Abstract
ATP mediates intercellular communication. Mechanical stress and changes in cell volume induce ATP release from various cell types, both secretory and non-secretory. In the present study, we stressed Xenopus oocytes with a hypertonic solution enriched in mannitol (300 mm). We measured simultaneously ATP release and ionic currents from a single oocyte. A decrease in cell volume, the activation of an inward current and ATP release were coincident. We found two components of ATP release: the first was associated with granule or vesicle exocytosis, because it was inhibited by tetanus neurotoxin, and the second was related to the inward current. A single exponential described the correlation between ATP release and the hypertonic-activated current. Gadolinium ions, which block mechanically activated ionic channels, inhibited the ATP release and the inward current but did not affect the decrease in volume. Oocytes expressing CFTR (cystic fibrosis transmembrane regulator) released ATP under hypertonic shock, but ATP release was significantly inhibited in the first component: that related to granule exocytosis. Since the ATP measured is the balance between ATP release and ATP degradation by ecto-enzymes, we measured the nucleoside triphosphate diphosphohydrolase (NTPDase) activity of the oocyte surface during osmotic stress, as the calcium-dependent hydrolysis of ATP, which was inhibited by more than 50 % in hypertonic conditions. The best-characterized membrane protein showing NTPDase activity is CD39. Oocytes injected with an antisense oligonucleotide complementary to CD39 mRNA released less ATP and showed a lower amplitude in the inward current than those oocytes injected with water.
Purinergic signalling is almost ubiquitous. It is well known that ATP is kept inside cells because it is highly hydrophilic and does not permeate through lipidic cell membranes. However, ATP release has been reported in various cell types. Neuronal cells release ATP during synaptic transmission (Silinsky & Redman, 1996; Vizi et al. 2000), and it is generally supposed that all synaptic vesicles contain ATP, which is co-released with the main neurotransmitter, albeit in some cases some synaptic vesicles may only contain ATP (Bodin & Burnstock, 2001). Several laboratories have recorded ATP release from other non-neuronal cells such as astrocytes (Cotrina et al. 1998; Guthrie et al. 1999; Newman, 2001; Verderio & Matteoli, 2001; Stout et al. 2002) and from skeletal muscle fibres (Forrester, 1972). The purinergic signalling is especially relevant in the heart-vascular system, where P2 receptors have been detected in epithelial and smooth muscle fibres. This and the presence of P2 receptors in the same cells strongly suggest an autocrine purinergic loop. Endothelial cells also release ATP during shear stress or under hypotonic challenge (Yegutkin et al. 2000; Hashimoto et al. 2001; Buxton et al. 2001; Koyama et al. 2001; Hisadome et al. 2002; Schwiebert et al. 2002). Mechanically induced release of ATP has been confirmed in every cell type studied (Grygorczyk & Guyot, 2001). The sensitivity to gadolinium suggests that ATP release is related to some type of mechanically activated ionic channel(s) (Hamill & Martinac, 2001).
Purinergic receptors have been described in the ovarian follicle of the amphibian Xenopus laevis (Lotan et al. 1982; 1986; Arellano et al. 1996; King et al. 1996a,b) and also in granulosa and luteal cells from pigs or humans (Kamada et al. 1994). Moreover, ionic currents activated or regulated via purinergic receptors have been described in Xenopus follicles (Arellano et al. 1998; Pérez-Sanmartín et al. 2000) and mouse cumulus cell-enclosed oocytes (Arellano et al. 2002). Although the stimulation of these receptors and ion channels has been implicated in the development of the oocyte (Eppig et al. 1985; Billig & Rosberg, 1986; Downs et al. 1986), the cellular mechanisms involved are poorly understood.
Xenopus oocytes release ATP in resting conditions, but they increase the efflux of ATP when expressing purinergic PY receptors (Nakamura & Strittmatter, 1996) and CFTR (cystic fibrosis transmembrane regulator; Jiang et al. 1998), and under local mechanical stress (Maroto & Hamill, 2001) and hyperpolarizing pulses (Bodas et al. 2000). Some endogenous currents of Xenopus oocytes are linked to mechanically gated channels (Zhang & Hamill, 2000). Currents activated by hyperpolarization or hypertonic solutions share some common features: they are inward currents in oocytes clamped at −60 mV, they are non-selective and, finally, they are permeable to large cations and anions (Zhang & Hamill, 2000). Since we have already demonstrated that hyperpolarization pulses induce the release of ATP (Bodas et al. 2000), we aimed to explore the release of ATP in oocytes shrunk by hypertonic solutions that activate an inward current.
METHODS
Solutions and chemicals
Isotonic Ringer solution (NR, also Normal Ringer solution, 238 mosmol l−1) contained (mm): 115 NaCl, 2 KCl, 10 (Hepes/NaOH), 1.8 CaCl2, 1.8 MgCl2 (pH 7.4). Modified Barth's solution (MBS) contained (mm): 88 NaCl, 1 KCl, 2.4 NaHCO3, 20 Hepes, 0.82 MgSO4, 0.33 Ca(NO3)2, 0.41 CaCl2 (pH 7.5), supplemented with 100 IU ml−1 of penicillin and 0.1 mg ml−1 of streptomycin. Hypertonic solution (HS) was prepared by adding 300 mm mannitol to NR, which resulted in an osmolarity of 543 mosmol l−1, which was measured in a 5520 Wescor Osmometer.
Preparation of Xenopus oocytes
Mature female frogs (Xenopus laevis) were purchased from Centre d'Elevage de Xenopes (Montpellier, France). Frogs were maintained alive and handled in accordance with the EU laws and under the supervision of the Council for Animal Research Ethics of University of Barcelona. Oocytes were prepared as follows: frogs were anaesthetized in cold distilled water containing 1.7 g l−1 of tricaine (ethyl 3-aminobenzoate methanesulfonic acid, Sigma). Ovarian sacs were extracted by sterile surgical procedures and placed in NR. The frogs were immediately placed in a small water tank until they started to swim. Approximately 3 months after the first surgery, the frogs underwent contralateral surgery and were killed by decapitation. Oocytes of stages V and VI were collected using fine tip forceps and kept at 16–17 °C in MBS. The follicle envelope was removed by collagenase (Sigma, 1A; 0.5 mg ml−1) treatment in NR for 45–50 min. Electrophysiological measurements were made 3–48 h after defolliculation.
Electrophysiological records
Whole-cell data were recorded using a two-microelectrode voltage-clamp configuration. The voltage and the current microelectrodes were filled with KCl (3 m) and had resistances ranging from 1 to 2 MΩ. The bath electrode was an Ag-AgCl pellet that made contact with the recording solutions through an agar bridge. Membrane potential and current were digitized by a PC computer through a Digidata-1200A (Axon Instruments) converter. The board was controlled by the Whole Cell Analysis Program (kindly provided by John Dempster, University of Strathclyde, UK). The signal was filtered at half the frequency of acquisition. Solutions were changed by gravity and controlled by electrovalves (ALA, Scientific Instruments, New York).
Preparation of luminescent reaction
A vial of luciferase extract lantern from Photinus pyralis (Sigma) was resuspended in 1 ml of HS and desalted in a 10 ml 10 DG-Biorad column equilibrated with HS. 200 µl of d-luciferin (2.5 mg ml−1, Boehringer Mannheim) was added to the eluate and the resulting suspension was centrifuged for 30 s in a bench-top centrifuge. The eluate was frozen and maintained at −20 °C until use. An aliquot was thawed on ice and maintained at 4 °C until it was added to the recording chamber (125 µl).
