Abstract
Inspiratory activity of the hypoglossal nerve (XIIn) often precedes that of the phrenic nerve (PHRn). By manipulating artificial respiration, this preceding activity (pre-I XIIn activity) can be lengthened or isolated prematurely (decoupled XIIn activity) without developing into overt PHRn-associated inspiratory bursts. We hypothesized that these pre-I and decoupled XIIn activities, collectively termed ‘XIIn-w/o-PHRn activity’, reflect certain internal states of the respiratory centre at the period just prior to the transition from the expiratory phase to the inspiratory phase. In decerebrate, neuromuscularly blocked and artificially ventilated rats, the firing properties of medullary respiratory neurones were examined during the period of the XIIn-w/o-PHRn activity. The majority of the inspiratory neurones examined could be classified into two types: one was active (XIIn-type) and the other was inactive (PHRn-type) during the XIIn-w/o-PHRn period. On the other hand, augmenting expiratory (E-AUG) neurones of the Bötzinger complex (BOT) and the caudal ventral respiratory group (VRG) fired intensively during this period. Their firing stopped at the onset of the overt inspiratory bursts in the XIIn and PHRn, suggesting that BOT E-AUG neurones inhibit PHRn-type, but not XIIn-type, inspiratory neurones. We hypothesize that XIIn-type inspiratory activity facilitates the phase change from expiration to inspiration, through activation of certain inspiratory neurones that inhibit the firing of BOT E-AUG neurones and generation of the overt inspiratory bursts in XIIn-type and PHRn-type inspiratory neurones.
Respiration is a coordinated movement of pump muscles and upper airway muscles. Inspiratory and expiratory activities in the upper airway muscles are closely synchronized with the corresponding activities of pump muscles. However, close inspection has revealed that the onset of inspiratory discharges in the cranial nerves frequently precedes phrenic nerve (PHRn) discharges (Cohen, 1975; Fukuda & Honda, 1982a; Sica et al. 1984; Hwang & St John, 1987; Peever et al. 2001). In a preceding study (Saito et al. 2002b) we analysed the discharge patterns of the hypoglossal nerve (XIIn) and PHRn by experimentally controlling the onset times of their inspiratory discharges. We were able to lengthen the preceding XIIn activity (pre-I XIIn activity) and even isolate premature XIIn activity decoupled from PHRn activity (decoupled XIIn activity). Intracellular analysis of XIIn motoneurones during such XIIn activity without accompanying PHRn activity (XIIn-w/o-PHRn activity) has shown that all XIIn motoneurones with inspiratory activity are excited whereas those with expiratory activity are suppressed during the XIIn-w/o-PHRn activity (Saito et al. 2002b). Thus, the XIIn-w/o-PHRn activity, which emerges in the expiratory phase if strictly defined based on PHRn discharge, may reflect the internal inspiratory activity of the respiratory centre. In addition, the XIIn-w/o-PHRn activity interacts with fictive swallowing elicited by electrical stimulation of the superior laryngeal nerve (SLn) in the same way that the overt inspiratory activity interacts with fictive swallowing (Saito et al. 2002a,b), further suggesting a close relationship of the XIIn-w/o-PHRn activity to the central inspiratory activity.
We assumed that the period of the pre-I XIIn activity corresponds to the preparatory period for the expiration-inspiration transition. In order to obtain further information about the neuronal mechanisms occurring during this period, we analysed firing properties of brainstem respiratory neurones, including possible premotor neurones to XIIn and PHRn motoneurones. For this purpose, the method used previously (Saito et al. 2002b) to exaggerate or even isolate the pre-I XIIn activity, which normally fuses immediately into the subsequent overt inspiratory activity and therefore is not conspicuous, was most useful. We hypothesized that (1) there are at least two types of brainstem inspiratory neurones that drive XIIn and PHRn motoneurones and (2) augmenting expiratory (E-AUG) neurones of the Bötzinger complex (BOT), which are glycinergic (Schreihofer et al. 1999) and inhibitory to inspiratory neurones at the late expiratory phase (Ezure, 1990; Jiang & Lipski, 1990), are the key neurones that differentiate the two types of firing patterns. In fact, different control by BOT E-AUG neurones of XIIn vs. PHRn activity has been suggested (Peever et al. 2001). Thus, the aims of the present study were to record from inspiratory and expiratory neurones of the dorsal respiratory group (DRG), the ventral respiratory group (VRG) and the BOT, to analyse their firing properties during the XIIn-w/o-PHRn activity, and to gain insight into the neuronal mechanism of the phase change from expiration to inspiration.
METHODS
Experimental procedures were performed in accordance with the Guiding Principles for the Care and Use of Animals in the Field of Physiological Science (Physiological Society of Japan, 2001). The experiments were reviewed and approved by the Animal Care and Use Committee of the Tokyo Metropolitan Institute for Neuroscience.
Surgical procedure
Experiments were conducted on 21 adult male rats (385–580 g). The animals were initially anaesthetized with sodium pentobarbitone (Nembutal, 60 mg kg−1; i.p.). When necessary, supplementary doses (about 5 mg kg−1 h−1; i.v.) were given throughout surgery before decerebration (see below).
The trachea was intubated, and cannulae were placed in the femoral artery and vein for blood pressure monitoring and drug administration, respectively. The rats were placed in a stereotaxic frame, initially in the supine position. The XIIn on one side was cut distally and mounted in a bipolar cuff electrode for recording and stimulation. In 11 experiments, similar bipolar cuff electrodes were attached to the cut SLn on both sides for stimulation. The exposed XIIn and SLn were covered with Vaseline jelly, thin plastic films and skin flaps. The animals were then rotated to the prone position and supported by hip pins and a clamp placed on an upper thoracic vertebra.
Precollicular decerebration was performed after craniotomy. The brain rostral to the transection was removed by suction. No further anaesthetics were given after decerebration, since this rendered the animal insentient. The decerebration was normally completed within 3 h after the anaesthetic induction. The dorsal surface of the medulla was exposed by partial cerebellectomy for recording purposes. In 10 experiments, after laminectomy, stainless needle electrodes were fixed in the ventrolateral quadrant of the C2/C3 spinal cord bilaterally for stimulation. The C4/C5 PHRn was cut distally, mounted on a bipolar recording electrode, and immersed in oil pools. The animals were injected with the neuromuscular blocker pancuronium bromide (Mioblock, Sankyo, Tokyo; 0.15 mg kg−1 h−1) and artificially ventilated. A bilateral pneumothorax was made and a positive end-expiratory pressure (PEEP) (1–2 cmH2O) was steadily applied and dynamically changed during the neural recordings. Blood pressure was monitored and kept above 80 mmHg; pressor agents (10 % dextran, 1–2 ml kg−1; phenylephrine hydrochloride, Neo-Synesin, Kowa, Tokyo, 2–3 mg kg−1) were intravenously administered when necessary. Tracheal pressure, end-tidal CO2 (kept at 4–6 %) and rectal temperature (kept at 36–37 °C) were monitored.
