both studies, systemic infusion of the selective β2-agonist was associated with significant increases in heart rate and cardiac output (Gratze et al. 1999) or blood pressure (Hoit et al. 2000), and the magnitude of these responses also differed among genotypes. With such systemic effects, it is likely that compensatory cardiovascular reflexes were engaged. Hence, it is difficult to determine whether the observed differences were due to direct vasodilator effects of the β2-agonists or differences in reflex responses.
Based upon genome-wide linkage analyses, we previously identified the long arm of chromosome 5 (5q33.3 to 34) as a region containing one or more genes influencing blood pressure level in Rochester, MN, USA (Krushkal et al. 1998, 1999). After cataloguing DNA sequence variation in the linked region, we found that the Gly16 and Glu27 variants of the β2-adrenoceptor were associated with higher blood pressure levels and the diagnosis of hypertension among young and middle-aged Caucasians (Bray et al. 2000). Additional studies have also reported increased frequency of the Gly16 variant in hypertensive patients (Kotanko et al. 1997); however, others found no relationship (Candy et al. 2000; Herrmann et al. 2000; Jia et al. 2000; Xie et al. 2000; Kato et al. 2001) or that the Arg16 variant was associated with higher blood pressure level and a family history of hypertension (Timmermann et al. 1998; Bengtsson et al. 2001). Although mean diastolic blood pressure was higher and heart rate was lower in the Gly16 than Arg16 homozygotes in the present sample, these differences did not achieve statistical significance. Thus, while considerable evidence justifies the conclusion that polymorphic variation in the β2-adrenoceptor gene contributes to interindividual differences in measures of intermediate physiology relevant to the regulation of blood pressure, the mapping from any particular allelic variant to these intermediate traits and, in turn, to the development of hypertension remains uncertain. Further characterization of additional DNA sequence variation in the β2-adrenoceptor gene may help refine understanding of these relationships (Drysdale et al. 2000).
In summary, this study adds to an accumulating body of evidence that variation in the β2-receptor gene contributes to interindividual differences in vascular responses to stimulation of β2-adrenoceptors, which may mediate previously reported associations of β2-adrenoceptor polymorphisms with differences in blood pressure level and the diagnosis of hypertension. A major new finding is that the Arg16 → Gly polymorphism may influence vasodilator responses to β2-adrenergic agonists through differences in endothelial generation of nitric oxide.
Acknowledgments
This work was supported by US Public Health Service grants U01-HL54464, R01-HL53330, R01-HL46493, R01-HL63328 and funds from the Mayo Foundation. The investigators also appreciate support from the personnel and facilities of the Mayo General Clinical Research Center funded by US Public Health Service grant M01-RR00585.
References
- Bengtsson K, Orho-Melander M, Melander O, Lindblad U, Ranstam J, Rastam L, Groop L. β2-Adrenergic receptor gene variation and hypertension in subjects with type 2 diabetes. Hypertension. 2001;37:1303–1308. doi: 10.1161/01.hyp.37.5.1303. [DOI] [PubMed] [Google Scholar]
- Bray MS, Krushkal J, Li L, Ferrell R, Kardia S, Sing CF, Turner ST, Boerwinkle E. Positional genomic analysis identifies the beta(2)-adrenergic receptor gene as a susceptibility locus for human hypertension. Circulation. 2000;101:2877–2882. doi: 10.1161/01.cir.101.25.2877. [DOI] [PubMed] [Google Scholar]
- Candy G, Samani N, Norton G, Woodiwiss A, Radevski I, Wheatley A, Cockcroft J, Hall IP. Association analysis of beta2 adrenoceptor polymorphisms with hypertension in a Black African population. J Hypertens. 2000;18:167–172. doi: 10.1097/00004872-200018020-00006. [DOI] [PubMed] [Google Scholar]
