Abstract
Two forms of long-term depression (LTD) of excitatory synaptic transmission have been identified in the mammalian CNS, which are induced by the synaptic activation of N-methyl-d-aspartate (NMDA) and metabotropic glutamate (mGlu) receptors, respectively. The mGlu receptor-dependent form of LTD can be activated by application of 3,5-dihydroxyphenylglycine (DHPG), a group I selective mGlu receptor agonist. DHPG-induced LTD is increasingly being used to investigate the mechanisms of mGlu receptor-dependent LTD. However, recent experiments have argued for both a pre- and postsynaptic locus of expression of DHPG-induced LTD. In the present study we report that DHPG-induced LTD is not associated with changes in the sensitivity of CA1 neurons to bath applied AMPA. Furthermore, in contrast to homosynaptic LTD, DHPG-induced LTD is also not associated with changes in sensitivity to focally uncaged l-glutamate. These data do not support the notion that DHPG-induced LTD requires a modification of AMPA receptors, such as their internalisation, but are compatible with a presynaptic mechanism of expression.
Long-term depression (LTD) and long-term potentiation (LTP) are the subject of intense investigation since it is believed that these forms of synaptic plasticity are involved in a variety of physiological functions, including learning and memory (Bliss & Collingridge, 1993; Bear & Abraham, 1996). Two main forms of LTD have been identified in the mammalian CNS, which are distinguished on the basis of their induction mechanisms. One form, known as homosynaptic LTD, requires the activation of NMDA receptors, Ca2+ entry into the postsynaptic neuron and the activation of serine/threonine protein phosphatases, and is believed to be expressed postsynaptically (Bear & Abraham, 1996). The second form involves activation of mGlu receptors but the enzymes and expression mechanisms involved have not been so clearly identified (Anwyl, 1999; Kemp & Bashir, 2001). Both forms of LTD can be induced synaptically; the former is usually induced by delivering a prolonged period of low frequency stimulation (LFS) and the latter by delivering a similarly prolonged period of paired-pulse stimulation (see Kemp & Bashir, 2001).
A means of studying mGlu receptor-dependent LTD is to transiently activate mGlu receptors with a group I mGlu receptor agonist, such as 3,5-dihydroxyphenylglycine (DHPG) (Palmer et al. 1997; Camodeca et al. 1999; Fitzjohn et al. 1999). This, so-called, DHPG-induced LTD occludes with synaptically induced mGlu receptor-dependent LTD (Huber et al. 2001) and is being used increasingly to investigate signalling and expression mechanisms of this type of synaptic plasticity (Fitzjohn et al. 2001; Huber et al. 2001; Kleppisch et al. 2001; Schnabel et al. 2001; Snyder et al. 2001; Watabe et al. 2001; Xiao et al. 2001; Faas et al. 2002; Moult et al. 2002; Rush et al. 2002). Unlike NMDA receptor-dependent LTD, DHPG-induced LTD does not require either Ca2+ (Fitzjohn et al. 2001) or serine/threonine protein phosphatases (Schnabel et al. 2001) but involves activation of Gαq (Kleppisch et al. 2001), protein synthesis (Huber et al. 2001), p38 mitogen-activated protein kinase (Rush et al. 2002) and tyrosine dephosphorylation (Moult et al. 2002). The locus of expression of DHPG-induced LTD is highly controversial with evidence for both presynaptic (Fitzjohn et al. 2001; Watabe et al. 2001; Faas et al. 2002) and postsynaptic changes (Snyder et al. 2001; Xiao et al. 2001). Here we provide evidence that DHPG induces LTD without affecting sensitivity to either bath applied AMPA or rapidly uncaged l-glutamate.
