Abstract
It is well established that the hypothalamic-pituitary-adrenal responses to immune stressors are sexually dimorphic in rodents (females > males), but the underlying mechanism is still unclear. To investigate the mechanism, in this study we examined whether the sex steroid environment affects the following variables in male and female rats: (1) plasma levels of ACTH, interleukin (IL)-1β, IL-6 and tumour necrosis factor-α (TNF-α) after systemic lipopolysaccharide (LPS) administration; (2) static concentrations of corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP) in the mediobasal hypothalamus (MBH) and those of ACTH in the anterior pituitary (AP); and (3) the binding characteristics of IL-1β, IL-6 and TNF-α in the MBH and AP. LPS-induced ACTH release was significantly higher in female than in male rats, and this sexual difference was abolished by performing gonadectomy in both sexes. Administration of physiological doses of testosterone and oestradiol to gonadectomized males and females, respectively, restored the altered ACTH responses to normal. Changes in the sex steroid milieu did not affect plasma cytokine responses to LPS, tissue contents of CRH, AVP and ACTH, or the IL-6 binding characteristics in the MBH and AP. However, the number of IL-1β and TNF-α binding sites, but not their binding affinities, in the MBH showed significant changes according to altered sex hormone milieu, in the same direction as the LPS-induced ACTH response. These results suggest that the hypothalamic sensitivity to peripheral IL-1β and TNF-α may be an important mechanism underlying the sexually dimorphic ACTH response to LPS in rats.
It is well known that the hypothalamic-pituitary-adrenal (HPA) responses to inflammatory stimuli and other types of stressors are sexually dimorphic in rodents, with females having higher hormonal responses than males (Kuhn & Francis, 1997; Rivier, 1999; Spinedi et al. 2002). The major proportion of this sexual dichotomy in the immune-HPA function is certainly mediated by the sex-based difference in the gonadal steroid environment, because gonadectomy and sex hormone treatment can profoundly modulate the HPA axis responses to lipopolysaccharide (LPS) and proinflammatory cytokines. The stimulation of the HPA axis by LPS is primarily mediated by the release of interleukin (IL)-1, IL-6 and tumour necrosis factor-α (TNF-α). Although these three cytokines can all activate every level of the HPA axis, it is generally agreed that the acute-phase ACTH response to these cytokines is principally mediated by the release of corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP) from the hypothalamus, which are two major secretagogues of ACTH (Turnbull & Rivier, 1999).
The mechanism underlying these neuroimmunoendocrine sex differences is still unclear, but may involve sex steroid modulation of CRH and AVP secretion, of circulating cytokine responses, or of cytokine binding in the pituitary and/or hypothalamus. In the present study, we examined in male and female rats whether the manipulation of the gonadal steroid milieu affects the release of ACTH, IL-1β, IL-6 and TNF-α induced by LPS, the anterior pituitary (AP) ACTH and hypothalamic CRH and AVP concentrations, and the binding characteristics of the three cytokines in the AP and hypothalamus. We thus obtained data suggesting that sex steroid modulation of IL-1β and TNF-α binding sites in the hypothalamus may be an important mechanism underlying the sexually dimorphic- and gonadal hormone-dependent ACTH response to LPS.
METHODS
Animals
All the following procedures were approved by the Ethical Committee for Animal Experimentation of the International University of Health and Welfare. Animals were maintained in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Adult male and female rats of the Wistar strain were used. They were housed in an air-conditioned room with controlled lighting (light 08:00–20:00 h) and were given free access to laboratory chow and tap water. All the following surgeries and the first injections of sex steroids were carried out 14 days before experimentation. Throughout the whole surgical procedure, we confirmed that the animals were under deep anaesthesia by the presence of constricted pupils and areflexia. From male rats, three groups were prepared: bilaterally orchidectomized (ODX), sham ODX and ODX + testosterone (T) groups. ODX and sham ODX were performed under ether anaesthesia when the animals were 9–10 weeks of age (body weight, 280–330 g). Until used for experimentation, the ODX + T group received a daily s.c. injection of testosterone (5 mg (kg body wt)−1) dissolved in sesame oil. This dose of testosterone is physiological, as reported previously (Watanobe & Takebe, 1987a). The ODX and sham ODX groups were both treated daily with sesame oil only. All these treatments were started on the day of ODX or sham ODX. From female rats, five groups were prepared: bilaterally ovariectomized (OVX), sham OVX, OVX + oestradiol (E2), OVX + progesterone (P) and OVX + E2 + P groups. OVX and sham OVX were performed under ether anaesthesia when the animals were 9–10 weeks of age (body weight, 220–240 g). Until used for experimentation, the OVX + E2, OVX + P and OVX + E2 + P groups received a daily s.c. injection of E2 benzoate (2.5 µg (kg body wt)−1), progesterone (5 mg (kg body wt)−1), or a combination of these, respectively. These doses of E2 and progesterone are within the physiological range, as reported previously (Watanobe & Takebe, 1987a). Both steroids were dissolved in sesame oil for injection. The OVX and sham OVX groups were treated daily with sesame oil only. All these treatments were started on the day of OVX or sham OVX. In the sham OVX rats, we checked vaginal smears daily after surgery, and confirmed that all the animals displayed normal oestrous cycles of 4–5 days duration. This group was used for experimentation in the dioestrous stage of the oestrous cycle. Separate groups of male and female rats with the above treatments were used for Experiments I-III.
Drugs
T, E2 benzoate, P and LPS (Escherichia coli, serotype O127: B8) were all purchased from Sigma Chemical Company (St Louis, MO, USA). Rat recombinant IL-1β, IL-6 and TNF-α were purchased from BioSource International (Camarillo, CA, USA).
Experiment I: LPS administration
To allow for sequential blood sampling under unanaesthetized conditions, the animals were implanted under ether anaesthesia with a jugular vein catheter filled with heparin solution (10 units ml−1) 2 days prior to the experiment. At 08:00 h on the day of the experiment, an extension of the jugular vein catheter was installed and filled with heparinized saline. After leaving the animals unstressed for 3 h, at 11:00 h all the above-mentioned eight groups were given an i.v. bolus injection of LPS (100 µg (kg body wt)−1) dissolved in normal saline (vehicle) or vehicle alone (control). LPS administration was done 12 h after the last injection of sex steroid(s) or oil. Blood samples (300 µl) were drawn immediately before (time zero), and at 30, 60, 90, 120, 150 and 180 min after the injection. Only at time zero, an additional 200–300 µl of blood was drawn to also measure sex steroid(s). To prevent the loss of circulating plasma volume, the same volume of normal saline was injected i.v. immediately after each blood collection. The blood was collected in prechilled plastic tubes containing EDTA-2Na (2.5 mg ml−1 blood), centrifuged, and the plasma was stored at −70 °C until assayed for ACTH, cytokines (IL-1β, IL-6 and TNF-α) and sex steroids. At the end of the experiment, the animals were killed by rapid decapitation.
Experiment II: measurement of hormones in the hypothalamus, AP and plasma
Twelve hours after the final injection of sex steroid(s) or oil, all the eight experimental groups of male and female rats were rapidly decapitated between 11:00 and 12:00 h. Trunk blood was collected in ice-cold plastic tubes containing EDTA-2Na (2.5 mg ml−1 blood) and centrifuged. The plasma was stored at −70 °C for the measurement of sex steroids. The brains and APs were quickly removed. Each brain was placed on a glass plate on ice, and the mediobasal hypothalamus (MBH) was dissected out. The MBH was limited by the optic chiasm anteriorly, the hypothalamic fissures laterally, the mamillary bodies posteriorly, and by a horizontal line drawn 3 mm above the median eminence (containing the paraventricular nucleus) dorsally. The preparation of the MBH for CRH and AVP assays, and of the AP for ACTH assay, was carried out according to the method we have previously reported (Watanobe & Takebe, 1987a). Briefly, the tissue was individually homogenized in 200 µl of ice-cold 0.1 n HCl with a Teflon-glass homogenizer (20 strokes). The homogenate was centrifuged at 1000 g for 30 min at 4 °C, and the supernatant was stored at −70 °C until assayed for hormones and protein concentrations.
