Abstract
Neuronal nicotinic α7 subunits have been found in chick and rat skeletal muscle during development and denervation. In the present study, reverse transcriptase-polymerase chain reaction was used to detect α7 subunit mRNA in denervated mouse muscle. To determine whether the α7 subunit forms functional nicotinic acetylcholine receptors (nAChRs) in muscle, choline was used to induce a membrane depolarization because choline has been considered a specific agonist of α7-containing (α7*) nAChRs. We found, however, that choline (3–10 mm) also weakly activates muscle nAChRs. After inhibiting muscle nAChRs with a specific muscle nAChR inhibitor, α-conotoxin GI (αCTxGI), choline was used to activate the α7* nAChRs on muscle selectively. Four weeks after denervation, rapid application of choline (10 mm) elicited a substantial depolarization in the presence of αCTxGI (0.1 µm). This component of the depolarization was never present in denervated muscles obtained from mutant mice lacking the α7 subunit (i.e. α7-null mice). The depolarization component that is resistant to αCTxGI was antagonized by pancuronium (3–10 µm) and by a 4-oxystilbene derivative (F3, 0.1–0.5 µm) at concentrations considered highly specific for α7* nAChRs. Another selective α7 antagonist, methyllycaconitine (0.05–5 µm), did not strongly inhibit this choline-induced depolarization. Furthermore, the choline-sensitive nAChRs showed little desensitization over 10 s of application with choline (10–30 mm). These results indicate that functional α7* nAChRs are significantly present on denervated muscle, and that these receptors display unusual functional and pharmacological characteristics.
Nicotinic acetylcholine receptors (nAChRs) are pentameric membrane proteins that form cation-selective ion channels (McGehee & Role, 1995; Lindstrom et al. 1996; Role & Berg, 1996; Albuquerque et al. 1997; Dani, 2001). There are two broad classes of nAChRs based on their general location: muscle nAChRs composed of α1, β1, δ, and γ or ε subunits, and neuronal nAChRs formed by α2-α10 and β2-β4 subunits (Cordero-Erausquin et al. 2000). This classification is not definitive, however, because neuronal nAChRs have been found in non-neuronal cells (Grando et al. 1995; Navaneetham et al. 1997; Flora et al. 2000), and the α1 muscle nAChR subunit has been found in chick ciliary ganglia (Pugh et al. 1995).
The expression of nAChRs in skeletal muscle is regulated by neural activity during development (Hall & Sanes, 1993). In the embryonic state, the most abundantly expressed nAChR is a muscle-type receptor composed of α12β1γδ. Small quantities of neuronal nAChR subunits have also been found in chick (α4, α5, α7, β4) and rat (α7) muscle (Corriveau et al. 1995; Romano et al. 1997; Fischer et al. 1999). As the neuromuscular junction develops, the ε subunit replaces the γ subunit in the muscle nAChR, and neuronal nAChR subunits are no longer expressed. Upon denervation, the γ subunit replaces the ε subunit in the muscle nAChR, and the neuronal α7 subunit is expressed again (Romano et al. 1997; Fischer et al. 1999).
The α7 subunit can form a homomeric nAChR channel when expressed in Xenopus oocytes (Séguéla et al. 1993). α7 homomeric channels are highly permeable to Ca2+ and are inhibited by α-bungarotoxin (α-BgTx) (Séguéla et al. 1993; Zhang et al. 1994; Castro & Albuquerque, 1995). Several biochemical and biophysical studies have demonstrated that the native α-BgTx-binding sites are composed solely of α7 subunits in rat neuronal cells (Chen & Patrick, 1997; Rangwala et al. 1997). However, the α7 subunit can participate in heteromeric nAChRs in chick retinal ganglion neurons (α7α8* nAChR; Anand et al. 1993) and in chick sympathetic neurons (α3α7* and α3α5α7* nAChRs; Yu & Role, 1998b). In Xenopus oocytes, heterologously expressed α7 subunits can also contribute to the formation of heteromeric nAChRs, such as α7β1 γδ (Helekar et al. 1994) and α7β3 (Palma et al. 1999). Thus, the native α7* nAChRs are not necessarily homogeneous in the nervous system. There might be cell-specific regulatory mechanisms that control the composition and function of α7* nAChRs (see Cooper & Millar, 1997).
Recently, it was proposed that the α7 subunit might form a functional nAChR in the denervated muscle of the rat (Fischer et al. 1999), but its function was not fully identified. In the present study, we confirm the expression of the α7 subunit in denervated mouse muscle by reverse transcriptase-polymerase chain reaction (RT-PCR). Using mutant mice that lack the α7 subunit (α7-null mice), we provide evidence indicating that the α7 subunit contributes to a nAChR in muscle that has an unusual pharmacology.
METHODS
Animals
C57BL/6J mice were bred and kept in the animal-care facilities of our institution. Animals were housed (3–5 per cage) under a 12 h-12 h light-dark cycle with free access to water and food. In some electrophysiological recordings (Figs 2, 4 and 6), we used mice lacking the α7 subunit. These α7-null mice were generated by mating heterozygous parents. The offspring were genotyped by PCR (Orr-Urtreger et al. 1997) using the following primers: α7(+), CCT GGT CCT GCT GTG TTA AAC TGC TTC; α7(-), CTG CTG GGA AAT CCT AGG CAC ACT TGA G; and Neo-3(-), CTG ATC GAC AAG ACC GGC TTC CAT CC. PCR was performed as follows: 94 °C, 5 min; 30 cycles of 94 °C, 1 min; 68 °C, 1 min; 72 °C, 1.5 min; then 72 °C, 5 min. The α7-null (-/-) mutant mice were compared to their α7 (+/+) wild-type siblings. After PCR, 30 µl of the PCR products were run on a 2 % agarose gel. The wild-type band (primers α7(+) and α7(-)) was 435 bp, the mutant band (primers α7(+) and Neo-3(-)) was 750 bp.
Figure 2. Choline and ACh evoke depolarizations mediated by muscle-type nAChRs at the endplate of innervated muscle.
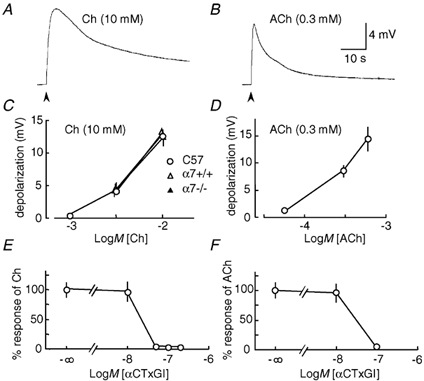
Rapid agonist applications were made to fibres from 3 mouse diaphragm muscles. A, trace showing the averaged response to 10 mm choline (Ch; n = 16). The arrowhead indicates agonist application. B, averaged response to 0.3 mm ACh (n = 12) C, concentration-response curves for choline from C57BL/6J mice (n = 4–16), α7+/+ (n = 13–21) and α7-/- mice (n = 9–21). D, concentration-response curve of ACh from C57BL/6J mice (n = 5–12). The peak amplitude of the depolarizations evoked by agonists was calculated and then averaged. E, concentration-response curve demonstrating inhibition by α-conotoxin GI (αCTxGI) of the depolarization evoked by 10 mm choline (n = 3–8). F, concentration-response curve demonstrating inhibition by αCTxGI of the depolarization evoked by 0.3 mm ACh (n = 4–12). These responses were obtained 20 min after treatment with αCTxGI. The peak amplitude of depolarization was normalized as a percentage of the control response observed without αCTxGI. Data values in C-F represent the means ± s.e.m.
