Abstract
Current evidence indicates that the physiological functions of inflammatory cells are highly sensitive to their microenvironment, which is partially determined by the inflammatory cells and their potential targets. In the present investigation, interactions between neutrophils, macrophages and muscle cells that may influence muscle cell death are examined. Findings show that in the absence of macrophages, neutrophils kill muscle cells in vitro by superoxide-dependent mechanisms, and that low concentrations of nitric oxide (NO) protect against neutrophil-mediated killing. In the absence of neutrophils, macrophages kill muscle cells through a NO-dependent mechanism, and the presence of target muscle cells causes a three-fold increase in NO production by macrophages, with no change in the concentration of inducible nitric oxide synthase. Muscle cells that are co-cultured with both neutrophils and macrophages in proportions that are observed in injured muscle show cytotoxicity through a NO-dependent, superoxide-independent mechanism. Furthermore, the concentration of myeloid cells that is necessary for muscle killing is greatly reduced in assays that use mixed myeloid cell populations, rather than uniform populations of neutrophils or macrophages. These findings collectively show that the magnitude and mechanism of muscle cell killing by myeloid cells are modified by interactions between muscle cells and neutrophils, between muscle cells and macrophages and between macrophages and neutrophils.
The physiological functions of myeloid cells are highly sensitive to the microenvironment, so that the function of a single myeloid cell type in isolation would probably differ from the function of that same cell type in the presence of other myeloid cell populations. For example, activated macrophages are a rich source of nitric oxide (NO) production and NO may either potentiate or inhibit neutrophil-mediated cytotoxicity (Moilanen & Vapaatalo, 1995). Potential mechanisms through which increased cytotoxicity could occur include the generation of highly reactive, cytotoxic peroxynitrite through NO reaction with superoxide (Gryglewski et al. 1986), or through increased activation of neutrophil myeloperoxidase by NO (Abu-Soud & Hazen, 2000) to produce more toxic free radicals. However, NO may also inhibit neutrophil-mediated cytotoxicity if it were to inactivate nicotinamide adenine dinucleotide phosphate in neutrophils (Clancy et al. 1992) and thereby decrease superoxide production, or if it were to induce neutrophil apoptosis (Fortenberry et al. 1990; Blaylock et al. 1998; Ward et al. 2000). Thus, the cytotoxic capacity of any selected myeloid cell population may be modified by the presence of other myeloid cell populations, although this expectation has not been tested experimentally.
In the present investigation, we tested the hypothesis that mixed myeloid cell populations and uniform populations of myeloid cells differ in their cytolytic capacity and in the mechanisms through which they induce cytolysis. We compare the mechanisms through which neutrophils and macrophages lyse target cells when assayed as individual populations or in a combined population of neutrophils and macrophages that simulates the co-localization of these myeloid cells which commonly occurs in tissues following acute injuries. Muscle cells are used as targets in the present investigation because muscle injury and modified muscle use lead to extravasation of large numbers of activated neutrophils and macrophages, which coincides with the appearance of muscle membrane lesions (Tidball et al. 1999). Thus, identification of interactions between neutrophils and macrophages in cytotoxicity assays with muscle may provide insights into cytolytic mechanisms that occur in inflamed muscle in vivo. In addition, the time course of invasion and relative proportions of neutrophils and macrophages in muscle experiencing modified loading are known (Tidball et al. 1999), so that in vitro assays can be designed to reflect the relative proportions of myeloid cell populations that occur in vivo.
The present investigation also provides new insights into the capacity of macrophages to cause muscle damage. Typically, muscle macrophages are expected to play a primarily beneficial role in muscle following injury or modified use. Observations that support this role for macrophages include: (1) their invasion into injured tissues coincides with the onset of tissue repair (Hopkinson-Woolley et al. 1994; St Pierre & Tidball, 1994), (2) they phagocytose tissue debris, (3) they can induce apoptosis in neutrophils (Meszaros et al. 2000), which may play a role in attenuating neutrophil-mediated damage, and (4) some macrophage subpopulations are capable of generating unknown factors that can promote tissue repair (Robertson et al. 1993; Massimino et al. 1997). However, recent observations showed that the depletion of macrophages from inflamed, dystrophic muscle reduced muscle membrane lesions (Wehling et al. 2001). This latter observation indicated that macrophages have the capacity to cause muscle membrane damage, although the mechanisms through which they can cause muscle lysis was unknown. In the present investigation, we have shown that macrophages lyse muscle cells through nitric oxide-dependent mechanisms, and that their killing is potentiated by neutrophils and by the target muscle cells themselves.
METHODS
Cell culture
L6 rat myotubes were used for all cytotoxicity assays. L6 cells were plated in 96-well plates in 10 % fetal bovine serum (FBS) in Dulbecco's modified Eagle's media (DMEM) until confluent, and then placed in serum-free media overnight to induce withdrawal from the cell cycle and promote fusion to myotubes. The cells were then returned to 10 % FBS in DMEM for an additional 2 days, and then subjected to serum withdrawal overnight, to further promote fusion. They were then returned to complete media for approximately 4 days before use in cytotoxicity assays. At the end of this period, microscopic examination indicated that approximately 75 % of the myonuclei appeared to be located in myotubes.