Experiment III: cytokine binding assays
Tissue preparation
Between 11:00 and 12:00 h, 12 h after the final injection of sex steroid(s) or oil, all the above eight groups of rats were rapidly decapitated. Trunk blood was collected in ice-cold plastic tubes containing EDTA-2Na (2.5 mg ml−1 blood) and centrifuged. The plasma was stored at −70 °C for the measurement of sex steroid(s). The MBH and the AP were quickly removed in the same manner as in Experiment II. The preparation of receptor membranes from the MBH and the AP was performed according to methods previously described by Katsuura et al. (1988) and ourselves (Watanobe & Takebe, 1987a,b,c) with minor modifications. Briefly, each tissue was individually homogenized in ice-cold buffer (40 mm Tris, 7 mm KCl, 2 mm MgCl2, 0.1 % bovine serum albumin, 5 % bacitracin; pH 7.4) using a Teflon-glass homogenizer (20 strokes). The homogenate was centrifuged at 1000 g for 10 min at 4 °C, and the resultant supernatant was further centrifuged at 48 000 g for 30 min at 4 °C. The pellet was resuspended in the same buffer and centrifuged at 48 000 g for 10 min at 4 °C. After discarding the supernatant, the pellet was stored at −70 °C until used.
Iodination of cytokines
Rat recombinant IL-1β, IL-6 and TNF-α were all iodinated using the method reported by Bolton & Hunter (1973). The specific activities of the radioiodinated cytokines were 110–170, 70–150 and 45–80 µCi µg−1 for IL-1β, IL-6 and TNF-α, respectively.
Radioreceptor assays for cytokines
The protocols of radioreceptor assays for IL-1β, IL-6 and TNF-α were determined, incorporating methods described elsewhere (Katsuura et al. 1988; Cornfield & Sills, 1991; Elisasser et al. 1991; Kinouchi et al. 1991). Optimum conditions for the binding studies were determined from preliminary experiments. On the day of the experiment, frozen pellets of the MBH and AP membranes were thawed and resuspended with a vortex in the above-mentioned buffer. To enable binding studies for all three cytokines to be carried out on the same samples, several pellets were pooled to give a tissue suspension that contained about 400 µg protein per tube. An aliquot of this suspension was reserved for future precise determination of protein concentration. A 100 µl aliquot of tissue suspension and 100 µl of the above buffer were incubated with 100 µl of the respective iodinated cytokine at eight concentrations each. 125I-IL-1β,125I-IL-6 and 125I-TNF-α were used in the ranges 0.2–15.8, 0.2–6.9 and 0.6–.2 nm, respectively. Incubation was carried out in triplicate for 150 min at 4 °C in 12 × 75 mm plastic tubes with periodical vortexing. Parallel incubations were performed in the presence of a 1000-fold excess of unlabelled respective cytokine. The bound radioactivity was separated from the free by centrifugation at 14 000 g for 15 min. The supernatant was aspirated and the bound radioactivity in the pellet was counted by a gamma counter. Specific binding was calculated as the difference between total binding (in the absence of non-radioactive cytokine) minus non-specific binding (in the presence of a 1000-fold excess of non-radioactive cytokine).
Assays
Tissue protein concentrations were determined utilizing the BCA Protein Assay Reagent (Pierce, Rockford, Ill, USA). ACTH levels in the plasma and AP were measured using an immunoradiometric assay kit produced by Mitsubishikagaku Biochemicals (Tokyo, Japan); the sensitivity was 5.0 pg ml−1. Rat IL-1β, IL-6 and TNF-α levels in the plasma were determined by specific enzyme-linked immunosorbent assay kits purchased from BioSource International. The sensitivities of these assays were 3, 8 or 4 pg ml−1, respectively. CRH radioimmunoassay (RIA) was carried out according to the method reported previously (Watanobe & Takebe, 1993); the sensitivity was 1.0 pg tube−1. AVP was measured using an RIA kit purchased from Mitsubishikagaku Biochemicals; the sensitivity was 0.1 pg tube−1. Plasma concentrations of T, E2 and P were determined by respective RIA kits, all of which were produced by DPC Corp. (Los Angeles, CA, USA). The sensitivities of these assays were 5 ng dl−1, 8 pg ml−1 and 0.1 ng ml−1, respectively. In all these assays, both intra- and interassay coefficients of variation were less than 10 %.
Data analysis
Secretory responses of ACTH were expressed in two ways: the time-line graph, and the area under the response curve (AUC) that was calculated using the trapezoidal rule. To analyse the cytokine binding data, Scatchard analysis was employed to determine the Kd and the number of binding sites (Bmax) with the use of linear regression (Scatchard, 1949). The Hill plot (Cornish-Bowden & Koshland, 1975) was used to determine cooperative interactions in the ligand-binding isotherm.
Results were expressed as the mean ± s.e.m. For the purpose of detecting significant alterations within groups, data of individual experimental groups were analysed by two-way ANOVA with repeated measures. One-way ANOVA was used to compare data among different groups. When significant F values were obtained, a Bonferroni multiple comparisons test was performed. Differences were considered significant if P was smaller than 0.05.
RESULTS
Experiment I: effects of sex, gonadectomy and sex steroid replacement on the LPS-induced ACTH release
Table 1 shows the plasma concentrations of sex steroids in the eight experimental groups examined. The plasma T levels in both the sham ODX and ODX + T groups were very similar to our recent data obtained from intact adult male rats (Watanobe & Schiöth, 2002). The ODX group had non-detectable levels of T, which indicates a complete ODX. The plasma concentrations of E2 and P in both the sham OVX and OVX + E2 + P groups indicated that these two groups had a physiological ovarian steroid environment corresponding to that found during the dioestrous stage of the oestrous cycle (Freeman, 1988). The levels of E2 in the OVX + E2 group and those of P in the OVX + P group were also within the physiological ranges of the respective hormones that are found during dioestrus. The detectable amount of plasma P in both the OVX and OVX + E2 groups may probably have been of adrenal origin, as already described elsewhere (Freeman, 1988).
Table 1.
Plasma concentrations of sex steroids in the eight experimental groups
| Sex | Group | No. | [T] (ng dl−1) | [E2] (pg ml−1) | [P] (ng ml−1) |
|---|---|---|---|---|---|
| M | Sham ODX | 10 | 172 ± 15 | n.d. | n.d. |
| ODX | 9 | <5 | n.d. | n.d. | |
| ODX + T | 11 | 183 ± 17 | n.d. | n.d. | |
| F | Sham OVX | 12 | n.d. | 30 ± 3 | 5.1 ± 0.3 |
| OVX | 10 | n.d. | <8 | 0.8 ± 0.1* | |
| OVX + E2 | 9 | n.d. | 29 ± 2 | 0.7 ± 0.1* | |
| OVX + P | 11 | n.d. | <8 | 5.3 ± 0.4 | |
| OVX + E2 + P | 9 | n.d. | 31 ± 2 | 5.4 ± 0.3 |
T, testosterone; E2, oestradiol; P, progesterone; ODX, orchidectomy; OVX, ovariectomy; n.d., not determined.
Statistically significant vs. the other female groups.
Figure 1 shows the temporal profiles of plasma ACTH, IL-1β, IL-6 and TNF-α after LPS administration in the three male groups. Although not shown in Fig. 1, i.v. administration of vehicle alone did not significantly affect ACTH secretion in any group during the entire period of observation (47 ± 7 pg ml−1 (the lowest time point value) to 62 ± 8 pg ml−1 (the highest time point value)). Basal ACTH levels were statistically not different among the sham ODX (52 ± 7 pg ml−1), ODX (65 ± 8 pg ml−1) and ODX + T (48 ± 6 pg ml−1) groups. In all three groups, LPS produced significantly higher ACTH titres than vehicle during the period 30–180 min, and ACTH reached its peak at time 60 min. It is worth noting that the ODX group had significantly higher ACTH levels than the sham ODX group between 30 and 150 min, and peak ACTH titres at time 60 min were 1.8-fold higher (P < 0.02) in the former group than in the latter. This potentiating effect of ODX was completely abolished by supplementing T to ODX rats (ODX + T group). Also, when evaluated according to the ACTH AUC, similar intergroup differences in the ACTH response to LPS were observed (Fig. 3). The ACTH AUC after LPS in the ODX group was 1.7-fold higher (P < 0.05) than that in both the sham ODX and ODX + T groups. Plasma IL-1β was undetectable in every control animal. IL-6 and TNF-α were measurable in the three control groups, but did not change significantly during the entire period of sampling (13 ± 7 pg ml−1 (the lowest time point value) to 26 ± 10 pg ml−1 (the highest time point value) for IL-6; 36 ± 13 pg ml−1 (the lowest) to 63 ± 21 pg ml−1 (the highest) for TNF-α). Compared with the vehicle, LPS produced significantly higher levels of all three cytokines, but there were differences in their temporal secretory patterns. In every male group, IL-1β and IL-6 both reached a peak 90 min after LPS administration, and TNF-α 60 min after. Over the experiment, there were no significant intergroup differences in the plasma cytokine responses.
