Abstract
High frequency electrical stimulation of deep brain structures (DBS) has been effective at controlling abnormal neuronal activity in Parkinson's patients and is now being applied for the treatment of pharmacologically intractable epilepsy. The mechanisms underlying the therapeutic effects of DBS are unknown. In particular, the effect of the electrical stimulation on neuronal firing remains poorly understood. Previous reports have showed that uniform electric fields with both AC (continuous sinusoidal) or DC waveforms could suppress epileptiform activity in vitro. In the present study, we tested the effects of monopolar electrode stimulation and low-duty cycle AC stimulation protocols, which more closely approximate those used clinically, on three in vitro epilepsy models. Continuous sinusoidal stimulation, 50 % duty-cycle sinusoidal stimulation, and low (1.68 %) duty-cycle pulsed stimulation (120 μs, 140 Hz) could completely suppress spontaneous low-Ca2+ epileptiform activity with average thresholds of 71.11 ± 26.16 μA, 93.33 ± 12.58 μA and 300 ± 100 μA, respectively. Continuous sinusoidal stimulation could also completely suppress picrotoxin- and high-K+-induced epileptiform activity with either uniform or localized fields. The suppression generated by the monopolar electrode was localized to a region surrounding the stimulation electrode. Potassium concentration and transmembrane potential recordings showed that AC stimulation was associated with an increase in extracellular potassium concentration and neuronal depolarization block; AC stimulation efficacy was not orientation-selective. In contrast, DC stimulation blocked activity by membrane hyperpolarization and was orientation-selective, but had a lower threshold for suppression.
Intractable seizures in human epilepsy are, by definition, resistant to pharmacological therapy. Although surgical resection of the seizure focus is a potential treatment option in some patients, ablation of neuronal tissue is irreversible and can be associated with serious complications. A reversible and adjustable alternative to resection, deep brain electrical stimulation (DBS) of discrete brain structures such as the cerebellum (Cooper et al. 1973), locus coeruleus (Faber & Vladyka, 1983) and thalamic centromedian nucleus (Velasco et al. 2000), has been effective in alleviating seizures in humans. However, the mechanisms of DBS remain unknown and stimulation protocols are, therefore, determined empirically and not optimized to modulate any specific physiological parameter.
In vitro, constant DC electric fields have been used to suppress high-K+- (Gluckman et al. 1996) and low-Ca2+- (Ghai, 1998; Warren & Durand, 1998) induced epileptiform bursting by directly hyperpolarizing pyramidal neurons. However, the efficiency of the suppression is highly dependent on the orientation of the exogenous electric field relative to the dendritic-somatic axis of the neuron (Ghai et al. 2000). Furthermore, DC stimulation, because it is charge unbalanced, can result in electrode corrosion and tissue damage (Agnew & McCreery, 1990a; Gluckman et al. 2001). We recently demonstrated that 50 Hz continuous sinusoidal electric fields, applied via large parallel wire ‘field’ electrodes (which uniformly stimulate the entire encompassed brain tissue), could suppress epileptiform activity in vitro (Bikson et al. 2001).
The goals of the present study were: (1) to determine the feasibility of local suppression of epileptiform activity using AC fields applied with monopolar electrodes, which more closely approximate field distributions generated in clinical DBS; (2) to test the inhibitory effects of low-duty cycle and clinically relevant stimulation protocols; and (3) to directly compare the efficacy and clinical practicality of locally applied DC versus AC fields. The mechanisms, spatial extent, and orientation dependence of local AC and DC stimulation protocols were studied using extracellular, intracellular and ion-sensitive recordings. The results of this report demonstrate that clinically relevant stimulation configurations and protocols can suppress neuronal activity by inducing depolarization block, mediated in part by local extracellular potassium accumulation.
METHODS
Preparation of hippocampal slices and perfusion
All experiments were performed in the CA1 or CA3 regions of hippocampal brain slices prepared from Sprague-Dawley rats (175–250 g). Rats were anaesthetized with ethyl ether and decapitated. The experimental protocol was reviewed and approved by the Institution Animal Care and Use Committee. The brain was rapidly removed and one hemisphere glued to the stage of a Vibroslicer (Vibroslice, Campden Instruments Ltd, London, UK) Slicing was carried out in cold (3–4 °C), oxygenated sucrose-based artificial cerebrospinal fluid (ACSF) consisting of (mm): sucrose 220, KCl 3, NaH2PO4 1.25, MgSO4 2, NaHCO3 26, CaCl2 2, dextrose 10. The resulting 350 μm thick slices were immediately transferred to a holding chamber with ‘normal’ ACSF consisting of (mm): NaCl 124, KCl 3.75, KH2PO4 1.25, CaCl2 2, MgSO4 2, NaHCO3 26, dextrose 10, held at room temperature and bubbled with 95 % O2-5 % CO2.
