Abstract
The electrical and synaptic properties of myenteric neurones in normal and inflamed guinea-pig distal colons were evaluated by intracellular microelectrode recording. Chronic inflammation was established 6 days following administration of trinitrobenzene sulfonic acid (TNBS). In S neurones, inflammation only altered synaptic inputs as the amplitude of fast excitatory postsynaptic potentials were significantly larger (31 ± 2 mV compared to 20 ± 1 mV) and they were more likely to receive slow excitatory synaptic input (85% compared to 55%). AH neurones displayed altered electrical properties in colitis compared to control tissues: they generated more action potentials during a maximal depolarising current pulse (7 ± 1 compared to 1.6 ± 0.2); they had a smaller afterhyperpolarisation (9 ± 2 mV s compared to 20 ± 2 mV s); and they were more likely to receive fast excitatory synaptic input (74% compared to 17%), possess spontaneous activity (46% compared to 3%), and generate anodal break action potentials (58% compared to 19%). Although the resting membrane potential, input resistance and action potential characteristics were unaltered in AH neurones from inflamed tissues, they exhibited an enhanced Cs+-sensitive rectification of the current–voltage relationship. This suggests that the increase in excitability of AH neurones may involve a colitis-induced augmentation of the hyperpolarisation-activated cation current (Ih) in these cells. An increased excitability, selectively in AH neurones, suggests that the afferent limb of intrinsic motor reflexes is disrupted in the inflamed colon and this may contribute to dysmotility associated with inflammatory diseases.
Significant progress has been made in resolving the subpopulations of enteric neurones that carry out various functions in the guinea-pig intestines. On the basis of their neurochemical expression and projections, subtypes of neurones characterised electrophysiologically as ‘S’ neurones have been characterised as anally and orally projecting interneurones, as well as excitatory and inhibitory motor neurones (Brookes, 2001). In the distal colon, it is likely that some S neurones are stretch-sensitive and therefore may serve as primary afferent neurones as well (Spencer et al. 2002, 2003). Neurones characterised electrophysiologically as ‘AH’ neurones have been described as multifunctional elements at the afferent limb of intrinsic reflex circuitry (Costa et al. 1986; Gershon et al. 1994; Furness et al. 1995; Kunze et al. 1995; Lomax et al. 1999). The AH neurones of the myenteric plexus have been identified as intrinsic primary afferents (IPANs) in the ileum, as they respond to mucosal stimulation (Bertrand et al. 1997; Furness et al. 1998; Kunze & Furness, 1999) and stretch (Kunze et al. 1998, 2000). AH neurones also serve as interneurones because they form interconnected, self-reinforcing networks that act to synchronise motor events in the bowel (Wood, 1994b; Kunze & Furness, 1999; Thomas et al. 2000). Because AH neurones of the colon have almost identical electrical, morphological, chemical coding and axonal projection characteristics to those in the ileum (Wade & Wood, 1988a,b; Lomax et al. 1999, 2001; Wada-Takahashi & Tamura, 2000; Tamura et al. 2001), it has been proposed that distal colonic AH neurones serve as IPANs as well.
Changes in enteric nervous system (ENS) structure and function have been observed in several models of intestinal inflammation (for review see Castro, 1992; Wood, 1992; Sharkey & Parr, 1996). Alterations in the electrical properties of enteric neurones have been observed in animal models of inflammation that involve active parasitic infections (Palmer et al. 1998) or allergen-induced responses (Frieling et al. 1994). However, because inflammatory bowel disease (IBD) involves a cell-mediated immune response (Kim & Berstad, 1992; Fedorak, 1995), which is different from mechanisms that mediate inflammation in those models, it is important to identify changes in the electrical and synaptic properties of enteric neurones in a model that more closely resembles human IBD.
The trinitrobenzene sulfonic acid (TNBS) model of inflammation has been widely used in rats and mice, and the extent of the inflammation is very reproducible. It has acute and chronic phases and is immunologically and histopathologically comparable to IBD, with features similar to Crohn's disease (Kim & Berstad, 1992; Fedorak, 1995). In addition, there are motility changes similar to those seen in IBD (Morteau et al. 1993). The present study was undertaken to elucidate what changes occur in the electrical and synaptic properties of myenteric neurones of the guinea-pig distal colon during TNBS-induced inflammation.
METHODS
Animal preparations
All methods used in this study were approved by the University of Vermont Animal Care and Use Committee. Adult guinea-pigs (Charles River, Montreal, Canada) of either sex, weighing 250–350 g, were housed in metal cages with soft bedding. The animals had access to food and water ad libitum and were maintained at 23–24 °C on a 12:12 h light-dark cycle.
In order to generate inflammation in the distal colon, guinea-pigs were anaesthetised with isoflurane (induced at 4%, maintained on 1.5% in oxygen) and 0.3 ml of trinitrobenzene sulfonic acid (TNBS; 25 mg ml−1) in 30% ethanol was delivered into the lumen of the colon through a polyethylene catheter inserted rectally 7 cm proximal to the anus. Control animals remained naïve until tissue collection or they received 0.3 ml of intracolonic saline (0.9% NaCl) under anaesthesia. Animals were maintained in a controlled environment for 6 days after TNBS or saline administration. At the time of tissue collection, animals were deeply anaesthetised with isoflurane and exsanguinated. The severity of colitis was assessed by weight change and macroscopic colonic damage scoring. Six days following saline or TNBS administration, animals in the two groups gained 39 ± 2 g and 27 ± 3 g, respectively (P < 0.01t test). The criteria for scoring of gross morphological damage have been described previously (McCafferty et al. 1997). A single administration of TNBS/ethanol in the guinea-pig distal colon caused regional inflammation that was characterised by ulceration, hyperaemia, adhesions and oedema that were similar to previous reports in rat (Morris et al. 1989) and mouse (Neurath et al. 1995). Macroscopic damage scores revealed that 6 days following administration, the colons of TNBS-treated animals remained significantly damaged (mean score of 4.9 ± 0.3, n= 37) compared to controls (mean score of 0.82 ± 0.02, n= 53; P < 0.001, t test). The characteristics of inflammation 6 days post-TNBS were consistent with a chronic inflammatory state.
Tissue preparation
The distal colon, identified as the part of the colon between the hypogastric flexure and the pelvic brim, was removed and placed in iced Krebs solution (mm: NaCl, 121; KCl, 5.9; CaCl2, 2.5; MgCl2, 1.2; NaHCO3, 25; NaH2PO4, 1.2; and glucose, 8; aerated with a 95%O2/5%CO2; all from Sigma, St Louis, MO, USA). Nifedipine (5 μm) and atropine (200 nm)(Sigma) were added to eliminate smooth muscle contraction. The mucosa, submucosa and circular muscle of the colon were removed with forceps exposing the myenteric plexus on the longitudinal smooth muscle. The preparation was then moved to a 2.5 ml recording chamber.