Figure 1. Effects of ODX and T replacement on plasma ACTH and cytokine responses to an i.v. bolus administration of LPS (100 µg (kg body wt)−1) in male rats.
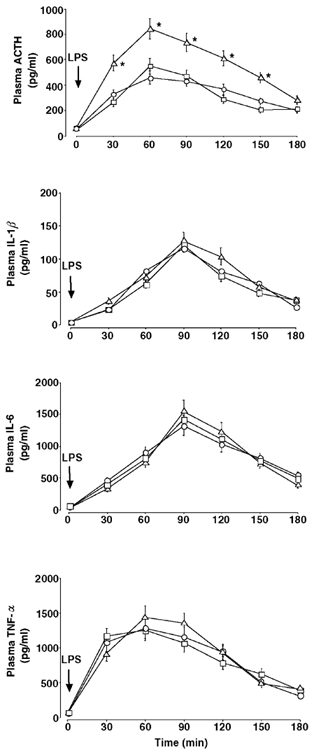
○, sham ODX; Δ, ODX; □, ODX + T. For abbreviations, see Table 1. *Statistically significant vs. the other two groups.
Figure 3. Effects of gonadectomy and sex steroid replacement on the integrated ACTH secretion (area under the curve, AUC) induced by LPS (100 µg (kg body wt)−1, i.v. bolus) in male and female rats.
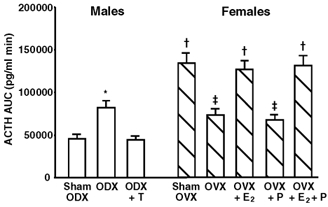
For abbreviations, see Table 1. *Statistically significant vs. the other male groups. †Statistically significant vs. the OVX, OVX + P and the three male groups. ‡Statistically significant vs. the sham ODX and ODX + T groups.
Figure 2 shows the temporal profiles of ACTH and the three cytokines in the plasma after LPS injection in the five female groups. As in the male rats, i.v. injection of vehicle alone was without effect on ACTH secretion in any female group over the experiment (53 ± 10 pg ml−1 (the lowest time point value) to 71 ± 11 pg ml−1 (the highest time point value)). Basal ACTH levels in the sham OVX, OVX, OVX + E2, OVX + P and OVX + E2 + P groups were 78 ± 9, 62 ± 7, 73 ± 8, 56 ± 7 and 70 ± 8 pg ml−1, respectively, all of which were statistically not different from each other. In agreement with previous studies by other investigators (Spinedi et al. 1994; Chisari et al. 1995), the basal ACTH concentrations in the sham OVX, OVX + E2 and OVX + E2 + P groups were slightly higher than those in the sham ODX group (52 ± 7 pg ml−1; Fig. 1), although these differences did not reach statistical significance. In all female groups, LPS significantly elevated plasma ACTH concentrations above the values after vehicle alone, and the hormone reached its peak 60 min postadministration. The ACTH response to LPS in the OVX group was significantly lower than that in the sham OVX group between 30–120 min, and peak ACTH levels at time 60 min were 41 % lower (P < 0.05) in the former group than in the latter. This decrease in ACTH release after OVX was not affected by supplementing P only (OVX + P group), but was restored to the level of the sham OVX group by the treatments containing E2 (OVX + E2 and OVX + E2 + P groups). Similar intergroup differences in the ACTH response among the female groups were also observed when they were compared according to the ACTH AUC (Fig. 3). The integrated ACTH responses to LPS in the sham OVX, OVX + E2 and OVX + E2 + P groups were 1.7–2.0-fold higher (P < 0.05) than those in the OVX and OVX + P groups. In addition, there was a clear sex difference in the LPS-induced ACTH secretion. Both the peak ACTH level and the ACTH AUC were significantly (P < 0.01) higher in the sham OVX than in the sham ODX group by 3.0 or 2.9 times, respectively (Figs 1–3). Plasma cytokine data in the female rats were very similar to those in the male groups (Fig. 1). In the five female groups given vehicle alone, IL-1β was consistently undetectable and neither IL-6 nor TNF-α, of which levels were similar to the above-mentioned male data, changed significantly over the experiment (data not shown). LPS administration significantly stimulated the release of all three cytokines in every female group (Fig. 2). The values and time course of each cytokine were statistically indistinguishable among all female groups, nor was there any significant difference from the male rat data (Fig. 1).
Figure 2. Effects of OVX and E2 and/or P replacement on plasma ACTH and cytokine responses to an i.v. bolus administration of LPS (100 µg (kg body wt)−1) in female rats.
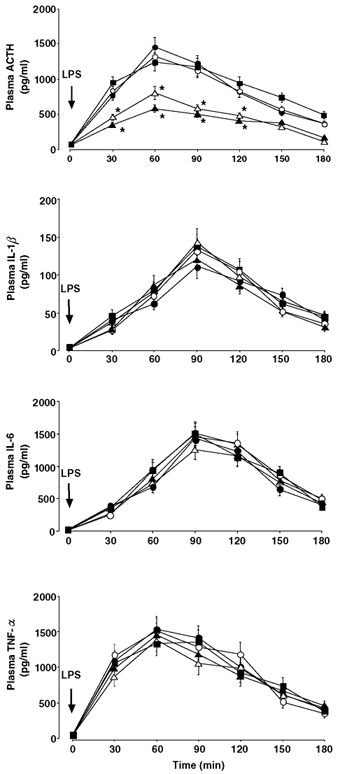
○, sham OVX; Δ, OVX; •, OVX + E2; ▴, OVX + P; ▪, OVX + E2 + P. For abbreviations, see Table 1. *Statistically significant vs. the sham OVX, OVX + E2 and OVX + E2 + P groups.
Experiment II: effects of sex, gonadectomy and sex steroid replacement on the MBH CRH and AVP and AP ACTH contents
Plasma concentrations of sex steroids in the eight experimental groups were very similar to those in Experiment I (data not shown). As shown in Table 2, gonadectomy and sex steroid replacement were without effect on the MBH CRH and AVP or the AP ACTH contents in either sex, nor was there any significant sex-based difference in any variable.
Table 2.
Tissue contents of CRH and AVP in the MBH and those of ACTH in the AP in the eight experimental groups
| Sex | Group | No. | MBH [CRH] (ng (mg protein)−1) | MBH [AVP] (ng (mg protein)−1) | AP [ACTH] (μg (mg protein)−1) |
|---|---|---|---|---|---|
| M | Sham ODX | 9 | 1.06 ± 0.15 | 1.78 ± 0.21 | 0.89 ± 0.11 |
| ODX | 10 | 1.01 ± 0.08 | 1.65 ± 0.14 | 0.96 ± 0.13 | |
| ODX + T | 9 | 1.18 ± 0.14 | 1.83 ± 0.18 | 0.83 ± 0.09 | |
| F | Sham OVX | 10 | 1.21 ± 0.13 | 1.88 ± 0.13 | 0.79 ± 0.08 |
| OVX | 11 | 1.04 ± 0.09 | 1.90 ± 0.21 | 0.81 ± 0.10 | |
| OVX + E2 | 9 | 1.15 ± 0.16 | 1.76 ± 0.15 | 0.92 ± 0.12 | |
| OVX + P | 10 | 1.03 ± 0.08 | 1.70 ± 0.11 | 0.86 ± 0.13 | |
| OVX + E2 + P | 12 | 1.25 ± 0.12 | 1.68 ± 0.19 | 0.77 ± 0.11 |
CRH, corticotropin-releasing hormone; AVP, arginine vasopressin; MBH, mediobasal hypothalamus; AP, anterior pituitary. For other abbreviations, see Table 1.
Experiment III: effects of sex, gonadectomy and sex steroid replacement on the characteristics of cytokine binding in the MBH and AP
Plasma levels of sex hormones in all the male and female groups were very similar to those in Experiments I and II (data not shown).