After > 1 h of recovery, a slice was transferred to a standard interface recording chamber with ‘normal’ ACSF at 35 ± 0.5 °C, and a warmed, humidified 95 % O2-5 % CO2 vapour maintained over the exposed surface of the slice. After 10 min, slices were perfused with ‘low calcium’ ACSF (low-Ca2+), ‘high potassium’ ACSF (high-K+), or picrotoxin-containing ACSF. Low-Ca2+ ACSF consisted of (mm): NaCl 124, KCl 4.75, KH2PO4 1.25, CaCl2 0.2, MgSO4 1.5, NaHCO3 26, dextrose 10.0. High-K+ ACSF consisted of (mm): NaCl 124, KCl 8.5, NaH2PO4 1.25, CaCl2 1.5, MgSO4 1.5, NaHCO3 26, dextrose 10.0. Picrotoxin ACSF was made by adding 100 μm picrotoxin to normal ACSF (with 0.5 mm MgSO4). Prolonged incubation (up to 60 min) in these solutions resulted in spontaneous epileptiform activity in the CA1 or CA3 regions of the hippocampus.
Generation and application of local and uniform electric fields
‘Local’ current was delivered to the tissue via a tungsten monopolar electrode (Warren & Durand, 1998). Unless otherwise stated, the recording and stimulation electrodes were positioned in the stratum pyramidale, ∼50 μm apart, such that injected anodal current resulted in a hyperpolarization of pyramidal cell somas (Warren & Durand, 1998). ‘Uniform’ electric fields were generated across individual slices by passing current between two parallel AgCl-coated silver wires placed on the surface of the ACSF in the interface chamber (Ghai, 1998). AC stimulation was continuous sinusoidal 50 Hz current with zero DC offset unless otherwise specified. The waveforms applied to the electrode were generated by a function generator (Wavetek Arbitrary Waveform Generator 395, Wavetek Wandel Goltermann, Swabia Court Triangle Park, NC, USA), and converted to a current by a stimulus isolation unit (A-M Systems analog isolator 2200, A-M Systems, Inc., Carlsborg, WA, USA).
Electrical recording and data analysis
Extracellular recordings were made with 150 mm NaCl-filled glass microelectrodes (5–10 MΩ). The recordings were obtained from the stratum pyramidale in the CA1/CA3 area. Intracellular recordings were obtained with sharp glass micropipettes (50–100 MΩ) filled with 2 m potassium acetate. Potassium-selective microelectrodes were constructed using established methods described elsewhere (Amman, 1986). We utilized N,N-dimethyltrimethylsilylamine (Fluka Chemicals, Buchs, Switzerland) to silanize the electrode tips, and the Fluka 60398 potassium-selective membrane solution, which contains the potassium ionophore valinomycin. The potassium-selective microelectrodes were filled with 150 mm KCl. Electrodes were calibrated in 0.1, 1, 10 and 100 mm KCl with the separate solution method (Amman, 1986). Only electrodes of 95 % Nernstian slope and time constant, τ, < 200 ms were used. Electrodes were at least 1000-fold more selective to potassium than sodium. During intracellular and potassium measurements, the voltage recorded by a field electrode placed in the somatic layer (within 100 μm of the sharp/potassium-selective electrode) was subtracted to remove the field artifact and provide a true transmembrane or potassium concentration measurement.
All signals were amplified with an Axoprobe-1A amplifier (Axon Instruments), low-pass filtered (10 Hz field and potassium, 1 kHz sharp) with an FLA-01 (Cygnus Technology, Inc., Delaware Water Gap, PA, USA), and stored on videotape via an A/D converter (Sony PCM-501ES). Results are reported as means ±s.d.; n represents the number of slices.
RESULTS
Effect of localized 50 Hz sinusoidal electrical stimulation on low-Ca2+-, picrotoxin- and high-K+-induced epileptiform activity
The effect of 50 Hz local sinusoidal currents generated by a monopolar electrode on both synaptic (picrotoxin and high-K+) and non-synaptic (low-Ca2+) epileptiform activity was tested. ‘Threshold’ stimulation amplitude was defined as the minimum field size required to completely suppress bursting for the duration of the stimulus. Fig. 1A shows an example of the effect of suprathreshold electric fields on spontaneous low-Ca2+ activity in the CA1 region. Suprathreshold stimulation blocked bursting for the duration of the stimulus (1 min) and for up to 2 min after termination of stimulation (71.11 ± 26.16 μA, n= 21). The stimulation also resulted in a 1–5 mV negative shift in the baseline field potential. There was no long-term effect on low-Ca2+ burst amplitude, duration, or frequency observed at any stimulus intensity or duration, suggesting that the stimulation did not induce tissue damage.
Figure 1. Suppression of synaptic and non-synaptic epileptiform activity by AC electric fields.
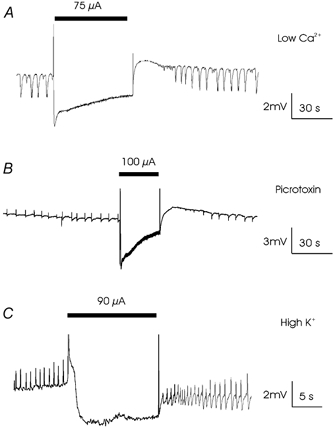
Effect of suprathreshold 50 Hz sinusoidal fields on spontaneous activity induced by perfusion of slices with low-Ca2+ ACSF (A), picrotoxin ACSF (B) or high-K+ ACSF (C). In this and all subsequent figures the bar indicates the duration of electric field application.
