Abstract
Whole-cell patch-clamp recordings of GABAergic IPSCs were made from cholinergic interneurones in slices of striatum from developing rats aged 21–60 days postnatal. In addition, the Ca2+ channel subtypes involved in synaptic transmission, as well as dopamine (DA)-induced presynaptic inhibition, were investigated pharmacologically with development by bath application of Ca2+ channel blockers and DA receptor agonists. The IPSC amplitude was reduced by ω-conotoxin GVIA (ω-CgTX) or ω-agatoxin TK (ω-Aga-TK) across the whole age range, suggesting that multiple types of Ca2+ channels mediate transmission of the synapse. The IPSC fraction reduced by ω-CgTX significantly decreased, whereas that reduced by ω-Aga-TK remained unchanged with development. DA or quinpirole, a D2-like receptor agonist, presynaptically reduced the IPSC amplitude throughout development. The DA-induced inhibition decreased with age in parallel with the decrease in N-type Ca2+ channels. DA showed no further inhibition of IPSCs after the inhibitory effect of ω-CgTX had reached steady state throughout development. These results demonstrate that there is a functional link between presynaptic N-type Ca2+ channels and D2-like DA receptors at inhibitory synapses in the striatum. They also demonstrate that the suppression of GABAergic transmission by D2-like receptors is mediated by modulation of N-type Ca2+ channels and decreases in parallel with the developmental decline in the contribution of N-type Ca2+ channels to exocytosis.
It has been suggested that the interaction between acetylcholine (ACh) and dopamine (DA) in the basal ganglia has a crucial role in the control of voluntary movements, and disorder of the interaction could cause basal ganglia-related disorders such as Parkinson's disease (Lehmann & Langer, 1983; Calabresi et al. 2000; Kaneko et al. 2000). The primary source of ACh in the basal ganglia is cholinergic interneurones in the striatum (Bolam et al. 1984; Phelps et al. 1985), which receive dopaminergic fibres originating in the substantia nigra pars compacta (Kubota et al. 1987). Although several studies have reported postsynaptic DA receptor-mediated modulation of activity of striatal cholinergic interneurones (Aosaki et al. 1998; Yan et al. 1997), little information is available on the DA-induced modulation of synaptic transmission onto these neurones. It has been recently demonstrated that activation of D2-like DA receptors at the GABAergic terminals onto cholinergic interneurones in the striatum of young rats inhibits GABA release (Pisani et al. 2000; Momiyama & Koga, 2001).
Fast synaptic transmission in the mammalian central nervous system is mediated by multiple types of voltage-dependent Ca2+ channels, including N-, P/Q- and R-types (Takahashi & Momiyama, 1993; Wheeler et al. 1994; Wu et al. 1998), revealed by the use of Ca2+ channel subtype-specific toxins. However, the toxin sensitivity of inhibitory synapses has been examined in relatively few sites, including the hippocampal CA1 region (Lenz et al. 1998) and the medial superior olive nucleus (Barnes-Davies et al. 2001). The mechanism underlying the D2-like receptor-mediated presynaptic inhibition in the present GABAergic synapse is a selective blockade of N-type Ca2+ channels, although multiple types of Ca2+ channels are also involved in this synaptic transmission (Momiyama & Koga, 2001). Recent studies have demonstrated that N-type Ca2+ channels contribute to synaptic transmission only during the early postnatal stage in several central synapses, including GABAergic synapses in the cerebellum and thalamus, glycinergic synapses in the spinal dorsal horn, and glutamatergic synapses in the calyx of Held, where P-type channels dominate in mature animals (Iwasaki & Takahashi, 1998; Forsythe et al. 1998; Iwasaki et al. 2000). Therefore, the contribution of N-type Ca2+ channels to GABAergic synaptic transmission in the striatum might decrease with development. If the linkage between the N-type Ca2+ channels and D2-like receptor is tight, DA-induced presynaptic inhibition may also decline in association with the decrease in N-type channel contribution. The aim of the present study was to clarify these issues. The present results suggest that D2-like receptor-mediated presynaptic inhibition decreases in parallel with the decrease in N-type channel contribution. Preliminary data from the present study have been published in abstract form (Momiyama, 2001, 2002).
Methods
All experiments were performed according to the Guiding Principles for the Care and Use of Animals in the Field of Physiological Sciences of the Physiological Society of Japan (1998) and the UK Animals (Scientific Procedures) Act 1986. The experimental procedures have been previously described (Momiyama & Koga, 2001). Briefly, rats of postnatal day 21–60 (P21–60) were killed by decapitation under halothane anaesthesia, and then coronal slices containing the striatum (300 μm thick) were prepared. The composition of the cutting Krebs solution was as follows (mm): choline chloride, 120; KCl, 2.5; CaCl2, 0.5; MgCl2, 7; NaH2PO4, 1.25; NaHCO3, 26; d-glucose, 15; ascorbic acid, 1.3. Whole-cell recordings were made after 1 h incubation of the slices in standard Krebs solution of the following composition (mm): NaCl, 124; KCl, 3; CaCl2, 2.4; MgCl2, 1.2; NaH2PO4, 1; NaHCO3, 26; d-glucose, 10 (pH 7.4 when bubbled with 95 % O2-5 % CO2) at room temperature (21-25 °C). Patch pipettes made from standard-walled borosilicate glass capillaries (1.5 mm outer diameter; Clark Electromedical, Reading, UK) were filled with an internal solution of the following composition (mm): CsCl, 140; NaCl, 9; Cs-EGTA, 1; Cs-Hepes, 10; Mg-ATP, 2 (pH adjusted with 1 m CsOH).