Figure 4A shows the specific binding of 125I-IL-1β to plasma membrane preparations from the MBH and AP, respectively, of the sham ODX group. Incubation of the membrane receptors with increasing concentrations of 125I-IL-1β indicated that the binding was saturable in both the MBH and the AP. Scatchard analyses of these binding data yielded straight lines for both tissues, as shown in a typical experiment (Fig. 4B), which was replicated six times. Hill coefficients of unity revealed a single class of 125I-IL-1β binding site with no cooperativity in both the MBH and the AP. Also in the remaining two male and five female groups, similar binding studies were performed for both the MBH and the AP six times each. Of the total 125I-IL-1β added, its per cent specific binding in the MBH ranged from 0.5 to 13 %, depending on the sex, sex steroid status and the concentration of 125I-IL-1β added. In the AP, the per cent specific binding of 125I-IL-1β varied from 0.3 to 1.8 %, which was subject only to the applied concentration of 125I-IL-1β. Table 3 shows the results of the replicated experiments in all the eight groups. The Kd value was not statistically different between the MBH and the AP, and was unaffected by any change in the gonadal steroid milieu in either tissue in either sex. Significant intergroup differences were observed in the Bmax of 125I-IL-1β in the MBH. The Bmax in the MBH of the ODX group was significantly (P < 0.05) higher than that of the sham ODX and ODX + T groups, by 1.7 and 1.9 times, respectively. As regards the female rats, the Bmax of 125I-IL-1β in the MBH was 1.6 times higher (P < 0.05) in the sham OVX group than in both the OVX and OVX + P groups. This reduction in the Bmax by OVX was abolished by administering E2 to the OVX animals (OVX + E2 and OVX + E2 + P groups). A comparison between the sham ODX and sham OVX rats indicated that the latter group had a 2.6-fold higher (P < 0.01) Bmax of 125I-IL-1β in the MBH than the former. Differing from the MBH, the AP tissue did not show any significant sex or intergroup differences in the 125I-IL-1βBmax.
Figure 4. Specific binding of 125I-IL-1β to membrane preparations of the MBH and AP from sham ODX rats.
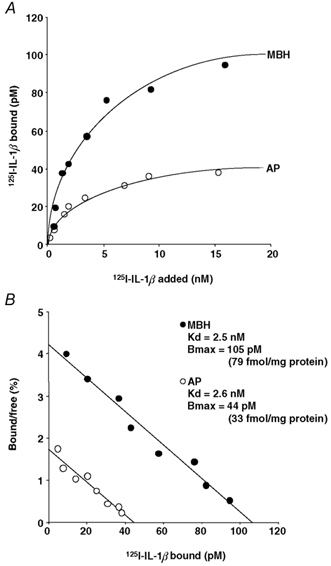
A, saturation curve for specific 125I-IL-1β binding as a function of ligand concentration. B, Scatchard plot of the binding data. In this figure and in Figs 5 and 6, each value is the mean of triplicate determinations from a typical experiment that was replicated six times with similar results. The same binding studies were also performed for the remaining seven experimental groups. Kd, equilibrium dissociation constant; Bmax, number of binding sites. For abbreviations, see Tables 1 and 2.
Table 3.
Characteristics of 125I-IL-1β binding to MBH and AP homogenates in the eight experimental groups
| MBH | AP | ||||
|---|---|---|---|---|---|
| Sex | Group | Kd (nM) | Bmax (fmol (mg protein)−1) | Kd (nM) | Bmax (fmol (mg protein)−1) |
| M | Sham ODX | 2.4 ± 0.3 | 82 ± 9 | 2.5 ± 0.2 | 30 ± 4 |
| ODX | 2.2 ± 0.2 | 143 ± 16* | 2.4 ± 0.3 | 33 ± 5 | |
| ODX + T | 2.5 ± 0.3 | 76 ± 8 | 2.5 ± 0.3 | 29 ± 3 | |
| F | Sham OVX | 2.3 ± 0.3 | 213 + 27† | 2.3 ± 0.3 | 28 ± 4 |
| OVX | 2.6 ± 0.4 | 130 ± 14‡ | 2.5 ± 0.3 | 31 ± 3 | |
| OVX + E2 | 2.4 ± 0.3 | 260 ± 23‡ | 2.6 ± 0.2 | 32 ± 4 | |
| OVX + P | 2.4 ± 0.2 | 135 ± 18‡ | 2.3 ± 0.2 | 33 ± 3 | |
| OVX + E2 + P | 2.5 ± 0.2 | 220 ± 25† | 2.4 ± 0.2 | 34 ± 4 | |
Six separate binding experiments were performed in each group. For abbreviations, see Tables 1 and 2 and Fig. 4.
Statistically significant vs. the other male groups.
Statistically significant vs. the OVX, OVX + P and the three male groups.
Statistically significant vs. the sham ODX and ODX + T groups.
Figure 5A shows the results of a typical experiment of 125I-IL-6 binding to the MBH and AP tissues from the sham ODX group, which was replicated six times each. Both tissues showed specific and saturable binding of 125I-IL-6. A Scatchard plot of these binding data was linear in both the MBH and the AP, indicating the presence of a single type of binding site for 125I-IL-6 (Fig. 5B). Also, in the remaining seven groups, similar binding experiments were conducted for both the MBH and the AP six times each. Of the total 125I-IL-6 added, the per cent specific binding in the MBH and the AP was 0.6–3 % and 0.5–2.5 %, respectively, which varied depending on the applied concentration of 125I-IL-6 only. Table 4 shows the data from the replicated binding studies in all the eight groups examined. Differing from the 125I-IL-1β binding, the Bmax of 125I-IL-6 in the MBH was unaffected by sex or the manipulation of sex steroid environment. The Kd in the MBH and both the Kd and Bmax in the AP were all statistically not different among all groups, as in the case of 125I-IL-1β binding (Table 3).
Figure 5. Specific binding of 125I-IL-6 to membrane preparations of the MBH and AP from sham ODX rats.
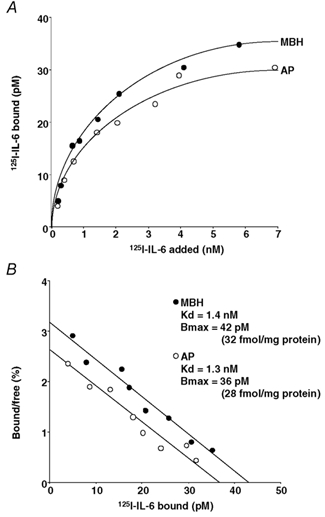
A, saturation curve for specific 125I-IL-6 binding as a function of ligand concentration. B, Scatchard plot of the binding data. For abbreviations, see Tables 1 and 2 and Fig. 4.
Table 4.
Characteristics of 125I-IL-6 binding to MBH and AP homogenates in the eight experimental groups
| MBH | AP | ||||
|---|---|---|---|---|---|
| Sex | Group | Kd (nM) | Bmax (fmol (mg protein)−1) | Kd (nM) | Bmax (fmol (mg protein)−1) |
| M | Sham ODX | 1.3 ± 0.2 | 34 ± 4 | 1.3 ± 0.1 | 31 ± 3 |
| ODX | 1.4 ± 0.2 | 31 ± 3 | 1.2 ± 0.1 | 28 ± 4 | |
| ODX + T | 1.4 ± 0.1 | 32 ± 3 | 1.4 ± 0.2 | 33 ± 4 | |
| F | Sham OVX | 1.2 ± 0.1 | 29 ± 3 | 1.2 ± 0.1 | 32 ± 4 |
| OVX | 1.2 ± 0.2 | 28 ± 4 | 1.3 ± 0.2 | 30 ± 2 | |
| OVX + E2 | 1.4 ± 0.2 | 33 ± 4 | 1.4 ± 0.2 | 29 ± 3 | |
| OVX + P | 1.3 ± 0.2 | 32 ± 3 | 1.5 ± 0.2 | 32 ± 3 | |
| OVX + E2 + P | 1.2 ± 0.2 | 31 ± 4 | 1.3 ± 0.1 | 27 ± 4 | |
Figure 6A shows the data from a representative experiment of 125I-TNF-α binding to the MBH and AP tissues from the sham ODX group, which was replicated six times each. It was revealed that both tissues have specific, saturable and a single-type binding site for 125I-TNF-α (Fig. 6B). Also, in the remaining seven groups, similar binding experiments were conducted for both the MBH and the AP six times each. Of the total 125I-TNF-α added, its per cent specific binding in the MBH varied between 0.6 and 15 %, depending on the sex, gonadal steroid milieu and the applied concentration of 125I-TNF-α. In the AP, the per cent specific binding of 125I-TNF-α ranged between 0.3 and 2.5 %, which was subject only to the concentration of 125I-TNF-α added. Table 5 shows the data from the replicated binding studies conducted for both the MBH and the AP in all the male and female groups. The Kd value was not statistically different between the MBH and the AP, and was unaffected by any alterations in the sex steroid milieu in either tissue in either sex. Significant intergroup differences were observed in the Bmax of 125I-TNF-α in the MBH. The ODX group had a significantly higher (P < 0.05) Bmax in the MBH than the sham ODX and ODX + T groups by 2.2 and 1.9 times, respectively. In female rats, 125I-TNF-αBmax in the MBH was significantly higher (P < 0.05) in the sham OVX group than in both the OVX and OVX + P groups, by 1.7 and 1.6 times, respectively. This OVX-induced decrease in the MBH Bmax was reinstated to the level of the sham OVX group after the treatments containing E2 (OVX + E2 and OVX + E2 + P groups). There was a clear sexual difference in the 125I-TNF-αBmax in the MBH, which was 3.5 times higher (P < 0.01) in the sham OVX than in the sham ODX group. In contrast to the MBH, Bmax values in the AP were similar across sexes and groups. These data of 125I-TNF-α binding in both tissues were very similar to those of 125I-IL-1β binding (Table 3).