Recording and stimulation
Activities from the PHRn and XIIn were amplified, full-wave rectified and low-pass filtered (time constant 10 ms). Brainstem neuronal activity was recorded extracellularly with glass micropipettes filled with 3 m KCl solution saturated with Fast Green FCF dye (DC impedance, 1–1.2 MΩ). For later histological reconstruction, recording sites in each experiment were occasionally marked with dye.
In the BOT and the VRG, only neurones with antidromic activation from the brainstem and/or the spinal cord were included for analysis. In the DRG, XIIn motoneurones were excluded by antidromic activation from the XIIn. Stimulating electrodes of the brainstem were similar to those for recording, but with a lower impedance (about 0.8 MΩ). A stimulating electrode was positioned in the caudal VRG within the area 1–1.5 mm caudal to the obex and 1.3–2.0 mm lateral to the midline. The stimulation was applied along a track at the depth 1–2.3 mm and the intensity was less than 30 μA (rectangular pulse of 150 μs duration). When antidromic spikes were not evoked from the first track, one or two more tracks within the same area were examined. The side of stimulation was either contralateral or ipsilateral to the recorded neurone, although both contralateral and ipsilateral sides were occasionally stimulated. The ipsilateral stimulation was applied only for neurones recorded in the BOT area, i.e. for neurones recorded at the level 1.5 mm or more rostral to the obex, thus minimizing the possibility of stimulating the axons of vagal motoneurones (Kanjhan et al. 1995), which leave the brainstem at the same rostrocaudal level of the somata of origin (Zheng et al. 1991; Saito et al. 2002c). For neurones recorded around the level of the obex or more caudally, only spinal cord projections were examined. The intensity of the spinal cord stimulation was 100–500 μA (rectangular pulse of 150 μs duration).
Fictive swallowing could be evoked by electrical stimulation of the SLn with either single rectangular pulses, trains of 5–11 pulses at 200–300 Hz, or continuous trains of constant current pulses (150 μs duration) between 35 and 55 Hz (see Saito et al. 2002a).
At the end of each experiment, the rat was killed by i.v. injection of anaesthetic (Nembutal, 50 mg kg−1) followed by cessation of ventilation. The brainstem was removed and fixed in 4 % paraformaldehyde. After fixation, serial frozen sections of the brainstem (100 μm thick in the frontal plane) were made. Recording sites were reconstructed on the basis of the dye marks. The anatomical structures were identified using the atlas by Paxinos & Watson (1998). All signals were monitored on a thermal array recorder (8M15, NEC-Sanei, Tokyo, Japan) and oscilloscopes. In addition, most data were stored on magnetic tape (PCM recorder; PC-216A, Sony Precision Technology, Tokyo, Japan; sampling rate 50 μs) for subsequent off-line analysis.
RESULTS
Dissociation between XIIn and PHRn activities was generated by manipulating the ventilatory condition and the corresponding activities of medullary respiratory neurones were analysed. The respiratory neurones (n= 178) examined were not cranial motoneurones and consisted of inspiratory neurones (n= 133) and expiratory neurones (n= 45).
Hypoglossal vs. phrenic nerve activity
To produce the dissociation between XIIn and PHRn inspiratory activities, we either altered PEEP levels dynamically or stopped the ventilation temporarily, maintaining the lung inflation at certain levels as exemplified in Fig. 1 (see also Saito et al. 2002b). The XIIn activity preceding the PHRn activity (pre-I XIIn activity) was markedly exaggerated by increasing PEEP (Fig. 1Ba) or by maintaining lung inflation (Fig. 1Bc). When the pre-I XIIn activity was augmented by increased PEEP, small XIIn activities that were not followed by an overt burst of XIIn and PHRn inspiratory activity (decoupled XIIn activity) were often observed (Fig. 1Ba). These activities were easily elicited by large PEEPs that suppressed overt inspiratory bursts for a prolonged period.
Figure 1. Dissociation between hypoglossal nerve (XIIn) and phrenic nerve (PHRn) activities.
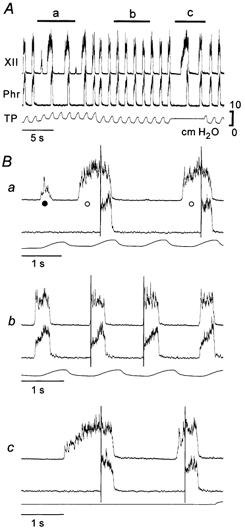
A, relationship between XIIn activity and PHRn activity can be modified by changing positive end-expiratory pressure (PEEP) levels and maintaining lung inflation. Responses indicated by a, b and c in A are expanded in the corresponding traces in B. XII: integrated XIIn activity; Phr: integrated PHRn activity; TP: tracheal pressure. In Ba, pre-I XIIn and decoupled XIIn activities are indicated by ○ and •, respectively. Vertical lines in B show the onset of PHRn activity.
Under normal ventilatory conditions, both XIIn and PHRn inspiratory activities started at the nadir and terminated at around the peak of tracheal pressure (Fig. 1Bb). During phasic lung inflations with increased PEEP, the onset of the pre-I XIIn and decoupled XIIn activities consistently coincided with the nadir of tracheal pressure, but the overt bursts of XIIn and PHRn activity tended to start at around the peak of tracheal pressure. The decoupled XIIn activity was terminated at the rising phase of tracheal pressure, and therefore this activity was evoked only during phasic lung inflations. During maintained lung inflation, once started, XIIn activity was never isolated but was always followed by overt XIIn and PHRn inspiratory bursts (Fig. 1Bc). The rising slope of the PHRn inspiratory discharge was steeper when the pre-I XIIn activity was larger than when the pre-I XIIn activity was small or absent (Fig. 2).
Figure 2. Different onset slope of phrenic nerve (PHRn) inspiratory activity.
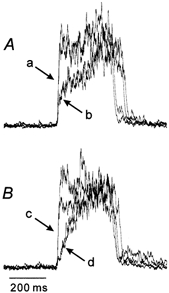
Two traces (arrow a in A) during increased PEEP from Fig. 1Ba and two traces (arrow b in A) during control PEEP from Fig. 1Bb are superimposed. Two traces (arrow c in B) during maintained lung inflation at 5 cmH2O from Fig. 1Bc and two traces (arrow d in B) during the subsequent episode of lung deflation to atmospheric pressure (not shown) are superimposed.
