Abstract
We investigated long-term cardiovascular effects in the offspring of sheep exposed to prenatal dexamethasone (DM). We assessed in vitro vascular responsiveness and evaluated endothelial nitric oxide synthase (eNOS) message and protein levels in femoral muscle removed from 5-month-old sheep. Dexamethasone was administered i.m. to pregnant ewes as 3 weekly courses (4 × 2 mg at 12 h intervals), starting on day 103 of gestation (term ≈149 days). Ewes were allowed to lamb. At 5 months of age a carotid catheter was placed for blood pressure measurement and hamstring muscle was removed from the lambs under general anaesthesia. We demonstrate that following prenatal DM exposure in the 5-month-old offspring: (1) blood pressure is unchanged; (2) as previously reported in the fetus, sensitivity to endothelin-1 (ET) is increased; (3) acetylcholine-induced relaxation is increased; (4) l-NAME suppressible vasodilatory response to ET is abolished; (5) there is no change in endothelium-independent vasodilatation; and (6) there is no change in eNOS RNA and protein levels, when compared to saline treated controls. We speculate that decreased agonist-induced NO release is not due to alteration in gene expression, but is more likely to be a post-transcriptional event. In summary, the lack of a difference in resting mean arterial pressure (MAP) between DM and control lambs indicates that the compensation we have previously demonstrated in the fetus following glucocorticoid exposure persists to 5 months postnatal age. Compensation is likely due to non-NO-dependent mechanisms, since no evidence was found of upregulated NOS.
Several studies, conducted in different species, indicate that exposure to glucocorticoids in utero at levels higher than appropriate for the specific stage of embryonic or fetal maturation acutely causes an increase in fetal blood pressure (Derks et al. 1997; Anwar et al. 1999; Docherty et al. 2001a; Koenen et al. 2002), and in the longer term causes fetal growth retardation in rats (Benediktsson et al. 1993), sheep (Jobe et al. 1998), non-human primates (Novy & Walsh, 1983), and also humans (French et al. 1999). There is accumulating evidence from rat studies that glucocorticoid exposure at higher than normal endogenous levels in utero leads to altered cardiovascular function in adult life (Benediktsson et al. 1993; Levitt et al. 1996; Langley-Evans, 1997). To date, these findings have not been substantially replicated in other larger mammals. One study of sheep exposed at a very early stage of development to a short course of DM reported an elevation of blood pressure in postnatal life that persists to 5 years of age (Dodic et al. 1999). A second study, also in the sheep, found that blood pressure was slightly reduced in young lambs following fetal exposure to betamethasone, and was not different from that of controls at 1 year of age (Moss et al. 2001). Given that the betamethasone group in this study also received progesterone to prevent premature labour, it is difficult to draw generalized conclusions from the data given. There are no studies on peripheral vascular function following antenatal glucocorticoids. This is important since the major determinants of systemic blood pressure are the small resistance arteries (Christensen & Mulvany, 2001).
We have previously shown that DM elevates MAP, reduces body weight, increases vascular responses to endothelin-1 (ET) and recruits compensatory mechanisms in fetal sheep (Derks et al. 1997; Anwar et al. 1999; Docherty et al. 2001a; Molnar et al. 2002). In the present study, our goal was to investigate persistent effects of DM administered to pregnant sheep at a stage equivalent to the therapy used for women in preterm labour on the peripheral vasculature in the offspring (NIH Consensus Development Panel, 1995). Because ET mediates vasodilatation via NO production following binding to the endothelin B (ET-B) receptor, we have evaluated in vitro vascular responses and eNOS RNA and protein levels in the femoral vasculature from 5-month-old offspring of sheep exposed to three weekly courses of DM in utero. We hypothesized that repeated maternal administration of DM, used at a gestational age similar to that at which treatment is recommended in human pregnancy, has long-term effects on NO-mediated vasodilatation and vasoconstrictor responsiveness to ET in the femoral vasculature of the offspring.
METHODS
Animals
Rambouillet-Columbia crossbred ewes (Ovis aries) carrying single fetuses of known gestational age were studied. The experimental protocol was approved by the Institutional Animal Care and Use Committee at Cornell University. All facilities were approved by the American Association for the Accreditation of Laboratory Animal Care.
