Abstract
Hippocampal CA3 pyramidal cells receive two independent afferents from the enthorinal cortex, i.e. a direct input via the temporoammonic pathway (TA, perforant path) and an indirect input via the mossy fibres (MF) of dentate granule cells. In spite of past suggestions that the TA is assigned an important role in exciting the pyramidal cells, little is known about their physiological properties. By surgically making an incision through the sulcus hippocampi and a small part of the dentate molecular layer, we succeeded in isolating TA-mediated monosynaptic responses in CA3 stratum lacunosum-moleculare. The TA–CA3 synaptic transmission was completely blocked by a combination of d,l-2-amino-5-phosphonopentanoic acid (AP5) and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), NMDA and non-NMDA receptor antagonists, respectively, and displayed paired-pulse facilitation and NMDA receptor-dependent long-term potentiation, which are all typical of glutamatergic synapses. We next addressed the heterosynaptic interaction between TA–CA3 and MF-CA3 synapses. The TA–CA3 transmission was partially attenuated by single-pulse MF pre-stimulation at inter-pulse intervals of up to 70 ms. However, surprisingly, burst stimulation of the MF alone induced long-lasting facilitation of TA–CA3 synaptic efficacy. This non-Hebbian form of synaptic plasticity was efficiently prevented by local application of AP5 into the MF synapse-rich area. Therefore, MF-activated NMDA receptors are responsible for the heterosynaptic modification of TA–CA3 transmission, and thereby, the history of MF activity may be etched into TA–CA3 synaptic strength. Our findings predict a novel form of spatiotemporal information processing in the hippocampus, i.e. a use-dependent intersynaptic memory transfer.
CA3 pyramidal cells form an autoassociative network with tens of thousands of counterparts in both the ipsilateral and contralateral hippocampi via their associational/ commissural (AC) axons (Amaral & Witter, 1995). This network serves as a pivotal input device of the hippocampus; two distinct inputs from the enthorinal cortex converge on the apical dendrite of a CA3 pyramidal cell (Amaral & Witter, 1995). One consists of axons arising from layer II/III enthorinal cortical neurons, termed the temporoammonic (TA) pathway. At the distal portion of the apical dendrite in stratum lacunosum-moleculare, each CA3 pyramidal cell synapses directly with about four thousand TA afferents. The enthorinal cells also send their axon collaterals to the dentate gyrus, in which granule cells relay neocortical information to CA3 via their mossy fibre (MF) axons. Thus, the MF is an indirect input pathway from the enthorinal cortex to the hippocampus. A target pyramidal cell makes synaptic contacts with only a few dozen MF axons, most of which are aligned in the proximal segment of the apical dendrite in the stratum lucidum (Henze et al. 2000).
Unlike any other excitatory synapses, MF-CA3 synapses exhibit several unique properties, e.g. giant presynaptic terminals, multiple transmitter release sites and prominent paired-pulse facilitation (Henze et al. 2000). They display long-term potentiation (LTP) of synaptic efficacy in response to high frequency stimulation, but exceptionally, the induction is independent of NMDA receptor activation (Nicoll & Malenka, 1995). Thus, the functional significance of MF postsynaptic NMDA receptors remains unclear.
The direct TA input transmits significant cortical information to the hippocampus. The denervation of MFs spares selective firing of CA3 pyramidal cells for particular locations in animal's environment (McNaughton et al. 1989), suggesting that ‘place’ representation is rendered principally by TA signal. Indeed, the TA input is capable of driving CA3 pyramidal cells to fire, probably more efficiently than MFs (Urban et al. 2001). Nonetheless, less information is available on TA–CA3 synaptic properties, the main reason being the difficulty in isolating TA–CA3 monosynaptic responses. Most of the previous studies on TA–CA3 synapses have utilized anaesthetized animals (Yeckel & Berger, 1990; Wu & Leung 1998; Do et al. 2002; Martinez et al. 2002), yet these in vivo experiments could not exclude a possible contamination with polysynaptic components. Unfortunately, this is the case in entorhino-hippocampal slice preparations (Berzhanskaya et al. 1998; Urban & Barrionuevo, 1998; McMahon & Barrionuevo, 2002), in which simple TA stimulation would unexpectedly cause disynaptic activation of CA3 pyramidal cells through recruitment of dentate granule cells via the perforant path. In the present study, therefore, we establish a method of recording TA monosynaptic responses in vitro and find that the TA net activity is dynamically regulated by heterosynaptic MF signals. Here we report that NMDA receptors at MF synapses help to inscribe the suprathreshold MF activity into a plastic change in TA–CA3 synaptic efficacy. Thus, the present study has important implication for network operation in the CA3 region.
Methods
Materials
d,l-2-Amino-5-phosphonopentanoic acid (AP5), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and NMDA were purchased from Sigma (St Louis, MO, USA). [2S,2′R,3′R]-2-(2′3’-dicarboxycyclopropyl)glycine (DCG IV) was obtained from Tocris (Cookson, Ballwin, MO, USA). Tetrodotoxin was from Wako Chemicals (Osaka, Japan).
Hippocampal slice preparation
According to the Japanese Pharmacological Society guide for the care and use of laboratory animals (Ikegaya & Matsuki, 2002), postnatal 17- to 27-day-old Wistar/ST rats (SLC, Shizuoka, Japan) were deeply anaesthetized with ether and immediately decapitated as described previously (Ueno et al. 2002). Briefly, the brain was quickly removed and immersed in ice-cold artificial cerebrospinal fluid (ACSF), consisting of (mm): 124 NaCl, 25 NaHCO3, 3 KCl, 1.24 KH2PO4, 1.4 MgSO4, 2.2 CaCl2, 10 glucose and 7 µm picrotoxin, continuously bubbled with 95 % O2 and 5 % CO2. Transverse hippocampal slices (400 µm thick) were prepared using a ZERO-1 vibratome (Dosaka, Osaka, Japan).
