Abstract
Sour taste is elicited by acids. How taste cells transduce sour taste is controversial because acids (specifically protons) have diverse effects on cell membranes. Consequently, it is difficult to differentiate between events related to sour taste transduction per se and unrelated effects of protons. We have studied acid taste transduction in mouse taste buds using a lingual slice preparation where it is possible to measure changes in pH and [Ca2+]i simultaneously in taste cells. Focal application of citric acid or HCl to the apical tips of taste buds produced widespread acidification of the entire taste bud. Citric acid was effective at a pH of ∼4, but HCl only at a pH of ∼1.5. Despite acidification of the whole taste bud, only a select few taste cells exhibited Ca2+ responses. Acid-evoked Ca2+ responses were dose dependent in a range consistent with them being sour-taste responses. Cells exhibiting acid-evoked Ca2+ responses also responded to KCl depolarization. Acid-evoked Ca2+ responses were blocked by Ba2+ (2 mm) and Cd2+ (500 μm), suggesting that acid responses are generated by Ca2+ influx through depolarization-gated Ca2+ channels. Removing extracellular Ca2+ reduced acid-evoked Ca2+ responses, but depleting intracellular Ca2+ stores with thapsigargin had no effect, suggesting that acid taste responses are generated by an influx of extracellular Ca2+. Neither Cs+ (500 μm) nor amiloride (100 μm) affected acid-evoked Ca2+ responses, suggesting that neither hyperpolarization-activated cyclic nucleotide-gated cation (pacemaker) channels nor epithelial Na+ channels, respectively, transduce sour taste. Collectively, the results indicate that acids, especially weak acids, acidify the taste bud and evoke depolarization-induced Ca2+ entry into a select subset of taste cells. The primary transducer protein(s) for sour taste remain undiscovered.
Sourness is one of the primary taste sensations and sour taste stimuli are uniquely comprised of acids (Miyamoto et al. 1998; DeSimone et al. 2001). It is generally accepted that sour taste is caused by the action of protons on taste receptor cells, although the precise mechanism is controversial. A variety of transduction mechanisms have been proposed. The most straightforward explanation is that taste cells simply respond to protons acting on the extracellular membrane of the apical tip of the cell that is exposed in the taste pore. Such a direct action of protons on the apical tips of taste cells would suggest that taste responses would be graded according to the pH of the acid stimulus. However, this is generally not true: neither the perception of sour taste nor nerve responses to acid tastants is predictably related to stimulus pH (Ganzevles & Kroeze, 1987; Ogiso et al. 2000). Instead, for a given pH, weak acids such as acetic and citric acid are perceived as more sour than strong acids such as H2SO4 and HCl (Richards 1898; Makhlouf & Blum, 1972). Furthermore, DeSimone and co-workers (DeSimone et al. 2001) have found that weak acids acidify taste cells to a greater extent than do strong acids. They interpreted this to mean that molecules of a weak acid penetrate a cell more readily than protons; once inside the taste cells, the weak acid dissociates to acidify the cell. Thus, intracellular acidification has been termed the primary stimulus for sour taste (DeSimone et al. 2001).
We have used Ca2+ imaging of taste receptor cells in a semi-intact preparation of the lingual epithelium in which taste buds remain intact, to measure responses to acid taste stimuli. By measuring changes in both pH and [Ca2+]i in taste buds and taste cells, we show that acid stimuli applied focally to the taste pore cause widespread acidification of the taste bud, but that only a few taste receptor cells respond to the acid stimuli.
METHODS
Tissue preparation
All experimental protocols were approved by the University of Miami Care and Use Committee. We obtained lingual slices containing the vallate papilla from adult (n= 56) DBA/2 mice as described in Caicedo et al. (2002). Animals were killed by exposure to CO2, followed by cervical dislocation.
Fluorescent indicator dyes (see below) were injected iontophoretically as described previously (Caicedo & Roper, 2001; Caicedo et al. 2002). Single or multibarrelled micropipettes were used to deliver taste stimuli directly to the pore region of a selected taste bud (see Fig. 1A–C). The advantage of this technique over the use of isolated taste cells or taste buds is that it mimics the natural situation; only the apical tips of the intact taste cells are exposed to a taste stimulus in the taste pore and the basolateral portions of taste cells remain intact in their natural milieu within the epithelium (Fig. 1A–C). Taste buds could be stimulated repeatedly with a variety of taste stimuli and viable responses could be collected from taste cells for up to 7 h. Data were typically collected at 2 s intervals for approximately 45 s, although longer recordings (up to 3 min) could be made without any adverse effects on the cells. Photobleaching was minimal, but all recordings were routinely corrected to account for this (see below).
Figure 1. Calcium imaging and pH imaging of taste buds using scanning laser confocal microscopy.