Neurones in the dorsolateral part of the striatum (Momiyama & Koga, 2001) were viewed via a microscope with a water-immersion optic (Olympus BX50WI, Olympus, Tokyo, Japan). Whole-cell recordings were made using a patch-clamp amplifier (Axopatch 200B, Axon Instruments, Foster City, CA, USA) from 102 large neurones identified as cholinergic according to their size and membrane properties (Jiang & North, 1991; Kawaguchi, 1992; Momiyama & Koga, 2001).
After the whole-cell configuration was made, synaptic currents were evoked by focal stimulation at −60 mV with a stimulating glass electrode containing 1 m NaCl in the presence of 6-cyano-7-nitroquinoxalline-2,3-dione (CNQX, 5 μm), d-(-)-2-amino-5-phosphonopentanoic acid (d-AP5, 25 μm) and strychnine (0.5 μm) to block NMDA-, non-NMDA- and glycine receptor-mediated components, respectively. The mean distance between the recorded cell and the stimulating electrode was 50.0 ± 1.50 μm (n = 102). There were no significant (P > 0.05) differences in the amplitude of the synaptic currents between ages. The evoked synaptic currents were reversibly blocked by bath application of bicuculline (10 μm) in all of the nine cells examined (data not shown), confirming that they were GABAA receptor-mediated inhibitory postsynaptic currents (IPSCs; Momiyama & Koga, 2001). Experiments were carried out at room temperature (21-25 °C).
Data were stored on digital audiotapes using a DAT recorder (PC204AX, Sony, Japan). IPSCs were digitized off-line at 10 kHz (low-pass-filtered at 2 kHz with an 8-pole Bessel filter) using pCLAMP 8 (Axon Instruments) software. The effects of drugs on the evoked IPSCs were assessed by averaging IPSC amplitudes for 100 s (20 traces) during the peak response of drug and comparing this value with the averaged amplitude of 20 traces just before drug application. Statistical analysis was carried out using both Student's two-tailed t test and the non-parametrical Mann-Whitney U test. For all statistics, 0.05 was used as the confidence limit. Data are expressed as means ± s.e.m.
Drugs were stored in frozen stock solution and dissolved in the perfusing solution just before application at the final concentration. All drugs were bath applied. Trans-(-)-4aR-4,4a,5,6,7,8,8a,9octahydro-5-propyl-1H-pyrazolo[3,4-g]quinoline hydrochloride (quinpirole) was purchased from Research Biochemicals International (Natick, MA, USA). CNQX and d-AP5 were from Tocris Cookson (Bristol, UK). Dopamine, bicuculline methochloride and strychnine were from Sigma (St Louis, MO, USA). Synthetic ω-conotoxin GVIA (ω-CgTX) and ω-agatoxin TK (ω-Aga-TK) were from Alamone labs (Jerusalem, Israel). These toxins were dissolved in oxygenated solution containing cytochrome c (1 mg ml−1; Sigma).
Results
Developmental decline in ω-CgTX sensitivity of GABAergic IPSCs in striatal cholinergic interneurones
The relative magnitude of inhibition by an N-type channel blocker ω-CgTX (3 μm) at P21-29 was 53.5 ± 3.1 % (n = 16). The remaining fraction of IPSCs was largely blocked by subsequent application of a P/Q-type channel blocker, ω-Aga-TK (200 nm). The ω-CgTX-sensitive IPSC fraction decreased with age (Fig. 2). Figure 1A shows the effect of ω-CgTX and subsequent application of ω-Aga-TK on the IPSCs evoked in a striatal cholinergic interneurone of a P53 rat. Bath application of ω-CgTX (3 μm) showed little effect, the remaining fraction being largely blocked by subsequent application of ω-Aga-TK (200 nm). The relative magnitude of inhibition by ω-CgTX (3 μm) at P30-39, P40-49 and P51-60 was 46.8 ± 4.4 % (n = 16), 35.7 ± 8.0 % (n = 5) and 26.6 ± 3.2 % (n = 14), respectively (Fig. 2).
Figure 2. Summary of the developmental changes in the effects of ω-CgTX and ω-Aga-TK on the IPSCs in striatal cholinergic interneurones.
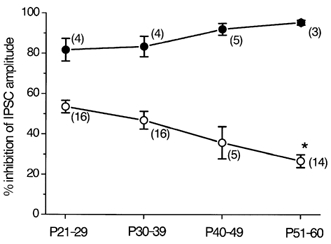
The ω-CgTX-sensitive fraction (○) and ω-Aga-TK-sensitive fraction (•) of the IPSCs are plotted at different postnatal ages. Each point shows the mean ± s.e.m. derived from 3-16 cells, as indicated in parentheses. *P < 0.05 compared with the value of P21-29 or P30-39.