Electrophysiological recording
Slices were preincubated in a 95 % O2-5 % CO2-saturated ACSF for at least 1 h at 32 °C, placed in an interface recording chamber and perfused with the same ACSF (32 °C). Test stimuli were delivered to TA every 30 s through bipolar tungsten electrodes positioned near the hippocampal fissure, and field excitatory postsynaptic potentials (fEPSPs) were recorded from CA3 stratum lacunosum-moleculare by a glass microelectrode filled with 0.15 mNaCl (resistance ≈1 Ω). The stimulus intensity was set to produce fEPSPs with a half-maximal slope, and the baseline was recorded for at least 30 min to ensure the stability of the response. In some experiments, two other stimulating electrodes were positioned in the stratum granulosum and CA3 stratum radiatum for conditioned stimulation of MF and AC, respectively (stimulus intensity 300 µA). Either AP5 or NMDA was applied at a rate of ≈300 µl min−1 through a local perfusion pipette (280 µm inner diameter) positioned within ≈100 µm of the slice surface of the stratum lucidum. In the experiments shown in Fig. 7, slices were attached onto a MED-P515A probe (Alpha MED Sciences, Chuo-ku, Tokyo, Japan) with a wired mesh weighing 140 mg and perfused with ACSF. Two of the planar microelectrodes out of the 64 available were used to stimulate TA and MF (bipolar constant current pulses, 15–50 µA, 100 µs). The fEPSPs evoked at the remaining 62 sites were simultaneously recorded at a 20 kHz sampling with a MED64 multichannel recording system (Alpha MED Sciences; Oka et al. 1999; Shimono et al. 2000). Synaptic responses were evaluated by measuring changes in the maximal slopes of fEPSPs, but the fEPSP amplitudes were adopted for positive-going (source) responses (Fig. 6) or ‘small’ negative-going (sink) responses (less than 0.2 mV of peak amplitude) (Figs 2, 3A and 4) because it was hard to estimate their slopes precisely. Unless otherwise specified, slices were continuously disinhibited by a low concentration of picrotoxin (7 µm) in order to induce homosynaptic LTP at TA synapses (McMahon & Barrionuevo, 2002). This dose is insufficient for complete blockade of GABAergic inhibition, but neither the probability of LTP induction nor the degree of LTP magnitude was changed in the range 7 to 120 µm picrotoxin; the effect was preliminarily checked at concentrations ranging from 0.5 to 120 µm (data not shown). Because we hoped that our experiments were conducted under physiological conditions, the present study adopted ACSF containing 7 µm picrotoxin.
Figure 7. MF activation induces heterosynaptic LTP of TA–CA3, but not TA-CA1, transmission.
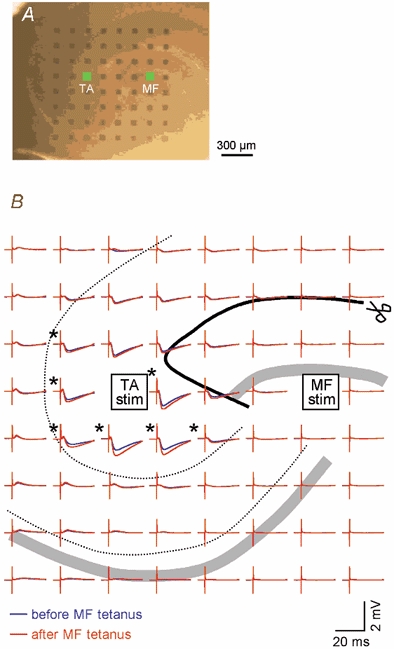
A, micrograph of a hippocampal slice placed onto a 8 × 8 electrode-array probe, with interelectrode spacing of 150 µm, centred in the apical dendritic field of CA3 pyramidal cells. The electrodes cover the stratum lacunosum-moleculare of CA1 as well as CA3. B, samples of TA-evoked fEPSPs immediately before (blue) and 60 min after (red) MF tetanization (100 Hz for 1 s). Each trace represents an average waveform of 10 successive responses recorded by the location-matched electrode in A. TA stimulation and MF tetanization were applied through the electrodes indicated by the green squares in A. The acceptable level of a defined LTP is a more than 18.7 % increase in fEPSP slopes (asterisks), which corresponds to 2.58 ×s.d. of baseline responses (P < 0.01). MF tetanization induced heterosynaptic TA–CA3 LTP but did not affect TA-CA1 fEPSPs. Experiments were repeated with a different seven slices, producing the same results. The scissors and thick black line indicate the incision made in this slice.
Figure 6. No heterosynaptic interaction between other combinations of three CA3 afferents.
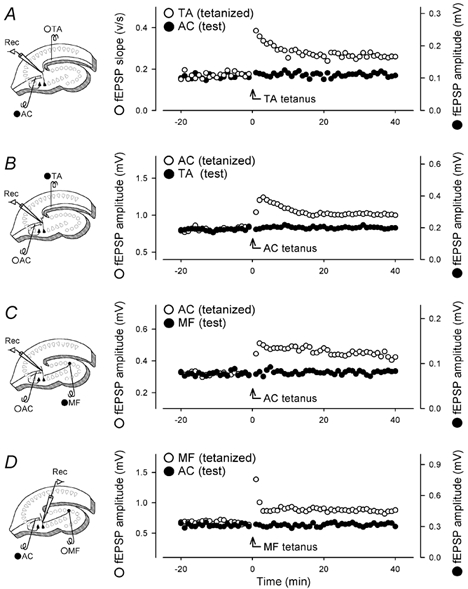
Two different afferents were alternatively stimulated at 15 s intervals, and tetanic stimulation (100 Hz for 1 s) was delivered to one pathway (open circles) at time 0. A, field responses were recorded from the stratum lacunosum-moleculare. Thus, stimulation of the TA and AC results in a negative-going (sink) field potential and a positive-going (source) field potential, respectively. The afferents to produce sink potentials were set as a tetanized pathway, that is, the tetanized pathway (open circles) was TA, and the control pathway (closed circles) was AC. B, field responses were recorded from the stratum radiatum. The tetanized pathway was AC, and the test pathway was TA. C, field responses were recorded from the stratum radiatum. The tetanized pathway was AC, and the test pathway was MF. D, field responses were recorded from the stratum lucidum. The tetanized pathway was MF, and the test pathway was AC. Although MF-evoked responses did not display marked post-tetanic potentiation, we confirmed that 2 µm DCG IV inhibited these responses. Experiments were repeated with 3–5 slices, producing similar results. Data show one representative case. We did not conduct normalization or averaging because the baseline values, particularly of the test pathway, varied considerably among experiments.
Figure 2. Pharmacological characterization of TA–CA3 fEPSPs.