Figure 6. Specific binding of 125I-TNF-α to membrane preparations of the MBH and AP from sham ODX rats.
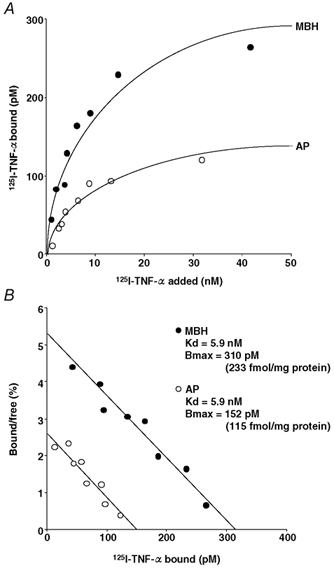
A, saturation curve for specific 125I-TNF-α binding as a function of ligand concentration. B, Scatchard plot of the binding data. For abbreviations, see Tables 1 and 2 and Fig. 4.
Table 5.
Characteristics of 125I-TNF-α binding to MBH and AP homogenates in the eight experimental groups
| MBH | AP | ||||
|---|---|---|---|---|---|
| Sex | Group | Kd (nM) | Bmax (fmol (mg protein)−1) | Kd (nM) | Bmax (fmol (mg protein)−1) |
| M | Sham ODX | 5.9 ± 0.5 | 230 ± 21 | 5.8 ± 0.5 | 110 ± 16 |
| ODX | 5.7 ± 0.4 | 512 ± 48* | 5.7 ± 0.6 | 118 ± 13 | |
| ODX + T | 5.8 ± 0.6 | 263 ± 31 | 5.9 ± 0.5 | 109 ± 14 | |
| F | Sham OVX | 6.0 ± 0.5 | 810 ± 78† | 5.6 ± 0.6 | 113 ± 12 |
| OVX | 5.7 ± 0.6 | 483 ± 52‡ | 5.7 ± 0.5 | 120 ± 15 | |
| OVX + E2 | 5.8 ± 0.4 | 836 ± 91† | 5.9 ± 0.4 | 116 ± 11 | |
| OVX + P | 6.0 ± 0.5 | 502 ± 41‡ | 5.8 ± 0.5 | 107 ± 13 | |
| OVX + E2 + P | 5.9 ± 0.4 | 825 ± 83† | 5.5 ± 0.5 | 115 ± 12 | |
Six separate binding experiments were performed in each group. For abbreviations, see Tables 1 and 2 and Fig. 4.
Statistically significant vs. the other male groups.
Statistically significant vs. the OVX, OVX + P and the three male groups.
Statistically significant vs. the sham ODX and ODX + T groups.
DISCUSSION
It has been repeatedly shown that ACTH and corticosterone responses to LPS and IL-1 are sexually dimorphic in rodents, with females having greater responses than males (Spinedi et al. 1992, 1994, 2002; Frederic et al. 1993; Rivier, 1994; Hadid et al. 1995; Watanobe et al. 1996). In addition, we have recently reported that ACTH and corticosterone responses are also greater in female than in male rats after administration of TNF-α, but not IL-6 (Watanobe, 2002). The distinct sexual dimorphism in the LPS-induced ACTH release found in the present study mostly agrees with these previous reports. However, this is not entirely the case for the influences of gonadectomy and sex hormone treatments. We found that the ACTH response to LPS was significantly enhanced by ODX, and that it reverted to normal after administration of a physiological dose of testosterone. These data are in agreement with a previous study in mice by other investigators (Hadid et al. 1995). The potentiation by ODX of ACTH responses to IL-1β and TNF-α has also been reported (Rivier, 1994; Watanobe et al. 1996; Watanobe, 2002). With regard to female rats, we found that OVX significantly diminished the LPS-induced ACTH release, and that this effect was completely abolished by supplementing E2 but not P. These changes in ACTH secretion after OVX are, however, at odds with previous data reported by Spinedi et al. (1992) and Rivier (1994) using mice and rats, respectively. Both groups reported that OVX led to a significant stimulation of corticosterone release induced by LPS and IL-1β, respectively. Although we have no clear explanations for this discrepancy, there are several possibilities. First, sex steroids may differentially affect ACTH and corticosterone responses to immune stressors under certain circumstances, as found by Rivier upon IL-1β administration (Rivier, 1994). Second, a species difference may exist between mice and rats with regard to the immune-HPA axis sensitivity to gonadal steroids. Frederic et al. (1993) reported that ODX did not affect IL-1-induced corticosterone secretion in mice, a finding that contrasts with the significant potentiation in rats of corticosterone (Rivier, 1994) and ACTH (Watanobe et al. 1996) responses to IL-1. To make matters even more complicated, Frederic et al. (1993) also reported that the effects of gonadectomy on the LPS-induced corticosterone responses varied among different strains of mice. Third, differences in inherent properties of LPS and cytokines as immune stressors might also account for the inconsistent findings among various reports. LPS is known to increase the synthesis and secretion of an array of proinflammatory and anti-inflammatory cytokines. These cytokines can exert stimulatory or inhibitory effects on the HPA axis on their own, and there also exist multiple interactions among different individual cytokines (Turnbull & Rivier, 1999). It is thus apparent that the final outcome of the HPA axis modulation by LPS is determined by the net effect of the interactions among various cytokines.
Spinedi et al. (1992, 1994) were the first to report the sexually dimorphic ACTH and corticosterone responses to LPS in rats and mice, respectively. Although Hadid et al. (1995) reported that ODX potentiates the LPS-induced ACTH release in mice, our present study is the first to examine the effects of gonadectomy and sex hormone treatment on ACTH secretion induced by this immune stressor in rats. Our data strongly indicate that physiological levels of testosterone and oestradiol exert significant inhibitory and stimulatory influences, respectively, on the LPS-induced ACTH secretion in rats.
The present data of plasma IL-1β, IL-6 and TNF-α after LPS administration are very similar to those in our previous report in terms of time course and magnitude (Kakizaki et al. 1999). For all three cytokines, we found no significant sex or intergroup differences in either the temporal profile or the magnitude. These results indicate that IL-1β, IL-6 and TNF-α in the periphery do not participate in the gonadal steroid modulation of the LPS-induced ACTH secretion. However, Spinedi et al. (1992) reported in an early study that plasma TNF-α responses to LPS were enhanced by both ODX and OVX in mice. As they found that the gonadectomy-induced rise in TNF-α secretion coexisted with that of corticosterone, they suggested that the facilitated release of TNF-α may, at least in part, mediate the enhanced corticosterone responses after ODX and OVX. However, in a subsequent study using the same animal species, the same investigators were unable to confirm the gonadectomy-induced stimulation of TNF-α responses (Hadid et al. 1995). Although they did not discuss the reason for this discrepancy, and thus the details are unclear, we think it possible, on the basis of our present data, that there may be a species difference in the endotoxin-stimulated release of cytokines.