Suprathreshold stimulation also blocked picrotoxin-induced bursting for the duration of the stimulus (30 s) and for up to 2 min after termination of stimulation (65.32 ± 25.81 μA, n= 11) and caused a 1–5 mV shift in the field potential (Fig. 1B). Figure 1C shows an example of the effect of suprathreshold applied current on spontaneous high-K+ bursting in the CA3 region. Suprathreshold stimulation blocked bursting for the duration of the stimulus (10 s, 88.33 ± 20.21 μA, n= 3). As above, application of suprathreshold fields was always associated with a 1–5 mV negative shift in the field potential.
Effects of low-duty cycle waveforms on suppression
We modified the 50 Hz sinusoidal stimulation protocol to be 1 s-on and 1 s-off and found low calcium-induced epileptiform activity could still be completely suppressed by this 50 % duty cycle waveform (93.33 ± 12.58 μA, n= 3). The characteristics of during-stimulus negative shift and after-stimulus refractory period were similar to those generated by 100 % duty cycle stimulation (Fig. 2A). A biphasic pulse train (120 μs, 140 Hz, duty cycle 1.68 %) used clinically (Schachter & Saper, 1998) was also effective in blocking epileptiform activity (300 ± 100 μA, n= 3). The negative potential shift during the stimulation and post-stimulus refractory period was similar to the phenomena observed during 100 % duty cycle sinusoidal stimulation (Fig. 2B).
Figure 2. Suppression of low calcium-induced epileptiform activity by low-duty cycle waveforms.
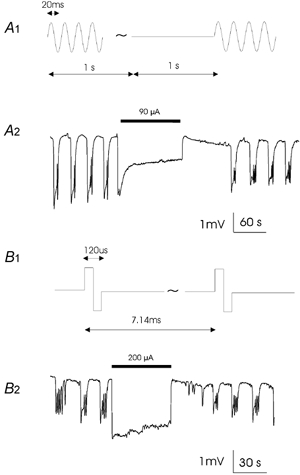
A, suppression of epileptiform activity (A2) by the stimulation using a 50 Hz sinusoidal field (A1) with 50 % duty cycle (1 s-on and 1 s-off). B, suppression of epileptiform activity (B2) by the stimulation using a biphasic train (B1) used clinically (120 s, 140 Hz, duty cycle 1.68 %).
Extracellular potassium concentration and transmembrane potential during AC and DC suppression
In order to compare the underlying mechanism of AC and DC suppression of epileptiform activity, we measured both extracellular potassium concentration and the intracellular voltage during local AC and DC stimulation. Consistent with data obtained using field stimulation (Bikson et al. 2001), suppression of low-Ca2+ activity with AC stimulation (Fig. 3A1) was always associated with a substantial increase (2.5 mm± 0.5, n= 5) in extracellular potassium concentration (Fig. 3A2). Termination of the stimulus was followed by a transient decrease in extracellular potassium concentration below the baseline level, during which activity was also suppressed. Bursting resumed as extracellular potassium levels returned to baseline. Similar results were obtained with experiments performed in both picrotoxin and potassium preparations (data not shown).
Figure 3. Extracellular potassium recordings during AC and DC stimulation.
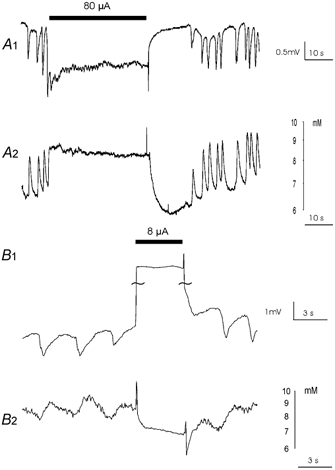
A1, low Ca2+ epileptiform activity was suppressed by a locally applied suprathreshold AC field. A2, the stimulation resulted in a transient increase in extracellular potassium. B1, inhibition of epileptiform activity by a DC pulse. B2, the potassium concentration was maintained at its lowest during the application of DC electric field.
Short DC anodal pulses (3–5 s) also completely blocked the low-Ca2+ activity (Fig. 3B1, 3.78 ± 2.54 μA, n= 7). In this case, the large step increase induced in the field potential was due to a ‘stimulus artifact’ rather than an endogenously generated field shift. Epileptiform activity returned immediately after the termination of DC stimulation. Consistent with previous data using field stimulation (Ghai et al. 2000), the potassium wave was also completely abolished when the voltage events were suppressed (Fig. 3B2, n= 3). Similar results were observed in picrotoxin- and potassium-induced activities (data not shown).