Figure 1. Effects of ω-CgTX and ω-Aga-TK on the GABAergic IPSCs in striatal cholinergic interneurones of adult rats.
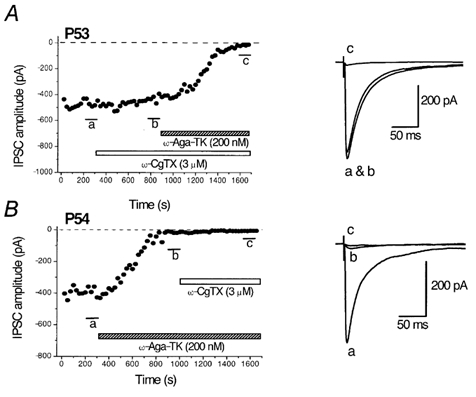
A, time course of the effects of ω-CgTX (3 μm) and subsequent application of ω-Aga-TK (200 nm) on the amplitude of IPSCs evoked in striatal cholinergic interneurones of a P53 rat. B, time course of the effects of ω-Aga-TK (200 nm) and subsequent application of ω-CgTX (3 μm) on the amplitude of IPSCs in a P54 rat. IPSCs were evoked at 0.2 Hz at −60 mV. Each point represents the mean amplitude of five consecutive responses. Blockers were applied during the indicated periods. Superimposed traces on the right are averages of 20 consecutive IPSCs during the indicated periods.
When ω-Aga-TK (200 nm) was applied first to P21-29 rats, IPSCs evoked in striatal cholinergic interneurones were inhibited by 81.7 ± 5.6 % (n = 4, Fig. 2), and the remaining fraction was further diminished by ω-CgTX (3 μm). As shown in Fig. 1B, ωAga-TK (200 nm) largely diminished the IPSCs at P54. The ω-Aga-TK-sensitive fraction at P30-39, P40-49 and P51-60 was 83.5 ± 5.1 % (n = 4), 92.1 ± 2.9 % (n = 5) and 95.3 ± 1.2 % (n = 3), respectively (Fig. 2). The ω-Aga-TK-sensitive fraction apparently increased with age (Fig. 2). However, the differences were not statistically significant.
The inhibition of the evoked IPSCs by subsequent application of both ω-CgTX (3 μm) and ω-Aga-TK (200 nm) remained unchanged until P60: by 95.4 ± 0.93 % (n = 13), 94.0 ± 0.89 % (n = 13), 95.3 ± 1.7 % (n = 4) and 96.0 ± 1.2 % (n = 4), at P21-29, P30-39, P40-49 and P51-60, respectively.
Developmental decrease in dopamine-induced inhibition of IPSCs
In young rats (P12-17), activation of presynaptic D2-like receptors inhibits GABA release onto striatal cholinergic interneurones by selectively blocking N-type Ca2+ channels (Momiyama & Koga, 2001). To investigate whether D2-like receptor-mediated presynaptic inhibition also changes with age, the effects of DA and quinpirole, a D2-like receptor agonist, were examined on the IPSCs during age range to P60. Figure 3Aa shows the effect of DA (30 μm) on the evoked IPSCs in a striatal cholinergic interneurone in a P26 rat. DA inhibited the IPSCs in a reversible manner. The DA-induced inhibition at P21-29 was 41.5 ± 4.4 % (n = 10), which was similar to that of P12-17 rats (Momiyama & Koga, 2001). The DA-induced inhibition of IPSCs decreased with age. As shown in Figure 3Ab, DA had little or no effect on the IPSCs in rats over P50. DA (30 μm) inhibited the IPSCs by 29.6 ± 5.5 % (n = 9) at P30-39, 21.4 ± 3.8 % (n = 7) at P40-49 and 9.7 ± 3.0 % (n = 12) at P51-60 (Fig. 4A).
Figure 3. Developmental decline in the inhibitory effects of dopamine (DA) and quinpirole on the IPSCs in striatal cholinergic interneurones.
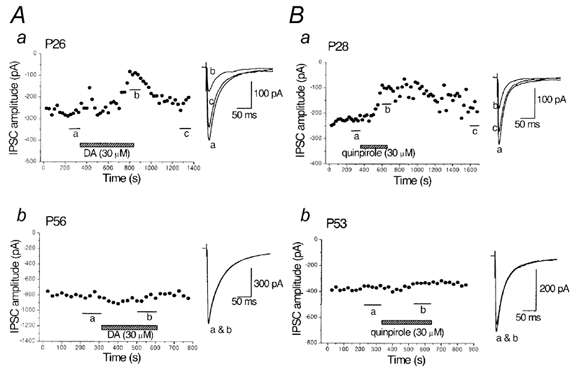
A, time course of the inhibitory effect of DA (30 μm) on the evoked IPSCs in striatal cholinergic interneurones obtained from P26 rats (a) and lack of DA-induced inhibition in a P56 rat (b). B, time course of the inhibitory effect of quinpirole (30 μm) on the IPSCs in a P28 rat (a) and lack of effect in a P53 rat (b). IPSCs were evoked at 0.2 Hz at −60 mV. Each point represents the mean amplitude of five consecutive responses. DA or quinpirole was applied during the indicated periods. Traces to the right show averaged IPSCs of 20 consecutive responses during the indicated periods.