Measurement and analysis of ATP release
We followed a procedure described elsewhere (Bodas et al. 2000). Briefly, oocytes were placed in the recording chamber and the two electrodes were inserted. After the chamber was equilibrated with HS (1–2 min), a small volume (5–20 µl) of luciferin-luciferase mixture was added to the recording chamber, which was completely light-proof. The light generated by the enzymatic reaction was transmitted through six optic fibres placed in front of the six planes of the recording cuvette. The end of the optic fibres was focused in front of a photomultiplier (Hamamatsu, R374) and controlled by a slit. The resulting electric signal was amplified in a P16 Grass amplifier (USA), filtered at 5 Hz in a Bessel filter (Frequency Devices, USA) and collected by the Digidata-1200A converter (Axon Instruments). To calculate the amount of ATP released, a known dose of ATP was added through a third pipette, with an electronic nanoinjector (Nanoject, Drummond). This dose was usually registered 2–6 min after starting the electrophysiological recording in HS, which was made at room temperature (22–23 °C).
To analyse ATP release, we integrated the light signal over a 1 min period and the amount of ATP enclosed in the area was calculated by comparison with the dose signal.
In some experiments, the light chain of tetanus toxin (L-TeNT) was injected 20–30 h before recording. The final concentration in the oocytes was around 10 nm. Tetanus toxin was prepared and kindly provided by Professor J. Blasi (University of Barcelona).
Estimation of Xenopus oocyte volume
Oocytes placed in a Petri dish containing NR were observed on an inverted microscope (Olympus CK 40) and the images digitized through an Olympus DP10 camera. Individual oocytes were transferred to a new Petri dish containing HS. Images were recorded for the period indicated. Assuming that oocytes are spherical in shape, cell volume was 4/3πr3, where r is the radius. At t = 0, the volume of the oocyte was taken as 100 %.
NTPDase activity (ecto-ATPase activity)
The NTPDase activity of defolliculated Xenopus oocytes was assessed as described elsewhere (Ziganshin et al. 1995). Groups of six oocytes were placed in 96 ELISA wells, in a volume of 100 µl of NR. Two groups of oocytes were assayed in the presence of NR or HS. In both cases, a subgroup of oocytes was incubated in the corresponding media, and calcium was replaced by 1 mm EGTA. Oocytes were first washed and subsequently incubated in the above medium for 30 min at room temperature. Media were then carefully replaced by fresh solutions supplemented or not by 100 µm ATP. After an additional 30 min incubation period, the media were removed and their phosphate contents were determined using the malachite green method (Lanzetta et al. 1979). Each condition was assayed in triplicate and results are shown as the means ± s.e.m. from four independent experiments. In some experiments, the mannitol in HS was replaced by sucrose or sorbitol. In other experiments, NTPDase activity was assayed in potato apyrase (grade VII, Sigma).
Expression of human CFTR in Xenopus oocytes
Plasmid containing full length cDNA for CFTR (pBS6.2-CFTR) was generously supplied by Professor Johanna Rommens (Department of Genetics, Hospital for Sick Children, Toronto, Canada). The plasmid (10 µg) was linearized with Xho I (Promega) and the resultant product was used for mRNA synthesis in vitro using the mCAP RNA Capping Kit (Stratagene). The mRNA obtained was injected (50 nl, 1–2 mg ml−1) into oocytes 3 days before membrane current recordings. CFTR-activated ionic currents were elicited with NR supplemented with 100 µm 8-Br-cAMP (Sigma), 1 mm IBMX (3-isobutyl-1-methylxanthine, Sigma) and 1 µm forskolin (Sigma). Only oocytes exhibiting the activated current were further stimulated with the hypertonic protocol.
Injection of DNA antisense oligonucleotide complementary to CD39 mRNA in Xenopus oocytes
An antisense oligonucleotide complementary to CD39 mRNA (antiCD39): 5′-TGTTGGTCAAGTTCAGCATGTAGCCCAG-3′ was designed from consensus sequences of human, rat, mouse, cow, chicken and Torpedo marmorata and synthesized by Invitrogen-Life Technologies. The oligonucleotide was re-hydrated in ultra-pure water and injected (50 nl, 2 mg ml−1) into oocytes 40–72 h before membrane current recordings.
Statistical analysis
Curves were fitted using Sigma Plot (SPSS). Statistical analysis was performed using Sigma Stat software (SPSS). Unless otherwise specified, values given in the text correspond to the mean ± s.e.m. When comparing two-group means of normally distributed data, the Student’ s t test was used. Otherwise, the Mann-Whitney rank sum test was applied. A significance level of P < 0.05 was adopted in all comparisons.
RESULTS
Oocytes in hypertonic (543 mosmol l−1) conditions showed a reversible decrease in volume of 13 ± 3 % (n = 6), and reached a steady state within ≈10 min, depending on the oocyte batch (Fig. 1A). During shrinkage, the oocytes released ATP (Fig. 1B). When they were recorded individually in voltage-clamp conditions with a membrane voltage (Vm) of −70 mV, the hypertonic medium induced an inward current and ATP release (Fig. 2). The time course of currents varied from one oocyte to another. At the beginning of recording, the amount of ATP released was apparently independent of the amplitude of the current. However, after 8 min, this correlation fitted an exponential curve (ATP release = a × exp(b × current), where b was 10−4 and a was 116; R = 0.997). The total amount of ATP released at the end of minute 20 of recording was 7 ± 2 pmol (n = 24).
Figure 1. Macroscopic changes induced by hypertonic shock in Xenopus oocytes.

A, reduction in cell volume (%) in 6 oocytes. B, example of ATP release measured in a single oocyte by means of the luciferin-luciferase reaction. This oocyte was not penetrated with fine-tipped electrodes. The trace corresponds to changes in light intensity, reflecting the presence of ATP in the medium. Just at the beginning of the recording, a known dose of ATP (0.6 pmol) was added to evaluate the amount of ATP released by the oocyte.
Figure 2. Simultaneous recording of ATP release and ionic currents from a single oocyte stressed by hypertonic solution.
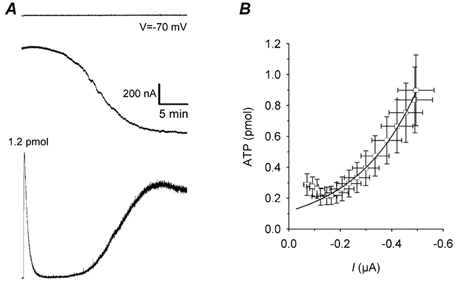
A, single oocyte superfused in hypertonic solution 2 min before the experiment. Upper trace: membrane voltage. Middle trace: membrane current. Bottom trace: ATP release. Note that in the light trace, we added a known dose of ATP (1.2 pmol) to evaluate the release of ATP. B, example illustrating a plot of ATP release vs. current and time (each point represents the ATP release vs. current amplitude during each minute of recording from minute 4 to minute 20). An exponential curve fitted the data obtained between minute 10 and minute 20 (n = 20).
The effect of the hypertonic solution applied for periods of 20 min was reversible (Fig. 3) and the initial volume was restored a short time after returning the oocytes to the isotonic solution. A second challenge with hypertonic solution reduced the cellular volume again, reaching the same values as recorded in the first perfusion. However, the hypertonic shock-activated inward current was blocked after a second hypertonic shock and ATP release was also inhibited. This inhibitory effect was not observed when oocytes were perfused with hypertonic solution for shorter periods such as 10 min. In these cases, hypertonic-activated current and ATP release after a second hypertonic stimulus were slightly inhibited (data not shown).