We investigated the effect of AC and DC stimulation on membrane potential with sharp electrode intracellular recording. Consistent with data using field stimulation (Bikson et al. 2001), during picrotoxin-induced bursting, suprathreshold local AC stimulation caused a tonic depolarization (21 mV ± 5, n= 4) in the membrane potential (Fig. 4A1). The tonic depolarization was seen clearly after a notch filter (45–55 Hz) was used to remove the 50 Hz stimulation artifact (Fig. 4A2). No action potentials were observed during suprathreshold AC stimulation. DC stimulation also suppressed action potentials but generated hyperpolarization (27 mV ± 5, n= 3) of the membrane potential (Fig. 4B).
Figure 4. Transmembrane potential recordings during AC and DC stimulation.
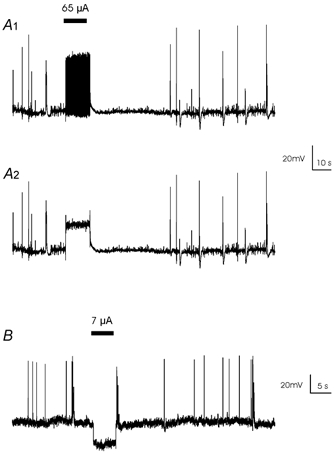
A1, transmembrane potential during the application of AC electric fields. A2, removal of a 50 Hz artifact through a notch filter shows that during field application the neuron was depolarized. B, transmembrane potential recording shows neurons were hyperpolarized during the application of an anodic DC pulse.
Spatial selectivity of suppression using uniform and local stimulation
In contrast to uniform electric fields, the electric field generated by a point source attenuates with distance and thus the threshold for suppression should increase with distance. The spatial extent of uniform (global) AC electric field- and localized AC current injection-induced suppression of epileptiform activity was compared in the picrotoxin model. Spontaneous activity was monitored in two sites with field electrodes separated by 500 μm. When a uniform electric field was applied across the slice using parallel wire electrodes, epileptiform activity was suppressed in both sites by a suprathreshold field (Fig. 5Aa1 and 2, 160 ± 36 mV mm−1, n= 5). In contrast, when 50 Hz sinusoidal current was injected locally through a monopolar electrode, activity was successfully blocked in the region close to the stimulation site (Fig. 5Bb2), but was not completely suppressed in the remote region (Fig. 5Bb1).
Figure 5. Effect of globally and locally applied electric fields on the suppression.
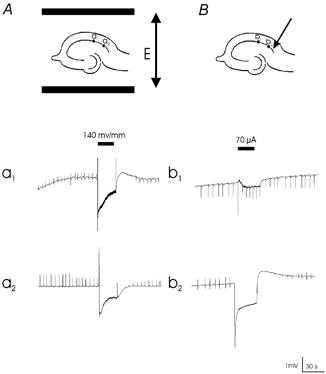
An electric field (E) was generated either by a pair of parallel wire electrodes (A) or by a monopolar electrode (B). Two field electrodes were separated by 500 μm. A, picrotoxin-induced epileptiform activity was blocked in both sites (a1 and a2). B, stimulation blocked bursting recorded by the proximal electrode (b2), but failed to completely block activity recorded by the distal electrode (b1).
The spatial selectivity of both AC and DC local stimulation was quantified by placing the monopolar electrode at progressively larger distances from the recording electrode in the CA1 region (Fig. 6A). The mean threshold for suppression of low calcium activity using either AC or DC stimulation current was measured at 50 μm, 167 μm, 333 μm and 500 μm (Fig. 6B and C). As was expected for both AC and DC stimulation, suppression threshold increased with distance. Both curves could be fitted by a polynomial function. ANOVA tests showed that for both AC and DC suppression, thresholds were significantly dependent on distances from the recording electrode (P < 0.01, Sokal & Rohlf, 1981). When distance was increased from 50 μm to 500 μm, the threshold for DC stimulation increased by 170 ± 149 % while AC stimulation threshold increased by 222 ± 141 % (n= 15), indicating that AC stimulation was more localized than DC stimulation.
Figure 6. Dependence of the threshold of AC and DC electric field to block activity on distance.
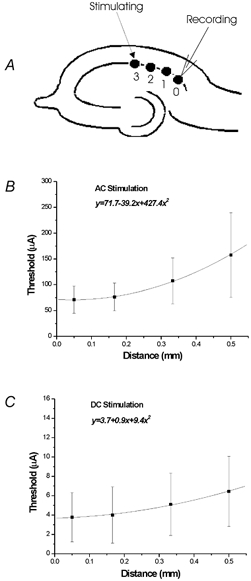
A, recording electrode was positioned in the CA1a of the hippocampus and the stimulation electrode was moved further away gradually. The threshold was determined for AC (B) and DC stimulation (C) at distances of 50, 167, 333 and 500 μm.