Figure 4. Summarized plots showing the developmental decreases in DA- and quinpirole-induced inhibition of the IPSCs.
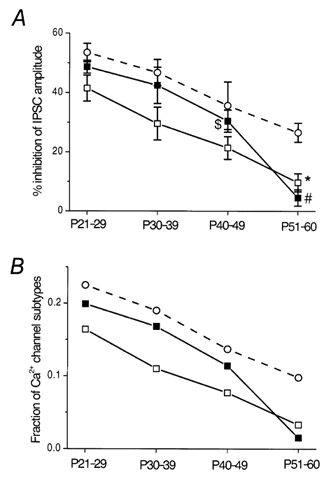
A, summarized effects of DA (30 μm, □) and quinpirole (30 μm, ▪) on the evoked IPSCs at different postnatal ages, compared with the effect of ω-CgTX (○, derived from Fig. 2). Each point shows the mean ± s.e.m. derived from 3-16 cells. *P < 0.05 compared with the value of P21-29 or P30-39. #P < 0.05 compared with the value of P21-29, P30-39 or P40-49. $P < 0.05 compared with the value of P21-29. B, developmental declines in the estimated fractions of Ca2+ channels involved in the IPSC inhibition by DA (□) or quinpirole (▪), compared with the change in the fraction of N-type channels (○). The fractions were calculated assuming a third-power relation between presynaptic Ca2+ concentration and postsynaptic response amplitude.
Quinpirole-induced inhibition of the IPSCs also decreased with age. Figure 3Ba shows the inhibitory effect of quinpirole (30 μm) on the IPSCs in a P28 rat, and Figure 3Bb shows the lack of quinpirole-induced effect in a P53 rat. The quinpirole (30 μm)-induced inhibition of IPSCs was 48.7 ± 2.2 % (n = 4) at P21-29, 42.5 ± 6.11 % (n = 3) at P30-39, 30.5 ± 3.7 % (n = 3) at P40-49, and 4.5 ± 2.8 % (n = 3) at P51-60 (Fig. 4A).
A non-linear relationship between presynaptic calcium concentration and transmitter release has been reported in several central synapses (Takahashi & Momiyama, 1993; Momiyama & Koga, 2001). Assuming a power function for the relationship, the relative amplitude of postsynaptic currents remaining after application of ω-CgTX (A) can be described as A = (1 – a)m, where a represents a fraction of the presynaptic Ca2+ channel subtypes sensitive to ω-CgTX (N-type) and m is the power coefficient (Takahashi & Momiyama, 1993; Momiyama & Koga, 2001). Assuming m = 3, the value a at P21-29, P30-39, P40-49 and P51-60 was 0.225, 0.190, 0.137 and 0.098, respectively (Fig. 4B). According to this relationship, the fractions of Ca2+ channels involved in DA- or quinpirole-induced inhibition of the evoked IPSCs could be calculated. The fraction sensitive to DA at P21-29, P30-39, P40-49 and P51-60 was 0.164, 0.110, 0.077 and 0.033, respectively (Fig. 4B). Similarly, the quinpirole-sensitive fraction at P21-29, P30-39, P40-49 and P51-60 was 0.199, 0.168, 0.114 and 0.015, respectively (Fig. 4B).
Parallel decrease in ω-CgTX and DA sensitivity during development
In order to investigate whether or not ω-CgTX sensitivity and D2-like receptor-mediated presynaptic inhibition decrease in parallel with development, and whether the coupling between these two molecules is tight or not during developmental stages, the effects of ω-CgTX and DA were examined in the same neurones at different ages of rats.
Figure 5 shows the effect of DA before and after the application of ω-CgTX in P26 (Fig. 5A) and P56 (Fig. 5B) rats. ω-CgTX (3 μm) was applied after the IPSCs had recovered from the inhibition induced by the initial application of DA (30 μm). DA was applied again after the ω-CgTX-induced inhibition had reached steady state (Momiyama & Koga, 2001). DA inhibited the IPSCs by 40.3 ± 5.1 % (n = 7), 18.0 ± 3.9 % (n = 4) and 8.3 ± 3.0 % (n = 4) at P25-28, P37-40 and P55-56, respectively (Fig. 6A). The ω-CgTX-induced inhibition in the respective groups of neurones was 51.8 ± 6.2 % (n = 7), 37.3 ± 8.2 % (n = 4) and 25.5 ± 4.0 % (n = 4) at P25-28, P37-40 and P55-56, respectively (Fig. 6A). As shown in Fig. 5A and B, DA had no further inhibitory effect on the IPSCs after the ω-CgTX-induced effect had reached steady state at P26 or P56, as was the case in younger rats (Momiyama & Koga, 2001). The DA-induced inhibition after ω-CgTX application was 1.1 ± 0.42 % (n = 7), 0.67 ± 0.39 % (n = 4) and 1.6 ± 0.54 % (n = 4) at P25-28, P37-40 and P55-56, respectively (Fig. 6A). Figure 6B shows the relationship between DA- and ω-CgTX-induced inhibitory effects in the 15 neurones described above (seven cells at P25-28, four cells at P37-40 and four cells at P55-56). The relation between the effects of DA and ω-CgTX was significant (r = 0.83, P < 0.001, Fig. 6B), both decreasing in parallel with age.