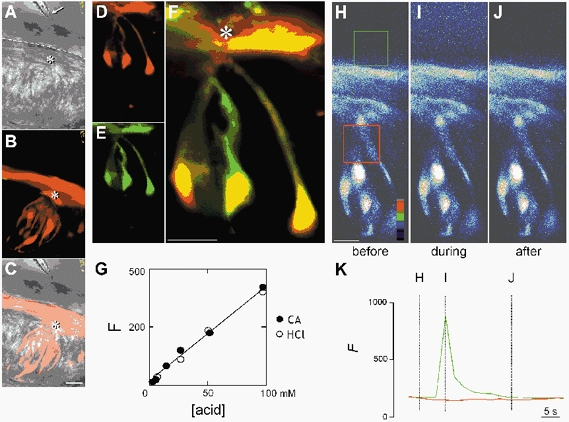
A–C, positioning a micropipette to deliver acid taste stimuli. A, bright-field micrograph showing the tip of a micropipette (arrow) containing a taste stimulus, positioned ∼50 μm from the taste pores (asterisk) of taste buds in slices of lingual tissue. The dashed line indicates epithelial (mucosal) surface. B, fluorescence image (568 nm) corresponding to A showing a single taste bud in which several taste cells have been loaded with the fluorescent Ca2+ indicator dye, Calcium Orange (CaO, see Methods for details). Note how an adherent layer of CaO marks the superficial epithelial boundary. C, merged images of A and B. Scale bars = 20 μm. D-F, loading cells with CaO to measure changes in [Ca2+]i, and 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein dextran (BCECF-D) to measure changes in pH simultaneously within the same cells. Three taste cells are visible in this focal plane. D, fluorescence image (568 nm) of CaO (red). E, fluorescence image (488 nm) of BCECF-D (green). F, merged images of D and E. An asterisk indicates the taste pore. Scale bars = 10 μm. G, serial dilution of stimulus solutions containing a mixture of 100 mm citric acid (CA; •) or HCl (○) and 200 μm lucifer yellow (LY). The linear relationship between LY fluorescence intensity (F) and acid concentration was used to calculate the stimulus concentration in the experiment (see Methods). H-J, solutions applied focally to the taste pore did not penetrate into the epithelial interstitial spaces. Pseudocolour images of a taste bud loaded with Calcium Green dextran (CGD), captured before, during and after delivery of citric acid (100 mm) to a taste bud. K, mean fluorescence level calculated for boxes in H. Red, tissue within the lingual slice (see H); green, bath solution (see H). Note the marked increase in fluorescence in the bath solution near the taste bud pore (green box/tracing and see I), but no fluorescence signal within the taste tissue (red box/tracing and see I).
Cells loaded with the Ca2+ indicator dye Calcium Green-1 dextran (CGD; 10 000 MW; Molecular Probes, Eugene, OR, USA), the pH indicator dye 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein dextran (BCECF-D; Molecular Probes), and/or the AM form of BCECF (BCECF-AM) were excited at 488 nm with an argon laser attached to an Olympus Fluoview scanning confocal microscope. Cells loaded with the Ca2+ indicator dye, Calcium Orange (CaO; tetrapotassium salt; Molecular Probes), were excited at 568 nm with a krypton laser. Additional details about the imaging procedure can be found in Caicedo et al. (2000, 2002).
To allow comparison of recordings from cells with different resting fluorescence levels, data from individual cells were normalized to the pre-stimulation fluorescence value and are presented as relative fluorescence change ΔF/F in arbitrary units, where F denotes the resting fluorescence level. Changes in [Ca2+]i and pH are both reported in this manner.
Assessment of changes in pH in taste tissue
Changes in pH were monitored using single-wavelength estimation of pH shifts with the pH-sensitive dye, BCECF (Schwiening & Willoughby, 2002). Normally, the pH of a solution can be determined from the single-wavelength fluorescence if the fluorescence intensity of the dye can be measured in its completely unprotonated and completely protonated form under the same conditions in which the actual fluorescence intensity is measured. Because this is difficult to achieve in living cells, it is extremely difficult to calibrate a single-wavelength fluorescent dye for absolute pH. However, because BCECF has a pKa of 6.98 and its fluorescence intensity (FBCECF) approaches zero at low pH, a change in pH can be described as:
(cf. Schwiening & Willoughby, 2002).
This equation reveals that pH is a monotonic function of ΔFBCECF/F0 (where F0 is the pre-stimulation baseline level of fluorescence). Thus, we were able to use shifts in BCECF fluorescence as a qualitative indicator of changes in pH, because a decrease in BCECF fluorescence reflects a decrease in pH.
Taste cells were loaded with BCECF-D iontophoretically (1 mm), as described above for Ca2+-sensitive dyes. BCECF-D was usually loaded into taste cells together with a Ca2+-sensing dye (see below) to measure changes in [Ca2+]i at the same time as pH shifts (Fig. 1D–F). This did not affect the loading efficacy of either dye. Iontophoresis of BCECF-D usually loaded 20–30 % of cells in a taste bud. To measure the change in pH in the entire taste bud, slices were incubated in the dark in a standard Tyrode solution (see below) containing 2 mm BCECF-AM for 30 min. This resulted in most of the cells within the taste bud containing BCECF-AM (see Fig. 2B).
Figure 2. Effects of stimulating taste buds with citric acid or HCl on pH in the taste bud.
