Abstract
The external intercostal muscles in humans show marked regional differences in respiratory effect, and this implies that their action on the lung during breathing is primarily determined by the spatial distribution of neural drive among them. To assess this distribution, monopolar electrodes were implanted under ultrasound guidance in different muscle areas in six healthy individuals and electromyographic recordings were made during resting breathing. The muscles in the dorsal portion of the third and fifth interspace showed phasic inspiratory activity with each breath in every subject. However, the muscle in the ventral portion of the third interspace showed inspiratory activity in only three subjects, and the muscle in the dorsal portion of the seventh interspace was almost invariably silent. Also, activity in the ventral portion of the third interspace, when present, and activity in the dorsal portion of the fifth interspace were delayed relative to the onset of activity in the dorsal portion of the third interspace. In addition, the discharge frequency of the motor units identified in the dorsal portion of the third interspace averaged (mean ± s.e.m.) 11.9 ± 0.3 Hz and was significantly greater than the discharge frequency of the motor units in both the ventral portion of the third interspace (6.0 ± 0.5 Hz) and the dorsal portion of the fifth interspace (6.7 ± 0.4 Hz). The muscle in the dorsal portion of the third interspace started firing simultaneously with the parasternal intercostal in the same interspace, and the discharge frequency of its motor units was even significantly greater (11.4 ± 0.3 vs. 8.9 ± 0.2 Hz). These observations indicate that the distribution of neural inspiratory drive to the external intercostals in humans takes place along dorsoventral and rostrocaudal gradients and mirrors the spatial distribution of inspiratory mechanical advantage.
It is well established that the internal intercostal muscles of the parasternal region (the so-called parasternal intercostals) elevate the ribs and expand the lung when they contract (De Troyer & Kelly, 1982; De Troyer et al. 1996, 1998); however, the action of the external intercostals remains uncertain, particularly in humans. The conventional view, based on the theory proposed in the mid-1700s by Hamberger (1749), maintains that these muscles also have an inspiratory action on the ribs and the lung. However, this view has not been proven, and recent measurements of the respiratory displacements of the ribs in normal subjects, combined with measurements of muscle orientation and muscle mass in cadavers, have led to the conclusion that the respiratory effect of the external intercostals (that is, the change in airway pressure produced by the muscles during a maximal isolated contraction against a closed airway) shows marked topographic differences (Wilson et al. 2001). Thus, the muscles in the dorsal portion of the rostral segments of the ribcage have a large inspiratory effect, in agreement with the theory of Hamberger (1749); this inspiratory effect would be even greater than that of the parasternal intercostals. However, this effect decreases markedly and progressively in the ventral and the caudal direction, such that the muscles in the ventral portion of the caudal segments of the ribcage have an expiratory, rather than an inspiratory, effect. The implication is that the action of the external intercostals on the lung during breathing would be primarily determined by the topographic distribution of neural drive among them.
Taylor (1960) performed extensive electromyographic (EMG) recordings from the intercostal muscles in the ventrolateral aspect of the ribcage in many healthy individuals, and he concluded that the external intercostals are activated only by inspiratory efforts but are usually inactive during resting breathing. However, recent studies have indicated that, in the dog, the topographic distribution of neural drive among the intercostal muscles is closely matched to the topographic distribution of respiratory effect (De Troyer & Legrand, 1995; Legrand et al. 1996; Legrand & De Troyer, 1999). The muscle areas with the greatest inspiratory effect thus receive the greatest neural drive during the inspiratory phase of the breathing cycle, and those with a smaller inspiratory effect receive little or no inspiratory drive. Based on this principle, one would therefore predict that in humans: (1) the inspiratory drive to the external intercostals in the rostral segments of the ribcage is greater in the dorsal than the ventral portion; (2) the inspiratory drive to the external intercostals in the dorsal portion of the ribcage is greater in the rostral than the caudal segments; and (3) the inspiratory drive to the external intercostals in the dorsal portion of the rostral segments is greater than that to the parasternal intercostals. Although Campbell & Newsom Davis (1970), referring to experiments by Sears and Newsom Davis, has suggested that the external intercostals in the dorsal aspect of the rostral segments of the human ribcage do contract during resting inspiration, there are no data in the literature with which these predictions can be compared. Therefore, we set out to test them.
Methods
The studies were carried out in six healthy men of 33–51 years of age. The subjects gave informed consent to the procedures which conformed with the Declaration of Helsinki and were approved by the human ethics committee of the University of New South Wales. Three subjects had previously participated in many respiratory experiments and two of them were aware of the hypotheses underlying the studies, but three subjects were naive and had little or no prior experience as respiratory subjects. Before the study, these subjects were just told that the purpose was to obtain electrical recordings from several muscles of the ribcage compartment of the chest wall.
Preliminary studies
Anteroposterior radiographs of the chest were obtained in two subjects at resting end-expiration to locate precisely the dorsal portion of the rostral interspaces relative to the scapula and to define anatomical landmarks for subsequent insertion of needle electrodes. With the subjects standing and maintaining their arms along the sides of the body, the dorsal portion of the third interspace projected about 1–2 cm cranial and 2 cm medial relative to the medial border of the spine of the scapula. This site, which corresponds to the muscle area just medial to the angle of the ribs, could be located by palpation easily, and it was then evaluated in all six subjects with a 5 MHz ultrasound linear probe (Acuson 128 X/P, Computed Sonography System; Acuson Inc., Mountain View, CA, USA). While the probe was placed perpendicular to the skin, the external intercostal muscle was clearly visualized in each subject, so its thickness and its depth from the skin surface could be measured with a built-in digital calliper. Depending on the subject's height and muscularity, the thickness of the external intercostal ranged between 7 and 10 mm, and the depth of its external layer relative to the skin surface was between 22 and 30 mm. Three experimental protocols were then followed.
Experiment 1
The six subjects were first studied to compare the magnitude of neural inspiratory drive to the dorsal versus the ventral portion of the external intercostal muscle in the third interspace. Each subject was seated comfortably with all restrictive garments removed. The respiratory displacements of the chest wall were monitored by inductance plethysmography (Respitrace; Ambulatory Monitoring, Ardsley, NY, USA). The respitrace bands were positioned around the ribcage at the level of the nipples and around the abdomen at the level of the umbilicus, respectively, and the gains on the two signals were adjusted by using the conventional isovolume manoeuvre (Chadha et al. 1982). No extraneous movements were permitted after this calibration procedure. The subject was then grounded with a large flexible strap on the right shoulder, and recordings of external intercostal EMG activity were made.