Myeloid cells were collected from rat peritoneal spaces at 16–20 h following sodium caseinate injection. Rats were killed by injection of sodium pentobarbital prior to collection of the peritoneal exudate. All experimental protocols and use of animals were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the UCLA Institutional Animal Care and Use Committee. Pelleted cells from the peritoneal exudate were resuspended in 0.85 % ammonium chloride to lyse erythrocytes and then again pelleted by centrifugation at 200 g for 10 min at 4 °C. The cells were then resuspended in Hanks’ balanced salt solution (HBSS), overlaid on Histopaque 1077 (Sigma, St Louis) and then centrifuged at 400 g for 45 min at 4 °C. Macrophages were then collected at the HBSS-Histopaque interface, and neutrophils were collected from pelleted cells. The purity of the macrophage and neutrophil preparations was assessed microscopically in hematoxylin-stained preparations of cells isolated on the Histopaque gradient that were then adhered to microscope slides by centrifugation (Cytospin, Shandon, USA).
Cytotoxicity assays
Cytotoxicity assays were modifications of the techniques of Hohlfeld & Engel (1990). L6 myotubes were incubated for 2 h in HBSS containing 51Cr and 0.25 % FBS, and then washed in HBSS twice before use in cytotoxicity assays. L6 myotubes were co-cultured for 24 h with either neutrophils, macrophages or neutrophils and macrophages together in HBSS containing 0.25 % FBS and 400 µm arginine, after which the medium was collected and 51Cr release into the medium assayed by scintillation counting. Macrophages and neutrophils in mixed myeloid cell assays were used at a 10:1 macrophage to neutrophil ratio, because this represents the relative proportion of these cells in inflamed muscle at the stage when extensive muscle membrane lysis occurs (Tidball et al. 1999). The conventional expression of the ratio of target cells to effector cells used in cytotoxicity assays was not used in this investigation, because that value cannot be determined accurately when using multinucleated myotubes as target cells. Muscle cells that were used as targets in these assays were induced to withdraw from the cell cycle and to differentiate, which resulted in their fusion into multinucleated myotubes. Accurate measurement of the total number of myotubes in each cytotoxicity experiment was not possible, because the size of myotubes and the number of myotubes per well were highly variable. Therefore, we normalized the data as number of effector cells mm−2 of culture well surface area, because the bottom of each well used in cytotoxicity assays was covered with a monolayer of myotubes.
Myeloid cells were activated in some assays by the addition of phorbol 12-myristate 13-acetate (PMA) or by the addition of N-formyl methionine-leucine-phenylalanine (fMLP) and platelet activating factor (PAF) to the media at the onset of co-culturing. In addition, the following reagents were added to the co-cultures in various experiments (to perturb selected enzyme or free radical function): l-nitro-arginine methyl ester (l-NAME; to inhibit NO production by all nitric oxide synthase (NOS) isoforms); superoxide dismutase (SOD; to dismutate superoxide); S-methyl thiourea (SMT; to selectively inhibit inducible NOS (iNOS)). Cytotoxicity was expressed as a percentage of total lysis by setting 0 % as the chromium released spontaneously by L6 myotubes incubated for 24 h in the absence of myeloid cells. 100 % cytotoxicity was set at the chromium release into the media by L6 myotubes incubated with 0.1 % Triton X-100 in HBSS. All assays were performed at least six times in an experiment and each value is expressed as the mean with its standard error (s.e.m.). Each experiment was repeated several times. Each experiment under a given set of conditions yielded similar results, although the data for only one representative experiment for each set of conditions are presented. Values were compared using Student's t test. The 0.01 level was taken to indicate statistical significance.
Western analysis
The presence and relative concentrations of inducible nitric oxide synthase (iNOS), neuronal NOS (nNOS) and endothelial NOS (eNOS) in myotube cultures in the presence or absence of macrophages or neutrophils was assessed in Western blots according to previously described techniques (Koh & Tidball, 2000). Antibodies to each NOS isoform were obtained from Transduction Labs (San Diego, CA, USA).
Assays for nitric oxide
NO concentration was measured by a modification of the Greiss reaction (Amano & Noda, 1995).
Assays for superoxide
Superoxide concentration was determined by superoxide-dismutase-inhibitable reduction of ferricytochrome C (Talpain et al. 1995).