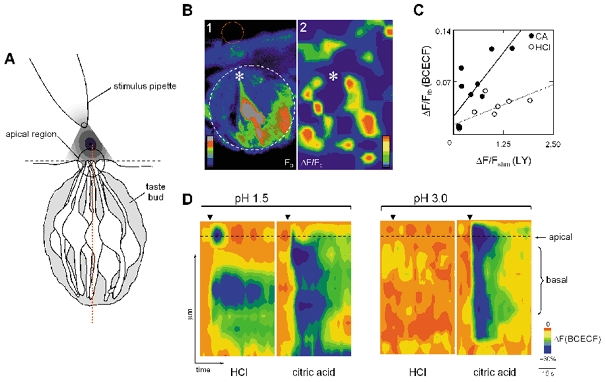
A, schematic drawing of a taste bud, illustrating focal application of taste stimuli. The vertical dashed line through the middle of the taste bud (red) shows the region selected for measuring BCECF fluorescence intensity (i.e. linescan) in D during acid stimulation. B, focal application of citric acid or HCl to the taste pore (pH ∼3) (dashed red line in B1) caused acidification throughout a taste bud (dashed white line in B1). B1, pseudoscale image fluorescence intensity (FBCECF) in a taste bud loaded with BCECF-D and BCECF-AM. The asterisk indicates the taste pore. Dashed lines indicate the regions in which fluorescence intensity was measured to obtain the data presented in C. B2, pseudocolour image of the difference in BCECF fluorescence intensity before and during peak response to citric acid (ΔF/F). The greatest decreases in pH are shown in red, according to the pseudocolour scale shown. C, acidification of the taste bud was directly related to the stimulus concentration at the taste pore. Plot of the stimulus strength at the taste pore (ΔF/Fstim; n= 3 taste buds) versus change in pH of the taste bud (ΔF/Ftb) for citric acid (CA, •) and HCl (○). Continuous line, least squares regression for CA; dashed line, regression for HCl. D, acidification of the taste bud in response to focal application of citric acid or HCl at pH 1.5 or 3.0. Changes in pH along the linescan (red dashed line shown in A) versus time (abscissa) are indicated by a pseudocolour scale in which cool colours blue) represent acidification (inset). Arrowhead, time of stimulus delivery. Dashed lines show mucosal surface (see A).
Measurement of changes in [Ca2+]i
To measure changes in [Ca2+]i simultaneously with changes in pH, we used CaO as a Ca2+ indicator and BCECF as a pH indicator. Because CaO is excited at 550 nm and BCECF at 490 nm, we were able to simultaneously image changes in [Ca2+]i and pH in the same cells (Fig. 1D–F).
To examine the changes in [Ca2+]i in response to acid stimuli in more detail and to construct concentration-response relationships, we measured changes in [Ca2+]i independent of pH by loading taste cells with CGD. CGD was preferred to CaO for measuring changes in [Ca2+]i alone because Ca2+ signals recorded using CGD have a greater signal-to-noise ratio (Haugland, 1996).
Sour stimuli
We selected citric acid as a representative weak acid, although other weak acids such as acetic acid gave qualitatively similar results (data not shown). The standard citric acid stimulus solution was 100 mm (pH ∼3 unadjusted) in standard Tyrode solution. We used HCl as a representative strong acid.
To compare the actions of citric acid and HCl at equivalent pH, we prepared three HCl solutions of pH 1.5, 3 and 7 by titrating standard Tyrode buffer (pH 7.2) with 1 N HCl. An equivalent series of citric acid solutions (pH 1.5, 3 and 7) was prepared by titrating 100 mm citric acid (pH 3) with HCl (for pH 1.5) or NaOH (for pH 7).
Tracking stimulus intensity
Acid solutions were applied focally to taste bud pores from a puffer pipette positioned ∼50 μm from the taste pore (see Fig. 1C) and pressure ejection with a picospritzer (General Valve, East Hanover, NJ, USA). In some experiments, tastants were applied by using a seven-barrel focal stimulus micropipette. The intensities of stimuli were varied by altering ejection duration, pressure, distance from the taste pore and bath flow rate. Taste stimuli were applied at ∼5 min intervals to avoid cellular adaptation.
To measure the concentration of the tastant at the taste pore, each stimulus solution included lucifer yellow CH (potassium salt, 200 μm; Molecular Probes), a fluorescent dye that is insensitive to pH. We prepared a calibration solution containing the acid stimulus and 200 μm lucifer yellow and verified that as the stimulus was diluted, there was a linear decrease in lucifer yellow fluorescence intensity. The linear relationship between lucifer yellow fluorescence intensity and dilution of the acid can was used to calculate the stimulus concentration at the taste pore, as:
where F is the lucifer yellow fluorescence intensity, a is the intercept and b the slope of the calibration curve (Fig. 1G). Since lucifer yellow does not penetrate the epithelial tissue (Fig. 1H–K), the fluorescence from the tracer dye did not interfere with the fluorescence signal of ion-sensitive dyes within the epithelial tissue. Therefore, the pH of the tissue and [Ca2+]i could be measured at the same time as the stimulus concentration at the taste pore was measured with lucifer yellow.
Perfusion solutions
Standard Tyrode solution consisted of (mm): 135 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 5 NaHCO3, 10 Hepes, 10 glucose and 10 sodium pyruvate. For nominally Ca2+-free Tyrode solution, 137 NaCl and 0 CaCl2 were used. All other constituents were the same. For the high-K+ Tyrode solution, 90 NaCl and 50 KCl were used. Cd2+ (500 μm), acetylcholine (200 μm), thapsigargin (5 μm), Cs+ (500 μm) and amiloride (100 μm) were added to standard Tyrode solution. Ba2+ was applied at 2 mm by equimolar substitution for Ca2+ in the standard Tyrode solution. For all solutions (with the exception of the acidic test solutions described above), the pH was adjusted to 7.2 using NaOH or HCl and the osmolarity was adjusted to ∼335 mosmol l−1 by varying the concentration of NaCl. All chemicals were obtained from Sigma (St Louis, MO, USA).
All solutions other than acid tastants were applied via bath perfusion. Complete bath exchange was accomplished in ∼20 s. After exchanging a perfusion solution, the tissue was allowed to equilibrate in the new solution for at least 5 min before further experimentation.
Data analysis
Statistical tests of significance (paired Student's t test or ANOVA with appropriate post hoc tests) were applied to compare response amplitudes of ΔF/F responses for different treatments, where the amplitude was defined as being equal to the highest (for [Ca2+]i) or lowest (for pH) value of ΔF/F within a recording period. For averaging, responses were aligned at the initiation of the rising phase.