Methods
Two different electrophysiological recording techniques were used to record from CA1 neurons. Rat hippocampal slices were obtained in accordance with the Animals (Scientific Procedures) Act 1986. In the first series of experiments, grease-gap recording from slices obtained from adult rats was used, as described previously (Palmer et al. 1997). In brief, adult female Wistar rats, of between 150 and 180 g in weight, were anaesthetised with halothane and decapitated. Transverse slices (400 μm in thickness), from which the CA3 regions were removed, were placed in ACSF comprising (mm): NaCl 124; KCl 3; NaHCO3 26; NaH2PO4 1.25; MgSO4 1; CaCl2 2; d-glucose 10, equilibrated with 95 % O2-5 % CO2. Individual slices were perfused with Mg2+ free ACSF at a rate of 2 ml min−1, at 28 °C. The Schaffer collateral- commissural pathway was stimulated at 0.033 Hz and field EPSPs recorded using a data acquisition program (Anderson & Collingridge, 2001). AMPA (1 μm) was applied by addition to the perfusing medium for 2 min.
In the second series of experiments, visually guided whole-cell patch-clamp recordings were obtained from either the cell soma or the proximal apical dendrite of CA1 neurons in slices obtained from 12- to 15-day-old male Sprague-Dawley rats, as described previously (Benke et al. 1998; Eder et al. 2002). In brief, rats were anaesthetised with isoflurane and decapitated. Slices were perfused at room temperature (22-24 °C) with ACSF comprising (mm): NaCl 125; KCl 2.5; NaHCO3 25; NaH2PO4 1.25; MgCl2 1; CaCl2 2; d-glucose 25, equilibrated with 95 % O2-5 % CO2. The patch solution comprised (mm): potassium gluconate 130, KCl 5, EGTA 0.5, Mg-ATP 2, Hepes 10, d-glucose 5. The access resistance of the electrodes was 18 ± 1 MΩ and 38 ± 1 MΩ for the somatic and dendtritic recordings, respectively. The Schaffer collateral- commissural pathway was stimulated at 0.033 Hz, using a bipolar metal electrode.
In these experiments, caged l-glutamate was released by focal photolysis, as described previously (Dodt et al. 1999, 2002; Eder et al. 2002). In brief, the beam of an argon ion UV laser was focused by the objective (60 ×, 0.9 NA, Olympus) onto a small spot (1 μm in diameter) positioned on a dendrite approximately 50-100 μm from the soma. Laser stimulation was delivered once every 30 s, 15 s after synaptic stimulation. Once a stable whole-cell recording had been obtained the ‘caged-glutamate’, γ-CNB (α-carboxy-2-nitrobenzyl)-caged glutamate (Molecular Probes) was added to the perfusate at a concentration of 0.25-0.5 mm, and the solution recirculated (6.5 ml volume). This had no discernible effect on neurons per se. l-Glutamate was released by brief laser pulses (3 ms; wavelength 351-364 nm; effective intensity 3-5 mW) applied at regular intervals throughout the experiment.
Results
Extracellular recording experiments
The first set of experiments was performed using extracellular recording techniques to record fEPSPs from the CA1 region of hippocampal slices. Perfusion of (SR)-DHPG (100 μm, 20 min) resulted in a substantial LTD. Thus, 30 min following washout of DHPG synaptic responses were depressed to 32 ± 8 % of control. In contrast, in the same experiments depolarisations induced by perfusion of AMPA (1 μm; 2 min) were not significantly affected by DHPG (95 ± 9 % of control; n = 5; Fig. 1). These data suggest that DHPG-induced LTD is mediated presynaptically. However, although DHPG induces LTD globally and does not require concomitant synaptic activity (Fitzjohn et al. 1999; Huber et al. 2001) it is not possible to fully discount the possibility that bath applied AMPA and synaptically released l-glutamate are sampling separate populations of AMPA receptors that are differentially regulated by DHPG.
Figure 1. DHPG-induced LTD does not affect responses to bath applied AMPA.
