Abstract
This study investigated the role of neuropeptide Y (NPY) in mediating cardiovascular responses to reduced oxygenation in the late gestation ovine fetus by: (1) comparing the effects on the cardiovascular system of an exogenous infusion of NPY with those elicited by moderate or severe reductions in fetal oxygenation; and (2) determining the effect of fetal i.v. treatment with a selective NPY-Y1 receptor antagonist on the fetal cardiovascular responses to acute moderate hypoxaemia. Under general anaesthesia, 14 sheep fetuses (0.8–0.9 of gestation) were surgically prepared with vascular and amniotic catheters. In 5 of these fetuses, a Transonic flow probe was also implanted around a femoral artery. Following at least 5 days of recovery, one group of fetuses (n = 9) was subjected to a 30 min treatment period with exogenous NPY (17 μg kg−1 bolus plus 0.85 μg kg−1 min−1 infusion). In this group, fetal blood pressure and heart rate were monitored continuously and the distribution of the fetal combined ventricular output was assessed via injection of radiolabelled microspheres before and during treatment. The second group of fetuses instrumented with the femoral flow probe (n = 5) were subjected to a 3 h experiment consisting of 1 h of normoxia, 1 h of hypoxaemia, and 1 h of recovery during a slow i.v. infusion of vehicle. One or two days later, the acute hypoxaemia protocol was repeated during fetal i.v. treatment with a selective NPY-Y1 receptor antagonist (50 μg kg−1bolus + 1.5 μg kg−1 min−1 infusion). In these fetuses, fetal arterial blood pressure, heart rate and femoral vascular resistance were recorded continuously. The results show that fetal treatment with exogenous NPY mimics the fetal cardiovascular responses to asphyxia, and that treatment of the sheep fetus with a selective NPY-Y1 receptor antagonist does not affect the fetal cardiovascular response to acute moderate hypoxaemia. These results support a greater role for NPY in mediating the fetal cardiovascular responses to acute asphyxia than to acute moderate hypoxaemia.
In the sheep fetus during late gestation, episodes of reduced oxygenation evoke an integrated cardiovascular response that facilitates fetal survival during the period of adversity (Cohn et al. 1974; Giussani et al. 1994a). However, the pattern and magnitude of the fetal defence response to reduced oxygen delivery vary according to the severity of the challenge. In response to acute moderate hypoxaemia, reducing the fetal arterial oxygen pressure (Pa,O2) from ≈24 to ≈12 mmHg, the fetal cardiovascular responses include bradycardia, an increase in arterial blood pressure and an increase in femoral vascular resistance (Boddy et al. 1974; Giussani et al. 1994a). The increase in femoral vascular resistance contributes to peripheral vasoconstriction. This aids the redistribution of the fetal combined ventricular output (CVO) in favour of the adrenal, myocardial and cerebral circulations, which undergo vasodilatation during hypoxaemia (Cohn et al. 1974; Reller et al. 1995; Green et al. 1996; McCrabb & Harding, 1996; van Bel et al. 1997). In response to acute asphyxia, secondary to reduced uterine blood flow, and leading to reductions in the fetal Pa,O2 below 12 mmHg with associated acidaemia, generalised vasoconstriction occurs. This causes reductions in blood flow to even the cerebral, myocardial and adrenal circulations (Yaffe et al. 1987; Jensen et al. 1999; Bennet et al. 2002).
Control of the fetal cardiovascular responses to acute hypoxaemia and acute asphyxia involves neural and endocrine components. The bradycardia and the initial femoral vasoconstriction during acute hypoxaemia are triggered by the same carotid chemoreflex, as both can be abolished by selective carotid, but not aortic, chemodenervation (Bartelds et al. 1993; Giussani et al. 1993). During acute asphyxia, selective chemodenervation attenuates the initial fall in heart rate but does not totally prevent bradycardia (Jensen & Hanson, 1995; Jensen et al. 1999). During acute hypoxaemia and acute asphyxia, fetal bradycardia is largely mediated by vagal efferents and peripheral vasoconstriction by α-adrenergic efferents (Cohn et al. 1974; Reuss et al. 1982; Parer, 1984; Giussani et al. 1993; Jensen & Hanson, 1995; Giussani et al. 1997; Jensen et al. 1999; Bennet et al. 2002). Once initiated, fetal peripheral vasoconstriction is augmented during hypoxaemia and asphyxia by slower release into the fetal circulation of classical vasoactive hormones such as adrenaline and arginine vasopressin (Alexander et al. 1974; Jones & Robinson, 1975; Giussani et al. 1994b; Jensen & Hanson, 1995; Jensen et al. 1999; Bennet et al. 2002; Gardner et al. 2002). However, the contribution made by more recently discovered vasoconstrictor agents, such as the neuropeptides, to fetal cardiovascular function either during basal conditions or in response to acute hypoxaemia or asphyxia remains unclear.
Neuropeptide Y (NPY) is a 36 amino acid peptide from the family of pancreatic polypeptides. The peptide was discovered and isolated from porcine brain preparations and its molecular structure was reported to be highly conserved across species (Tatemoto et al. 1982). Neuropeptide Y is co-localised with noradrenaline in sympathetic nerve terminals and it is also present in the adrenal medulla in mammals (Allen et al. 1983; Lundberg et al. 1983; Ekblad et al. 1984). At the synaptic terminal, NPY is stored in large dense vesicles and it may be co-released with noradrenaline from sympathetic varicosities depending on the frequency and pattern of nerve stimulation (Bloom et al. 1988). In the adult, NPY is known to have a wide variety of actions; however, one of its earliest actions described in the literature is of potent and long-lasting vasoconstriction, either via a direct effect on NPY-Y1 receptors in peripheral circulations or by potentiation of the vasopressor effects of constrictor agents, such as catecholamines (Edvinsson et al. 1984; Franco-Cereceda et al. 1985; Corder et al. 1986; Waeber, 1990; Balasubramaniam, 1997). One previous study measured changes in fetal plasma NPY levels during basal and acute hypoxaemic conditions (Fletcher et al. 2000). In that study, it was reported that the levels of NPY increased significantly in fetal plasma during acute hypoxaemia. However, the role of this increase in plasma NPY during episodes of reduced oxygenation in the fetus remains unknown.
Therefore this study tested the hypothesis that increases in circulating plasma concentrations of NPY play a role in the ovine fetal cardiovascular responses to acute oxygen deprivation during late gestation by: (1) comparing the effects on fetal heart rate, blood pressure and the redistribution of the combined ventricular output of an exogenous infusion of NPY with those of acute moderate or severe hypoxaemia; and (2) determining the effect of fetal intravenous treatment with a selective NPY-Y1 receptor antagonist on the fetal cardiovascular responses to a acute moderate hypoxaemia.
Methods
Animals
A total of 14 sheep fetuses of known gestational age and divided into two experimental groups were used. Group I fetuses were used to investigate the effects of fetal intravenous treatment with exogenous NPY on the fetal cardiovascular system, and Group II fetuses were used to investigate the effects of fetal intravenous treatment with a selective NPY-Y1 receptor antagonist on the fetal cardiovascular response to acute moderate hypoxaemia. Group I comprised nine Merino sheep fetuses, which were surgically prepared at 0.8-0.9 of gestation. Group II comprised five Welsh Mountain sheep fetuses, which were surgically prepared at 0.8 of gestation.