Electrophysiological recordings
Preparations were continuously perfused at 7 ml min−1 with Krebs solution containing nifedipine and atropine maintained at 37°C. Glass microelectrodes used for recording were filled to the shoulder with 1% (w/v) neurobiotin (Vector Laboratories, Burlingame, CA, USA) in 1.0 m KCl, and the remainder filled with 2.0 m KCl and had resistances in the range of 50–150 MΩ. Myenteric ganglia were visualised at × 200 with Hoffman modulation contrast optics through an inverted microscope (Nikon Diaphot, Melville, NY, USA) and individual myenteric neurones were randomly impaled. Transmembrane potential was measured with an Axoclamp-2A amplifier (Axon Instruments, Foster City, CA, USA) and electrical signals were acquired and analysed using MacLab Chart or Scope software (AD Instruments, Castle Hills, Australia). Synaptic activation of neurones was elicited by direct stimuli (a single pulse of 0.5 ms or a train of 0.5 ms pulses at 20 Hz for 2–4 s) applied to fibre tracts in interganglionic connectives with monopolar extracellular electrodes made from Teflon-insulated platinum wire. For some experiments, tetrodotoxin (TTX) or hexamethonium (Sigma, St Louis, MO, USA) were applied to preparations by addition to the circulating Krebs solution. Unhealthy cells were excluded from the study if the input resistance was below 50 MΩ or had an action potential that peaked at a level less than 0 mV. A similar proportion of neurones met exclusion criteria for each animal treatment group (control: 20%, 41/208 impalements; TNBS: 17%, 22/128 impalements; P > 0.05 Fisher's exact test).
Using criteria described previously for classifying neurones in the guinea-pig small intestine (Bornstein et al. 1994; Wood, 1994a), neurones in this study were classified as AH, S or nonspiking. The most important criteria for classifying an individual neurone as AH or S was the presence or absence, respectively, of a shoulder on the repolarising phase of the action potential (Schutte et al. 1995). Morphological characterisation, which was successful in 61% of recorded neurones, supported the electrophysiological classification. AH neurones always had a Dogiel type II morphology (multiaxonal) and S neurones always had a Dogiel type I or filamentous morphology (monoaxonal). While nonspiking neurones never generated action potentials in response to depolarising current pulses (0.5 s, 0.1–1 nA), they did receive subthreshold synaptic input and were included in the study only if the morphological classification as a neurone could be established. Therefore, this group may reflect an underestimate of the population of nonspiking neurones. The nomenclature of this classification scheme differs from previous studies in the guinea-pig distal colon (Wade & Wood, 1988a,b; Lomax et al. 1999; Wada-Takahashi & Tamura, 2000; Tamura et al. 2001). AH neurones in this study correspond to AH/type 2 neurones in previous studies and S neurones correspond to the combination of type 1, type 4 and type 5 neurones. Nonspiking neurones correspond to type 3 neurones previously described.
Because no differences were observed between neurones recorded from 40 naïve (26 AH neurones, 93 S neurones and 7 nonspiking neurones) and 13 saline-treated control animals (10 AH neurones, 26 S neurones and 5 nonspiking neurones), results from these neurones were grouped, and are hereafter referred to as control neurones.
Neuronal morphology and chemical coding
Tissues used in electrophysiological experiments were fixed overnight at 4 ° C in 0.1 m phosphate buffered saline (PBS; 0.1 m pH 7.4) containing 4% paraformaldehyde, and 0.2% picric acid. The tissue was washed with PBS and incubated for 2 h at room temperature with PBS containing 0.5% Triton X-100 and 4% normal goat serum. This solution was removed, and the sections were incubated overnight at room temperature with a 1:10000 dilution of rabbit anti-calbindin antiserum (SWANT, Bellinzona, Switzerland) in PBS containing 4% normal goat serum and 0.5% Triton X-100. Following three 15 min washes with PBS, tissue sections were incubated with a 1:400 dilution of goat anti-rabbit antiserum (Jackson ImmunoResearch, West Grove, PA, USA) and a 1:500 dilution of AMCA-conjugated strepavidin (Jackson ImmunoResearch) in PBS containing 0.5% Triton X-100 for 2 h. Following three 15 min washes with PBS, the tissue was mounted on glass slides and viewed on an Olympus AX70 fluorescence photomicroscope.
Data analyses
All electrophysiological properties were analysed offline on MacLab Chart or Scope software. Input resistances were determined by averaging the voltage deflection from three hyperpolarising current pulses (0.1–0.2 nA; 500 ms). The maximum number of action potentials per 500 ms depolarising current pulse was determined using pulses with a stimulus no higher than 1 nA. In S neurones, the maximum amplitude of the fast excitatory postsynaptic potential (fEPSP) in response to a supramaximal stimulus was determined by averaging the difference between the membrane potential (held to: control: −92 ± 2 mV, n= 37 and inflamed: −94 ± 2 mV, n= 27, by injecting hyperpolarising current) and the peak of the fEPSP of at least three evoked potentials elicited by focal stimulation of fibre tracts with single pulses (0.5 ms, 1–10 V). Afterhyperpolarisation (AHP) magnitude in AH neurones was determined by integrating the voltage more negative than resting membrane potential (RMP) over time until the membrane potential returned to resting levels (data points at 1 ms intervals; Prism v. 3.0a for Macintosh, GraphPad Software, La Jolla, CA, USA). The measured AHP was generated following a single action potential that peaked only after the offset of a single depolarising current pulse (0.4–0.7 nA; 0.2–5 ms). Antidromically stimulated action potentials were analysed to compare action potential characteristics in control and inflamed AH neurones. The maximum rates of depolarisation and repolarisation were determined from the derivative of the time-voltage trace. The duration of the action potentials were measured at a voltage that corresponded to half-repolarisation. Action potential amplitude was measured as the voltage difference between resting membrane potential and the peak of the action potential. The magnitudes of action potentials were determined by integrating the voltage greater than resting membrane potential over the time of the entire action potential (data points at 0.025 ms intervals; Prism). Differences between treatment groups were determined using a t test for continuous data and a Fisher's exact test or chi-square test for proportional data with a significance level of P < 0.05. All statistical analyses were conducted using Prism.
RESULTS
Electrical and synaptic properties of myenteric neurones in inflamed distal colon
Intracellular recordings were obtained from 167 neurones in 53 control colons and 106 neurones in 37 inflamed colons. Neurones that were studied were classified as AH neurones, S neurones or nonspiking neurones, as described in the Methods section. The electrophysiological properties of neurones from control animals were similar to those found in previous studies (Wade & Wood, 1988a,b; Lomax et al. 1999; Wada-Takahashi & Tamura, 2000; Tamura et al. 2001). The proportion of myenteric neurones from inflamed preparations classified into each of the three groups was unchanged compared to control populations (AH neurones: control, 36 neurones (22%); inflamed, 24 neurones (23%); S neurones: control, 119 neurones (71%); inflamed 77 neurones (73%;) and nonspiking neurones: control, 12 neurones (7%); inflamed 5 neurones (4%); P > 0.05, chi-square).
Nonspiking neurones
The electrophysiological properties of nonspiking neurones from inflamed animals were not different from those of controls. These neurones had similar resting membrane potentials (control: −49 ± 5 mV, n= 12; inflamed: −47 ± 5 mV, n= 5; P > 0.05 t test) and input resistances (control: 109 ± 15 MΩ, n= 12; inflamed: 134 ± 37 MΩ, n= 5; P > 0.05t test). Nonspiking neurones from control and inflamed preparations exhibited no spontaneous activity or anodal break action potentials. Focal stimulation of fibre tracts with single pulses (0.5 ms, 1–10 V) evoked fEPSPs in the majority of nonspiking neurones from both groups (control: 10/12 (83%); inflamed: 4/5 (80%); P > 0.05 Fisher's exact test). In response to 2–4 s trains of high-frequency fibre tract stimulation (20 Hz, 0.5 ms per pulse, 3–8 V), slow excitatory postsynaptic potentials (sEPSPs) (control, 7/12 (58%); inflamed, 3/4 (75%); P > 0.05 Fisher's exact test) as well as slow inhibitory postsynaptic potentials (sIPSPs)(control: 2/12 (17%); inflamed: 1/4 (25%); P > 0.05 Fisher's exact test) could be evoked in similar proportions of neurones from both groups.