- Cockcroft JR, Gazis AG, Cross DJ, Wheatley A, Dewar J, Hall IP, Noon JP. Beta(2)-adrenoceptor polymorphism determines vascular reactivity in humans. Hypertension. 2000;36:371–375. doi: 10.1161/01.hyp.36.3.371. [DOI] [PubMed] [Google Scholar]
- Creager MA, Gallagher SJ, Girerd XJ, Coleman SM, Dzau VJ, Cooke JP. l-arginine improves endothelium-dependent vasodilation in hypercholesterolemic humans. J Clin Invest. 1992;90:1248–1253. doi: 10.1172/JCI115987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes M, Chowienczyk PJ, Ritter JM. Effects of inhibition of the l-arginine/nitric oxide pathway on vasodilation caused by beta-adrenergic agonists in human forearm. Circulation. 1997;95:2293–2297. doi: 10.1161/01.cir.95.9.2293. [DOI] [PubMed] [Google Scholar]
- Dietz NM, Rivera JM, Eggener SE, Fix RT, Warner DO, Joyner MJ. Nitric oxide contributes to the rise in forearm blood flow during mental stress in humans. J Physiol. 1994;480:361–368. doi: 10.1113/jphysiol.1994.sp020366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishy V, Sofowora GG, Xie HG, Kim RB, Byrne DW, Stein CM, Wood AJ. The effect of common polymorphisms of the beta2-adrenergic receptor on agonist-mediated vascular desensitization. N Engl J Med. 2001;345:1030–1035. doi: 10.1056/NEJMoa010819. [DOI] [PubMed] [Google Scholar]
- Drysdale CM, McGraw DW, Stack CB, Stephens JC, Judson RS, Nandabalan K, Arnold K, Ruano G, Liggett SB. Complex promoter and coding region beta 2-adrenergic receptor haplotypes alter receptor expression and predict in vivo responsiveness. Proc Natl Acad Sci U S A. 2000;97:10483–10488. doi: 10.1073/pnas.97.19.10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelke KA, Halliwill JR, Proctor DN, Dietz NM, Joyner MJ. Contribution of nitric oxide and prostaglandins to reactive hyperemia in human forearm. J Appl Physiol. 1996;81:1807–1814. doi: 10.1152/jappl.1996.81.4.1807. [DOI] [PubMed] [Google Scholar]
- Feldman RD. Beta-adrenergic receptor alterations in hypertension – physiological and molecular correlates. Can J Physiol Pharmacol. 1987;65:1666–1672. doi: 10.1139/y87-261. [DOI] [PubMed] [Google Scholar]
- Feldman RD, Limbird LE, Nadeau J, Robertson D, Wood AJ. Leukocyte beta-receptor alterations in hypertensive subjects. J Clin Invest. 1984;73:648–653. doi: 10.1172/JCI111255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratze G, Fortin J, Labugger R, Binder A, Kotanko P, Timmermann B, Luft FC, Hoehe M R, Skrabal F. Beta-2 adrenergic receptor variants affect resting blood pressure and agonist-induced vasodilation in young adult Caucasians. Hypertension. 1999;33:1425–1430. doi: 10.1161/01.hyp.33.6.1425. [DOI] [PubMed] [Google Scholar]
- Green SA, Cole G, Jacinto M, Innis M, Liggett SB. A polymorphism of the human beta 2-adrenergic receptor within the fourth transmembrane domain alters ligand binding and functional properties of the receptor. J Biol Chem. 1993;268:23116–23121. [PubMed] [Google Scholar]
- Green SA, Turki J, Innis M, Liggett SB. Amino-terminal polymorphisms of the human beta 2-adrenergic receptor impart distinct agonist-promoted regulatory properties. Biochemistry. 1994;33:9414–9419. doi: 10.1021/bi00198a006. [DOI] [PubMed] [Google Scholar]
- Greenfield ADM, Whitney RJ, Mowbray JF. Methods for the investigation of peripheral blood flow. Br Med Bull. 1963;19:101–109. doi: 10.1093/oxfordjournals.bmb.a070026. [DOI] [PubMed] [Google Scholar]
- Herrmann V, Buscher R, Go MM, Ring KM, Hofer JK, Kailasam MT, O'Connor DT, Parmer RJ, Insel PA. Beta2-adrenergic receptor polymorphisms at codon 16, cardiovascular phenotypes and essential hypertension in whites and African Americans. Am J Hypertens. 2000;13:1021–1026. doi: 10.1016/s0895-7061(00)01188-2. [DOI] [PubMed] [Google Scholar]
- Hoit BD, Suresh DP, Craft L, Walsh RA, Liggett SB. Beta2-adrenergic receptor polymorphisms at amino acid 16 differentially influence agonist-stimulated blood pressure and peripheral blood flow in normal individuals. Am Heart J. 2000;139:537–542. doi: 10.1016/s0002-8703(00)90099-1. [DOI] [PubMed] [Google Scholar]