Effect of stimulating electrode location on suppression of epileptiform activity
To test the effect of the electrode location on blocking threshold, the monopolar electrode was placed either in the alveus or the stratum radiatum in slices exposed to low-Ca2+ solution. The distance from the recording site at pyramidal cell layer was identical for both stimulation locations (Fig. 7A). No significant threshold difference was found for AC stimulation (Fig. 7B, n= 9, P < 0.01). Anodal DC currents delivered in the stratum radiatum were not able to suppress activity, even at 250 % of amplitudes effective during alveus stimulation (Fig. 7C, n= 9). These data show that AC stimulation does not depend on the relative location of the electrode along the dendritic-somatic axis, while DC stimulation is highly orientation-dependent.
Figure 7. Effect of field orientation on suppression threshold.
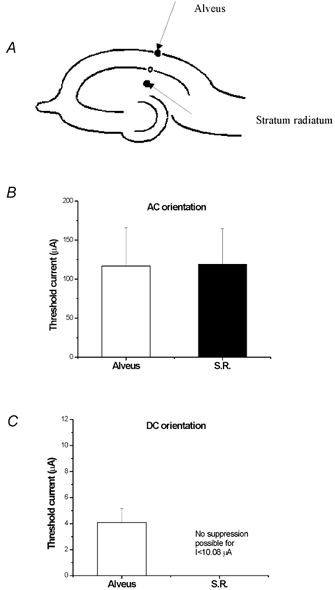
A, AC and DC electric fields were delivered through an electrode placed either in the alveus or the stratum radiatum. B, for the AC electric field, the threshold of suppressing activity using alveur stimulation was not significantly different from that using stratum radiatum (S.R.) stimulation. C, for the DC electric field, the epileptiform activity could only be blocked by alveur stimulation. It could not be blocked by an anodic pulse delivered at the stratum radiatum even if the applied current was 250 % of the effective one applied at the alveus.
DISCUSSION
Comparison between AC and DC stimulation for the suppression of epileptiform activity - mechanism and clinical relevance
Low-amplitude DC electric fields are effective in suppressing epileptiform activity in several in vitro models. The suppression thresholds are far below the values required to produce excitation (Warren & Durand, 1998), i.e. trigger neuronal firing. The inhibition mechanism is well established as membrane hyperpolarization (Durand & Bikson, 2001). There are several drawbacks associated with DC stimulation: (1) even at low current, erosion and electrochemical damage occur during continuous DC stimuli due to unbalanced charge (Brummer & Turner, 1977); (2) termination of DC stimulation generates immediate ‘excitation rebound’ of epileptiform activity, (Ghai et al. 2000) and (3) the suppression efficiency is highly dependent on the relative orientation between the electric field and the somatic-dendrite axis (Fig. 7; Jefferys, 1981; Chan et al. 1988; Gluckman et al. 1996; Durand & Bikson 2001) Thus, in vivo, the intended neuronal target must be anatomically uniform for DC stimulation to be effective. Moreover, inhibition (by hyperpolarization) could be changed into excitation (by depolarization) if the implanted electrode was slightly displaced.
AC stimulation is also highly effective at suppressing neuronal activity in various models of epilepsy in vitro and has several advantages over DC stimulation including: (1) termination of AC stimulation is often associated with a prolonged disruption of epileptiform activity (compare Fig. 3A with 3B and Fig. 4A with 4B); and (2) AC stimulation is not orientation-dependent (Fig. 7).
DBS, which uses exclusively AC stimulation, has been used to suppress seizure activity in humans (Cooper et al. 1973; Faber & Vladyka, 1983; Velasco et al. 2000). Although AC stimulation protocols require higher currents than DC stimulation (see above), DBS has been proven to be safe and well tolerated in patients with epilepsy (Fisher et al. 1992). Paradoxically, the clinical effect of high frequency stimulation of a targeted structure mimics the effect of lesioning the structure, which suggests that high frequency stimulation is inhibiting neuronal firing (Benazzouz et al. 1995).
It is well established that high frequency (AC) stimulation of excitable tissue results in extracellular potassium accumulation (Gardner-Medwin, 1983a,b; Gardner-Medwin & Nicholson, 1983; Bawin et al. 1986; Poolos et al. 1987; Kaila et al. 1997). This increase can, in turn, depolarize neurons sufficiently to tonically inactivate Na+ channels such that action potentials cannot be initiated (Traub et al. 1991; Hille 1992). While small increases in potassium can promote epileptiform activity, large increases could thus suppress spontaneous bursting (Rutecki et al. 1985). Consistent with our previous report using uniform electric fields (Bikson et al. 2001) the present study shows that local suppression of epileptiform activity in vitro with monopolar AC stimulation is always associated with a large increase in extracellular potassium and ‘depolarization block’ of neurons. Moreover, post-stimulus suppression is linked to an undershoot of potassium below baseline levels.