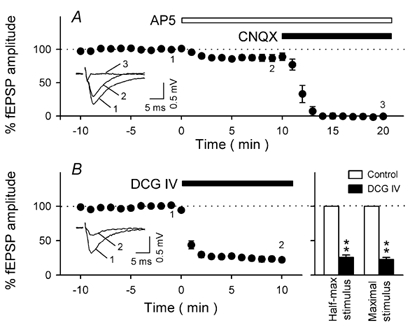
A, time courses of TA–CA3 responses following bath application of 50 µm AP5 alone and then both 50 µm AP5 and 20 µm CNQX. Application of AP5 decreased TA–CA3 fEPSPs by 11.1 ± 5.5 %, and the remaining component was completely abolished by additional perfusion with CNQX. These results indicate that basal neurotransmission at TA–CA3 synapses are mediated not only by AMPA receptors but also partly by NMDA receptors. Representative recordings at times −2, 8 and 20 min (marked 1, 2 and 3 in the figure) are shown in the inset. B, Effect of DCG IV on TA–CA3 fEPSPs. Bath perfusion with 1 µm DCG IV attenuated TA responses. Representative recordings at times −2 and 10 min (marked 1 and 2 in the figure) are shown in the inset. The data are summarized in the right panel. The TA was activated at a stimulus intensity that produced either half-maximal or maximal fEPSPs. The suppressive effect of DCG IV did not depend on stimulus intensity. Data represent means ± s.e.m. of 5 slices.
Figure 3. Homosynaptic plasticity of TA–CA3 transmission.
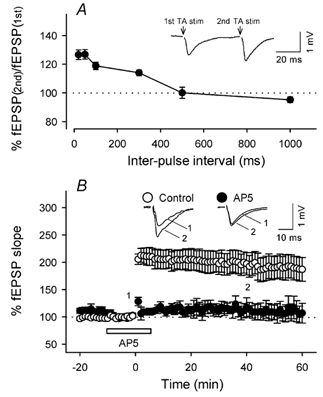
A, paired-pulse facilitation of TA–CA3 fEPSPs. The ordinate indicates the ratio of the second fEPSP amplitude to the first one at each inter-pulse interval (n = 3–7). A typical field response evoked by paired-pulse stimuli with a 50 ms interval is shown in the inset. B, time course of changes in fEPSP slopes following TA tetanic stimulation (100 Hz for 1 s). The tetanus was delivered to TA in the absence (open circles, n = 7) or presence (closed circles, n = 5) of 50 µm AP5. AP5 was continuously perfused from −10 min to 5 min (open bar). Representative recordings at times 0 and 40 min are shown in the inset. Data represent means ± s.e.m. of n cases.
Figure 4. Heterosynaptic modulation of TA–CA3 synaptic transmission by MF inputs.
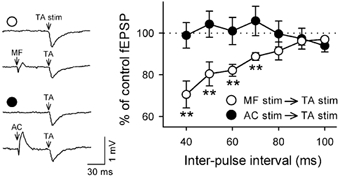
MF (open circles, n = 8) or AC (closed circles, n = 5) was stimulated (each 1 pulse at 300 µA) prior to TA stimulation. The ordinate indicates the ratio of TA-evoked fEPSPs following MF/AC stimulation to control fEPSPs without the prior conditioned stimulus. When the MF stimulus was immediately preceded by TA activation, TA–CA3 transmission was significantly depressed. The depression occurred as a function of the time by which the MF stimulus precedes the TA stimulus. The AC stimulus was virtually ineffective. Representative field potentials with (each lower trace) or without (each upper trace) the conditioned stimulus at an inter-pulse interval of 40 ms are shown in the inset. **P < 0.01vs. no conditioned stimulus. Data represent means ± s.e.m. of n cases.
Results
Isolation of TA monosynaptic responses
In all experiments, a cut was made along the sulcus hippocampi and across the edge of the dentate molecular layer using a small, curved scalpel under stereomicroscopic controls (Fig. 1A) in order to prevent contamination with monosynaptic and polysynaptic components via the classical perforant path, i.e. entorhino-dentate connections. In these slices, no apparent responses were any longer elicited in the dentate gyrus by stimulation of the enthorinal cortex (data not shown), indicating complete lesions of the perforant path. Under these conditions, single-pulse stimulation of the TA pathway evoked a characteristic negative field potential (Fig. 1B). The input-output relationship was constructed in the range 10–400 µA of stimulus intensity. The maximal slope (Fig. 1C), the peak amplitude (Fig. 1D) and the width at half height (Fig. 1E) of the negative potential increased with increasing stimulus intensity, but the latency from the TA stimulus to the sink peak was always around 5–6 ms and almost unchanged by the intensity (Fig. 1F), suggesting that the sink potentials reflect monosynaptic responses.
Figure 1. Recording of TA–CA3 fEPSPs.
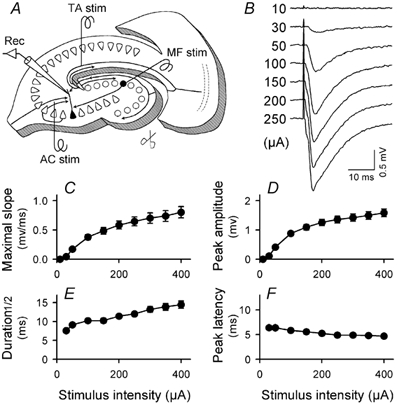
A, recording set up. A recording electrode was positioned in CA3 stratum lacunosum-moleculare of a slice incised along the sulcus hippocampi and across the edge of the dentate molecular layer (scissors). Three stimulating electrodes were placed in the proximate of the hippocampal fissure, the stratum granulosum and CA3 stratum radiatum to stimulate TA, MF and AC, respectively. B, representative traces of fEPSPs evoked by applying gradually increased intensity of the TA stimulus, ranging from 10 to 250 µA. C-F, input-output relationships of the slope of fEPSPs (C), the amplitude of fEPSPs (D), the width of fEPSPs at half-height (E) and the latency from TA stimulation to reaching the fEPSP peak (F). Almost no shift in the peak latency (only a 21.9 ± 18.7 % decrease) was detected by increasing stimulus intensity, confirming that the fEPSPs reflect TA–CA3 monosynaptic responses. All data represent means ± s.e.m. of 24 slices; where no error bars are apparent they have been obscured by the symbol.
The synaptic responses were slightly attenuated by bath perfusion with 50 µm AP5, a NMDA receptor antagonist, and completely abolished by additional application of 20 µm CNQX, a non-NMDA receptor antagonist (Fig. 2A). Therefore, they proved to be ionotropic glutamate receptor-mediated fEPSPs; the main component was produced by AMPA receptors, but NMDA receptors were also partly involved in the baseline transmission. This is consistent with a report of TA-CA1 synapse responses, which also contain relatively large NMDA receptor-mediated components (Otmakhova et al. 2002).