Assessment of macrophage subpopulation type
Peritoneal macrophages were collected by the same procedure used to generate macrophages for cytotoxicity assays and were then assayed to determine whether they expressed ED1 or ED2 antigen. ED1 antigen is expressed by phagocytic macrophages that are early invaders of injured muscle (Dijkstra et al. 1985; St Pierre & Tidball, 1994). ED2 antigen is expressed by non-phagocytic macrophages that are relatively late invaders of injured muscle (Dijkstra et al. 1985; St Pierre & Tidball, 1994). Peritoneal macrophages were adhered to microscope slides by centrifugation at 500 g for 3 min (Cytospin), and then air-dried, fixed in acetone and immunolabelled with either anti-ED1 or anti-ED2. The numbers of ED1-positive or ED2-positive macrophages were then assessed microscopically by counting all cells that were ED1-positive or ED2-positive cells in each of four randomly selected fields of 1.2 × 105 µm2 in each of four samples.
RESULTS
Macrophages and neutrophils lyse myotubes in vitro
Co-cultures of neutrophils and myotubes or macrophages and myotubes showed that each myeloid cell type was capable of myotube lysis. Although myoblasts were also present in the cultures as a minor fraction of the muscle cell population, microscopic examination of the cultures at the end of cytotoxicity assays showed no detectable enrichment in either myotubes or myoblasts in the surviving cell population. This indicated that there was no strong preference for the lysis of either myotubes or myoblasts in these assays. Antibody staining of the isolated peritoneal macrophages used in these assays was more than 90 % ED1-positive, and the majority of the remaining, ED1-negative cells were found to express ED2 antigen. Macrophage-mediated lysis of muscle cells was significantly elevated at 25 000 macrophages mm−2 in cultures to which exogenous activating agents were not added (8.2 % cytolysis in 24 h; s.e.m. = 2.0). However, the level of macrophage lysis was further elevated by activation with PMA (39 %; s.e.m. = 4.1) or by receptor-mediated activation using PAF and fMLP (13.4 %; s.e.m. = 2.4) (Fig. 1). Similarly, there was significant, neutrophil-mediated lysis of myotubes at concentrations of 25 000 neutrophils mm−2 in the absence of exogenous activation (4.2 %; s.e.m. = 0.8), or when neutrophils were activated with PMA (26.4 %; s.e.m. = 4.1), or PAF and fMLP (14.7 %; s.e.m. = 1.7) (Fig. 2).
Figure 1. Macrophages lyse muscle cells in vitro.
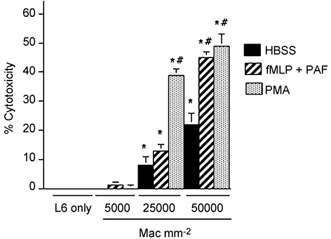
Results of chromium release cytotoxicity assays of rat ED1+ macrophage killing of L6 myotubes. Bars = s.e.m.n = 6 for each group. * Significantly different from L6 muscle cells only under same culture conditions at P < 0.01. # Significantly different from macrophage and muscle co-cultures with HBSS in the absence of exogenous activators. Mac = macrophages.
Figure 2. Neutrophils lyse muscle cells in vitro.
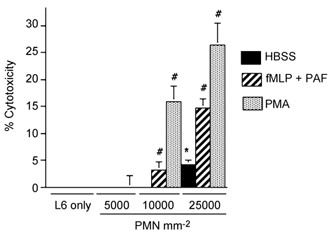
Results of chromium release cytotoxicity assays of rat neutrophil killing of L6 myotubes. Bars = s.e.m.n = 6 for each group. * Significantly different from muscle cells only under same culture conditions at P < 0.01. # Significantly different from neutrophil and muscle co-cultures with HBSS in the absence of exogenous activators. PMN = neutrophils.
Macrophages and neutrophils lyse muscle cells through free radical-mediated mechanisms
We assayed the identity of cytotoxic molecules that caused muscle cell lysis by selectively blocking production or by removing specific free radicals from the assay. Macrophage-induced myotube lysis was prevented or greatly reduced by the addition of the NOS inhibitor l-NAME, which indicated that NO is a primary cytotoxic molecule in macrophage-mediated lysis of muscle cells (Fig. 3). Addition of SOD to macrophage co-cultures to deplete superoxide had no effect on the amount of myotube lysis (data not shown). Conversely, addition of SOD to neutrophil co-cultures prevented significant increase in myotube lysis compared to myotube-only controls, which indicated that superoxide or a superoxide derivative functions as a primary cytolytic molecule in neutrophil-mediated lysis of muscle cells (Fig. 4).
Figure 3. NOS inhibition prevents muscle lysis in macrophage co-cultures.
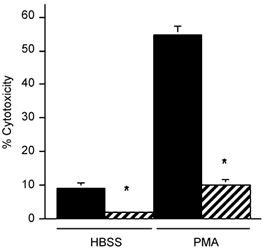
Cultures contained 25 000 macrophages mm−2. Cultures received no exogenous activator (HBSS) or were activated with PMA. Black bar = no l-NAME. Hatched bar = 150 µm l-NAME. * Significantly different from no l-NAME at P < 0.01. Bars = s.e.m.n = 6 for each group.
Figure 4. Superoxide dismutase decreases muscle lysis in neutrophil co-cultures.