Surgical procedures
Experiments on Group I fetuses were carried out at the Biomedical Sciences Institute, Faculty of Medicine, University of Chile. Experiments on Group II fetuses were carried out at the Department of Physiology, University of Cambridge, UK. Procedures in Chile were reviewed and approved by the Faculty of Medicine Ethics Committee of the University of Chile and were performed under the recommendations in the Guiding Principles for Research Involving Animals and Human Beings of the American Physiological Society. Procedures in Cambridge were licensed by the Home Office and were carried out following recommendations by the UK Animals (Scientific Procedures) Act, 1986.
In brief, food, but not water, was withheld from the pregnant ewes for 24 h prior to surgery. Under general anaesthesia (20 mg (kg body weight)−1i.v. sodium thiopentone, Intraval Sodium, for induction; 1.5 % halothane in 50:50 O2:N2O for maintenance) the gravid uterus was exposed via a midline laparotomy. In Group I (n = 9), the fetuses were partially exteriorised and catheters were inserted into a fetal carotid artery, femoral artery, femoral vein and into the amniotic cavity. The carotid artery catheter was advanced to the ascending aorta, and the femoral artery and vein catheters were advanced to the descending aorta and inferior vena cava, respectively. In Group II (n = 5), the fetuses were instrumented with a femoral artery and vein catheter, an amniotic catheter and an ultrasonic flow transducer (2RS with silicone flange; Transonic Inc., Ithaca, NY, USA). The flow transducer was implanted around the other fetal femoral artery just distal to the inguinal ligament and anchored to surrounding muscle with four stitches through the silicone flange (Giussani et al. 1993). In both groups, the uterine incisions were sewn together in layers and the abdomen and skin closed. All catheters were filled with heparinised saline (80 i.u. heparin ml−1 in 0.9 % NaCl), plugged with brass pins, and exteriorised (with the flow probe lead where appropriate) through a small incision in the maternal flank. Catheters and leads were then housed in a pouch sutured onto the maternal skin.
Post-operative care
Ewes were housed in individual pens, had free access to hay and water, and were fed concentrates twice daily. Antibiotics were administered post-operatively and daily for 3 days to the ewes (either 9–12 mg i.m. Depocillin; Mycofarm, Cambridge, UK or 1 million i.u. of penicillin, i.v., Penicilina G Sódica, Laboratorio Chile, Chile) and into the amniotic cavity (either 300 mg ampicillin, Penbritin, SmithKline Beecham Animal Health, Surrey, UK or 1 million i.u. of penicillin, Penicilina G Sódica and 500 mg of kanamycin, Canamicina Sulfato, Laboratorio Chile, Chile). Vascular catheters were maintained patent by daily flushing with heparinised saline (80 i.u. heparin ml−1 in 0.9 % NaCl).
Experimental procedures
At least 5 days of post-operative recovery were allowed before the commencement of any experiment.
Group I - NPY infusion study
This protocol consisted of three experimental periods of varying duration. Following 20 min of basal recordings, fetuses were treated i.v. with exogenous NPY at a dose of 17 µg kg−1 (4 nmol kg−1) bolus plus 0.85 µg kg−1 min−1 (0.2 nmol kg−1 min−1) infusion, dissolved in bovine albumin 0.1 % vehicle for a period of 30 min. The dose of NPY treatment was calculated from our own pilot experiments in which concentrations of NPY were measured in fetal sheep plasma during sustained and severe reductions in fetal oxygenation. These doses were also calculated considering the only report of the half-life (6 min) for NPY reported in adult cats (Lundberg et al. 1986) and the estimation of the distribution of NPY in the circulating blood volume of fetal sheep. At the end of the treatment, fetuses were monitored for a further 60 min.
Fetal arterial blood pressure (Statham P23Db, Hato Rey, Puerto Rico), venous pressure, amniotic pressure (Statham P23BB) and fetal heart rate (triggered from the arterial pressure pulse) were recorded continuously throughout the protocol on a polygraph (Gilson ICM-5, Emeryville, CA, USA). Fetal CVO and organ blood flows were determined prior to treatment (0 min) and at 10 (+10 min) and 30 min (+30 min) following the onset of treatment. Each time, 15 µm diameter radionuclide-labelled microspheres (57Co, 113Sn, 65Zn, respectively; 3M Company, St Paul and NEN-TRAC Dupont Company, Boston, MA, USA; 1.0–1.2 million) were injected into the inferior vena cava while reference samples were obtained from the ascending and descending aorta (Heymann et al. 1977). The rate at which the reference samples were drawn was 3.24 ml min−1 for 1.5 min. This method allows blood flow determination to all organs except the lung (Heymann et al. 1977).
Fetal arterial blood samples (0.5 ml) were taken into chilled, heparinised syringes before (0 min) during (+10 and +30 min) and after (+90 min) treatment for measurement of arterial pH, Pa,CO2 and Pa,O2 (BMS 3MK2 Blood Microsystem and PHM 73/Blood Gas Monitor, Radiometer, Copenhagen, Denmark; measurements corrected to 39 °C) and percentage saturation of haemoglobin and haemoglobin concentration (OSM2 Haemoximeter, Radiometer). Additional arterial blood samples (0.5 ml) were taken prior to treatment (0 min) and at 5 (+5 min), 10 (+10 min), 20 (+20 min), 30 (+30 min), 40 (+40 min) and 90 min (+90 min) following the onset of treatment for determination of plasma concentrations of NPY.
On completion of the experiments the ewe and fetus were anaesthetised with sodium thiopentone i.v. (1 g; Tiopental Sódico, Laboratorio Chile, Chile) and killed by i.v. injection of saturated potassium chloride.
Calculations
Post mortem, the uterus and individual fetal organs were dissected and weighed. All dissected material was carbonised, ground to coarse powder, placed in vials and counted with a multi-channel gamma pulse analyser (Minaxi 5000, Packard, Canberra, Australia). Microsphere mixing was considered appropriate when the percentage difference in calculated blood flow to either cerebral hemisphere and to either kidney was < 10 %. To minimise error in the calculation of organ blood flow, sufficient microspheres were injected to ensure a distribution of > 400 microspheres per organ (Heymann et al. 1977). In each fetus, blood flow to all organs was calculated by comparing the organ radioactivity with the activity and flow rate of the appropriate reference sample (ascending aorta for upper body organs and descending aorta for lower body organs). The fetal CVO was calculated as the sum of blood flow to all organs except the lungs (Heymann et al. 1977). Vascular resistance to each organ was calculated for each fetus by dividing perfusion pressure (arterial - venous pressure) at the time of microsphere injection by organ blood flow. In addition, blood oxygen content (O2 content) was calculated as follows:
Hormone analysis
Blood samples taken for hormone analysis were immediately centrifuged at 4000 r.p.m. for 4 min at 4°C. Plasma aliquots were snap frozen in liquid N2 and were stored at −70°C until required for assaying. Plasma concentrations of NPY were determined by radioimmunoassay. All samples were assayed in duplicate at the same time. The assay used rabbit anti-NPY antiserum (Peninsula Laboratory, Belmont, State, USA) and 125I-labelled porcine NPY. Separation of free and bound fractions was performed with dextran-coated charcoal. The assay could detect less than 1 pmol l−1 of NPY. The inter-assay coefficient of variation was 9.8 %.