Figure 4. Lack of the αCTxGI-resistant response to choline in denervated muscle from α7-null mice.
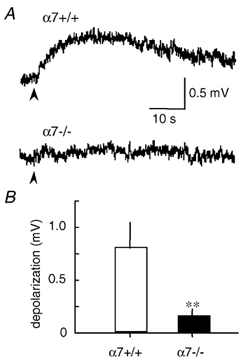
Choline-evoked depolarization was obtained after treatment with 0.1 µmαCTxGI in the denervated soleus muscles. A, traces showing averaged response to 10 mm choline in α7+/+ and α7-/- mice. Stimulation artifacts caused by the agonist applications (arrowheads) have been eliminated. B, peak amplitude of the αCTxGI-resistant depolarization by choline in α7+/+ and α7-/- mice. Data values represent the means ± s.e.m. (n = 17–24, 5–6 muscles, **P < 0.01, determined by unpaired t test).
Figure 6. αCTxGI-resistant depolarization present throughout prolonged application of choline in denervated muscle.
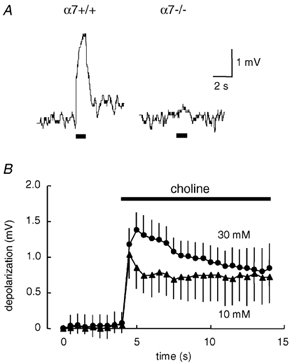
Choline-evoked depolarization was obtained after treatment with 0.1 µmαCTxGI in the denervated soleus muscles. A, traces showing typical recordings of the αCTxGI-resistant depolarization evoked by 1 s application of choline (10 mm) in denervated muscles of α7+/+ and α7-/- mice. Horizontal bars below the traces indicate 1 s application of choline. Similar results were obtained in 3 separate muscle fibres from 2 muscles of α7+/+ mice and in 5 separate muscle fibres from 2 muscles of α7-/- mice. B, time course of averaged αCTxGI-resistant responses to prolonged choline application in denervated muscles of C57BL/6 mice. The choline solution (10–30 mm) containing 0.1 µmαCTxGI was continuously applied to the recording area for 10 s via an outflow tube. The horizontal bar in the plot indicates 10 s application of choline. Data values represent the means ± s.e.m. (10 mm choline: ▴, n = 11, 4 muscles; 30 mm choline : •, n = 10, 6 muscles).
All procedures were approved by the Baylor College of Medicine Animal Research Committee and followed the ‘Using Animals in Intramural Research’ guidelines from the National Institutes of Health.
Muscle denervation
Mice (2–3 months old) were anaesthetized with a mixture of ketamine (112.5 mg kg−1), xylazine (5.7 mg kg−1) and acepromazine (1.1 mg kg−1) injected intraperitoneally. The hind limbs were surgically denervated by removing approximately 1 cm of the sciatic nerve just proximal to the bifurcation of the tibial and peroneal nerve. Four weeks after the denervation, soleus muscles were isolated.
RT-PCR
Total cellular RNA was extracted from mouse (2–4 months old) tissue using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA). The following sets of primers were used, as described previously (Léna et al. 1999; Song et al. 1999): α7 forward: 5′-GTG GAA CAT GTC TGA GTA CCC CGG AGT GAA-3′, α7 reverse: 5′-GAG TCT GCA GGC AGC AAG AAT ACC AGC A-3′, β-actin forward: 5′-TGG AAT CCT GTG GCA TCC ATG AAA C-3′, β-actin reverse: 5′-TAA AAC GCA GCT CAG TAA CAG TCC G-3′. These sets of primers were located on different exons to rule out genomic DNA amplification. RT-PCR for detection of α7 subunit and β-actin mRNA was performed in individual tubes using 0.3 µg total RNA and the SuperScript One-Step RT-PCR kit (Life Technologies, Grand Island, NY, USA) with the following conditions. The reverse transcriptase reaction was performed at 50 °C for 30 min. After an initial denaturation step at 94 °C for 2 min, 37 and 33 cycles (94 °C, 30 s; 60 °C, 45 s; 68 °C, 45 s) of PCR were run to obtain the α7 and β-actin products, respectively. The numbers of PCR cycles chosen were within the linear range of the amplification. The number of PCR cycles was chosen to obtain a band strong enough for easy quantification, but still faint enough to observe a linear increase in PCR product in the gel when more cycles were used. The number of PCR cycles that were tested for linearity was 35–41 and 33–40, respectively, before settling on the values given. Subsequently, 20 µl of each PCR product was run on 1 % agarose gels containing ethidium bromide. Fluorescence intensity from the PCR bands on the gels was measured using an imaging system (Alpha Innotech, San Leandro, CA, USA) with ChemiImager version 5.5 software. Data are expressed as means ± s.e.m. Statistical differences were determined by Student's unpaired t test (two tails).
Electrophysiology
Adult mice (2–4 months old) were anaesthetized with halothane and killed by decapitation. Diaphragm and soleus muscles were isolated with their tendons. The muscle preparation was stretched slightly beyond its resting tension, and was fixed with insect pins on rubber plates in a chamber. A modified Krebs solution (mm: NaCl 122, KCl 5.9, CaCl2 2.5, MgCl2 1.2, NaHCO3 15.5, and glucose 11.5, pH 7.4) was equilibrated with 95 % O2 and 5 % CO2, maintained at 32 ± 1 °C and perfused through the chamber (2 ml min−1). Intracellular recordings were performed in current-clamp mode using an Axopatch-1C (Axon Instruments, Foster City, CA, USA) with a glass micropipette filled with 3 m KCl (20 MΩ resistance). The microelectrode was inserted near the endplate on the surface fibres such that the rise times of the miniature endplate potentials were 1 ms or less. To minimize the differences among the preparations used, the membrane potential was corrected by passing currents into the muscle fibre through the recording pipettes. Experiments were conducted at a membrane potential of approximately −60 mV in innervated muscle. In the case of denervated muscle, the entire surface of the muscle exhibits supersensitivity to acetylcholine (ACh) (Cangiano, 1985). We recorded from regions mid-way along the muscle fibre, as described by Fischer et al. (1999). The resting membrane potential was lowered in denervated muscle, such that the experiments were conducted at a membrane potential of approximately −53 mV.
In order to measure the depolarization evoked by nicotinic agonists, such as choline and ACh, local agonist applications were made onto the muscle membrane 0.2 mm away from the tip of the recording electrode. The agonist was rapidly applied with outflow tubes (150 µm i.d. glass pipettes) mounted on a computer-controlled, high-speed motorized drive (Newport Corp., Irvine, CA, USA) (see Vernino et al. 1992, 1994). The duration of the local agonist application was 30 ms, unless otherwise mentioned. The external solution contained 1 µm atropine, a muscarinic ACh receptor antagonist, throughout the measurement to prevent any involvement of muscarinic receptors in the observed responses. To block muscle-type nAChRs, the preparations were often pretreated with α-conotoxin GI (αCTxGI) by bath application for at least 20 min before application of the nicotinic agonists. For the experiments investigating the pharmacological properties of the agonist-induced depolarization that was resistant to αCTxGI, pancuronium (20 min), methyllycaconitine (MLA, 20 min), F3 (30 min) or α-BgTx (30 min) was bath applied in combination with αCTxGI before recording. These antagonists were continuously present in the chamber during and after the local application of pure agonist solution (30 ms duration). We also performed experiments with the antagonist in both the bath and the outflow tube to rule out the possibility that the blocking effect of a nicotinic antagonist would be weakened as the pure choline solution was applied via the outflow tube. We investigated the choline response with MLA (50 nm to 1 µm) and αCTxGI (0.1 µm) added not only to the chamber but also to the choline solution (10–30 mm) in the outflow tube (Table 1). While testing for desensitization using long (1–10 s) choline applications, 0.1 µmαCTxGI was present both in the bath and in the outflow solution.