Prenatal dexamethasone treatment
Dexamethasone (2 mg; n = 6) or saline vehicle (n = 6) was administered i.m. to pregnant ewes as courses of four injections at 12 h intervals as described earlier (Molnar et al. 2002). Briefly, three weekly courses were given, starting on day 103 (0.7 gestation). The dose of DM was chosen following trials in which 2, 4 and 6 mg was administered without progesterone coverage. Delivery within 48 h of receiving the first four-injection course occurred in 84 and 100 % of animals in the 4 and 6 mg groups, respectively (n = 6 per group). All animals in the 2 mg group delivered at term. We have previously shown that the administration of a course of 2 mg DM increases fetal blood pressure with each injection and that the increases in blood pressure to the first course at 103–105 days gestation (dGA; 23 %), and to the second course at 110–112 dGA (30 %) (Koenen et al. 2001) were similar to that induced by direct infusion of DM to the fetus, which achieves an increase of 24 % (Derks et al. 1997). A 2 mg dose is one-third of the dose that women at risk of premature delivery receive to mature the fetal lung and corresponds to about 30 µg kg−1 on a weight-adjusted basis (NIH Consensus Development Panel, 1995).
After receiving prenatal DM or vehicle alone, ewes were allowed to lamb. At 4 months of age lambs underwent surgery to exteriorize the right carotid artery to allow subsequent percutaneous insertion of a carotid arterial catheter. Surgery was performed under isoflourane general anaesthesia using techniques previously described in details (Nathanielsz et al. 1980). Briefly, sheep were pretreated with 1 g ketamine and 1 mg glycopyrrolate i.m. and anaesthesia was induced with 4 % isofluorane for 4 min prior to tracheal intubation. Animals were allowed 7 days post-operative recovery. Lambs were returned to the farm after carotid plate placement surgery. At 5 months of age lambs (body weight: CTR: 30.7 ± 2.4 kg vs. DM: 31.5 ± 1.5 kg) were instrumented with percutaneous polyvinyl catheters inserted into the carotid artery and jugular vein. In addition a femoral flow probe (Transonics Inc., Ithaca, NY) was placed around the left femoral artery for blood flow measurements. On the seventh day post-surgery MAP was recorded for 60 min starting at 10:00 h using a pressure transducer (Cobe, Arvada, CO, USA) connected to the arterial catheter. Femoral arterial blood flow was recorded with an ultrasonic flow probe. Data were recorded using a data acquisition system (WinDAQ Pro, Dataq Instruments, Inc., Akron, OH, USA) at 20 Hz. MAP and femoral vascular resistance (FVR = MAP over femoral arterial blood flow) were calculated using the pressure and flow waveforms. Lambs were returned to the farm after the studies.
Tissue collection
At 5 months of age a hamstring muscle biopsy (1–2 cm3) obtained under general anaesthesia was used for in vitro myography, real-time PCR and immunohistochemistry.
Wire myography
Preparation of arteries
The muscles were immediately placed in ice-cold physiological salt solution (PSS). Vessels with intact endothelium with similar dimensions (length, CTR: 1.91 ± 0.03 mm vs. DM: 1.7 ± 0.09 mm; internal diameter, CTR: 374.4 ± 25 µm vs. DM: 371 ± 36 µm) were mounted on a small vessel wire myograph as described earlier (Molnar et al. 2002) (Danish MyoTechnology, Åarhus, Denmark). Vessels were bathed in PSS at 37 °C and aerated continuously with 5 % CO2-95 % O2 to achieve a pH of 7.4. The arteries were set to a predetermined internal circumference, which has been shown to be the optimum calibre for studies of the peripheral vasculature (Docherty et al. 2001b).
Protocol
The startup protocol and evaluation of vessel viability were conducted as described previously (Anwar et al. 1999). Briefly, each vessel was stimulated with 5 µm noradrenalin (NA), and 125 mm KCl in PSS, or a combination of both. Concentration response curves (CRCs) were performed to the ET (10−11 to 10−6m) in the absence and the presence of the NOS inhibitor Nω-nitro-l-arginine methylester (l-NAME; 100 µm) and to the endothelium-dependent acetylcholine (ACh; 10−9 to 10−5m); and to the endothelium-independent NO donor sodium nitroprusside (SNP; 10−10 to 10−5m). Responses to vasodilator agents were determined following a stable contraction to 5 µm NA.