S neurones
Almost all of the electrical and synaptic properties of S neurones remained unchanged in the inflamed colon. Properties that were unchanged when comparing control S neurones to those from inflamed tissues included resting membrane potential, input resistance, maximum number of action potentials during a 500 ms current pulse, or proportion of cells exhibiting spontaneous activity, anodal break action potentials, fEPSPs or sIPSPs (Table 1). A difference that was detected in the inflamed tissue was an increase in the proportion of S neurones receiving sEPSPs (Table 1). Another difference in S neurones of inflamed vs. control tissue was the maximum amplitude of the fEPSP. When neurones were held at approximately −90 mV to prevent activation of action potentials, the maximum amplitude of the fast EPSP in S neurones from inflamed tissue was significantly greater than that of S neurones in control tissue (Fig. 1).
Table 1.
Comparison of electrical and synaptic properties of S neurones from control and inflamed distal colon
| Control | Inflamed | |
|---|---|---|
| Resting membrane potential (mV) | −52 ± 1 (119) | −55 ± 1 (77) |
| Input resistance (MΩ) | 145 ± 11 (119) | 137 ± 12 (77) |
| Maximum number of action potentials during a 500 ms current pulse | 5 ± 1 (119) | 7 ± 1 (77) |
| Neurones exhibiting spontaneous activity | 35/119 (29%) | 30/77 (39%) |
| Neurones exhibiting anodal break action potentials | 32/116 (28%) | 30/72 (42%) |
| Neurones exhibiting fEPSPs | 115/118 (97%) | 75/77 (97%) |
| Neurones exhibiting sEPSPs | 54/98 (55%) | 56/66 (85%)* |
| Neurones exhibiting sIPSPs | 9/98 (9%) | 10/66 (15%) |
There were no differences in most electrical or synaptic properties in S neurones between control and inflamed tissue. There was an increased proportion of S neurones that received slow excitatory synaptic input. Data are means ±s.e.m. for (n) cells, or the proportion of cells exhibiting the given property. Continuous data were statistically analysed with a t test (all P > 0.05). Proportional data were statistically analysed with Fisher's exact test
P < 0.01 compared to control).
Figure 1. Amplitude of fast excitatory synaptic potentials in S neurones from control or inflamed distal colon.
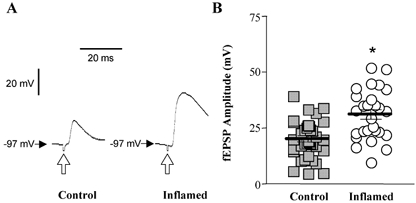
A, respresentative traces of fEPSPs elicited by fibre tract stimulation (0.5 ms 5.4 V; open arrows) in S neurones from control and inflamed tissue. Both traces are on the same voltage, current and time scale. B, scatter plot illustrating the maximum amplitude of fEPSPs in S neurones from TNBS-treated (open circles) or control colons (grey squares). Data points are displaced horizontally so that overlapping data points can be visualised. The lines demonstrate the mean ±s.e.m. for each treatment group (*P < 0.001 compared to control, t test; n= 37 for controls and 27 for inflamed).
It is possible that changes in the electrical properties of S neurones were not detected in inflamed tissue because S neurones represent a diverse population of neurones, and differences within a particular subset of S neurones may not be identified when S neurones are compared as a whole. Therefore, S neurones that were successfully filled with neurobiotin were classified into two subpopulations, those that projected orally (control, n= 31; inflamed, n= 23) and those that projected aborally (control, n= 29; inflamed, n= 23), and data from these subsets were analysed (Table 2). There were no differences in all electrical and synaptic properties between orally and aborally projecting S neurones from control tissues. Within the population of orally projecting S neurones, depolarising current pulses evoked more action potentials in inflamed tissue compared to controls. In addition, these neurones were more likely to exhibit spontaneous activity (including both spontaneous action potentials and fEPSPs) and anodal break action potentials. Within both subsets of S neurones, the amplitude of evoked fEPSPs from inflamed preparations was significantly higher than that of controls.
Table 2.
Comparison of electrical and synaptic properties of subpopulations of S neurones from control and inflamed distal colon
| Orally projecting S neurones | Aborally projecting S neurones | |||
|---|---|---|---|---|
| Control | Inflamed | Control | Inflamed | |
| Resting membrane potential (mV) | −51 ± 2 (31) | −54 ± 2 (23) | −52 ± 2 (29) | −54 ± 2 (23) |
| Input resistance (MΩ) | 139 ± 18 (31) | 163 ± 31 (23) | 128 ± 18 (29) | 113 ± 16 (23) |
| Maximum number of action potentials during a 500 ms current pulse | 5 ± 1 (31) | 12 ± 4 (23)* | 7 ± 2 (29) | 7 ± 2 (23) |
| Neurones exhibiting spontaneous activity | 7/31 (23%) | 13/23 (57%)* | 10/29 (34%) | 7/23 (30%) |
| Neurones exhibiting anodal break action potentials | 6/30 (20%) | 13/22 (59%)* | 9/29 (31%) | 8/21 (38%) |
| Neurones exhibiting fEPSPs | 29/30 (97%) | 23/23 (100%) | 28/29 (97%) | 21/23 (91%) |
| fEPSP amplitude (mV) | 22 ± 2 (13) | 30 ± 4 (9)* | 17 ± 3 (9) | 31 ± 4 (6)* |
| Neurones exhibiting sEPSPs | 14/26 (54%) | 16/21 (76%) | 15/23 (65%) | 18/21 (86%) |
| Neurones exhibiting sIPSPs | 4/26 (15%) | 4/21 (19%) | 4/23 (17%) | 4/21 (19%) |
There were no differences in electrical or synaptic properties between orally and aborally projecting S neurones in control tissue. In the population of orally projected S neurones, depolarising current pulses evoked more action potentials in inflamed tissue compared to controls. In addition, these neurones were more likely to exhibit spontaneous activity and anodal break action potentials. Within both subsets of S neurones, the amplitude of evoked fEPSPs from inflamed preparations was significantly higher than that of controls. Data are means ±s.e.m. for (n) cells, or the proportion of cells exhibiting the given property. Continuous data were statistically analysed with a t test and proportional data were statistically analysed with Fisher's exact test
P < 0.05).
AH neurones
Certain electrical and synaptic properties of AH neurones from inflamed colons, including resting membrane potential, input resistance, and the proportions of neurones that received sEPSPs and sIPSPs, were unaltered from control colons (Table 3). In addition, the proportion of AH neurones that were immunoreactive for calbindin in the inflamed colon remained unchanged as compared to controls (control, 19/24 (79%); inflamed, 15/19 (79%); P > 0.05, Fisher's exact test).
Table 3.
Comparison of electrical and synaptic properties of AH neurones from control and inflamed distal colon
| Control | Inflamed | |
|---|---|---|
| Resting membrane potential (mV) | −69 ± 2 (36) | −66 ± 2 (28) |
| Input resistance (MΩ) | 116 ± 11 (36) | 107 ± 9 (28) |
| Neurones exhibiting sEPSPs | 34/34 (100%) | 25/25 (100%) |
| Neurones exhibiting sIPSPs | 0/34 (0%) | 3/25 (12%) |
There were no differences in several electrical or synaptic properties in AH neurones between control and inflamed tissue. Data are means ±s.e.m. for (n) cells, or the proportion of cells exhibiting the given property. Continuous data were statistically analysed with a t test and proportional data were statistically analysed with Fisher's exact test (all P > 0.05).