Table 1.
No effect of methyllycaconitine on the αCTxGI-resistant depolarization by choline in denervated muscle
| Choline (mM) | MLA (μM) | Depolarization (mV) | n | Muscles tested |
|---|---|---|---|---|
| 10 | 0 | 0.72 ± 0.23 | 11 | 5 |
| 10 | 1 | 0.75 ± 0.26 n.s. | 11 | 4 |
| 30 | 0 | 2.31 ± 0.20 | 8 | 3 |
| 30 | 0.05 | 2.31 ± 0.28 n.s. | 9 | 3 |
The denervated muscles were pretreated with methyllcaconitine (MLA) in the presence of αCTxGI (0.1 μM). Then, the choline solution (10–30mM) containing MLA (in and αCTxGI 0.1 μM) was rapidly applied via the outflow tube. n.s., not significantly differ from the control response without MLA, determined by unpaired t test.
Data acquisition and analysis (Axobasic, Axon Instruments) were performed using a personal computer equipped with an interface board (TL-1, Axon Instruments). Electrophysiological responses were sampled at a rate of 67 Hz for 4096 sample points. To assess the effects of drugs, peak amplitudes were compared. We estimated the peak amplitude of an agonist-induced depolarization (mV) by calculating the difference between the resting membrane potential and the peak of the depolarization. The resting potential was obtained by averaging the baseline for 4 s just before applying the nicotinic agonist. Within each muscle, measurements were considered independent and contributed to the n value when new muscle fibres were used. The data were then averaged and expressed as means ± s.e.m. The number of muscle fibres tested was given as n, and the number of different muscles that were used is also given. Statistical differences were determined by Student's unpaired t test (two tails), unless otherwise mentioned.
Reagents
All chemicals were purchased from Sigma (St Louis, MO, USA), unless indicated. MLA was purchased from Research Biochemicals International (Natick, MA, USA). α-CTxGI was purchased from Calbiochem-Novabiochem (La Jolla, CA, USA). F3 was kindly provided by Dr C. Gotti (University of Milan, Italy).
RESULTS
Expression of nAChR α7 subunit mRNA in denervated m uscle
RT-PCR was performed to assess mRNA expression of β-actin and nAChR α7 subunit (Fig. 1). Since the expression level of β-actin in skeletal muscle is not modified after denervation (Anzai et al. 1996), the amount of α7 PCR product was normalized as a percentage of the β-actin level. A fragment of neuronal α7 subunit of the expected size was obtained in 4 out of 14 normal (innervated) soleus muscles (29 %) of adult mice. The expression level of α7 mRNA was not changed 2 weeks after denervation (data not shown). Four weeks after muscle denervation, the α7 PCR product was found in a higher proportion (7 out of 9 denervated muscles, 78 %), and the mean amplified levels were significantly increased from those in the innervated muscle (Fig. 1).
Figure 1. Semi-quantified expression level of the α7 nAChR subunit by RT-PCR.
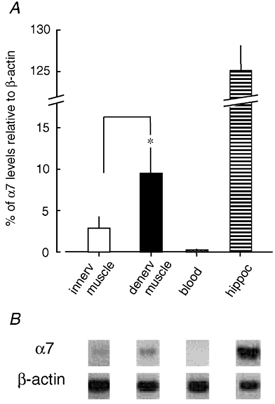
Total RNA was extracted from mouse innervated muscle (innerv), 4 week-denervated muscle (denerv), blood and hippocampus (hippoc). RT-PCR products for α7 subunit and β-actin were obtained simultaneously from each sample. A, the α7 mRNA level was normalized as a percentage of the β-actin mRNA level, which was considered to be 100 %. Data values represent the means ± s.e.m. (n = 6–14). A significant difference (*P < 0.05) existed between innervated and denervated muscles as determined by an unpaired t test. B, photo-images in the negative form demonstrating the RT-PCR product for α7 mRNA that yielded the expected band of 509 bp, and the product for β-actin mRNA that showed the expected band of 348 bp.
Although there is a report indicating that α7 mRNA is expressed in human mononuclear leukocytes (Kawashima & Fujii, 2000), no α7 subunit was detected from blood samples (200 µl per mouse, n = 10), which we used as a negative control. This result also indicates that α7 PCR products detected in muscle preparations were not derived from the blood cells contained in microvessels in muscle. On the other hand, a large amount of α7 PCR product was obtained in all hippocampal tissues isolated from mouse brain (n = 6). The hippocampus serves as a positive control because α7* nAChR is highly expressed there (Albuquerque et al. 1997; Jones et al. 1999; Dani, 2001; Ji et al. 2001). No differences were detected in the size of fragments amplified from muscle and hippocampal preparations. These negative and positive controls support the specificity of our detection of α7 mRNA in muscle.
Choline-evoked depolarizations mediated by muscle-type nAChRs in innervated muscle
Intracellular recordings were performed to investigate the possibility that the α7 subunit forms a functional nAChR in denervated skeletal muscle. All the nicotinic responses analysed in this study returned to the resting membrane potential within 3 min after stimulation, and were stable and repeatable.
Choline is a full agonist for α7* nAChRs, and is considered a very weak agonist for muscle-type nAChRs (Papke et al. 1996; Alkondon et al. 1997). However, the application of 10 mm choline elicited a depolarization at neuromuscular endplates in innervated diaphragm muscle of mice (Fig. 2A). The choline-evoked response developed slowly (time to peak, 5.0 ± 0.8 s, n = 18, 7 muscles) and recovered slowly (1/3 decay time or time to decline to 66.7 % of peak amplitude, 13.9 ± 2.3 s, n = 18, 7 muscles). The response was faster when 0.3 mm ACh was applied to the endplate: time to peak, 1.1 ± 0.2 s, n = 12, 3 muscles, P < 0.001; 1/3 decay time, 2.5 ± 0.2 s, n = 12, 3 muscles, P < 0.001 (Fig. 2B). It was necessary to use > 15-times higher concentrations of choline than ACh to obtain a similar amplitude of depolarization (Fig. 2C and D). Moreover, the peak amplitude of the choline-evoked depolarization from innervated muscles of α7+/+ mice was the same as that in α7-/- mice (Fig. 2C). These results indicate that the agonist activity of choline on muscle-type nAChRs is independent of α7* nAChRs.