Immunohistochemical studies indicated that group II metabotropic glutamate receptors are highly expressed in the terminal field of TA, i.e. the stratum lacunosum-moleculare of the Ammon's horn (Petralia et al. 1996; Shigemoto et al. 1997). In CA1, bath application of DCG IV (1 µm), an agonist of this type of metabotropic receptors, decreases TA-evoked synaptic responses by about 80 % (Kew et al. 2001). We confirmed that in CA3 as well, the same concentration of DCG IV induced a 77.7 % decrease in the fEPSPs (Table 1, Fig. 2B). It is possible that an AC-mediated component is disynaptically merged on TA-evoked fEPSPs when the TA pathway is strongly stimulated. Thus, we checked the effect of DCG IV on fEPSPs when the TA was maximally activated by stimulation at 400 µA. However, the suppressive effect of DCG IV did not depend on the intensity of TA stimulation (Fig. 2B). This indicates that our stimulus conditions produced few AC contaminations. DCG IV is shown to have no effect on AC-CA3 responses (Table 1; Ueno et al. 2002) but does inhibit MF-CA3 responses (Table 1; Kamiya et al. 1996). However, TA-evoked fEPSPs displayed only < 130 % of paired-pulse facilitation ratios (Table 1, Fig. 3A), while MF-CA3 transmission was characterized by a high value for this ratio (Table 1; Claiborne et al. 1993). Thus, the fEPSPs were not contaminated with a MF component. Because GABAergic transmission was not completely blocked in our experimental conditions, the remnant inhibitory influence might affect the ratio of paired-pulse facilitation. However, even when slices were completely disinhibited by 100 µm picrotoxin, the paired-pulse facilitation ratio of TA-evoked fEPSPs was 129.5 ± 6.1 % at a 50 ms interval (mean ± s.e.m. of 4 slices), which was almost the same as that at a low concentration of picrotoxin (Table 1). Taken together, we concluded that TA–CA3 monosynaptic fEPSPs were purely isolated by the optimized incision of entorhino-hippocampal slices.
Table 1.
Characteruzation of TA-, AC- and MF-evoked fEPSPs
| Stimulated pathway | % inhibition by DCG IV | % Paired-pulse facilitation |
|---|---|---|
| TA | 77.7 ± 3.3 (5) | 126.9 ± 3.3 (7) |
| AC | −4.7 ± 1.7 (4) | 124.2 ± 2.9 (4) |
| MF | 70.9 ± 4.8(4) | 247.9 ± 48.1 (4) |
Summary of the effect of 1–2 μm DCG IV and paired-pulse facilitation ratio of TA–CA3, AC–CA3 and MF–CA3 synaptic transmission. All fEPSPs were extracellularly recorded from the stratum lacunosum-molecular after single-pulse stimulation of the hippocampal fissure (TA) stratum radiatum (AC) and stratum granulosum (MF). The ratios of paired-pulse faciltation were measured at 50 ms interpulse interval. The number of slices tested is indicated in parentheses.
When the TA was tetanized at 100 Hz for 1 s fEPSPs were immediately enhanced, and homosynaptic LTP was induced (Fig. 3B). In the presence of 50 µm AP5, the tetanus caused no change in fEPSPs without slight post-tetanic potentiation in the immediate aftermath (Fig. 3B). Thus, the LTP is NMDA-receptor dependent.
Non-Hebbian interaction between MF and TA
Theoretical analyses have predicted that a functional interplay between MF and TA inputs is crucial for memory storage and retrieval in the hippocampus (Treves & Rolls, 1992; Lisman, 1999). Experimental evidence has revealed that these two signals are actively summed in the apical dendrite of a CA3 pyramidal cell (Urban & Barrionuevo, 1998). We therefore addressed the possible interaction of TA synaptic efficacy with MF and AC inputs.
As single-pulse simulation was delivered to stratum granulosum, a small ‘source’ signal emerged in the field potentials recorded from CA3 stratum lacunosum-moleculare (Fig. 4), which displayed a high degree of paired-pulse facilitation and was attenuated by DCG IV (Table 1). Thus, MF synapses were successfully excited under our conditions. When the TA stimulus was immediately preceded by the MF stimulus, the TA-evoked fEPSP was significantly depressed (Fig. 4). The depression occurred when the MF stimulus preceded the TA stimulus by up to 70 ms, suggesting temporal summation of the TA- and MF-elicited EPSPs (Fig. 4). In contrast, prestimulation of AC exerted no apparent effect on TA-evoked fEPSPs. The validity of the AC stimulus was readily confirmed by a larger source response elicited in the field potential, compared with the MF-evoked source signal (Fig. 4). This displayed a low ratio of paired-pulse facilitation and was resistant to DCG IV (Table 1).
After confirming the stability of the baseline responses for at least 30 min, burst stimulation (100 Hz for 1 s) was delivered to the MF in the absence of TA stimulation. Thereafter, surprisingly, TA-evoked fEPSPs increased gradually and reached a steady state after ≈5 min, and this enhancement was maintained for > 60 min (Fig. 5A). Thus, MF activation heterosynaptically induced long-lasting facilitation of TA–CA3 fEPSPs. This heterosynaptic LTP was completely abolished by transverse incision of the stratum oriens, pyramidale and lucidum of CA3c, which was made in order to transect the MF pathway (Fig. 5C). Thus, activation of MF synapses causes TA potentiation but it is also possible that MF tetanus elicited disynaptic AC activation via firing of CA3 pyramidal cells, indirectly affecting TA transmission. However, the non-associative form of LTP was not produced by a 100 Hz tetanus of AC (Fig. 5B and Fig. 6B), even when the stimulus intensity was increased up to 400 µA (n = 3, data not shown). We therefore concluded that heterosynaptic TA modulation is produced specifically by the MF afferents.
Figure 5. Heterosynaptic induction of TA–CA3 LTP by MF inputs.
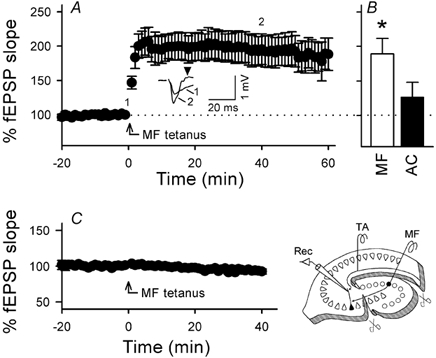
A, time courses of TA–CA3 responses following MF tetanization (100 Hz for 1 s). The tetanus was applied without TA stimulation. TA-evoked fEPSPs gradually increased up to about 200 % and were maintained for at least 60 min. Representative traces at times 0 and 40 min are shown in the inset. After MF tetanus, a TA stimulus that was previously ineffective to evoke a spike elicited spike-relevant responses (arrowhead) in most cases tested (> 90 %), which suggests that MF activation gates TA inputs. B, the average fEPSP slopes from 40 to 60 min after tetanic stimulation of MF (white column, n = 6) or AC (black column, n = 6). A significant increase in TA-evoked fEPSPs was obtained only for MF tetanization. C, in stratum lucidum-transected slices (as shown in the schematic drawing), MF tetanization (100 Hz for 1 s did not cause the induction of heterosynaptic LTP (n = 4). Data represent means ± s.e.m. of n cases.