In contrast to nonspiking and S neurones, many of the electrical and synaptic properties of AH neurones were significantly altered in the inflamed colon. AH neurones from inflamed colons exhibited slower accommodation than AH neurones from the normal colon (Fig. 2). The mean maximum number of action potentials per 500 ms current pulse was significantly greater in AH neurones from inflamed tissue compared to control. The median number of action potentials was also increased in the inflamed colon (6) as compared to control (1). AH neurones from inflamed colons were more likely to exhibit spontaneous action potentials (Fig. 3), and were more likely to exhibit anodal break action potentials (Fig. 4) compared to controls. The mean magnitude of the AHP was reduced in neurones from inflamed colons compared to controls (Fig. 5). Another difference that was detected in AH neurones from inflamed tissue, as compared to control tissue, was an increase in the proportion of neurones that received fEPSPs (Fig. 6). To determine if these fEPSPs were cholinergic, the nicotinic acetylcholine receptor antagonist, hexamethonium was applied to the bathing media during recordings from five AH neurones from inflamed preparations. In each of the five cells tested, hexamethonium (100 μM) completely abolished the fEPSP (Fig. 6).
Figure 2. Accomodation in AH neurones from control or inflamed distal colon.
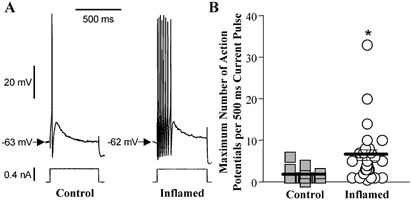
A, representative traces of a response to a 500 ms depolarising current pulse in AH neurones from control and inflamed tissue. Both traces are on the same voltage, current and time scale. B, scatter plot illustrating the maximum number of action potentials in response to a 500 ms depolarising current pulse in AH neurones from TNBS-treated (open circles) or control colons (grey squares). Data points are displaced horizontally so that overlapping data points can be visualised. The lines demonstrate the mean ±s.e.m. for each treatment group (*P < 0.001 compared to control, t test; n= 36 for controls and 28 for inflamed).
Figure 3. Spontaneous activity in AH neurones from control or inflamed distal colon.
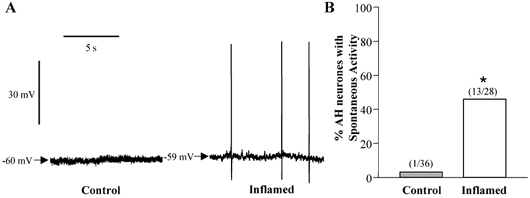
A, representative traces of baseline recordings in AH neurones from control and inflamed tissue. Both traces are on the same voltage and time scale. B, bar graph illustrating the proportion of AH neurones from TNBS-treated (open bar) or control colons (grey bar) that demonstrated spontaneous activity (*P < 0.001 compared to control, Fisher's exact test).
Figure 4. Anodal break action potentials in AH neurones from control or inflamed distal colon.
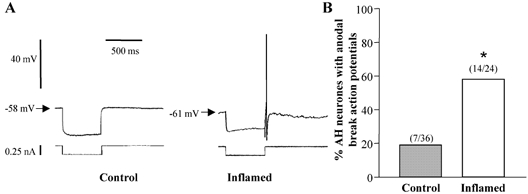
A, representative traces of responses at the offset of a 500 ms hyperpolarising current pulse in AH neurones from control and inflamed tissue. Both traces are on the same voltage, current and time scale. B, bar graph illustrating the proportion of AH neurones from TNBS-treated (open bar) or control colons (grey bar) that demonstrated anodal break action potentials following a hyperpolarising current pulse (*P < 0.001 compared to control, Fisher's exact test).
Figure 5. Slow afterhyperpolarisations in AH neurones from control or inflamed distal colon.
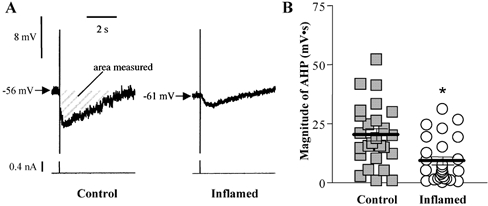
A, representative traces of AHP following single action potentials from control and inflamed tissue. Traces for both cells are on the same voltage, current and time scales. The magnitude of the AHP, determined by integrating the voltage less than resting membrane potential over the time of the entire AHP, for these representative neurones were: control, 25 mV s; and inflamed, 5 mV s. B, scatter plot illustrating the magnitude of the AHP in AH neurones from TNBS-treated (open circles) or control colons (grey squares). Data points are displaced horizontally so that overlapping data points can be visualised. The lines demonstrate the mean ±s.e.m. for each treatment group (*P < 0.001 compared to control, t test; n= 33 for controls and 26 for inflamed).
Figure 6. Fast EPSPs in AH neurones from control or inflamed distal colon.
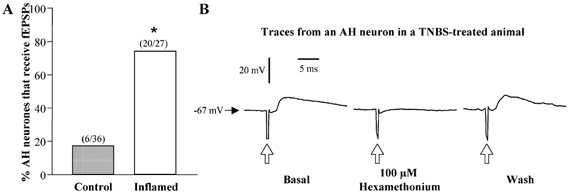
A, bar graph illustrating the proportion of AH neurones from TNBS-treated (open bar) or control colons (grey bar) that received fast excitatory synaptic input in response to focal stimulation of fibre tracts with single pulses (0.5 ms, 1–10 V) (*P < 0.001 compared to control, Fisher's exact test). B, respresentative trace of one of the five AH neurones from inflamed preparations whose fEPSPs elicited by fibre tract stimulation (0.5 ms 3.6 V; open arrows) were blocked by the addition of 100 μm hexamethonium to the bathing solution.
Potential mechanisms contributing to elevated excitability of AH neurones in colitis
A prominent feature of AH neurones, that is important in regulating the excitability of these cells, is the AHP. Three ionic conductances contribute to the magnitude of the AHP in AH neurones: a Ca2+-activated K+ conductance, a voltage-gated Ca2+ conductance and a hyperpolarisation-activated non-selective cation conductance (Galligan et al. 1990; Kunze et al. 1994; Rugiero et al. 2002; Vogalis et al. 2002). Because the AHP was dramatically reduced in AH neurones from inflamed preparations, we sought to examine whether the characteristics attributable to these ionic conductances were altered in inflammation. The most thoroughly examined of these is the Ca2+-activated K+ conductance that generates the prolonged AHP and maintains the resting membrane potential at a more hyperpolarised level. Increased excitability of AH neurones during electrically evoked sEPSPs or agonist-induced depolarisations is associated with a depolarisation and an increase in input resistance, which probably involves a decrease in the Ca2+-activated K+ conductance (Wood, 1994b). No changes in resting membrane potential or input resistance were detected in AH neurones from the inflamed colon, suggesting that the Ca2+-activated K+ conductance was unaltered in colitis. Therefore, we concentrated on the characteristics of these neurones that are associated with the voltage-activated Ca2+ conductance and the hyperpolarisation-activated non-selective cation current (Ih).