To verify that muscle-type nAChRs underlie the endplate depolarization by choline, innervated diaphragm muscles were pretreated with αCTxGI, which specifically inhibits muscle-type nAChRs expressed in Xenopus oocytes (IC50 = 20 nm) without affecting α7 homomeric nAChRs (Johnson et al. 1995). At the concentrations (10–100 nm) used previously (Johnson et al. 1995), αCTxGI inhibited both choline-evoked and ACh-evoked depolarizations (Fig. 2E and F). This result supports the conclusion that choline can activate muscle-type nAChRs to cause a depolarization of innervated muscle at neuromuscular endplates.
Nicotinic depolarizations in denervated muscle that are independent of muscle-type nAChRs
To investigate the function of α7* nAChRs in adult mouse muscle, αCTxGI was used to inhibit muscle-type nAChRs. αCTxGI (0.1 µm) almost completely inhibited choline-evoked depolarizations at the endplates of innervated mouse soleus muscle (Fig. 3A, upper trace). However, 4 weeks after muscle denervation, the application of 10 mm choline evoked a significant depolarization all along the surface of the muscle membrane even in the presence of 0.1 µmαCTxGI (Fig. 3A, lower trace). Most of the denervated muscle responded to the choline application (16 of 17 recordings, 5 muscles), and the averaged peak amplitude of depolarization was significantly increased when compared to the response from the innervated muscles (Fig. 3B). In addition, choline increased the peak amplitude of the αCTxGI-resistant depolarization in a concentration-dependent manner: the peak amplitude was 3.2-fold augmented by increasing the choline concentration from 10 to 30 mm (Table 1). Using a higher concentration of αCTxGI (1 µm) did not inhibit this component of the choline-evoked depolarization (Fig. 3B), indicating that muscle-type nAChRs do not mediate this component.
Figure 3. The αCTxGI-resistant depolarization by choline and ACh in denervated muscle of C57BL/6J mice.
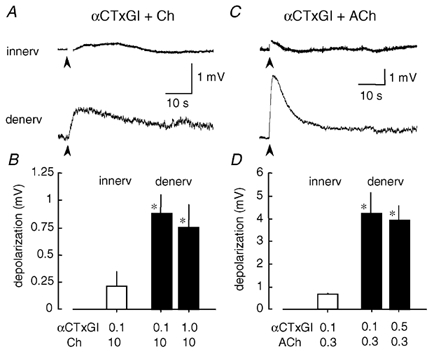
All the data were obtained after treatment with αCTxGI in mouse soleus muscles. A, traces showing averaged response to 10 mm choline (Ch) in the presence of 0.1 µmαCTxGI in the innervated (innerv) and denervated (denerv) muscles (n = 10–17, 4–5 muscles). Stimulation artifacts just after the agonist applications (arrowheads) are blanked. B, peak amplitude of 10 mm choline-evoked depolarization in the presence of αCTxGI (0.1–1 µm) in the innervated and denervated states. Data values represent the means ± s.e.m. (n = 10–17). C, traces demonstrating averaged response to 0.3 mm ACh in the presence of 0.1 µmαCTxGI in the innervated and denervated muscles. Stimulation artifacts are blanked (n = 3–6, 3 muscles). D, peak amplitude of 0.3 mm ACh-evoked depolarization in the presence of αCTxGI (0.1–0.5 µm) in the innervated and denervated states. Data values represent the means ± s.e.m. (n = 3–6). *Significantly different response (P < 0.05) to choline or ACh in the presence of 0.1 µmαCTxGI in the denervated muscles from those in the innervated muscle, determined by unpaired t test.
ACh also evoked a depolarization in the denervated soleus muscles in the presence of 0.1 µmαCTxGI (Fig. 3C). The peak amplitude of the depolarization was augmented by increasing ACh from 0.06 to 0.6 mm (not shown). On the other hand, the depolarization caused by ACh (0.3 mm) was not altered by increasing αCTxGI from 0.1 to 0.5 µm (Fig. 3D). The αCTxGI-resistant depolarization evoked by 0.3 mm ACh was 5-times larger than that evoked by 10 mm choline in denervated muscle (Fig. 3B and D). The ACh- choline relationship was different at the innervated muscle endplate where 0.3 mm ACh and 10 mm choline evoked similarly sized depolarizations from muscle-type junctional nAChRs (Fig. 2B and D).
The αCTxGI-resistant component differed from the muscle-type response in other ways. The time to peak and 1/3 decay time of the αCTxGI-resistant component were 9.5 ± 1.4 and 15.4 ± 3.7 s, respectively (n = 16, 5 muscles) when evoked by choline (10 mm) and were 2.0 ± 0.4 s (n = 6, 3 muscles, P < 0.01) and 3.5 ± 0.4 s (n = 6, 3 muscles, P < 0.05 determined by Student's unpaired one-tail t test) when evoked by ACh (0.3 mm). These times are significantly longer than those found with junctional nAChRs. For example, when compared with the innervated endplate response, the time to peak of the αCTxGI-resistant component in denervated muscle was 1.9-fold longer in 10 mm choline and 1.8-fold longer in 0.3 mm ACh. These results support the conclusion that, in denervated muscle treated with αCTxGI, the choline-evoked or ACh-evoked membrane depolarizations are not mediated by muscle-type nAChRs.
α7-Null mice lack the α-CTxGI-resistant depolarization evoked by choline in denervated muscle
We used α7-null (-/-) mutant mice to identify the nAChR subtype involved in the choline-evoked, αCTxGI-resistant depolarization. In wild-type littermates (α7+/+ mice), 10 mm choline (with 0.1 µmαCTxGI) elicited a depolarization in denervated soleus muscles (n = 17, 5 muscles; Fig. 4). This component of depolarization was statistically the same in amplitude as the response found with C57BL/6J wild-type mice (Fig. 3). In contrast, no significant αCTxGI-resistant depolarization in response to choline was ever observed with denervated soleus muscles from α7-null (-/-) mice (n = 24, 6 muscles; Fig. 4A and B). These results strongly support the idea that this αCTxGI-resistant depolarization in denervated muscle requires the α7 subunit and α7* nAChRs.
Pharmacological properties of the α-CTxGI-resistant depolarization
The pharmacological properties of the choline-evoked depolarizations in innervated diaphragm muscle (no αCTxGI) and denervated muscle (with αCTxGI) were compared. When pretreated with pancuronium (0.1–10 µm), a competitive nAChR antagonist, the peak amplitudes of both responses were diminished in a concentration-dependent manner (Fig. 5A), but the effective concentration ranges differed. The IC50 for the αCTxGI-resistant component in the denervated muscle was 2.1 µm, whereas the IC50 for the depolarization in the innervated muscle was 0.2 µm. The IC50 in denervated muscle was similar to the IC50 (3 µm) obtained by whole-cell patch-clamp recordings of ACh-induced α7* currents from rat hippocampal interneurons (not shown), whereas the IC50 in the innervated muscle was similar to that of junctional muscle-type (α1β1εδ) nAChRs expressed in Xenopus oocytes (Yost & Winegar, 1997).
Figure 5. Distinct pharmacological properties of the αCTxGI-resistant depolarization in denervated muscle and the junctional nicotinic response in innervated muscle.