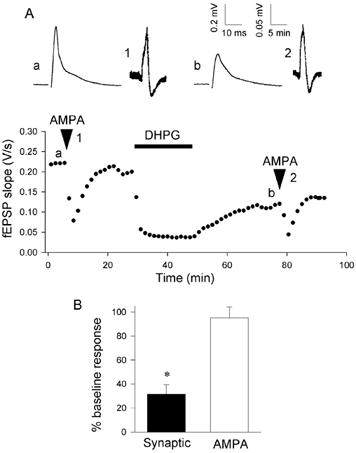
A, fEPSP slope vs. time. AMPA (1 μm, 2 min applied at times indicated by arrowheads) resulted in depolarisations and a consequential depression of the fEPSP. (SR)-DHPG (100 μm) resulted in a depression of fEPSPs but not AMPA-induced depolarisations. Representative fEPSPs (a, b) and AMPA-induced depolarisations (1, 2) are shown for the times indicated on the graph. B, pooled data from 5 experiments (*P < 0.05).
Focal glutamate responses
In the second set of experiments we therefore used photolysis to rapidly uncage l-glutamate over a small region of dendrite of a CA1 neuron (Dodt et al. 1999). Initially we wished to establish how closely we could mimic the synaptic activation of AMPA receptors in hippocampal slices, using this method. We found, using a laser intensity of 30 % maximum, that a 3 ms pulse focused on a 1 μm spot elicited a current that was similar, in both time course and amplitude, to synaptic currents induced in the same neurons by stimulation of Schaffer collateral- commissural fibres (see also Eder et al. 2002). Both responses were blocked in parallel by 5 μm NBQX (n = 6; Fig. 2A).
Figure 2. Homosynaptic LTD involves a parallel decrease in EPSC and focal glutamate responses.
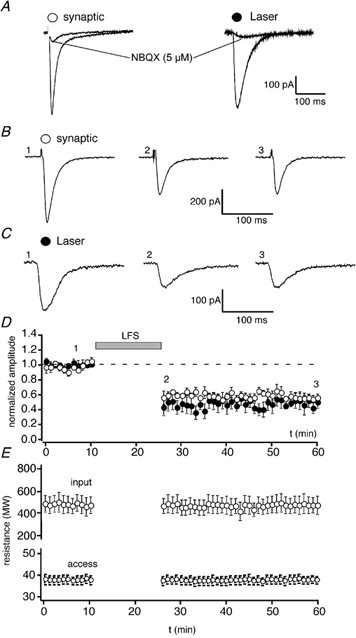
A, EPSCs (left) and current responses to uncaged glutamate (right) are blocked by NBQX (5 μm). B, EPSCs (averages of 4 successive traces) obtained before (1), shortly following (2) and 30 min following (3) the induction of LTD by LFS. C, inward currents induced by the uncaging of glutamate (3 ms, 3-5 mW, 1 μm laser spot diameter, size of uncaging area approx. 10 μm) at the corresponding time points for the same neuron. D, pooled data for 7 experiments plotting EPSC amplitude (open symbols) and laser-induced responses (filled symbols) vs. time. The numbers (1-3) refer to the times at which the traces (in B and C) were obtained. E, plots of input resistance (upper) and access resistance (lower) for these neurons.
Homosynaptic LTD
An important issue to establish is the extent to which focally released l-glutamate can detect alterations in AMPA receptors during synaptic plasticity. There is evidence that NMDA receptor-dependent homosynaptic LTD involves the internalisation of AMPA receptors (Carroll et al. 1999; Beattie et al. 2000). This is therefore a suitable form of plasticity with which to determine the sensitivity of the method. Indeed, previous studies have revealed that a form of NMDA receptor-dependent LTD in the hippocampus, induced by pairing depolarisation with photolysis of caged l-glutamate, is associated with a reduction in the amplitude of subsequent responses to uncaged l-glutamate (Kandler et al. 1998) and very similar findings have also been obtained in neocortex (Dodt et al. 1999; Eder et al. 2002).