Figure 3. Effect of two consecutive hypertonic shocks on cell volume, inward current and ATP release.
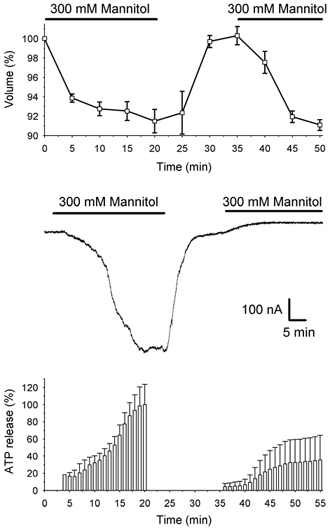
Upper graph: decrease in cell volume in 6 oocytes superfused twice (indicated with horizontal bars) in hypertonic solution. Middle trace: example of ionic currents recorded in one oocyte clamped at Vm=−70 mV in which two consecutive hypertonic superfusions (indicated with horizontal bars) were applied. Lower graph: percentage of ATP release with respect to the maximal release measured after the first period of 20 min of hypertonic perfusion (n = 3).
To test the possible exocytotic source of ATP, we treated some oocytes with tetanus neurotoxin (TeNT). Oocytes previously micro-injected with the light chain of tetanus neurotoxin (L-TeNT) did not show any significant change in the hypertonic shock-activated membrane currents (Fig. 4). However, the correlation between the current and the amount of ATP released could be fitted with an exponential function (ATP release = a × exp(b × current), where b was 10−4 and a was 25; R = 0.975). This fit included all points measured. The amount of ATP released at the beginning of the recording was decreased (compared with Fig. 2).
Figure 4. Effect of tetanus toxin on hypertonic shock-activated membrane current and ATP release.
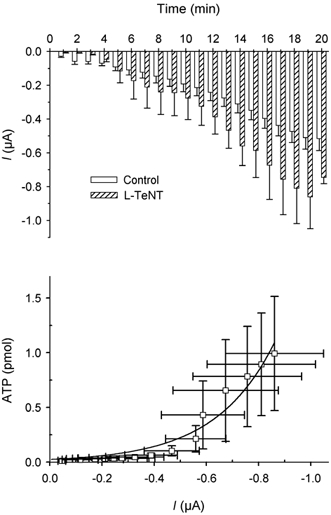
Upper graph: average of current amplitudes during 1 min of recording in non-injected oocytes (□, n = 33) and in L-TeNT injected oocytes ( , n = 4). Lower graph: plot of ATP release vs. current and time (each point represents the ATP release vs. current amplitude during each minute of recording from minute 4 to minute 20). An exponential function fitted the values of all the data represented. Compare the amount of ATP released in this condition and that in Fig. 2.
, n = 4). Lower graph: plot of ATP release vs. current and time (each point represents the ATP release vs. current amplitude during each minute of recording from minute 4 to minute 20). An exponential function fitted the values of all the data represented. Compare the amount of ATP released in this condition and that in Fig. 2.
We increased the cytoplasmatic ATP concentration by injecting 9.2 nl of K2ATP (250 mm, pH ≈ 7). In six oocytes we found an increase in the rate of ATP release (Fig. 5). The increase of ATP release was only recorded when oocytes were shrunken and the inward current was activated. When the same solution of ATP was injected into oocytes in isotonic conditions, no ATP release was detected (data not shown). In hypertonically stressed oocytes injected with ultra-pure water (9.2 nl), no increase in ATP was detected (Fig. 5).
Figure 5. Effect of intracellular ATP injection on ATP release and ionic current elicited by a hypertonic solution.
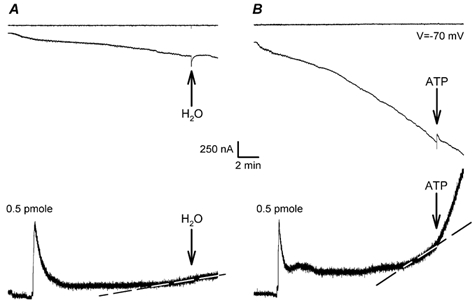
The cytoplasmic ATP concentration was increased by a direct injection of a small volume of an ATP solution. Examples of recordings in two oocytes superfused in hypertonic solution into which we injected 9.2 nl of distilled water (A) and 9.2 nl of 250 mm ATP (B). Upper traces: membrane voltage. Middle traces: membrane current. Bottom traces: ATP release. Note that in both light traces, we added a known dose of ATP (0.5 pmol) to evaluate the release of ATP. Arrows indicate the injection of solutions. Dashed lines indicate the slope of ATP release before injections.
To determine the influence of mechanically activated ionic channels, oocytes were treated with gadolinium. In all experiments, a known dose of ATP was applied in the presence of gadolinium, in order to check for any interference by gadolinium ions in the luciferin- luciferase-ATP reaction. When oocytes were preincubated for 10 min with an isotonic solution containing GdCl3 (200 µm), and then with a hypertonic solution containing the same concentration of GdCl3, we did not observe any change in the reduction of their volume (Fig. 6). Hypertonic shock-activated currents were significantly inhibited (66 %) after 20 min, throughout all the phases of the currents. The plot of ATP release vs. inward current is a flat horizontal line, revealing that the second component of ATP release (the ionic mechanism) was blocked, but the first component (the exocytotic mechanism) was not affected by gadolinium ions. We also explored the relationship between voltage and current in shrunken oocytes: the effect of gadolinium on the I-V ratio of the hypertonic shock-activated currents is summarized in Fig. 7. While the currents in hypertonic conditions were linear, we found a slight rectification in the presence of gadolinium. The reversal potential was −30 ± 1 mV in isotonic solution, −28 ± 1 mV in isotonic solution plus gadolinium, −23 ± 1 mV in hypertonic solution and −29 ± 1 mV in hypertonic solution plus gadolinium (n = 6, in all conditions).
Figure 6. Effect of gadolinium on cell volume, membrane current and ATP release during a hypertonic shock.
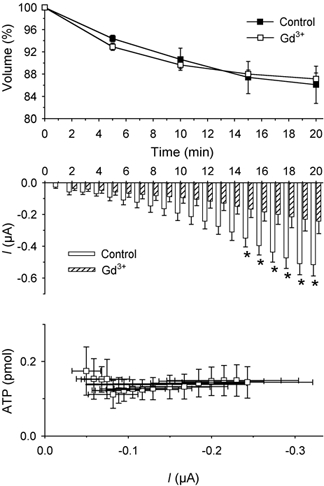
Upper graph: decrease in cell volume in oocytes superfused in hypertonic solution. The value at time 0 was set at 100 %. •, hypertonic solution (n = 6); ○, hypertonic solution plus 200 µm GdCl3 (n = 6). Middle graph: average of current amplitudes during 1 min of recording. □, non-treated oocytes (n = 33);  , gadolinium-treated oocytes (n = 11). *P < 0.05. Lower graph: plot of ATP release vs. current and time (each point represents the ATP release vs. current amplitude during each minute of recording from minute 4 to minute 20) showing the inhibition of ATP release and the hypertonic shock-activated currents.
, gadolinium-treated oocytes (n = 11). *P < 0.05. Lower graph: plot of ATP release vs. current and time (each point represents the ATP release vs. current amplitude during each minute of recording from minute 4 to minute 20) showing the inhibition of ATP release and the hypertonic shock-activated currents.