Group II - NPY receptor blockade during acute hypoxaemia study
After at least 5 days of post-surgical recovery, at 126 days of gestation, fetuses in Group II were subjected to a 3 h protocol divided into 3 periods of 60 min: 1 h normoxia, 1 h hypoxaemia and 1 h recovery during a slow fetal i.v. infusion of vehicle (80 i.u. heparin ml−1 in 0.9 % NaCl). A transparent polyethylene bag was placed over the ewe's head into which known concentrations of O2, N2 and CO2 were passed at a rate of ≈40 l min−1. Following 1 h of breathing air through the bag (normoxia), acute moderate hypoxaemia was induced in the mother for 1 h by switching the gas mixture to 9 % O2 in N2 with small amounts of CO2 (18 l min−1 air: 22 l min−1 N2: 1–2 l min−1 CO2). This reduced the fetal descending aortic Pa,O2 to ≈11–12 mmHg without alterating fetal Pa,CO2 from baseline. After 1 h of fetal isocapnic hypoxaemia the ewe was returned to breathing air for a further 60 min (recovery).
On a separate day (1 to 2 days later), isocapnic hypoxaemia was also induced during fetal i.v. treatment with a selective NPY-Y1 receptor antagonist [Cys31, Nva34, NPY(27–36)2]; Bachem UK Ltd; Essex, UK; Balasubramaniam et al. 1996; 50 µg kg−1bolus + 1.5 µg kg−1 min−1 infusion, dissolved in vehicle). Fetal treatment with the antagonist started 20 min before the onset of hypoxaemia and ran continuously until the end of the hypoxaemic challenge. Experiments involving fetal exposure to acute isocapnic hypoxaemia with and without the selective NPY-Y1 receptor antagonist were randomised.
The dose of the NPY-Y1 receptor antagonist employed in this study was also based on our own pilot experiments in three Group II fetuses. Two days prior to the first acute hypoxaemic protocol, increasing bolus doses of NPY (10, 20, 50, 100 µg, Bachem UK Ltd, Essex, UK) were administered into the fetal femoral artery (Fig. 1). The two largest doses produced a fall in femoral blood flow and an increase in femoral vascular resistance, similar to those measured in response to acute moderate hypoxaemia (Fig. 1). The dose of antagonist chosen for the protocol was double that needed to completely prevent the femoral vasoconstriction induced by the highest dose of exogenous NPY.
Figure 1. Efficacy of the NPY-Y1 receptor antagonist.
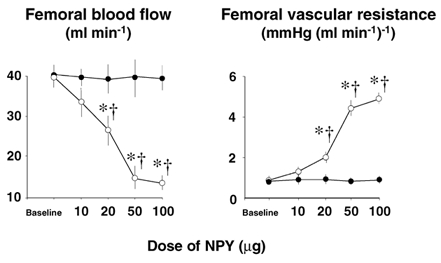
Values are means ± s.e.m. for absolute values of femoral blood flow and femoral vascular resistance responses to increasing i.a. bolus doses of NPY in fetuses before (○) and after (•) i.v. pre-treatment with the NPY-Y1 receptor antagonist for 5 min (25 µg kg−1bolus + 0.75µg kg−1 min−1 infusion). Antagonist treatment was stopped 5 min after each dose and cardiovascular variables were allowed to return to baseline before starting the antagonist treatment prior to the next highest dose of exogenous NPY. Significant differences (P < 0.05): * differences by post-hoc analysis indicating a significant main effect of time compared with baseline; † differences by post-hoc analysis indicating a significant main effect of treatment (two way ANOVA + Tukey test).
During any acute hypoxaemic experimental protocol, descending aortic blood samples (0.4 ml) were taken from the fetus at 15 (N15) and 45 min (N45) of normoxia, at 15 (H15) and 45 min (H45) of hypoxaemia and at 15 (R15) and 45 min (R45) of recovery, to determine blood gases, acid-base status, haemoglobin concentration and its percentage saturation with oxygen (ABL 5 and OSM 2; Radiometer; measurements corrected to 39 °C). An additional fetal arterial sample (0.3 ml) was taken 5 min after the onset of hypoxaemia to confirm that the fetal Pa,O2 had fallen to the desired level.
At the end of all experiments, the ewes and fetuses were humanely killed using a lethal dose of sodium pentobarbitone (200 mg (kg body weight)−1) Pentoject; Animalcare Ltd, York, UK). The position of the catheters was confirmed, the flow probe was removed and the fetuses were weighed.
Calibrated fetal arterial and amniotic pressures were measured using disposable pressure transducers (Cobe, Argon, Texas, USA) and pressure amplifiers developed at Cornell University. Femoral blood flow was measured on a T201 or T206 flow box (Transonics, Inc, Ithaca, NY). Fetal heart rate was derived from the femoral blood flow pulsatility. Fetal femoral vascular resistance was calculated by dividing fetal perfusion (arterial - venous) pressure by fetal femoral blood flow. All cardiovascular variables were recorded continuously using a data acquisition system that sampled all signals at a rate of 500 Hz. Cardiovascular analog data were digitised, displayed and stored every second on a hard drive by custom software (NI-DAQ, National Instruments, Austin, Texas) running on IBM compatible computers. Digital files were subsequently analysed using MS-Excel spreadsheets.
Statistical analyses
Data are expressed as the mean ± s.e.m. Data for blood flow distribution during the fetal intravenous treatment with exogenous NPY protocol in Group I fetuses are expressed as percentage change (Fig. 3 and Fig. 4). Cardiovascular variables during the acute hypoxaemia protocol in Group II fetuses were analysed as absolute data, changes from baseline, areas under the curve and as the slopes of the initial decrease in heart rate and the initial increase in femoral vascular resistance during acute hypoxaemia. Absolute values averaged every minute are shown in Fig. 5A and summary measures of these data are shown in Fig. 5B (Matthews et al. 1990). Values for the summary measures represent the mean ± s.e.m. for cardiovascular variables calculated over pre-determined time periods known to pick up the salient features of the fetal cardiovascular response to this challenge (Giussani et al. 1996, 2001): normoxia (1 h of baseline), early hypoxaemia (first 15 min of hypoxaemia), late hypoxaemia (remaining 45 min of hypoxaemia), early recovery (first 15 min of recovery) and late recovery (remaining 45 min of recovery). Comparisons of data within and between treatment groups were made using a two-way ANOVA followed by Tukey or Student-Newman-Keuls tests. For all statistical comparisons, differences were considered significant when P < 0.05.
Figure 3. Cardiac output and regional blood flows (A) and regional vascular resistances (B) during NPY infusion.