Voltage-gated Ca2+ conductance
Depolarising currents of the action potential of AH neurones consist of a TTX-sensitive Na+ current and a voltage-activated Ca2+ current. The Ca2+ component of the action potential initiates the increase in intracellular Ca2+ that activates the K+ conductance responsible for the AHP (Hillsley et al. 2000). In AH neurones, the prominent Ca2+ component of the action potential results in a characteristic shoulder on the repolarising phase and an overall broadening of the spike in these cells. Therefore, we measured various parameters of the action potential with the idea that a reduction in the Ca2+ current would be reflected by changes in the shape and magnitude of the action potential. The characteristics that were evaluated in antidromically elicited action potentials included the maximum rates of depolarisation and repolarisation, the duration of the action potentials at half-repolarisation, the action potential amplitude and integrated magnitude (Fig. 7). There were no differences in any of the measurements between inflamed and control AH neurones, suggesting that conductances active during the action potential, including voltage-gated Na+, Ca2+ and K+ currents are unaltered in colitis.
Figure 7. Action potentials in AH neurones from control or inflamed distal colon.
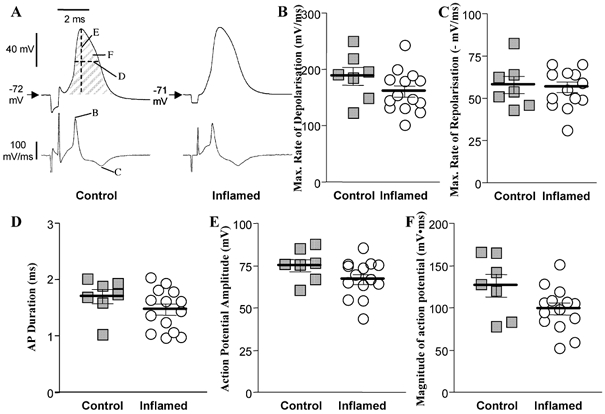
A, representative traces of antidromically elicited action potentials in AH neurones from control and inflamed tissue. Both traces are on the same voltage, current and time scale. Letters on the control voltage and derivitive traces indicate parameters that were measured and correspond to graphs shown in B−F. The magnitude of the action potentials for these representative neurones, determined by integrating the voltage greater than resting membrane potential over the time of the action potential, were: control, 129 mV ms; inflamed, 119 mV ms. B−F, scatter plots illustrating the maximum rate of depolarisation (B), maximum rate of repolarisation (C), duration at half-repolarisation (D), amplitude (E) and magnitude (F) of the action potentials in AH neurones from TNBS-treated (open circles) or control colons (grey squares). Data points are displaced horizontally so that overlapping data points can be visualised. The lines demonstrate the means ±s.e.m. for each treatment group (for all plots P > 0.05 compared to control, t test; n= 7 for controls and 14 for inflamed).
Hyperpolarisation-activated cation current (Ih)
Another property of AH neurones that can influence neuronal excitability, is a hyperpolarisation-activated cation current (Ih)(Galligan et al. 1990; Rugiero et al. 2002). Ih is active during the AHP of AH neurones, and its action opposes the hyperpolarising effect of the Ca2+-activated K+ conductance. Consistent with this, the amplitude of the AHP is increased in the presence of Cs+, which inhibits Ih (Galligan et al. 1990). Ih is detected as a characteristic sag in the hyperpolarising electrotonic potential when an anionic rectangular current pulse is applied via the intracellular recording electrode (Galligan et al. 1990).
A voltage-dependent sag was detected in the electrotonic potential during a hyperpolarising current pulse in AH neurones from both control and inflamed tissues, but was more pronounced in neurones from inflamed tissue (Fig. 8A). To determine whether Ih is altered in AH neurones of the inflamed colon, we tested whether the Cs+-sensitive component of the electrotonic potential was altered in colitis. Neurones were held at −50 mV and hyperpolarising and depolarising current pulses were injected to determine the current-voltage relationship in the absence and presence of Cs+. In these experiments, average voltage measurements were made during the first 25 ms (instantaneous voltage) and during the last 100 ms (steady state voltage) of a prolonged (900 ms) current pulse. Plots of the instantaneous and steady state voltage as a function of injected current were constructed for each cell, in the absence and presence of Cs+ (Fig. 8B). In the absence of Cs+, rectification was detected at voltages more negative than −70 mV in AH neurones from both control and inflamed tissues. The difference between the Cs+ and no Cs+ curves (in mV), was measured for control and inflamed cells, and these values were plotted as a function of voltage in the presence of Cs+. These relationships for the instantaneous and steady state voltages are plotted in Fig. 8C and D, respectively. There were no differences detected in the Cs+-sensitive instantaneous voltage. In the steady state voltage measurements, there was a significant augmentation of the Cs+-sensitive voltage at potentials more negative than −90 mV. In the presence of Cs+, the occurrence of anodal break action potentials was reduced, and the AHP was enhanced, in neurones from inflamed tissue (Fig. 8E and F).
Figure 8. Contribution of a Cs+-sensitive hyperpolarisation-activated conductance to excitability in AH neurones from inflamed distal colon.
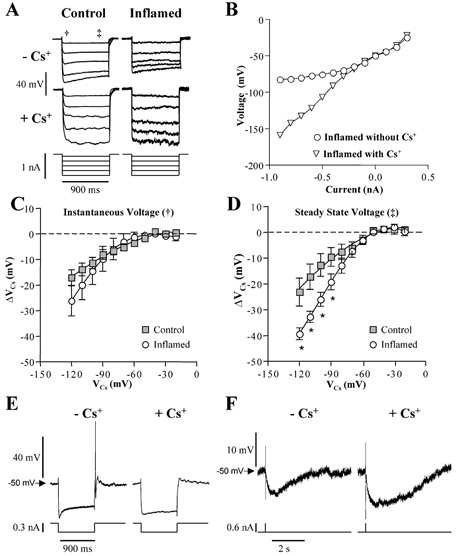
A, representative hyperpolarising electrotonic potentials from AH neurones from control and inflamed preparations in the absence and presence of 2 mm CsCl. Note that the depolarising sag in the electrotonic potential is more pronounced in traces from the neurone in inflamed tissue than control tissue, and that the amplitudes of electrotonic potentials were larger in the presence of CsCl. B, a representative current–voltage plot of the steady state electrotonic potentials elicited by intracellular injection of current at a holding potential of −50 mV. C and D, using plots like that shown in B, changes in voltage provoked by Cs+ (ΔVCs) were measured as the difference between Cs+ and no Cs+ lines and plotted as a function of cell voltages from the Cs+ graph (VCs). There were no differences detected in the Cs+-sensitive instantaneous voltage (measured at the time point indicated by † in A; P > 0.05 two-way ANOVA for repeated measures). In the steady state voltage measurements (measured at the time point indicated by ‡ in A), there was a significant augmentation of the Cs+-sensitive voltage at potentials more negative than −90 mV (*P < 0.05, two-way ANOVA for repeated measures, Bonferroni's multiple comparisons test). In inflamed tissue, application of CsCl to AH neurones reduced the occurence of anodal break action potentials (E), and increased the magnitude of the afterhyperpolarisation (F). E and F are representative examples from the same cell, held at −50 mV.
DISCUSSION
The aim of this study was to characterise the electrical and synaptic properties of myenteric neurones in the distal colon of guinea-pigs during TNBS-induced inflammation, and to identify potential mechanisms for differences relative to normal tissue. Results of this study indicate that dramatic changes occur in the electrical and synaptic properties of myenteric neurones of the inflamed colon, and that these changes are mainly found in one subpopulation of cells, AH neurones, which serve at the afferent end of the motor reflex circuitry in the intestines. Furthermore, data presented here suggest that the enhancement of AH neuronal excitability in inflamed tissue involves an increase in the hyperpolarisation-activated current in these cells. These changes in AH neuronal activity are likely to contribute to the dysmotility that is associated with inflammatory bowel disease (IBD).