The data of the content of CRH and AVP in the MBH and that of ACTH in the AP indicate that none of these variables is significantly affected by sex, gonadectomy or sex hormone treatment. These results are in agreement with previous reports by other investigators (Spinedi et al. 1994; Chisari et al. 1995; Hadid et al. 1995). It is thus very unlikely that alterations in the releasable pool of ACTH and the two major ACTH secretagogues are involved in the sex steroid modulation of the ACTH response to LPS. An array of studies have investigated the roles of gonadal steroids in regulating the biosynthesis of CRH and AVP in the hypothalamus. For CRH, several studies have reported a stimulatory effect of oestrogen on the neurohormone (Bohler et al. 1990; Vamvakopoulos & Chrousos, 1993; Patchev et al. 1995; Roy et al. 1999; Corchero et al. 2001), but conflicting reports also exist (Haas & George, 1988; Broad et al. 1995; Paulmyer-Lacroix et al. 1996). Similarly, although one group of investigators reported that testosterone is inhibitory to CRH synthesis (Handa et al. 1994), this was not confirmed by others (Viau & Meaney, 1996; Viau et al. 1999). Concerning the effects of sex steroids on AVP biosynthesis, a stimulatory action of oestrogen on the neuropeptide has been demonstrated by several groups (Greer et al. 1986; Forsling, 1993; Patchev et al. 1995), although such an effect was not reported by others (Van Tol et al. 1988; Nappi et al. 1997; Roy et al. 1999). De Vries and collaborators have reported that testosterone is stimulatory to AVP synthesis (van Leeuwen et al. 1985; de Vries et al. 1986, 1994; Wang & De Vries, 1995), but the opposite action was demonstrated by Viau & Meaney (1996). These discrepancies among the various reports may be attributable to the differences in animal species, sex and length of steroid treatment, because all these confounding variables may affect glucocorticoid secretion and its negative feedback effect on CRH, AVP and ACTH secretion (Patchev & Almeida, 1996). In addition, in terms of our present experimental design, it is important to bear in mind that tissue contents of CRH, AVP and also ACTH may not well reflect the rapidly releasable pool of these hormones, which is the more important parameter in acute hormonal responses. This is because tissue contents of hormones, in general, represent a difference between an increase by synthesis and a decrease by release, which thus raises the possibility that similar hormonal contents in different experimental groups could result if both the synthesis and release are altered to a similar degree but in an opposite direction.
Previous studies have demonstrated the existence of IL-1 binding sites or receptors in the AP (Haour et al. 1990; Ban et al. 1991; Marquette et al. 1995) and the hypothalamus (Farrar et al. 1987; Katsuura et al. 1988; Yabuuchi et al. 1994) in rats and mice. Similarly, specific binding sites or receptors for IL-6 and TNF-α in the AP and the hypothalamus have also been demonstrated in rats, mice and cows (Cornfield & Sills, 1991; Elisasser et al. 1991; Ohmichi et al. 1992; Schöbitz et al. 1992; Gadient & Otten, 1993; Wolvers et al. 1993). There are at least some inconsistencies among these studies, and none of them demonstrated cytokine binding sites or receptors simultaneously in both the AP and the hypothalamus. These discrepancies may derive from the difference in animal species used, varying techniques employed (radioreceptor assay, autoradiography and mRNA detection), the use of homologous vs. heterologous cytokines as radioligands in binding experiments, and also other differences in the experimental design. In the present study, using homologous cytokines as radioligands we detected saturable, specific and single-class binding sites for IL-1β, IL-6 and TNF-α in both the AP and the MBH from all eight rat groups. In the AP, neither the Bmax nor the Kd of each cytokine's binding was significantly affected by any change in the sex hormone environment. In the MBH, although IL-6 binding was similar among all eight groups with regard to both the Bmax and the Kd, there were clear intergroup differences in the IL-1β and TNF-αBmax values, which were not accompanied by significant intergroup differences in the Kd. It is interesting to note that these changes in the IL-1β and TNF-αBmax approximately paralleled those of the LPS-induced ACTH release, which also changed significantly according to the sex steroid milieu (see above). The significantly higher ACTH response in the sham OVX than in the sham ODX group coexisted with a higher Bmax of both IL-1β and TNF-α in the former group. The observed changes in the ACTH response induced by gonadectomy and sex hormone replacement were all associated with altered Bmax of both IL-1β and TNF-α in the same directions. All these results strongly suggest that sex steroid regulation of IL-1β and TNF-αBmax in the MBH may be an important mechanism underlying the sexually dimorphic ACTH response to LPS. The observation that the binding characteristics of IL-6 in both the MBH and the AP were not affected by the gonadal steroid milieu seems to concur with our recent report that the ACTH response to this cytokine is not sexually dimorphic (Watanobe, 2002).
We have previously reported that both IL-1β and TNF-α primarily act in the median eminence to stimulate the release of both CRH and AVP, which then culminates in the acute-phase secretion of ACTH from the AP (Watanobe et al. 1991; Watanobe & Takebe, 1992, 1993, 1994). The median eminence is a brain structure devoid of the blood-brain barrier and is also the most ventral part of the MBH (Broadwell & Brightman, 1976). Taken together, our present results make it very likely that the number of IL-1β and TNF-α binding sites in the MBH determines the central sensitivity to these cytokines of peripheral origin, which then regulates the amount of CRH and AVP released from the median eminence and reaching the AP.
We are unaware of any previous study reporting a differential regulation of the same cytokine receptors or binding sites between the hypothalamus and AP under any physiological or pathological condition. However, published studies have demonstrated that altered nutritional state can differentially affect the expression of insulin-like growth factor-I receptors in various tissues including the hypothalamus and AP in rats (Olchovsky et al. 1993; Takenaka et al. 1996). Although we have no clear explanation for the mechanism underlying the tissue-specific regulation of IL-1β and TNF-α receptors by gonadal steroids, the possibility remains that promotor sequences and/or post-translational modifications of these cytokine receptors may differ between the MBH and the AP.
In summary, in this study we examined the mechanism that may mediate the sex- and sex hormone-dependent ACTH responses to LPS in rats. We found that plasma levels of IL-1β, IL-6 and TNF-α, static concentrations of CRH and AVP in the MBH and those of ACTH in the AP, as well as IL-6 binding in the MBH and AP, do not play a role in the gonadal steroid modulation of ACTH secretion. Interestingly, however, the number of IL-1β and TNF-α binding sites in the MBH showed significant changes according to altered sex hormone milieu, in the same direction as the LPS-induced ACTH release. These results strongly suggest that the central sensitivity to peripheral IL-1β and TNF-α, the MBH Bmax of both of which can be modulated by sex hormones, may be an important mechanism underlying the sexually dimorphic ACTH response to endotoxin in rats.
Acknowledgments
This study was supported in part by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science to H.W. (nos 12671072 and 14571071), and from the International University of Health and Welfare to H.W.