Figure 5. Occlusion of DA-induced inhibition by ω-CgTX in young and adult rats.
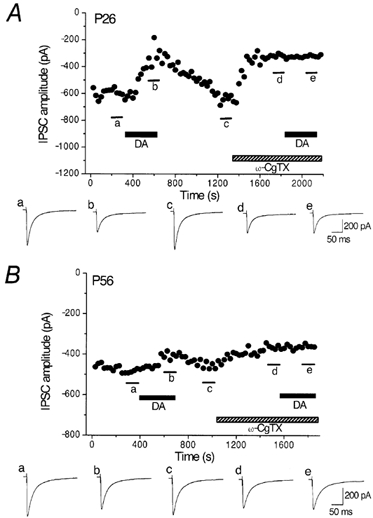
Top panels in A and B, effects of DA (30 μm) on the evoked IPSCs before and after application of ω-CgTX (3 μm) at P26 (A) and P56 (B). IPSCs were evoked at 0.2 Hz. Vh, −60 mV. Each point represents the mean amplitude of five consecutive IPSCs. DA and ω-CgTX were applied in the bath during the indicated periods. ω-CgTX was applied after IPSCs had recovered from the inhibition by an initial application of DA. DA was applied again after ω-CgTX-induced suppression had reached steady state. Bottom panels in A and B, averaged traces of 20 consecutive IPSCs during the indicated periods. Note the small effect of DA and ω-CgTX at P56.
Figure 6. Parallel decrease in DA- and ω-CgTX-induced inhibition with age.
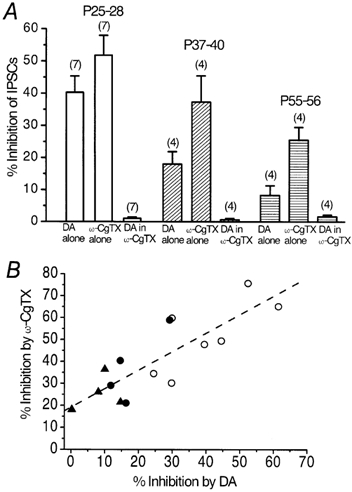
A, summarized histograms showing the inhibitory effects of DA (30 μm), ω-CgTX (3 μm) and DA in the presence of ω-CgTX examined in the same neurones at three different age ranges of rats. Note that, throughout the developmental stages, DA no longer inhibited the IPSCs after the effect of ω-CgTX had reached steady state (1.1 ± 0.42 % at P25-28, 0.67 ± 0.39 % at P37-40 and 1.6 ± 0.54 % at P55-56). Numbers of cells are indicated in parentheses. B, correlation between DA- and ω-CgTX-induced inhibition of the IPSCs examined in the same neurones. Each point represents one neurone summarized in A (○, P25-28; •, P37-40; ▴, P55-56). The dashed line was drawn by the least-squares method (r = 0.83, P < 0.001).
Discussion
The present study demonstrates that the contribution of N-type Ca2+ channels to GABAergic transmission onto cholinergic interneurones in the striatum, and D2-like receptor-mediated presynaptic inhibition of the transmission, decrease in parallel during postnatal development. It also demonstrates that presynaptic N-type Ca2+ channels are tightly linked with D2-like receptors as a specific target throughout the developmental stage.
Recent studies have reported that the contribution of N-type Ca2+ channels is limited during postnatal weeks 2-3 in several central synapses, whereas it remains unchanged in other synapses up to around P50 (Iwasaki & Takahashi, 1998; Iwasaki et al. 2000). The present results show that the GABAergic synapse onto striatal cholinergic interneurones has an intermediate profile, since the ω-CgTX-sensitive fraction of the IPSCs gradually decreased with age until P60, but did not absolutely disappear. Previous studies have suggested that glutamatergic synapses in the hippocampus undergo similar developmental change (Luebke et al. 1993; Wheeler et al. 1994). It has been reported, at the calyx of Held synapse, that N-type channels are located more distant from the transmitter release sites than P/Q-type channels (Wu et al. 1999), and that these remote N-type channels disappear with postnatal development (Iwasaki & Takahashi, 1998; Iwasaki et al. 2000). It remains to be elucidated whether or not N-type channels are located more distant from GABA release sites than P/Q-type channels, and whether such a change in the spatiotemporal profile of the Ca2+ channel domain occurs with development in the present striatal synapse.