We induced homosynaptic LTD using LFS either by pairing 60 shocks delivered at 0.5 Hz at −40 mV or by delivering 900 shocks at 1 Hz in bridge mode with no current injection. Whole-cell voltage-clamp recordings (-70 mV) were obtained locally from the dendritic region, where the photolysis was performed, and neighbouring fibres stimulated, as described previously (Benke et al. 1998). EPSCs and responses to glutamate photolytically released by laser illumination (3 ms duration) were obtained alternately throughout an experiment. LFS led to a reduction in amplitude of both the EPSCs and responses to focal l-glutamate. Indeed, both responses were depressed in parallel and to a very similar extent. EPSC amplitude was depressed to 57 ± 6 % and l-glutamate responses to 52 ± 9 % of control, 30 min following LFS (n = 7; Fig. 2). These data show that focally uncaged l-glutamate can be used to accurately monitor the behaviour of synaptic AMPA receptors during synaptic plasticity. Homosynaptic LTD was not associated with any change in the kinetics of EPSCs or in input resistance of the neurons. Access resistances were stable throughout.
DHPG-induced LTD
In the last set of experiments, we induced LTD by applying S-DHPG (30 μm; 20 min) to slices obtained from juvenile rats, as described previously (Fitzjohn et al. 1999). EPSCs and responses to focal glutamate were obtained alternately. DHPG induced a robust LTD in which EPSCs were depressed to 65 ± 6 % of control, 30 min following washout of the compound. In contrast, responses to focally uncaged l-glutamate were never depressed by this treatment; 30 min following washout of DHPG responses were 108 ± 6 % of control (n = 7; Fig. 3). DHPG-induced LTD was not associated with any change in the kinetics of EPSCs or in input resistance of the neurons. Access resistances were stable throughout.
Figure 3. DHPG-induced LTD does not involve a reduction in glutamate sensitivity.
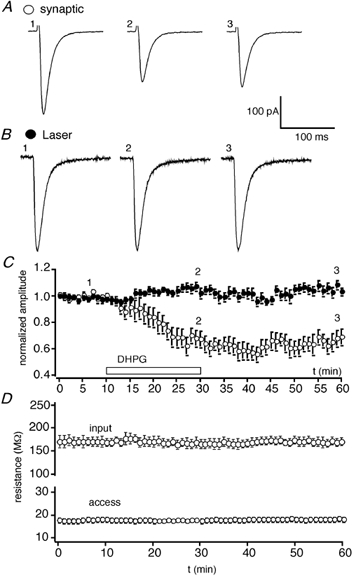
A, EPSCs (averages of 10 successive traces) obtained before (1), during (2) and 30 min following (3) the application of DHPG (30 μm; applied for time indicated by bar). B, inward currents induced by the uncaging of glutamate (same parameters as in Fig. 2) at the corresponding time points for the same neuron. C, pooled data for 7 experiments plotting EPSC amplitude (open symbols) and laser-induced responses (filled symbols) vs. time. The numbers (1-3) refer to the times at which the traces (in A and B) were obtained. D, plots of input resistance (upper) and access resistance (lower) for these neurons. In C and D, each point is the mean of 2 successive responses.
Discussion
In the present study we have found that homosynaptic LTD can be explained by a postsynaptic alteration in AMPA receptor function but DHPG-induced LTD cannot. We find a pronounced synaptic depression that is associated with no reduction in glutamate sensitivity, even when the application of glutamate is such that the response closely mimics the synaptic current. These data are most simply explained on the basis of postsynaptic and presynaptic expression mechanisms for NMDA and mGlu receptor-dependent LTD, respectively.
Homosynaptic LTD
In the course of validating the glutamate uncaging technique in hippocampal slices we found that homosynaptic LTD was associated with a decrease in glutamate sensitivity. Previous studies in the neocortex produced a similar result (Dodt et al. 1999; Eder et al. 2002). In addition, in the hippocampus it has been shown that a form of NMDA receptor-dependent LTD, elicited by pairing depolarisation with focal release of l-glutamate, is associated with a reduction in glutamate sensitivity (Kandler et al. 1998). These data demonstrate that the focal uncaging of glutamate provides a sensitive measure of alterations in postsynaptic AMPA receptor function during synaptic plasticity.