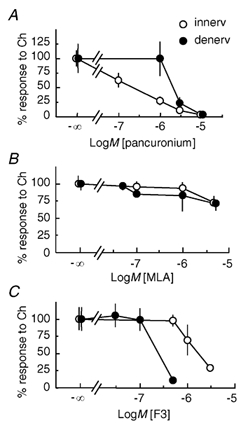
Choline-evoked depolarizations were obtained in either the innervated diaphragm muscles without αCTxGI (innerv, ○) or the denervated soleus muscles treated with 0.1 µmαCTxGI (denerv, •) in C57BL/6J mice. The inhibitors (pancuronium, MLA or F3) were applied first, before rapid agonist application was carried out. The concentration-response curves demonstrate the inhibitory effects of pancuronium (A), MLA (B) and F3 (C) on the depolarization induced by 10 mm choline (Ch). Peak amplitude of the depolarization was normalized as a percentage of the control response obtained without these blockers. Data values represent the means ± s.e.m. (A: ○, n = 4-8, 3 muscles; •, n = 7-9, 4 muscles; B: ○, n = 17-18, 3 muscles; •, n = 3-4, 3 muscles; C: ○, n = 3-5, 3 muscles; •, n = 6-8, 3 muscles).
In hippocampal neurons, MLA at 1–5 nm effectively inhibits α7 nAChRs (Alkondon et al. 1992; Palma et al. 1996; Ji et al. 2001). MLA at much higher concentrations (0.05–5 µm) did not strongly inhibit either the αCTxGI-resistant depolarization in the denervated muscle or the juntional response in the innervated muscle (Fig. 5B). MLA (5 µm) diminished the peak amplitude of both responses by only 30 %. In these experiments, choline was applied to the muscle fibre via an outflow tube, and the MLA was present in the chamber but not the outflow tube. To exclude the possibility that the MLA was being washed off as the choline solution arrived from the outflow tube, MLA (1 µm) and αCTxGI (0.1 µm) were added not only to the chamber but also to the choline solution (10–30 mm) in the outflow tube. The results were the same: the peak amplitude of the αCTxGI-resistant response with MLA was still not different from the control response without MLA (Table 1).
This surprisingly weak effect of MLA on the αCTxGI-resistant component led us to use another potent antagonist for α7* nAChRs, the 4-oxystilbene derivative F3 (Gotti et al. 1998, 2000). Pretreatment with F3 decreased the peak amplitude of the αCTxGI-resistant component. Both responses to choline were inhibited but with different concentration dependencies (Fig. 5C). As expected for an α7* nAChR (Gotti et al. 1998, 2000), the F3 concentrations used to inhibit the αCTxGI-resistant component (0.1–0.5 µm, IC50 = 0.26 µm) were 6-times lower than those used to inhibit the junctional nAChRs of innervated muscle (1–3 µm, IC50 = 1.6 µm).
To further characterize the difference between the junctional nAChRs and those mediating αCTxGI-resistant depolarization, we used α-BgTx, an antagonist of both α7* and muscle-type nAChRs. The peak amplitude of the αCTxGI-resistant depolarization evoked by 10 mm choline was significantly diminished by 0.1 µmα-BgTx. The control response without α-BgTx was 1. 02 ± 0.19 mV (n = 19, 7 muscles), and the test response with α-BgTx was 0.25 ± 0.09 mV (n = 13, 5 muscles, P < 0.01 determined by unpaired t test). The junctional nAChR responses evoked by 10 mm choline in the innervated muscle were completely blocked by 0.1 µmα-BgTx (n = 6, 3 muscles). Although both the junctional nAChRs and the putative α7* nAChR are inhibited by α-BgTx, the concentration dependence is slightly different. Thus, consistent with the functional data, the pharmacology supports the conclusion that the αCTxGI-resistant depolarization in denervated muscle is not mediated by typical muscle-type nAChRs.
Desensitization profile of the α-CTxGI-resistant depolarization
α7* nAChRs desensitize rapidly (< 100 ms) upon prolonged application of a high concentration of agonist (Alkondon et al. 1997). We therefore examined the effects of prolonged exposure to choline on the αCTxGI-resistant depolarization in denervated muscle. When the exposure time was increased to 1 s, 10 mm choline induced a sustained depolarization in denervated muscles of α7+/+ mice in the presence of 0.1 µmαCTxGI, whereas the choline response was nearly absent in α7-/- mice (Fig. 6A). When the exposure time was further increased to 10 s, the αCTxGI-resistant depolarization declined with time in denervated muscles of C57BL/6J mice (Fig. 6B): during the 10 s of application, the response to 10 mm choline decreased by 31 % from the peak amplitude, and the response to 30 mm choline decreased by 39 % from the peak amplitude. In other words, the depolarization only partially desensitized, and the majority of the current persisted throughout the 10 s choline application. Thus, unlike classical neuronal α7* nAChR responses, the αCTxGI-resistant depolarization did not exhibit a fast desensitization profile.
DISCUSSION
The present study demonstrates the expression of the α7 nAChR subunit in denervated mouse muscle by RT-PCR. The α7 subunit has previously been observed in denervated rat muscle by immunohistochemisty and in situ hybridization (Fischer et al. 1999), and during muscle cell differentiation (Campos-Caro et al. 2001). Electrophysiological experiments presented here indicate that α7* nAChRs are functional and partially mediate nicotinic responses in the denervated muscle. This conclusion was based on the use of the selective agonist choline, after inhibiting muscle-type nAChRs with αCTxGI. The conclusion was further supported because this nicotinic activity was never present in α7-null mice. Results obtained by comparing wild-type α7+/+ and α7-/- mice indicate that there is functional nicotinic activity via α7* nAChRs in these non-neuronal cells.
We carried out intracellular recordings with rapid application of choline and ACh. Although choline is a full agonist for α7* nAChRs (Papke et al. 1996), the selectivity is not perfect. In fact, choline acts as a weak agonist for muscle-type, α3β4, and α4β4 nAChRs expressed in Xenopus oocytes (Papke et al. 1996; Alkondon et al. 1997; Zwart & Vijverberg, 2000). It has been reported that choline causes depolarization of muscle membranes (Del Castillo & Katz, 1957; Portela et al. 1970), and that the choline-evoked depolarization is enhanced after the muscle denervation (Portela et al. 1969; Fischer et al. 1999). Choline also increases the intracellular Ca2+ concentration at the muscle endplates (Dezaki et al. 1999). These earlier studies observed the choline-induced currents mediated mainly by muscle-type nAChRs. After inhibiting muscle-type nAChRs, we showed there is another choline-induced conductance in denervated muscle, and that conductance requires the presence of the α7 subunit. Although it is possible that the α7 subunit acts to help produce a conductance without participating in the actual nAChR channel, our results strongly support the idea that functional α7* nAChRs are upregulated after muscle denervation.
We utilized αCTxGI, a muscle-type nAChR inhibitor, to determine that the choline-evoked depolarization from innervated muscles was almost exclusively mediated by junctional muscle-type nAChRs. Upon denervation, the subsequent nAChR supersensitivity observed with several nicotinic agonists (Cangiano, 1985) arises mainly from upregulation of the extrajunctional muscle nAChRs composed of α12β1γδ subunits (Hall & Sanes, 1993). The nicotinic activity on the surface of denervated muscle membrane was strongly diminished by αCTxGI at the same concentration (0.1 µm; Fig. 3) used to inhibit the mouse extrajunctional nAChRs expressed in oocytes (Johnson et al. 1995) and the junctional nAChRs in mouse innervated muscle (Fig. 2). αCTxGI appears to have a similar potency at these two types of α1-containing nAChRs expressed in muscle following denervation.