Several studies have reported that G-protein-coupled receptors such as adenosine receptors (Mogul et al. 1993; Umemiya & Berger, 1994), metabotropic receptors (Takahashi et al. 1996; Stefani et al. 1998), oxytocin receptors (Hirasawa et al. 2001) and DA receptors (Momiyama & Koga, 2001) selectively couple to the N- or P/Q-types of Ca2+ channels in the presynaptic terminals. The present study is the first report of developmental change in the modulation of central synaptic transmission by G-protein-coupled receptors in association with a change in the contribution of a certain Ca2+ channel subtype to transmission. A diverse spatial localization of DA receptors in striatal neurones has been suggested (Radnikow & Misgeld, 1998; Momiyama & Koga, 2001). In addition, several studies have reported changes in DA receptors, DA content or DA transporter density in the striatum during developmental stages comparable to those in the present study. D2-receptors increase at 3-4 weeks of age (Broaddus & Bennett, 1990). An increase in DA in the striatum occurs during postnatal weeks 2-3, and the content remains relatively constant after this period (Noisin & Thomas, 1988). D2-receptor mRNAs gradually increase until P16, then decline slightly, and a cluster pattern becomes manifest later in development (P16), although the hybridization signal is fairly evenly distributed throughout the striatum at earlier developmental stages (Chen & Weiss, 1991). DA transporter density increases until puberty (P50) and declines considerably and steadily thereafter to about one-quarter of the pubertal value at old age (Moll et al. 2000). These changes within the striatum could totally underlie the present decrease in DA-induced presynaptic inhibition. Therefore, developmental redistribution of Ca2+ channels in combination with changes in the presynaptic D2-like receptor expression, as well as their spatial relation, may contribute to the remodelling of the modulation of transmitter release.
What is the functional implication for the parallel decrease in the contribution of ω-CgTX-sensitive transmission and DA-induced presynaptic inhibition at the GABAergic synapse onto striatal cholinergic interneurones of developing rats? A number of neurochemical studies have shown DA-induced modulation of ACh release in the striatum (for review, see Di Chiara et al. 1994). In addition, it has been suggested that the balance between DA and ACh is essential for motor control, and that disruption of the balance could result in dysfunction, as observed in Parkinson's disease (Lehmann & Langer, 1983; Calabresi et al. 2000; Kaneko et al. 2000). Therefore, the present results indicate the relationship between motor control and ageing. Actually, an age-dependent differential response of DA systems to high doses of methamphetamine has been demonstrated; P40 rats did not manifest the long-term (7 day) DA-related extrapyramidal symptoms observed in P90 rats induced by high doses of methamphetamine (Kokoshka et al. 2000). Another implication for developing striatal synapses might be the expression of synaptic plasticity, since long-term depression (LTD) in corticostriatal glutamatergic synapses has been proposed as a cellular model for developmental and adult neuronal plasticity in the striatum (Calabresi et al. 1996). It has been shown that LTD expression is associated with a decrease in transmitter release from presynaptic terminals and that both the apparent probability of transmitter release and the magnitude of LTD decrease concomitantly with age to around P30 (Choi & Lovinger, 1997a,b). On the other hand, several studies have reported physiological functions of N-type Ca2+ channels other than synaptic transmission, during development or in mature animals. It has been demonstrated that N-type channels are involved in directed migration of immature neurones in the cerebellum before the establishment of synaptic circuits (Komuro & Rakic, 1992), and that the number of N-type channels increases upon synaptogenesis during early development (Vigers & Pfenninger, 1991). It has also been suggested that N-type Ca2+ channels are involved in nociceptive transmission in adult animals, since intrathecal administration of N-type channel antagonists produces antinociceptive effects (Chaplan et al. 1994; Omote et al. 1996), and an immunohistochemical study has demonstrated the localization of N-type channels in pain pathways of adult rats (Westenbroek et al. 1998). The present findings could add, in addition to these functions, another functional role of N-type channels in developing animals. Considering that P60 in the rat is the age between adolescence and young adulthood (Kokoshka et al. 2000), the present results suggest that N-type Ca2+ channels could be involved in the control of movements prominent especially in these or younger ages, such as vivid motion or complex behavioural patterns. They also indicate the possibility that pharmacological manipulation of N-type Ca2+ channels could be at least one of the therapeutic tools for basal ganglia-related disorders, depending on the age of the patient.
Acknowledgments
The author is grateful to Dr Tomoyuki Takahashi for comments on the manuscript. This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (no. 13680904), Core Research for Evolutional Science and Technology of Japan Science and Technology Corporation, and Astra Zeneca Research Grant 2001.