- Jia H, Sharma P, Hopper R, Dickerson C, Lloyd DD, Brown MJ. Beta2-adrenoceptor gene polymorphisms and blood pressure variations in East Anglian Caucasians. J Hypertens. 2000;18:687–693. doi: 10.1097/00004872-200018060-00005. [DOI] [PubMed] [Google Scholar]
- Kato N, Sugiyama T, Morita H, Kurihara H, Sato T, Yamori Y, Yazaki Y. Association analysis of beta(2)-adrenergic receptor polymorphisms with hypertension in Japanese. Hypertension. 2001;37:286–292. doi: 10.1161/01.hyp.37.2.286. [DOI] [PubMed] [Google Scholar]
- Kotanko P, Binder A, Tasker J, Defreitas P, Kamdar S, Clark AJ, Skrabal F, Caulfield M. Essential hypertension in African Caribbean associates with a variant of the beta2-adrenoceptor. Hypertension. 1997;30:773–776. doi: 10.1161/01.hyp.30.4.773. [DOI] [PubMed] [Google Scholar]
- Krushkal J, Ferrell R, Mockrin SC, Turner ST, Sing CF, Boerwinkle E. Genome-wide linkage analyses of systolic blood pressure using highly discordant siblings. Circulation. 1999;99:1407–1410. doi: 10.1161/01.cir.99.11.1407. [DOI] [PubMed] [Google Scholar]
- Krushkal J, Xiong M, Ferrell R, Sing CF, Turner ST, Boerwinkle E. Linkage and association of adrenergic and dopamine receptor genes in the distal portion of the long arm of chromosome 5 with systolic blood pressure variation. Hum Mol Genet. 1998;7:1379–1383. doi: 10.1093/hmg/7.9.1379. [DOI] [PubMed] [Google Scholar]
- Lang CC, Stein CM, Brown RM, Deegan R, Nelson R, He HB, Wood M, Wood AJ. Attenuation of isoproterenol-mediated vasodilatation in blacks. N Engl J Med. 1995;333:155–160. doi: 10.1056/NEJM199507203330304. [DOI] [PubMed] [Google Scholar]
- Oates JA, Brown NJ. Antihypertensive agents and the drug therapy of hypertension. In: Hardman JG, Limbird LE, Gilman AG, editors. The Pharmacological Basis of Therapeutics. New York: McGraw-Hill; 2001. pp. 871–900. [Google Scholar]
- Panza JA, Casino PR, Kilcoyne CM, Quyyumi AA. Role of endothelium-derived nitric oxide in the abnormal endothelium-dependent vascular relaxation of patients with essential hypertension. Circulation. 1993;87:1468–1474. doi: 10.1161/01.cir.87.5.1468. [DOI] [PubMed] [Google Scholar]
- Reihsaus E, Innis M, MacIntyre N, Liggett SB. Mutations in the gene encoding for the beta 2-adrenergic receptor in normal and asthmatic subjects. Am J Respir Cell Mol Biol. 1993;8:334–339. doi: 10.1165/ajrcmb/8.3.334. [DOI] [PubMed] [Google Scholar]
- Stein C, Nelson R, Deegan R, He H, Wood M, Wood A. Forearm beta adrenergic receptor-mediated vasodilation is impaired, without alteration of forearm norepinephrine spillover, in borderline hypertension. J Clin Invest. 1995;96:579–585. doi: 10.1172/JCI118070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svetkey LP, Timmons PZ, Emovon O, Anderson NB, Preis L, Chen YT. Association of hypertension with beta2- and alpha2c10-adrenergic receptor genotype. Hypertension. 1996;27:1210–1215. doi: 10.1161/01.hyp.27.6.1210. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Mattei P, Ghiadoni L, Sudano I, Salvetti A. Defective l-arginine-nitric oxide pathway in offspring of essential hypertensive patients. Circulation. 1996;94:1298–1303. doi: 10.1161/01.cir.94.6.1298. [DOI] [PubMed] [Google Scholar]
- Timmermann B, Mo R, Luft FC, Gerdts E, Busjahn A, Omvik P, Li GH, Schuster H, Wienker TF, Hoehe MR, Lund-Johansen P. Beta-2 adrenoceptor genetic variation is associated with genetic predisposition to essential hypertension: The Bergen Blood Pressure Study. Kidney Int. 1998;53:1455–1460. doi: 10.1046/j.1523-1755.1998.00926.x. [DOI] [PubMed] [Google Scholar]
- Xie HG, Stein CM, Kim RB, Gainer JV, Sofowora G, Dishy V, Brown NJ, Goree RE, Haines JL, Wood AJ. Human beta2-adrenergic receptor polymorphisms: no association with essential hypertension in black or white Americans. Clin Pharmacol Ther. 2000;67:670–675. doi: 10.1067/mcp.2000.106293. [DOI] [PubMed] [Google Scholar]