Firing properties of inspiratory neurones
Inspiratory neurones were recorded from the ventrolateral medulla (n = 56) and the dorsomedial medulla (n = 77). The neurones from the former area were located in the VRG and the BOT and those from the latter area were located in the DRG including the nucleus tractus solitarii (NTS); we call these two groups VRG neurones and DRG neurones, respectively. The VRG inspiratory neurones (Fig. 3) were antidromically activated by electrical stimulation of either the spinal cord or the brainstem. No attempts were made to analyse the detailed patterns of their axonal projections since the stimulation was used only to exclude cranial motoneurones. Although the inspiratory neurones of the DRG area (Fig. 4) were not examined for their projections to either the spinal cord or the brainstem, motoneurones that were antidromically activated from the XIIn were excluded.
Figure 3. Two types of ventral respiratory group (VRG) inspiratory neurones.
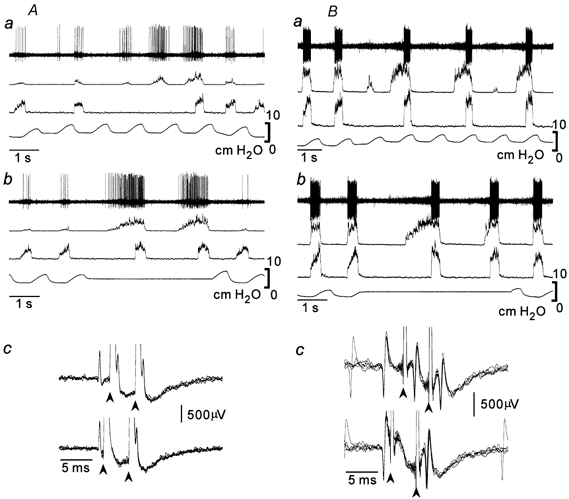
XIIn-type (A) and PHRn-type (B) inspiratory neurones during increased PEEP (Aa and Ba) and maintained lung inflation (Ab and Bb). These neurones were antidromically activated from the medulla (Ac and Bc). In the collision test (Ac and Bc), medullary stimuli of double shocks (arrowheads) were applied 1.7 ms (Ac, upper trace), 3 ms (Bc, upper trace), 0.7 ms (Ac, lower trace) and 1 ms (Bc, lower trace) after spontaneous spikes (five superimposed traces). Traces are (from the top) extracellular activity, XIIn activity, PHRn activity and tracheal pressure. The same arrangement is adopted in the subsequent figures.
Figure 4. Two types of DRG inspiratory neurones.

XIIn-type (A) and PHRn-type (B) inspiratory neurones during increased PEEP (a), maintained lung inflation (b) and lung deflation (c). During lung deflation the coupling between firing of PHRn-type inspiratory neurones and PHRn discharge became loose.
The majority of the inspiratory neurones could be classified into one of the two types of firing patterns (Table 1). One type (XIIn-type) of inspiratory neurone fired during both the XIIn-w/o-PHRn activity and the PHRn activity (n = 14 for VRG neurones; n = 45 for DRG neurones). The other type (PHRn-type) of neurone fired only during the PHRn activity (n = 39 for VRG neurones; n = 20 for DRG neurones). The XIIn-type neurones fired during both the pre-I and the decoupled XIIn activities (Fig 3 and Fig 4). In some inspiratory neurones, their firing and the XIIn or PHRn activity were loosely correlated, and these neurones were not classifiable (n = 3 for VRG neurones; n = 12 for DRG neurones). Although not all the inspiratory neurones of the VRG were examined for spinal projections, four XIIn-type neurones and 22 PHRn-type neurones projected to the spinal cord.
Table 1.
Classification of inspiratory neurones
| n | % of total | |
|---|---|---|
| DRG neurones | 77 | |
| XIIn-type | 45 | 58 |
| PHRn-type | 20 | 26 |
| Unclassifiable | 12 | 16 |
| VRG neurones | 56 | |
| XIIn-type | 14 | 25 |
| PHRn-type | 39 | 70 |
| Unclassifiable | 3 | 5 |
Augmenting expiratory neurones
Expiratory neurones with an augmenting firing pattern (E-AUG neurones) were recorded from the BOT and the VRG at the level 0–1.3 mm caudal to the obex, i.e. from the caudal VRG (cVRG). The BOT E-AUG neurones (n = 36) were antidromically activated from either the spinal cord or from the brainstem caudal to the obex (Fig. 5). The cVRG E-AUG neurones (n = 9) were antidromically activated from the spinal cord (Fig. 6). No attempts were made to identify the detailed projection patterns of their axons.
Figure 5. Augmenting expiratory (E-AUG) neurone of the Bötzinger complex (BOT).

Augmenting firing persisted during decoupled XIIn (A) and pre-I XIIn (B) activities and stopped at the PHRn inspiratory activity (see vertical lines in B). During lung deflation (C) this neurone stopped firing. It was antidromically activated from the ipsilateral caudal VRG (cVRG) (D). In the collision test (D), medullary stimuli (arrowheads) were applied 2 ms (upper trace) and 1 ms (lower trace) after spontaneous spikes (five superimposed traces). Note that firing of the E-AUG neurone was suppressed by phasic lung inflations (A and C).
Figure 6. Augmenting expiratory (E-AUG) neurone of the caudal ventral respiratory group (cVRG).
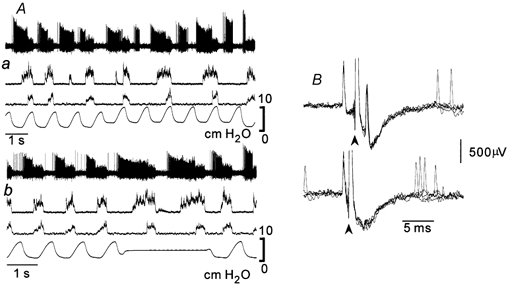
Augmenting firing continued during pre-I XIIn (Ab) and decoupled XIIn (Aa) activities and stopped at the PHRn inspiratory activity. This neurone was antidromically activated from the spinal cord (B). In the collision test (B), spinal cord stimuli (arrowheads) were applied 2 ms (upper trace) and 1 ms (lower trace) after spontaneous spikes (five superimposed traces).