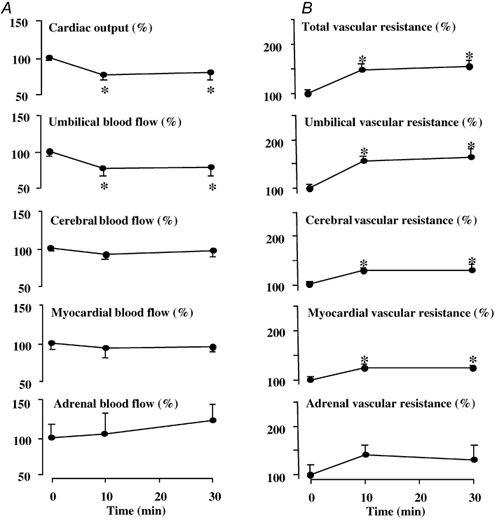
Values are expressed as the mean percentage change ± s.e.m. from baseline prior to treatment (0 min) and at +10 and +30 min following the onset of fetal intravenous treatment with NPY (17 µg kg−1bolus + 0.85µg kg−1 min−1 infusion). Significant differences are: *P < 0.05, differences by post-hoc analysis indicating a significant main effect of time (one-way repeated measures ANOVA + Student-Newman-Keuls test).
Figure 4. Blood flow (A) and vascular resistances (B) in peripheral circulations during NPY infusion.
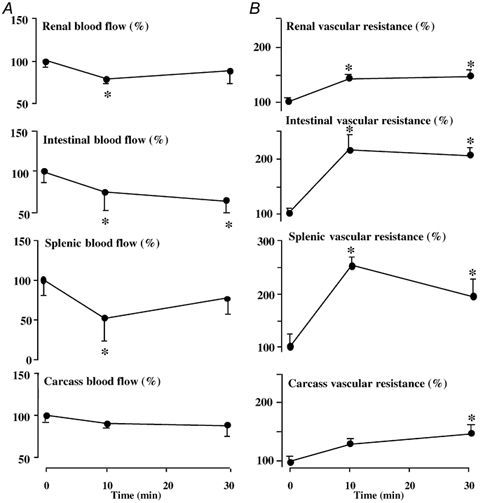
Values are expressed as the mean percentage change ± s.e.m. from baseline prior to treatment (0 min) and at +10 and +30 min following the onset of fetal intravenous treatment with (17 µg kg−1bolus + 0.85µg kg−1 min−1 infusion). Significant differences are: *P < 0.05, differences by post-hoc analysis indicating a significant main effect of time (one-way repeated measures ANOVA + Student-Newman-Keuls test).
Figure 5. Fetal cardiovascular responses to acute hypoxaemia with and without the selective NPY-Y1 receptor antagonist.
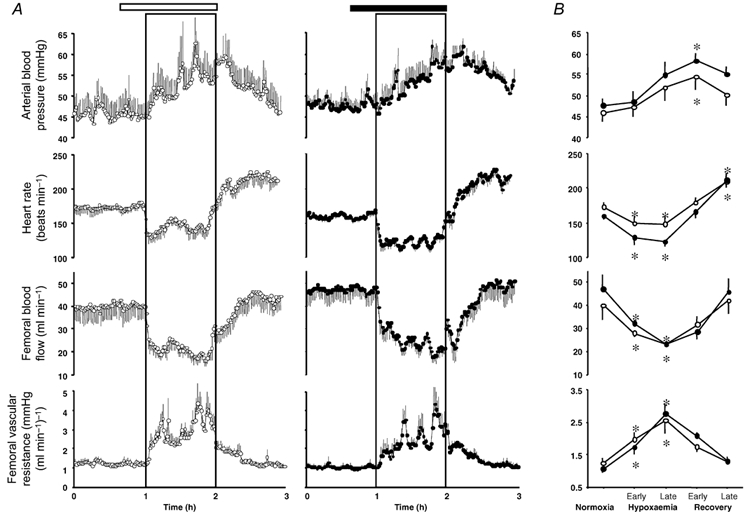
Values are means ± s.e.m. for cardiovascular variables of five fetuses calculated every minute of the experimental protocol (A) and the statistical summary for these cardiovascular data (B). Fetuses were infused with either saline (open horizontal bar) or with the NPY-Y1 receptor antagonist (filled horizontal bar) dissolved in saline. Treatment started 20 min prior to the onset of acute hypoxaemia (box) and ran continuously until the end of the hypoxaemic challenge. Values for the statistical summary represent the means ± s.e.m. for cardiovascular variables calculated for the following time periods: normoxia (1 h of baseline), early hypoxaemia (first 15 min of hypoxaemia), late hypoxaemia (remaining 45 min of hypoxaemia), early recovery (first 15 min of recovery), and late recovery (remaining 45 min of recovery). Significant differences were assessed using a two-way ANOVA followed by Tukey or Student-Newman-Keuls tests. Siginificant differences are: *P < 0.05, differences by post-hoc analysis indicating a significant main effect of time. There were no significant differences between groups.
Results
Group I - NPY infusion study
Fetal intravenous treatment with exogenous NPY elevated fetal plasma circulating concentrations of NPY from 8 ± 2 to 383 ± 67 pmol l−1 (mean ± s.e.m. prior to treatment and following treatment). Treatment of the sheep fetus with exogenous NPY led to mild, but significant falls in fetal pHa, Pa,O2 and saturation of haemoglobin with oxygen and a significant increase in haemoglobin concentration (Table 1). Fetal Pa,CO2 and blood oxygen content remained unaltered from baseline during treatment with NPY.
Table 1.
Fetal arterial blood gas and acid base status during the NPY infusion
| Basal | +10 min | +30 min | +90 min | |
|---|---|---|---|---|
| pHa | 7.40 ± 0.01 | 7.37 ± 0.01 | 7.37 ± 0.01 | 7.34 ± 0.02* |
| Pa,CO2 (mmHg) | 44.6 ± 1.6 | 45.2 ± 1.3 | 46.0 ± 1.1 | 47.0 ± 1.6 |
| Pa,O2 (mmHg) | 22.2 ± 0.9 | 20.2 ± 1.4* | 20.7 ± 1.0* | 20.8 ± 1.1* |
| Sat. Hb (%) | 49.5 ± 2.1 | 44.5 ± 2.3* | 43.5 ± 1.6* | 38.9 ± 2.5* |
| Hb (g dl−1) | 8.8 ± 0.5 | 9.3 ± 0.5* | 9.7 ± 0.5* | 10.5 ± 0.4* |
| O2cont (ml O2 dl−1) | 5.8 ± 0.4 | 5.5 ± 0.2 | 5.6 ± 0.2 | 5.4 ± 0.3 |
Values are means ±s.e.m. Fetuses (n = 9) were treated i.v. with NPY (17 μg kg–1bolus + 0.85μg kg–1 min–1 infusion) for 30 min. Arterial blood samples were take prior to treatment (0 min) and at 10 (+10 min), 30 (+30 min) and 90 (+90 min) minutes following the onset of treatment. Significant differences are:
P < 0.05, differences by post-hoc analysis indicating a significant main effect of time (one-way repeated measures ANOVA + the Student-Newman-Keuls test).
pHa, arterial pH; Pa,CO2. arterial CO2 partial pressure; Pa,O2, arterial O2 partial pressure, Sat. Hb, percentage saturation of haemoglobin with oxygen; Hb, haemoglobin concentration; O2 cont, arterial oxygen content.