Figure 7. I-V relationship of hypertonic shock-activated currents.
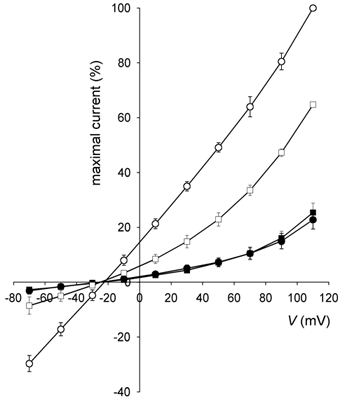
Current amplitudes are represented as a percentage of the amplitude of the current measured in non-treated oocytes in hypertonic medium at +110 mV. Circles: non-treated oocytes (n = 6) in isotonic (•) and hypertonic (○) media. Squares: gadolinium-treated oocytes (n = 6) in isotonic (▪) and hypertonic (□) media.
Some specific proteins like CFTR have been implicated in the extrusion of ATP from cells. Oocytes injected with 50–100 ng of mRNA encoding CFTR were tested with a solution containing 100 µm 8-Br-cAMP, 1 mm IBMX and 1 µm forskolin before being stimulated with hypertonic solutions. The current elicited by this solution was reversible and we did not detect any release of ATP. When the oocytes expressing CFTR were stressed by hypertonic solution, we found a significant (P < 0.05) decrease in the activated inward current (up to minute 15) and in ATP release (up to minute 9) (Fig 8). The plot of ATP released vs. current reveals an exponential function from minute 4 to minute 20 (ATP release = a × exp(b × current), where b was 10−4 and a was 48; R = 0.998).
Figure 8. Hypertonic shock-activated membrane currents and ATP release in oocytes expressing CFTR.
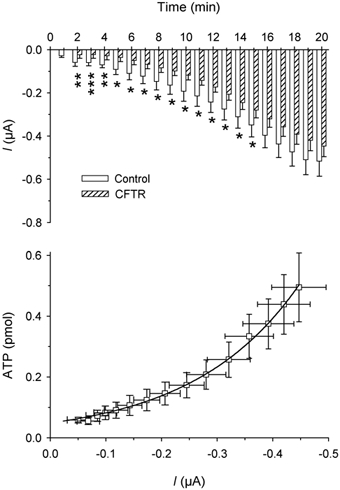
Upper graph: average of current amplitudes during 1 min of recording:. □, non-injected oocytes (n = 33);  , oocytes expressing CFTR (n = 17). *P < 0.05; **P < 0.01; ***P < 0.001. Lower graph: plot of ATP release vs. current and time (each point represents the ATP release vs. current amplitude during each minute of recording from minute 4 to minute 20). An exponential curve fitted all the data represented
, oocytes expressing CFTR (n = 17). *P < 0.05; **P < 0.01; ***P < 0.001. Lower graph: plot of ATP release vs. current and time (each point represents the ATP release vs. current amplitude during each minute of recording from minute 4 to minute 20). An exponential curve fitted all the data represented
The ATP measured above is the result of the equilibrium between ATP release and ATP degradation by ecto-enzymes such as NTPDases. The NTPDase activity of the intact oocyte surface was measured in isotonic and hypertonic conditions (Fig. 9) as the calcium-dependent ATPase activity. At low calcium concentrations (1 mm EGTA), the hydrolysis of ATP was much lower (0.03 ± 0.01 nmol inorganic phosphate (Pi) oocyte−1 (30 min)−1) than in the presence of 1.8 mm CaCl2 (0.28 ± 0.02 nmol Pi oocyte−1 (30 min)−1). After 30 min in hypertonic solution, the activity in medium containing 1 mm EGTA did not differ (0.03 ± 0.02 nmol Pi oocyte−1 (30 min)−1) from that measured in isotonic conditions. However, the calcium-dependent activity was reduced to 0.12 ± 0.02 nmol Pi. oocyte−1 (30 min)−1, which represents about a 56 % inhibition of NTPDase activity. To rule out the direct inhibition of NTPDase activity by mannitol, we assayed other NTPDases. NTPDase activity from potato was (112 ± 20) × 103 nmol (mg protein)−1 (30 min)−1 and (113 ± 20) × 103 nmol (mg protein)−1 (30 min)−1 when 300 mm mannitol was added (n = 3).
Figure 9. External NTPDase activity of oocytes during hypertonic shock.
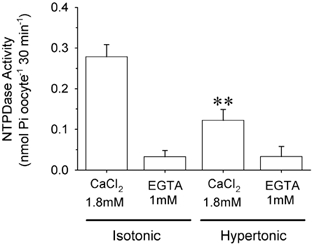
The bar histogram shows the NTPDase activity as ATP hydrolysis stimulated by calcium. We investigated the activity in isotonic and hypertonic conditions. Calcium-independent activity was very low and was insensitive to hypertonic shock. However, the calcium-stimulated activity was inhibited by 56 %. **P < 0.01 (n = 4).
Finally, we recorded ionic current and ATP release from CD39 antisense-injected oocytes (n = 13) and from water-injected oocytes (n = 8). In 13 of the 14 oocytes CD39 antisense oligonucleotide significantly reduced ATP release and the ionic current after 13 min of hypertonic exposure (Fig. 10).
Figure 10. Hypertonic shock-activated membrane currents and ATP release in oocytes injected with an antisense oligonucleotide complementary to CD39 mRNA.
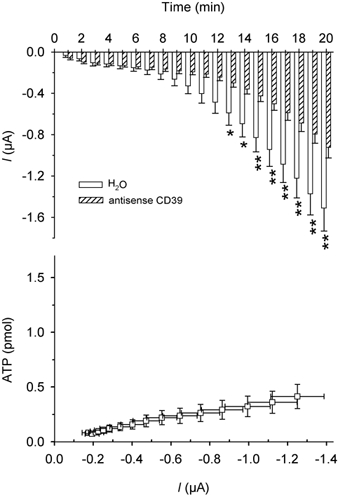
Upper graph: average of current amplitudes during 1 min of recording. □, water-injected oocytes (n = 8);  , CD39 antisense-injected oocytes (n = 13). *P < 0.05, **P < 0.01. Lower graph: plot of ATP release vs. current and time in CD39 antisense-injected oocytes (each point represents the ATP release vs. current amplitude during each minute of recording from minute 7 to minute 20).
, CD39 antisense-injected oocytes (n = 13). *P < 0.05, **P < 0.01. Lower graph: plot of ATP release vs. current and time in CD39 antisense-injected oocytes (each point represents the ATP release vs. current amplitude during each minute of recording from minute 7 to minute 20).
DISCUSSION
Cell volume regulation is a complex cellular response, that involves at least three steps (Montrose-Rafizadeh & Guggino, 1990). First, the cell must be able to detect changes in cell volume; second, it must initiate regulatory processes that alter intracellular solute content, and third, the cell must remember its initial volume and shut down the volume regulatory process. Mammalian cells show regulatory cell volume decrease under hypotonic conditions, but they do not always exhibit regulatory cell volume increase under hypertonic conditions (Montrose-Rafizadeh & Guggino, 1990).