Several different mechanisms could underlie the alteration in glutamate sensitivity during homosynaptic LTD. These include a reduction in glutamate affinity, an increase in desensitisation, a decrease in AMPA receptor single-channel conductance, lateral diffusion of AMPA receptors from synaptic to extrasynaptic sites or internalisation of AMPA receptors. A major alteration in AMPA receptor affinity or desensitisation, sufficient to account for the reduction in amplitude, would be associated with changes in kinetics of dendritically recorded synaptic currents. Such changes have not been observed (Lüthi et al. 1999). In addition, homosynaptic LTD does not involve an alteration in AMPA receptor single-channel conductance (Lüthi et al. 1999). Lateral diffusion of AMPA receptors to extrasynaptic sites is also unlikely to account for LTD, since focally uncaged l-glutamate will affect all AMPA receptors in the vicinity of the uncaging (estimated to be within a 10 μm sphere; Eder et al. 2002) including extrasynaptic receptors. The simplest explanation, therefore, is an internalisation of AMPA receptors. This conclusion is consistent with the observations that intracellular application of a peptide that disrupts AMPA receptor trafficking mimics LTD (Lüthi et al. 1999), that treatments that affect exocytosis and endocytosis disrupt LTD (Lüscher et al. 1999; Man et al. 2000) and that LTD induction protocols induce AMPA receptor internalisation in cultured hippocampal neurons (Carroll et al. 1999; Beattie et al. 2000; Ehlers, 2000).
The finding that the reduction in the l-glutamate response was very similar to that of the synaptic response suggests that all AMPA receptors in the vicinity of the uncaging are affected by LTD induction to a similar extent. We optimised the conditions so that the majority, if not all, of the synaptic receptors that are exposed to uncaged l-glutamate are activated during the LFS protocol. This was achieved by stimulating a band of fibres, using a large stimulating electrode positioned close by, which would be expected to activate all of the excitatory synapses innervating the region of the neuron exposed to uncaged l-glutamate. However, since the uncaged l-glutamate will also affect extrasynaptic receptors, this means that either synaptic AMPA receptors greatly outnumber extrasynaptic AMPA receptors on CA1 spines and neighbouring dendrites or that extrasynaptic receptors are also internalised during LTD.
DHPG-induced LTD
In contrast to the situation with homosynaptic LTD, we find that DHPG can induce LTD without affecting postsynaptic glutamate sensitivity. Since the system is sensitive enough to detect postsynaptic changes we can dismiss a number of possible mechanisms for the expression of DHPG-induced LTD, such as changes in AMPA receptor kinetics, AMPA receptor conductance and internalisation of AMPA receptors. One possible explanation is that AMPA receptors move laterally in the membrane from synaptic to extrasynaptic sites, where they would still sense focally released l-glutamate. Another possibility is that DHPG-induced LTD is expressed presynaptically. The latter conclusion is consistent with the findings that DHPG-induced LTD is associated with alterations in paired-pulse facilitation (Fitzjohn et al. 2001; Faas et al. 2002) and can be blocked by treatments that affect presynaptic K+ channels (Watabe et al. 2001).
The results of the present experiments are not readily compatible with the hypothesis that internalisation of AMPA receptors is the mechanism underlying the expression of DHPG-induced LTD (Snyder et al. 2001; Xiao et al. 2001). Further studies are required to explain these differences and to determine how DHPG, which is believed to trigger the plasticity postsynaptically (Huber et al. 2000; Watabe et al. 2001), leads to a prolonged inhibition of l-glutamate release.
Acknowledgments
This work was supported by the MRC and by the Deutsche Forschungsgemeinschaft (SFB 391).