Recordings were made first from the dorsal portion of the muscle by using a Teflon-coated monopolar electrode (Medelec DMG 50; Surrey, UK). The recordings were referenced to a surface electrode positioned 2–3 cm away, and the EMG signal was amplified and band-pass filtered below 53 Hz and above 3000 Hz. Since this area had been examined by ultrasonography, we could define in each individual subject the maximal permissible length of the needle track and marked this on the electrode. The needle was thus inserted perpendicular to the skin surface and advanced in small steps through the trapezius and rhomboid muscles and then into the external intercostal. Electromyographic activity was continuously monitored on a loudspeaker and an oscilloscope throughout the procedure, and once a site in the external intercostal was encountered that contained audible and visible single motor unit activity with an acceptable signal-to-noise ratio (> 3:1) during quiet breathing or mild voluntary hyperpnoea, the audio signal was removed and the subject was instructed to breathe quietly for 3–4 min through a mouthpiece connected to a pneumotachograph. The changes in lung volume (obtained by integration of the airflow signal), end-tidal PCO2 (measured with a small probe attached to the pneumotachograph), and ribcage and abdomen cross-sectional area were checked to ensure that the subject was actually breathing at rest. All signals were stored on computer via a Cambridge Electronic Design 1401 interface (Cambridge, UK) for subsequent analysis. After the run was completed, the needle was removed such that its tip was no longer in the external intercostal, and it was then reinserted at a slightly different angle or to a slightly different depth. In each subject, six to twelve sites were studied.
Recordings were then made from the ventral portion of the muscle in the third interspace. The overall experimental procedure was similar to that used for the dorsal portion, but the needle was inserted 10–15 mm lateral to the midclavicular line. Indeed, the initial chest radiographs had indicated that the costochondral junctions of ribs 3 and 4 were medial to this line, thus implying that a needle inserted more laterally should go through the external intercostal. When examined by ultrasonography, the depth of the muscle relative to the skin surface also ranged between 25 and 42 mm, but its thickness was only 2–3 mm. As for the dorsal portion of the muscle, five to twelve sites were recorded from the ventral portion of the third external intercostal muscle in each subject.
In five subjects, a mild transient ache was occasionally noted as the needle penetrated the rhomboid or the external intercostal, but this always subsided rapidly. One subject reported significant discomfort during the needle insertion in the dorsal area, well before it reached the external intercostal, and topical anaesthesia of the skin and superficial muscles was achieved with 2 ml of 1 % lignocaine (lidocaine). As a result, all EMG recordings were obtained while the subjects had minimal discomfort or none at all.
Experiment 2
Four subjects were subsequently studied, on a different day, to assess neural inspiratory drive to the dorsal portion of the external intercostal muscle in the third, fifth and seventh interspaces. The experimental procedure was similar to that used in Experiment (Expt) 1. In each subject, five to ten sites were studied in each muscle. In two subjects, recordings were also made from the ventral portion of the external intercostal in the fifth interspace.
Experiment 3
Four subjects were studied, on yet another day, to assess neural drive to the dorsal portion of the external intercostal muscle in the third interspace relative to the parasternal intercostal in the same interspace. After the recordings from the external intercostal were completed, the needle was inserted in the ventral part of the interspace at an angle of about 45 deg, as previously described (Gandevia et al. 1996). Here again, six to fourteen sites were recorded from each muscle in every subject.
Data analysis
The magnitude of neural inspiratory drive to a given area of external intercostal or parasternal intercostal muscle was assessed in three ways. First, all periods of resting breathing (corresponding to the different recording sites in the muscle) were played into a leaky integrator (decay time constant, 50 ms), and for each period, the presence or absence of phasic inspiratory activity in the integrated EMG signal (i.e. with a progressive increase in the signal during the inspiratory phase of the breathing cycle and a decrease in the signal during the expiratory phase) was noted. On the basis of this definition of phasic inspiratory activity, no sites were encountered with phasic expiratory activity. Second, 10 consecutive breaths from each period were examined to measure the time of onset (TO) of the integrated inspiratory EMG activity relative to the onset of inspiratory airflow. Inspiratory time (TI) during these breaths was also measured from the airflow signal. To allow comparison between the subjects, TO for each breath was expressed as a percentage of TI; this fraction was assigned a positive sign when activity started after the onset of inspiratory airflow and a negative sign when activity preceded the onset of airflow.
The third method used to quantify neural drive was based on the measurement of the firing rates of single motor units and has been previously described in detail (Gandevia et al. 1996; De Troyer et al. 1997). Thus, the periods of quiet breathing used for the first two analyses were played into a commercial neural analysis package (Spike 2; Cambridge Electronic Design), and trigger levels were set manually to capture all spikes with an appropriate signal-to-noise ratio. All spikes were subsequently recalled and manually sorted into one, two, three or four types or ‘templates’ based on their size and detailed morphology. The interactive software allowed: (1) updating of the mean shape of the template for each motor unit; (2) review of the frequency plots of each single motor unit, together with inspiratory flow and respiratory movements; and (3) superimposition of all spikes from a particular motor unit. Using this method, we could follow simultaneously the discharge of up to four single motor units during several breaths.
For each motor unit, the mean discharge frequency was measured from the plateau in the latter third of the inspiratory phase during several consecutive breaths. Units that discharged sporadically or very late in the breaths with less than three stable interdischarge intervals were noted but not included in the main analysis. Several units, particularly in the ventral portion of the external intercostal in the third interspace and the dorsal portion of the external intercostal in the fifth interspace, discharged tonically with an inspiratory modulation. Since this study was aimed at quantifying the magnitude of neural inspiratory drive, the discharge frequency of these units was taken as the difference between the frequency in the latter third of the inspiratory phase and the frequency during expiration.
For each muscle area in each subject, the values of TO/TI for a given recording site were averaged over the 10 breaths recorded from that site, and the mean values thus obtained were then averaged over all sites. The firing rates of the single motor units identified in the different recording sites of a given muscle area were also averaged over all units, and statistical comparisons between the different muscle areas were made in individual subjects by using Student's unpaired t tests. The changes in lung volume, inspiratory flow, and the pattern of ribcage and abdominal displacement were compared similarly. However, since three muscle areas were studied in Expt 2, comparison between them was made by analysis of variance (ANOVA) with repeated measures and multiple comparison testing of the mean values was performed, when appropriate, using Student-Newman-Keuls tests. The criterion for statistical significance was taken as P < 0.05.