Cell shrinkage elicited by a hyperosmotic solution promotes subsequent influx of ions and water; regulatory volume increase is mediated either by Na+-K+-Cl− cotransport or by the coupled activities of Na+-H+ exchange (NHE) and Cl−-HCO3− anion exchange (Goss et al. 2001). Several isoforms from mammalian and non-mammalian cells have been cloned, including Xenopus oocytes. To achieve a regulatory volume increase Xenopus oocytes must co-express the endogenous ion transporter xoNHE and at least the anion exchanger AE2 (Jiang et al. 1997), cloned from mouse (Grinstein et al. 1992). This indicates that Xenopus oocytes share the same first steps of cell volume regulation as mammalian cells and therefore the release of ATP induced by cell shrinkage should be a considered a general phenomenon related to volume adaptation.
Changes in cell volume are associated with ATP release, which is usually detected during cell swelling in hypotonic conditions (Hazama et al. 2000; Grygorczyk & Guyot, 2001; Koyama et al. 2001; Okada et al. 2001; Sabirov et al. 2001; Boudreault & Grygorczyk, 2002). Several reports have shown that hypotonic shock-activated currents are observed only in follicle-enclosed Xenopus oocytes (Arellano & Miledi, 1993, 1995; Ackerman et al. 1994; Aleu et al. 1997). We tested defolliculated Xenopus oocytes in hypotonic conditions (95 mosmol l−1) and we did not detect any release of ATP or activation of any ionic current. Moreover, we did not detect ATP release in oocytes in which even the vitelline envelope had been completely removed (data not shown). In contrast, oocytes challenged with hypertonic solution released ATP and showed a reduction in volume. We also recorded ATP release in oocytes that were not impaled by glass microelectrodes, which revealed that the release was not due to leakage around the electrodes during cell shrinkage. A hypertonic challenge in Xenopus oocytes also activated an inward current that has been described elsewhere (Zhang & Hamill, 2000) and is reminiscent of that activated by mechanical stress (Saitou et al. 2000).
The release of ATP was accompanied by activation of an inward current. While the effect of hypertonic solution on cell volume was reversible, even after a second application, activation of the inward current was totally blocked during a second hypertonic challenge and release of ATP, though still detectable, represented only 35 % of the amount of ATP released during the first hypertonic stimulus.
The hypertonic shock-activated inward current was completely reversible, but was blocked after a second hypertonic challenge, suggesting an association between the opening of the current and some disruption between the cytoskeleton and the plasma membrane. In fact, hypertonic solutions applied to Xenopus oocytes have been shown to induce the formation of individual cytoskeleton-free vesicles (Zhang et al. 2000). Such vesicles showed a reduced sensitivity to mechanically activated channels. The association between the cytoskeleton and mechanically activated channels has been reported in other experimental models such as neurons, in which the use of cytochalasin or colchicines disrupts the assembly of cytoskeleton and decreases the activity of mechanically activated channels (Cho et al. 2002).
The ATP release-current relationship described two major components of hypertonic shock-activated ATP release. The first component took place during the first 10 min of shrinkage, where the amount of ATP released and the amplitude of the inward current were not correlated. This indicates that a non-ionic process is involved, for example exocytosis. The second component began when oocyte volume reached a minimum. In this phase the amount of ATP released correlated with the amplitude of the inward current.
It is well known that exocytosis is inhibited by clostridial toxins (Rosseto et al. 2001; Aleu et al. 2002). To analyse the possible exocytotic origin of ATP release we used tetanus neurotoxin (TeNT). To verify the action of this toxin, we injected the light chain of TeNT (L-TeNT) instead of superfusing the oocytes with the holotoxin, because it is not known whether Xenopus oocytes express any receptors to clostridial toxins on their surface. We found that L-TeNT injected into oocytes inhibited the first component of ATP release induced by the hypertonic stimulus. In oocytes injected with L-TeNT, all points analysed fitted an exponential curve, and the fitted curves in L-TeNT-treated and non-L-TeNT-treated oocytes were very similar, suggesting that both describe the same cellular mechanism. Since only the release of ATP during shrinkage (the first component) is sensitive to L-TeNT, this component may correspond to an exocytosis-mediated release. In agreement with these results, a recent study provided evidence that ATP release from Xenopus oocytes stressed with local mechanical stimulation is sensitive to Brefeldin A, which interrupts the traffic of vesicles from the Golgi apparatus to the cell surface, suggesting an exocytotic pathway for ATP release (Maroto & Hamill, 2001). In other non-secretory cells also, such as those from the pig ureter epithelium (Knight et al. 2002), the release of ATP is associated with the exocytosis of small vesicles.
The second component of ATP release (once oocyte volume reached the minimum) was strongly inhibited by gadolinium, a classical blocker of mechanically activated ionic channels, but during the shrinking process (first component) there was no significant difference between control and gadolinium-treated oocytes: this implies that the first component of ATP release (the exocytotic process) is not affected by gadolinium. It has been reported that gadolinium also enhances ATP release during cell swelling owing to its membrane fusogenic activity (Boudreault & Grygorczyk, 2002), but we ruled out this possibility in Xenopus oocytes because, at the concentrations tested, ATP release did not increase.
During hypertonic challenge, gadolinium did not inhibit shrinkage of the oocytes, but the hypertonic shock-activated current was strongly inhibited. Similar results were obtained during the activation of osmoreceptors in supraoptic neurons (Oliet & Bourque, 1996) and the concentrations at which gadolinium was more active were in the range of 100–500 µm. Xenopus oocytes may thus be sensitive to hypertonic solutions in a manner similar to neurons. However, in oocytes the gadolinium-induced inhibition of the hypertonic-activated current was coincident with the inhibition of ATP release. The inhibitory effect of gadolinium on both the current and ATP release was observed throughout the period of current activation.
The reversal potential of the hypertonic-activated current was the same under all conditions tested and was very close to the reversal potential of Cl− in Xenopus oocytes calculated according to their intracellular Cl− concentration (Dascal, 1986). Gadolinium did not change the reversal potential in isotonic or hypertonic solutions. If ATP is released as a charged molecule, only a small fraction of the current (1 %) would be supported by the anionic ATP, and this net ATP current would be masked by the background noise of the amplifier. This explains why, when the intracellular concentration of ATP was increased, we recorded a clear increase in ATP release but not in current amplitude. An increase in ATP release was only detected when the inward current was activated and the oocyte had shrunk.
A link between ATP release and chloride flux has been demonstrated in Xenopus oocytes expressing functional CFTR (Jiang et al. 1998). We did not detect ATP release when activating CFTR with cAMP, as reported by other groups, supporting the view that CFTR is not permeable to ATP (Grygorczyk & Hanrahan, 1997; Watt et al. 1998; Braunstein et al. 2001). In oocytes injected with CFTR during the shrinking process, the current activated by the hypertonic stimulus was inhibited, but there was little effect on the ionic current recorded after the oocytes reached their minimum volumes. CFTR and L-TeNT inhibited ATP release during the first minutes of recording but not later, suggesting that they affect the same processses of ATP secretion. This would support one of the hypotheses formulated by Devidas & Guggino (1997), who propose that CFTR indirectly controls the exocytosis of ATP.