All of the E-AUG neurones, both BOT and cVRG neurones, were active during the pre-I XIIn and decoupled XIIn activities. Since the firing of these neurones was suppressed by phasic lung inflation (Feldman & Cohen, 1978; Kubin & Lipski, 1980; Manabe & Ezure, 1988), their firing was decreased or stopped at the peak of tracheal pressure. However, their firing was not specially modulated by the emergence of the XIIn-w/o-PHRn activity (Fig 5 and Fig 6). These neurones continued firing during the pre-I XIIn period and ceased firing suddenly at the start of the overt inspiratory activity. It is characteristic that their firing became more vigorous as the pre-I XII activity became larger during prolonged suppression of the overt inspiratory activity (Fig 5 and Fig 6).
Relation to swallowing activity
In 58 DRG inspiratory neurones (Table 2), the responses to fictive swallowing evoked by electrical stimulation of the SLn were examined (Fig. 7). Some neurones were active (n = 17, called ‘type 1’, Saito et al. 2002a) and other neurones were inactive (n = 41) during swallowingrelated XIIn bursts. Many of the 41 inactive neurones fired between the bursts (n = 26, ‘type 2’, Saito et al. 2002a). Out of the 32 XIIn-type inspiratory neurones, three were type 1 and 20 were type 2. Out of the 17 PHRn-type inspiratory neurones, 11 were type 1 and one was type 2.
Table 2.
Swallowing-related activity
| Tested | Type 1 | Suppressed (type 2) | |
|---|---|---|---|
| DRG neurones | 58 | 17 | 41 (26) |
| XIIn-type | 32 | 3 | 29 (20) |
| PHRn-type | 17 | 11 | 6 (1) |
| Unclassifiable | 9 | 3 | 6 (5) |
Responses to fictive swallowing were examined in these 58 of the 77 DRG neurones listed in Table 1.
Figure 7. Swallowing-related activities and dorsal respiratory group (DRG) inspiratory neurones.
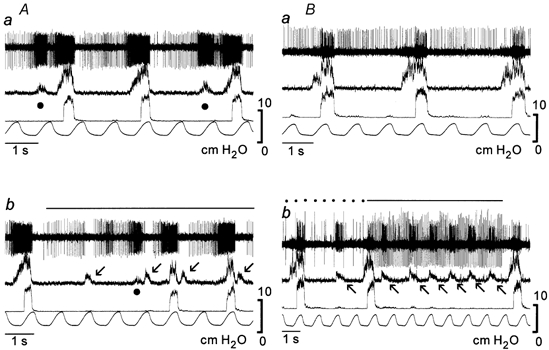
The firing of XIIn-type (A) inspiratory neurones was suppressed whereas that of PHRn-type (B) inspiratory neurones was activated during swallowing evoked by SLn stimulation (dots and lines over the traces). Arrows, swallows; •, decoupled XIIn activities.
We preliminarily recorded the activity of some VRG inspiratory neurones during swallowing, but could not find any swallowing-related activities in either XIIn-type or PHRn-type neurones (not shown).
Distribution of neurones
Figure 8A indicates the location of all neurones analysed (n= 162; 118 inspiratory and 45 expiratory) based on manipulator readings; unclassifiable inspiratory neurones were omitted. Although the laterality of the DRG and VRG neurones overlaps to some extent, all DRG neurones were recorded at a depth of less than 1.2 mm from the dorsal surface and the VRG neurones were recorded from a deeper area. The ratio of XIIn-type neurones to PHRn-type neurones was higher in the DRG than the VRG area. Though only a limited number of neurones were examined, histological examination (Fig. 8B) of dye marks showed that: (1) the DRG neurones were located in the NTS and in the reticular formation ventral to the NTS and lateral to the XIIn nucleus; (2) the VRG neurones were located in the ventrolateral medulla around the nucleus ambiguus, (3) the BOT E-AUG neurones were located in the area caudal to the facial nucleus and ventral to the compact formation of the nucleus ambiguus and (4) the cVRG E-AUG neurones were located in and around the nucleus ambiguus or the nucleus retroambiguus.
Figure 8. Distribution of neurones analysed.
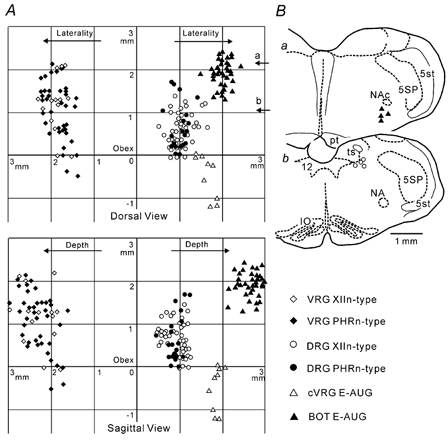
A, dorsal view (upper panel) and sagittal view (lower panel) of neurone locations based on the coordinates of manipulator readings. The origin is the obex. VRG inspiratory neurones are shown on the left and the other neurones are on the right. B, histological reconstruction based on dye marks at the BOT (Ba) and the DRG (Bb); the levels roughly correspond to arrows in A. IO, inferior olivary nucleus; NA, ambiguus nucleus; NAc, compact formation of ambiguus nucleus; pt, pyramidal tract; ts, solitary tract; 5SP, spinal trigeminal nucleus; 5st, spinal trigeminal tract; 12, hypoglossal nucleus.
DISCUSSION
The present results show: (1) that some inspiratory neurones start firing before the onset, but some neurones never fire until the time of onset, of the overt inspiratory phase; and (2) that BOT E-AUG neurones fire vigorously until the onset of the overt inspiratory phase, suggesting that these inhibitory neurones act on the latter but not on the former inspiratory neurones. These phenomena may be closely related to the brainstem process which occurs at the moment just prior to the expiration-inspiration transition.
Pre-I XIIn activity and role of BOT E-AUG neurones
The pre-I XIIn activity and the decoupled XIIn activity are tightly coupled with the inspiratory phase, presumably reflecting the activity of a type of brainstem inspiratory neurone, i.e. XIIn-type inspiratory neurones. In the previous study, we assumed that these two types of XII activities represent ‘latent inspiratory activities’ (Saito et al. 2002b). On the other hand, the extensive firing of the E-AUG neurones of both the BOT and the cVRG during the pre-I XIIn activity indicates that the period of the pre-I XIIn activity belongs to the late expiratory phase. The firing of cVRG E-AUG neurones strongly suggests that spinal expiratory motoneurones are also active (Kirkwood & Sears, 1973; Arita et al. 1987; Iscoe, 1998). Therefore, the pre-I XIIn period can be considered as the transitional period from the expiratory to the inspiratory phase.