Results
Dorsal vs. ventral external intercostal (Expt 1)
There were no adverse consequences of the procedures, and Table 1 shows the indices of the pattern of breathing during recordings from the dorsal and ventral portions of the external intercostal muscle in the third interspace in the six subjects. Tidal volume (VT) during the recordings from the dorsal portion averaged (mean ± s.e.m.) 0.71 ± 0.03 l and was similar to that during the recordings from the ventral portion (0.74 ± 0.06 l). Inspiratory duration (TI), mean inspiratory flow rate (VT /TI), and the ribcage contribution to VT during the recordings from the dorsal portion were also similar to those during the recordings from the ventral portion.
Table 1.
Pattern of breathing during electromyographic recordings
| Tidal volume | T1 | Inspiratory flow | Ribcage contribution to tidal volume | |
|---|---|---|---|---|
| (l) | (s) | (ls−1) | (%) | |
| Experiment 1 | ||||
| Dorsal external intercostal (3) | 0.71 ± 0.03 | 2.19 ± 0.32 | 0.35 ± 0.03 | 66.2 ± 4.3 |
| Ventral external intercostal (3) | 0.74 ± 0.06 | 2.39 ± 0.39 | 0.34 ± 0.04 | 68.0 ± 3.5 |
| Experiment 2 | ||||
| Dorsal external intercostal (3) | 0.71 ± 0.10 | 2.12 ± 0.29 | 0.36 ± 0.09 | 66.7 ± 4.1 |
| Dorsal external intercostal (5) | 0.76 ± 0.10 | 2.24 ± 0.33 | 0.36 ± 0.07 | 66.6 ± 4.0 |
| Dorsal external intercostal (7) | 0.74 ± 0.07 | 2.29 ± 0.31 | 0.34 ± 0.05 | 67.4 ± 4.6 |
| Experiment 3 | ||||
| Dorsal external intercostal (3) | 0.68 ± 0.03 | 1.92 ± 0.17 | 0.36 ± 0.04 | 65.2 ± 6.7 |
| Parasternal intercostal (3) | 0.74 ± 0.04 | 2.10 ± 0.11 | 0.36 ± 0.03 | 66.4 ± 8.4 |
Values of Expt 1 are means ± s.e.m. for six subjects; values of Expts 2 and 3 are means ± s.e.m. for four subjects. T1, inspiratory time. Numbers in paren theses indicate the interspace recorded from.
Representative records of dorsal and ventral external intercostal EMG activity during resting breathing in two subjects are shown in Fig. 1 and Fig. 2. The dorsal portion of the muscle showed phasic inspiratory activity in every breath in every subject (Fig. 1A and Fig. 2A). This activity involved many motor units, appeared early after the onset of inspiratory airflow and persisted well after the cessation of inspiratory airflow. In two subjects, activity in several sites even started concomitantly with or shortly (15–70 ms) before the onset of inspiratory airflow. On the other hand, the ventral portion of the muscle in three subjects was silent throughout resting breathing and showed inspiratory discharges only when the increase in lung volume was voluntarily and substantially augmented (Fig. 1B). In the other three subjects, the ventral portion did show phasic inspiratory activity during resting breathing. Only one these subjects, however, had inspiratory activity in each site. In the other two, inspiratory activity was recorded from only a few sites and, when present, it was superimposed on tonic activity persisting throughout expiration. In addition, such inspiratory activity started after the onset of inspiratory airflow (Fig. 2B). As shown in Fig. 3, therefore, TO/TI was significantly (P < 0.002 or less) greater for the ventral than the dorsal portion in every subject. Whereas this fraction for the ventral portion in the six subjects averaged 84.1 ± 3.8 %, for the dorsal portion it was only 4.4 ± 0.8 %.
Figure 1. Examples of EMG activity recorded from the dorsal and ventral portions of the external intercostal muscle in the third interspace.
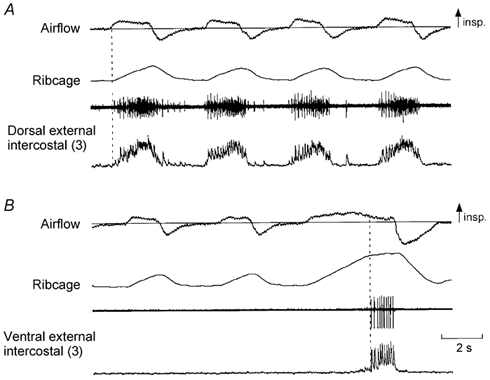
A, from top to bottom: traces of airflow, changes in ribcage cross-sectional area (increase upwards), and raw and integrated EMG activity recorded from the dorsal portion of the external intercostal muscle in the third interspace in a representative subject. B, traces obtained during recording from the ventral portion of the muscle in the same subject. The dorsal portion of the muscle (A) shows multi-unit activity in phase with inspiration and only slightly delayed relative to the onset of inspiratory airflow (vertical dashed line), but the ventral portion of the muscle (B) is electrically silent during resting breathing and fires only at the end of a large, voluntary inspiration (third breath in B, vertical dashed line). In this and subsequent figures the numbers in parentheses indicate the interspace concerned.
Figure 2. Records from the dorsal and ventral portions of the external intercostal muscle in the third interspace in a subject with inspiratory activity in the ventral portion during quiet breathing.
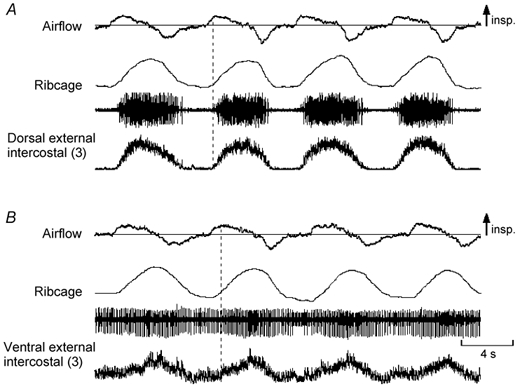
Same conventions as in Fig. 1. The two portions of the muscle in this subject are active during resting inspiration. However, whereas inspiratory activity in the dorsal portion (A) starts shortly after the onset of inspiratory airflow, inspiratory activity in the ventral portion (B) starts later. In addition, such inspiratory activity is superimposed on tonic activity persisting throughout expiration. Note also that this recording (B) contains far-field contamination during the expiratory pause; this expiratory activity arises from different single motor units, presumably in the internal intercostal muscle, and is associated with a decrease in the end-expiratory position of the ribcage.