RESULTS
We assessed whether focally applied citric acid and HCl alter the intracellular pH of taste buds in lingual slices. When applied to the taste pore at pH ∼1.5–4, citric acid readily decreased the pH of intracellular spaces in taste buds (Fig. 2A and B). HCl at pH ∼1.5 (but not at lower values) had a similar effect. We surmise that extracellular pH within the taste bud underwent similar changes, but we could not specifically discriminate interstitial signals. The bulk of the acid-sensitive dye was intracellular. Acidification was not due to direct exposure of the basolateral membranes of taste cells to the acid stimulus, as indicated by the fact that the tracer dye (lucifer yellow) delivered together with the stimulus does not penetrate the taste tissue (see Fig. 1K). These results suggest that acids penetrate the taste epithelium to reach the basolateral membranes of taste cells within the taste bud. The pH of the acid stimulus at the taste pore was directly related to the extent of acidification of the taste bud, as indicated by the significant correlation between these two parameters (Fig. 2C). At equivalent pH, citric acid was much more effective than HCl in acidifying the taste bud (Fig. 2D), consistent with the ability of fully protonated acids (i.e. weak acids) to permeate tissue membranes more easily than protons (Lyall et al. 2001).
We next tested the ability of weak and strong acids to stimulate taste cells, as indicated by transient increases in [Ca2+]i (see Caicedo et al. 2000). Interestingly, despite the widespread acidification of taste cells throughout the whole of the taste bud, focally applied acids caused Ca2+ responses in only a subset of taste cells (Fig. 3A–C). We determined that 24.6 % (17/69) of taste cells responded to acid stimulation. This corresponds well with data published by Caicedo et al. (2002). The amplitudes of Ca2+ responses were directly related to ΔpHi in this subset of cells (Fig. 3D). Furthermore, the amplitude of the Ca2+ responses depended on the concentration of the tastants applied (Fig. 4; Table 1).
Figure 3. Citric acid acidifies all cells within a taste bud, but only some of these cells respond with an increase in [Ca2+]i.
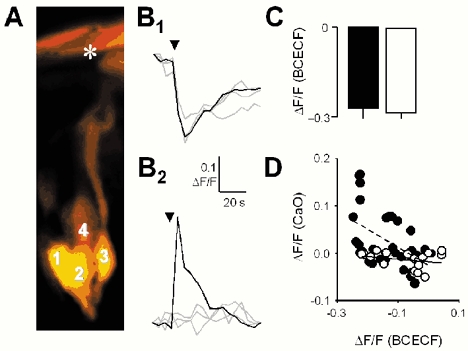
A, a single taste bud within which four individual taste cells are visible. The asterisk indicates the taste pore. The cells were loaded with CaO (red) and BCECF-D (green) to measure simultaneously changes in [Ca2+]i and intracellular pH. The red and green channels have been merged in this image and overlapping red and green is visible as yellow. Fluorescence was quantified in cells 1–4. B, the tracings are changes in intracellular pH (B1) and [Ca2+]i (B2) in response to focally applied citric acid (arrowhead) in the four cells indicated in the taste bud in A. Each of the cells responded to citric acid with a decrease in intracellular pH (B1), but only one cell (cell 4) also exhibited an increase in [Ca2+]i (B2, black line). There was no change in [Ca2+]i in the remaining three cells (grey lines), despite a decrease in pH in these cells. C, mean (±s.e.m.) decrease in pH in citric-acid-responsive (▪) and non-responsive (□) cells (n= 5 cells). D, scatterplot of the change in pH (abscissa) versus the change in [Ca2+]i (ordinate) in a single representative taste cell that did (•) and another that did not (○) exhibit a Ca2+ response to citric acid. The dashed line is the least-squares regression through the •s. The continuous line is the regression through the ○s.
Figure 4. Ca2+ responses of taste cells to focally applied acid are concentration-dependent.
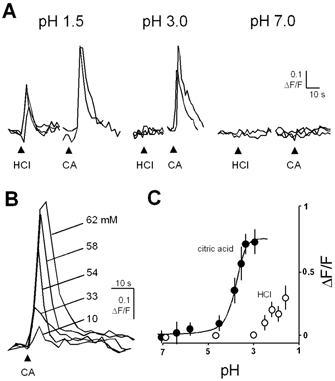
A, representative tracings of Ca2+ responses to citric acid and HCl at different stimulus pH values. Tracings at each pH represent several recordings obtained from the same taste cell. Arrowheads indicate stimulus delivery. B, Ca2+ responses from a single representative taste cell stimulated with different concentrations of citric acid. C, concentration-response relationship of Ca2+ responses (mean ±s.e.m.) versus concentration of citric acid (•, n= 187 responses from 31 cells) and HCl (○, n= 26 responses from 5 cells).
Table 1.
Changes in Ca2+ in response to different concentrations of tastants and different pH values
| Ca2+ response amplitudeb | |||
|---|---|---|---|
| Stimulus pHa | [H+]a | Citric acid | HCl |
| 1.5 | 32 mm | 0.14 ± 0.02 | 0.15 ± 0.02 |
| 3.0 | 1 mm | 0.18 ± 0.03 | 0.01 ± 0.00* |
| 7.0 | 100 μm | 0.03 ± 0.04 | 0.04 ± 0.02 |
The pH at the taste pore was not measured but was inevitably greater than that in the pipette (i.e. [H+] was lower) due to stimulus dilution in the bath solution. These data are responses of the same cells to citric acid and HCl applied sequentially.