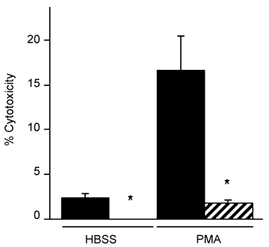
Cultures contained 10 000 neutrophils mm−2. Cultures received no exogenous activator (HBSS) or were activated with PMA. Black bar = no SOD. Hatched bar = 500 U ml−1 SOD. * Significantly different from no SOD at P < 0.01. Bars = s.e.m.n = 6 for each group.
NO inhibits neutrophil-induced cytotoxicity
Addition of l-NAME to neutrophil co-cultures caused significant increases in myotube lysis, (Fig. 5). Addition of 1 mm l-NAME to muscle cultures that contained 5000 neutrophils mm−2 yielded 16.8 % muscle lysis (s.e.m. = 3.0), which is similar to the level of muscle lysis attained by receptor-activated neutrophils at five times higher neutrophil concentration in the absence of NOS inhibitors. This finding indicates that NO protects muscle cells from neutrophil-mediated cytolysis. However, the protective or cytotoxic effects of NO on muscle are dose-dependent. Measurements using the Greiss reaction (Amano & Noda, 1995) showed that NO concentration was 0.9 µm (s.e.m. = 0.03; n = 5) in neutrophil and muscle co-cultures in which the protective effect of NO was observed. In contrast, a NO concentration of 22.9 µm (s.e.m. = 0.7; n = 6) occurred in macrophage and muscle co-cultures in which NO was cytotoxic.
Figure 5. NOS inhibition increases muscle lysis in muscle and neutrophil co-cultures.
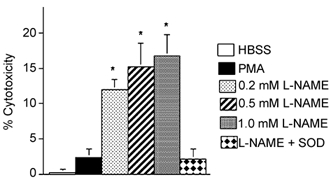
Cultures contained 5000 neutrophils mm−2. * Significantly different from HBSS at P < 0.01. Bars = s.e.m.n = 6 for each group.
NOS inhibition increases superoxide concentration in neutrophil cultures
Our observations that neutrophil-mediated cytotoxicity occurred through superoxide-dependent mechanisms and that NOS inhibition increased neutrophil cytotoxicity, suggested that NOS inhibition could increase superoxide concentration in neutrophil cultures. In testing this possibility, we found that the addition of l-NAME to muscle and neutrophil co-cultures significantly increased superoxide concentration (Fig. 6). Non-activated neutrophil co-cultures showed approximately a 2-fold increase in superoxide concentration, while fMLP/PAF- or PMA-activated neutrophil cultures showed approximately 40 % and 35 % increases in superoxide concentration respectively (Fig. 6). Addition of SOD prevented the potentiation of neutrophil cytotoxicity caused by the addition of L-NAME to neutrophil cultures (Fig. 5). Together, these data indicate that NO protects muscle from neutrophil-mediated lysis by reducing the superoxide concentration below toxic levels.
Figure 6. NOS inhibition increases superoxide concentration in neutrophil-muscle co-cultures.
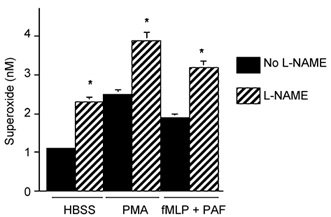
Cultures contained 5000 neutrophils mm−2. * Significantly different from no l-NAME controls at P < 0.01. Superoxide values represent nanomoles of superoxide produced in 30 min by 106 cells. Bars = s.e.m.
Neutrophils promote macrophage-induced cytotoxicity
Although the results of the cytotoxicity assays that used single effector cell populations showed that either neutrophils or macrophages alone can lyse muscle cells, those experimental conditions may not appropriately model inflamed muscle in which mixed populations of neutrophils and ED1-positive macrophages are present. Previous work (Tidball et al. 1999) has shown that there are approximately ten times more ED1-positive macrophages than neutrophils in inflamed muscle at the stage of injury when much muscle membrane lysis occurs. We tested whether mixed myeloid cell populations in which there was a 10:1 ratio of macrophages to neutrophils could affect the cytotoxicity of the system. These assays employed 500 neutrophils mm−2 and 5000 macrophages mm−2, which was substantially less than the concentrations of inflammatory cells that produced muscle lysis with a single effector cell population (Fig. 1 and Fig. 2). Addition of l-NAME or SOD to co-cultures containing these low concentrations of single effector cell populations caused no measurable cytotoxicity (data not shown).
The addition of a relatively low concentration of neutrophils to co-cultures of macrophages and muscle cells greatly increased muscle cell lysis (Fig. 7). Also, the addition of l-NAME, but not SOD, caused a large and significant decrease in cytotoxicity. These data indicate that neutrophils may promote macrophage-mediated cytolysis and that NO-dependent processes are the primary cytolytic mechanisms in mixed myeloid populations that contain neutrophils and macrophages.