Figure 3. Onset time of inspiratory activity in the dorsal and ventral portions of the external intercostal muscle in the third interspace.
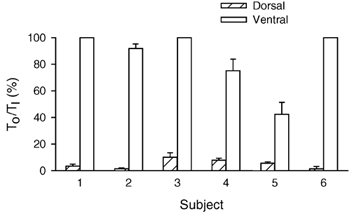
Data are mean ± s.e.m. values of onset time obtained in six subjects during quiet breathing. For each portion of the muscle in each subject, onset time (TO) is expressed as a percentage of inspiratory time (TI). When breathing quietly, subjects 1, 3 and 6 never had any inspiratory activity in the ventral portion.
In each subject, 12–22 single motor units were identified in the dorsal portion of the external intercostal in the third interspace. These 109 units showed a rhythmic behaviour as shown in Fig. 4, with a phasic inspiratory increase in the instantaneous discharge frequency; only two of these units (1.8 %) discharged tonically throughout expiration. Nine to 13 motor units were also recorded from the ventral portion of the muscle in the three subjects who had phasic inspiratory activity there (subjects 2, 4 and 5 in Fig. 3). All these units also showed an increase in discharge frequency during inspiration, but 14 of them (44 %) continued to discharge throughout expiration with a firing rate of 6.6 ± 0.3 Hz (range, 4.2- 8.5 Hz).
Figure 4. Measurement of the firing rates of single motor units.
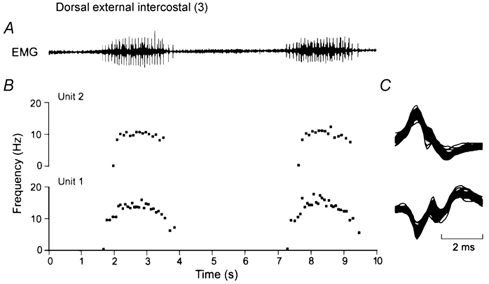
A shows a representative record of inspiratory EMG activity obtained in the dorsal portion of the external intercostal muscle in the third interspace for two consecutive breaths. Two single motor units (denoted Units 1 and 2) were clearly identified in this record, and the instantaneous discharge frequencies of these two units are shown in B. All the action potentials from the two units are superimposed in C. Note that all the spikes in C are identical, thus indicating that they originate in a single motor unit. Note also in B that the discharge frequency of both units increases rapidly in the first part of inspiration and plateaus in the last two-thirds of inspiration; the discharge frequency of Unit 1 then declines gradually in the first part of expiration, whereas the discharge frequency of Unit 2 stops rather abruptly after the end of inspiration.
In the three subjects with phasic inspiratory activity in both the dorsal and ventral portions of the muscle, a total of 58 motor units in the dorsal portion and 32 motor units in the ventral portion were thus studied, and the histograms of their discharge rates at end-inspiration (i.e. excluding tonic expiratory firing; see Methods) are shown in Fig. 5. The discharge frequency of the units in the dorsal portion was significantly greater (P < 0.001) than that of the units in the ventral portion in every subject. For the three subjects, units in the ventral portion thus had a mean discharge frequency of 6.0 ± 0.5 Hz, whereas units in the dorsal portion had a discharge frequency of 11.9 ± 0.3 Hz. The discharge frequency of the 51 units recorded in the dorsal portion of the three subjects without ventral activity (subjects 1, 3 and 6 in Fig. 3) was slightly smaller but still amounted to 10.1 ± 0.2 Hz.
Figure 5. Firing frequencies of motor units in the dorsal and ventral portions of the external intercostal muscle in the third interspace.
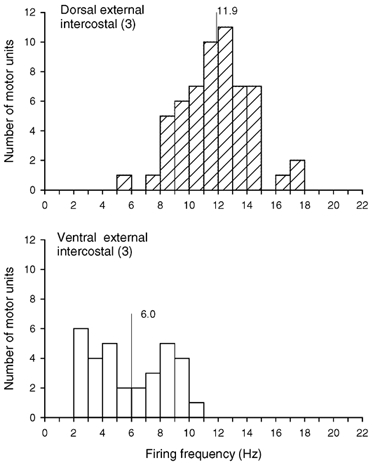
Histograms of the inspiratory modulation of the discharge frequencies of all motor units identified in the dorsal (top) and ventral (bottom) portions of the muscle in the three subjects who had phasic inspiratory activity in both portions during quiet breathing (subjects 2, 4 and 5 in Fig. 3). Bin width, 1.0 Hz. Mean values are indicated on each histogram. For units which fired tonically throughout expiration, the firing frequency is taken as the difference in discharge rate between inspiration and expiration (see Methods).
Dorsal external intercostal in spaces 3, 5 and 7 (Expt 2)
The indices of the pattern of breathing during periods in which the recordings from the dorsal portion of the external intercostal muscle in the third, fifth and seventh interspaces were made in the four subjects are summarized in Table 1, and representative EMG recordings from the three muscles in one subject are shown in Fig. 6. There was no difference between the three conditions (three muscles) with respect to VT, TI, VT/TI, or the ribcage contribution to VT. However, whereas all subjects had phasic inspiratory activity in the third and fifth interspaces in every breath (Fig. 6A and B), only one subject (subject no. 3 in Fig. 3) had regular phasic inspiratory activity in the seventh interspace. In two subjects, this muscle was silent in all but a few breaths (Fig. 6C), and, in one subject, it commonly showed continuous tonic activity without any respiratory modulation; in each subject, however, inspiratory discharges were recorded in each site during large, voluntary inspirations (Fig. 6C). The ventral portion of the external intercostal muscle in the fifth interspace was also silent during quiet breathing in the two subjects studied.
Figure 6. Examples of EMG activity recorded from the dorsal portion of the external intercostal muscle in the third, fifth and seventh interspaces.