Treatment of the sheep fetus with NPY had pronounced effects on fetal blood pressure, heart rate, cardiac output and changes in blood flow and vascular resistance in regional circulations. Fetal treatment with NPY led to a marked and sustained increase in fetal systemic blood pressure and a transient fall in fetal heart rate (Fig. 2). Whilst treatment of the sheep fetus with NPY led to a significant fall in cardiac output and significant falls in blood flow to the umbilical, renal, splenic and intestinal circulations, blood flow to the cerebral, myocardial, adrenal and carcass circulations were maintained during NPY treatment (Fig. 3 and Fig. 4). The pronounced increase in arterial blood pressure coupled with these changes in cardiac output and regional blood flows led to a significant increase in total vascular resistance and vascular resistance in all regional circulations with the exception of the adrenal vascular bed (Fig. 3 and Fig. 4). However, the percentage increments in vascular resistance in the peripheral circulations (renal: 46 ± 13 %; intestinal: 118 ± 26 %; splenic: 152 ± 17 %; carcass: 46 ± 16 %) were greater than those calculated in the cerebral (29 ± 12 %) and myocardial (24 ± 9 %) vascular beds during fetal treatment with exogenous NPY (Fig. 3 and Fig. 4).
Figure 2. Fetal arterial blood pressure and heart rate responses to NPY infusion.
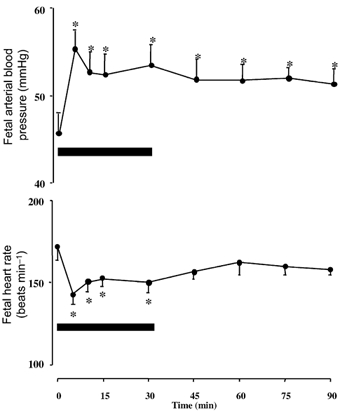
Values are means ± s.e.m. at 5, 10, 15, 30, 45, 60, 75 and 90 min after the onset of fetal intravenous treatment with NPY (17 µg kg−1bolus + 0.85µg kg−1 min−1 infusion). Significant differences are: *P < 0.05, differences by post-hoc analysis indicating a significant main effect of time (one-way repeated measures ANOVA plus Student-Newman-Keuls test).
Group II - NPY blockade during acute hypoxaemia study
Fetal basal arterial blood gas status was similar before infusion with either vehicle or the NPY-Y1 receptor antagonist during normoxia (Table 2). Intravenous treatment of the sheep fetus with the NPY-Y1 receptor antagonist during normoxia had no effect on arterial blood gas status (Table 2).
Table 2.
Fetal arterial blood gas status during acute hypoxaemia with and without the NPY-Y, receptor antagonist
| Normoxia | Hypoxaemia | Recovery | |||||
|---|---|---|---|---|---|---|---|
| N15 | N45 | H15 | H45 | R15 | R45 | ||
| PHa | Saline | 7.35 ± 0.01 | 7.35 ± 0.01 | 7.32 ± 0.02 | 7.27 ± 0.02* | 7.25 ± 0.03* | 7.28 ± 0.01* |
| Antagonist | 7.34 ± 0 | 7.34 ± 0 | 7.31 ± 0.02 | 7.28 ± 0.04* | 7.25 ± 0.02* | 7.27 ± 0.02* | |
| Pa,CO2 (mmHg) | Saline | 51.0 ± 1.8 | 51.7 ± 1.5 | 51.2 ± 1.4 | 51.7 ± 0.7 | 49.5 ± 1.5 | 50.0 ± 1.3 |
| Antagonist | 51.6 ± 0.9 | 51.4 ± 0.6 | 52.4 ± 1.1 | 52.6 ± 1.3 | 51.4 ± 1.1 | 49.6 ± 0.9 | |
| Pa,O2 (mmHg) | Saline | 21.3 ± 0.9 | 21.7 ± 0.8 | 11.8 ± 0.3* | 12.2 ± 0.3* | 23.3 ± 1.7 | 21.3 ± 1.6 |
| Antagonist | 22.2 ± 0.8 | 22.2 ± 1.0 | 11.8 ± 0.4* | 12.4 ± 0.6* | 25.6 ± 1.2 | 21.6 ± 1.9 | |
| ABE (mequiv 1−1) | Saline | 2.0 ± 0.3 | 2.0 ± 0.3 | −0.5 ± 0.8 | −4.7 ± 1.3* | −8.2 ± 2.0* | −4.8 ± 1.5* |
| Antagonist | 1.8 ± 0.5 | 1.4 ± 0.4 | −0.4 ± 0.7 | −4.4 ± 1.8* | −9.6 ± 1.3* | −5.8 ± 0.9* | |
| Sat. Hb (%) | Saline | 61.6 ± 2.9 | 62.7 ± 2.3 | 29.4 ± 1.6* | 30.3 ± 1.9* | 58.2 ± 3.4 | 57.5 ± 3.5 |
| Antagonist | 60.5 ± 1.1 | 58.6 ± 2.5 | 30.2 ± 1.7* | 28.9 ± 1.9* | 59.9 ± 2.1 | 56.5 ± 3.3 | |
| Hb (g dl−1) | Saline | 8.9 ± 0.5 | 8.9 ± 0.5 | 9.9 ± 0.5* | 9.7 ± 0.5* | 8.6 ± 0.5 | 8.3 ± 0.4 |
| Antagonist | 8.0 ± 0.4 | 8.5 ± 0.4 | 9.4 ± 0.4* | 9.5 ± 0.3* | 8.7 ± 0.3 | 8.7 ± 0.3 | |
Values are means ± s.e.m. taken at 15 (N15) and 45 (N45) minutes of normoxia, 15 (H15) and 45 (H45) minutes of hypoxaemia and 15 (R15) and 45 (R45) minutes of recovery for 5 fetuses during vehicle infusion and during treatment with the selective NPY-Y1 receptor antagonist. Treatment started 20 min prior to the onset of acute hypoxaemia and ran continuously until the end of the hypoxaemic challenge (bar). Significant differences are:
P < 0.05, differences by post-hoc analysis indicating a significant main effect of time (one-way repeated measures ANOVA + Tukey test).
pHa, arterial pH; Pa,CO2 arterial CO2 partial pressure; Pa,O2, arterial O2 partial pressure; ABE, acid–base excess; Sat. Hb., percentage saturation of haemoglobin with oxygen; Hb, haemoglobin concentration.
In hypoxaemia, fetal descending aortic Pa,O2 fell rapidly to similar values during vehicle infusion or treatment with the NPY-Y1 receptor antagonist (Table 2). Fetal hypoxaemia during vehicle or antagonist infusion occurred without significant alterations in fetal Pa,CO2 from baseline, but was accompanied by progressive metabolic acidaemia. Fetal pHa and base excess fell during hypoxaemia and recovery following fetal infusion with either vehicle or the NPY-Y1 receptor antagonist (Table 2). The magnitude of the changes in fetal pHa and base excess during hypoxaemia and recovery were similar in the two groups.
During fetal infusion with vehicle, acute hypoxaemia rapidly elicited fetal bradycardia, a pronounced and sustained increase in fetal arterial blood pressure and an abrupt fall in fetal femoral blood flow which remained depressed for most of the duration of the hypoxaemic insult (Fig. 5). This fall in femoral blood flow and the increase in arterial blood pressure resulted in a marked increase in calculated fetal femoral vascular resistance, which remained elevated until the end of hypoxaemia. While fetal arterial blood pressure, femoral blood flow and femoral vascular resistance returned towards baseline values following the end of hypoxaemia, a rebound tachycardia developed during the recovery period (Fig. 5).