REFERENCES
- Ban E, Milon G, Prudhomme N, Fillion G, Haour F. Receptors for interleukin-1 (α and β) in mouse brain: mapping and neuronal localization in hippocampus. Neuroscience. 1991;43:21–30. doi: 10.1016/0306-4522(91)90412-h. [DOI] [PubMed] [Google Scholar]
- Bohler HC, Zoeller RT, King JC, Rubin BS, Weber R, Merriam GR. Corticotropin releasing hormone mRNA is elevated on the afternoon of proestrus in the parvocellular paraventricular nuclei of the female rat. Mol Brain Res. 1990;8:259–262. doi: 10.1016/0169-328x(90)90025-9. [DOI] [PubMed] [Google Scholar]
- Bolton AE, Hunter WM. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973;133:529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broad KD, Keverne EB, Kendrick KM. Corticotropin releasing factor mRNA expression in the sheep brain during pregnancy, parturition and lactation and following exogenous progesterone and oestrogen treatment. Mol Brain Res. 1995;29:310–316. doi: 10.1016/0169-328x(94)00260-l. [DOI] [PubMed] [Google Scholar]
- Broadwell RD, Brightman MW. Entry of peroxidase into neurons of the central and peripheral nervous systems from extracerebral and cerebral blood. J Comp Neurol. 1976;166:257–283. doi: 10.1002/cne.901660302. [DOI] [PubMed] [Google Scholar]
- Chisari A, Carino M, Perone M, Gaillard RC, Spinedi E. Sex and strain variability in the rat hypothalamo-pituitary-adrenal (HPA) axis function. J Endocrinol Invest. 1995;18:25–33. doi: 10.1007/BF03349692. [DOI] [PubMed] [Google Scholar]
- Corchero J, Manzanares J, Fuentes JA. Role of gonadal steroids in the corticotropin-releasing hormone and proopiomelanocortin gene expression response to Delta (9)-tetrahydrocannabinol in the hypothalamus of the rat. Neuroendocrinology. 2001;74:185–192. doi: 10.1159/000054685. [DOI] [PubMed] [Google Scholar]
- Cornfield LJ, Sills MA. High affinity interleukin-6 binding sites in bovine hypothalamus. Eur J Pharmacol. 1991;202:113–115. doi: 10.1016/0014-2999(91)90263-p. [DOI] [PubMed] [Google Scholar]
- Cornish-Bowden A, Koshland DE. Diagnostic uses of the Hill (Logit and Nernst) plots. J Mol Biol. 1975;95:201–212. doi: 10.1016/0022-2836(75)90390-3. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Duetz W, Buijs RM, van Heerikhuize J, Vreeburg JT. Effects of androgens and estrogens on the vasopressin and oxytocin innervation of the adult rat brain. Brain Res. 1986;399:296–302. doi: 10.1016/0006-8993(86)91519-2. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Wang Z, Bullock NA, Numan S. Sex differences in the effects of testosterone and its metabolites on vasopressin messenger RNA levels in the bed nucleus of the stria terminalis of rats. J Neurosci. 1994;14:1789–1794. doi: 10.1523/JNEUROSCI.14-03-01789.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elisasser TH, Caperna TJ, Fayer R. Tumor necrosis factor-α affects growth hormone secretion by a direct pituitary interaction. Proc Soc Exp Biol Med. 1991;198:547–554. doi: 10.3181/00379727-198-43287. [DOI] [PubMed] [Google Scholar]
- Farrar WL, Kilian PL, Ruff MR, Hill JM, Pert CB. Visualization and characterization of interleukin 1 receptors in brain. J Immunol. 1987;139:459–463. [PubMed] [Google Scholar]
- Forsling ML. The influence of gonadal steroids on the release and actions of neurohypophysial hormones. Regul Pept. 1993;45:193–196. doi: 10.1016/0167-0115(93)90205-m. [DOI] [PubMed] [Google Scholar]
- Frederic F, Oliver C, Wollman E, Delhaye-Bouchaud N, Mariani J. IL-1 and LPS induce a sexually dimorphic response of the hypothalamo-pituitary-adrenal axis in several mouse strains. Eur Cytokine Netw. 1993;4:321–329. [PubMed] [Google Scholar]
- Freeman ME. The ovarian cycle of the rat. In: Knobil E, Neill J, editors. The Physiology of Reproduction. New York: Raven Press; 1988. pp. 1893–1928. [Google Scholar]
- Gadient RA, Otten U. Differential expression of interleukin-6 (IL-6) and interleukin-6 receptor (IL-6R) mRNAs in rat hypothalamus. Neurosci Lett. 1993;153:13–16. doi: 10.1016/0304-3940(93)90065-s. [DOI] [PubMed] [Google Scholar]
- Greer ER, Caldwell JD, Johnson MF, Prange AJ, Pedersen CA. Variations in concentration of oxytocin and vasopressin in the paraventricular nucleus of the hypothalamus during the estrous cycle in rats. Life Sci. 1986;38:2311–2318. doi: 10.1016/0024-3205(86)90638-7. [DOI] [PubMed] [Google Scholar]
- Haas DA, George S. Gonadal regulation of corticotropin-releasing factor immunoreactivity in hypothalamus. Brain Res Bull. 1988;20:361–367. doi: 10.1016/0361-9230(88)90065-2. [DOI] [PubMed] [Google Scholar]
- Hadid R, Spinedi E, Daneva T, Grau G, Gaillard RC. Repeated endotoxin treatment decreases immune and hypothalamo-pituitary-adrenal axis responses: Effects of orchidectomy and testosterone therapy. Neuroendocrinology. 1995;62:348–355. doi: 10.1159/000127024. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O'Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994;28:464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Haour FG, Ban EM, Milon GM, Baran D, Fillion GM. Brain interleukin 1 receptors: characterization and modulation after lipopolysaccharide injection. Prog Neuroendocrinimmunol. 1990;3:196–204. [Google Scholar]
- Kakizaki Y, Watanobe H, Kohsaka A, Suda T. Temporal profiles of interleukin-1β, interleukin-6, and tumor necrosis factor-α in the plasma and hypothalamic paraventricular nucleus after intravenous or intraperitoneal administration of lipopolysaccharide in the rat: Estimation by push-pull perfusion. Endocr J. 1999;46:487–496. doi: 10.1507/endocrj.46.487. [DOI] [PubMed] [Google Scholar]
- Katsuura G, Gottschall PE, Arimura A. Identification of a high-affinity receptor for interleukin-1beta in rat brain. Biochem Biophys Res Commun. 1988;156:61–67. doi: 10.1016/s0006-291x(88)80805-2. [DOI] [PubMed] [Google Scholar]
- Kinouchi K, Brown G, Pasternak G, Donner DB. Identification and characterization of receptors for tumor necrosis factor-α in the brain. Biochem Biophys Res Commun. 1991;181:1532–1538. doi: 10.1016/0006-291x(91)92113-x. [DOI] [PubMed] [Google Scholar]
- Kuhn C, Francis R. Gender difference in cocaine-induced HPA axis activation. Neuropsychopharmacol. 1997;16:399–407. doi: 10.1016/S0893-133X(96)00278-3. [DOI] [PubMed] [Google Scholar]
- Marquette C, Van Dan AM, Ban E, Laniece P, Crumeyrolle-Arias M, Fillion G, Berkenbosch F, Haour F. Rat interleukin-1β binding sites in rat hypothalamus and pituitary gland. Neuroendocrinology. 1995;62:362–369. doi: 10.1159/000127026. [DOI] [PubMed] [Google Scholar]
- Nappi RE, Bonneau MJ, Rivest S. Influence of the estrous cycle on c-fos and CRH gene transcription in the brain of endotoxin-challenged female rats. Neuroendocrinology. 1997;65:29–46. doi: 10.1159/000127162. [DOI] [PubMed] [Google Scholar]
- Ohmichi M, Hirota K, Koike K, Kurachi H, Ohtsuka S, Matsuzaki N, Yamaguchi M, Miyake A, Tanizawa O. Binding sites for interleukin-6 in the anterior pituitary gland. Neuroendocrinology. 1992;55:199–203. doi: 10.1159/000126115. [DOI] [PubMed] [Google Scholar]
- Olchovsky D, Song J, Gelato MC, Sherwood J, Spatola E, Bruno JF, Berelowitz M. Pituitary and hypothalamic insulin-like growth factor-I (IGF-I) and IGF-I receptor expression in food-deprived rats. Mol Cell Endocrinol. 1993;93:193–198. doi: 10.1016/0303-7207(93)90123-2. [DOI] [PubMed] [Google Scholar]
- Patchev VK, Almeida OF. Gonadal steroids exert facilitating and “buffering” effects on glucocorticoid-mediated transcriptional regulation of corticotropin-releasing hormone and corticosteroid receptor genes in rat brain. J Neurosci. 1996;16:7077–7084. doi: 10.1523/JNEUROSCI.16-21-07077.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patchev VK, Hayashi S, Orikasa C, Almeida OF. Implications of estrogen-dependent brain organization for gender differences in hypothalamo-pituitary-adrenal regulation. FASEB J. 1995;9:419–423. doi: 10.1096/fasebj.9.5.7896013. [DOI] [PubMed] [Google Scholar]