Refers to the solution in the focal stimulus pipette.
Expressed as δF/F.
P < 0.05, paired Student's t test for HCl versus citric acid.
To determine the source of Ca2+ in acid responses, we removed Ca2+ from the bath. Citric-acid-induced Ca2+ responses were significantly reduced when [Ca2+]i was nominally ∼0 (Fig. 5A). By contrast, depletion of intracellular Ca2+ stores with thapsigargin had no effect on citric acid responses (Fig. 5B). As a control for thapsigargin, we tested responses to acetylcholine, which has been shown to stimulate muscarinic acetylcholine receptors and elicit intracellular Ca2+ release in murine taste cells (Ogura, 2002). Thapsigargin effectively abolished responses to acetylcholine in the same preparations in which acid responses were unaffected (Fig. 5B). These data indicate that the increase in [Ca2+]i in taste cells following focal acid stimulation is due to Ca2+ entry and does not involve release of Ca2+ from intracellular stores.
Figure 5. Ca2+ responses of taste cells to focally applied citric acid require entry of extracellular Ca2+.
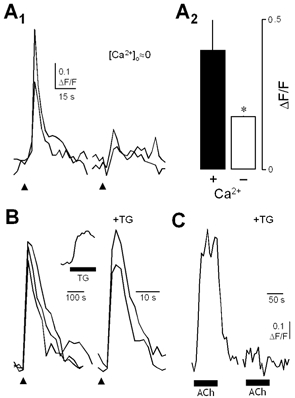
A1, reducing [Ca2+]o by bathing with Ca2+-free Tyrode solution reduced the amplitude of the Ca2+ response to citric acid (pH 3). Superimposed traces from two representative taste cells, stimulated focally with citric acid (arrowhead) either in the presence of normal or reduced extracellular Ca2+ (nominally 0 mm). A2, mean (±s.e.m.) amplitude of responses to citric acid in normal (+) or reduced (−) external [Ca2+] (*P < 0.05 for control versus Ca2+-free response amplitude, n= 5). Removing extracellular Ca2+ did not inhibit the increase in [Ca2+]i in response to acetylcholine (ACh; data not shown). B, thapsigargin (TG, horizontal bar, 5 μm) caused a sustained increase in [Ca2+]i in taste cells. The inset shows changes in [Ca2+]i in a representative taste cell. TG had no effect on Ca2+ responses to focally applied citric acid (pH 3; P > 0.05 for control versus thapsigargin-treated response amplitude, n= 3). Tracings show Ca2+ responses in a taste cell that was stimulated repeatedly with citric acid (arrowhead) before and after bath-applied TG (5 μm). C, by contrast, TG blocked Ca2+ responses evoked by acetylcholine (horizontal bars), which is known to elicit intracellular Ca2+ release in murine taste cells (Ogura, 2002). Data in C were obtained from the same cell shown in B. ACh was bath applied.
Consistent with this interpretation, we found a strong association between acid-induced Ca2+ responses and Ca2+ responses elicited by KCl-induced depolarization. Of the taste cells that responded to citric acid, 94 % (16/17) also responded to bath-applied KCl (50 mm). This is important because bath-applied KCl does not evoke Ca2+ responses in all taste cells. We found that 55 % of cells responded to KCl, which is similar to the incidence (45 %) reported by Caicedo et al. (2000). In addition, blocking K+ channels with Ba2+ prevented KCl-induced and citric-acid-induced Ca2+ responses alike (Fig. 6A). Finally, treating cells with the voltage-gated Ca2+ channel (VGCC) blocker, Cd2+, eliminated taste cell responses to citric acid (Fig. 6B), suggesting that acid-induced Ca2+ entry occurs via VGCCs.
Figure 6. Ba2+ and Cd2+ block the Ca2+ responses evoked by focally applied citric acid, but Cs+ and amiloride (Amil) have no effect.
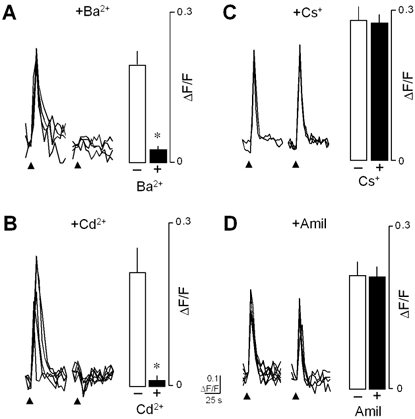
A, superimposed Ca2+ responses in a taste cell stimulated repeatedly with citric acid (pH 3, arrowheads) either before or after 2 mm Ba2+ in the bath. Mean (±s.e.m.) amplitudes of Ca2+ responses to citric acid shown in the bar graphs. (*P < 0.05 for control Ca2+ responses versus responses in the presence of 2 mm Ba2+, n= 7 cells in each column.) B, similarly, superimposed Ca2+ responses evoked by repeated focal stimulation with citric acid (pH 3, arrowhead) before and after 0.5 mm Cd2+ in the bath. Mean (±s.e.m.) amplitudes of responses shown in bar graphs (*P < 0.05 for control responses versus responses in presence of Cd2+, n= 4 cells in each column). C, superimposed Ca2+ responses evoked by repeated focal stimulation with citric acid (pH 3, arrowheads) before and after 1 mm Cs+ in the bath. Mean (±s.e.m.) amplitudes of responses are shown in the bar graphs (P > 0.05 for control responses versus responses in presence of Cs+, n= 3 cells in each column). D, superimposed Ca2+ responses evoked by repeated focal stimulation with citric acid (pH 3, arrowhead) before and after 100 μm amiloride in the bath. Mean (±s.e.m.) amplitudes of responses are shown in the bar graphs (P > 0.05 for control responses versus responses observed in the presence of amiloride, n= 4 cells in each column).
