Abstract
Programming of the hypothalamo-pituitary-adrenal (HPA) axis during prenatal and early postnatal life may explain, in part, the association between low birth weight (BW) and the increased incidence of cardiovascular and metabolic disease in later life. This study examined the effect of natural variations in BW on HPA axis function in juvenile and adult pigs. Low (< 1.47 kg) and high (> 1.53 kg) BW pure-bred Large White piglets from 15 litters were studied at 3 (n = 47) and 12 (n = 17) months of age. At each age, HPA axis function was tested by hypoglycaemic challenge (i.v. insulin; 0.5 IU (kg body weight)−1) and ACTH challenge (i.v. Synacthen, 2 µg (kg body weight)−1). At 3 months of age, adrenal size, the ratio of adrenal cortical to medullary area and stimulated cortisol concentrations were elevated in pigs that were of low BW and that remained small after birth. At 12 months of age, thinness at birth was associated with elevated adrenal responsiveness to insulin-induced hypoglycaemia. These results are consistent with the hypothesis that impaired fetal and early postnatal growth are associated with altered HPA axis function in later life.
Epidemiological studies in man have shown that impaired fetal growth resulting in low birth weight (BW) is associated with an increased risk of cardiovascular and metabolic diseases in adult life (Barker et al. 1989; Phillips et al. 1994). In addition, poor growth in the first year of life, followed by rapid weight gain, further increases the risk of adult-onset degenerative diseases such as coronary heart disease (Eriksson et al. 2001). These epidemiological observations have therefore led to the hypothesis that adult disease may originate in early life (Barker et al., 1989) as the result of tissue programming by environmental influences acting at critical periods of development (Widdowson & McCance, 1975).
In part, the association between low BW and the increased incidence of cardiovascular and metabolic disease in later life may be due to hyperactivity or resetting of the hypothalamo-pituitary-adrenal (HPA) axis in response to stress in prenatal and early postnatal life. The HPA axis is functional in utero and is known to affect fetal growth and the development of individual fetal tissues and organ systems (Fowden, 1995). It has also been shown to influence metabolism and cardiovascular function in the fetus during late gestation (Macdonald et al. 1983; Tangalakis et al. 1992). In rats, pre- and postnatal manipulations, such as maternal restraint stress or daily removal of pups from their mother in early postnatal life, programme life-long changes in HPA axis activity and can either enhance or impair responsiveness depending on the timing and duration of the insult (Meaney et al. 1989; Henry et al. 1994). Certainly, low BW in humans is associated with increased urinary glucocorticoid excretion in children and with elevated fasting cortisol concentrations in children and adults from a number of different populations (Clark et al. 1996; Phillips et al. 2000).
BW varies 2–3-fold amongst littermates in normally fed sows which provides a naturally occurring form of growth retardation with less genetic variation than seen in man and other monotocous species. Low BW pigs are also of disproportionate body shape, showing evidence of brain sparing as assessed by an increased brain to liver ratio (Bauer et al. 1998). This ratio is a good index of asymmetrical growth retardation during late gestation. At 3 months of postnatal age, low BW and disproportionate body shape at birth are associated with elevated basal blood pressure in pigs (Poore et al. 2002a). The present study examined the effect of natural variations in BW on basal and stimulated HPA axis function in juvenile (3-month-old) and adult (12-month-old) pigs. Responses of the HPA axis to insulin-induced hypoglycaemia and ACTH challenge were examined.
METHODS
Animals
Pure-bred Large White pigs were obtained from sows mated to a single boar and which were allowed to farrow normally at term (115 ± 2 days). Fifteen litters from nine sows were used in this study. Sows were fed a standard diet (15 % protein; 12.6 MJ kg−1 digestible energy; ABN, Peterborough, UK) at least 4 weeks prior to conception (2 kg day−1) and during gestation and lactation (2.5–3 kg day−1) such that nutritional requirements were satisfied according to standard guidelines (Agricultural and Food Research Council, 1990). Water was provided ad libitum. Piglets were provided with straw bedding and infrared heat lamps from birth until weaning at 4–5 weeks of age. Weaner piglets were housed in groups and fed ad libitum on a standard pig diet (creep feed; 20 % protein; H & C Beart Ltd, Kings Lynn, UK) until the first studies were performed at 3 months of age. From this time, pigs were housed individually adjacent to their siblings and fed according to their size (0.5 kg per 30 kg body weight, twice a day at 08.00 and 17.00 h; Agricultural and Food Research Council, 1990). At the completion of all studies at 3 months of age, pigs were returned to group housing (ad libitum feeding of 20 % protein pig creep) until at 4–5 months of age, prior to puberty, pigs were again housed individually and fed on the adult 15 % protein diet for the remainder of the study.
At birth, all piglets in each litter were weighed and a set of morphometric measurements were made: head length (snout- between ears), crown rump length (between ears-base of tail, CRL) and abdominal circumference (AC). The average BW of all piglets born to all litters was 1.50 ± 0.02 kg (n = 170) and the 95 % confidence interval of the mean was 1.47–1.53 kg. Piglets whose BWs fell within the confidence interval of the mean were excluded from the study. Forty-seven piglets remained and were assigned to one of two groups, those with BW lower than the 95 % confidence interval of the mean were defined as ‘low BW’ pigs (< 1.47 kg at birth) and those higher than the 95 % confidence interval of the mean were defined as ‘high BW’ pigs (> 1.53 kg at birth). The range of birth weights in the low BW group was 0.80–1.40 kg (n = 22) and in the high BW group was 1.65–2.40 kg (n = 25). Approximately similar numbers of each sex were selected (low BW females, n = 15; low BW males, n = 7; high BW females, n = 13; high BW males, n = 12). Selected pigs were weighed and measured again at 1, 3 and 12 months of age. It was not possible to obtain all data from all animals: the number of observations for each experimental data set is indicated in the legend of each table or figure.