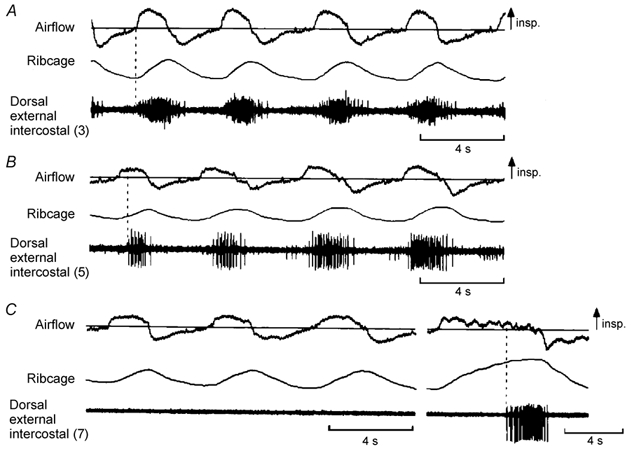
Same conventions as in Fig. 1, except that only raw EMG activity is shown. Traces obtained in a representative subject (subject no. 1 in Fig. 3). As in Figs 1 and 2, the muscle in the third interspace (A) shows multi-unit activity in phase with inspiration. The muscle in the fifth interspace (B) also shows phasic inspiratory activity, but this activity starts later than that in the third interspace relative to the onset of inspiratory airflow. The muscle in the seventh interspace (C) is electrically silent during quiet breathing (first three breaths) and fires only at the end of a large, voluntary inspiration (last breath; vertical dashed line).
Although the dorsal portion of the external intercostal in the third and the fifth interspaces was invariably active during resting inspiration, the two muscles had different TO values. This difference is also illustrated in Fig. 6, and Fig. 7 shows the values of TO/TI measured for the three interspaces in the individual subjects. TO/TI was smaller (P < 0.05 or less) in the third interspace than in the fifth in three of four subjects. Also, TO/TI was smaller (P < 0.001) in the fifth interspace than in the seventh in every subject, including subject no. 3. For the four subjects, TO/TI thus averaged 8.7 ± 1.7 % in the third interspace, 35.7 ± 3.6 % in the fifth interspace and 91.3 ± 2.7 % in the seventh interspace.
Figure 7. Onset time of inspiratory activity in the dorsal portion of the external intercostal muscle in the third, fifth and seventh interspaces.
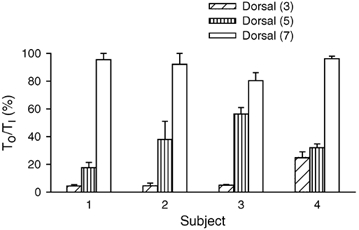
Data are mean ± s.e.m. values of onset time obtained in four subjects during quiet breathing. Onset time (TO) for each muscle is expressed as a percentage of inspiratory time (TI).
A total of 61 single motor units (12–18 units per subject) were identified in the external intercostal of the third interspace in this experiment, and 63 motor units (11–18 units per subject) were sampled from the muscle in the fifth interspace. All units increased their firing rates in phase with inspiration, and histograms of these inspiratory firing frequencies are shown in Fig. 8. The discharge frequency of the units in the third interspace was greater (P < 0.001) than the discharge frequency of the units in the fifth interspace in every subject, such that for the four subjects, the discharge frequency of motor units in the third and fifth interspaces averaged 11.3 ± 0.4 and 6.7 ± 0.4 Hz, respectively. Whereas only three units in the third interspace (5 %) continued to discharge throughout expiration, 27 units in the fifth interspace (43 %) did so with a tonic firing rate of 6.6 ± 0.4 Hz (range, 2.7–9.7 Hz).
Figure 8. Firing frequencies of motor units in the dorsal portion of the external intercostal muscle in the third and fifth interspaces.
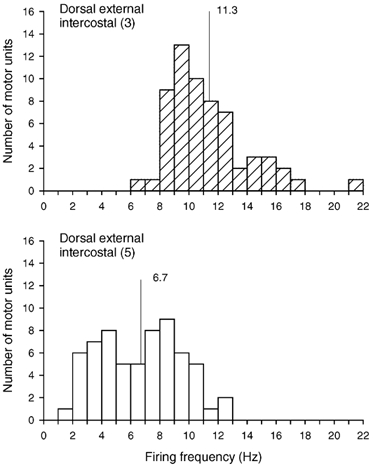
Histograms of the inspiratory modulation of the discharge frequencies of all motor units identified in the dorsal portion of the external intercostal muscle in the third (top) and fifth (bottom) interspaces in four subjects breathing at rest. Bin width, 1.0 Hz. Mean values are indicated on each histogram.
Dorsal external intercostal vs. parasternal intercostal (Expt 3)
The four subjects had a similar pattern of breathing when recordings were made from the dorsal portion of the external intercostal in the third interspace and from the parasternal intercostal (Table 1), and they invariably showed phasic inspiratory EMG activity in both muscles. Activity in either muscle involved many motor units and started early after the onset of inspiratory airflow. The TO/TI ratio for the external intercostal and parasternal intercostal muscles in the four subjects thus averaged 7.1 ± 1.7 and 12.5 ± 1.9 %, respectively.
A total of 79 motor units (12–26 units per subject) were identified in the external intercostal in this experiment, and 86 motor units (16–24 units per subject) were identified in the parasternal intercostal. All units in both muscles increased their discharge rates during inspiration. However, although the two pools of units had similar discharge frequencies in one subject (external intercostal, 10.7 ± 0.6 Hz; parasternal intercostal, 10.6 ± 0.4 Hz; n.s.), three subjects had greater discharge frequencies in the external intercostal (P < 0.005 or less). As shown in Fig. 9, the firing frequency of the external intercostal motor units in the four subjects thus averaged 11.4 ± 0.3 Hz, but the firing frequency of all the parasternal intercostal motor units was only 8.9 ± 0.2 Hz.
Figure 9. Firing frequencies of motor units in the dorsal portion of the external intercostal and parasternal intercostal muscles in the third interspace.
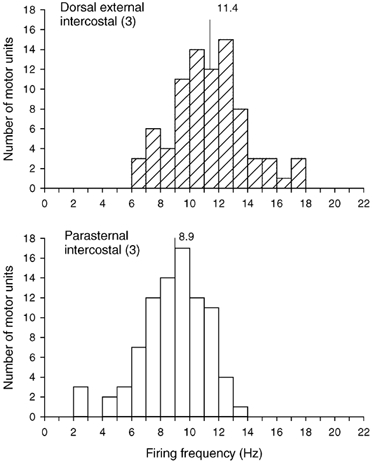
Histograms of the inspiratory modulation of the discharge frequencies of all motor units identified in the dorsal portion of the external intercostal muscle in the third interspace (top) and the parasternal intercostal muscle in the same interspace (bottom) in four subjects breathing at rest. Bin width, 1.0 Hz. Mean values are indicated on each histogram.