Are potassium transients sufficient to induce depolarization block? Under normal conditions a tonic depolarization of ∼20 mV will suppress regenerative action potentials in most classes of neurons (Hille, 1992). Potassium-selective microelectrodes measure an average potassium concentration at the interface of the bath and slice surface, and so underestimate the potassium concentration at the surface of the cells during endogenous potassium release. Current clamp (Takahashi & Tsuruhara, 1987; Traynelis & Dingledine, 1988) and voltage clamp (Chvatal et al. 1999) recordings from glial cells, as well as modelling studies (Hounsgaard & Nicholson, 1983), approximate this underestimate at 25–75 %. Thus, in this study, the 2 mm increase measured by potassium-selective electrodes during suprathreshold stimulation could reflect a ∼6 mm rise at the surface of cells which would result in depolarization block. Moreover, pro-convulsant conditions can significantly reduce the level of depolarization required for action potential block (Hahn & Durand, 2001). Potassium levels in the brain during normal function, though not during seizures (Pumain et al. 1985; Jefferys, 1995), are lower than those used in the present report. Induction of depolarization block at ‘normal’ potassium levels would presumably require high stimulation intensities. Lastly, because in vitro the bath fluid can serve as a potassium sink, larger potassium rises might be expected in vivo (Krnjevic et al. 1980; Pumain et al. 1985; Jefferys, 1995).
Alternatively, other factors could contribute to depolarization block of neurons in response to high frequency stimulation. Stimulus-induced reductions in extracellular Ca2+ (Krnjevic et al. 1980) would depolarize neurons and shift the activation characteristics of ion channels to facilitate depolarization block at lower potentials. However, Ca2+ reductions are not sufficient in themselves to induce depolarization block, since epileptiform activity persists under reduced Ca2+ conditions. Stimulus-induced increases in intracellular K+ would depolarize neurons while large increases in intracellular Cl− could result in ‘depolarizing GABA’ (Bragin et al. 1999). The latter would promote further extracellular potassium release, though could not play a role during suppression of low-Ca2+ or picrotoxin activity. Further studies are required to determine the contribution of each of these factors in inducing depolarization block. There is no evidence suggesting these other factors are sufficient in themselves to induce depolarization block, however, they would all be expected to: (1) promote extracellular potassium accumulation and (2) reduce the level of extracellular potassium required to induce depolarization block.
Although extracellular potassium accumulation and intracellular recordings during DBS are currently not available, clinical high frequency stimulation would be expected to induce large extracellular potassium transients (Krnjevic et al. 1980) which, in turn, would have a dramatic effect on neuronal function. Our results suggest that DBS may suppress neuronal firing (mimicking the effect of a lesion), and hence the symptoms of epilepsy and Parkinson's disease, by a depolarization block mechanism mediated, at least in part, by a potassium rise.
Comparison between uniform and local stimulation for the block of epileptiform activity - clinical relevance
Stimulation using both large parallel (uniform field) electrodes and monopolar electrodes is effective in suppressing epileptiform activity. Uniform electric fields affect the entire tissue between the electrodes and, in vitro, do not have to be in direct contact with the targeted tissue, thereby minimizing the electrochemical damage (Durand & Bikson, 2001). While this method is convenient in a brain slice preparation, clinical implementation might be difficult because of the anatomical constraints of placing two large electrodes across a targeted structure. In addition, in vivo, the field electrodes would inevitably be in contact with nervous tissue. In contrast, monopolar electrodes are more selective (Fig. 5) and could be placed directly within a seizure focus.
Low-duty cycle stimulation and clinical relevance
In clinical practice, low-duty cycle stimulation protocols are used mainly to minimize tissue damage and improve battery efficiency (Agnew & McCreery, 1990b; Benabid et al. 2000). In the present study, low-duty cycle (1.68–50 %) AC fields were capable of completely suppressing epileptiform activity in all slices in which 100 % duty cycle was also effective. Moreover, lowering the duty cycle from 100 % to 50 % resulted in only a small increase in suppression threshold (far less than double) and a suppression protocol used clinically with 1.68 % duty cycle resulted in only ∼5-fold increase in threshold. Thus, the results of this study suggest that a lower-duty cycle stimulation protocol could effectively suppress activity with less charge delivered.
In conclusion, our results show that localized AC stimulation can suppress epileptiform activity in a localized region in vitro. The effective duty cycle and frequency are comparable with those used in clinical DBS (Loddenkemper et al. 2001). Compared with DC stimulation, AC stimulation was not orientation-dependent, was associated with extracellular potassium accumulation, and was more spatially selective. DC suppression is generated as a result of membrane hyperpolarization while AC suppression results from depolarization block. The results are consistent with the hypothesis that neuronal suppression by DBS is due to a depolarization block of neurons (partially) as a result of raised extracellular potassium concentration.
Acknowledgments
We thank Dr John E. Fox and Alicia Jensen for reading this manuscript and providing a number of suggestions. This work is supported by a Whitaker Development Award and NIH grant no. 1R01 NS40785-01.