The BOT E-AUG neurones fire vigorously until their firing stops suddenly at the onset of the overt inspiratory activity of the PHRn and the XIIn. Since the BOT E-AUG neurones inhibit brainstem inspiratory neurones (Jiang & Lipski, 1990; Ezure, 1990; Tian et al. 1999; Schreihofer et al. 1999), it is plausible that the overt inspiratory activity of the PHRn as well as the XIIn emerges only after the E-AUG neurones stop their firing. The steeper onset slope of PHRn discharge in association with the augmented pre-I XIIn activity may result from a stronger disinhibitory effect from BOT E-AUG neurones compared with the case of small or absent pre-I XIIn activity. On the other hand, the pre-I XIIn and decoupled XIIn activities seem to be free from the inhibition caused by BOT E-AUG neurones, in accordance with previous reports (Fukuda & Honda, 1988; Peever et al. 2001). We aimed to investigate the details of these phenomena by recording from brainstem respiratory neurones. Under normal ventilatory conditions, the pre-I XIIn activity is short, followed immediately by the overt XIIn and PHRn inspiratory activities. In the present study, we lengthened the duration of the pre-I XIIn activity by suppressing the start of the overt inspiratory activity and have designed a suitable condition to analyse the neuronal mechanisms of this critical period.
During the course of this study, many decrementing expiratory (E-DEC) neurones (Manabe & Ezure, 1988; Ezure, 1990; Saito et al. 2002c) or post-inspiratory neurones (Richter et al. 1986; Schwarzacher et al. 1991; Zheng et al. 1991) were recorded from the BOT and the VRG. However, these neurones were discarded since they hardly fired during the period of the XIIn-w/o-PHRn activity and no meaningful information could be obtained.
Two types of brainstem inspiratory neurones
In the present experiments, although motoneurones of the cranial nerves were excluded, a loosely defined selection of the inspiratory neurones from the DRG and the VRG was made. In contrast to the clearly defined E-AUG neurones, the classification of inspiratory neurones in the rat is generally not simple. The firing patterns of the inspiratory neurones studied were not uniform. Some fired with a strong burst but some fired sparsely during the inspiratory phase. Many neurones correspond to augmenting inspiratory neurones, but many neurones, in particular those with weak firing, are not suitable to judge whether they are augmenting or not. However, apparently decrementing inspiratory neurones are not included in the present study. Furthermore, the present XIIn-type inspiratory neurones may be classified into either expiratory-inspiratory phase-spanning neurones (Cohen, 1969; Sun et al. 1998) or inspiratory neurones depending on whether the pre-I XIIn activity is present or not.
With the above-mentioned reservations, the majority of the inspiratory neurones could be classified into either XIIn-type or PHRn-type neurones. Presumably, the PHRn-type inspiratory neurones are inhibited but the XIIn-type neurones are not inhibited by BOT E-AUG neurones. This neural organization may explain XIIn and PHRn discharges, since the sampled inspiratory neurones may include the premotor neurones of XIIn and PHRn motoneurones. In fact, some of the PHRn-type inspiratory neurones of the VRG project to the spinal cord and therefore may drive PHRn motoneurones (Lipski et al. 1994; Tian & Duffin, 1996). Furthermore, some of the XIIn-type inspiratory neurones of the DRG are located in the area where premotor neurones of XIIn motoneurones are distributed (Amri et al. 1990; Ono et al. 1998; Cunningham & Sawchenko, 2000; Peever et al. 2002). Some of the XIIn-type neurones of the VRG may also be premotor neurones of XIIn motoneurones (Amri & Car, 1988; Ezure et al. 1993; Lipski et al. 1994). Peever et al. (2001) have shown that XIIn motoneurones and PHRn motoneurones are controlled differently via different premotor inspiratory neurones and that PHRn motoneurones are inhibited by BOT E-AUG neurones, but XIIn motoneurones are not inhibited by the same neurones. The present results are complementary to the results of Peever et al. (2001) and suggest that the premotor neurones of XIIn and PHRn motoneurones are controlled differently by BOT E-AUG neurones.
It should also be noted that neuronal populations other than the premotor neurones of the XIIn might be included in the XIIn-type inspiratory neurones. Since cranial nerves other than the XIIn also show their own distinctive discharge pattern (Fukuda & Honda, 1982a,b, 1983; Sica et al. 1984), some of the XIIn-type neurones might be related to other cranial nerves. In addition, some others may be related to the inspiratory neurones that are hypothesized, with their firing preceding the inspiratory phase, to contribute to the expiratory to inspiratory phase change process (Connelly et al. 1992; Sun et al. 1998; Ballanyi et al. 1999).
Interaction between inspiration and swallowing
In the previous studies we suggested the presence of inhibitory interaction between the decoupled XIIn activity and swallowing activity (Saito et al. 2002b), which is similar to the interaction between the overt inspiratory activity and swallowing activity (Saito et al. 2002a). Such interaction has been revealed in the firing pattern of the present DRG inspiratory neurones. It is characteristic that the majority of type 2 neurones are inhibited at swallowing bursts and many of them are XIIn-type neurones. This may suggest that the XIIn-type inspiratory neurones do not function during swallowing although they function as premotor neurones of XIIn motoneurones during respiration. These facts suggest a segregation of the two populations of XIIn premotor neurones that provide the respiratory and swallowing activities.
The fact that the ratio of neurones that fire between XIIn swallowing bursts is higher in XIIn-type than in PHRn-type neurones is consistent with the firing pattern of BOT E-AUG neurones during swallowing. These latter neurones fire between XIIn swallowing bursts in rats (Y. Saito, K. Ezure & I. Tanaka, unpublished observations), in a manner similar to that seen in cats (Oku et al. 1994). That is, XIIn-type inspiratory neurones, which are not inhibited by BOT E-AUG neurones, can fire between XIIn swallowing bursts. The mutual inhibition between inspiration and swallowing might be produced via XIIn-type inspiratory neurones. From this latter assumption, we hypothesize that the XIIn-type inspiratory neurones may act on the swallowing pattern generator, while the PHRn-type inspiratory neurones may be passively influenced by the same pattern generator. Further examination of the activities of respiratory neurones during the pre-I XIIn and decoupled XIIn activities and during swallowing may provide a more detailed view of the interaction between respiration and swallowing.