Although there was a suggestion that fetal treatment with the NPY-Y1 receptor antagonist led to persistent bradycardia and a slower rise in femoral vascular resistance during acute hypoxaemia, detailed statistical analyses of the data failed to show any significant difference in the fetal cardiovascular response to acute hypoxaemia with and without NPY-Y1 receptor antagonism (Fig. 5).
Discussion
One of the most important functions of NPY in the physiology of the adult animal is of potent and long-lasting vasoconstriction (Edvinsson et al. 1984; Franco-Cereceda et al. 1985; Corder et al. 1986; Waeber, 1990; Balasubramaniam, 1997). Although there are several reports of changes in adult plasma NPY levels in multiple species under various physiological challenges, including asphyxia (Lundberg et al. 1986), hypoglycaemia (Han et al. 1997), hypobaric hypoxia (Cheng et al. 1992) and haemorrhagic hypovolaemia (Rudehill et al. 1987), only one previous study has measured changes in NPY concentrations in the fetus under basal and stimulated conditions (Fletcher et al. 2000). In that study, it was reported that ovine maternal basal plasma NPY concentration was approximately three times that measured in the fetus. However, while maternal plasma NPY concentration remained unchanged from baseline, significant increments in fetal plasma NPY concentration occurred during acute hypoxaemia. The increase in fetal plasma NPY concentration in the absence of changes in maternal plasma NPY concentration suggested that the NPY released in response to the hypoxaemic challenge was of fetal rather than maternal origin. Furthermore, additional data presented by Fletcher et al. (2000) showed that fetal treatment with clinical doses of the synthetic steroid dexamethasone greatly enhanced both the peripheral vasoconstrictor and the fetal plasma NPY responses to acute hypoxaemia. Values for plasma NPY concentration were correlated to femoral vascular resistance values irrespective of fetal treatment, with the greatest levels of both variables being achieved during hypoxaemia in dexamethasone-treated fetuses (Fletcher et al. 2000). Combined past data, therefore, suggested that the increase in circulating plasma NPY in response to acute oxygen deprivation was a strong candidate for mediating, at least in part, peripheral vasoconstrictor responses in the late gestation ovine fetus.
The present study tested the hypothesis that increased concentrations of NPY have a role in the fetal cardiovascular defence to acute oxygen deprivation by designing a two-pronged approach. First, the study characterised the redistribution of the fetal combined ventricular output during exogenous NPY treatment. Second, the study determined the effects of a selective NPY-Y1 receptor antagonist on the fetal peripheral vasoconstrictor response to acute moderate hypoxaemia. The results of the study show that exogenous intravenous treatment of the late gestation sheep fetus with NPY produces bradycardia, hypertension and a generalised increase in organ vascular resistance, including the myocardial and cerebral vascular beds. However, treatment of the late gestation sheep fetus with a selective NPY-Y1 receptor antagonist did not affect basal cardiovascular function, or any of the fetal cardiovascular responses to acute moderate hypoxaemia.
In the present study, treatment of the sheep fetus with the calculated dose of NPY increased fetal plasma NPY to concentrations similar to those measured in severely hypoxaemic sheep fetuses in our pilot experiments. However, these concentrations were ≈30 times greater than those measured in fetal sheep in response to acute moderate hypoxaemia (Fletcher et al. 2000). Therefore, it is possible that fetal treatment with lower doses of NPY, resulting in circulating NPY concentrations similar to those measured during acute moderate hypoxaemia, may not elicit the marked and generalised vasoconstriction. Interestingly, in the present study, the relatively higher doses of NPY treatment in the sheep fetus not only led to hypertension, bradycardia and vasoconstriction in all peripheral vascular beds, but it also produced significant reductions in fetal cardiac output and in umbilical blood flow. It is likely that the powerful vasoconstrictor effects of NPY in the sheep fetus largely contributed to the increase in arterial blood pressure, via an increase in total peripheral vascular resistance, and to the fall in cardiac output, via both an increase in cardiac afterload and a fall in heart rate. Reller et al. (1987) reported that in the late gestation sheep fetus, the right ventricle is immature relative to the left ventricle, and that increases in afterload produce dramatic reductions in right ventricular stroke volume, which largely contribute to a fall in cardiac output. The mechanism mediating the fall in heart rate following treatment with NPY is probably a baroreflex response. The significant reduction in umbilical blood flow is also a possible explanation for the slight fall in pH, Pa,O2 and haemoglobin saturation during treatment with NPY. The significant increase in haemoglobin concentration may have buffered the reduction in fetal oxygenation so that oxygen content remained unchanged from baseline, and it may also represent an effect of NPY on red cell number, secondary to haemoconcentration and/or emptying of venous capacitance vessels.
Since NPY is known to be co-localised with noradrenaline in postganglionic sympathetic nerve terminals innervating the peripheral vasculature (Lundberg et al. 1983; Ekblad et al. 1984) and to be present in high concentrations in the adult mammalian CNS (Gray & Morley, 1986) and adrenal medulla (Allen et al. 1983), it is possible that the source of the increase in circulating NPY in response to acute stress in the adult (Lundberg et al. 1986; Rudehill et al. 1987; Cheng et al. 1992; Han et al. 1997) or fetal (Fletcher et al. 2000) animal is both neural and endocrine. However, despite the relatively high adrenal medullary NPY activity, adrenalectomy in adult rats had no effect on basal NPY or on the increment in plasma NPY in response to acute psychological stress (Mormede et al. 1990). In addition, experiments in intact calves (Bloom et al. 1988) and adrenalectomised, weaned lambs (Bloom et al. 1989) have provided evidence for a negligible role of the adrenal medulla in contributing to the increase in circulating NPY concentrations during splanchnic nerve stimulation. Taken together, these data suggest that adrenal medullary NPY contributes little to the increment in plasma NPY observed during splanchnic nerve stimulation and certain types of acute stress. Furthermore, overspill from perivascular sympathetic nerve terminals is the most likely source of plasma NPY levels under stressful conditions in the adult or fetal animal.
In the present study, the dose of the selective NPY-Y1 receptor antagonist chosen was twice that needed to completely block the marked femoral vasoconstriction induced by the highest bolus dose of exogenous NPY in pilot experiments. Despite using this relatively high dose, it is possible that the circulating concentration of the selective antagonist achieved was sufficient to block any endocrine actions of NPY on the fetal circulation, but insufficient to antagonise the much greater synaptic concentrations of NPY during acute hypoxaemia.
Finally, since in the experiments using the selective NPY-Y1 receptor antagonist only femoral blood flow was monitored as an index of peripheral blood flow, a remaining possibility that needs consideration is that the antagonist could have prevented peripheral vasoconstrictor responses to acute hypoxaemia, which are not well-represented by the femoral vascular bed. However, we do not support this possibility as the femoral vascular bed has been validated to be a good index of peripheral vasoconstrictor responses to acute hypoxaemia in the fetus, in preparations where peripheral flow was measured using both the microsphere technique and ultrasound flow probes (Giussani et al. 1996). In addition in the present study, treatment of the sheep fetus with the selective NPY-Y1 receptor antagonist did not affect the increase in fetal arterial blood pressure, a response that represents an increase in total peripheral vascular resistance.