The membrane protein CD39 is also related to ATP transport through the cell plasma membrane, as suggested elsewhere (Wang et al. 1998; Bodas et al. 2000; Abraham et al. 2001). CD39 has two transmembrane domains and a small hydrophobic loop (Wang et al. 1998), which is analogous to other ionic channels. CD39 is a member of the family of type 1 NTPDases, since it hydrolyses ATP or ADP in a calcium-dependent manner (see Zimmermann, 2000 for review). Endogenous NTPDase activity has been described in Xenopus oocytes (Ziganshin et al. 1995; Bodas et al. 2000; Aguilar et al. 2001). In our study, NTPDase activity was inhibited by more than 50 % at the end of the period of hypertonic challenge. This inhibition is likely to contribute to the exponential increase in ATP detected because the probability of ATP being degraded decreases with time. We ruled out a direct effect of mannitol, since mannitol had no inhibitory effect on the NTPDase activity of potato apyrase. The amino acid sequence responsible for the activity of NTPDase has been conserved in all types of NTPDases, including potato apyrase, so any direct inhibitory effects of mannitol would be apparent in all of them. The inhibition of NTPDase activity may also be due to endocytosis of the endogenous CD39, which has been associated with caveolae (Koziak et al. 2000). However, under the condition of hypertonic stimulus, we detected a release of ATP that was sensitive to clostridial toxins, indicating an increase in the rate of exocytosis. Since the increase in ATP release is coincident with inhibition of NTPDase activity, and we have suggested that CD39 is involved in plasma membrane ATP transport (Bodas et al. 2000), CD39 may have two conformations: one in which it behaves as a hydrolysing enzyme and another in which it behaves as a transporter. In hypertonic conditions, a significant part of the endogenous CD39 of oocytes would adopt the transporter conformation, thus reducing the number of molecules acting as NTPDase. The experiments performed in oocytes injected with CD39 antisense oligonucleotide showed a decrease in ATP and also in inward current (second component), reinforcing the view that CD39 is involved, directly or indirectly, in ATP release induced by hypertonic stress. Moreover these experiments also relate the transport of ATP to the inward current. In addition, our group has demonstrated that in Xenopus oocytes expressing human CD39 there is an increase in the hyperpolarizing-activated current associated with ATP release (Bodas et al. 2000).
Our studies have focused entirely on ATP release induced by hypertonic stress, but ATP release may also be induced in Xenopus oocytes by mechanical stress (Maroto & Hamill, 2001) and hyperpolarizing pulses (Bodas et al. 2000). Other mechanisms implicated in ATP release involve hemi-gap junctions, as recently described in astrocytes (Stout et al. 2002). Xenopus oocytes express endogenous hemi-gap channels Cx38 (Ebihara, 1996), and they may be involved in ATP release in this system.
Finally, we conclude that ATP release is induced by hypertonic stress in Xenopus oocytes via two different mechanisms: one related to exocytosis and the other related to an ionic current. CD39 is, directly or indirectly, implicated in this process.
Acknowledgments
This study was supported by the Ministerio de Ciencia y Tecnología (DGI) of the Spanish Government, the CIRIT of the Generalitat de Catalunya, the Fundació La Marató de TV3 and the Fundació August Pi i Sunyer. We thank Professor Blasi for the gift of L-TeNT and helpful comments. We also thank the Language Advisory Service of the Universitat de Barcelona for linguistic help. The Whole Cell Analysis Program was kindly provided by John Dempster (University of Strathclyde, Scotland, UK). Finally, we are indebted to Professor Johanna Rommens, Department of Genetics, Hospital for Sick Children, Toronto, Canada, for the generous gift of cDNA encoding CFTR.
REFERENCES
- Abraham EH, Sterling KM, Kim RJ, Saliknova AY, Huffman HB, Crockett MA, Johnston N, Parker HW, Boyle WE, Jr, Hartov A, Demidenko E, Efird J, Kahn J, Grubman SA, Jefferson DM, Robson SC, Thakar JH, Lorico A, Rappa G, Sartorelli AC, Okunieff P. Erythrocyte membrane ATP binding cassette (ABC) proteins: MRP1 and CFTR as well as CD39 (ecto-apyrase) involved in RBC ATP transport and elevated blood plasma ATP of cystic fibrosis. Blood Cell Mol Dis. 2001;27:165–180. doi: 10.1006/bcmd.2000.0357. [DOI] [PubMed] [Google Scholar]
- Ackerman MJ, Wickman KD, Clapham DE. Hypotonicity activates a native chloride current in Xenopus oocytes. J Gen Physiol. 1994;103:153–179. doi: 10.1085/jgp.103.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar JS, Reyes R, Asensio AC, Oaknin S, Rotllan P, Miledi R. Ectoenzymatic breakdown of diadenosine polyphosphates by Xenopus laevis oocytes. Eur J Biochem. 2001;268:1289–1297. doi: 10.1046/j.1432-1327.2001.01987.x. [DOI] [PubMed] [Google Scholar]
- Aleu J, Blasi J, Solsona C, Marsal J. Calcium-dependent acetylcholine release from Xenopus oocytes: simultaneous ionic current and acetylcholine release recordings. Eur J Neurosci. 2002;16:1442–1448. doi: 10.1046/j.1460-9568.2002.02208.x. [DOI] [PubMed] [Google Scholar]
- Aleu J, Ivorra I, Lejarreta M, González-Ros JM, Morales A, Ferragut JA. Functional incorporation of P-glycoprotein into Xenopus oocyte plasma membrane fails to elicit a swelling-evoked conductance. Biochem Bioph Res Co. 1997;237:407–412. doi: 10.1006/bbrc.1997.7150. [DOI] [PubMed] [Google Scholar]
- Arellano RO, Gary E, Miledi R. Cl− currents activated via purinergic receptors in Xenopus follicles. Am J Physiol. 1998;274:C333–340. doi: 10.1152/ajpcell.1998.274.2.C333. [DOI] [PubMed] [Google Scholar]
- Arellano RO, Martnez-Torres A, Garay E. Ionic currents activated via purinergic receptors in the cumulus cell-enclosed mouse oocyte. Biol Reprod. 2002;67:837–846. doi: 10.1095/biolreprod.102.003889. [DOI] [PubMed] [Google Scholar]
- Arellano RO, Miledi R. Novel Cl− currents elicited by follicle stimulating hormone and acetylcholine in follicle-enclosed Xenopus oocytes. J Gen Physiol. 1993;102:833–857. doi: 10.1085/jgp.102.5.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arellano RO, Miledi R. Functional role of follicular cells in the generation of osmolarity-dependent Cl− currents in Xenopus follicles. J Physiol. 1995;488:351–357. doi: 10.1113/jphysiol.1995.sp020971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arellano RO, Woodward RM, Miledi R. Ion Channels. Vol. 4. New York: Plenum press; 1996. Ion channels and membrane receptors in follicle-enclosed Xenopus oocytes; pp. 203–259. [DOI] [PubMed] [Google Scholar]