At 9–10 weeks of age, before the morning feed, selected pigs were tranquilised with azaperone (Janssen Pharmaceuticals Ltd, Oxford, UK; 5 mg (kg body weight)−1i.m. for pigs > 20 kg) or diazepam (Phoenix Pharmaceuticals Ltd, Gloucester, UK; 2 mg (kg body weight)−1i.m. for pigs < 20 kg), each in combination with ketamine (Fort Dodge Animal Health Ltd, Southampton, UK; 10 mg (kg body weight)−1i.m.) and then anaesthetised with halothane (3–6 % in O2). Catheters were inserted under aseptic conditions into the dorsal aorta and vena cava via the femoral vessels and were exteriorised via a small incision on the animal's back. Pigs were kept in protective coats made of elastic tubing (Tubigrip, Seton Healthcare Group, Oldham, UK) to protect the catheters. Post-operative recovery was monitored, without analgesia, and pigs were seen to restore normal feeding patterns and behaviour immediately after recovery from surgery. Antibiotic treatment was administered on the day of surgery (Depocillin; procaine benzylpenicillin, 15 mg (kg body weight)−1; Depocillin Mycofarm Ltd, Cambridge, UK and Duphatrim; trimethoprim, 2.5 mg kg−1 with sulfadiazine, 12.5 mg kg−1; Fort Dodge Animal Health Ltd; i.m.), for 3 days following surgery and every 2–3 days thereafter (Duphatrim alone, i.v.). During the experimental period, feeding patterns, behaviour and weight gain were monitored to ensure continued animal wellbeing.
Seventeen pigs had their catheters and coats removed at the completion of the experiments at 3 months of age. Several weeks after implantation, normal animal movement caused catheters to be worked out of blood vessels such that they could be easily removed without anaesthesia. These pigs were then studied again at 10–12 months of age (low BW females, n = 5; low BW males, n = 4; high BW females, n = 4; high BW males, n = 4). Femoral artery and vein catheters were inserted in the previously un-operated leg using aseptic conditions under general anaesthesia (sodium pentobarbitone; Rhône Mérieux Ltd, Harlow, UK; 20 mg kg−1i.v., following tranquilisation with azaperone; 5 mg (kg body weight)−1i.m.).
All procedures were carried out in accordance with the regulations of the UK Home Office Animals (Scientific Procedures) Act, 1986.
Experimental protocol
All animals were allowed at least 2 days for recovery from surgery. For measurement of basal hormone concentrations, up to three blood samples were collected from each animal over several days, during baseline conditions, and the results were averaged. Insulin and ACTH challenges were performed following an overnight fast, each at least 2 days apart.
Insulin challenge
Porcine insulin (0.5 IU (kg body weight)−1; Novo Nordisk Pharmaceuticals Ltd, Crawley, UK) was administered intravenously as a bolus dose and arterial blood samples (2–4 ml into chilled EDTA tubes) were collected for analysis of glucose, ACTH and cortisol concentrations at −30 and −15 min, immediately before (0 min) and at 5, 10, 15, 30, 45, 60, 90 and 120 min after insulin administration. At −30, 0, 5, 15, 30, 60, 90 and 120 min, samples (1 ml) were collected into chilled heparinised tubes containing EGTA (5 µmol (ml blood)−1 and glutathione (40 µmol (ml blood)−1) for analysis of catecholamine concentrations. All blood samples were centrifuged immediately for 5 min at 4 °C and, after measurement of plasma glucose concentrations, the plasma was stored at −20 °C (samples for ACTH and cortisol analysis) or −80 °C (samples for catecholamine analysis).
ACTH challenge
Synthetic ACTH1–24 (2.0 µg (kg body weight)−1; Synacthen, Alliance Pharmaceuticals Ltd, Chippenham, UK) was administered intravenously as a bolus dose and arterial blood samples (2 ml into chilled EDTA tubes) were collected for analysis of cortisol concentrations at −30 and −15 min and immediately before (0 min) and at 5, 10, 20, 30, 60, 90 and 120 min after ACTH administration. Blood samples were treated as described for the insulin challenge.
Biochemical analyses
Total plasma cortisol concentrations were measured by radioimmunoassay as previously described (Silver et al. 1983). Tritiated cortisol (TRK 407) was purchased from Amersham Biosciences UK Ltd (Little Chalfont, UK) and the cortisol antibody (Pink 72) was a generous gift from the Tenovus Institute for Cancer Research (University of Wales, College of Medicine, Cardiff). The intra-assay coefficients of variation for the cortisol assay were 5.0 % at the level of 27.4 ± 0.4 ng ml−1 and 13.6 % at the level of 5.6 ± 0.2 ng ml−1. The inter-assay coefficient of variation for the cortisol assay was 10 % at the level of 23.6 ± 0.3 ng ml−1 and the minimum detectable dose was 0.4 ng ml−1. Plasma ACTH concentrations were measured by a commercially available radioimmunoassay kit (Diasorin Ltd, Wokingham, UK). The inter-assay coefficient of variation for the ACTH assay was 7 % at the level of 147.6 ± 5.4 pg ml−1, the intra-assay coefficient of variation was 14 % at the level of 45.4 ± 2.0 pg ml−1 and the minimum detectable dose was 3.0 pg ml−1.
Plasma catecholamine (noradrenaline and adrenaline) concentrations were determined by HPLC using electrochemical detection (Silver et al. 1982). Samples were prepared by absorption of 250 µl of plasma onto acid-washed alumina and 20 µl aliquots of the 100 µl perchloric acid elutes were injected onto the column. Dihydroxybenzylamine was added as the internal standard to each plasma sample before absorption. Recovery ranged from 63- 97 % and all catecholamine values were corrected for their respective recovery. The inter-assay coefficients of variation for noradrenaline and adrenaline were 6.2 and 7.3 %, respectively, and the minimum detectable dose was 10 pg ml−1.
Tissue collection
At the end of the 3-month-old experimental period, pigs not required for adult studies (low BW females, n = 8; low BW males, n = 2; high BW females, n = 4; high BW males, n = 8) were killed by a lethal dose of sodium pentobarbitone (200 mg (kg body weight)−1, i.v.). Adrenal glands were collected from each animal, weighed and fixed immediately in 10 % formalin. Adrenal glands were also collected in a similar manner from adult pigs at the end of the 12-month-old experimental period (low BW females, n = 5; low BW males, n = 4; high BW females, n = 3; high BW males, n = 2).
Adrenal morphometry
Following fixation, adrenal glands were embedded in paraffin wax and sectioned (7 µm), transverse to the long axis, in the mid-glandular region. Sections were stained using haematoxylin and eosin for examination under light microscopy. The areas represented by the adrenal cortex and the adrenal medulla were calculated from a digital image of each section using a computer package (Image-Pro Plus). The ratios of cortical to medullary area from nine sections were averaged from each animal.