After inhibiting muscle-type nAChRs with αCTxGI in the denervated muscle, the remaining choline-evoked response arose from an α7* nAChR with an unusual pharmacology. Pancuronium was a less potent blocker of the αCTxGI-resistant depolarization (putative α7* nAChRs) than of the muscle-type, junctional nAChR response. The difference in the inhibition by F3 between muscle-type nAChRs and the nAChR type mediating the αCTxGI-resistant depolarization further supports the participation of α7. F3 is a ligand that inhibits α7* nAChRs with nanomolar affinity, but inhibits muscle-type nAChRs only at higher concentrations. The IC50 values of F3 for chick and rat α7-homomeric nAChRs expressed in oocytes are 119 and 1 nm, respectively (Gotti et al. 1998, 2000), whereas the IC50 for muscle-type nAChRs is 3 µm (Dr C. Gotti, University of Milan, Italy, personal communication). These IC50 values of F3 for α7 nAChRs and muscle-type nAChRs are comparable to those obtained in this study for the αCTxGI-resistant response to choline (264 nm) and for the junctional nAChR response (1.6 µm), respectively.
The lack of inhibition by MLA, a potent α7-homomeric nAChR antagonist in neurons, of the αCTxGI-resistant component is of considerable interest. No antagonism by MLA has been reported for the native α7* nAChR responses in chick ventral lateral geniculate nucleus (Guo et al. 1998; Guo & Chiappinelli, 2002), in chick sympathetic ganglion (Yu & Role, 1998a,b) and in rat dorsal motor nucleus (Ferreira et al. 2001). Furthermore, a recent paper describing a choline-sensitive nicotinic response in the lateral geniculate nucleus (Guo & Chiappinelli, 2002) may be relevant to our results, because both those responses persist during prolonged choline exposure, in contrast to typical α7 receptors, which desensitize rapidly (Alkondon et al. 1997). Although the molecular basis for these atypical pharmacological properties is unknown, heteromeric α7* nAChRs have been proposed to explain the unique characteristics. It has been proposed that the native α3α7* nAChR expressed in chick sympathetic ganglion neurons is sensitive to α-BgTx but not to MLA, whereas an α3α5α7* nAChR is sensitive to MLA but not to α-BgTx (Yu & Role, 1998a,b). The α7 subunit can also co-assemble with muscle-type subunits to form α72β1γδ nAChRs under some conditions when expressed in Xenopus oocytes (Helekar et al. 1994), but in chick embryonic muscle the α7 subunit was not found in combination with α1, β1 and δ subunits (Corriveau et al. 1995; Romano et al. 1997). Since several neuronal nAChR subunits (α4, β2 and α5) have been found in mammalian muscle (Sala et al. 1996; Flora et al. 2000), there could be other possible combinations for heteromeric nAChRs. Accordingly, our data are consistent with denervated muscle expressing putative heteromeric α7* nAChR(s) with a low sensitivity to MLA.
Post-translational processing is another factor that could modulate the α7* nAChR function. Homomeric α7 nAChRs display multiple charged forms and different conformations in rat brain and in PC12 cells (Rakhilin et al. 1999; Drisdel & Green, 2000). These studies suggest that muscle-specific processing events may induce conformational changes in the α7 subunit that decrease the sensitivity to MLA. Alternatively, other more unlikely possibilities cannot be eliminated, such as the following: the αCTxGI-resistant effect of choline might be mediated by an unknown type(s) of nAChR(s) whose expression is regulated by the α7 gene product.
The functional significance of α7* nAChRs in the denervated muscle remains to be elucidated, but the following are reasonable speculations. Because α7* nAChRs have a much higher calcium permeability than muscle-type nAChRs (Decker & Dani, 1990; Vernino et al. 1992, 1994; Séguéla et al. 1993; Castro & Albuquerque, 1995), the activity-dependent Ca2+ signals arising from α7* nAChRs may have a role in the changes associated with denervation. After the breakdown of the motor nerve, the denervated muscle expresses extrajunctional nAChRs to a large extent, and exhibits supersensitivity to innervation by ‘foreign’ nerves (Cangiano, 1985). α7* nAChR activity may also contribute to the reception of chemical signals from the foreign nerves. Furthermore, Ca2+ influx through α7* nAChRs may induce a specific proteolytic activity, such as Ca2+-activated neutral protease (CANP; Connold et al. 1986), and promote remodelling of the extracellular matrix molecules. This process could help to expedite the redistribution of molecules that form the postsynaptic transmembrane complex (see Daniels, 1997).
Acknowledgments
Dr Daoyun Ji (Baylor College of Medicine, USA) provided patch-clamp data from hippocampal interneurons and commented on the manuscript. Dr Cecilia Gotti (University of Milan, Italy) generously provided F3 as a gift. This work was supported by grants from the National Institute on Drug Abuse (DA09411 and DA12661), the National Institute of Neurological Disorders and Stroke (NS21229), and the Smoking Research Foundation (Japan) to Dr Ikuko Kimura and H.T. and by Grant-in-Aid (14771274) for Scientific Research from the Japanese Ministry of Science, Culture, Sports and Technology (to H.T.).
REFERENCES
- Albuquerque EX, Alkondon M, Pereira EF, Castro NG, Schrattenholz A, Barbosa CT, Bonfante-Cabarcas R, Aracava Y, Eisenberg HM, Maelicke A. Properties of neuronal nicotinic acetylcholine receptors: pharmacological characterization and modulation of synaptic function. J Pharmacol Exp Ther. 1997;280:1117–1136. [PubMed] [Google Scholar]
- Alkondon M, Pereira EFR, Cortes WS, Maelicke A, Albuquerque EX. Choline is a selective agonist of α7 nicotinic acetylcholine receptors in the rat brain neurons. Eur J Neurosci. 1997;9:2734–2742. doi: 10.1111/j.1460-9568.1997.tb01702.x. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Wonnacott S, Albuquerque EX. Blockade of nicotinic currents in hippocampal neurons defines methyllycaconitine as a potent and specific receptor antagonist. Mol Pharmacol. 1992;41:802–808. [PubMed] [Google Scholar]
- Anand R, Peng X, Lindstrom J. Homomeric and native alpha 7 acetylcholine receptors exhibit remarkably similar but non-identical pharmacological properties, suggesting that the native receptor is a heteromeric protein complex. FEBS Lett. 1993;327:241–246. doi: 10.1016/0014-5793(93)80177-v. [DOI] [PubMed] [Google Scholar]