References
- Aosaki T, Kiuchi K, Kawaguchi Y. Dopamine D1-like receptor activation excites rat striatal large aspiny neurons in vitro. J Neurosci. 1998;18:5180–5190. doi: 10.1523/JNEUROSCI.18-14-05180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes-Davies M, Owens S, Forsythe ID. Calcium channels triggering transmitter release in the rat medial superior olive. Hear Res. 2001;162:134–145. doi: 10.1016/s0378-5955(01)00378-1. [DOI] [PubMed] [Google Scholar]
- Bolam P, Wainer BH, Smith AD. Characterization of cholinergic neurons in the rat neostriatum. A combination of choline acetyltransferase immunocytochemistry, Golgi-impregnation and electron microscopy. Neurosci. 1984;12:711–718. doi: 10.1016/0306-4522(84)90165-9. [DOI] [PubMed] [Google Scholar]
- Broaddus WC, Bennett JP., Jr Postnatal development of striatal dopamine function. I. An examination of D1 and D2 receptors, adenylate cyclase regulation and presynaptic dopamine markers. Brain Res Mol Brain Res. 1990;52:265–271. doi: 10.1016/0165-3806(90)90244-s. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Centonze D, Gubellini P, Pisani A, Bernardi G. Acetylcholine-mediated modulation of striatal function. Trends Neurosci. 2000;23:120–126. doi: 10.1016/s0166-2236(99)01501-5. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Pisani A, Mercuri NB, Bernardi G. The corticostriatal projection: from synaptic plasticity to dysfunctions of the basal ganglia. Trends Neurosci. 1996;19:19–24. doi: 10.1016/0166-2236(96)81862-5. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Pogrel JW, Yaksh TL. Role of voltage-dependent calcium channel subtypes in experimental tactile allodynia. J Pharmacol Exp Ther. 1994;269:1117–1123. [PubMed] [Google Scholar]
- Chen JF, Weiss B. Ontogenetic expression of D2 dopamine receptor mRNA in rat corpus striatum. Brain Res Dev Brain Res. 1991;63:95–104. doi: 10.1016/0165-3806(91)90070-y. [DOI] [PubMed] [Google Scholar]
- Choi S, Lovinger DM. Decreased probability of neurotransmitter release underlies striatal long-term depression and postnatal development of corticostriatal synapses. Proc Natl Acad Sci U S A. 1997a;94:2665–2670. doi: 10.1073/pnas.94.6.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Lovinger DM. Decreased frequency but not amplitude of quantal synaptic responses associated with expression of corticostriatal long-term depression. J Neurosci. 1997b;17:8613–8620. doi: 10.1523/JNEUROSCI.17-21-08613.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Morelli M, Consolo S. Modulatory functions of neurotransmitters in the striatum: ACh/dopamine/NMDA interactions. Trends Neurosci. 1994;17:228–233. doi: 10.1016/0166-2236(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Forsythe ID, Tsujimoto T, Barnes-Davies M, Cuttle MF, Takahashi T. Inactivation of presynaptic calcium current contributes to synaptic depression at a fast central synapse. Neuron. 1998;20:797–807. doi: 10.1016/s0896-6273(00)81017-x. [DOI] [PubMed] [Google Scholar]
- Hirasawa M, Kombian SB, Pittman QJ. Oxytocin retrogradely inhibits evoked, but not miniature, EPSCs in the rat supraoptic nucleus: role of N- and P/Q-type calcium channels. J Physiol. 2001;532:595–607. doi: 10.1111/j.1469-7793.2001.0595e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki S, Momiyama A, Uchitel OD, Takahashi T. Developmental changes in calcium channel types mediating central synaptic transmission. J Neurosci. 2000;20:59–65. doi: 10.1523/JNEUROSCI.20-01-00059.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki S, Takahashi T. Developmental changes in calcium channel types mediating synaptic transmission in rat auditory brainstem. J Physiol. 1998;509:419–423. doi: 10.1111/j.1469-7793.1998.419bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z-G, North RA. Membrane properties and synaptic responses of rat striatal neurones in vitro. J Physiol. 1991;443:533–553. doi: 10.1113/jphysiol.1991.sp018850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko S, Hikida T, Watanabe D, Ichinose H, Nagatsu T, Kreitman RJ, Pastan I, Nakanishi S. Synaptic integration mediated by striatal cholinergic interneurons in basal ganglia function. Science. 2000;289:633–637. doi: 10.1126/science.289.5479.633. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Large aspiny cells in matrix of the rat neostriatum in vitro: physiological identification, relation to the compartments and excitatory postsynaptic currents. J Neurophysiol. 1992;67:1669–1682. doi: 10.1152/jn.1992.67.6.1669. [DOI] [PubMed] [Google Scholar]
- Kokoshka JM, Fleckenstein AE, Wilkins DG, Hanson GR. Age-dependent differential responses of monoaminergic systems to high doses of methamphetamine. J Neurochem. 2000;75:2095–2102. doi: 10.1046/j.1471-4159.2000.0752095.x. [DOI] [PubMed] [Google Scholar]
- Komuro H, Rakic P. Selective role of N-type calcium channels in neuronal migration. Science. 1992;257:806–809. doi: 10.1126/science.1323145. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Inagaki S, Shimada S, Kito S, Eckenstein F, Tohyama M. Neostriatal cholinergic neurons receive direct synaptic inputs from dopaminergic axons. Brain Res. 1987;413:179–184. doi: 10.1016/0006-8993(87)90167-3. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Langer SZ. The striatal cholinergic interneuron: synaptic target of dopaminergic terminals? Neurosci. 1983;10:1105–1120. doi: 10.1016/0306-4522(83)90102-1. [DOI] [PubMed] [Google Scholar]