Figure 7. NOS inhibition reduces muscle lysis in co-cultures with macrophages and neutrophils.
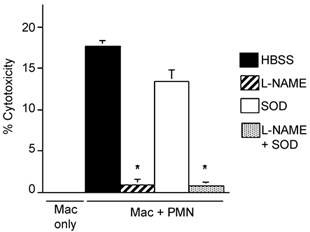
All cultures contained confluent myotubes to which either macrophages only (5000 macrophages mm−2) or both macrophages (5000 macrophages mm−2) and neutrophils (500 neutrophils mm−2) were added. SOD was added at 500 U ml−1 in some cultures. l-NAME was added at 150 µm to some cultures. * Significantly different from macrophage and neutrophil co-cultures in HBSS only (P < 0.01). Bars = s.e.m.n = 6 for each sample. Mac = macrophages. PMN = neutrophils.
NO generation in co-cultures is promoted by interactions between myotubes and macrophages
NO production in macrophage and L6 myotube co-cultures was approximately three times greater than the sum of NO produced by myotubes when cultured alone plus NO produced by macrophages alone (Fig. 8). Western analysis showed that L6 myotubes expressed nNOS but no detectable eNOS or iNOS (data not shown), and that iNOS was the only detectable NOS isoform expressed by macrophages under these conditions (Fig. 9). In addition, immunohistochemistry for iNOS distribution in macrophage co-cultures showed no detectable iNOS on myotubes, but strong antibody binding to macrophages (Fig. 10). Thus, the potential sources for NO in this system are muscle nNOS or macrophage iNOS. We tested which of these possibilities was the primary source of NO in macrophage and muscle co-cultures by adding an iNOS -selective inhibitor, SMT, to co-cultures and measuring changes in NO concentration. Addition of SMT reduced NO levels in co-cultures to concentrations that were similar to concentrations measured in muscle cultures alone (Fig. 8). This indicates that macrophages are responsible for the large increase in NO production when macrophages are co-cultured with muscle cells and that low levels of NO production in the presence of SMT may reflect NO generation by nNOS in myotubes.
Figure 8. The presence of target muscle cells increases NO production by macrophages.
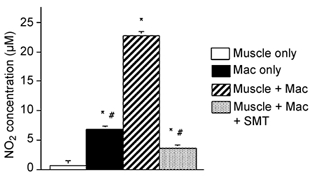
Values are µm of nitrite in culture media as an indicator of NO production. Cultures contained confluent myotubes only, macrophages (Mac) only (5000 macrophages mm−2) or both macrophages (5000 macrophages mm−2) and myotubes. SMT (1 mm) were added to inhibit production of NO by iNOS. * Significantly different from muscle only at P < 0.01. # Significantly different from muscle and macrophage co-culture at P < 0.01. Bars = s.e.m.n = 6 for each sample.
Figure 9. Western analysis for iNOS in cells cultured for 24 h.
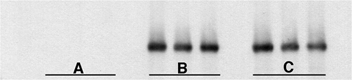
A, confluent myotubes only. B, 5000 macrophages mm−2 only. C, confluent myotubes with 5000 macrophages mm−2. Each lane contains total protein from cells in 3 wells of a 96-well plate.
Figure 10. Anti-iNOS labelling of L6 myotubes after co-culturing with macrophages for 24 h.
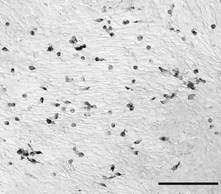
Nomarski optics were used for contrast. Only mononucleated cells which resembled macrophages appeared labelled with anti-iNOS. Bar = 100 µm.
These findings indicated that the elevation in NO production in macrophage and muscle co-cultures could result from either an increase in iNOS concentration, or an increase in iNOS activity in macrophages. Western analysis showed no increase in iNOS concentration in macrophages from macrophage and muscle co-cultures, compared to macrophages alone (Fig. 9). Collectively, these data show that myotubes promote the activity rather than the expression of iNOS in macrophages to levels approximately three times higher than NO production by macrophages in the absence of myotubes.
DISCUSSION
The major finding of the present investigation is that the cytolytic capacity of myeloid cells and the mechanisms through which target cells are lysed can differ profoundly in mixed myeloid cell populations, compared to uniform populations of effector cells. For example, the observation presented here which shows that neutrophils lyse muscle cells through superoxide-dependent mechanisms is consistent with other in vitro findings (Simchowitz & Spilberg, 1979; Markey et al. 1990; Srinivasan et al. 1997); however, no significant lysis through superoxide-dependent mechanisms was detected if macrophages were also present at macrophage/neutrophil ratios that occur in muscle injury and inflammation. Instead, the addition of low concentrations of neutrophils to macrophage and muscle co-cultures resulted in macrophage activation and promoted NO-dependent cytotoxicity at myeloid cell concentrations that are substantially lower than concentrations that are required for muscle cell lysis by uniform myeloid populations. Although it is feasible that the increase in cell lysis that occurs with elevated NO production in these cultures reflects changes in NO-mediated signalling, rather than NO-mediated cytotoxicity, that scenario is not consistent with other findings concerning NO cytotoxicity. NO functions as a signalling molecule at concentrations that typically range from 10 nm to 1 µm (Stamler & Meissner, 2001), but is cytotoxic at concentrations that exceed a few micromolar NO and may reach 20 µm NO (Wink et al. 1996). These latter, high values were attained in our cytotoxicity assays in which macrophage-mediated, NAME-inhibited cytotoxicity occurred.