References
- Anderson WW, Collingridge GL. The LTP program: A data acquisition program for on-line analysis of long-term potentiation and other synaptic events. J Neurosci Meth. 2001;108:71–83. doi: 10.1016/s0165-0270(01)00374-0. [DOI] [PubMed] [Google Scholar]
- Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Rev. 1999;29:93–120. doi: 10.1016/s0165-0173(98)00050-2. [DOI] [PubMed] [Google Scholar]
- Bear MF, Abraham WC. Long-term depression in the hippocampus. Ann Rev Neurosci. 1996;19:437–462. doi: 10.1146/annurev.ne.19.030196.002253. [DOI] [PubMed] [Google Scholar]
- Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, Von Zastrow M, Malenka RC. Regulation of AMPA receptor endocytosis by a signalling mechanism shared with LTD. Nature Neurosci. 2000;3:1291–1300. doi: 10.1038/81823. [DOI] [PubMed] [Google Scholar]
- Benke TA, Lüthi A, Isaac JTR, Collingridge GL. Modulation of AMPA receptor unitary conductance by synaptic activity. Nature. 1998;393:793–797. doi: 10.1038/31709. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Camodeca N, Breakwell NA, Rowan JJ, Anwyl R. Induction of LTD by activation of group 1 mGluR in the dentate gyrus in vitro. Neuropharmacology. 1999;38:1597–1606. doi: 10.1016/s0028-3908(99)00093-3. [DOI] [PubMed] [Google Scholar]
- Carroll RC, Lissin DV, Von Zastrow M, Nicoll RA, Malenka RC. Rapid redistribution of glutamate receptors contributes to long-term depression in hippocampal cultures. Nature Neurosci. 1999;2:454–460. doi: 10.1038/8123. [DOI] [PubMed] [Google Scholar]
- Dodt H-U, Eder M, Frick A, Zieglgänsberger W. Precisely localized LTD in the neocortex revealed by infrared-guided laser stimulation. Science. 1999;286:110–113. doi: 10.1126/science.286.5437.110. [DOI] [PubMed] [Google Scholar]
- Dodt H-U, Eder M, Schierloh A, Zieglgänsberger W. Infrared-guided laser stimulation of neurons in brain slices. Science's STKE. 2002;120:pl2. doi: 10.1126/stke.2002.120.pl2. [DOI] [PubMed] [Google Scholar]
- Eder M, Zieglgänsberger W, Dodt H-U. Neocortical long-term potentiation and long-term depression: Site of expression investigated by infrared-guided laser stimulation. J Neurosci. 2002;22:7558–7568. doi: 10.1523/JNEUROSCI.22-17-07558.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28:511–525. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- Faas GC, Adwanikar H, Gereau RW, IV, Saggau P. Modulation of presynaptic calcium transients by metabotropic glutamate receptor activation: A differential role in acute depression of synaptic transmission and long-term depression. J Neurosci. 2002;22:6885–6890. doi: 10.1523/JNEUROSCI.22-16-06885.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzjohn SM, Kingston AE, Lodge D, Collingridge GL. DHPG-induced LTD in area CA1 of juvenile rat hippocampus: characterization and sensitivity to novel mGlu antagonists. Neuropharmacology. 1999;38:1577–1583. doi: 10.1016/s0028-3908(99)00123-9. [DOI] [PubMed] [Google Scholar]
- Fitzjohn SM, Palmer MJ, May JE, Neeson A, Morris SAC, Collingridge GL. A characterisation of long-term depression induced by metabotropic glutamate receptor activation in the rat hippocampus in vitro. J Physiol. 2001;537:421–430. doi: 10.1111/j.1469-7793.2001.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Kayer MS, Bear MR. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288:1254–1256. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- Huber KM, Roder JC, Bear MF. Chemical induction of mGluR5- and protein synthesis-dependent long-term depression in hippocampal area CA1. J Neurophysiol. 2001;86:321–325. doi: 10.1152/jn.2001.86.1.321. [DOI] [PubMed] [Google Scholar]