Statistics
All results are expressed as means ± standard error of the mean (s.e.m.). For each experiment, the areas (AUC) under the cortisol (both challenges), ACTH, noradrenaline and adrenaline (insulin challenge only) response curves (integrated plasma concentrations after insulin or ACTH administration (from 5–120 min) above the mean pre-insulin or pre-ACTH administration (from −30 to 0 min) concentrations) were calculated. The relationships between two factors, the measured dependent variables vs. particular independent variables, were tested using separate correlation analyses. The effects of group (BW) and time were tested using multifactorial analyses of variance (ANOVA). Where appropriate, Tukey's post hoc tests were applied to identify significant differences between means. Statistical analyses were performed using SigmaStat Statistical Software version 2.0. Student's unpaired t tests were used to identify differences between two factors. Unless otherwise stated, the effect of BW or the relationships between two factors were not different in male and female pigs and results shown therefore include the male and female data combined. For all statistical tests, significance was accepted when P < 0.05.
RESULTS
Postnatal growth
Morphometric parameters measured at birth, 3 and 12 months of age in low and high BW are presented in Table 1. There were significant differences in body mass index (BMI; (body weight) CRL−2), head length:current weight (CW) and head length:AC between low and high BW pigs at 3 months, but not 12 months of age (Table 1). At 3 months of age, low BW pigs remained significantly smaller than their high BW littermates (Table 1). By 12 months of age, there was no longer a difference in current weight between low and high BW male pigs (low BW: 168.8 ± 13.9 kg, n = 4; high BW: 176.3 ± 9.9 kg, n = 4), but this difference remained in female pigs (low BW: 140.0 ± 5.5 kg, n = 5; high BW: 162.5 ± 6.3 kg, n = 4; P < 0.05). Absolute postnatal growth rates (GR; weight gained (day)−1) from birth to 1 month and from birth to 3 months of age were significantly less in low than in high BW pigs (Table 1). The relative increase in body weight (fractional GR; weight gained per day per starting kg) in low BW pigs was significantly greater than in high BW pigs during suckling (birth to 1 month) and between 3 and 12 months of age, but not in the immediate post-weaning period (1–3 months; Table 1). BW significantly determined GRs from birth to 1 month and from birth to 3 months (R = +0.71, P < 0.001, n = 47 and R = +0.50, P < 0.001, n = 47, respectively), as well as fractional GRs from birth to 1 month and from 3 to 12 months (R = −0.40, P < 0.01, n = 47 and R = −0.85, P < 0.001, n = 47, respectively). GR from birth to 12 months was significantly associated with BW in female (R = +0.68, P < 0.05, n = 9) but not male pigs (R = +0.07, n = 8).
Table 1.
Body weights, morphometric measurement ratios (at birth and at 3 and 12 months of age) and postnatal growth rates in low and high BW pigs
| Low BW | High BW | |
|---|---|---|
| At birth | ||
| BW (kg) | 1.13 ± 0.04d | 1.90 ± 0.04 |
| BMI (kg CRL−2) | 17.7 ± 0.31d | 21.4 ± 0.51 |
| head length:BW (cm kg−1) | 8.78 ± 0.36d | 5.73 ± 0.14 |
| head length:AC | 0.449 ± 0.017a | 0.403 ± 0.010 |
| At 3 months | ||
| CW (kg) | 23.5 ± 1.9d | 37.2 ± 2.5 |
| BMI (kg CRL−2) | 40.9 ± 2.4a | 51.6 ± 2.7 |
| head length:CW (cm kg−1) | 0.99 ± 0.09a | 0.63 ± 0.08 |
| head length:AC | 0.339 ± 0.014a | 0.303 ± 0.010 |
| At 12 months | ||
| CW (kg) | 152.8 ± 8.1* | 169.4 ± 6.9 |
| BMI (kg CRL−2) | 81.5 ± 3.8 | 82.0 ± 6.5 |
| head length:CW (cm kg−1) | 0.223 ± 0.016 | 0.260 ± 0.035 |
| head length:AC | 0.268 ± 0.015 | 0.331 ± 0.046 |
| GR 0–1 months (kg day−1) | 0.237 ± 0.012d | 0.350 ± 0.014 |
| GR 0–3 months (kg day−1) | 0.284 ± 0.020d | 0.452 ± 0.030 |
| GR 0–12 months (kg day−1) | 0.476 ± 0.034 | 0.541 ± 0.025 |
| Fractional GR 0–1 months (kg day−1 kg−1) | 0.175 ± 0.008b | 0.144 ± 0.009 |
| Fractional GR 1–3 months (kg day−1 kg−1) | 0.046 ± 0.003 | 0.056 ± 0.003 |
| Fractional GR 3–12 months (kg day−1 kg−1) | 0.026 ± 0.002c | 0.016 ± 0.002 |
Values are means ± S.E.M. AC, abdominal circumference; BMI, body mass index; BW, birth weight; CRL, crown rump length; CW, current weight; GR, growth rate. Low BW vs. high BW (unpaired t test):
P < 0.05
P < 0.01
P < 0.005
P < 0.001
different effect of BW in male and female pigs: refer to text.
At 3 months of age: low BW, n = 22; high BW, n = 25. At 12 months of age: low BW, n = 9; high BW, n = 7.
Adrenal weights and morphometry
Juvenile pigs
Postnatal adrenal growth, as measured by either adrenal size, expressed in relation to body weight, or the area occupied by the adrenal cortex, relative to adrenal medullary area, was significantly greater in low than in high BW pigs at 3 months of age (Fig. 1). BW was directly associated with the adrenal:CW ratio (R = −0.56, P < 0.01, n = 22). Both the adrenal:CW and cortex:medulla ratio were significantly related to disproportionate body shape at birth, as measured by the head length:BW ratio (R = +0.61, P < 0.005, n = 22 and R = +0.52, P < 0.05, n = 22, respectively) and the head length:AC ratio (R = +0.58, P < 0.005 and R = +0.56, P < 0.05, respectively; n = 22).
Figure 1. Adrenal size and morphometry in juvenile and adult pigs.
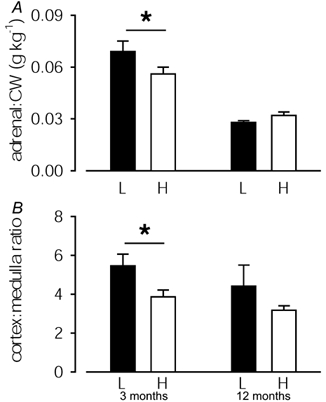
The ratios of adrenal weight:current weight (CW) and cortical:medullary area in low (L; ▪) and high (H; □) BW pigs at 3 and 12 months of age. At 3 months of age, adrenal:CW (P < 0.01) and cortex:medulla ratios (P < 0.05) in low BW pigs were significantly greater than in high BW pigs, as indicated by asterisks.