- Anzai K, Kobayashi S, Kotake H, Murakami H, Korematsu K, Nonaka I. Neural BC1 RNA in mouse skeletal muscle is a denervation-induced RNA whose expression is developmentally regulated. Neurosci Lett. 1996;216:81–84. doi: 10.1016/0304-3940(96)12981-5. [DOI] [PubMed] [Google Scholar]
- Campos-Caro A, Carrasco-Serrano C, Valor LM, Ballesta JJ, Criado M. Activity of the nicotinic acetylcholine receptor α5 and α7 subunit promoters in muscle cells. DNA Cell Biol. 2001;20:657–666. doi: 10.1089/104454901753340640. [DOI] [PubMed] [Google Scholar]
- Cangiano A. Denervation supersensitivity as a model for the neural control of muscle. Neuroscience. 1985;14:963–971. doi: 10.1016/0306-4522(85)90268-4. [DOI] [PubMed] [Google Scholar]
- Castro NG, Albuquerque EX. α-Bungarotoxin-sensitive hippocampal nicotinic receptor channel has a high calcium permeability. Biophys J. 1995;68:516–524. doi: 10.1016/S0006-3495(95)80213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DN, Patrick JW. The α-bungarotoxin-binding nicotinic acetylcholine receptor from rat brain contains only the α7 subunit. J Biol Chem. 1997;272:24024–24029. doi: 10.1074/jbc.272.38.24024. [DOI] [PubMed] [Google Scholar]
- Connold AL, Evers JV, Vrbova G. Effects of low calcium and protease inhibitors on synapse elimination during postnatal development in the rat soleus muscle. Brain Res. 1986;393:99–107. doi: 10.1016/0165-3806(86)90069-6. [DOI] [PubMed] [Google Scholar]
- Cooper ST, Millar NS. Host cell-specific folding and assembly of the neuronal nicotinic acetylcholine receptor α7 subunit. J Neurochem. 1997;68:2140–2151. doi: 10.1046/j.1471-4159.1997.68052140.x. [DOI] [PubMed] [Google Scholar]
- Cordero-Erausquin M, Marubio LM, Klink R, Changeux J-P. Nicotinic receptor function; new perspectives from knockout mice. Trends Pharmacol Sci. 2000;21:211–217. doi: 10.1016/s0165-6147(00)01489-9. [DOI] [PubMed] [Google Scholar]
- Corriveau RA, Romano SJ, Conroy WG, Oliva L, Berg DK. Expression of neuronal acetylcholine receptor genes in vertebrate skeletal muscle during development. J Neurosci. 1995;15:1372–1383. doi: 10.1523/JNEUROSCI.15-02-01372.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA. Overview of nicotinic receptors and their roles in the central nervous system. Biol Psychiatry. 2001;49:166–174. doi: 10.1016/s0006-3223(00)01011-8. [DOI] [PubMed] [Google Scholar]
- Daniels MP. Intracellular communication that mediates formation of the neuromuscular junction. Mol Neurobiol. 1997;14:143–170. doi: 10.1007/BF02740654. [DOI] [PubMed] [Google Scholar]
- Decker ER, Dani JA. Calcium permeability of the nicotinic acetylcholine receptor: the single-channel calcium influx is significant. J Neurosci. 1990;10:3413–3420. doi: 10.1523/JNEUROSCI.10-10-03413.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Castillo J, Katz B. Interaction at end-plate receptors between different choline derivatives. Proc R Soc Lond B. 1957;146:369–381. doi: 10.1098/rspb.1957.0018. [DOI] [PubMed] [Google Scholar]
- Dezaki K, Tsuneki H, Kimura I. Methyllycaconitine-sensitive neuronal nicotinic receptor-operated slow Ca2+ signal by local application or perfusion of ACh at the mouse neuromuscular junction. Neurosci Res. 1999;33:17–24. doi: 10.1016/s0168-0102(98)00109-6. [DOI] [PubMed] [Google Scholar]
- Drisdel RC, Green WN. Neuronal α-bungarotoxin receptors are α7 subunit homomers. J Neurosci. 2000;20:133–139. doi: 10.1523/JNEUROSCI.20-01-00133.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira M, Ebert SN, Perry DC, Yasuda RP, Baker CM, Davila-Garcia MI, Kellar KJ, Gillis RA. Evidence of a functional α7-neuronal nicotinic receptor subtype located on motoneurons of the dorsal motor nucleus of the vagus. J Pharmacol Exp Ther. 2001;296:260–269. [PubMed] [Google Scholar]
- Fischer U, Reinhardt S, Albuquerque EX, Maelicke A. Expression of functional α7 nicotinic acetylcholine receptor during mammalian muscle development and denervation. Eur J Neurosci. 1999;11:2856–2864. doi: 10.1046/j.1460-9568.1999.00703.x. [DOI] [PubMed] [Google Scholar]
- Flora A, Schulz R, Benfante R, Battaglioli E, Terzano S, Clementi F, Fornasari D. Transcriptional regulation of the human α5 nicotinic receptor subunit gene in neuronal and non-neuronal tissues. Eur J Pharmacol4. 2000;393:85–95. doi: 10.1016/s0014-2999(00)00040-6. [DOI] [PubMed] [Google Scholar]
- Gotti C, Balestra B, Moretti M, Rovati GE, Maggi L, Rossoni G, Berti F, Villa L, Pallavicini M, Clementi F. 4-Oxystilbene compounds are selective ligands for neuronal nicotinic αbungarotoxin receptors. Br J Pharmacol. 1998;124:1197–1206. doi: 10.1038/sj.bjp.0701957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C, Carbonnelle E, Moretti M, Zwart R, Clementi F. Drugs selective for nicotinic receptor subtypes; a real possibility or a dream? Behav Brain Res. 2000;113:183–192. doi: 10.1016/s0166-4328(00)00212-6. [DOI] [PubMed] [Google Scholar]
- Grando SA, Horton R, Pereira EF, Diethelm-Okita BM, George PM, Albuquerque EX, Conti-Fine BM. A nicotinic acetylcholine receptor regulating cell adhesion and motility is expressed in human keratinocytes. J Invest Dermatol. 1995;105:774–781. doi: 10.1111/1523-1747.ep12325606. [DOI] [PubMed] [Google Scholar]
- Guo J-Z, Chiappinelli VA. A novel choline-sensitive nicotinic receptor subtype that mediates enhanced GABA release in the chick ventral lateral geniculate nucleus. Neuroscience. 2002;110:505–513. doi: 10.1016/s0306-4522(01)00579-6. [DOI] [PubMed] [Google Scholar]
- Guo J-Z, Tredway TL, Chiappinelli VA. Glutamate and GABA release are enhanced by different subtypes of presynaptic nicotinic receptors in the lateral geniculate nucleus. J Neurosci. 1998;18:1963–1969. doi: 10.1523/JNEUROSCI.18-06-01963.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall ZW, Sanes JR. Synaptic structure and development: the neuromuscular junction. Cell. 1993;72:99–121. doi: 10.1016/s0092-8674(05)80031-5. [DOI] [PubMed] [Google Scholar]
- Helekar SA, Char D, Neff S, Patrick J. Prolyl isomerase requirement for the expression of functional homo-oligomeric ligand-gated ion channels. Neuron. 1994;12:179–189. doi: 10.1016/0896-6273(94)90162-7. [DOI] [PubMed] [Google Scholar]
- Ji D, Lape R, Dani JA. Timing and location of nicotinic activity enhances or depresses hippocampal synaptic plasticity. Neuron. 2001;31:131–141. doi: 10.1016/s0896-6273(01)00332-4. [DOI] [PubMed] [Google Scholar]
- Johnson DS, Martinez J, Elgoyhen AB, Heinemann SF, McIntosh JM. α-Conotoxin Im1 exhibits subtype-specific nicotinic acetylcholine receptor blockade: Preferential inhibition of homomeric α7 and α9 receptors. Mol Pharmacol. 1995;48:194–199. [PubMed] [Google Scholar]