Hyperpolarization-activated cyclic nucleotide-gated cation (HCN) channels have been implicated in sour taste transduction (Stevens et al. 2001). Therefore, we assessed the effect of the HCN channel blocker Cs+ on acid-evoked Ca2+ responses. Treating taste cells with Cs+ (500 μm) had no effect on responses to acid (Fig. 6C), suggesting that HCN channels are not involved.
Acid-sensing ion channels (ASIC), members of the MDEG/degenerin/epithelial Na+ channel (ENaC) family, have also been postulated to transduce sour taste (Ugawa et al. 1998; Lin et al. 2002). Many members of this family of ion channels are blocked by amiloride. However, as illustrated in Fig. 6D, bath-applied amiloride (100 μm) had no effect of the Ca2+ responses to acid stimuli. These data suggest that amiloride-sensitive ENaCs are not responsible for Ca2+ responses to acids in mouse taste cells.
DISCUSSION
The present study was undertaken to investigate mechanisms underlying sour (acid) taste transduction. We measured changes in intracellular pH and Ca2+ simultaneously in murine taste buds exposed to acid taste stimuli. We found that focal application at the taste pore of weak and strong acids to intact taste buds in thick slices of lingual tissue acidified the whole taste bud. However, whereas > 90 % of taste cells were acidified within a particular taste bud in response to a sour stimulus, only a few of these cells exhibited acid-evoked increases in [Ca2+]i. These data suggest that a select subset of taste cells possess the molecular machinery to transduce acid (sour) stimuli.
Ca2+ responses evoked by focally applied citric acid and HCl in taste cells were consistent with there being a measure of sour taste transduction per se, and not merely a general cellular response to acidification. First, only a subset of taste cells manifests Ca2+ responses, despite widespread acidification. Second, Ca2+ responses were concentration dependent and, most importantly, the behavioural sensitivity to citric acid and HCl in mice (Bachmanov et al. 1996) is similar to the concentrations that elicited Ca2+ responses in taste cells in the present study. Third, pharmacological manipulations such as amiloride treatment had similar effects on behavioural assays for sour taste and acid-evoked Ca2+ responses in taste cells; specifically, amiloride does not reduce sour taste (Ossebaard & Smith, 1996; Tennissen & McCutcheon, 1996) nor block the Ca2+ responses evoked by focal stimulation with acids. The sole exception is that acid taste in hamsters, but not other species tested (including humans), is reduced by amiloride (Gilbertson & Gilbertson, 1994.) Collectively, these data support the interpretation that Ca2+ responses reflect taste mechanisms and therefore can be used to investigate taste transduction.
It has been proposed that intracellular acidification of taste cells could act as the primary stimulus for sour taste (Lyall et al. 2001). According to this interpretation, fully protonated (and therefore electrically neutral) acid molecules such as H3-citrate cross the plasma membrane and dissociate intracellularly to acidify the cytoplasm. This challenges the view that protons act extracellularly on the exposed chemosensitive tips of taste receptor cells and that the effective stimulus is [H+] (i.e. pH) in the taste pore. Our results support those of Lyall et al. (2001). Equal proton concentrations in the taste pore (i.e. equal pH) did not evoke equal responses from taste cells. A weak (i.e. undissociated) acid, citric acid, was much a more potent stimulus than a strong (i.e. dissociated) acid, HCl, at the same pH. Furthermore, we found a linear relationship between intracellular acidification and Ca2+ responses. Focally applied citric acid decreased pH throughout the taste bud. When applied at a sufficiently high concentration, HCl did the same, presumably by elevating [H+] sufficiently in the taste pore to drive protons across transcellular and paracellular pathways into the taste bud (DeSimone et al. 1993; Simon et al. 1993; Stewart et al. 1998). Although we could not specifically distinguish interstitial spaces within the taste bud, we presume that triprotic citric acid (i.e. undissociated) molecules penetrate both extra- and intracellular spaces in the taste bud and acidify both compartments. These observations indicate that it is not possible to distinguish the ultimate site of action of protons in transducing sour taste other than to say that this site presumably is on the basolateral membranes, either intra- or extracellular.
In the present study, almost all cells that manifest acid-evoked Ca2+ responses also showed Ca2+ responses when depolarized with KCl. In addition, blocking VGCCs prevented Ca2+ responses in these same cells. Taken together, these data suggest that acid-evoked Ca2+ responses are caused by protons acting at a basolateral site to depolarize taste cells. Subsequently, this depolarization could open VGCCs and lead to Ca2+ influx. This interpretation is supported by observations that acid stimuli elicit inward current and depolarize taste cells in mammals (Sato & Beidler, 1982; Gilbertson et al. 1992; Lin et al. 2002).