Adult pigs
By 12 months of age, the ratios of adrenal:CW and cortex:medulla were no longer significantly different between low and high BW pigs (Fig. 1), although the adrenal:CW ratio tended (R = +0.54, P = 0.059, n = 13) to be determined by thinness (BMI) at birth.
Basal hormone concentrations
Juvenile pigs
At 3 months of age, there were no significant differences between low and high BW pigs in basal plasma ACTH, cortisol, noradrenaline or adrenaline concentrations (Fig. 2) or in the ratio of basal cortisol:ACTH (low BW: 1.55 ± 0.27, n = 16; high BW: 1.13 ± 0.16, n = 12). However, low BW was associated with elevated basal noradrenaline concentrations (R = −0.34, P < 0.05, n = 28). Disproportionate body shape at birth (increased head length:BW) also significantly predicted increased basal noradrenaline concentrations, but in male (R = +0.51, P < 0.05, n = 16) not female pigs (R = +0.28, n = 12).
Figure 2. Basal plasma ACTH, cortisol and catecholamine concentrations.
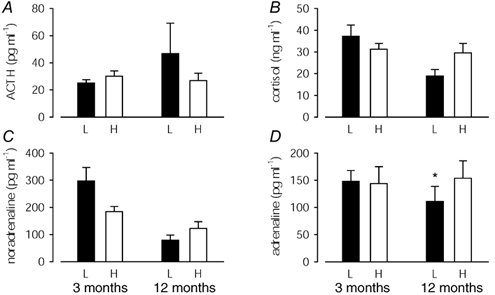
Basal plasma ACTH (A), cortisol (B), noradrenaline (C) and adrenaline (D) concentrations in low (L; ▪) and high (H; □) BW pigs at 3 and 12 months of age. * Different effect of BW in male and female pigs: refer to text.
Adult pigs
At 12 months of age, there were no significant differences between low and high BW pigs in basal plasma ACTH or noradrenaline concentrations (Fig. 2A and C), however there were tendencies for basal cortisol concentrations (Fig. 2B; P = 0.057) and the basal cortisol:ACTH ratio (low BW: 0.81 ± 0.17, high BW: 1.37 ± 0.25; P = 0.074) to be reduced in low BW compared with high BW pigs. There was no effect of BW on basal adrenaline concentrations overall (Fig. 2D), however adrenaline concentrations were significantly reduced in low BW female (low BW: 59 ± 18 pg ml−1, n = 5; high BW: 218 ± 73 pg ml−1, n = 4; P < 0.05), but not male pigs (low BW: 175 ± 41 pg ml−1, n = 4; high BW: 112 ± 23 pg ml−1, n = 4). The ratio of basal cortisol:basal ACTH was determined by the adrenal:CW ratio (R = +0.74, P < 0.005, n = 17). Thinness at birth was associated with reduced basal adrenaline concentrations (R = +0.49, P < 0.05, n = 17), but when analysed by sex this relationship was significant only in adult female pigs (females: R = +0.62, P < 0.05, n = 9; males: R = −0.05, n = 8).
Insulin challenge
Juvenile pigs
Plasma glucose concentrations during insulin challenge at 3 months of age were not different between low and high BW pigs (Fig. 3A). There were no significant differences between low and high BW pigs in plasma ACTH, noradrenaline and adrenaline concentrations during insulin challenge at 3 months of age (Fig. 3B, D and E). However, the cortisol response to insulin-induced hypoglycaemia in low BW pigs was significantly greater than in high BW pigs (Fig. 3C) and there was a significant (P < 0.05) difference in the cortisol AUC between the groups (low BW: 4.57 ± 0.83 µg min ml−1; high BW: 2.57 ± 0.40 µg min ml−1). There was no effect of BW on ACTH AUC (low BW: 5.19 ± 0.85 ng min ml−1; high BW: 4.67 ± 0.85 ng min ml−1), noradrenaline AUC (low BW: 22 ± 5 ng min ml−1; high BW: 15 ± 2 ng min ml−1) or adrenaline AUC (low BW: 126 ± 20 ng min ml−1; high BW: 91 ± 18 ng min ml−1) at 3 months of age.
Figure 3. Plasma glucose, ACTH, cortisol and catecholamine concentrations during insulin challenge.
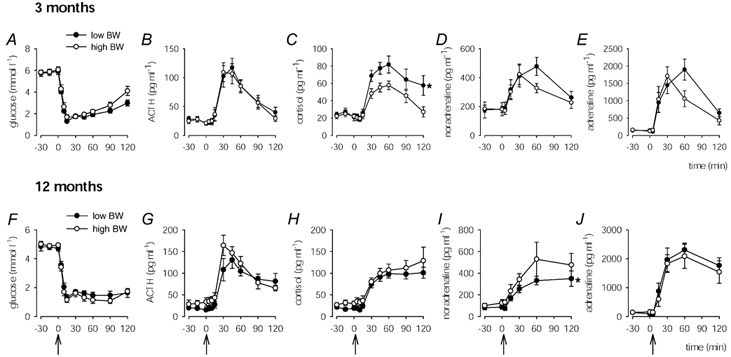
Plasma glucose (A and F), ACTH (B and G), cortisol (C and H), noradrenaline (D and I) and adrenaline (E and J) concentrations in low (•) and high (○) BW pigs prior to and following insulin administration (0.5 IU (kg body weight)−1; i.v.) at time 0 min (arrows) at 3 (A-E) and 12 (F-J) months of age. At 3 months of age, cortisol concentrations during insulin challenge in low BW pigs were significantly (P < 0.005) greater than in high BW pigs (* 2-way ANOVA). At 12 months of age, noradrenaline concentrations during insulin challenge in low BW pigs were significantly (P < 0.05) lower than in high BW pigs (* 2-way ANOVA).
The cortisol response (AUC) to hypoglycaemia was determined by current adrenal size and relative cortical area (Table 2). These effects remained significant only in male (R = +0.85, P < 0.001, n = 16 and R = +0.77, P < 0.05, n = 16, respectively) but not female pigs (R = +0.01, n = 16 and R = +0.15, n = 12, respectively). Also in males only, disproportionate body shape (head length:BW) at birth predicted significantly increased cortisol AUC and adrenaline AUC (males: R = +0.54, P < 0.05, n = 16 and R = +0.49, P < 0.05, n = 16, respectively; females: R = +0.42, n = 12 and R = +0.24, n = 12, respectively).