The present investigation also shows that target cells themselves can promote the cytotoxicity of myeloid cells (Fig. 11). The levels of NO production in muscle cell co-cultures with macrophages were three times greater than the total NO production when the two cell types were cultured separately. There was no measurable increase in the concentration of NOS in macrophages in muscle co-cultures, which indicates that the increase in NO production in co-cultures resulted from an increase in NOS activity. Although it is feasible that the increased NO was generated by either iNOS in macrophages or by nNOS in muscle cells in the co-cultures, the observation that the iNOS-selective inhibitor SMT reduced NO production in the co-cultures to levels that were similar to those observed in muscle cell cultures alone, indicates that iNOS was the source of increased NO production. This muscle cell-induced increase in NO production by macrophages without an increase in iNOS expression suggests that iNOS activity can be regulated at the post-translational level in macrophages under these conditions. Previous investigations have provided clear evidence that iNOS activity is regulated through transcriptional controls (Radomski et al. 1990), but other findings have indicated that iNOS activity can also be regulated post-translationally. For example, increases in tyrosine phosphorylation of iNOS have been shown to coincide with rapid increases in iNOS activity (Pan et al. 1996), which may reflect a post-translational influence on iNOS activity.
Figure 11. Diagram representing the interactions between myeloid cells and target muscle cells that were identified in the present investigation.
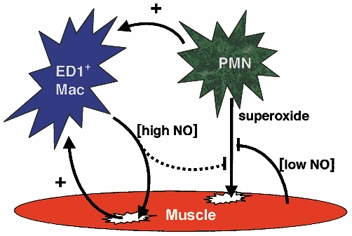
ED1-positive macrophages lyse muscle cells by NO-dependent mechanisms, while neutrophils lyse muscle cells through a superoxide-dependent mechanism that can be inhibited by NO released from muscle, and possibly from macrophages. Macrophage (Mac) activation can be promoted (+) by neutrophils, and by interactions with the target cells.
Although the high concentrations of NO that are generated by activated macrophages are highly cytotoxic to muscle cells, our findings show that lower levels of NO production can protect against neutrophil-mediated cytotoxicity. This conclusion is consistent with the dual functionality of NO that has been characterized in other systems (Palmer et al. 1992; Wink et al. 1993; Gupta et al. 1997). The ability of NO to either protect or promote cytotoxicity in a concentration-dependent way has been explained, at least in part, by the variety of reactive intermediate molecules that can be formed, depending on the stoichiometry of NO, superoxide and other free radicals in the tissue (Miles et al. 1996; Wink et al. 1996). This NO protective effect against superoxide-dependent cytotoxicity that is induced by muscle may have particular relevance to muscle injuries that occur in increased muscle use following periods of disuse. Although increased muscle use can promote neutrophil invasion, it also increases both the expression and activity of nNOS by muscle, which could serve a protective function against neutrophil-mediated damage (Tidball et al. 1998).
Recent findings (Zhuang et al. 2001) have shown that modification of NO concentration does not always affect the susceptibility of muscle cells to lysis by free radicals in vitro. Rando and his colleagues (Zhuang et al. 2001) showed that muscle cells from nNOS null mice did not experience higher levels of cytolysis than wild-type muscle cells when exposed to paraquat, which generates superoxide intracellularly, or quinone, which causes increased production of free radicals, or sodium nitroprusside, which generates NO. In addition, the same investigators tested whether adenoviral transfection of muscle cells from dystrophin-deficient mdx mice with nNOS cDNA would influence susceptibility to free radical-mediated lysis. Mdx muscle cells were examined because they express little NOS. Expression of the adenoviral NOS had no effect on cell lysis when challenged with paraquat, which indicated that modulation of NOS expression and NO concentration were not sufficient to affect susceptibility to superoxide-mediated lysis. The findings of the present investigation, combined with those of this previous work (Zhuang et al. 2001), indicate that NO can protect against superoxide-mediated lysis of muscle by neutrophils, but not effectively protect against superoxide produced as a consequence of some pharmacological manipulations.