- Kandler K, Katz LC, Kauer JA. Focal photolysis of caged glutamate produces long-term depression of hippocampal glutamate receptors. Nature Neurosci. 1998;1:119–123. doi: 10.1038/368. [DOI] [PubMed] [Google Scholar]
- Kemp N, Bashir ZI. Long-term depression: a cascade of induction and expression mechanisms. Prog Neurobiol. 2001;65:339–365. doi: 10.1016/s0301-0082(01)00013-2. [DOI] [PubMed] [Google Scholar]
- Kleppisch T, Voigt V, Allman R, Offermanns S. Gαq-Deficient mice lack metabotropic glutamate receptor-dependent long-term depression but show normal long-term potentiation in the hippocampal CA1 region. J Neurosci. 2001;21:4943–4948. doi: 10.1523/JNEUROSCI.21-14-04943.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher C, Xia H, Beattie EC, Carroll RC, Von Zastrow M, Malenka RC, Nicoll RA. Role of AMPA receptor cycling in synaptic transmission and plasticity. Neuron. 1999;24:649–658. doi: 10.1016/s0896-6273(00)81119-8. [DOI] [PubMed] [Google Scholar]
- Lüthi A, Chittajallu R, Duprat F, Palmer MJ, Benke TA, Kidd FL, Henley JM, Isaac JTR, Collingridge GL. Hippocampal LTD expression involves a pool of AMPARs regulated by the NSF-GluR2 interaction. Neuron. 1999;24:389–399. doi: 10.1016/s0896-6273(00)80852-1. [DOI] [PubMed] [Google Scholar]
- Man YH, Lin JW, Ju WH, Ahmadian G, Liu L, Becker LE, Sheng M, Wang YT. Regulation of AMPA receptor-mediated synaptic transmission by clathrin-dependent receptor internalization. Neuron. 2000;25:649–662. doi: 10.1016/s0896-6273(00)81067-3. [DOI] [PubMed] [Google Scholar]
- Moult PR, Schnabel R, Kilpatrick IC, Bashir ZI, Collingridge GL. Tyrosine dephosphorylation underlies DHPG-induced LTD. Neuropharmacology. 2002;43:175–180. doi: 10.1016/s0028-3908(02)00110-7. [DOI] [PubMed] [Google Scholar]
- Palmer MJ, Irving AJ, Seabrook GR, Jane DE, Collingridge GL. The Group 1 mGlu receptor agonist DHPG induces a novel form of LTD in the CA1 region of the hippocampus. Neuropharmacology. 1997;36:1517–1532. doi: 10.1016/s0028-3908(97)00181-0. [DOI] [PubMed] [Google Scholar]
- Rush AM, Wu JW, Rowan MJ, Anwyl R. Group I metabotropic glutamate receptor (mGluR)-dependent long-term depression mediated via p38 mitogen-activated protein kinase is inhibited by previous high frequency stimulation and activation of mGluRs and protein kinase C in the rat dentate gyrus in vitro. J Neurosci. 2002;22:6121–6128. doi: 10.1523/JNEUROSCI.22-14-06121.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabel R, Kilpatrick IC, Collingridge GL. Protein phosphatase inhibitors facilitate DHPG-induced LTD in the CA1 region of the hippocampus. Br J Pharmacol. 2001;132:1095–1101. doi: 10.1038/sj.bjp.0703905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Philpot BD, Huber KM, Dong X, Fallon JR, Bear MF. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nature Neurosci. 2001;4:1079–1085. doi: 10.1038/nn746. [DOI] [PubMed] [Google Scholar]
- Watabe AM, Carlisle HJ, O'Dell TJ. Postsynaptic induction and presynaptic expression of Group 1 mGluR-dependent LTD in the hippocampal CA1 region. J Neurophysiol. 2001;87:1395–1403. doi: 10.1152/jn.00723.2001. [DOI] [PubMed] [Google Scholar]
- Xiao MY, Zhou Q, Nicoll RA. Metabotropic glutamate receptor activation causes a rapid redistribution of AMPA receptors. Neuropharmacology. 2001;41:664–671. doi: 10.1016/s0028-3908(01)00134-4. [DOI] [PubMed] [Google Scholar]