In the present study, the recordings of individual neurones were randomly obtained, so the proportion of neurones can be used to estimate the relative abundance of each cell type. Although there were no changes in the proportions of cell types, this does not preclude the possibility that there was neuronal cell loss during inflammation. In a previous study, Sanovic et al. (1999) found that a similar hapten-induced model of inflammation in rats caused a persistent loss of neurones. Although the present results do not rule out neuronal loss during TNBS-induced colitis in guinea-pigs, it indicates that any such loss is unlikely to be limited to an electrophysiologically defined subset of cells.
Inflammation was associated with an increased proportion of AH neurones that receive fast excitatory synaptic transmission. Because Dogiel type II neurones express nicotinic acetylcholine receptors under normal conditions (Kirchgessner & Liu, 1998; Galligan et al. 2000) it is likely that this effect is due to an increased efficiency of synaptic communication. AH neurones in the guinea-pig distal colon are cholinergic (Lomax & Furness, 2000) and probably form networks with other AH neurones (Wood, 1994b); therefore, it is possible that this is a result of increased excitability in AH neurones. Support for this concept is the observation that the fEPSPs in S neurones, which arise from AH neurones, had larger amplitudes in inflamed tissue. Although this could be due to postsynaptic changes, it could also be due to increased release of acetylcholine. Likewise, the increased proportion of S neurones that receive sEPSPs is likely to be a downstream effect of increased excitability in AH neurones because AH neurones probably mediate sEPSPs in S neurones through the release of tachykinins. Previous studies involving measurement of neurotransmitter release have reported decreased release in inflamed intestinal segments (Collins et al. 1992); however, these investigations have involved release from whole tissue, and include neurotransmitter release from motor neurones, whereas the findings reported here were obtained at single synaptic junctions between neurones.
Unlike the AH neurones, which underwent significant changes in the inflamed colon, the electrical properties of most S neurones in the inflamed colon were comparable to those in the normal colon. One factor that may contribute to this difference is that AH neurones extend projections to the lamina propria of the mucosa whereas S neurones do not (Neunlist et al. 1999). In TNBS colitis, inflammation is centred in the lamina propria; therefore, the processes of AH neurones, but not S neurones, pass directly into the region in which inflammatory mediators and trophic factors, released as part of the immune response, are likely to be at their highest concentrations. Indeed, it has been demonstrated that extrinsic afferent neurones that project to the mucosa of the gut also undergo changes in response to TNBS-induced inflammation (Sengupta et al. 1999; Moore et al. 2001). When S neurones were divided into subpopulations, based on axonal projection patterns, those that projected in an oral direction were relatively excitable, compared to controls, as they demonstrated slower accommodation, more frequent anodal break action potentials and spontaneous activity. Orally projecting S neurones probably include excitatory motor neurones and ascending interneurones (Brookes, 2001); additional studies will be required to determine if one or both of these subpopulations are altered in colitis.
The increased excitation observed in AH neurones in the present study, e.g. increased occurrence of spontaneous activity, anodal break action potentials and fEPSPs, and decreased accommodation and AHP, is reminiscent of that observed in a study of AH neurones in the jejunum of Trichinella spiralis-infected guinea-pigs (Palmer et al. 1998) with some notable exceptions. In the Trichinella model, the hyperexcitability observed in the AH neurones was accompanied by a depolarised membrane potential, increased input resistance and reduced duration of the action potential, all of which were not observed in the present study. These differences may be attributable to the type of inflammatory stimulus (infectious vs. immune cell-mediated) or the location of these neurones in the guinea-pig jejunum, but serve to underscore the concept that several possible mechanisms contribute to the level of excitability of AH neurones.
Two distinct properties of AH neurones, the resting membrane potential and the AHP, involve the activity of Ca2+-activated K+ conductances. These conductances may involve the same K+ channel because they have similar pharmacological properties (they are blocked by charybdotoxin and iberiotoxin, but not by apamin) (Kunze et al. 1994; Rugiero et al. 2002; Vogalis et al. 2002) and acute application of excitatory agonists such as substance P suppress these conductances (Grafe et al. 1980; Johnson et al. 1980). Interestingly, in the inflamed colon, we detected a significant attenuation of the AHP, but no change in the resting membrane potential or input resistance. Therefore, it is quite possible that the difference in excitability of AH neurones in the inflamed vs. normal colon is due to changes in conductance(s) other than the Ca2+-activated K+ conductance.
The activation of the prolonged AHP in AH neurones is initiated by the influx of extracellular calcium through N-type voltage-gated Ca2+ channels during the action potential, which triggers calcium-induced calcium release (CICR) through ryanodine receptors from intracellular stores (Hillsley et al. 2000; Vogalis et al. 2001; Rugiero et al. 2002). The resulting increase in intracellular Ca2+ leads to the activation of Ca2+-activated K+ channels and, in turn, the AHP. In AH neurones, the Ca2+ component of the action potential results in a shoulder on the repolarising phase and a broadening of the spike. In the current study, several features of the AH neurone action potential were evaluated, including rate of rise, rate of fall, duration, amplitude and integrated magnitude, and none of these features were altered in colitis. These data suggest that the decrease in the AHP of AH neurones in inflamed tissue, and associated increase in excitability, involve a mechanism other than changes in Ca2+ influx. Future investigations will be necessary to discern whether inflammation is associated with changes in mobilisation from intracellular stores that could contribute to the decreased magnitude of the AHP in TNBS colitis.
Another conductance that influences the magnitude of the AHP in AH neurones, and therefore neuronal excitability, is a Cs+-sensitive cation current that is activated by membrane hyperpolarisation (Ih) (Galligan et al. 1990). This conductance is triggered by the after-spike hyperpolarisation and persists throughout the prolonged AHP. Therefore, Ih opposes the hyperpolarising action of activated Ca2+-activated K+ channels, and attenuates the amplitude of the AHP. While there are no previous reports of a modulation of Ih in AH neurones by synaptic inputs or application of neuroactive compounds, modulation of this conductance has been observed in other systems (for review see Ludwig et al. 1999; Santoro & Tibbs, 1999; Gauss & Seifert, 2000) and it is clear that the excitability of AH neurones would be enhanced or suppressed by an increase or decrease in this conductance. Data presented here suggest that there is a sustained augmentation of Ih in AH neurones of the inflamed colon. The characteristic sag in the electrotonic potential during a hyperpolarising current pulse was larger in neurones of the inflamed colon. Furthermore, the Cs+-sensitive rectification of the current- voltage relationship in AH neurones was significantly larger in inflamed tissue, and AH neurones from inflamed tissue were less excitable when Ih was blocked by Cs+. These data support the novel concept that inflammation results in an enhancement of Ih, and that modulation of this conductance is a means of altering the excitability of these cells. While mechanisms responsible for this change have not been identified, a recent study indicates that prolonged (24 h) exposure of AH neurones to prostaglandin E2 ethanolamide, a stable analogue of prostaglandin E2, leads to an attenuated AHP and increased excitability of guinea-pig distal colonic myenteric AH neurones (Manning et al. 2002).
Altered colonic motility associated with inflammatory bowel disease contributes significantly to the symptoms of inflammatory bowel disease. The inflamed colon behaves as a semirigid tube, such that decreased motor reflexes paralleled with variable secretion result in diarrhoea or constipation (O'Brien & Phillips, 1996). Because AH neurones are situated at the afferent end of the intrinsic neural circuitry of the bowel, enhanced excitability of AH neurones would probably lead to changes in the efficacy of motor reflexes. With AH neurones generating spontaneous activity in the inflamed bowel, the fidelity of output from these cells, which is normally limited to responses to physiological stimuli, would be diminished, thereby leading to changes in reflex activity.