Drugs
All PSS reagents and NA (A-9512), l-NAME (N-5751), ACh (A-6500) and SNP (S-0501) were acquired from Sigma Chemical (St Louis, MO, USA). Endothelin-1 (H-6995) was obtained from Bachem (Torrance, CA, USA). Stock solutions were made up in distilled H2O. Endothelin-1 stock solution was dissolved in ethanol. Stock solutions were frozen as aliquots at −20 °C and thawed as required. Dilutions were made in PSS on the day of each experiment and kept on ice before use. Dexamethasone (Azium) was obtained from Schering, NY, USA.
Immunohistochemistry
Femoral muscle tissue was immediately placed in 10 % buffered formalin phosphate. Paraffin sections (6 µm) were serially mounted onto Superfrost Plus slides (Fisher Scientific, Fair Lawn, NJ, USA) and stained using the Vecta Stain Elite peroxidase/ diaminobenzidine method (Hsu et al. 1981). Briefly, slides were cleaned of paraffin and rehydrated. Sections were blocked for endogenous peroxidase with 0.5 % peroxidase in methanol. Sections were incubated in the primary antibody (1:300; mouse monoclonal anti-eNOS: N30020, Transduction Laboratories, Lexington, KY, USA) overnight at 4 °C. The biotinylated secondary antibody and avidin-biotin complex (Vector Lab, Burlingame, CA, USA) were applied for 60 min each. Slides were incubated in diaminobenzidine chromogen solution to visualize antibody binding. Light Green counter-stain was used at 1:8 dilution for 90 s. Sections were dehydrated in ethanol and coverslipped for light microscopy. Each group was subjected to the same conditions.
Image acquisition and staining intensity analysis
Images were acquired with a Nikon Eclipse E600 microscope with Spot software (Diagnostic Instruments, Inc.) at one time, under the same conditions. Intensity of eNOS-positive staining was evaluated with SimplePCI software (Compix Inc., Imaging Systems, Cranberry Township, PA, USA). Three vessels of equal size (100–400 µm diameter) per animal at ×20 magnification were analysed in a blind fashion.
Real-time reverse transcription polymerase chain reaction (RT-PCR)
RNA isolation
Total RNA was isolated from snap-frozen tissue. Tissue samples of femoral muscle were homogenized in RNA-Stat60 (Tel-Test, Friendswood, TX, USA) and RNA was isolated according to the manufacturer's instructions. RNA concentration and quality were determined spectrophotometrically at 260 and 280 nm. Samples were run on a 2% agarose gel to check the integrity of RNA.
RT-PCR
RNA was assayed using a one-step RT-PCR kit (TaqMan; Heid et al. 1996). RT-PCR was conducted on an Applied Biosystem 7700 Sequence Detector using fluorescence-labelled internal probes for the genes of interest and factor-VIII as an endogenous control, for the relative comparison between treated and non-treated groups. Total RNA (100 ng) was reverse transcribed in a 25 µl reaction containing 1 × TaqMan One-Step RT-PCR Master Mix Reagents Kit, forward and reverse primers (50 nm) and TaqMan probe (250 nm). Probes were obtained from Applied Biosystems (Foster City, CA, USA). All primers were obtained from Sigma Genosys (Woodlands, TX, USA). ABI Prism Primer Express software was used to design the optimal TaqMan probe and primer combinations (eNOS accession number: AF201926; factor VIII: AF180523). The primers used were: factor-VIII 5′-pol (5′GTTTCCTCCCAGAGTGCCCTA3′) and 3′ pol (5′5'TGGCGATGTTAAAAAGTTGATCC3′); eNOS 5′-pol (5′ACAGCTTTCAGCTCGCCAAG3′) and 3′-pol (5′CTTCTGCTCGTTTTCCAGGTG3′). A fluorescent probe, which contained a Fam reporter molecule attached to the 5′ end and a Tamra quencher linked at the 3′ end was used: factor-VIII 5′CATCAGTCATGTACAGAAGACTGTGTTTGTAGAGTCA3′; eNOS 5′CACGCCGCCACAGTGTCTTCAT3′. For each reaction the amount of fluorescence was detected as the function of the quantity of a reporter dye (Fam) that was released. A validation experiment was performed demonstrating equal efficiencies of the gene of interest and endogenous control amplification over different initial template concentrations. For the relative quantification of gene expression the comparative threshold cycle (CT) method was employed as described in User Bulletin 2 for ABI Prism 7700 Sequence Detection System). CT represents the PCR cycle at which an increase in reporter fluorescence above a background signal can first be detected (10 times the standard deviation of the baseline). First, endogenous control CT values were subtracted from the gene of interest CT values to derive a ΔCT value. The relative expression of the gene of interest was then evaluated using the expression 2-ΔΔCT, where the value for ΔΔCT was obtained by subtracting the ΔCT of the calibrator from each ΔCT, using the mean of the saline control as the calibrator. All samples were assayed in separate tubes in one run together with the endogenous control.