REFERENCES
- Agnew W, McCreery D. Neural Protheses: Fundamental Studies. Englewood Cliffs, NJ: Prentice-Hall; 1990a. [Google Scholar]
- Agnew WF, McCreery DB. Considerations for safety with chronically implanted nerve electrodes. Epilepsia. 1990b;31:S27–32. doi: 10.1111/j.1528-1157.1990.tb05845.x. [DOI] [PubMed] [Google Scholar]
- Amman D. Ion-Selective Microelectrodes: Principles, Design and Application. Berlin, Heidelberg, New York, Tokyo: Springer-Verlag; 1986. [Google Scholar]
- Bawin SM, Sheppard AR, Mahoney MD, Abu Assal M, Adey WR. Comparison between the effects of extracellular direct and sinusoidal currents on excitability in hippocampal slices. Brain Res. 1986;362:350–354. doi: 10.1016/0006-8993(86)90461-0. [DOI] [PubMed] [Google Scholar]
- Benabid AL, Koudsie A, Pollak P, Kahane P, Chabardes S, Hirsch E, Marescaux C, Benazzouz A. Future prospects of brain stimulation. Neurol Res. 2000;22:237–246. doi: 10.1080/01616412.2000.11740666. [DOI] [PubMed] [Google Scholar]
- Benazzouz A, Piallat B, Pollak P, Benabid AL. Responses of substantia nigra pars reticulata and globus pallidus complex to high frequency stimulation of the subthalamic nucleus in rats: electrophysiological data. Neurosci Lett. 1995;189:77–80. doi: 10.1016/0304-3940(95)11455-6. [DOI] [PubMed] [Google Scholar]
- Bikson M, Lian J, Hahn PJ, Stacey WC, Sciortino C, Durand DM. Suppression of epileptiform activity by high frequency sinusoidal fields in rat hippocampal slices. J Physiol. 2001;531:181–191. doi: 10.1111/j.1469-7793.2001.0181j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr, Wilson CL, Fried I, Mathern GW. Hippocampal and entorhinal cortex high-frequency oscillations (100–500 Hz) in human epileptic brain and in kainic acid-treated rats with chronic seizures. Epilepsia. 1999;40:127–137. doi: 10.1111/j.1528-1157.1999.tb02065.x. [DOI] [PubMed] [Google Scholar]
- Brummer SB, Turner MJ. Electrochemical considerations for safe electrical stimulation of the nervous system with platinum electrodes. IEEE Trans Biomed Eng. 1977;24:59–63. doi: 10.1109/TBME.1977.326218. [DOI] [PubMed] [Google Scholar]
- Chan CY, Hounsgaard J, Nicholson C. Effects of electric fields on transmembrane potential and excitability of turtle cerebellar Purkinje cells in vitro. J Physiol. 1988;402:751–771. doi: 10.1113/jphysiol.1988.sp017232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chvatal A, Anderova M, Ziak D, Sykova E. Glial depolarization evokes a larger potassium accumulation around oligodendrocytes than around astrocytes in gray matter of rat spinal cord slices. J Neurosci Res. 1999;56:493–505. doi: 10.1002/(SICI)1097-4547(19990601)56:5<493::AID-JNR5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Cooper IS, Amin I, Gilman S. The effect of chronic cerebellar stimulation upon epilepsy in man. Trans Am Neurol Assoc. 1973;98:192–196. [PubMed] [Google Scholar]
- Durand DM, Bikson M. Suppression and control of epileptiform activity by electrical stimulation: A review. Proceedings of the IEEE, Special Issue on Neural Engineering; Merging Engineering and Neuroscience. 2001;89:1065–1082. [Google Scholar]
- Faber J, Vladyka V. Antiepileptic effect of electric stimulation of the locus coeruleus in man. Act Nerv Super (Praha) 1983;25:304–308. [PubMed] [Google Scholar]
- Fisher RS, Uematsu S, Krauss GL, Cysyk BJ, McPherson R, Lesser RP, Gordon B, Schwerdt P, Rise M. Placebo-controlled pilot study of centromedian thalamic stimulation in treatment of intractable seizures. Epilepsia. 1992;33:841–851. doi: 10.1111/j.1528-1157.1992.tb02192.x. [DOI] [PubMed] [Google Scholar]
- Gardner-Medwin AR. Analysis of potassium dynamics in mammalian brain tissue. J Physiol. 1983a;335:393–426. doi: 10.1113/jphysiol.1983.sp014541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner-Medwin AR. A study of the mechanisms by which potassium moves through brain tissue in the rat. J Physiol. 1983b;335:353–374. doi: 10.1113/jphysiol.1983.sp014539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner-Medwin AR, Nicholson C. Changes of extracellular potassium activity induced by electric current through brain tissue in the rat. J Physiol. 1983;335:375–392. doi: 10.1113/jphysiol.1983.sp014540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghai RS. OH, USA: Case Western Reserve University; 1998. Electric field suppression of spontaneous, low calcium epileptiform activity in the CA1 region of rat hippocampal slices. M Sc thesis. [Google Scholar]
- Ghai RS, Bikson M, Durand DM. Effects of applied electric fields on low-calcium epileptiform activity in the CA1 region of rat hippocampal slices. J Neurophysiol. 2000;84:274–280. doi: 10.1152/jn.2000.84.1.274. [DOI] [PubMed] [Google Scholar]