Although the results of this investigation are particularly relevant to understanding the potential mechanisms through which myeloid cells can promote muscle damage following acute injury, they may also help increase understanding of inflammatory cell-mediated damage in some muscle diseases. Recent investigations (Rando et al. 1998) have shown that muscle cells in culture are highly sensitive to free radical-mediated damage, and the susceptibility to damage increases significantly if the muscle does not express dystrophin, a cytoskeletal, membrane-associated protein. Dystrophin deficiency causes the progressive muscle inflammation and wasting that occurs in Duchenne muscular dystrophy. Recent findings have shown that depletion of macrophages from the mdx mouse model of Duchenne muscular dystrophy causes a significant reduction in muscle membrane lesions (Wehling et al. 2001). That observation suggests the possibility that macrophages may promote the injury of dystrophic muscle through mechanisms identified in the present study.
Acknowledgments
This work was supported by grants from the NIH (AR40343, AR/HD47721, AR47855-01), Muscular Dystrophy Association, USA, and NASA (NAG5-4837). Katharine Wen provided superb technical assistance.
REFERENCES
- Abu-Soud HM, Hazen SL. Nitric oxide modulates the catalytic activity of myeloperoxidase. J Biol Chem. 2000;275:5425–5430. doi: 10.1074/jbc.275.8.5425. [DOI] [PubMed] [Google Scholar]
- Amano F, Noda T. Improved detection of nitric oxide radical (NO) production in an activated macrophage culture with a radical scavenger, carboxy PTIO, and Griess reagent. FEBS Letters. 1995;368:425–428. doi: 10.1016/0014-5793(95)00700-j. [DOI] [PubMed] [Google Scholar]
- Blaylock MG, Cuthbertson BH, Galley HF, Ferguson N, Webster NR. The effect of nitric oxide and peroxynitrite on apoptosis in human polymorphonuclear leukocytes. Free Rad Biol Med. 1998;25:748–752. doi: 10.1016/s0891-5849(98)00108-7. [DOI] [PubMed] [Google Scholar]
- Clancy RM, Leszcynska-Piziak J, Abramson SB. Nitric oxide, an endothelial cell relaxation factor, inhibits neutrophil superoxide anion production via a direct action on the NADPH oxidase. J Clin Invest. 1992;90:1116–1121. doi: 10.1172/JCI115929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra CD, Dopp EA, Joling P, Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunol. 1985;54:589–599. [PMC free article] [PubMed] [Google Scholar]
- Fortenberry JD, Owens ML, Brown LA. S-nitrosoglutathione enhances neutrophil DNA fragmentation and cell death. Am J Physiol. 1990;276:L435–442. doi: 10.1152/ajplung.1999.276.3.L435. [DOI] [PubMed] [Google Scholar]
- Gryglewski RJ, Palmer RM, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986;320:454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Gupta MP, Evanoff V, Hart CM. Nitric oxide attenuates hydrogen peroxide-mediated injury to porcine pulmonary artery endothelial cells. Am J Physiol. 1997;272:L1133–1141. doi: 10.1152/ajplung.1997.272.6.L1133. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R, Engel AG. Lysis of myotubes by alloreactive cytotoxic T cells and natural killer cells. Relevance to myoblast transplantation. J Clin Invest. 1990;86:370–374. doi: 10.1172/JCI114711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkinson-Woolley J, Hughes D, Gordon S, Martin P. Macrophage recruitment during limb development and wound healing in the embryonic and foetal mouse. J Cell Sci. 1994;107:1159–1167. doi: 10.1242/jcs.107.5.1159. [DOI] [PubMed] [Google Scholar]
- Koh TJ, Tidball JG. Nitric oxide inhibits calpain-mediated proteolysis of talin in skeletal muscle cells. Am J Physiol Cell Physiol. 2000;279:C806–812. doi: 10.1152/ajpcell.2000.279.3.C806. [DOI] [PubMed] [Google Scholar]
- Markey BA, Phan SH, Varani J, RyaN US, Ward PA. Inhibition of cytotoxicity by intracellular superoxide dismutase supplementation. Free Rad Biol Med. 1990;9:307–314. doi: 10.1016/0891-5849(90)90005-4. [DOI] [PubMed] [Google Scholar]
- Massimino ML, Rapizzi E, Cantini M, Dalla LiberaL, Mazzoleni F, Arslan P, Carraro U. ED2+ macrophages increase selectively myoblast proliferation in muscle cultures. Biochem Biophys Res Commun. 1997;235:754–759. doi: 10.1006/bbrc.1997.6823. [DOI] [PubMed] [Google Scholar]
- Meszaros AJ, Reichner JS, Albina JE. Macrophage-induced neutrophil apoptosis. J Immunol. 2000;165:435–441. doi: 10.4049/jimmunol.165.1.435. [DOI] [PubMed] [Google Scholar]