Expiratory to inspiratory phase change and influence from lung stretch receptors
Since the pre-I XIIn and the decoupled XIIn activities are greatly modified by inputs from lung stretch receptors, and since these phenomena are closely related to the expiratory to inspiratory phase change process, it is worth summarizing the relation between these XIIn activities and lung inflation in the following six points. (1) The overt inspiratory activities of the XIIn and the PHRn normally start at the nadir and stop at the rising phase of tracheal pressure. These correspond to the Hering-Breuer inflation and deflation reflexes (Cohen, 1979). That is, the waxing and waning of inputs from slowly adapting lung stretch receptors (SARs) may account for the phenomena (Cohen, 1979). (2) When XIIn and PHRn activities are dissociated by increased PEEP, the pre-I XIIn activity and the decoupled XIIn activity start at the nadir of tracheal pressure but the overt PHRn inspiratory activity starts at around the peak of tracheal pressure. This point indicates that SARs operate on the respiratory system primarily via XIIn-type inspiratory neurones. In addition, points (1) and (2) suggest that inputs from rapidly adapting lung stretch receptors (RARs), which facilitate inspiration (Coleridge & Coleridge, 1986; Ezure & Tanaka, 2000), may trigger the overt inspiratory phase. This idea is based on the fact that RARs normally active at lung deflation become activated at lung inflation during increased PEEP (Yu et al. 1987; Lipski et al. 1991; Ezure et al. 1998). (3) During maintained lung inflation, the decoupled XIIn activity is never evoked, because once started, XIIn activity augments gradually and always fuses into the overt XIIn inspiratory burst. This point is closely related to point (1) and indicates that phasic lung afferent inputs are necessary to inhibit the ongoing pre-I XIIn activity in contrast to the overt inspiratory activity, which can stop without such afferent inputs. (4) When a series of decoupled activities is evoked before the overt inspiratory bursts, the amplitude of the decoupled XIIn activity increases toward the end of the expiratory phase. This may arise from the fact that the threshold for the phase change from expiration to inspiration decreases with time (Cohen & Feldman, 1977). (5) The larger the pre-I XIIn activity, the steeper the onset slope of the following PHRn activity. This point might reflect the inhibitory rebound, i.e. the release from BOT E-AUG neurone-induced inhibition of PHRn-type inspiratory neurones, although the possibility also exists that PHRn motoneurones are already excited from a sub-population of XIIn-type neurones that project to the spinal cord. (6) Since the augmented pre-I XIIn activity is observed in vagotomized animals (Fukuda & Honda, 1982b; Peever et al. 2001), the dissociated XIIn and PHRn activities are generated not only by lung stretch receptors but also by other factors such as chemoreceptors (Fukuda & Honda, 1982b, 1983, 1988).
We put forward the following hypothesis for the neuronal mechanisms that take place at the expiratory to inspiratory phase change, which is compatible with the hypothesis that the neurones free from inhibition by BOT E-AUG neurones trigger the initiation of the expiratory to inspiratory transition (Ezure, 1990). At the late expiratory phase, activates in both BOT E-AUG neurones and XIIn-type inspiratory neurones develop gradually towards the expiration-inspiration transition. Some subtype of XIIn-type inspiratory neurones may be important for the expiratory to inspiratory phase change process, which generates the overt inspiratory bursts and triggers the neurone firing that inhibits BOT E-AUG neurones whose firing interferes with the inspiratory phase. Such neurones can be expiratory-inspiratory phase-spanning neurones (Cohen, 1969; Smith et al. 1990; Connelly et al. 1992; Sun et al. 1998; Ballanyi et al. 1999) or constant inspiratory neurones (Ezure et al. 1989; Ezure, 1990), which are located in the pre-BOT area and are possibly included in the present data. The sudden discharge of these inspiratory neurones may excite decrementing inspiratory neurones that in turn inhibit BOT E-AUG neurones and also PHRn-type inspiratory neurones. Both decrementing and constant inspiratory neurones are located predominantly in the pre-BOT area, inhibitory and excitatory, respectively (Ezure et al. 1989; Ezure, 1990), and may be involved in the critical process of the phase change from expiration to inspiration.
Correspondence between in vivo and in vitro studies
Some data obtained in neonatal in vitro preparations seem comparable to the present data and provide useful information about the neuronal organisation of the medullary respiratory network. Firstly, neonatal inspiratory neurones are classified into subtypes in the en bloc rat medulla (type-I, type-II and type-III; Onimaru et al. 1997) based on the differential timing of synaptic activity. The present XIIn-type neurones may correspond to type-I neurones which have pre-I activities whereas the PHRn-type neurones may correspond to type-II and type-III neurones which do not have pre-I activities. Secondly, type-III neurones are inhibited at the late expiratory phase possibly by ‘pre-I neurones’, a type of expiratory neurone that is active before and after the inspiratory phase (Onimaru et al. 1997). This resembles the assumed inhibition of PHRn-type neurones by BOT E-AUG neurones (see below). Thirdly, SAR afferents modulate the respiratory rhythm in vitro in neonatal rats in a similar manner to that seen in vivo in mature animals (Mellen & Feldman, 2001). Phasic lung inflations transform the firing patterns in various subsets of respiratory neurones in vitro and shorten the inspiratory phase similarly to the present results.
The firing pattern (Fig. 5) and distribution (Fig. 8) of BOT E-AUG neurones raise the possibility that the neonatal pre-I neurones (Onimaru et al. 1997) develop into E-AUG neurones of the mature BOT. Since pre-I neurones form a subset of expiratory neurones and are located in the rostral ventrolateral medulla rostral to the pre-BOT (Onimaru et al. 1997; Ballanyi et al. 1999), i.e. in the BOT (for definition, see Kanjhan et al. 1995), only E-AUG and E-DEC neurones in the adult BOT could correspond to neonatal pre-I neurones. However, in vitro experiments suggest that mature E-DEC neurones correspond to another subset of respiratory neurones, called ‘biphasic neurones’ (Mellen & Feldman, 2001). These neurones receive excitation from SARs in a manner similar to that of mature E-DEC neurones (Hayashi et al. 1996; Manabe & Ezure, 1988; Saito et al. 2002c). In fact during phasic lung inflations pre-I neurones resemble the firing pattern of the mature E-AUG neurones (Mellen & Feldman, 2001). On the other hand, some pre-I neurones are suggested to be excitatory (Onimaru et al. 1997). We cannot specify corresponding expiratory neurones in the mature BOT at present. However, if such pre-I neurones are not inhibited at the inspiratory phase (Onimaru et al. 1997; Ballanyi et al. 1999), they could correspond to expiratory-inspiratory phase-spanning neurones (Smith et al. 1990; Connelly et al. 1992; Sun et al. 1998) or XIIn-type inspiratory neurones, which may participate in the phase change from expiration to inspiration. Finally, however, it should be emphasized that at present the information about the correspondence between mature in vivo and neonatal in vitro neurones is largely speculative and more direct evidence is eagerly awaited.