- Gluckman BJ, Neel EJ, Netoff TI, Ditto L, Spano ML, Schiff SJ. Electric field suppression of epileptiform activity in hippocampal slices. J Neurophysiol. 1996;76:4202–4205. doi: 10.1152/jn.1996.76.6.4202. [DOI] [PubMed] [Google Scholar]
- Gluckman B, Nguyen H, Weinstein S, Schiff S. Adaptive electric field control of epileptic seizures. J Neurosci. 2001;21:590–600. doi: 10.1523/JNEUROSCI.21-02-00590.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn PJ, Durand DM. Bistability dynamics in simulations of neural activity in high-extracellular-potassium conditions. J Comput Neurosci. 2001;11:5–18. doi: 10.1023/a:1011250329341. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. Sunderland, MA, USA: Sinauer Associates; 1992. [Google Scholar]
- Hounsgaard J, Nicholson C. Potassium accumulation around individual purkinje cells in cerebellar slices from the guinea-pig. J Physiol. 1983;340:359–388. doi: 10.1113/jphysiol.1983.sp014767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferys JGR. Influence of electric fields on the excitability of granule cells in guinea-pig hippocampal slices. J Physiol. 1981;319:143–152. doi: 10.1113/jphysiol.1981.sp013897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferys JGR. Nonsynaptic modulation of neuronal activity in the brain: electric currents and extracellular ions. Physiol Rev. 1995;75:689–783. doi: 10.1152/physrev.1995.75.4.689. [DOI] [PubMed] [Google Scholar]
- Kaila K, Lamsa K, Smirnov S, Taira T, Voipio J. Long-lasting GABA-mediated depolarization evoked by high-frequency stimulation in pyramidal neurons of rat hippocampal slice is attributable to a network-driven, bicarbonate-dependent K+ transient. J Neurosci. 1997;17:7662–7672. doi: 10.1523/JNEUROSCI.17-20-07662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjevic K, Morris ME, Reiffenstein RJ. Changes in extracellular Ca2+ and K+ activity accompanying hippocampal discharges. Can J Physiol Pharmacol. 1980;58:579–582. doi: 10.1139/y80-097. [DOI] [PubMed] [Google Scholar]
- Loddenkemper T, Pan A, Neme S, Baker KB, Rezai AR, Dinner DS, Montgomery EB, Jr, Luders HO. Deep brain stimulation in epilepsy. J Clin Neurophysiol. 2001;18:514–532. doi: 10.1097/00004691-200111000-00002. [DOI] [PubMed] [Google Scholar]
- Poolos NP, Mauk MD, Kocsis JD. Activity-evoked increases in extracellular potassium modulate presynaptic excitability in the CA1 region of the hippocampus. J Neurophysiol. 1987;58:404–416. doi: 10.1152/jn.1987.58.2.404. [DOI] [PubMed] [Google Scholar]
- Pumain R, Menini C, Heinemann U, Louvel J, Silva-Barrat C. Chemical synaptic transmission is not necessary for epileptic seizures to persist in the baboon Papio papio. Exp Neurol. 1985;89:250–258. doi: 10.1016/0014-4886(85)90280-8. [DOI] [PubMed] [Google Scholar]
- Rutecki PA, Lebeda FJ, Johnston D. Epileptiform activity induced by changes in extracellular potassium in hippocampus. J Neurophysiol. 1985;54:1363–1374. doi: 10.1152/jn.1985.54.5.1363. [DOI] [PubMed] [Google Scholar]
- Schachter SC, Saper CB. Vagus nerve stimulation. Epilepsia. 1998;39:677–686. doi: 10.1111/j.1528-1157.1998.tb01151.x. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry: The Principles and Practise of Statistics in Biological Research. New York: W.H. Freeman; 1981. pp. 208–371. [Google Scholar]
- Takahashi T, Tsuruhara H. Slow depolarizing potentials recorded from glial cells in the rat superficial dorsal horn. J Physiol. 1987;388:597–610. doi: 10.1113/jphysiol.1987.sp016633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Wong RK, Miles R, Michelson H. A model of a CA3 hippocampal pyramidal neuron incorporating voltage-clamp data on intrinsic conductances. J Neurophysiol. 1991;66:635–650. doi: 10.1152/jn.1991.66.2.635. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Dingledine R. Potassium-induced spontaneous electrographic seizures in the rat hippocampal slice. J Neurophysiol. 1988;59:259–276. doi: 10.1152/jn.1988.59.1.259. [DOI] [PubMed] [Google Scholar]
- Velasco M, Velasco F, Velasco AL, Jimenez F, Brito F, Marquez I. Acute and chronic electrical stimulation of the centromedian thalamic nucleus: modulation of reticulo-cortical systems and predictor factors for generalized seizure control. Arch Med Res. 2000;31:304–315. doi: 10.1016/s0188-4409(00)00085-0. [DOI] [PubMed] [Google Scholar]
- Warren RJ, Durand DM. Effects of applied currents on spontaneous epileptiform activity induced by low calcium in the rat hippocampus. Brain Res. 1998;806:186–195. doi: 10.1016/s0006-8993(98)00723-9. [DOI] [PubMed] [Google Scholar]