Discussion
The amplitude of neural drive to respiratory muscles in humans is difficult to quantify. Previous attempts to evaluate this amplitude involved measuring EMG activity during breathing with surface electrodes and expressing it relative to ‘maximal’ values obtained during inhalation to total lung capacity (TLC) (e.g. Grassino et al. 1978; Goldman et al. 1978; Juan et al. 1984; Sinderby et al. 1998). We could have used a similar method with intramuscular electrodes to compare neural drive in the different areas of the external intercostal muscle. However, this method would have three major drawbacks. First, it would assume that the different muscle areas are recruited similarly during inspiration to TLC, and studies of the distribution of neural drive among the parasternal and external intercostal muscles in dogs have suggested that this assumption may not be justified. Specifically, although activity in the different inspiratory muscle areas increased when neural drive was augmented by CO2 or by increased inspiratory mechanical loads, the topographic differences in activity that were present during resting breathing persisted with all changes in lung volume and with all loads (De Troyer & Legrand, 1995; Legrand et al. 1996; Legrand & De Troyer, 1999). It is unlikely, therefore, that the different areas of external intercostal would be driven equally during an inspiration to TLC. Second, Kim et al. (1985) have shown in isolated strips from the canine diaphragm that changes in muscle length introduce substantial alterations in EMG activity independent of neural drive. A similar effect of muscle length has been demonstrated during supramaximal stimulation of the phrenic nerves with single twitches in normal humans (Gandevia & McKenzie, 1986): the maximal compound muscle action potentials increased 2- to 10-fold when lung volume was increased from residual volume to TLC (i.e. when diaphragmatic muscle length was reduced by ~40 %). Since the different areas of external intercostal muscle in humans shorten by different amounts during inspiration to TLC (Wilson et al. 2001), it would therefore be expected that they would show different artifactual changes in EMG activity during the manoeuvre. Finally, with such large respiratory excursions, it would be difficult to eliminate cross-contamination from neighbouring muscles. Thus, activity from the rhomboid and latissimus dorsi muscles might contaminate recordings from the dorsal areas of the external intercostal, and activity from the pectoralis and serratus anterior muscles might contaminate recordings from the ventral areas.
To avoid these drawbacks, we therefore sampled from many sites within individual muscle areas during resting breathing and measured, for each site, the frequency with which inspiratory activity was recorded and the time of onset of activity relative to inspiratory airflow. Indeed, in the dog, the areas of parasternal and external intercostal muscle that receive the greatest inspiratory drive also have the propensity to fire earlier at the onset of inspiration (De Troyer & Legrand, 1995; Legrand et al. 1996; Legrand & De Troyer, 1999). Furthermore, when the neural drive to the canine external intercostals is increased by ribcage vibration, their motor units begin firing earlier (Leduc et al. 2000). In addition, single motoneurones are known to fire at higher frequencies with increasing neural drive (e.g. Binder et al. 1996), and studies in patients with severe chronic obstructive pulmonary disease (Gandevia et al. 1996; De Troyer et al. 1997) and in healthy individuals (Gandevia et al. 1999) have shown that the discharge frequencies of motor units in all inspiratory muscles increase in response to increases in respiratory drive. Given that the fibre-type compositions of the parasternal intercostal muscles and the entire external intercostals in humans are similar (Mizuno & Secher, 1989) and their force-frequency characteristics are likely to be similar (Farkas et al. 1985), it is therefore reasonable to assume that the different pools of external and parasternal intercostal motoneurones have similar properties and, hence, that a muscle area with a greater neural drive might also show higher motor unit firing rates. Thus, by measuring the frequency of inspiratory activity during resting breathing, the onset time of this activity, and the peak discharge frequency of large numbers of clearly identified single motor units, we assessed for each muscle area three distinct indices of neural drive that were not affected by activation of adjacent muscles, were free of recording artefact due to changes in muscle length, and did not need to be normalized to the level of drive observed under another respiratory manoeuvre.
These measurements required that the subjects breathe through a mouthpiece, wear a noseclip, and have a needle electrode in the chest. Consequently, even though the data were obtained with little or no discomfort, the subjects were aware of their breathing and one cannot exclude the possibility that the natural (automatic) pattern of muscle activation was affected by central mechanisms. Nonetheless, the observations obtained in the different subjects and in the different experimental sessions in any given subject were consistent.
The external intercostal muscle in the dorsal portion of the third interspace showed phasic inspiratory activity with every breath in every subject (Figs 1, 2 and 6), whereas the ventral portion of the muscle in the same interspace showed no activity at all during resting breathing in three of six subjects and occasional inspiratory activity in two subjects. Similarly, the two subjects in whom recordings were made from the dorsal and ventral portions of the fifth interspace had invariable phasic inspiratory activity in the dorsal portion and no activity in the ventral portion. Moreover, the three subjects who had inspiratory activity in both the dorsal and the ventral portion of the muscle in the third interspace showed a definite time lag, ranging from 1.2 s (subject 5 in Fig. 3) to 2.6 s (subject 2), between the onset of activity in the dorsal and the ventral portion. The firing rates of motor units in these two muscle areas were also strikingly different (Fig. 5). For these three subjects, the discharge frequencies of motor units in the dorsal portion averaged 11.9 Hz. The corresponding value for the three subjects who had inspiratory activity only in the dorsal portion was 10.1 Hz, and these values are very close to those previously measured in the diaphragm. Iscoe et al. (1976) have reported that, during resting inspiration, phrenic motor axons in the cat have a mean discharge frequency of 11.4 ± 3.8 Hz, and in two separate studies in normal humans, we have found that, during resting breathing, diaphragmatic motor units discharge with a mean frequency of 10.5 ± 2.4 and 11.0 ± 2.7 Hz (De Troyer et al. 1997; Gandevia et al. 1999). On the other hand, the inspiratory modulation of the motor units recorded from the ventral portion of the external intercostal in our subjects was only 6.0 Hz. Yet, the periods during which the recordings from the dorsal and ventral portion were made were characterized by a similar tidal volume and a similar inspiratory flow rate (Table 1). The ribcage contribution to tidal volume was also similar, thus indicating that these differences in timing and discharge frequency cannot be attributed to differences in the pattern of breathing. Although the three indices of neural drive used in this study are based on different measurements, they thus lead to the same conclusion that, in normal humans studied in the laboratory, the neural inspiratory drive to the external intercostals is distributed along a dorso-ventral gradient.