Since protons affect virtually every class of ion channel (Hille, 1992), it is difficult to determine exactly what proton-sensitive target(s) are selectively expressed by the subset of acid-responsive taste cells we observed in our experiments. There are several classes of ion channels that have been proposed to mediate sour taste, including ENaCs (Gilbertson et al. 1992; Gilbertson & Gilbertson, 1994), pacemaker channels (HCNs; Stevens et al. 2001), two-pore-domain K+ channels (KCNKs; Lin et al. 2002), and acid-sensitive ion channels (MDEG1, ASIC2b; Ugawa et al. 1998; Liu & Simon, 2001; Lin et al. 2002). Amiloride has been used in an attempt to differentiate among the candidate sour transduction channels. Amiloride inhibits many ENaC and ASIC/MDEG channels, although splice variants and variations in subunit composition of multimeric channels can confer amiloride resistance (Waldmann & Lazdunski, 1998). Topically applied amiloride does not alter the taste of acids in humans, although it does affect the sourness of NaCl solutions (Ossebaard & Smith, 1996). Nor does amiloride alter responses of chorda tympani nerve fibres to acid taste stimuli in rats (Lyall et al. 2002) or primates (Hellekant et al. 1997). The exception to these findings is the observation that amiloride blocks acid taste in hamsters (but not other species tested; Hettinger & Frank, 1990; Gilbertson & Gilbertson, 1994). The effects of amiloride in hamsters have been interpreted as supporting a role for ENaCs in sour taste transduction in certain species, but not as a general mechanism. Our findings show an absence of effect of amiloride in acid-evoked Ca2+ responses in taste cells, consistent with amiloride-insensitive mechanisms. HCN channels also fail to explain acid taste transduction in our experiments. Cs+, a potent blocker of HCN channels, had no effect on acid-evoked Ca2+ responses in taste cells. We are unaware of any behavioural tests of acid taste using mixtures containing Cs+ salts. This leaves KCNK channels as candidate sour transducer channels (Lin et al. 2002). Some members of this family of ion channels are sensitive to extracellular protons, others to intracellular acidification. Since it was not possible to distinguish between intra- and extracellular acidification in the basolateral compartment of taste cells, we have no further information with which to focus the search. Additional experiments are needed to identify which transducer proteins are expressed by acid-sensitive taste cells.
Figure 7. Proposed model for the action of strong (top) and weak (bottom) acid taste stimuli on taste buds.
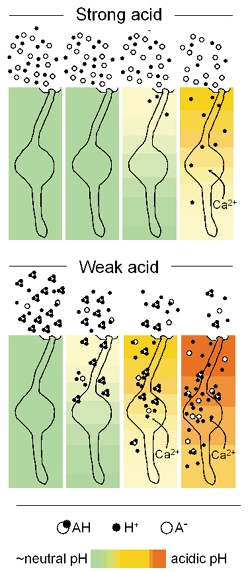
At a sufficiently acid pH, protons from fully dissociated (strong) acid permeate and acidify taste buds. However, undissociated molecules of weak acid (shown here, triprotic acetic acid) more effectively penetrate lingual tissue, release proportionately more protons, and acidify taste buds to a greater degree. In only a subset of taste cells, this acidification elicits a Ca2+ response (not depicted). AH, undissociated (protonated) acid. A−, dissociated
Acknowledgments
This work was supported by NIH grants DC00374 (S.D.R.) and NIDCD DC0425-01 (A.C.).
REFERENCES
- Bachmanov AA, Tordoff MG, Beauchamp GK. Ethanol consumption and taste preferences in C57BL/6ByJ and 129/J mice. Alcohol Clin Exp Res. 1996;20:201–206. doi: 10.1111/j.1530-0277.1996.tb01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caicedo A, Jafri MS, Roper SD. In situ Ca2+ imaging reveals neurotransmitter receptors for glutamate in taste receptor cells. J Neurosci. 2000;20:7978–7985. doi: 10.1523/JNEUROSCI.20-21-07978.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caicedo A, Kim K-K, Roper SD. Individual mouse taste cells respond to multiple chemical stimuli. J Physiol. 2002;544:501–509. doi: 10.1113/jphysiol.2002.027862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caicedo A, Roper SD. Taste receptor cells that discriminate between bitter stimuli. Science. 2001;291:1557–1560. doi: 10.1126/science.291.5508.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSimone JA, Lyall V, Heck GL, Feldman GM. Acid detection by taste receptor cells. Respir Physiol. 2001;129:231–245. doi: 10.1016/s0034-5687(01)00293-6. [DOI] [PubMed] [Google Scholar]
- DeSimone JA, Ye Q, Heck GL. Ion pathways in the taste bud and their significance for transduction. Ciba Found Symp. 1993;179:218–229. doi: 10.1002/9780470514511.ch14. [DOI] [PubMed] [Google Scholar]
- Ganzevles PG, Kroeze JH. Effects of adaptation and cross-adaptation to common ions on sourness intensity. Physiol Behav. 1987;40:641–646. doi: 10.1016/0031-9384(87)90111-9. [DOI] [PubMed] [Google Scholar]
- Gilbertson DM, Gilbertson TA. Amiloride reduces the aversiveness of acids in preference tests. Physiol Behav. 1994;56:649–654. doi: 10.1016/0031-9384(94)90221-6. [DOI] [PubMed] [Google Scholar]
- Gilbertson TA, Avenet P, Kinnamon SC, Roper SD. Proton currents through amiloride-sensitive Na channels in hamster taste cells. Role in acid transduction. J Gen Physiol. 1992;100:803–824. doi: 10.1085/jgp.100.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugland R. Handbook of Fluorescent Probes and Research Chemicals. Eugene, OR, USA: Molecular Probes; 1996. [Google Scholar]
- Hellekant G, Danilova V, Ninomiya Y. Primate sense of taste: behavioral and single chorda tympani and glossopharyngeal nerve fiber recordings in the rhesus monkey, Macaca mulatta. J Neurophysiol. 1997;77:978–993. doi: 10.1152/jn.1997.77.2.978. [DOI] [PubMed] [Google Scholar]
- Hettinger TP, Frank ME. Specificity of amiloride inhibition of hamster taste responses. Brain Res. 1990;513:24–34. doi: 10.1016/0006-8993(90)91085-u. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA, USA: Sinauer; 1992. [Google Scholar]
- Lin W, Ogura T, Kinnamon SC. Acid-activated cation currents in rat vallate taste receptor cells. J Neurophysiol. 2002;88:133–141. doi: 10.1152/jn.2002.88.1.133. [DOI] [PubMed] [Google Scholar]
- Lin W, Rao S, Kinnamon S, Gilbertson T. Evidence for expression of TASK-like K+ channels in rat taste cells. 2002. Association of Chemoreception Sciences Annual Meeting, Sarasota, FL, USA.