Table 2.
Correlation coefficients (R) between areas (AUC) under the ACTH, cortisol, noradrenaline and adrenaline curves during insulin challenge at 3 and 12 months of age and birth weight, morphometric measurement ratios at birth and adrenal size and morphometry
| ACTH AUC | Cortisol AUC | Noradrenaline AUC | Adrenaline AUC | |||||
|---|---|---|---|---|---|---|---|---|
| 3 months | 12 months | 3 months | 12 months | 3 months | 12 months | 3 months | 12 months | |
| At birth | ||||||||
| BW | −0.10 | −0.10 | −0.31 | −0.41 | −0.19 | +0.10 | −0.23 | −0.45 |
| BMI | −0.12 | +0.08 | −0.20 | −0.58a | −0.18 | +0.09 | −0.05 | −0.46 |
| head length:BW | +0.05 | +0.10 | +0.32 | +0.28 | −0.01 | −0.18 | +0.33 | +0.27 |
| head length:AC | +0.32 | +0.12 | +0.19 | −0.16 | +0.14 | −0.42 | +0.19 | −0.17 |
| At 3 months | At 12 months | At 3 months | At 12 months | At 3 months | At 12 months | At 3 months | At 12 months | |
| Adrenal:CW | +0.66a | −0.31 | +0.64a | −0.32 | +0.82d | +0.15 | +0.72c | −0.46 |
| Cortex:medulla | +0.48 | +0.21 | +0.65a | −0.27 | +0.70b | −0.32 | +0.85d | +0.07 |
AC, abdominal circumference; AUC, area under curve; BMI, body mass index; BW, birth weight; CW, current weight.
P < 0.05
P < 0.01
P < 0.001
P < 0.005.
At 3 months of age, n = 28; at 12 months of age, n = 17.
Adult pigs
Plasma glucose concentrations during insulin challenge at 12 months of age were not different between low and high BW pigs (Fig. 3F). There were no significant differences between low and high BW pigs in plasma ACTH, cortisol and adrenaline concentrations during the insulin challenge (Fig. 3G, H and J). However, the noradrenaline response to insulin-induced hypoglycaemia in low BW pigs was significantly lower than in high BW pigs (Fig. 3I). Overall, there were no effects of BW on ACTH AUC (low BW: 8.14 ± 0.99 ng min ml−1; high BW: 8.01 ± 0.93 ng min ml−1), cortisol AUC (low BW: 7.40 ± 0.91 µg min ml−1; high BW: 5.17 ± 1.29 µg min ml−1), noradrenaline AUC (low BW: 24 ± 4 ng min ml−1; high BW: 24 ± 7 ng min ml−1) or adrenaline AUC (low BW: 200 ± 26 ng min ml−1; high BW: 129 ± 35 ng min ml−1) at 12 months of age. However, adrenaline AUC was significantly increased in low BW male (low BW: 161 ± 18 ng min ml−1, n = 4; high BW: 71 ± 5 ng min ml−1, n = 4; P < 0.005), but not female pigs (low BW: 230 ± 34 ng min ml−1, n = 5; high BW: 206 ± 57 ng min ml−1, n = 4). Thinness at birth (BMI) significantly determined increased cortisol AUC (Table 2) at 12 months of age. There were no relationships between cortisol AUC and adrenal size or cortical area (Table 2), except in females where cortisol AUC was significantly enhanced in those pigs with a reduced ratio of adrenal cortex:medulla (females: R = −0.90, P < 0.05, n = 5; males: R = −0.07, n = 6).
ACTH challenge
Juvenile pigs
At 3 months of age, there was a significant (P < 0.005) interaction between time and plasma cortisol concentrations during the ACTH challenge, indicating a greater response in low than in high BW pigs (Fig. 4A). However, cortisol AUC in low BW pigs (5.08 ± 0.44 µg min ml−1) was not different to that in high BW pigs (4.34 ± 0.65 µg min ml−1). Cortisol AUC was significantly determined by adrenal size and relative cortical area in male (R = +0.72, P < 0.05, n = 11 and R = +0.81, P < 0.05, n = 11, respectively) but not female pigs (R = −0.33, n = 11 and R = −0.16, n = 11, respectively).
Figure 4. Plasma cortisol concentrations during ACTH challenge.
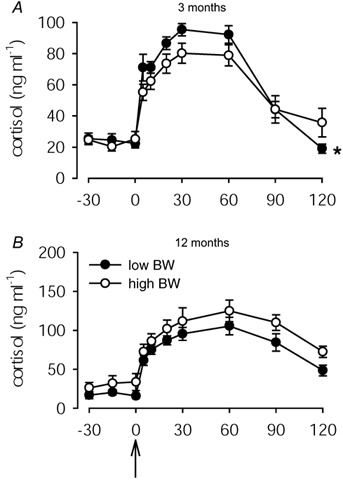
Plasma cortisol concentrations in low (•) and high (○) BW pigs prior to and following ACTH administration (2.0 µg (kg body weight)−1; i.v.) at time 0 min (arrow) at 3 (A) and 12 (B) months of age. At 3 months of age, cortisol concentrations in low BW pigs were significantly (P < 0.005) greater than in high BW pigs (* 2-way ANOVA).
Adult pigs
At 12 months of age, plasma cortisol concentrations during the ACTH challenge were not different between low and high BW pigs (Fig. 4B), and the total cortisol response (AUC) in low BW pigs (8.14 ± 1.05 µg min ml−1) was not different to that in high BW pigs (8.80 ± 1.15 µg min ml−1). Cortisol AUC following ACTH administration was not related to thinness (BMI) at birth (R = −0.21, n = 13), adrenal size (R = −0.11, n = 11) or cortical area (R = −0.49, n = 12). However, cortisol AUC was significantly negatively related to the ratio of adrenal cortex:medulla in female (R = −0.93, P < 0.01, n = 6) but not male pigs (R = −0.50, n = 6).
DISCUSSION
This study has characterised the effect of birth weight on postnatal HPA axis function in the pig, a species in which BW normally varies 2–3-fold within each litter. The results show that low BW in pigs, within the natural range of birth weights, is associated with changes in HPA axis function in later life. Postnatal growth patterns were, in part, determined by birth size in this species and these, together with the sex of the piglet, may also be implicated in the development of adult HPA function.