Data analysis
Data are represented as a mean ± standard error of the mean (s.e.m.; n = 6 in each group throughout). Significance was assessed by Student's two-tailed t test for parametric data at P < 0.05. Wall tension is expressed as millinewtons per millimetre of artery length. Sensitivity to the agonist is expressed as the negative log of the effective concentration required to produce 50 % of maximum effect. Sensitivity was calculated from each CRC by fitting the Hill equation using Prism (GraphPad Software, San Diego, CA, USA). The experimenter was unaware of treatment groups during data collection.
RESULTS
Blood pressure
There was no significant difference in resting MAP between saline (CTR) and DM-exposed groups (CTR, 77 ± 3.8 vs. DM, 80 ± 2.7 mmHg; P = 0.14). Power analysis revealed that we would need ≈300 more animals in each group, assuming similar standard deviations and an 80 % chance of detecting a difference at α = 0.05.
Femoral vascular resistance
There was no significant difference in FVR (CTR, 1.23 ± 0.03 vs. DM, 1.24 ± 0.03 mmHg (ml min−1)−1 between the groups.
Constrictor responses
Tension developed in response to 5 µm NA (CTR, 2.37 ± 0.69 vs. DM, 1.74 ± 0.19 mN mm−1) or 125 mm potassium (CTR, 4.63 ± 0.48 vs. DM, 4.94 ± 0.5 mN mm−1) and was not different between groups. All arteries produced concentration-dependent vasoconstriction to ET. The maximum tension developed to ET was similar in vessels from saline- and DM-exposed animals (CTR, 4.92 ± 0.09 vs. DM, 4.99 ± 0.24 mN mm−1). Sensitivity to ET was enhanced in the DM-exposed group (CTR, 8.21 ± 0.01 vs. DM, 8.35 ± 0.04; P = 0.006; Fig. 1). Following 1 h incubation of one vessel in each group with 100 µm l-NAME, a competitive inhibitor of NOS, an ET CRC was constructed and compared with a CRC to ET performed on a separate vessel without l-NAME preincubation (higher concentrations of l-NAME did not produce greater inhibition in preliminary experiments). l-NAME had no effect on basal tone in either group. Blocking NO production by l-NAME increased sensitivity to ET in controls. In contrast l-NAME did not change ET sensitivity in the DM-exposed group (Fig. 1).
Figure 1. Effect of l-NAME on endothelin-1 (ET) sensitivity.
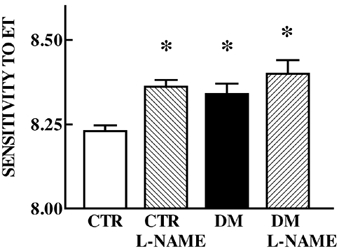
Sensitivity to ET without or with l-NAME (hatched bars) preincubation in femoral arteries of 5-month-old offspring of saline (CTR, open bar, n = 6) and dexamethasone-exposed (DM, filled bar, n = 6) sheep. Mean ± s.e.m.; * different from CTR, P < 0.05.
Relaxation responses
Tension developed to 5 µm NA prior to relaxation was similar in vessels from CTR- and DM-exposed animals.
Endothelium-dependent relaxation
Acetylcholine produced concentration-dependent relaxation. Relaxation to ACh was greater in arteries from DM-exposed animals both in terms of maximal relaxation (CTR, 67 ± 9.6 vs. DM, 93 ± 6.3 %) and slope of the relaxation curves (CTR, −0.38 ± 0.1 vs. DM, −0.96 ± 0.15) (Fig. 2A).
Figure 2. Relaxation to acetylcholine (ACH; A) and sodium nitroprusside (SNP; B).