- Liu L, Simon SA. Acidic stimuli activate two distinct pathways in taste receptor cells from rat fungiform papillae. Brain Res. 2001;923:58–70. doi: 10.1016/s0006-8993(01)03190-0. [DOI] [PubMed] [Google Scholar]
- Lyall V, Alam RI, Phan DQ, Ereso GL, Phan TH, Malik SA, Montrose MH, Chu S, Heck GL, Feldman GM, De Simone JA. Decrease in rat taste receptor cell intracellular pH is the proximate stimulus in sour taste transduction. Am J Physiol Cell Physiol. 2001;281:C1005–1013. doi: 10.1152/ajpcell.2001.281.3.C1005. [DOI] [PubMed] [Google Scholar]
- Lyall V, Alam RI, Phan TH, Phan DQ, Heck GL, De Simone JA. Excitation and adaptation in the detection of hydrogen ions by taste receptor cells: a role for cAMP and Ca2+ J Neurophysiol. 2002;87:399–408. doi: 10.1152/jn.00331.2001. [DOI] [PubMed] [Google Scholar]
- Makhlouf GM, Blum AL. Kinetics of the taste response to chemical stimulation: a theory of acid taste in man. Gastroenterology. 1972;63:67–75. [PubMed] [Google Scholar]
- Miyamoto T, Fujiyama R, Okada Y, Sato T. Salty and sour transduction. Multiple mechanisms and strain differences. Ann N Y Acad Sci. 1998;855:128–133. doi: 10.1111/j.1749-6632.1998.tb10554.x. [DOI] [PubMed] [Google Scholar]
- Ogiso K, Shimizu Y, Watanabe K, Tonosaki K. Possible involvement of undissociated acid molecules in the acid response of the chorda tympani nerve of the rat. J Neurophysiol. 2000;83:2776–2779. doi: 10.1152/jn.2000.83.5.2776. [DOI] [PubMed] [Google Scholar]
- Ogura T. Acetylcholine increases intracellular Ca2+ in taste cells via activation of muscarinic receptors. J Neurophysiol. 2002;87:2643–2649. doi: 10.1152/jn.2002.87.6.2643. [DOI] [PubMed] [Google Scholar]
- Ossebaard CA, Smith DV. Amiloride suppresses the sourness of NaCl and LiCl. Physiol Behav. 1996;60:1317–1322. doi: 10.1016/s0031-9384(96)00258-2. [DOI] [PubMed] [Google Scholar]
- Richards TW. The relation of the taste of acids to their degree of dissociation. Am Chem J. 1898;20:121–126. [Google Scholar]
- Sato M, Beidler LM. The response characteristics of rat taste cells to four basic taste stimuli. Comp Biochem Physiol. 1982;73:1–10. doi: 10.1016/0300-9629(82)90083-4. [DOI] [PubMed] [Google Scholar]
- Schwiening CJ, Willoughby D. Depolarization-induced pH microdomains and their relationship to calcium transients in isolated snail neurones. J Physiol. 2002;538:371–382. doi: 10.1113/jphysiol.2001.013055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SA, Holland VF, Benos DJ, Zampighi GA. Transcellular and paracellular pathways in lingual epithelia and their influence in taste transduction. Microsc Res Tech. 1993;26:196–208. doi: 10.1002/jemt.1070260303. [DOI] [PubMed] [Google Scholar]
- Stevens DR, Seifert R, Bufe B, Muller F, Kremmer E, Gauss R, Meyerhof W, Kaupp UB, Lindemann B. Hyperpolarization-activated channels HCN1 and HCN4 mediate responses to sour stimuli. Nature. 2001;413:631–635. doi: 10.1038/35098087. [DOI] [PubMed] [Google Scholar]
- Stewart RE, Lyall V, Feldman GM, Heck GL, De Simone JA. Acid-induced responses in hamster chorda tympani and intracellular pH tracking by taste receptor cells. Am J Physiol. 1998;275:C227–238. doi: 10.1152/ajpcell.1998.275.1.C227. [DOI] [PubMed] [Google Scholar]
- Tennissen AM, MCCutcheon NB. Anterior tongue stimulation with amiloride suppresses NaCl saltiness, but not citric acid sourness in humans. Chem Senses. 1996;21:113–120. doi: 10.1093/chemse/21.2.113. [DOI] [PubMed] [Google Scholar]
- Ugawa S, Minami Y, Guo W, Saishin Y, Takatsuji K, Yamamoto T, Tohyama M, Shimada S. Receptor that leaves a sour taste in the mouth. Nature. 1998;395:555–556. doi: 10.1038/26882. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Lazdunski M. H+-gated cation channels: neuronal acid sensors in the NaC/DEG family of ion channels. Curr Opin Neurobiol. 1998;8:418–424. doi: 10.1016/s0959-4388(98)80070-6. [DOI] [PubMed] [Google Scholar]