- Luebke JI, Dunlap K, Turner TJ. Multiple calcium channel types control glutamatergic synaptic transmission in the hippocampus. Neuron. 1993;11:895–902. doi: 10.1016/0896-6273(93)90119-c. [DOI] [PubMed] [Google Scholar]
- Mogul DJ, Adams ME, Fox AP. Differential activation of adenosine receptors decreases N-type but potentiates P-type Ca2+ current in hippocampal CA3 neurons. Neuron. 1993;10:327–334. doi: 10.1016/0896-6273(93)90322-i. [DOI] [PubMed] [Google Scholar]
- Moll GH, Mehnert C, Wicker M, Bock N, Rothenberger A, Rüther E, Huether G. Age-associated changes in the densities of presynaptic monoamine transporters in different regions of the rat brain from early juvenile to late adulthood. Brain Res Dev Brain Res. 2000;119:251–257. doi: 10.1016/s0165-3806(99)00182-0. [DOI] [PubMed] [Google Scholar]
- Momiyama T. Developmental changes in calcium channel subtypes in GABAergic transmission onto rat striatal cholinergic interneurons. Neurosci Res Suppl. 2001;25:S176. [Google Scholar]
- Momiyama T. Developmental changes in calcium channel subtypes and D2-like receptor-mediated presynaptic inhibition in GABAergic transmission onto rat striatal cholinergic interneurones. Abst 3rd Forum Eur Neurosci. 2002;115(6) [Google Scholar]
- Noisin EL, Thomas WE. Ontogeny of dopaminergic function in the rat midbrain tegmentum, corpus striatum and frontal cortex. Brain Res Dev Brain Res. 1988;41:241–252. doi: 10.1016/0165-3806(88)90186-1. [DOI] [PubMed] [Google Scholar]
- Omote K, Kawamata M, Satoh O, Iwasaki H, Namiki A. Spinal antinociceptive action of N-type voltage-dependent calcium channel blocker and the synergistic interaction with morphine. Anesthesiol. 1996;84:636–643. doi: 10.1097/00000542-199603000-00019. [DOI] [PubMed] [Google Scholar]
- Phelps PE, Houser CR, Vaughn JE. Immunocytochemical localization of choline acetyltransferase within the rat neostriatum: a correlated light and electron microscopic study of cholinergic neurons and synapses. J Comp Neurol. 1985;238:286–307. doi: 10.1002/cne.902380305. [DOI] [PubMed] [Google Scholar]
- Pisani A, Bonsi P, Centonze D, Calabresi P, Bernardi G. Activation of D2-like dopamine receptors reduces synaptic inputs to striatal cholinergic interneurons. J Neurosci. 2000;20 doi: 10.1523/JNEUROSCI.20-07-j0003.2000. RC69 (1–6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani A, Spadoni F, Bernardi G. Group III metabotropic glutamate receptor agonists modulate high voltage-activated Ca2+ currents in pyramidal neurons of the adult rat. Exp Brain Res. 1998;119:237–244. doi: 10.1007/s002210050337. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Forsythe ID, Tsujimoto T, Barnes-Davies M, Onodera K. Presynaptic calcium current modulation by a metabotropic glutamate receptor. Science. 1996;274:594–597. doi: 10.1126/science.274.5287.594. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Momiyama A. Different types of calcium channels mediate central synaptic transmission. Nature. 1993;366:156–158. doi: 10.1038/366156a0. [DOI] [PubMed] [Google Scholar]
- Umemiya M, Berger AJ. Activation of adenosine A1 and A2 receptors differentially modulates calcium channels and glycinergic synaptic transmission in rat brainstem. Neuron. 1994;13:1439–1446. doi: 10.1016/0896-6273(94)90429-4. [DOI] [PubMed] [Google Scholar]
- Vigers AJ, Pfenninger KH. N-type and L-type calcium channels are present in nerve growth cones. Numbers increase on synaptogenesis. Brain Res Dev Brain Res. 1991;60:197–203. doi: 10.1016/0165-3806(91)90048-n. [DOI] [PubMed] [Google Scholar]
- Westenbroek RE, Hoskins L, Catterall WA. Localization of Ca2+ channel subtypes on rat spinal motor neurons, interneurons, and nerve terminals. J Neurosci. 1998;18:6319–6330. doi: 10.1523/JNEUROSCI.18-16-06319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DB, Randall A, Tsien RW. Roles of N-type and Q-type Ca2+ channels in supporting hippocampal synaptic transmission. Science. 1994;264:107–111. doi: 10.1126/science.7832825. [DOI] [PubMed] [Google Scholar]
- Wu L-G, Borst JGG, Sakmann B. R-type Ca2+ currents evoke transmitter release at a rat central synapse. Proc Natl Acad Sci U S A. 1998;95:4720–4725. doi: 10.1073/pnas.95.8.4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L-G, Westenbroek RE, Borst JGG, Catterall WA, Sakmann B. Calcium channel types with distinct presynaptic localization couple differentially to transmitter release in single calyx-type synapses. J Neurosci. 1999;19:726–736. doi: 10.1523/JNEUROSCI.19-02-00726.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Song W-J, Surmeier DJ. D2 dopamine receptors reduce N-type Ca2+ currents in rat neostriatal cholinergic interneurons through a membrane-delimited, protein-kinase-C-insensitive pathway. J Neurophysiol. 1997;77:1003–1015. doi: 10.1152/jn.1997.77.2.1003. [DOI] [PubMed] [Google Scholar]