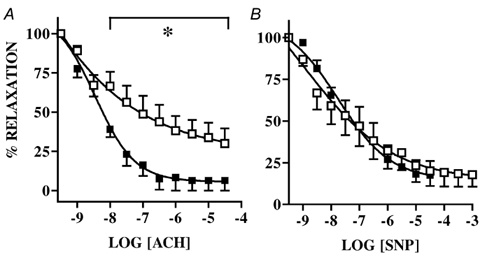
Relaxation to ACH and SNP in femoral arteries of 5-month-old offspring of saline (□, n = 6) and dexamethasone-exposed (▪, n = 6) sheep. Mean ± s.e.m.; *P < 0.05.
Endothelium-independent relaxation
There was no difference in response to SNP (Fig. 2B).
Anti-eNOS immunohistochemistry
Anti-eNOS staining was found in the endothelium of blood vessels. The pattern of staining was not different between groups and there was no difference in staining intensity relative to background (CTR, 146.45 ± 2.6 vs. DM, 152.3 ± 2.2).
RT-PCR
There was no difference in eNOS message from femoral muscle of control and DM-exposed animals (CTR, 1.1 ± 0.2 vs. DM, 1.8 ± 0.6).
DISCUSSION
In the present study we have demonstrated that exposure of the fetus to three courses of DM administered to the pregnant ewe at weekly intervals has persistent effects on the peripheral vasculature of the offspring at 5 months of age. Using the technique of small vessel wire myography, we have previously shown that DM administered in this manner results in an increase in the sensitivity developed in response to ET in the fetus (Molnar et al. 2002). In this paper we show that this effect of DM persists at least to 5 months of age and to our knowledge is the first demonstration of the programming effect of prenatal glucocorticoids on postnatal vascular function. Similar to our observations in the fetus (Molnar et al. 2002), when NOS is inhibited with l-NAME the vasoconstrictor effect of ET is enhanced in CTR but not in treated animals at 5 months postnatal age. In other words, DM permanently decreased ET-B receptor-mediated vasodilatation that occurs via the NOS system. Acetylcholine-induced relaxation was increased in arteries from offspring of DM-exposed sheep but not in the CTR group. There was no change in response to the NO donor SNP. Synthesis of eNOS demonstrated by immunostaining and RT-PCR was not altered by prior DM exposure.
Endothelial cells play a key role in cardiovascular regulation by producing a number of potent vasoactive agents, including NO the most potent vasodilator (Furchgott & Zawadzki, 1980) and ET the most potent vasoconstrictor (Clozel et al. 1993). In vitro studies have suggested close interaction between ET and NO (Cao et al. 1994; Ikeda et al. 1997), suggesting that imbalance between the two may contribute to an alteration in vascular tone characteristic of hypertension. Endothelin-1 binding to ET-A receptors on vascular smooth muscle leads to vasoconstriction (Rubanyi & Polokoff, 1994). ET-B receptors are expressed on endothelial cells and to a much lesser extent on vascular smooth muscle cells (Haynes et al. 1995) and result in vasodilatation by increasing the production of NO. Blocking NO production augments the vasoconstrictor property of ET (Lerman et al. 1992). Nitric oxide inhibits the synthesis of ET both in vivo and in cultured endothelial cells. Furthermore, NO facilitates dissociation of ET and its receptor-ligand complex, thereby terminating the effects of ET. It also shortens the duration of contraction by accelerating the return of intracellular Ca2+ to its basal concentration (Rubanyi & Polokoff, 1994). A dysfunction of the vascular endothelium has been implicated in the pathophysiology of a number of cardiovascular diseases, particularly essential hypertension (Ferro & Webb, 1997). Endothelial cell dysfunction brought on by glucocorticoids may alter the response of blood vessels to circulating endocrine factors and pharmacological agents that, in turn, contribute to increased peripheral resistance and raised blood pressure.
We used wire myography to evaluate agonist-induced NOS activity. Wire myography is a powerful tool for evaluating in vitro vascular resistance of arterial function for two major reasons. First, it demonstrates functional performance of the vessels in a way that is not done by traditional techniques. Second, by isolating the vessel from in vivo homeostatic responses the direct effects of agonists on vascular responsiveness can be evaluated. We compared ET responses in the absence and presence of a NOS inhibitor, l-NAME. Blocking NOS had no effect on basal vascular tone in either group. As we have previously shown in the fetus, sensitivity to ET increased in the presence of l-NAME in controls but not in the DM-exposed group, indicating blunted ET-induced NOS activity following DM exposure.