Pigs were studied at 3 months of age, as pre-pubertal juveniles, and at 12 months of age, as young adults. At 3 months of age, low BW pigs remained smaller than their high BW littermates, despite an early period of catch-up growth in low BW pigs during suckling (first postnatal month). In all adult pigs, there was a second phase of increased fractional growth rate between 3 and 12 months of age, although only the male, and not the female, low BW pigs were able to catch up to high BW pigs in current body weight.
HPA axis function in juvenile pigs
Cortisol responses to ACTH and insulin-induced hypoglycaemia were elevated in low BW pigs. Similar hyperactivity of the HPA axis in postnatal life, as measured by increased ACTH and corticosteroid output to stressors, has also been demonstrated in other experimental animals after intrauterine compromise induced by maternal handling and undernutrition, for example (Henry et al. 1994; McCormick et al. 1995; Hanson et al. 1999). In addition, in human populations, low BW has been shown to be associated with increased adrenocortical function in 9-year-old children (Clark et al. 1996) and with increased fasting cortisol concentrations in adults (Phillips et al. 1998). This upregulation of postnatal HPA function in association with low BW in pigs and other species may reflect changes in the HPA axis at the level of the hypothalamus, pituitary or adrenal gland itself.
At 3 months of age, the adrenal to body weight ratio was directly associated with low BW and disproportionate shape at birth. These observations suggest that the enhanced adrenal size known to occur with low body weight in fetal and newborn pigs persists for at least 3 months after birth (Wise et al. 1991; Klemcke et al. 1993). The increase in adrenal size and cortical area may therefore explain, in part, the exaggerated cortisol response to challenges in low BW pigs, particularly in male pigs in which cortisol AUC was directly predicted by adrenal size and cortical area. However, factors other than adrenal size may contribute to the increase in stimulated cortisol secretion in low BW juvenile pigs, as the increased cortisol AUC was not related to adrenal size or relative cortical area in females. In fetal sheep, adverse intrauterine conditions lead to changes in steroidogenic enzyme levels and/or ACTH receptors in the adrenal gland, which may alter basal and stimulated cortisol secretion (Ross et al. 2000; Hawkins et al. 2001). Changes at the adrenal level therefore contribute to the enhanced responsiveness of the HPA axis in juvenile pigs of low BW, although the relative importance of increases in adrenal growth, ACTH receptor density and/or steroidogenic enzyme levels to the heightened responsiveness remains unclear.
There was no direct evidence for changes in HPA function at the level of the hypothalamus and pituitary in juvenile pigs of low BW. Neither basal nor stimulated ACTH concentrations were affected by body weight and proportions at birth. In previous studies in rats, there is evidence for postnatal changes in pituitary pro-opiomelanocortin (POMC) expression after prenatal compromise (Weinstock et al. 1992). The current observation that the cortisol response (AUC) to endogenous, but not exogenous, ACTH was greater in low BW pigs suggests that pituitary POMC translation or processing may be altered in the low BW animals with an increase in the proportion of bioactive to immuno-reactive ACTH released from the pituitary gland. Further studies are therefore needed to determine whether low BW affects pituitary storage and secretion of bioactive ACTH in juvenile pigs.
Although the catecholamine responses to insulin-induced hypoglycaemia were not affected by BW, elevated basal plasma noradrenaline concentrations in juvenile pigs were associated with low BW. An enhanced adrenaline response to insulin administration was also observed in male pigs that had a disproportionate shape at birth. These observations suggest that poor intrauterine growth affects development of the sympathetic nervous system in the pig. Similar findings on the effects of intrauterine growth retardation or stress have been made in fetal rats, sheep and chick embryos (Hiraoka et al. 1991; Gagnon et al. 1994; Ruijtenbeek et al. 2000). In these species, the increased catecholamine concentrations associated with fetal growth retardation or stress have been attributed to enhanced sensitivity of the peripheral nervous system, a greater density of sympathetic innervation and to an increase in the adrenal content of phenylethanolamine N-methyltransferase (PNMT), which converts noradrenaline to adrenaline (Simonetta et al. 1997; Kennedy & Ziegler, 2000; Ruijtenbeek et al. 2000). In rats, the adverse effects of prenatal stress on sympatho-adrenal development persist after birth and led to enhanced stress-induced sympathetic activation in the adult offspring (Weinstock et al. 1998). In the current study, the elevated noradrenaline concentrations in the low BW juvenile pigs may explain, in part, the raised arterial blood pressure observed in these animals. (Poore et al. 2002a).
In the current study, birth size also determined postnatal growth patterns and, hence, postnatal HPA function may reflect a complex interaction between pre- and postnatal influences. Indeed, the increased stimulated HPA activity observed in 3-month-old low BW pigs may be induced by the postnatal environment, regardless of fetal experience. In rats, prenatal and postnatal manipulations have independent but opposite effects on adult HPA function (Meaney et al. 1989; Henry et al. 1994). In addition, the effects of the prenatal environment can be reversed by postnatal conditions in this species (Maccari et al. 1995). Since HPA function is known to be influenced by social interaction within groups of animals (Sapolsky, 1983), HPA activity in the pigs may have been affected, to some degree, by the normal competition between littermates housed in groups until 3 months of age. However, post-weaning, all pigs in the current study were fed ad libitum and, hence, low BW pigs were as likely to gain access to sufficient food as high BW pigs. Indeed, during suckling, the fractional growth rate of low BW pigs was greater than high BW pigs, which suggests that low BW pigs were sufficiently well fed to attempt catch up growth.