As for the third interspace, the external intercostal muscle in the dorsal portion of the fifth interspace always showed phasic inspiratory activity. However, the onset of activity in this area was commonly delayed relative to the onset of activity in the dorsal portion of the third interspace (Fig. 7). Also, the inspiratory discharge frequency of motor units in the fifth interspace was substantially lower than that of motor units in the third interspace (Fig. 8). The mean discharge frequency of motor units in the dorsal portion of the fifth interspace (6.7 Hz) was, in fact, only slightly greater than the mean discharge frequency of motor units in the ventral portion of the third interspace. In addition, the external intercostal muscle in the dorsal portion of the seventh interspace was almost invariably silent during resting breathing (Fig. 6). Thus, neural drive to the external intercostals in our subjects was also distributed along a rostro-caudal gradient, and this gradient, combined with the dorso-ventral gradient, suggests that, as in the dog (De Troyer et al. 1999; Legrand & De Troyer, 1999), the topographic distribution of neural inspiratory drive to these muscles in humans is matched to the topographic distribution of inspiratory effect.
The relationship between the magnitude of inspiratory effect and the magnitude of neural inspiratory drive for the four areas of external intercostal muscle investigated in this study is more specifically examined in Fig. 10A (filled circles). The values for inspiratory effect in this figure were derived from the previous studies of Wilson et al. (2001), and the values for neural drive were obtained by multiplying the mean motor unit firing rate (data shown in Fig. 5 and Fig. 8) by the time during quiet inspiration over which the motor units discharged. Although the two sets of data were collected from different subjects, there was a close linear relationship (coefficient of correlation, r = 0.994) between them, and this strengthens the view that a powerful link exists between the inspiratory effect of a particular area of external intercostal muscle and the degree to which it is driven during inspiration. It should be appreciated, however, that the two sets of data were also obtained with the subjects in different body positions, so the slope of this relationship is only indicative. Specifically, the current electromyographic studies were performed with the subjects seated, whereas the values for inspiratory effect were obtained with the subjects in the supine position. To the extent that the respiratory effect of a given intercostal muscle area depends on ribcage compliance and that ribcage compliance in humans increases during a change from the supine to the seated position (Estenne et al. 1985), it is likely that the different areas of external intercostal muscle, particularly in the third and fifth interspaces, have greater inspiratory effects in this position. If the values for inspiratory effect in the seated position could be determined, the actual relationship could therefore be less steep. Conversely, increases in inspiratory mechanical load would increase neural inspiratory drive to the external intercostals, such that the relationship might be steeper.
Figure 10. Relationship between inspiratory effect and neural drive for the external intercostal muscles in humans.
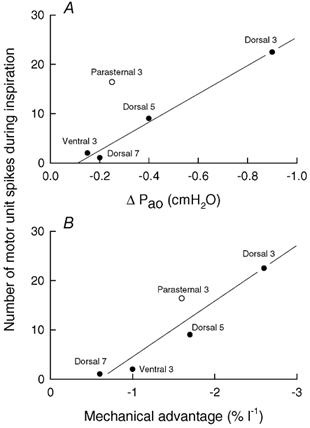
The filled circles in A correspond to the four areas of external intercostal muscle investigated in this study (dorsal portion of the third, fifth and seventh interspaces, and ventral portion of the third interspace), while the open circle corresponds to the parasternal intercostal in the third interspace. The values of inspiratory effect (ΔPao) were derived from De Troyer et al. (1998) and Wilson et al. (2001), and the values of neural drive during quiet inspiration were obtained by multiplying the mean firing rate of the individual motor units by the fraction of inspiration during which the motor units were firing and by the mean duration of inspiration (2.1 s); neural drive is therefore expressed as the mean number of motor unit spikes during inspiration. There is a close linear relationship (r = 0.994) between the data for the external intercostal muscles, but the data for the parasternal intercostal lie well above this relationship. However, when the values of neural drive are plotted against the values of mechanical advantage (B), a linear relationship (r = 0.931) fits all data well.
The data obtained for the parasternal intercostal in the third experiment of the study, however, lay well above the relationship for the external intercostals (open circle in Fig. 10A). Indeed, the mean firing rate of motor units in the parasternal intercostal in the third interspace was lower than the mean firing rate of motor units in the dorsal portion of the external intercostal in the same interspace (Fig. 9), but the two muscles were found to fire almost simultaneously at the onset of inspiration. As a result, the neural drive to the parasternal intercostal was greater than anticipated on the basis of its inspiratory effect. The reason for this difference is uncertain, but Wilson & De Troyer (1992, 1993) have shown that the respiratory effect of a given muscle is determined by the product of two variables, namely the mass of the muscle and its mechanical advantage (that is, the respiratory effect per unit muscle mass and unit maximum active stress). As in the dog (De Troyer et al. 1999), the distribution of muscle mass for the external intercostals in humans mirrors the distribution of mechanical advantage, such that the muscle areas with a greater inspiratory mechanical advantage also have a greater mass (Wilson et al. 2001). In contrast, the inspiratory mechanical advantage of the parasternal intercostal in the third interspace is about two-thirds of the mechanical advantage of the external intercostal in the dorsal portion of the same interspace (De Troyer et al. 1998; Wilson et al. 2001), but its mass is much lower. In other words, the difference in inspiratory effect between these two muscles is primarily related to the difference in mass, and indeed, when neural drive is plotted against mechanical advantage (Fig. 10B), the parasternal and external intercostals appear to yield a unique relationship (r = 0.931). Consequently, the idea could be offered that the distribution of neural drive among the different intercostal muscles is closely matched to the distribution of mechanical advantage, rather than the distribution of respiratory effect, but the question then would arise as to why the parasternal intercostal muscles in humans are not thicker. No answer to this teleological question can be provided at this stage.