Acetylcholine is a common agonist used to evaluate endothelium-dependent relaxation. In many cases it is a good measure of NO production as well. However, some authors report that ACh relaxation is not solely NO mediated. In both wild-type and eNOS-knockout mice, prostaglandins are the main mediators of the ACh-induced vasodilatation (Godecke et al. 1998). This appears to be the case in our animals. We have previously concluded that NO synthesis in the ovine femoral vasculature is not the predominant pathway mediating vasodilatation in response to ACh. In this study, ACh-induced relaxation was increased in arteries from DM-exposed sheep. We know that differences in NO sensitivity are not contributing to the observed effect, since exposing the same vessels to the NO donor SNP produced the same vasodilatation. In other words, DM exposure upregulated endothelium-dependent vasorelaxant activity without altering responsiveness to NO donors. We conclude that NOS-independent pathways (e.g. prostacyclins) are upregulated after prior prenatal DM exposure at 5 months of postnatal age. Mean blood pressure was elevated by 3 mmHg in the animals exposed to glucocorticoid as fetuses but was not significant (P = 0.14). The lack of effect of DM exposure on systemic blood pressure possibly reflects homeostatic compensatory mechanisms that we hypothesize will break down as the animal ages leading to hypertension. Interestingly, some studies report temporary hypotension at 3 months after prenatal maternal betamethasone administration (Moss et al. 2001), perhaps indicative of upregulated vasodilator activity and an altered trajectory in the development of postnatal blood pressure.
The major source of NO in the peripheral vasculature is the endothelial isoform (eNOS). NOS regulation is under tight and complicated control, involving gene transcription, post-translational modification, intracellular localization and activation (Papapetropoulos et al. 1999). Since downregulated eNOS synthesis is a well-documented component in endothelial dysfunction we would have predicted that both eNOS gene expression and protein would have been decreased in our model. However, we did not find any differences in eNOS gene expression or protein levels. It therefore seems likely that the glucocorticoid-induced blunted NO production is a post-translational event. Mattei et al. (1997) suggested that impairment in the basal release of NO is probably secondary to hypertension, while the alteration in agonist-induced endothelium-dependent vasodilatation seems to be the primary defect caused both by an alteration of the l-arginine-NO pathway and the production of endothelium-dependent constrictor factors (Mattei et al. 1997). We conclude that prenatal DM exposure permanently decreased NOS activity, which eventually would increase postnatal susceptibility to hypertension.
In conclusion, we have demonstrated that three courses of DM repeated at weekly intervals between 0.7 and 0.8 % of gestation in sheep, and using a lower dose than is used in human pregnancy, have permanent effects on femoral vascular function in the offspring. Sensitivity to ET increased in the presence of l-NAME in controls but not in the DM-exposed group, indicating blunted ET-induced NO synthesis following DM exposure. The lack of difference in anti-eNOS staining and message indicates that the ET-induced NO production is presumably a post-translational event rather than a result of decreased eNOS synthesis. We have also shown increased relaxation to ACh in the DM-exposed group. Since response to SNP was unchanged we conclude that DM exposure upregulates endothelium-dependent vasorelaxant activity without altering responsiveness to NO donors. There were no differences in resting MAP or FVR between DM-exposed animals and controls at 5 months of age. We speculate that later in life, there is a limited ability to increase compensatory vasodilatation perhaps similar to the exhaustion atrophy seen in other systems that are hyperactive for a long period. As a result vascular tone increases and hypertension supervenes.
Acknowledgments
This work was supported by NIH grants: HL21350 and HL55416. D. C. Howe was in receipt of a SHERT travelling fellowship. We thank X.-Y. Ding and S. V. Koenen for their helpful assistance with the animal preparations.