- Paulmyer-Lacroix O, Hery M, Pugeat M, Grino M. The modulatory role of estrogens on corticotropin-releasing factor gene expression in the hypothalamic paraventricular nucleus of ovariectomized rats: Role of the adrenal gland. J Neuroendocrinol. 1996;8:515–519. doi: 10.1046/j.1365-2826.1996.04835.x. [DOI] [PubMed] [Google Scholar]
- Rivier C. Stimulatory effect of interleukin-1 β on the hypothalamic-pituitary-adrenal axis of the rat: Influence of age, gender and circulating sex steroids. J Endocrinol. 1994;140:365–372. doi: 10.1677/joe.0.1400365. [DOI] [PubMed] [Google Scholar]
- Rivier C. Gender, sex steroids, corticotropin-releasing factor, nitric oxide, and the HPA response to stress. Pharmacol Biochem Behav. 1999;64:739–751. doi: 10.1016/s0091-3057(99)00148-3. [DOI] [PubMed] [Google Scholar]
- Roy BN, Reid RL, Van Vugt DA. The effects of estrogen and progesterone on corticotropin-releasing hormone and arginine vasopressin messenger ribonucleic acid levels in the paraventricular nucleus and supraoptic nucleus of the rhesus monkey. Endocrinology. 1999;140:2191–2198. doi: 10.1210/endo.140.5.6684. [DOI] [PubMed] [Google Scholar]
- Scatchard G. The attractions of proteins for small molecules and ions. Ann NY Acad Sci. 1949;51:660–672. [Google Scholar]
- Schöbitz B, Voorhuis DA, De Kloet ER. Localization of interleukin 6 mRNA and interleukin 6 receptor mRNA in rat brain. Neurosci Lett. 1992;136:189–192. doi: 10.1016/0304-3940(92)90046-a. [DOI] [PubMed] [Google Scholar]
- Spinedi E, Gaillard RC, Chisari A. Sexual dimorphism of neuroendocrine-immune interactions. In: Gaillard RC, editor. Neuroendocrine-Immune Interactions. Basel, Switzerland: Karger; 2002. pp. 91–107. [DOI] [PubMed] [Google Scholar]
- Spinedi E, Salas M, Chisari A, Perone M, Carino M, Gaillard RC. Sex differences in the hypothalamo-pituitary-adrenal axis response to inflammatory and neuroendocrine stressors. Neuroendocrinology. 1994;60:609–617. doi: 10.1159/000126804. [DOI] [PubMed] [Google Scholar]
- Spinedi E, Suescun MO, Hadid R, Daneva T, Gaillard RC. Effects of gonadectomy and sex hormone therapy on the endotoxin-stimulated hypothalamo-pituitary-adrenal axis: Evidence for a neuroendocrine-immunological sexual dimorphism. Endocrinology. 1992;131:2430–2436. doi: 10.1210/endo.131.5.1330501. [DOI] [PubMed] [Google Scholar]
- Takenaka A, Takahashi S, Noguchi T. Effect of protein nutrition on insulin-like growth factor-I (IGF-I) receptor in various tissues of rats. J Nutr Sci Vitaminol (Tokyo) 1996;42:347–357. doi: 10.3177/jnsv.42.347. [DOI] [PubMed] [Google Scholar]
- Turnbull AV, Rivier CL. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiol Rev. 1999;79:1–71. doi: 10.1152/physrev.1999.79.1.1. [DOI] [PubMed] [Google Scholar]
- Vamvakopoulos NC, Chrousos GP. Evidence of direct estrogenic regulation of human corticotropin-releasing hormone gene expression. Potential implications for the sexual dimorphism of the stress response and immune/inflammatory reaction. J Clin Invest. 1993;92:1896–1902. doi: 10.1172/JCI116782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen FW, Caffe AR, De Vries GJ. Vasopressin cells in the bed nucleus of the stria terminalis of the rat: sex differences and the influence of androgens. Brain Res. 1985;325:391–394. doi: 10.1016/0006-8993(85)90348-8. [DOI] [PubMed] [Google Scholar]
- Van Tol HH, Bolwerk EL, Liu B, Burbach JP. Oxytocin and vasopressin gene expression in the hypothalamo-neurohypophyseal system of the rat during the estrous cycle, pregnancy, and lactation. Endocrinology. 1988;122:945–951. doi: 10.1210/endo-122-3-945. [DOI] [PubMed] [Google Scholar]
- Viau V, Chu A, Soriano L, Dallman MF. Independent and overlapping effects of corticosterone and testosterone on corticotropin-releasing hormone and arginine vasopressin mRNA expression in the paraventricular nucleus of the hypothalamus and stress-induced adrenocorticotropic hormone release. J Neurosci. 1999;19:6684–6693. doi: 10.1523/JNEUROSCI.19-15-06684.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viau V, Meaney MJ. The inhibitory effect of testosterone on hypothalamic-pituitary-adrenal responses to stress is mediated by the medial preoptic area. J Neurosci. 1996;16:1866–1876. doi: 10.1523/JNEUROSCI.16-05-01866.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, De Vries GL. Androgen and estrogen effects on vasopressin messenger RNA expression in the medial amygdaloid nucleus in male and female rats. J Neuroendocrinol. 1995;7:827–831. doi: 10.1111/j.1365-2826.1995.tb00722.x. [DOI] [PubMed] [Google Scholar]
- Watanobe H. Sexual dimorphism in the pituitary-adrenal response to tumor necrosis factor-α, but not to interleukin-6, in the rat. Brain Res Bull. 2002;57:151–155. doi: 10.1016/s0361-9230(01)00735-3. [DOI] [PubMed] [Google Scholar]
- Watanobe H, Anzai J, Nigawara T, Habu S, Takebe K. Effects of gender and gonadectomy on ACTH response to interleukin-1β in the rat: Comparison with the modulation of ACTH response to immobilization stress. Neuroimmunomodulation. 1996;3:254–258. doi: 10.1159/000097279. [DOI] [PubMed] [Google Scholar]
- Watanobe H, Sasaki S, Takebe K. Evidence that intravenous administration of interleukin-1 stimulates corticotropin-releasing hormone secretion in the median eminence of freely moving rats: Estimation by push-pull perfusion. Neurosci Lett. 1991;133:7–10. doi: 10.1016/0304-3940(91)90044-t. [DOI] [PubMed] [Google Scholar]
- Watanobe H, Schiöth HB. Postnatal profile of plasma leptin concentrations in male and female rats: Relation with the maturation of the pituitary-gonadal axis. Regul Pept. 2002;105:23–28. doi: 10.1016/s0167-0115(01)00370-6. [DOI] [PubMed] [Google Scholar]
- Watanobe H, Takebe K. Role of postnatal gonadal function in the determination of thyrotropin (TSH) releasing hormone-induced TSH response in adult male and female rats. Endocrinology. 1987a;120:1711–1718. doi: 10.1210/endo-120-5-1711. [DOI] [PubMed] [Google Scholar]
- Watanobe H, Takebe K. Involvement of postnatal gonads in the maturation of dopaminergic regulation of prolactin secretion in male rats. Endocrinology. 1987b;120:2205–2211. doi: 10.1210/endo-120-6-2205. [DOI] [PubMed] [Google Scholar]
- Watanobe H, Takebe K. Involvement of postnatal gonads in the maturation of dopaminergic regulation of prolactin secretion in female rats. Endocrinology. 1987c;120:2212–2219. doi: 10.1210/endo-120-6-2212. [DOI] [PubMed] [Google Scholar]
- Watanobe H, Takebe K. Intravenous administration of tumor necrosis factor-α stimulates corticotropin releasing hormone secretion in the push-pull cannulated median eminence of freely moving rats. Neuropeptides. 1992;22:81–84. doi: 10.1016/0143-4179(92)90058-5. [DOI] [PubMed] [Google Scholar]
- Watanobe H, Takebe K. Intrahypothalamic perfusion with interleukin-1-beta stimulates the local release of corticotropin-releasing hormone and arginine vasopressin and the plasma adrenocorticotropin in freely moving rats: A comparative perfusion of the paraventricular nucleus and the median eminence. Neuroendocrinology. 1993;57:593–599. doi: 10.1159/000126412. [DOI] [PubMed] [Google Scholar]
- Watanobe H, Takebe K. Effects of intravenous administration of interleukin-1-beta on the release of prostaglandin E2, corticotropin-releasing factor, and arginine vasopressin in several hypothalamic areas of freely moving rats: Estimation by push-pull perfusion. Neuroendocrinology. 1994;60:8–15. doi: 10.1159/000126714. [DOI] [PubMed] [Google Scholar]
- Wolvers DA, Marquette C, Berkenbosch F, Haour F. Tumor necrosis factor-α: specific binding sites in rodent brain and pituitary gland. Eur Cytokine Netw. 1993;4:377–381. [PubMed] [Google Scholar]
- Yabuuchi K, Minami M, Katsumata S, Satoh M. Localization of type I interleukin-1 receptor mRNA in the rat brain. Mol Brain Res. 1994;27:27–36. doi: 10.1016/0169-328x(94)90180-5. [DOI] [PubMed] [Google Scholar]