HPA axis function in adult pigs
At 12 months of age, heightened cortisol responses to insulin-induced hypoglycaemia were associated with thinness at birth, not BW per se. Since neither the ACTH response to insulin nor the cortisol response to exogenous ACTH in adult pigs were related to BW or thinness at birth, the exaggerated cortisol response to insulin is likely to reflect a change in the relative levels of bioactive ACTH to total immuno-reactive ACTH. These observations suggest that the increased adrenal responsiveness to exogenous ACTH seen in the 3-month-old low BW pigs does not persist into young adult life. However, the mechanisms involved in the enhanced HPA axis activity in adulthood may be sex linked. Indeed, in males, the association between the cortisol response to insulin and adrenal size and cortical area seen in low BW pigs at 3 month of age was lost in adult pigs. Similarly, the increase in adrenal size and relative cortical area seen in low BW juvenile pigs was not observed at 12 months of age. In fact, the association between thinness at birth and increased cortisol AUC to insulin in adult pigs occurred despite a tendency for a reduced adrenal size in those animals that were thin at birth. In females, the lack of any relationship between cortisol AUC and adrenal size at 3 months of age, and the inverse relationships between relative cortical area and both the cortisol responses to insulin-induced hypoglycaemia and to exogenous ACTH, suggests that intra-adrenal mechanisms may play a more important role in the development of enhanced HPA axis activity than in males. The enhanced HPA activity associated with poor intrauterine growth in the pig, therefore, appears to be due largely to changes at the adrenal level at 3 months of age but may reflect a combination of alterations at the hypothalamic-pituitary and adrenal levels at 12 months of age, possibly in a sex-specific manner. This increase in hypothalamic-pituitary control of HPA activity with increasing age, particularly in males, may be due, in part, to resetting of the HPA axis in response to the reactive hypercortisolaemia seen in early postnatal life. Alternatively, it may reflect the inability of the adrenal gland to keep pace with the overall growth rate after the initial period of accelerated growth in the first few months of life.
In contrast to the findings in 3-month-old pigs, there was no effect of BW on basal noradrenaline concentrations in adult pigs. At 12 months of age, basal adrenaline concentrations were reduced in low BW pigs and were related to thinness at birth, but only in females. Noradrenaline responses to insulin-induced hypoglycaemia were reduced in the low BW adult pigs and, in males, this was accompanied by an increase in the adrenaline response. These observations suggest that changes in adrenal PNMT expression and/or activity may persist into adult life in a sex-specific manner. In low BW males that were disproportionate in body shape at birth, PNMT activity appears to be increased at both 3 and 12 months of age whereas, in low BW females, PNMT activity appears reduced in adults under basal conditions. In part, the sex-related differences in catecholamine secretion may reflect the failure of low BW females to catch up in body weight by 12 months of age.
Using a new model for the study of fetal origins of adult disease, this study has demonstrated that postnatal HPA axis function may be altered by a complex interaction between size and shape at birth, the postnatal patterns of growth and the sex of the individual. Since sex differences were observed at 3 months of age before the onset of gonadal sex steroid production at puberty, programming of the HPA axis in utero appears to occur in a sexually dimorphic manner. The increase in HPA activity observed in low BW pigs may have important implications for the functioning of other physiological systems and may provide a mechanism for early life programming of adult hypertension and glucose intolerance. Indeed, birth weight has recently been shown to be negatively associated with arterial pressure and glucose tolerance in juvenile and adult pigs, respectively (Poore et al. 2002a, b).
Acknowledgments
This work is supported by The Wellcome Trust. We are grateful to Paul Hughes for surgical assistance, Sue Nicols and Vicky Johnson for care of the animals, Malcolm Bloomfield for catecholamine measurements and Mel Quy for preparation of adrenal sections.
REFERENCES
- Agricultural Food and Research Council. Technical committee on response to nutrients, Report No. 4, Nutrient Requirements of sows and boars. Nutrition Abstracts and Reviews, Series B: Livestock and Feeding. 1990;60:383–406. [Google Scholar]
- Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298:564–567. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer R, Walter B, Hoppe A, Gaser E, Lampe V, Kauf E, Zwiener U. Body weight distribution and organ size in newborn swine (Sus scrofa domestica) - a study describing an animal model for asymmetrical intrauterine growth retardation. Exp Toxicol Pathol. 1998;50:59–65. doi: 10.1016/S0940-2993(98)80071-7. [DOI] [PubMed] [Google Scholar]
- Clark PM, Hindmarsh PC, Shiell AW, Law CM, Honour JW, Barker DJ. Size at birth and adrenocortical function in childhood. Clin Endocrinol. 1996;45:721–726. doi: 10.1046/j.1365-2265.1996.8560864.x. [DOI] [PubMed] [Google Scholar]
- Eriksson JG, Forsen T, Tuomilehto J, Osmond C, Barker DJ. Early growth and coronary heart disease in later life: longitudinal study. BMJ. 2001;322:949–953. doi: 10.1136/bmj.322.7292.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowden AL. Endocrine regulation of fetal growth. Reprod Fertil Devel. 1995;7:351–363. doi: 10.1071/rd9950351. [DOI] [PubMed] [Google Scholar]
- Gagnon R, Challis J, Johnston L, Fraher L. Fetal endocrine responses to chronic placental embolization in the late-gestation ovine fetus. Am J Obstet Gynecol. 1994;170:929–938. doi: 10.1016/s0002-9378(94)70309-4. [DOI] [PubMed] [Google Scholar]
- Hanson MA, Hawkins P, Ozaki T, Steyn C, Matthews SG, Noakes D, Poston L. Effects of experimental dietary manipulation during early pregnancy on cardiovascular and endocrine function in fetal sheep and young lambs. In: O'Brien P, Wheeler T, Barker D, editors. Fetal Programming. Influences on Development and Disease in Later Life. London: RCOG Press; 1999. pp. 365–373. [Google Scholar]