REFERENCES
- Anwar MA, Schwab M, Poston L, Nathanielsz PW. Betamethasone-mediated vascular dysfunction and changes in hematological profile in the ovine fetus. Am J Physiol. 1999;276:H1137–1143. doi: 10.1152/ajpheart.1999.276.4.H1137. [DOI] [PubMed] [Google Scholar]
- Benediktsson R, Lindsay RS, Noble J, Seckl JR, Edwards CR. Glucocorticoid exposure in utero: new model for adult hypertension. Lancet. 1993;341:339–341. doi: 10.1016/0140-6736(93)90138-7. [DOI] [PubMed] [Google Scholar]
- Cao WB, Zeng ZP, Zhu YJ, Luo WC, Cai BQ. Inhibition of nitric oxide synthesis increases the secretion of endothelin-1 in vivo and in cultured endothelial cells. Chin Med J (Engl) 1994;107:822–826. [PubMed] [Google Scholar]
- Christensen KL, Mulvany MJ. Location of resistance arteries. J Vasc Res. 2001;38:1–12. doi: 10.1159/000051024. [DOI] [PubMed] [Google Scholar]
- Clozel M, Breu V, Burri K, Cassal JM, Fischli W, Gray GA, Hirth G, Loffler BM, Muller M, Neidhart W. Pathophysiological role of endothelin revealed by the first orally active endothelin receptor antagonist. Nature. 1993;365:759–761. doi: 10.1038/365759a0. [DOI] [PubMed] [Google Scholar]
- Derks JB, Giussani DA, Jenkins SL, Wentworth RA, Visser GH, Padbury JF, Nathanielsz PW. A comparative study of cardiovascular, endocrine and behavioural effects of betamethasone and dexamethasone administration to fetal sheep. J Physiol. 1997;499:217–226. doi: 10.1113/jphysiol.1997.sp021922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty CC, Kalmar-Nagy J, Engelen M, Koenen SV, Nijland M, Kuc RE, Davenport AP, Nathanielsz PW. Effect of in vivo fetal infusion of dexamethasone at 0. 75 GA on fetal ovine resistance artery responses to ET-1. Am J Physiol Regul Integr Comp Physiol. 2001a;281:R261–268. doi: 10.1152/ajpregu.2001.281.1.R261. [DOI] [PubMed] [Google Scholar]
- Docherty CC, Kalmar-Nagy J, Engelen M, Nathaniels PW. Development of fetal vascular responses to endothelin-1 and acetylcholine in the sheep. Am J Physiol Regul Integr Comp Physiol. 2001b;280:R554–562. doi: 10.1152/ajpregu.2001.280.2.R554. [DOI] [PubMed] [Google Scholar]
- Dodic M, Peers A, Coghlan JP, May CN, Lumbers E, Yu Z, Wintour EM. Altered cardiovascular haemodynamics and baroreceptor-heart rate reflex in adult sheep after prenatal exposure to dexamethasone. Clin Sci (Colch) 1999;97:103–109. [PubMed] [Google Scholar]
- Ferro CJ, Webb DJ. Endothelial dysfunction and hypertension. Drugs. 1997;53(suppl. 1):30–41. doi: 10.2165/00003495-199700531-00006. [DOI] [PubMed] [Google Scholar]
- French NP, Hagan R, Evans SF, Godfrey M, Newnham JP. Repeated antenatal corticosteroids: size at birth and subsequent development. Am J Obstet Gynecol. 1999;180:114–121. doi: 10.1016/s0002-9378(99)70160-2. [DOI] [PubMed] [Google Scholar]
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Godecke A, Decking UK, Ding Z, Hirchenhain J, Bidmon HJ, Godecke S, Schrader J. Coronary hemodynamics in endothelial NO synthase knockout mice. Circ Res. 1998;82:186–194. doi: 10.1161/01.res.82.2.186. [DOI] [PubMed] [Google Scholar]
- Haynes WG, Strachan FE, Webb DJ. Endothelin ETA and ETB receptors cause vasoconstriction of human resistance and capacitance vessels in vivo. Circulation. 1995;92:357–363. doi: 10.1161/01.cir.92.3.357. [DOI] [PubMed] [Google Scholar]
- Heid CA, Stevens J, Livak KJ, Williams PM. Real time quantitative PCR. Genome Res. 1996;6:986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- Hs SM, Raine L, Fanger H. The use of antiavidin antibody and avidin-biotin-peroxidase complex in immunoperoxidase technics. Am J Clin Pathol. 1981;75:816–821. doi: 10.1093/ajcp/75.6.816. [DOI] [PubMed] [Google Scholar]