Using our experimental system, we also examined the interaction between other CA3 inputs. The tetanization of the TA pathway did not affect AC-CA3 fEPSPs (Fig. 6A). Likewise, AC tetanus did not change MF-CA3 fEPSPs (Fig. 6C) nor did MF tetanus affect AC-CA3 fEPSPs (Fig. 6D). Thus, there was no heterosynaptic interaction between TA → AC, AC → MF or MF → AC. In contrast with Fig. 6D, Bradler & Barrionuevo (1990) have indicated that burst stimulation of the MFs induced long-lasting facilitation of AC-CA3 transmission. This phenomenon probably resulted from disynaptic recruitment of AC-CA3 synapses, and we therefore consider that in a broad sense, their finding represents one form of ‘homosynaptic’ LTP induction. This difference in our and others’ observations means that under our experimental conditions, the MFs were more purely stimulated, and thus the undesirable contamination of the disynaptic AC component was minimal. Taken together, the non-Hebbian interplay seems likely to be specific between MF → TA.
Because recording with a single glass electrode alone could not precisely estimate the spatial distribution of the areas that were influenced by MF tetanization, we sought to determine the spatial spread of the non-Hebbian LTP by using an 8 × 8 electrode-array multichannel probe (Fig. 7A). Instead of the proximity of the hippocampal fissure, the middle part of stratum lacunosum-moleculare was stimulated to activate TA synapses efficiently because these microelectrodes were thin and planar. We confirmed again that MF tetanization induced heterosynaptic LTP in fEPSPs recorded from CA3 stratum lacunosum-moleculare (Fig. 7B). No potentiation was observed in fEPSPs in CA1 stratum lacunosum-moleculare, i.e. TA-CA1 synaptic responses (Fig. 7B), suggesting that MF-induced, heterosynaptic LTP was restricted to TA–CA3 synapses.
To determine whether NMDA receptors at MF synapses are involved in the non-Hebbian LTP, AP5 was locally applied into CA3 stratum lucidum, pyramidale and oriens, which contain the terminal field of the MF. The area that drugs were perfused over is illustrated in Fig. 8. In this experimental system, we recorded MF-evoked source responses with amplitude 0.11 ± 0.04 mV, whereas the responses were 0.13 ± 0.03 mV in amplitude in Fig. 5 (means ± s.e.m. of 5 and 6 slices, respectively, P > 0.1). Therefore, almost the same amount of MFs were stimulated in the experiments illustrated in Fig. 8 and Fig. 5. The local application of 2 µm DCG IV attenuated MF-evoked responses by 72.2 ± 11.8 % without apparently affecting TA-evoked fEPSPs (0.0 ± 1.7 %, means ± s.e.m. of 4 slices). In addition, the local application of 50 µm AP5 did not alter the baseline responses nor inhibit TA tetanus-induced homosynaptic LTP (Fig. 8A). These data indicate that the local perfusion could spatially separate MF and TA synaptic regions. Under these well-controlled conditions, local AP5 prevented the induction of MF-induced TA LTP (Fig. 8A). This form of LTP is, therefore, dependent on activation of heterosynaptic NMDA receptors. To examine whether or not NMDA receptor activation alone elicits the non-Hebbian LTP, the agonist NMDA, dissolved in Mg2+-free ACSF containing 1 µm tetrodotoxin, was locally applied without MF activation. The NMDA perfusion did not affect TA-evoked fEPSPs at as high a concentration as 1 mm (Fig. 8B).
Figure 8. Requirement of activation of NMDA receptors at MF synapses for non-Hebbian TA–CA3 LTP.
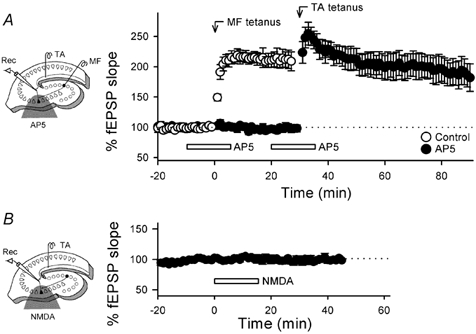
A, effect of local application of AP5 on MF-induced TA–CA3 LTP. The half-tone meshing in the schematic drawing indicates the drug-perfused area, which includes the stratum lucidum, a MF-terminal zone. Two tetani (each 100 Hz for 1 s) were delivered to MF and then TA at time 0 and 30 min, respectively, in the absence (open circles) or presence (closed circles) of 50 µm AP5 (n = 6). AP5 was twice applied through a silicon microtube each for 15 min during the time indicated by the open bars. AP5 inhibited the induction of MF-evoked, heterosynaptic LTP but not of TA-evoked, homosynaptic LTP. No changes in basal responses were produced by AP5, confirming that the local application successfully separated the TA- and MF-synaptic zones. B, effect of local application of NMDA on TA-evoked fEPSPs. Mg2+-free solution containing 1 mm NMDA and 1 µm tetrodotoxin was locally applied for 15 min, causing no apparent change in TA synaptic efficacy (n = 4). Data represent means ± s.e.m. of n cases.
Discussion
Accumulating evidence indicates that direct cortical input plays an important role in hippocampal function. Rats with lesions of intrahippocampal CA3-CA1 connections display normal location-specific activity of CA1 pyramidal cells and spatial recognition task performance (Brun et al. 2002), while TA lesions result in severe disruption of spatial memory encoding and retrieval (Lee & Kesner, 2001). Thus, the direct TA system is essential for place recognition as well as place-specific neuronal activity. Previous studies on the TA pathway have focused mainly on synapses onto CA1 neurons (Empson & Heinemann; 1995; Levy et al. 1998; Dvorak-Carbone & Schuman; 1999), yet less is known about the properties of TA–CA3 transmission. Electrophysiological studies have recently revealed that TA-CA1 synapses do not only exhibit NMDA-dependent LTP but their activity can also modulate the input efficacy from CA3 and thereby gate information outputs from the hippocampus (Remondes & Schuman, 2002). In CA3, on the other hand, three major afferents, i.e. MF, AC and TA, terminate on the same apical dendrite, forming a more complicated laminar network (Amaral & Witter, 1995). Elucidating the computational properties of the CA3 local circuit is, therefore, critical for understanding of hippocampal information processing. Indeed, theoretical analyses have proposed that a dynamic interplay of these CA3 afferents contributes to the efficacy of memory storage and retrieval (Treves & Rolls, 1992; Lisman, 1999). We have shown for the first time that prior MF activation causes transient depression of TA–CA3 synaptic efficacy, while conversely, burst stimulation of MF elicits long-lasting facilitation of TA–CA3 fEPSPs. No heterosynaptic interaction was detected for AC stimulation. Thus, TA–CA3 transmission is selectively susceptible to MF synaptic activity.