In summary, the data show that fetal intravenous treatment with exogenous NPY mimicked the fetal cardiovascular responses to acute severe hypoxaemia or severe asphyxia, but treatment of the sheep fetus with a selective NPY-Y1 receptor antagonist did not affect basal cardiovascular function nor the fetal cardiovascular responses to acute moderate hypoxaemia. Combined, these data do not support a strong role for NPY in the control of basal cardiovascular function or in mediating peripheral vasoconstrictor responses to acute moderate stress mediated via endocrine mechanisms in the late gestation ovine fetus. However, the data cannot exclude the possibility that NPY may contribute to peripheral vasoconstrictor responses to acute moderate stress mediated via neural mechanisms, or to acute severe stress mediated via neural or endocrine mechanisms. The authors speculate a greater role of NPY in the fetal cardiovascular defence to asphyxia, contributing in particular to the intense vasoconstriction measured under this condition.
Acknowledgments
The authors are grateful to Carlos Muñoz and Paul Hughes for technical assistance and to Sue Nicholls and Vicky Johnston for the care of the animals. This work was supported by FONDECYT 1010636, Chile; Glaxo-SmithKline, Research Triangle Park, NC, USA; Tommy's, the Baby Charity and The Department of Physiology at the University of Cambridge, UK. Andrew J. W. Fletcher was supported by the Foster Studentship, Department of Physiology, University of Cambridge, UK. Dino A. Giussani is a Fellow of The Lister Institute for Preventive Medicine, UK.
References
- Alexander DP, Bashore RA, Britton HG, Forsling ML. Ternal and fetal arginine vasopressin in the chronically catheterised sheep. Biol Neonate. 1974;25:242–248. doi: 10.1159/000240696. [DOI] [PubMed] [Google Scholar]
- Allen JM, Adrian TE, Polak JM, Bloom SR. Neuropeptide Y (NPY) in the adrenal gland. J Auton Nerv Syst. 1983a;9:559–563. doi: 10.1016/0165-1838(83)90013-9. [DOI] [PubMed] [Google Scholar]
- Balasubramaniam A. Neuropeptide Y family of hormones: receptor subtypes and antagonists. Peptides. 1997;18:445–457. doi: 10.1016/s0196-9781(96)00347-6. [DOI] [PubMed] [Google Scholar]
- Balasubramaniam A, Zhai W, Sheriff S, Tao Z, Chance WT, Fischer JE, Eden P, Taylor J. Bis (31/31′)Cys31, Trp32, Nva34] NPY-(31–36): a specific NPY-Y1 receptor antagonist. J Med Chem. 1996;39:811–813. doi: 10.1021/jm950811r. [DOI] [PubMed] [Google Scholar]
- Bartelds B, van Bel F, Teitel DF, Rudolph AM. Carotid, not aortic, chemoreceptors mediate the fetal cardiovascular response to acute hypoxemia in lambs. Pediatr Res. 1993;34:51–55. doi: 10.1203/00006450-199307000-00013. [DOI] [PubMed] [Google Scholar]
- Bennet L, Westgate JA, Gluckman PD, Gunn AJ. Fetal responses to asphyxia. In: Stevenson DK, Sunshine P, editors. Fetal and Neonatal Brain Injury: Mechanisms, Management, and the Risks of Practice. 2. UK: Cambridge University Press; 2002. [Google Scholar]
- Bloom SR, Edwards AV, Jones CT. The adrenal contribution to the neuroendocrine responses to splanchnic nerve stimulation in conscious calves. J Physiol. 1988;397:513–526. doi: 10.1113/jphysiol.1988.sp017016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom SR, Edwards AV, Jones CT. Neuroendocrine responses to stimulation of the splanchnic nerves in bursts in conscious, adrenalectomized, weaned lambs. J Physiol. 1989;417:79–89. doi: 10.1113/jphysiol.1989.sp017791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddy K, Jones CT, Mantell C, Ratcliffe JG, Robinson JS. Changes in plasma ACTH and corticosteroid of the maternal and fetal sheep during hypoxia. Endocrinology. 1974;94:588–591. doi: 10.1210/endo-94-2-588. [DOI] [PubMed] [Google Scholar]
- Cheng JT, Chen CF, Shum AY, Wang JY, Chen HI. Increase of plasma neuropeptide Y-like immunoreactivity following chronic hypoxia in the rat. Neurosci Lett. 1992;140:211–214. doi: 10.1016/0304-3940(92)90105-g. [DOI] [PubMed] [Google Scholar]
- Cohn EH, Sacks EJ, Heymann MA, Rudolph AM. Cardiovascular responses to hypoxemia and acidemia in fetal lambs. Am J Obstet Gynecol. 1974;120:817–824. doi: 10.1016/0002-9378(74)90587-0. [DOI] [PubMed] [Google Scholar]
- Corder R, Lowry PJ, Wilkinson SJ, Ramage AG. Comparison of the haemodynamic actions of neuropeptide Y, angiotensin II and noradrenaline in anaesthetised cats. Eur J Pharmacol. 1986;121:25–30. doi: 10.1016/0014-2999(86)90388-2. [DOI] [PubMed] [Google Scholar]
- Edvinsson L, Ekblad E, Hakanson A, Wahlestedt C. Neuropeptide Y potentiates the effect of various vasoconstrictor agents on rabbit blood vessels. Br J Pharmacol. 1984;83:519–525. doi: 10.1111/j.1476-5381.1984.tb16516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblad E, Edvinsson L, Wahlestedt C, Uddman R, Hakanson R, Sundler F. Neuropeptide Y co-exists and co-operates with noradrenaline in perivascular nerve fibers. Regul Pept. 1984;8:225–235. doi: 10.1016/0167-0115(84)90064-8. [DOI] [PubMed] [Google Scholar]
- Franco-Cereceda A, Lundberg JM, Dahlöf C. Neuropeptide Y and sympathetic control of heart contractility and coronary vascular tone. Acta Physiol Scand. 1985;124:361–369. doi: 10.1111/j.1748-1716.1985.tb07671.x. [DOI] [PubMed] [Google Scholar]
- Fletcher AJW, Edwards CMB, Gardner DS, Fowden AL, Giussani DA. Neuropeptide Y in the sheep fetus: Effects of acute hypoxemia and dexamethasone during late gestation. Endocrinology. 2000;141:3976–3982. doi: 10.1210/endo.141.11.7770. [DOI] [PubMed] [Google Scholar]
- Gardner DS, Fletcher AJ, Bloomfield MR, Fowden AL, Giussani DA. Effects of prevailing hypoxaemia, acidaemia or hypoglycaemia upon the cardiovascular, endocrine and metabolic responses to acute hypoxaemia in the ovine fetus. J Physiol. 2002;540:351–366. doi: 10.1113/jphysiol.2001.013434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani DA, Gardner DS, Cox DR, Fletcher AJW. Purinergic contribution to the circulatory, metabolic and adrenergic responses to acute hypoxemia in fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2001;280:R678–688. doi: 10.1152/ajpregu.2001.280.3.R678. [DOI] [PubMed] [Google Scholar]