- Ikeda U, Yamamoto K, Maeda Y, Shimpo M, Kanbe T, Shimada K. Endothelin-1 inhibits nitric oxide synthesis in vascular smooth muscle cells. Hypertension. 1997;29:65–69. doi: 10.1161/01.hyp.29.1.65. [DOI] [PubMed] [Google Scholar]
- Jobe AH, Wada N, Berry LM, Ikegami M, Ervin MG. Single and repetitive maternal glucocorticoid exposures reduce fetal growth in sheep. Am J Obstet Gynecol. 1998;178:880–885. doi: 10.1016/s0002-9378(98)70518-6. [DOI] [PubMed] [Google Scholar]
- Koenen SV, Mecenas CA, Smith GS, Jenkins S, Nathanielsz PW. Effects of maternal betamethasone administration on fetal and maternal blood pressure and heart rate in the baboon at 0. 7 of gestation. Am J Obstet Gynecol. 2002;186:812–817. doi: 10.1067/mob.2002.121654. [DOI] [PubMed] [Google Scholar]
- Koenen SV, Nijland M, Jenkins SL, Nathanielsz PW. Fetal blood pressure response to repeated maternal dexamethasone administration at 0. 68 and 0.75 gestation. J Soc Gynecol Invest. 2001;8:108. [Google Scholar]
- Langley-Evans SC. Maternal carbenoxolone treatment lowers birthweight and induces hypertension in the offspring of rats fed a protein-replete diet. Clin Sci (Lond) 1997;93:423–429. doi: 10.1042/cs0930423. [DOI] [PubMed] [Google Scholar]
- Lerman A, Sandok EK, Hildebrand FL, Jr, Burnett JC., Jr Inhibition of endothelium-derived relaxing factor enhances endothelin- mediated vasoconstriction. Circulation. 1992;85:1894–1898. doi: 10.1161/01.cir.85.5.1894. [DOI] [PubMed] [Google Scholar]
- Levitt NS, Lindsay RS, Holmes MC, Seckl JR. Dexamethasone in the last week of pregnancy attenuates hippocampal glucocorticoid receptor gene expression and elevates blood pressure in the adult offspring in the rat. Neuroendocrinology. 1996;64:412–418. doi: 10.1159/000127146. [DOI] [PubMed] [Google Scholar]
- Mattei P, Virdis A, Ghiadoni L, Taddei S, Salvetti A. Endothelial function in hypertension. J Nephrol. 1997;10:192–197. [PubMed] [Google Scholar]
- Molnar J, Nijland MJ, Howe DC, Nathanielsz PW. Evidence for microvascular dysfunction after prenatal dexamethasone at 0. 7, 0.75, and 0.8 gestation in sheep. Am J Physiol Regul Integr Comp Physiol. 2002;283:R561–567. doi: 10.1152/ajpregu.00031.2002. [DOI] [PubMed] [Google Scholar]
- Moss TJ, Sloboda DM, Gurrin LC, Harding R, Challis JR, Newnham JP. Programming effects in sheep of prenatal growth restriction and glucocorticoid exposure. Am J Physiol Regul Integr Comp Physiol. 2001;281:R960–970. doi: 10.1152/ajpregu.2001.281.3.R960. [DOI] [PubMed] [Google Scholar]
- Nathanielsz PW, Bailey A, Poore ER, Thorburn GD, Harding R. The relationship between myometrial activity and sleep state and breathing in fetal sheep throughout the last third of gestation. Am J Obstet Gynecol. 1980;138:653–659. doi: 10.1016/0002-9378(80)90083-6. [DOI] [PubMed] [Google Scholar]
- NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. Effect of corticosteroids for fetal maturation on perinatal outcomes. JAMA. 1995;273:413–418. doi: 10.1001/jama.1995.03520290065031. [DOI] [PubMed] [Google Scholar]
- Novy MJ, Walsh SW. Dexamethasone and estradiol treatment in pregnant rhesus macaques: effects on gestational length, maternal plasma hormones, and fetal growth. Am J Obstet Gynecol. 1983;145:920–931. doi: 10.1016/0002-9378(83)90841-4. [DOI] [PubMed] [Google Scholar]
- Papapetropoulos A, Rudic RD, Sessa WC. Molecular control of nitric oxide synthases in the cardiovascular system. Cardiovasc Res. 1999;43:509–520. doi: 10.1016/s0008-6363(99)00161-3. [DOI] [PubMed] [Google Scholar]
- Rubanyi GM, Polokoff MA. Endothelins: molecular biology, biochemistry, pharmacology, physiology and pathophysiology. Pharmacol Rev. 1994;46:325–415. [PubMed] [Google Scholar]