In the first part of the present study, we have established a method for isolating TA-mediated monosynaptic responses, in which slices were surgically incised through the sulcus hippocampi and the edge of dentate molecular layer in order to cleave the classical perforant path. Without this incision, stimulation of the stratum granulosum would not only excite MFs but also antidromically activate the perforant path, which would in turn cause direct activation of TA synapses. Likewise, stimulation of the hippocampal fissure would result in orthodromic and antidromic activation of granule cells. These undesirable contaminations with monosynaptic and polysynaptic components might lead to different observations. McMahon & Barrionuevo (2002) showed that MF tetanization, the conditions of which might directly stimulate a substantial number of TA synapses as well, caused a transient heterosynaptic depression of TA–CA3 fEPSPs, but we found no evidence for such depression. With our method that ensures separate stimulation of TA and MF, we reproducibly found that the tetanization led to heterosynaptic facilitation of TA–CA3 fEPSPs, i.e. non-Hebbian LTP.
Cellular basis for non-Hebbian interaction between MF and TA
How does MF activation induce LTP at distant synapses? MF and TA axons innervate the same apical dendrite of a CA3 pyramidal cell. Because TA-CA1 synapses were unaffected by MF tetanus, the MF appears to interact with TA synapses via its postsynaptic dendrite. Recent evidence indicates that synapses are capable of influencing others via dendritic action potentials, which can bidirectionally propagate across the length of the dendrites (Stuart et al. 1997). The dendritic spikes participate in the induction of associative and cooperative synaptic plasticity at TA-CA1 synapses (Magee & Johnston 1997; Golding et al. 2002). Strong MF activation probably generates postsynaptic spiking and thereby may modulate TA synaptic efficacy. Recent evidence shows that postsynaptic depolarization alone induces long-term changes in MF-CA3 synaptic strength (Berretta et al. 1999, 2000). This non-Hebbian interaction may be mediated by intrasynaptic ephaptic feedback (Berretta et al. 2000; Kasyanov et al. 2000). It is possible that this mechanism operates at TA–CA3 synapses, although it does not appear to work at AC-CA3 synapses (Berretta et al. 2000).
Another possibility can also be raised. The dendrites contain the intracellular Ca2+ stores, the surface of which expresses ryanodine and inositol trisphosphate receptors (Berridge, 1998). Both the receptors evoke Ca2+ release from the stores in response to a rise of intracellular Ca2+ itself, yielding Ca2+-mediated, regenerative spikes across the endoplasmic reticular membrane (Berridge, 1998). The intracellular Ca2+ wave propagates as slowly as 40 µm s−1 but can reach the distal part of the dendrite (Berridge, 1998), probably regulating heterosynaptic plasticity in the hippocampus (Nishiyama et al. 2000). Kapur et al. (2001) have demonstrated that burst stimulation of MFs evokes Ca2+ release from the internal stores in the thorny excrescences of CA3 pyramidal cells, i.e. MF synaptic spines, and this Ca2+ signal spreads into the adjacent dendrite. It is plausible, therefore, that intracellular Ca2+ spikes, produced by MF activation, are transduced to TA synapses, resulting in TA LTP. Kullmann et al. (1992) reported that at CA1 synapses, a rise in postsynaptic Ca2+ alone is sufficient to cause a transient (< 30 min) increase in synaptic efficacy and that presynaptic co-activation converts this transient potentiation into a sustained form. Importantly, the heterosynaptic induction of our TA LTP did not require TA stimulation, but under our conditions, spontaneous TA activity remained intact. Evaluating the contribution of basal TA activity to TA–CA3 synaptic changes is now underway in our laboratory. Further investigation would be necessary to clarify the inter-synaptic dynamics between MF and TA inputs
Although NMDA receptors are present at MF postsynaptic sites (Weisskopf & Nicoll, 1995; Vogt et al. 2000; Reid et al. 2001), they are not indispensable for the induction of MF-CA3 LTP, which may rather be explained by presynaptic mechanisms alone (Nicoll & Malenka, 1995). During development, NMDA receptors at MF synapses begin to function before AMPA receptors emerge (Ikegaya et al. 2002). Their activity may, therefore, be required for subsequent acquisition of AMPA receptors. Even in the adult hippocampus, however, the MF synaptic NMDA receptors are kept active (Weisskopf & Nicoll, 1995; Vogt et al. 2000; Reid et al. 2001). Therefore, their functional significance has remained unclear. The present study demonstrated that MF-induced TA LTP was inhibited by pharmacological blockade of NMDA receptors in the MF synapse-rich area. MF activation might also disynaptically recruit AC axons to some extent. Indeed, Bradler & Barrionuevo (1990) reported that MF tetanus leads to heterosynaptic LTP induction at AC-CA3 synapses (but see Fig. 6D). However, AC tetanus per se was proven virtually ineffective in inducing a plastic change in TA fEPSPs. We conclude, therefore, that NMDA receptors at MF synapses are responsible for the heterosynaptic LTP. Exogenous application of NMDA to MF synapses did not induce TA LTP. Thus, activation of MF synaptic NMDA receptors is necessary but not sufficient to trigger TA LTP. These receptors may serve as a threshold detector of MF activity for the non-Hebbian LTP.
Functional significance of non-Hebbian TA LTP in CA3 circuits
Non-Hebbian forms of synaptic plasticity, e.g. the spread of LTP beyond synapses, have recently been implicated as an integral part of global changes in neural networks (Bi & Poo, 2001). Our findings of a use-dependent LTP propagation through postsynaptic dendrites, therefore, provide novel insights into information processing by the CA3 circuit. Cortical information is doubly conveyed into CA3 via MF and TA, but the MF signal is previously processed in the dentate network. Our present study suggests that when a CA3 pyramidal cell receives a strengthened MF signal via the prior dentate processing, the TA input into the same neuron is also reinforced, thereby acquiring a more potent ability to excite the neuron. Thus, the dentate local circuits may gate direct cortical input into the hippocampus. In this case, all TA synapses may be equally affected since their presynaptic activity is apparently not required. This places strong limitation on the kind of information storage that is possible by such a mechanism. However, the lifespan of MFs is perhaps extremely short (Gould et al. 2001) and thus, the concomitant loss of information encoded in their synapses must be transferred to more stable storages. MF NMDA receptor-triggered, non-Hebbian TA LTP may represent such a heterosynaptic memory transfer system. The strength of TA–CA3 synapses may epitomize the history of MF activity.