In summary, these data provide a characterisation of myenteric neurones in the inflamed distal colon of the guinea-pig and suggest that AH neurone excitability is enhanced during chronic TNBS-induced inflammation. Because this animal model closely approximates human inflammatory bowel disease (Kim & Berstad, 1992; Fedorak, 1995), it is possible that enhanced excitability of enteric neurones exists in Crohn's disease and ulcerative colitis.
Acknowledgments
This work was supported by NIH grants F32DK60382 (D.R.L.) NS26995 (G.M.M.) and DK62267 (G.M.M.), and a grant from the Crohn's and Colitis Foundation of Canada (K.A.S., G.M.M.). K.A.S. is an Alberta Heritage Foundation for Medical Research Medical Scientist. The authors wish to thank Drs Rodney L. Parsons and Edward Parr for their helpful discussion.
REFERENCES
- Bertrand PP, Kunze WA, Bornstein JC, Furness JB, Smith ML. Analysis of the responses of myenteric neurons in the small intestine to chemical stimulation of the mucosa. Am J Physiol. 1997;273:G422–435. doi: 10.1152/ajpgi.1997.273.2.G422. [DOI] [PubMed] [Google Scholar]
- Bornstein JC, Furness JB, Kunze WA. Electrophysiological characterization of myenteric neurons: how do classification schemes relate? J Auton Nerv Syst. 1994;48:1–15. doi: 10.1016/0165-1838(94)90155-4. [DOI] [PubMed] [Google Scholar]
- Brookes SJ. Classes of enteric nerve cells in the guinea-pig small intestine. Anat Rec. 2001;262:58–70. doi: 10.1002/1097-0185(20010101)262:1<58::AID-AR1011>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Castro GA. Intestinal physiology in the parasitized host: integration, disintegration, and reconstruction of systems. Ann N Y Acad Sci. 1992;664:369–379. doi: 10.1111/j.1749-6632.1992.tb39775.x. [DOI] [PubMed] [Google Scholar]
- Collins SM, Hurst SM, Main C, Stanley E, Khan I, Blennerhassett P, Swain M. Effect of inflammation of enteric nerves: Cytokine-induced changes in neurotransmitter content and release. Ann NY Acad Sci. 1992;664:415–424. doi: 10.1111/j.1749-6632.1992.tb39780.x. [DOI] [PubMed] [Google Scholar]
- Costa M, Furness JB, Gibbins IL. Chemical coding of enteric neurons. Prog Brain Res. 1986;68:217–239. doi: 10.1016/s0079-6123(08)60241-1. [DOI] [PubMed] [Google Scholar]
- Fedorak RN. Naturally occurring and experimental models of inflammatory bowel disease. In: Kirsner J, Shorter R, editors. Inflammatory Bowel Disease. Baltimore: Williams and Wilkins; 1995. pp. 71–95. [Google Scholar]
- Frieling T, Cooke HJ, Wood JD. Neuroimmune communication in the submucous plexus of guinea pig colon after sensitization to milk antigen. Am J Physiol. 1994;267:G1087–1093. doi: 10.1152/ajpgi.1994.267.6.G1087. [DOI] [PubMed] [Google Scholar]
- Furness JB, Kunze WA, Bertrand PP, Clerc N, Bornstein JC. Intrinsic primary afferent neurons of the intestine. Prog Neurobiol. 1998;54:1–18. doi: 10.1016/s0301-0082(97)00051-8. [DOI] [PubMed] [Google Scholar]
- Furness JB, Young HM, Pompolo S, Bornstein JC, Kunze WA, McConalogue K. Plurichemical transmission and chemical coding of neurons in the digestive tract. Gastroenterology. 1995;108:554–563. doi: 10.1016/0016-5085(95)90086-1. [DOI] [PubMed] [Google Scholar]
- Galligan JJ, Lepard KJ, Schneider DA, Zhou X. Multiple mechanisms of fast excitatory synaptic transmission in the enteric nervous system. J Auton Nerv Syst. 2000;81:97–103. doi: 10.1016/s0165-1838(00)00130-2. [DOI] [PubMed] [Google Scholar]
- Galligan JJ, Tatsumi H, Shen KZ, Surprenant A, North RA. Cation current activated by hyperpolarization (IH) in guinea pig enteric neurons. Am J Physiol. 1990;259:G966–972. doi: 10.1152/ajpgi.1990.259.6.G966. [DOI] [PubMed] [Google Scholar]
- Gauss R, Seifert R. Pacemaker oscillations in heart and brain: a key role for hyperpolarization-activated cation channels. Chronobiol Int. 2000;17:453–469. doi: 10.1081/cbi-100101057. [DOI] [PubMed] [Google Scholar]
- Gershon MD, Kirchgessner AL, Wade PR. Functional anatomy of the enteric nervous system. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. New York: Raven Press; 1994. pp. 381–422. [Google Scholar]
- Grafe P, Mayer CJ, Wood JD. Synaptic modulation of calcium-dependent potassium conductance in myenteric neurones in the guinea-pig. J Physiol. 1980;305:235–248. doi: 10.1113/jphysiol.1980.sp013360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillsley K, Kenyon JL, Smith TK. Ryanodine-sensitive stores regulate the excitability of AH neurons in the myenteric plexus of guinea-pig ileum. J Neurophysiol. 2000;84:2777–2785. doi: 10.1152/jn.2000.84.6.2777. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Katayama Y, North RA. Slow synaptic potentials in neurones of the myenteric plexus. J Physiol. 1980;301:505–516. doi: 10.1113/jphysiol.1980.sp013220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Berstad A. Experimental colitis in animal models. Scand J Gastroenterol. 1992;27:529–537. doi: 10.3109/00365529209000116. [DOI] [PubMed] [Google Scholar]
- Kirchgessner AL, Liu MT. Immunohistochemical localization of nicotinic acetylcholine receptors in the guinea pig bowel and pancreas. J Comp Neurol. 1998;390:497–514. [PubMed] [Google Scholar]
- Kunze WA, Bornstein JC, Furness JB. Identification of sensory nerve cells in a peripheral organ (the intestine) of a mammal. Neuroscience. 1995;66:1–4. doi: 10.1016/0306-4522(95)00067-s. [DOI] [PubMed] [Google Scholar]
- Kunze WA, Bornstein JC, Furness JB, Hendriks R, Stephenson DS. Charybdotoxin and iberiotoxin but not apamin abolish the slow after-hyperpolarization in myenteric plexus neurons. Pflugers Arch. 1994;428:300–306. doi: 10.1007/BF00724511. [DOI] [PubMed] [Google Scholar]
- Kunze WA, Clerc N, Furness JB, Gola M. The soma and neurites of primary afferent neurons in the guinea-pig intestine respond differentially to deformation. J Physiol. 2000;526:375–385. doi: 10.1111/j.1469-7793.2000.00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze WA, Furness JB. The enteric nervous system and regulation of intestinal motility. Annu Rev Physiol. 1999;61:117–142. doi: 10.1146/annurev.physiol.61.1.117. [DOI] [PubMed] [Google Scholar]
- Kunze WA, Furness JB, Bertrand PP, Bornstein JC. Intracellular recording from myenteric neurons of the guinea-pig ileum that respond to stretch. J Physiol. 1998;506:827–842. doi: 10.1111/j.1469-7793.1998.827bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax AE, Bertrand PP, Furness JB. Electrophysiological characteristics distinguish three classes of neuron in submucosal ganglia of the guinea-pig distal colon. Neuroscience. 2001;103:245–255. doi: 10.1016/s0306-4522(00)00545-5. [DOI] [PubMed] [Google Scholar]