- Billig H, Rosberg S. Gonadotropin depression of adenosine triphosphate levels and interaction with adenosine in rat granulosa cells. Endocrinology. 1986;118:645–652. doi: 10.1210/endo-118-2-645. [DOI] [PubMed] [Google Scholar]
- Bodas E, Aleu J, Pujol G, Martin-Satué M, Marsal J, Solsona C. ATP crossing the cell plasma membrane generates an ionic current in Xenopus oocytes. J Biol Chem. 2000;275:20268–20273. doi: 10.1074/jbc.M000894200. [DOI] [PubMed] [Google Scholar]
- Bodin P, Burnstock G. Purinergic signalling: ATP release. Neurochem Res. 2001;26:959–969. doi: 10.1023/a:1012388618693. [DOI] [PubMed] [Google Scholar]
- Boudreault F, Grygorczyk R. Cell swelling-induced ATP release and gadolinium-sensitive channels. Am J Physiol Cell Physiol. 2002;282:C219–226. doi: 10.1152/ajpcell.00317.2001. [DOI] [PubMed] [Google Scholar]
- Braunstein GM, Roman RM, Clancy JP, Kudlow BA, Taylor AL, Shylonsky VG, Jovov B, Peter K, Jilling T, Ismailov II, Benos DJ, Schwiebert LM, Fitz JG, Schwiebert EM. Cystic fibrosis transmembrane conductance regulator facilitates ATP release by stimulating a separate ATP release channel for autocrine control of cell volume regulation. J Biol Chem. 2001;276:6621–6630. doi: 10.1074/jbc.M005893200. [DOI] [PubMed] [Google Scholar]
- Buxton IL, Kaiser RA, Oxhorn BC, Cheek DJ. Evidence supporting the Nucleotide Axis Hypothesis: ATP release and metabolism by coronary endothelium. Am J Physiol Heart Circ Physiol. 2001;281:H1657–1666. doi: 10.1152/ajpheart.2001.281.4.H1657. [DOI] [PubMed] [Google Scholar]
- Cho H, Shin J, Shin CY, Lee SY, Oh U. Mechanosensitive ion channels in cultured sensory neurons of neonatal rats. J Neurosci. 2002;22:1238–1247. doi: 10.1523/JNEUROSCI.22-04-01238.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrina ML, Lin JH, Nedergaard M. Cytoskeletal assembly and ATP release regulate astrocytic calcium signaling. J Neurosci. 1998;18:8794–8804. doi: 10.1523/JNEUROSCI.18-21-08794.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascal N. The use of Xenopus oocytes for the study of ion channels. CRC Crit Rev Biochem. 1986;22:317–387. doi: 10.3109/10409238709086960. [DOI] [PubMed] [Google Scholar]
- Devidas S, Guggino WB. The cystic fibrosis transmembrane conductance regulator and ATP. Curr Opin Cell Biol. 1997;9:547–552. doi: 10.1016/s0955-0674(97)80032-4. [DOI] [PubMed] [Google Scholar]
- Downs SM, Coleman DL, Eppig JJ. Maintenance of murine oocyte meiotic arrest: uptake and metabolism of hypoxanthine and adenosine by cumulus cell-enclosed and denuded oocytes. Dev Biol. 1986;117:174–183. doi: 10.1016/0012-1606(86)90359-3. [DOI] [PubMed] [Google Scholar]
- Ebihara L. Xenopus connexin38 forms hemi-gap-junctional channels in the nonjunctional plasma membrane of Xenopus oocytes. Biophys J. 1996;71:742–748. doi: 10.1016/S0006-3495(96)79273-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppig JJ, Ward-Bailey PF, Coleman DL. Hypoxanthine and adenosine in murine ovarian follicular fluid: concentrations and activity in maintaining oocyte meiotic arrest. Biol Reprod. 1985;33:1041–1049. doi: 10.1095/biolreprod33.5.1041. [DOI] [PubMed] [Google Scholar]
- Forrester T. An estimate of adenosine triphosphate release into the venous effluent from exercising human forearm muscle. J Physiol. 1972;224:611–628. doi: 10.1113/jphysiol.1972.sp009915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss GG, Jiang L, Vandorpe DH, Kieller D, Chernova MN, Robertson M, Alper SL. Role of JNK in hypertonic activation of Cl(-)-dependent Na(+)/H(+). exchange in Xenopus oocytes. Am J Physiol Cell Physiol. 2001;281:C1978–1990. doi: 10.1152/ajpcell.2001.281.6.C1978. [DOI] [PubMed] [Google Scholar]
- Grinstein S, Woodside M, Sardet C, Pouyssegur J, Rotin D. Activation of the Na+/H+ antiporter during cell volume regulation. Evidence for a phosphorylation-independent mechanism. J Biol Chem. 1992;267:23823–23828. [PubMed] [Google Scholar]
- Grygorczyk R, Guyot A. Osmotic swelling-induced ATP release: a new role for tyrosine and Rho-kinases? J Physiol. 2001;532:582. doi: 10.1111/j.1469-7793.2001.0582e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grygorczyk R, Hanrahan JW. CFTR-independent ATP release from epithelial cells triggered by mechanical stimuli. Am J Physiol. 1997;272:C1058–1066. doi: 10.1152/ajpcell.1997.272.3.C1058. [DOI] [PubMed] [Google Scholar]
- Guthrie PB, Knappenberger J, Segal M, Bennett MV, Charles AC, Kater SB. ATP released from astrocytes mediates glial calcium waves. J Neurosci. 1999;19:520–528. doi: 10.1523/JNEUROSCI.19-02-00520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Martinac B. Molecular basis of mechanotransduction in living cells. Physiol Rev. 2001;81:685–740. doi: 10.1152/physrev.2001.81.2.685. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Shinozuka K, Sasaki T, Tanaka N, Hossain S, Kubota Y, Tamura K, Shido O, Kunitomo M. Nicorandil-induced ATP release in endothelial cells of rat caudal artery is associated with increase in intracellular Ca(2+) Eur J Pharmacol. 2001;416:179–183. doi: 10.1016/s0014-2999(01)00867-6. [DOI] [PubMed] [Google Scholar]
- Hazama A, Fan HT, Abdullaev I, Maeno E, Tanaka S, Ando-Akatsuka Y, Okada Y. Swelling-activated, cystic fibrosis transmembrane conductance regulator-augmented ATP release and Cl− conductances in murine C127 cells. J Physiol. 2000;523:1–11. doi: 10.1111/j.1469-7793.2000.t01-6-00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisadome K, Koyama T, Kimura C, Droogmans G, Ito Y, Oike M. Volume-regulated anion channels serve as an auto/paracrine nucleotide release pathway in aortic endothelial cells. J Gen Physiol. 2002;119:511–520. doi: 10.1085/jgp.20028540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Chernova MN, Alper SL. Secondary regulatory volume increase conferred on Xenopus oocytes by expression of AE2 anion exchanger. Am J Physiol. 1997;272:C191–202. doi: 10.1152/ajpcell.1997.272.1.C191. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Mak D, Devidas S, Schwiebert EM, Bragin A, Zhang Y, Skach WR, Guggino WB, Foskett JK, Engelhardt JF. Cystic fibrosis transmembrane conductance regulator-associated ATP release is controlled by a chloride sensor. J Cell Biol. 1998;143:645–657. doi: 10.1083/jcb.143.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada S, Blackmore PF, Oehninger S, Gordon K, Hodgen GD. Existence of P2-purinoceptors on human and porcine granulosa cells. J Clin Endocr Metab. 1994;78:650–656. doi: 10.1210/jcem.78.3.8126137. [DOI] [PubMed] [Google Scholar]
- King BF, Pintor J, Wang S, Ziganshin AU, Ziganshina LE, Burnstock G. A novel P1 purinoceptor activates an outward K+ current in follicular oocytes of Xenopus laevis. J Pharmacol Exp Ther. 1996a;276:93–100. [PubMed] [Google Scholar]