- Jones S, Sudweeks S, Yakel JL. Nicotinic receptors in the brain: correlating physiology with function. Trends Neurosci. 1999;22:555–561. doi: 10.1016/s0166-2236(99)01471-x. [DOI] [PubMed] [Google Scholar]
- Kawashima K, Fujii T. Extraneuronal cholinergic system in lymphocytes. Pharmacol Ther. 2000;86:29–48. doi: 10.1016/s0163-7258(99)00071-6. [DOI] [PubMed] [Google Scholar]
- Léna C, De Kerchove D'Exaerde A, Cordero-Erausquin M, Le Novère N, Arroyo-Jimenez MM, Changeux J-P. Diversity and distribution of nicotinic acetylcholine receptors in the locus ceruleus neurons. Proc Natl Acad Sci U S A. 1999;96:12126–12131. doi: 10.1073/pnas.96.21.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom J, Anand R, Gerzanich V, Peng X, Wang F, Wells G. Structure and function of neuronal nicotinic acetylcholine receptors. Prog Brain Res. 1996;109:125–137. doi: 10.1016/s0079-6123(08)62094-4. [DOI] [PubMed] [Google Scholar]
- McGehee DS, Role LW. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annu Rev Physiol. 1995;57:521–546. doi: 10.1146/annurev.ph.57.030195.002513. [DOI] [PubMed] [Google Scholar]
- Navaneetham D, Penn A, Howard JF, Jr, Conti-Fine BM. Expression of the alpha 7 subunit of the nicotinic acetylcholine receptor in normal and myasthenic human thymuses. Cell Mol Biol. 1997;43:433–442. [PubMed] [Google Scholar]
- Orr-Urtreger A, Göldner FM, Saeki M, Lorezo I, Goldberg L, De Biasi M, Dani JA, Patrick JW, Beaudet AL. Mice deficient in the α7 neuronal nicotinic acetylcholine receptor lack α-bungarotoxin binding sites and hippocampal fast nicotinic currents. J Neurosci. 1997;17:9165–9171. doi: 10.1523/JNEUROSCI.17-23-09165.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma E, Bertrand S, Binzoni T, Bertrand D. Neuronal nicotinic α7 receptor expressed in Xenopus oocytes presents five putative binding sites for methyllycaconitine. J Physiol. 1996;491:151–161. doi: 10.1113/jphysiol.1996.sp021203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma E, Maggi L, Barabino B, Eusebi F, Ballivet M. Nicotinic acetylcholine receptors assembled from the α7 and β3 subunits. J Biol Chem. 1999;274:18335–18340. doi: 10.1074/jbc.274.26.18335. [DOI] [PubMed] [Google Scholar]
- Papke RL, Bencherif M, Lippiello P. An evaluation of neuronal nicotinic acetylcholine receptor activation by quaternary nitrogen compounds indicates that choline is selective for the α7 subtype. Neurosci Lett. 1996;213:201–204. doi: 10.1016/0304-3940(96)12889-5. [DOI] [PubMed] [Google Scholar]
- Portela A, Pérez RJ, Nallar R, Pérez JC, Stewart P, Vicente JA, Lonchampt P. Denervation effects on choline depolarization of muscle membrane. Experientia. 1969;25:143–144. doi: 10.1007/BF01899087. [DOI] [PubMed] [Google Scholar]
- Portela A, Pérez RJ, Vaccari J, Pérez JC, Stewart P. Muscle membrane depolarization by acetylcholine, choline and carbamylcholine, near and remote from motor end-plates. J Pharmacol Exp Ther. 1970;175:476–482. [PubMed] [Google Scholar]
- Pugh PC, Corriveau RA, Conroy WG, Berg DK. Novel subpopulation of neuronal acetylcholine receptors among those binding α-bungarotoxin. Mol Pharmacol. 1995;47:717–725. [PubMed] [Google Scholar]
- Rakhilin S, Drisdel RC, Sagher D, McGehee DS, Vallejo Y, Green WN. α-Bungarotoxin receptors contain α7 subunits in two different disulfide-bonded conformations. J Cell Biol. 1999;146:203–217. doi: 10.1083/jcb.146.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangwala F, Drisdel RC, Rakhilin S, Ko E, Atluri P, Harkins AB, Fox AP, Salman SB, Green WN. Neuronal α-bungarotoxin receptors differ structurally from other nicotinic acetylcholine receptors. J Neurosci. 1997;17:8201–8212. doi: 10.1523/JNEUROSCI.17-21-08201.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Role LW, Berg DK. Nicotinic receptors in the development and modulation of CNS synapses. Neuron. 1996;16:1077–1085. doi: 10.1016/s0896-6273(00)80134-8. [DOI] [PubMed] [Google Scholar]
- Romano SJ, Pugh PC, McIntosh JM, Berg DK. Neuronal-type acetylcholine receptors and regulation of α7 gene expression in vertebrate skeletal muscle. J Neurobiol. 1997;32:69–80. [PubMed] [Google Scholar]
- Sala C, Kimura I, Santoro G, Kimura M, Fumagalli G. Expression of two neuronal nicotinic receptor subunits in innervated and denervated adult rat muscle. Neurosci Lett. 1996;215:71–74. [PubMed] [Google Scholar]
- Séguéla P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain α7 a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D-K, Im Y-B, Jung J-S, Suh H-W, Huh S-O, Song J-H, Kim Y-H. Central injection of nicotine increases hepatic and splenic interleukin 6 (IL-6) mRNA expression and plasma IL-6 levels in mice: involvement of the peripheral sympathetic nervous system. FASEB J. 1999;13:1259–1267. doi: 10.1096/fasebj.13.10.1259. [DOI] [PubMed] [Google Scholar]
- Vernino S, Amador M, Luetje CW, Patrick J, Dani JA. Calcium modulation and high calcium permeability of neuronal nicotinic acetylcholine receptors. Neuron. 1992;8:127–135. doi: 10.1016/0896-6273(92)90114-s. [DOI] [PubMed] [Google Scholar]
- Vernino S, Rogers M, Radcliffe KA, Dani JA. Quantitative measurement of calcium flux through muscle and neuronal nicotinic acetylcholine receptors. J Neurosci. 1994;14:5514–5524. doi: 10.1523/JNEUROSCI.14-09-05514.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost CP, Winegar BD. Potency of agonists and competitive antagonists on adult- and fetal-type nicotinic acetylcholine receptors. Cell Mol Neurobiol. 1997;17:35–50. doi: 10.1023/a:1026325020191. [DOI] [PubMed] [Google Scholar]
- Yu CR, Role LW. Functional contribution of the α7 subunit to multiple subtypes of nicotinic receptors in embryonic chick sympathetic neurones. J Physiol. 1998a;509:651–665. doi: 10.1111/j.1469-7793.1998.651bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CR, Role LW. Functional contribution of the α5 subunit to neuronal nicotinic channels expressed by chick sympathetic ganglion neurones. J Physiol. 1998b;509:667–681. doi: 10.1111/j.1469-7793.1998.667bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z-W, Vijayaraghavan S, Berg DK. Neuronal acetylcholine receptors that bind α-bungarotoxin with high affinity function as ligand-gated ion channels. Neuron. 1994;12:167–177. doi: 10.1016/0896-6273(94)90161-9. [DOI] [PubMed] [Google Scholar]
- Zwart R, Vijverberg HPM. Potentiation and inhibition of neuronal α4 β4 nicotinic acetylcholine receptors by choline. Eur J Pharmacol. 2000;393:209–214. doi: 10.1016/s0014-2999(00)00002-9. [DOI] [PubMed] [Google Scholar]