Acknowledgments
We thank Mr H. Jiko and Mr A. Shimizu (Alpha MED Sciences, Chuo-ku, Tokyo, Japan) for their technical support of 8 × 8 multi-electrode recording from hippocampal slices. This work was supported in part by Grant-in-Aid for Science Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan and by the Research Grant for Longevity Science (13–2) from the Ministry of Health, Labor and Welfare of Japan.
References
- Amaral DG, Witter MP. Hippocampal formation. In: Paxinos G, editor. The Rat Nervous System. San Diego: Academic Press; 1995. pp. 443–492. [Google Scholar]
- Berretta N, Rossokhin AV, Cherubini E, Astrelin AV, Voronin LL. Long-term synaptic changes induced by intracellular tetanization of CA3 pyramidal neurons in hippocampal slices from juvenile rats. Neuroscience. 1999;93:469–477. doi: 10.1016/s0306-4522(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Berretta N, Rossokhin AV, Kasyanov AM, Sokolov MV, Cherubini E, Voronin LL. Postsynaptic hyperpolarization increases the strength of AMPA-mediated synaptic transmission at large synapses between mossy fibers and CA3 pyramidal cells. Neuropharmacology. 2000;39:2288–2301. doi: 10.1016/s0028-3908(00)00076-9. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- Berzhanskaya J, Urban NN, Barrionuevo G. Electrophysiological and pharmacological characterization of the direct perforant path input to hippocampal area CA3. J Neurophysiol. 1998;79:2111–2118. doi: 10.1152/jn.1998.79.4.2111. [DOI] [PubMed] [Google Scholar]
- Bi G, Poo M. Synaptic modification by correlated activity: Hebb's postulate revisited. Annu Rev Neurosci. 2001;24:139–166. doi: 10.1146/annurev.neuro.24.1.139. [DOI] [PubMed] [Google Scholar]
- Bradler JE, Barrionuevo G. Heterosynaptic correlates of long-term potentiation induction in hippocampal CA3 neurons. Neuroscience. 1990;35:265–271. doi: 10.1016/0306-4522(90)90080-n. [DOI] [PubMed] [Google Scholar]
- Brun VH, Otnass MK, Molden S, Steffenach HA, Witter MP, Moser MB, Moser EI. Place cells and place recognition maintained by direct entorhinal-hippocampal circuitry. Science. 2002;296:2243–2246. doi: 10.1126/science.1071089. [DOI] [PubMed] [Google Scholar]
- Claiborne BJ, Xiang Z, Brown TH. Hippocampal circuitry complicates analysis of long-term potentiation in mossy fiber synapses. Hippocampus. 1993;3:115–121. doi: 10.1002/hipo.450030202. [DOI] [PubMed] [Google Scholar]
- Do VH, Martinez CO, Martinez JL, Jr, Derrick BE. Long-term potentiation in direct perforant path projections to the hippocampal CA3 region in vivo. J Neurophysiol. 2002;87:669–678. doi: 10.1152/jn.00938.2000. [DOI] [PubMed] [Google Scholar]
- Dvorak-Carbone H, Schuman EM. Patterned activity in stratum lacunosum moleculare inhibits CA1 pyramidal neuron firing. J Neurophysiol. 1999;82:3213–22. doi: 10.1152/jn.1999.82.6.3213. [DOI] [PubMed] [Google Scholar]
- Empson RM, Heinemann U. The perforant path projection to hippocampal area CA1 in the rat hippocampal-entorhinal cortex combined slice. J Physiol. 1995;484:707–720. doi: 10.1113/jphysiol.1995.sp020697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding NL, Staff NP, Spruston N. Dendritic spikes as a mechanism for cooperative long-term potentiation. Nature. 2002;418:326–331. doi: 10.1038/nature00854. [DOI] [PubMed] [Google Scholar]
- Gould E, Vail N, Wagers M, Gross CG. Adult-generated hippocampal and neocortical neurons in macaques have a transient existence. Proc Natl Acad Sci USA. 2001;98:10910–10917. doi: 10.1073/pnas.181354698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henze DA, Urban NN, Barrionuevo G. The multifarious hippocampal mossy fiber pathway: a review. Neuroscience. 2000;98:407–427. doi: 10.1016/s0306-4522(00)00146-9. [DOI] [PubMed] [Google Scholar]
- Ikegaya Y, Koyama R, Yamada MK, Nishiyama N, Matsuki N. Rapid regrowth of hippocampal mossy fibres and preceding maturation of NMDA receptor-mediated neurotransmission. Eur J Neurosci. 2002;15:1859–1862. doi: 10.1046/j.1460-9568.2002.02035.x. [DOI] [PubMed] [Google Scholar]
- Ikegaya Y, Matsuki N. Vasopressin induces emesis in Suncus murinus. Jpn J Pharmacol. 2002;89:324–326. doi: 10.1254/jjp.89.324. [DOI] [PubMed] [Google Scholar]
- Kamiya H, Shinozaki H, Yamamoto C. Activation of metabotropic glutamate receptor type 2/3 suppresses transmission at rat hippocampal mossy fibre synapses. J Physiol. 1996;493:447–455. doi: 10.1113/jphysiol.1996.sp021395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur A, Yeckel M, Johnston D. Hippocampal mossy fiber activity evokes Ca2+ release in CA3 pyramidal neurons via a metabotropic glutamate receptor pathway. Neuroscience. 2001;107:59–69. doi: 10.1016/s0306-4522(01)00293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasyanov AM, Maximov VV, Byzov AL, Berretta N, Sokolov MV, Gasparini S, Cherubini E, Reymann KG, Voronin LL. Differences in amplitude-voltage relations between minimal and composite mossy fibre responses of rat CA3 hippocampal neurons support the existence of intrasynaptic ephaptic feedback in large synapses. Neuroscience. 2000;101:323–336. doi: 10.1016/s0306-4522(00)00366-3. [DOI] [PubMed] [Google Scholar]
- Kew JN, Ducarre JM, Pflimlin MC, Mutel V, Kemp JA. Activity-dependent presynaptic autoinhibition by group II metabotropic glutamate receptors at the perforant path inputs to the dentate gyrus and CA1. Neuropharmacology. 2001;40:20–27. doi: 10.1016/s0028-3908(00)00118-0. [DOI] [PubMed] [Google Scholar]
- Kullmann DM, Perkel DJ, Manabe T, Nicoll RA. Ca2+ entry via postsynaptic voltage-sensitive Ca2+ channels can transiently potentiate excitatory synaptic transmission in the hippocampus. Neuron. 1992;9:1175–1183. doi: 10.1016/0896-6273(92)90075-o. [DOI] [PubMed] [Google Scholar]