- Giussani DA, McGarrigle HHG, Spencer JAD, Moore PJ, Bennet L, Hanson MA. Effect of carotid denervation on plasma vasopressin levels during acute hypoxia in the late-gestation sheep fetus. J Physiol. 1994b;477:81–87. doi: 10.1113/jphysiol.1994.sp020173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani DA, Riquelme RA, Moraga FA, Mcgarrigle HHG, Gaete CR, Sanhueza EM, Hanson MA, Llanos AJ. Chemoreflex and endocrine components of cardiovascular responses to acute hypoxemia in the llama fetus. Am J Physiol. 1996;271:R73–83. doi: 10.1152/ajpregu.1996.271.1.R73. [DOI] [PubMed] [Google Scholar]
- Giussani DA, Spencer J, Hanson MA. Fetal cardiovascular reflex responses to hypoxaemia. Fetal Matern Med Rev. 1994a;6:17–37. [Google Scholar]
- Giussani DA, Spencer JA, Moore PJ, Bennet L, Hanson MA. Afferent and efferent components of the cardiovascular reflex responses to acute hypoxia in term fetal sheep. J Physiol. 1993;461:431–449. doi: 10.1113/jphysiol.1993.sp019521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani DA, Unno N, Jenkins SL, Wentworth RA, Derks JB, Collins JH, Nathanielsz PW. Dynamics of cardiovascular responses to repeated partial umbilical cord compression in late-gestation sheep fetus. Am J Physiol. 1997;273:H2351–2360. doi: 10.1152/ajpheart.1997.273.5.H2351. [DOI] [PubMed] [Google Scholar]
- Gray TS, Morley JE. Neuropeptide Y: Anatomical distribution and possible function in mammalian nervous system. Life Sci. 1986;38:389–401. doi: 10.1016/0024-3205(86)90061-5. [DOI] [PubMed] [Google Scholar]
- Green LR, Bennet L, Hanson MA. The role of nitric oxide synthesis in cardiovascular responses to acute hypoxia in the late gestation sheep fetus. J Physiol. 1996;497:271–277. doi: 10.1113/jphysiol.1996.sp021766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Chen X, Wu YM, Naes L, Westfall T. Elevated neuropeptide Y gene expression and release during hypoglycemic stress. Peptides. 1997;18:1335–1340. doi: 10.1016/s0196-9781(97)00212-x. [DOI] [PubMed] [Google Scholar]
- Heymann MA, Payne DB, Hoffman JI, Rudolph AM. Blood flow measurements with radionuclide-labeled particles. Prog Cardiovasc Dis. 1977;20:55–79. doi: 10.1016/s0033-0620(77)80005-4. [DOI] [PubMed] [Google Scholar]
- Jensen A, Garnier Y, Berger R. Dynamics of fetal circulatory responses to hypoxia and asphyxia. Eur J Obstet Gynaecol Reprod Biol. 1999;84:155–1572. doi: 10.1016/s0301-2115(98)00325-x. [DOI] [PubMed] [Google Scholar]
- Jensen A, Hanson MA. Circulatory responses to acute asphyxia in intact and chemodenervated fetal sheep near term. Reprod Fertil Dev. 1995;7:1351–1359. doi: 10.1071/rd9951351. [DOI] [PubMed] [Google Scholar]
- Jones CT, Robinson RO. Plasma catecholamines in fetal and adult sheep. J Physiol. 1975;285:381–393. doi: 10.1113/jphysiol.1975.sp010960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg JM, Fried G, Pernow J, Theodorsson-norheim E. Co-release of neuropeptide Y and catecholamines upon adrenal activation in the cat. Acta Physiol Scand. 1986;126:231–238. doi: 10.1111/j.1748-1716.1986.tb07810.x. [DOI] [PubMed] [Google Scholar]
- Lundberg JM, Terenius L, Hokfelt T, Goldstein M. High levels of neuropeptide Y in peripheral noradrenergic neurons in various mammals including man. Neurosci Lett. 1983;42:167–172. doi: 10.1016/0304-3940(83)90401-9. [DOI] [PubMed] [Google Scholar]
- McCrabb GJ, Harding R. Role of nitric oxide in the regulation of cerebral blood flow in the ovine fetus. Clin Exp Pharmacol Physiol. 1996;23:855–860. doi: 10.1111/j.1440-1681.1996.tb01133.x. [DOI] [PubMed] [Google Scholar]
- Matthews JNS, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. Br Med J. 1990;300:230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormede P, Castagne V, Rivet JM, Gaillard R, Corder R. Involvement of neuropeptide Y in neuroendocrine stress responses. Central and peripheral studies. J Neural Transm. 1990;29(suppl):65–75. doi: 10.1007/978-3-7091-9050-0_8. [DOI] [PubMed] [Google Scholar]
- Parer JT. Effect of atropine on heart rate and oxygen consumption of the hypoxic fetus. Am J Obstet Gynecol. 1984;148:1118–1122. doi: 10.1016/0002-9378(84)90638-0. [DOI] [PubMed] [Google Scholar]
- Reller MD, Burson MA, Lohr JL, Morton MJ, Thornburg KL. Nitric oxide is an important determinant of coronary flow at rest and during hypoxemic stress in fetal lambs. Am J Physiol. 1995;269:H2074–2081. doi: 10.1152/ajpheart.1995.269.6.H2074. [DOI] [PubMed] [Google Scholar]
- Reller MD, Morton MJ, Reid DL, Thornburg KL. Fetal lamb ventricles respond differently to filling and arterial pressures and to in utero ventilation. Pediatr Res. 1987;22:621–626. doi: 10.1203/00006450-198712000-00001. [DOI] [PubMed] [Google Scholar]
- Reuss ML, Parer JT, Harris JL, Kreuger TR. Hemodynamic effects of alpha-adrenergic blockade during hypoxia in fetal sheep. Am J Obstet Gynecol. 1982;142:410–415. doi: 10.1016/s0002-9378(16)32381-x. [DOI] [PubMed] [Google Scholar]
- Rudehill A, Olcen M, Sollevi A, Hamberger B, Lundberg JM. Release of neuropeptide Y upon haemorrhagic hypovolaemia in relation to vasoconstrictor effects in the pig. Acta Physiol Scand. 1987;131:517–523. doi: 10.1111/j.1748-1716.1987.tb08271.x. [DOI] [PubMed] [Google Scholar]
- Tatemoto K, Carlquist M, Mutt V. Neuropeptide Y -a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature. 1982;296:659–660. doi: 10.1038/296659a0. [DOI] [PubMed] [Google Scholar]
- Waeber B. Neuropeptide Y: a missing link? Hosp Pract. 1990:101–120. doi: 10.1080/21548331.1990.11704037. November 15. [DOI] [PubMed] [Google Scholar]
- Yaffe H, Parer JT, Block BS, Llanos AJ. Cardiorespiratory responses to graded reductions of uterine blood flow in the sheep fetus. J Dev Physiol. 1987;9:325–336. [PubMed] [Google Scholar]