- Hawkins P, Hanson MA, Matthews SG. Maternal undernutrition in early gestation alters molecular regulation of the hypothalamic-pituitary adrenal axis in the ovine fetus. J Neuroendocrinol. 2001;13:855–861. doi: 10.1046/j.1365-2826.2001.00709.x. [DOI] [PubMed] [Google Scholar]
- Henry C, Kabbaj M, Simon H, Le Moal M, Maccari S. Prenatal stress increases the hypothalamo-pituitary-adrenal axis response in young and adult rats. J Neuroendocrinol. 1994;6:341–345. doi: 10.1111/j.1365-2826.1994.tb00591.x. [DOI] [PubMed] [Google Scholar]
- Hiraoka T, Kudo T, Kishimoto Y. Catecholamines in experimentally growth-retarded rat fetus. Asia Oceania J Obstet Gynaecol. 1991;17:341–348. doi: 10.1111/j.1447-0756.1991.tb00284.x. [DOI] [PubMed] [Google Scholar]
- Kennedy B, Ziegler M. Ontogeny of epinephirine metabolic pathways in the rat: role of glucocorticoids. Int J Develop Neurosci. 2000;18:53–59. doi: 10.1016/s0736-5748(99)00106-9. [DOI] [PubMed] [Google Scholar]
- Klemcke HG, Lunstra DD, Brown-Borg HM, Borg KE, Christenson RK. Association between low birth weight and increased adrenocortical function in neonatal pigs. J Anim Sci. 1993;71:1010–1018. doi: 10.2527/1993.7141010x. [DOI] [PubMed] [Google Scholar]
- Maccari S, Piazza PV, Kabbaj M, Barbazanges A, Simon H, Le Moal M. Adoption reverses the long-term impairment in glucocorticoid feedback induced by prenatal stress. J Neurosci. 1995;15:110–116. doi: 10.1523/JNEUROSCI.15-01-00110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald AA, Colenbrander B, Wensing CJ. The effects of gestational age and chronic fetal decapitation on arterial blood pressure in the pig fetus. Eur J Obstet Gynecol Reprod Biol. 1983;16:63–70. doi: 10.1016/0028-2243(83)90221-6. [DOI] [PubMed] [Google Scholar]
- McCormick C, Smythe J, Sharma S, Meaney MJ. Sex-specific effects of prenatal stress on hypothalamic-pituitary-adrenal responses to stress and brain glucocorticoid receptor density in adult rats. Devel Brain Res. 1995;84:55–61. doi: 10.1016/0165-3806(94)00153-q. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH, Viau V, Sharma S, Sarrieau A. Neonatal handling alters adrenocortical negative feedback sensitivity and hippocampal type II glucocorticoid receptor binding in the rat. Neuroendocrinol. 1989;50:597–604. doi: 10.1159/000125287. [DOI] [PubMed] [Google Scholar]
- Phillips DI, Barker DJ, Fall CH, Seckl JR, Whorwood CB, Wood PJ, Walker BR. Elevated plasma cortisol concentrations: a link between low birth weight and the insulin resistance syndrome? J Clin Endocrinol Metab. 1998;83:757–760. doi: 10.1210/jcem.83.3.4634. [DOI] [PubMed] [Google Scholar]
- Phillips DI, Barker DJ, Hales CH, Hirst S, Osmond C. Thinness at birth and insulin resistance in adult life. Diabetologia. 1994;37:150–154. doi: 10.1007/s001250050086. [DOI] [PubMed] [Google Scholar]
- Phillips DI, Walker BR, Reynolds RM, Flanagan DE, Wood PJ, Osmond C, Barker DJ, Whorwood CB. Low birth weight predicts elevated plasma cortisol concentrations in adults from 3 populations. Hypertension. 2000;35:1301–1306. doi: 10.1161/01.hyp.35.6.1301. [DOI] [PubMed] [Google Scholar]
- Poore KR, Forehead AJ, Gardner DS, Giussani DA, Fowden AL. The effects of birth weight on basal cardiovascular function in pigs at 3 months of age. J Physiol. 2002a;539:969–978. doi: 10.1113/jphysiol.2001.012926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poore KR, Fowden AL. The effect of birth weight on glucose tolerance in pigs at 3 and 12 months of age. Diabetologia. 2002b;45:1247–1254. doi: 10.1007/s00125-002-0849-y. [DOI] [PubMed] [Google Scholar]
- Ross JT, Phillips ID, Simonetta G, Owens JA, Robinson JS, McMillen IC. Differential effects of placental restriction on IGF-II, ACTH receptor and steroidogenic enzyme mRNA levels in the foetal sheep adrenal. J Neuroendocrinol. 2000;12:79–85. doi: 10.1046/j.1365-2826.2000.00429.x. [DOI] [PubMed] [Google Scholar]
- Ruijtenbeek K, Le Noble FA, Janssen GM, Kessels CG, Fazzi GE, Blanco CE, De Mey JG. Chronic hypoxia stimulates periarterial sympathetic nerve development in chicken embryo. Circulation. 2000;102:2892–2897. doi: 10.1161/01.cir.102.23.2892. [DOI] [PubMed] [Google Scholar]
- Sapolsky R. Individual differences in cortisol secretory patterns in the wild baboon: role of negative feedback sensitivity. Endocrinol. 1983;113:2263–2267. doi: 10.1210/endo-113-6-2263. [DOI] [PubMed] [Google Scholar]
- Silver M, Barnes RJ, Comline RS, Burton GJ. Placental blood flow: some fetal and maternal cardiovascular adjustments during gestation. J Reprod Fertil Suppl. 1982;31:139–160. [PubMed] [Google Scholar]
- Silver M, Comline RS, Fowden AL. Fetal and maternal endocrine changes during the induction of parturition with the PGF analogue, cloprostenol, in chronically catheterized sows and fetuses. J Dev Physiol. 1983;5:307–321. [PubMed] [Google Scholar]
- Simonetta G, Rourke AK, Owens JA, Robinson JS, McMillen IC. Impact of placental restriction on the development of the sympathoadrenal system. Ped Res. 1997;42:805–811. doi: 10.1203/00006450-199712000-00015. [DOI] [PubMed] [Google Scholar]
- Tangalakis K, Lumbers ER, Moritz KM, Towstoless MK, Wintour EM. Effect of cortisol on blood pressure and vascular reactivity in the ovine fetus. Exp Physiol. 1992;77:709–717. doi: 10.1113/expphysiol.1992.sp003637. [DOI] [PubMed] [Google Scholar]
- Weinstock M, Matlina E, Maor GI, Rosen H, McEwen BS. Prenatal stress selectively alters the reactivity of the hypothalamic-pituitary adrenal system in the female rat. Brain Res. 1992;595:195–200. doi: 10.1016/0006-8993(92)91049-k. [DOI] [PubMed] [Google Scholar]
- Weinstock M, Poltyrev T, Schorer-Apelbaum D, Men D, McCarty R. Effect of prenatal stress on plasma corticosterone and catecholamines in response to footshock in rats. Physiol Behav. 1998;64:439–444. doi: 10.1016/s0031-9384(98)00056-0. [DOI] [PubMed] [Google Scholar]
- Widdowson EM, McCance RA. A review: new thoughts on growth. Ped Res. 1975;9:154–156. doi: 10.1203/00006450-197503000-00010. [DOI] [PubMed] [Google Scholar]
- Wise T, Stone RT, Vernon MW. Relationships of serum estriol, cortisol and albumin concentrations with pig weight at 110 days of gestation and at birth. Biol Neonate. 1991;59:114–119. doi: 10.1159/000243331. [DOI] [PubMed] [Google Scholar]