- Lee I, Kesner RP. Selective lesions of the perforant path in CA3 disrupt both encoding and retrieval of spatial memory. Soc Neurosci Abstr. 2001;315:5. [Google Scholar]
- Levy WB, Desmond NL, Zhang DX. Perforant path activation modulates the induction of long-term potentiation of the Schaffer collateral-hippocampal CA1 response: theoretical and experimental analyses. Learn Mem. 1998;4:510–518. doi: 10.1101/lm.4.6.510. [DOI] [PubMed] [Google Scholar]
- Lisman JE. Relating hippocampal circuitry to function: recall of memory sequences by reciprocal dentate-CA3 interactions. Neuron. 1999;22:233–242. doi: 10.1016/s0896-6273(00)81085-5. [DOI] [PubMed] [Google Scholar]
- McMahon DB, Barrionuevo G. Short- and long-term plasticity of the perforant path synapse in hippocampal area CA3. J Neurophysiol. 2002;88:528–533. doi: 10.1152/jn.2002.88.1.528. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA, Meltzer J, Sutherland RJ. Hippocampal granule cells are necessary for normal spatial learning but not for spatially-selective pyramidal cell discharge. Exp Brain Res. 1989;76:485–496. doi: 10.1007/BF00248904. [DOI] [PubMed] [Google Scholar]
- Magee JC, Johnston D. A synaptically controlled, associative signal for Hebbian plasticity in hippocampal neurons. Science. 1997;275:209–213. doi: 10.1126/science.275.5297.209. [DOI] [PubMed] [Google Scholar]
- Martinez CO, Do VH, Martinez JL, Derrick BE. Associative long-term potentiation (LTP).among extrinsic afferents of the hippocampal CA3 region in vivo. Brain Res. 2002;940:86–94. doi: 10.1016/s0006-8993(02)02598-2. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Malenka RC. Contrasting properties of two forms of long-term potentiation in the hippocampus. Nature. 1995;377:115–118. doi: 10.1038/377115a0. [DOI] [PubMed] [Google Scholar]
- Nishiyama M, Hong K, Mikoshiba K, Poo MM, Kato K. Calcium stores regulate the polarity and input specificity of synaptic modification. Nature. 2000;408:584–588. doi: 10.1038/35046067. [DOI] [PubMed] [Google Scholar]
- Oka H, Shimono K, Ogawa R, Sugihara H, Taketani M. A new planar multielectrode array for extracellular recording: application to hippocampal acute slice. J Neurosci Methods. 1999;93:61–67. doi: 10.1016/s0165-0270(99)00113-2. [DOI] [PubMed] [Google Scholar]
- Otmakhova NA, Otmakhov N, Lisman JE. Pathway-specific properties of AMPA and NMDA-mediated transmission in CA1 hippocampal pyramidal cells. J Neurosci. 2002;22:1199–1207. doi: 10.1523/JNEUROSCI.22-04-01199.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Niedzielski AS, Wenthold RJ. The metabotropic glutamate receptors, mGluR2 and mGluR3, show unique postsynaptic, presynaptic and glial localizations. Neuroscience. 1996;7:949–976. doi: 10.1016/0306-4522(95)00533-1. [DOI] [PubMed] [Google Scholar]
- Reid CA, Fabian-Fine R, Fine A. Postsynaptic calcium transients evoked by activation of individual hippocampal mossy fiber synapses. J Neurosci. 2001;21:2206–2214. doi: 10.1523/JNEUROSCI.21-07-02206.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remondes M, Schuman EM. Direct cortical input modulates plasticity and spiking in CA1 pyramidal neurons. Nature. 2002;416:736–740. doi: 10.1038/416736a. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor PJ, Neki A, Abe T, Nakanishi S, Mizuno N. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J Neurosci. 1997;17:7503–7522. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimono K, Brucher F, Granger R, Lynch G, Taketani M. Origins and distribution of cholinergically induced beta rhythms in hippocampal slices. J Neurosci. 2000;20:8462–8473. doi: 10.1523/JNEUROSCI.20-22-08462.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G, Spruston N, Sakmann B, Hausser M. Action potential initiation and backpropagation in neurons of the mammalian CNS. Trends Neurosci. 1997;20:125–131. doi: 10.1016/s0166-2236(96)10075-8. [DOI] [PubMed] [Google Scholar]
- Treves A, Rolls ET. Computational constraints suggest the need for two distinct input systems to the hippocampal CA3 network. Hippocampus. 1992;2:189–199. doi: 10.1002/hipo.450020209. [DOI] [PubMed] [Google Scholar]
- Ueno S, Tsukamoto M, Hirano T, Kikuchi K, Yamada MK, Nishiyama N, Nagano T, Matsuki N, Ikegaya Y. Mossy fiber Zn2+ spillover modulates heterosynaptic N-methyl-d-aspartate receptor activity in hippocampal CA3 circuits. J Cell Biol. 2002;158:215–220. doi: 10.1083/jcb.200204066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban NN, Barrionuevo G. Active summation of excitatory postsynaptic potentials in hippocampal CA3 pyramidal neurons. Proc Natl Acad Sci USA. 1998;95:11450–11455. doi: 10.1073/pnas.95.19.11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban NN, Henze DA, Barrionuevo G. Revisiting the role of the hippocampal mossy fiber synapse. Hippocampus. 2001;11:408–417. doi: 10.1002/hipo.1055. [DOI] [PubMed] [Google Scholar]
- Vogt K, Mellor J, Tong G, Nicoll R. The actions of synaptically released zinc at hippocampal mossy fiber synapses. Neuron. 2000;26:187–196. doi: 10.1016/s0896-6273(00)81149-6. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Nicoll RA. Presynaptic changes during mossy fibre LTP revealed by NMDA receptor-mediated synaptic responses. Nature. 1995;376:256–259. doi: 10.1038/376256a0. [DOI] [PubMed] [Google Scholar]
- Wu K, Leung LS. Monosynaptic activation of CA3 by the medial perforant path. Brain Res. 1998;797:35–41. doi: 10.1016/s0006-8993(98)00334-5. [DOI] [PubMed] [Google Scholar]
- Yeckel MF, Berger TW. Feedforward excitation of the hippocampus by afferents from the entorhinal cortex: redefinition of the role of the trisynaptic pathway. Proc Natl Acad Sci USA. 1990;87:5832–5836. doi: 10.1073/pnas.87.15.5832. [DOI] [PMC free article] [PubMed] [Google Scholar]