Acknowledgments
We express our thanks to Ms Kishimoto for her technical assistance. This work was partly supported by a Grant-in-Aid for Scientific Research for K.E. and Y.S. from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
REFERENCES
- Amri M, Car A. Projections from the medullary swallowing center to the hypoglossal motor nuclei: a neuroanatomical and electrophysiological study in sheep. Brain Res. 1988;441:119–126. doi: 10.1016/0006-8993(88)91389-3. [DOI] [PubMed] [Google Scholar]
- Amri M, Car A, Roman C. Axonal branching of medullary swallowing neurones projecting on the trigeminal and hypoglossal motor nuclei: demonstration by electrophysiological and fluorescent double labeling techniques. Exp Brain Res. 1990;81:384–390. doi: 10.1007/BF00228130. [DOI] [PubMed] [Google Scholar]
- Arita H, Kogo N, Koshiya N. Morphological and physiological properties of caudal medullary expiratory neurons of the cat. Brain Res. 1987;401:258–266. doi: 10.1016/0006-8993(87)91410-7. [DOI] [PubMed] [Google Scholar]
- Ballanyi K, Onimaru H, Homma I. Respiratory network function in the isolated brainstem-spinal cord of newborn rats. Prog Neurobiol. 1999;59:583–634. doi: 10.1016/s0301-0082(99)00009-x. [DOI] [PubMed] [Google Scholar]
- Cohen MI. Discharge patterns of brain-stem respiratory neurones during Hering-Breuer reflex evoked by lung inflation. J Neurophysiol. 1969;32:356–374. doi: 10.1152/jn.1969.32.3.356. [DOI] [PubMed] [Google Scholar]
- Cohen MI. Phrenic and recurrent laryngeal discharge patterns and the Hering-Breuer reflex. Am J Physiol. 1975;228:1489–1496. doi: 10.1152/ajplegacy.1975.228.5.1489. [DOI] [PubMed] [Google Scholar]
- Cohen MI. Neurogenesis of respiratory rhythm in the mammal. Physiol Rev. 1979;59:1105–1173. doi: 10.1152/physrev.1979.59.4.1105. [DOI] [PubMed] [Google Scholar]
- Cohen MI, Feldman JL. Models of respiratory phase-switching. Fed Proc. 1977;36:2367–2374. [PubMed] [Google Scholar]
- Coleridge HM, Coleridge JCG. Reflexes evoked from tracheobronchial tree and lungs. In: Cherniack NS, Widdicombe JG, editors. Handbook of Physiology, section 3, The Respiratory System, Control of Breathing. II. Washington: American Physiological Society; 1986. pp. 395–429. [Google Scholar]
- Connelly CA, Dobbins EG, Feldman JL. Pre-Bötzinger complex in cats: respiratory neuronal discharge patterns. Brain Res. 1992;590:337–340. doi: 10.1016/0006-8993(92)91118-x. [DOI] [PubMed] [Google Scholar]
- Cunningham ET, Sawchenko PE. Dorsal medullary pathways subserving oromotor reflexes in the rat: implications for the central neural control of swallowing. J Comp Neurol. 2000;417:448–466. [PubMed] [Google Scholar]
- Ezure K. Synaptic connections between medullary respiratory neurons and considerations on the genesis of respiratory rhythm. Prog Neurobiol. 1990;35:429–450. doi: 10.1016/0301-0082(90)90030-k. [DOI] [PubMed] [Google Scholar]
- Ezure K, Manabe M, Otake K. Excitation and inhibition of medullary inspiratory neurons by two types of burst inspiratory neurons in the cat. Neurosci Lett. 1989;104:303–308. doi: 10.1016/0304-3940(89)90593-4. [DOI] [PubMed] [Google Scholar]
- Ezure K, Oku Y, Tanaka I. Location and projection of one type of swallowing interneurons in cat medulla. Brain Res. 1993;632:216–224. doi: 10.1016/0006-8993(93)91156-m. [DOI] [PubMed] [Google Scholar]
- Ezure K, Tanaka I. Lung inflation inhibits rapidly adapting receptor relay neurons in the rat. Neuroreport. 2000;11:1709–1712. doi: 10.1097/00001756-200006050-00023. [DOI] [PubMed] [Google Scholar]
- Ezure K, Tanaka I, Miyazaki M. Inspiratory inhibition of pulmonary rapidly adapting receptor relay neurons in the rat. Neurosci Lett. 1998;258:49–52. doi: 10.1016/s0304-3940(98)00858-1. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Cohen MI. Relation between expiratory duration and rostral medullary expiratory neuronal discharge. Brain Res. 1978;141:172–178. doi: 10.1016/0006-8993(78)90627-3. [DOI] [PubMed] [Google Scholar]
- Fukuda Y, Honda Y. Differences in respiratory neural activities between vagal (superior laryngeal), hypoglossal and phrenic nerves in the anesthetized rat. Jpn J Physiol. 1982a;32:387–398. doi: 10.2170/jjphysiol.32.387. [DOI] [PubMed] [Google Scholar]
- Fukuda Y, Honda Y. Roles of vagal afferents on discharge patterns and CO2-responsiveness of efferent superior laryngeal, hypoglossal, and phrenic respiratory activities in anesthetized rats. Jpn J Physiol. 1982b;32:689–698. doi: 10.2170/jjphysiol.32.689. [DOI] [PubMed] [Google Scholar]
- Fukuda Y, Honda Y. Effects of hypocapnia on respiratory timing and inspiratory activities of the superior laryngeal, hypoglossal, and phrenic nerves in the vagotomized rat. Jpn J Physiol. 1983;33:733–742. doi: 10.2170/jjphysiol.33.733. [DOI] [PubMed] [Google Scholar]
- Fukuda Y, Honda Y. Modification by chemical stimuli of temporal difference in the onset of inspiratory activity between vagal (superior laryngeal) or hypoglossal and phrenic nerves of the rat. Jpn J Physiol. 1988;38:309–319. doi: 10.2170/jjphysiol.38.309. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Coles SK, McCrimmon DR. Respiratory neurons mediating the Breuer-Hering reflex prolongation of expiration in rat. J Neurosci. 1996;16:6526–6536. doi: 10.1523/JNEUROSCI.16-20-06526.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J-C, St John WM. Alterations of hypoglossal motoneuronal activities during pulmonary inflations. Exp Neurol. 1987;97:615–625. doi: 10.1016/0014-4886(87)90118-x. [DOI] [PubMed] [Google Scholar]
- Iscoe S. Control of abdominal muscles. Prog Neurobiol. 1998;56:433–506. doi: 10.1016/s0301-0082(98)00046-x. [DOI] [PubMed] [Google Scholar]
- Jiang C, Lipski J. Extensive monosynaptic inhibition of ventral respiratory group neurons by augmenting neurons in the Bötzinger complex in the cat. Exp Brain Res. 1990;81:639–648. doi: 10.1007/BF02423514. [DOI] [PubMed] [Google Scholar]