- King BF, Wang S, Burnstock G. P2 purinoceptor-activated inward currents in follicular oocytes of Xenopus laevis. J Physiol. 1996b;494:17–28. doi: 10.1113/jphysiol.1996.sp021472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight GE, Bodin P, De Groat WC, Burnstock G. ATP is released from guinea pig ureter epithelium on distension. Am J Physiol Renal Physiol. 2002;282:F281–288. doi: 10.1152/ajprenal.00293.2000. [DOI] [PubMed] [Google Scholar]
- Koyama T, Oike M, Ito Y. Involvement of Rho-kinase and tyrosine kinase in hypotonic stress-induced ATP release in bovine aortic endothelial cells. J Physiol. 2001;532:759–769. doi: 10.1111/j.1469-7793.2001.0759e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koziak K, Kaczmarek E, Kittel A, Sevigny J, Blusztajn JK, Schulte Am, Esch J, 2nd, Imai M, Guckelberger O, Goepfert C, Qawi I, Robson SC. Palmitoylation targets CD39/endothelial ATP diphosphohydrolase to caveolae. J Biol Chem. 2000;275:2057–2062. doi: 10.1074/jbc.275.3.2057. [DOI] [PubMed] [Google Scholar]
- Lanzetta PA, Alvarez LJ, Reinach PS, Candia OA. An improved assay for nanomole amounts of inorganic phosphate. Anal Biochem. 1979;100:95–97. doi: 10.1016/0003-2697(79)90115-5. [DOI] [PubMed] [Google Scholar]
- Lotan I, Dascal N, Cohen S, Lass Y. Adenosine-induced slow ionic currents in the Xenopus oocyte. Nature. 1982;296:572–574. doi: 10.1038/298572a0. [DOI] [PubMed] [Google Scholar]
- Lotan I, Dascal N, Cohen S, Lass Y. ATP-evoked membrane responses in Xenopus oocytes. Pflugers Arch. 1986;406:158–162. doi: 10.1007/BF00586677. [DOI] [PubMed] [Google Scholar]
- Maroto R, Hamill OP. Brefeldin A block of integrin-dependent mechanosensitive ATP release from Xenopus oocytes reveals a novel mechanism of mechanotransduction. J Biol Chem. 2001;276:23867–23872. doi: 10.1074/jbc.M101500200. [DOI] [PubMed] [Google Scholar]
- Montrose-Rafizadeh C, Guggino WB. Cell volume regulation in the nephron. Annu Rev Physiol. 1990;52:761–772. doi: 10.1146/annurev.ph.52.030190.003553. [DOI] [PubMed] [Google Scholar]
- Nakamura F, Strittmatter SM. P2Y1 purinergic receptors in sensory neurons: contribution to touch-induced impulse generation. Proc Natl Acad Sci U S A. 1996;93:10465–10470. doi: 10.1073/pnas.93.19.10465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA. Propagation of intercellular calcium waves in retinal astrocytes and Muller cells. J Neurosci. 2001;21:2215–2223. doi: 10.1523/JNEUROSCI.21-07-02215.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y, Maeno E, Shimizu T, Dezaki K, Wang J, Morishima S. Receptor-mediated control of regulatory volume decrease (RVD) and apoptotic volume decrease (AVD) J Physiol. 2001;532:3–16. doi: 10.1111/j.1469-7793.2001.0003g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliet SH, Bourque CW. Gadolinium uncouples mechanical detection and osmoreceptor potential in supraoptic neurons. Neuron. 1996;16:175–181. doi: 10.1016/s0896-6273(00)80034-3. [DOI] [PubMed] [Google Scholar]
- Pérez-Sanmartín AL, Miledi R, Arellano RO. Activation of volume-regulated Cl− channels by ACh and ATP in Xenopus follicles. J Physiol. 2000;525:721–734. doi: 10.1111/j.1469-7793.2000.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetto O, Seveso M, Caccin P, Schiavo G, Montecucco C. Tetanus and botulinum neurotoxins: turning bad guys into good by research. Toxicon. 2001;39:27–41. doi: 10.1016/s0041-0101(00)00163-x. [DOI] [PubMed] [Google Scholar]
- Sabirov RZ, Dutta AK, Okada Y. Volume-dependent ATP-conductive large-conductance anion channel as a pathway for swelling-induced ATP release. J Gen Physiol. 2001;118:251–266. doi: 10.1085/jgp.118.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou T, Ishikawa T, Obara K, Nakayama K. Characterization of whole-cell currents elicited by mechanical stimulation of Xenopus oocytes. Pflugers Arch. 2000;440:858–865. doi: 10.1007/s004240000337. [DOI] [PubMed] [Google Scholar]
- Schwiebert LM, Rice WC, Kudlow BA, Taylor AL, Schwiebert EM. Extracellular ATP signalling and P2X nucleotide receptors in monolayers of primary human vascular endothelial cells. Am J Physiol Cell Physiol. 2002;282:C289–301. doi: 10.1152/ajpcell.01387.2000. [DOI] [PubMed] [Google Scholar]
- Silinsky EM, Redman RS. Synchronous release of ATP and neurotransmitter within milliseconds of a motor nerve impulse in the frog. J Physiol. 1996;492:815–822. doi: 10.1113/jphysiol.1996.sp021348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout CE, Costantin JL, Naus CC, Charles AC. Intercellular calcium signalling in astrocytes via ATP release through connexin hemichannels. J Biol Chem. 2002;277:10482–10488. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- Wang TF, Ou Y, Guidotti G. The transmembrane domains of ectoapyrase (CD39) affect its enzymatic activity and quaternary structure. J Biol Chem. 1998;273:24814–24821. doi: 10.1074/jbc.273.38.24814. [DOI] [PubMed] [Google Scholar]
- Watt WC, Lazarowski ER, Boucher RC. Cystic fibrosis transmembrane regulator-independent release of ATP. Its implications for the regulation of P2Y2 receptors in airway epithelia. J Biol Chem. 1998;273:14053–14058. doi: 10.1074/jbc.273.22.14053. [DOI] [PubMed] [Google Scholar]
- Verderio C, Matteoli M. ATP mediates calcium signaling between astrocytes and microglial cells: modulation by IFN-gamma. J Immunol. 2001;166:6383–6391. doi: 10.4049/jimmunol.166.10.6383. [DOI] [PubMed] [Google Scholar]
- Vizi ES, Nitahara K, Sato K, Sperlagh B. Stimulation-dependent release, breakdown, and action of endogenous ATP in mouse hemidiaphragm preparation: the possible role of ATP in neuromuscular transmission. J Autonom Nerv Syst. 2000;81:278–284. doi: 10.1016/s0165-1838(00)00129-6. [DOI] [PubMed] [Google Scholar]
- Yegutkin G, Bodin P, Burnstock G. Effect of shear stress on the release of soluble ecto-enzymes ATPase and 5′-nucleotidase along with endogenous ATP from vascular endothelial cells. Brit J Pharmacol. 2000;129:921–926. doi: 10.1038/sj.bjp.0703136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Gao F, Popov VL, Wen JW, Hamill OP. Mechanically gated channel activity in cytoskeleton-deficient plasma membrane blebs and vesicles from Xenopus oocytes. Zhang Y & Hamill OP (2000). Calcium-, voltage- and osmotic stress-sensitive currents in Xenopus oocytes and their relationship to single mechanically gated channels. J Physiol. 2000;523:83–99. doi: 10.1111/j.1469-7793.2000.t01-1-00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- J Physiol. 523:117–130. [Google Scholar]
- Ziganshin AU, Ziganshina LE, King BE, Burnstock G. Characteristics of ecto-ATPase of Xenopus oocytes and the inhibitory actions of suramin on ATP breakdown. Pflugers Arch. 1995;429:412–418. doi: 10.1007/BF00374157. [DOI] [PubMed] [Google Scholar]
- Zimmermann H. Extracellular metabolism of ATP and other nucleotides. N-S Arch Pharmacol. 2000;362:299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]