- Lomax AE, Furness JB. Neurochemical classification of enteric neurons in the guinea-pig distal colon. Cell Tissue Res. 2000;302:59–72. doi: 10.1007/s004410000260. [DOI] [PubMed] [Google Scholar]
- Lomax AE, Sharkey KA, Bertrand PP, Low AM, Bornstein JC, Furness JB. Correlation of morphology, electrophysiology and chemistry of neurons in the myenteric plexus of the guinea-pig distal colon. J Auton Nerv Syst. 1999;76:45–61. doi: 10.1016/s0165-1838(99)00008-9. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Zong X, Hofmann F, Biel M. Structure and function of cardiac pacemaker channels. Cell Physiol Biochem. 1999;9:179–186. doi: 10.1159/000016315. [DOI] [PubMed] [Google Scholar]
- McCafferty DM, Wallace JL, Sharkey KA. Effects of chemical sympathectomy and sensory nerve ablation on experimental colitis in the rat. Am J Physiol. 1997;272:G272–280. doi: 10.1152/ajpgi.1997.272.2.G272. [DOI] [PubMed] [Google Scholar]
- Manning BP, Sharkey KA, Mawe GM. Effects of prostaglandin E2 in guinea pig colonic myenteric ganglia. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1388–1397. doi: 10.1152/ajpgi.00141.2002. [DOI] [PubMed] [Google Scholar]
- Moore BA, Hill CE, Vanner SJ. The intrinsic firing pattern of nociceptive sensory neurons innervating guinea pig ileum in enhanced by TNBS-ileitis. Gastroenterology. 2001;120:A56. [Google Scholar]
- Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803. [PubMed] [Google Scholar]
- Morteau O, More J, Pons L, Bueno L. Platelet-activating factor and interleukin 1 are involved in colonic dysmotility in experimental colitis in rats. Gastroenterology. 1993;104:47–56. doi: 10.1016/0016-5085(93)90834-y. [DOI] [PubMed] [Google Scholar]
- Neunlist M, Dobreva G, Schemann M. Characteristics of mucosally projecting myenteric neurones in the guinea-pig proximal colon. J Physiol. 1999;517:533–546. doi: 10.1111/j.1469-7793.1999.0533t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath MF, Fuss I, Kelsall BL, Stuber E, Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995;182:1281–1290. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien MD, Phillips SF. Colonic motility in health and disease. Gastroenterol Clin North Am. 1996;25:147–162. doi: 10.1016/s0889-8553(05)70369-1. [DOI] [PubMed] [Google Scholar]
- Palmer JM, Wong-Riley M, Sharkey KA. Functional alterations in jejunal myenteric neurons during inflammation in nematode-infected guinea pigs. Am J Physiol. 1998;275:G922–935. doi: 10.1152/ajpgi.1998.275.5.G922. [DOI] [PubMed] [Google Scholar]
- Rugiero F, Gola M, Kunze WA, Reynaud JC, Furness JB, Clerc N. Analysis of whole-cell currents by patch clamp of guinea-pig myenteric neurones in intact ganglia. J Physiol. 2002;538:447–463. doi: 10.1113/jphysiol.2001.013051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanovic S, Lamb DP, Blennerhassett MG. Damage to the enteric nervous system in experimental colitis. Am J Pathol. 1999;155:1051–1057. doi: 10.1016/S0002-9440(10)65207-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro B, Tibbs GR. The HCN gene family: molecular basis of the hyperpolarization-activated pacemaker channels. Ann N Y Acad Sci. 1999;868:741–764. doi: 10.1111/j.1749-6632.1999.tb11353.x. [DOI] [PubMed] [Google Scholar]
- Schutte IW, Kroese AB, Akkermans LM. Somal size and location within the ganglia for electrophysiologically identified myenteric neurons of the guinea pig ileum. J Comp Neurol. 1995;355:563–572. doi: 10.1002/cne.903550406. [DOI] [PubMed] [Google Scholar]
- Sengupta JN, Snider A, Su X, Gebhart GF. Effects of kappa opioids in the inflamed rat colon. Pain. 1999;79:175–185. doi: 10.1016/s0304-3959(98)00175-4. [DOI] [PubMed] [Google Scholar]
- Sharkey KA, Parr EJ. The enteric nervous system in intestinal inflammation. Can J Gastroenterol. 1996;10:335–341. [Google Scholar]
- Spencer NJ, Hennig GW, Smith TK. A rhythmic motor pattern activated by circumferential stretch in guinea-pig distal colon. J Physiol. 2002;545:629–648. doi: 10.1113/jphysiol.2002.028647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer NJ, Hennig GW, Smith TK. Stretch-activated neuronal pathways to longitudinal and circular muscle in guinea-pig distal colon. Am J Physiol Gastrointest Liver Physiol. 2003;284:G231–241. doi: 10.1152/ajpgi.00291.2002. [DOI] [PubMed] [Google Scholar]
- Tamura K, Ito H, Wade PR. Morphology, electrophysiology, and calbindin immunoreactivity of myenteric neurons in the guinea pig distal colon. J Comp Neurol. 2001;437:423–437. doi: 10.1002/cne.1293. [DOI] [PubMed] [Google Scholar]
- Thomas EA, Bertrand PP, Bornstein JC. A computer simulation of recurrent, excitatory networks of sensory neurons of the gut in guinea-pig. Neurosci Lett. 2000;287:137–140. doi: 10.1016/s0304-3940(00)01182-4. [DOI] [PubMed] [Google Scholar]
- Vogalis F, Furness JB, Kunze WA. Afterhyperpolarization current in myenteric neurons of the guinea pig duodenum. J Neurophysiol. 2001;85:1941–1951. doi: 10.1152/jn.2001.85.5.1941. [DOI] [PubMed] [Google Scholar]
- Vogalis F, Harvey JR, Furness JB. TEA- and apamin-resistant K(Ca) channels in guinea-pig myenteric neurons: slow AHP channels. J Physiol. 2002;538:421–433. doi: 10.1113/jphysiol.2001.012952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada-Takahashi S, Tamura K. Actions of reactive oxygen species on AH/type 2 myenteric neurons in guinea pig distal colon. Am J Physiol Gastrointest Liver Physiol. 2000;279:G893–902. doi: 10.1152/ajpgi.2000.279.5.G893. [DOI] [PubMed] [Google Scholar]
- Wade PR, Wood JD. Electrical behavior of myenteric neurons in guinea pig distal colon. Am J Physiol. 1988a;254:G522–530. doi: 10.1152/ajpgi.1988.254.4.G522. [DOI] [PubMed] [Google Scholar]
- Wade PR, Wood JD. Synaptic behavior of myenteric neurons in guinea pig distal colon. Am J Physiol. 1988b;255:G184–190. doi: 10.1152/ajpgi.1988.255.2.G184. [DOI] [PubMed] [Google Scholar]
- Wood JD. Gastrointestinal neuroimmune interactions. In: Holle GE, Wood JD, editors. Physiology of the Gastrointestinal Tract. Amsterdam: Excerpta Medica; 1992. pp. 607–615. [Google Scholar]
- Wood JD. Application of classification schemes to the enteric nervous system. J Auton Nerv Syst. 1994a;48:17–29. doi: 10.1016/0165-1838(94)90156-2. [DOI] [PubMed] [Google Scholar]
- Wood JD. Physiology of the enteric nervous system. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. New York: Raven Press; 1994b. pp. 423–482. [Google Scholar]