- Kanjhan R, Lipski J, Kruszewska B, Rong W. A comparative study of pre-sympathetic and Bötzinger neurons in the rostral ventrolateral medulla (RVLM) of the rat. Brain Res. 1995;699:19–32. doi: 10.1016/0006-8993(95)00814-7. [DOI] [PubMed] [Google Scholar]
- Kirkwood PA, Sears TA. Monosynaptic excitation of thoracic expiratory motoneurones from lateral respiratory neurones in the medulla of the cat. J Physiol. 1973;234:87–89P. [PubMed] [Google Scholar]
- Kubin L, Lipski J. Properties of the rostral NRA expiratory neurones projecting to the contralateral NTS group. Neurosci Lett Suppl. 1980;5:S141. [Google Scholar]
- Lipski J, Ezure K, Wong She RB. Identification of neurons receiving input from pulmonary rapidly adapting receptors in the cat. J Physiol. 1991;443:55–77. doi: 10.1113/jphysiol.1991.sp018822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipski J, Zhang X, Kruszewska B, Kanjhan R. Morphological study of long axonal projections of ventral medullary inspiratory neurons in the rat. Brain Res. 1994;640:171–184. doi: 10.1016/0006-8993(94)91871-6. [DOI] [PubMed] [Google Scholar]
- Manabe M, Ezure K. Decrementing expiratory neurons of the Bötzinger complex. I. Response to lung inflation and axonal projection. Exp Brain Res. 1988;72:150–158. doi: 10.1007/BF00248510. [DOI] [PubMed] [Google Scholar]
- Mellen NM, Feldman JL. Phasic vagal sensory feedback transforms respiratory neuron activity in vitro. J Neurosci. 2001;21:7363–7371. doi: 10.1523/JNEUROSCI.21-18-07363.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oku Y, Tanaka I, Ezure K. Activity of bulbar respiratory neurons during fictive coughing and swallowing in the decerebrate cat. J Physiol. 1994;480:309–324. doi: 10.1113/jphysiol.1994.sp020361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Arata A, Homma I. Neuronal mechanisms of respiratory rhythm generation: an approach using in vitro preparation. Jpn J Physiol. 1997;47:385–403. doi: 10.2170/jjphysiol.47.385. [DOI] [PubMed] [Google Scholar]
- Ono T, Ishikawa Y, Kuroda T, Nakamura Y. Swallowing-related perihypoglossal neurons projecting to hypoglossal motoneurons in the cat. J Dent Res. 1998;77:351–360. doi: 10.1177/00220345980770020301. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. Orlando, FL, USA: Academic Press; 1998. [Google Scholar]
- Peever JH, Mateika JH, Duffin J. Respiratory control of hypoglossal motoneurons in the rat. Euro J Physiol. 2001;442:78–86. doi: 10.1007/s004240000502. [DOI] [PubMed] [Google Scholar]
- Peever JH, Shen L, Duffin J. Respiratory pre-motor control of hypoglossal motoneurons in the rat. Neurosci. 2002;110:711–722. doi: 10.1016/s0306-4522(01)00594-2. [DOI] [PubMed] [Google Scholar]
- Richter DW, Ballantyne D, Remmers JE. How is the respiratory rhythm generated? A model. News Physiol Sci. 1986;1:109–112. [Google Scholar]
- Saito Y, Ezure K, Tanaka I. Swallowing-related activities of respiratory and non-respiratory neurones in the nucleus of solitary tract in the rat. J Physiol. 2002a;540:1047–1060. doi: 10.1113/jphysiol.2001.014985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Ezure K, Tanaka I. Difference between hypoglossal and phrenic activities during lung inflation and swallowing in the rat. J Physiol. 2002b;541:183–193. doi: 10.1113/jphysiol.2002.022566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Tanaka I, Ezure K. Morphology of the decrementing expiratory neurons in the brainstem of the rat. Neurosci Res. 2002c;44:141–153. doi: 10.1016/s0168-0102(02)00095-0. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Stornetta RL, Guyenet PG. Evidence for glycinergic respiratory neurons: Bötzinger neurons express mRNA for glycinergic transporter 2. J Comp Neurol. 1999;407:583–597. doi: 10.1002/(sici)1096-9861(19990517)407:4<583::aid-cne8>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Schwarzacher SW, Wilhelm Z, Anders K, Richter DW. The medullary respiratory network in the rat. J Physiol. 1991;435:631–644. doi: 10.1113/jphysiol.1991.sp018529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sica AL, Cohen MI, Donnelly DF, Zhang H. Hypoglossal motoneuron responses to pulmonary and superior laryngeal afferent inputs. Respir Physiol. 1984;56:339–357. doi: 10.1016/0034-5687(84)90069-0. [DOI] [PubMed] [Google Scholar]
- Smith JC, Greer JJ, Liu G, Feldman JL. Neural mechanisms generating respiratory pattern in mammalian brain-stem cord in vitro. I. Spatiotemporal patterns of motor and medullary neuron activity. J Neurophysiol. 1990;64:1149–1169. doi: 10.1152/jn.1990.64.4.1149. [DOI] [PubMed] [Google Scholar]
- Sun Q-J, Goodchild AK, Chalmers JP, Pilowsky PM. The pre-Bötzinger complex and phase-spanning neurons in the adult rat. Brain Res. 1998;809:204–213. doi: 10.1016/s0006-8993(98)00872-5. [DOI] [PubMed] [Google Scholar]
- Tian G-F, Duffin J. Spinal connections of ventral-group bulbospinal inspiratory neurons studied with cross-correlation in the decerebrate rat. Exp Brain Res. 1996;111:178–186. doi: 10.1007/BF00227296. [DOI] [PubMed] [Google Scholar]
- Tian G-F, Peever JH, Duffin J. Bötzinger-complex, bulbospinal expiratory neurones monosynaptically inhibit ventral-group respiratory neurones in the decerebrate rat. Exp Brain Res. 1999;124:173–180. doi: 10.1007/s002210050612. [DOI] [PubMed] [Google Scholar]
- Yu J, Coleridge JCG, Coleridge HM. Influence of lung stiffness on rapidly adapting receptors in rabbits and cats. Respir Physiol. 1987;68:161–176. doi: 10.1016/s0034-5687(87)80003-8. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Barillot JC, Bianchi AL. Are the post-inspiratory neurons in the decerebrate rat cranial motoneurons or interneurons? Brain Res. 1991;551:256–266. doi: 10.1016/0006-8993(91)90940-w. [DOI] [PubMed] [Google Scholar]