References
- Binder MD, Heckman CK, Powers RK. Handbook of Physiology, section 12, Exercise, Regulation and Integration of Multiple Systems. Oxford: Oxford University Press; 1996. The physiological control of motoneuron activity; pp. 3–53. [Google Scholar]
- Campbell EJM, Newsom Davis J. The intercostal muscles and other muscles of the rib cage. In: Campbell EJM, Agostoni E, Newsom Davis J, editors. The Respiratory Muscles. Mechanics and Neural Control. 2. Philadelphia: W.B. Saunders; 1970. pp. 161–174. [Google Scholar]
- Chadha TS, Watson H, Birch S, Jenouri GA, Schneider AW, Cohn MA, Sackner MA. Validation of respiratory inductance plethysmography using different calibration procedures. Am Rev Respir Dis. 1982;125:644–649. doi: 10.1164/arrd.1982.125.6.644. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Kelly S. Chest wall mechanics in dogs with acute diaphragm paralysis. J Appl Physiol. 1982;53:373–379. doi: 10.1152/jappl.1982.53.2.373. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Leeper JB, McKenzie DK, Gandevia SC. Neural drive to the diaphragm in patients with severe COPD. Am J Resp Crit Care Med. 1997;155:1335–1340. doi: 10.1164/ajrccm.155.4.9105076. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Legrand A. Inhomogeneous activation of the parasternal intercostals during breathing. J Appl Physiol. 1995;79:55–62. doi: 10.1152/jappl.1995.79.1.55. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Legrand A, Gevenois PA, Wilson TA. Mechanical advantage of the human parasternal intercostal and triangularis sterni muscles. J Physiol. 1998;513:915–925. doi: 10.1111/j.1469-7793.1998.915ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Troyer A, Legrand A, Wilson TA. Rostrocaudal gradient of mechanical advantage in the parasternal intercostal muscles of the dog. J Physiol. 1996;495:239–246. doi: 10.1113/jphysiol.1996.sp021588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Troyer A, Legrand A, Wilson TA. Respiratory mechanical advantage of the canine external and internal intercostal muscles. J Physiol. 1999;518:283–289. doi: 10.1111/j.1469-7793.1999.0283r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estenne M, Yernault JC, De Troyer A. Rib cage and diaphragm-abdomen compliance in humans: effects of age and posture. J Appl Physiol. 1985;59:1842–1848. doi: 10.1152/jappl.1985.59.6.1842. [DOI] [PubMed] [Google Scholar]
- Farkas GA, Decramer M, Rochester DF, De Troyer A. Contractile properties of intercostal muscles and their functional significance. J Appl Physiol. 1985;59:528–535. doi: 10.1152/jappl.1985.59.2.528. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Gorman RB, McKenzie DK, De Troyer A. Effects of increased ventilatory drive on motor unit firing rates in human inspiratory muscles. Am J Resp Crit Care Med. 1999;160:1598–1603. doi: 10.1164/ajrccm.160.5.9904023. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Leeper JB, McKenzie DK, De Troyer A. Discharge frequencies of parasternal intercostal and scalene motor units during breathing in normal and COPD subjects. Am J Resp Crit Care Med. 1996;153:622–628. doi: 10.1164/ajrccm.153.2.8564108. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, McKenzie DK. Human diaphragmatic EMG: changes with lung volume and posture during supramaximal phrenic stimulation. J Appl Physiol. 1986;60:1420–1428. doi: 10.1152/jappl.1986.60.4.1420. [DOI] [PubMed] [Google Scholar]
- Goldman MD, Grassino A, Mead J, Sears TA. Mechanics of the human diaphragm during voluntary contraction: dynamics. J Appl Physiol. 1978;44:840–848. doi: 10.1152/jappl.1978.44.6.840. [DOI] [PubMed] [Google Scholar]
- Grassino A, Goldman MD, Mead J, Sears TA. Mechanics of the human diaphragm during voluntary contraction: statics. J Appl Physiol. 1978;44:829–839. doi: 10.1152/jappl.1978.44.6.829. [DOI] [PubMed] [Google Scholar]
- Hamberger GE. De Respirationis Mechanismo et usu Genuino. Iena: 1749. [Google Scholar]
- Juan G, Calverley P, Talamo C, Schnader J, Roussos C. Effect of carbon dioxide on diaphragmatic function in human beings. N Engl J Med. 1984;310:874–879. doi: 10.1056/NEJM198404053101402. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Druz WS, Sharp JT. Effect of muscle length on electromyogram in a canine diaphragm strip preparation. J Appl Physiol. 1985;58:1602–1607. doi: 10.1152/jappl.1985.58.5.1602. [DOI] [PubMed] [Google Scholar]
- Leduc D, Brunko E, De Troyer A. Response of the canine inspiratory intercostal muscles to chest wall vibration. Am J Resp Crit Care Med. 2000;161:510–516. doi: 10.1164/ajrccm.161.2.9901032. [DOI] [PubMed] [Google Scholar]
- Legrand A, Brancatisano A, Decramer M, De Troyer A. Rostrocaudal gradient of electrical activation in the parasternal intercostal muscles of the dog. J Physiol. 1996;495:247–254. doi: 10.1113/jphysiol.1996.sp021589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand A, De Troyer A. Spatial distribution of external and internal intercostal activity in dogs. J Physiol. 1999;518:291–300. doi: 10.1111/j.1469-7793.1999.0291r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno M, Secher NH. Histochemical characteristics of human expiratory and inspiratory intercostal muscles. J Appl Physiol. 1989;67:592–598. doi: 10.1152/jappl.1989.67.2.592. [DOI] [PubMed] [Google Scholar]
- Sinderby C, Beck J, Spahija J, Weinberg J, Grassino A. Voluntary activation of the human diaphragm in health and disease. J Appl Physiol. 1998;85:2146–2158. doi: 10.1152/jappl.1998.85.6.2146. [DOI] [PubMed] [Google Scholar]
- Taylor A. The contribution of the intercostal muscles to the effort of respiration in man. J Physiol. 1960;151:390–402. doi: 10.1113/jphysiol.1960.sp006446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TA, Legrand A, Gevenois PA, De Troyer A. Respiratory effects of the external and internal intercostal muscles in humans. J Physiol. 2001;530:319–330. doi: 10.1111/j.1469-7793.2001.0319l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TA, De Troyer A. Effect of respiratory muscle tension on lung volume. J Appl Physiol. 1992;73:2283–2288. doi: 10.1152/jappl.1992.73.6.2283. [DOI] [PubMed] [Google Scholar]
- Wilson TA, De Troyer A. Respiratory effect of the intercostal muscles in the dog. J Appl Physiol. 1993;75:2636–2645. doi: 10.1152/jappl.1993.75.6.2636. [DOI] [PubMed] [Google Scholar]