- Miles AM, Bohle DS, Glassbrenner PA, Hansert B, Wink DA, Grisham MB. Modulation of superoxide-dependent oxidation and hydroxylation reactions by nitric oxide. J Biol Chem. 1996;271:40–47. doi: 10.1074/jbc.271.1.40. [DOI] [PubMed] [Google Scholar]
- Moilanen E, Vapaatalo H. Nitric oxide in inflammation and immune response. Ann Med. 1995;27:359–367. doi: 10.3109/07853899509002589. [DOI] [PubMed] [Google Scholar]
- Palmer RM, Bridge L, Foxwell NA, Moncada S. The role of nitric oxide in endothelial cell damage and its inhibition by glucocorticoids. Br J Pharmacol. 1992;105:11–12. doi: 10.1111/j.1476-5381.1992.tb14202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan J, Burgher KL, Szczepanik AM, Ringheim GE. Tyrosine phosphorylation of inducible nitric oxide synthase: implications for potential post-translational regulation. Biochem J. 1996;314:889–894. doi: 10.1042/bj3140889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski MW, Palmer RM, Moncada S. Glucocorticoids inhibit the expression of an inducible, but not the constituitive, nitric oxide synthase in vascular endothelial cells. Proc Natl Acad Sci U S A. 1990;87:10043–10047. doi: 10.1073/pnas.87.24.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando TA, Disatnik MH, Franco A. Muscle cells from mdx mice have an increased susceptibility to oxidative stress. Neuromuscul Disorders. 1998;8:14–21. doi: 10.1016/s0960-8966(97)00124-7. [DOI] [PubMed] [Google Scholar]
- Robertson TA, Maley MaL, Grounds MD, Papadimitriou J. The role of macrophages in skeletal muscle regeneration with particular reference to chemotaxis. Exp Cell Res. 1993;207:321–331. doi: 10.1006/excr.1993.1199. [DOI] [PubMed] [Google Scholar]
- Simchowitz L, Spilberg I. Evidence for the role of superoxide radicals in neutrophil-mediated cytotoxicity. Immunol. 1979;37:301–309. [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Buchweitz J, Ganey PE. Alteration by fluramide of neutrophil response to stimulation. Biochem Pharmacol. 1997;53:1179–1185. doi: 10.1016/s0006-2952(97)00099-3. [DOI] [PubMed] [Google Scholar]
- Stamler JS, Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol Rev. 2001;81:209–237. doi: 10.1152/physrev.2001.81.1.209. [DOI] [PubMed] [Google Scholar]
- St Pierre BA, Tidball JG. Differential response of macrophage subpopulations to soleus muscle reloading after hindlimb suspension. J Appl Physiol. 1994;77:290–297. doi: 10.1152/jappl.1994.77.1.290. [DOI] [PubMed] [Google Scholar]
- Talpain E, Armstrong RA, Coleman RA, Vardey CJ. Characterization of the PGE receptor subtype mediating inhibition of superoxide production in human neutrophils. Br J Pharmacol. 1995;114:1459–1465. doi: 10.1111/j.1476-5381.1995.tb13370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidball JG, Berchenko E, Frenette J. Macrophage invasion does not contribute to muscle membrane injury during inflammation. J Leukoc Biol. 1999;65:492–498. [PubMed] [Google Scholar]
- Tidball JG, Lavergne E, Lau KS, Spencer MJ, Stull JT, Wehling M. Mechanical loading regulates nitric oxide synthase expression and activity in developing and adult skeletal muscle. Am J Physiol. 1998;275:C260–266. doi: 10.1152/ajpcell.1998.275.1.C260. [DOI] [PubMed] [Google Scholar]
- Ward C, Wong TH, Murray J, Rahman I, Haslett C, Chilvers ER, Rossi AG. Induction of human neutrophil apoptosis by nitric oxide donors: evidence for a caspase-dependent, cyclic-GMP-independent, mechanism. Biochem Pharmacol. 2000;59:305–314. doi: 10.1016/s0006-2952(99)00329-9. [DOI] [PubMed] [Google Scholar]
- Wehling M, Spencer MJ, Tidball JG. A nitric oxide synthase transgene ameliorates muscular dystrophy in mdx mice. J Cell Biol. 2001;155:123–131. doi: 10.1083/jcb.200105110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink DA, Hanbauer J, Krishna MC, Degraff W, Gamson J, Mitchell JB. Nitric oxide protects against cellular damage and cytotoxicity from reactive oxygen species. Proc Natl Acad Sci U S A. 1993;90:9813–9817. doi: 10.1073/pnas.90.21.9813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink DA, Hanbauer I, GrishaM MB, Laval F, Nims RW, Laval J, Cook J, Pacelli R, Liebmann J, Krishna M, Ford PC, Mitchell JB. Chemical biology of nitric oxide: regulation and protective and toxic mechanisms. Curr Top Cell Regul. 1996;34:159–187. doi: 10.1016/s0070-2137(96)80006-9. [DOI] [PubMed] [Google Scholar]
- Zhuang W, Eby JC, Cheong M, Mohaatra PK, BredT DS, Disatnik M, Rando TA. The susceptibility of muscle cells to oxidative stress is independent of nitric oxide synthase expression. Muscle Nerve. 2001;24:502–511. doi: 10.1002/mus.1033. [DOI] [PubMed] [Google Scholar]


