Abstract
A diverse range of accessory proteins regulates the behaviour of most ligand- and voltage-gated ion channels. For glutamate receptor 6 (GluR6) kainate receptors, two unrelated proteins, concanavalin-A (Con-A) and postsynaptic density protein 95 (PSD-95), bind to extra- and intracellular domains, respectively, but are reported to exert similar effects on GluR6 desensitization behaviour. We have tested the hypothesis that distinct allosteric binding sites control GluR6 receptors via a common transduction pathway. Rapid agonist application to excised patches revealed that neither Con-A nor PSD-95 affect the onset of desensitization. The rate of desensitization elicited by 10 mm l-glutamate was similar in control (τfast= 5.5 ± 0.4 ms), Con-A-treated patches (τfast= 6.1 ± 0.5 ms) and patches containing PSD-95 and GluR6 receptors (τfast= 4.7 ± 0.6 ms). Likewise, the time course of recovery from GluR6 desensitization was similar in both control and Con-A conditions, whereas PSD-95 accelerated recovery almost twofold. Peak and steady-state (SS) dose-response relationships to glutamate were unchanged by lectin treatment (e.g. control, EC50(SS)= 31 ± 28 μmvs Con-A, EC50(SS)= 45 ± 9 μm, n= 6), suggesting that Con-A does not convert non-conducting channels with high agonist affinity into an open conformation. Instead, we demonstrate that the effects of Con-A on macroscopic responses reflect a shift in the relative contribution of different open states of the channel. In contrast, the effect of PSD-95 on recovery behaviour suggests that the association between kainate receptors and cytoskeletal proteins regulates signalling at glutamatergic synapses. Our results show that Con-A and PSD-95 regulate kainate receptors via distinct allosteric mechanisms targeting selective molecular steps in the transduction pathway.
For kainate receptors containing glutamate receptor 6 (GluR6) subunits, two unrelated proteins bind to different surfaces of the GluR6 receptor, but apparently regulate desensitization behaviour similarly. The plant lectin, concanavalin-A (Con-A), was the first of these ancillary proteins to be identified and has been used in numerous studies to block irreversibly the rapid response decline observed routinely during the agonist application (Mayer & Vyklicky, 1989; O'Dell & Christensen, 1989; Huettner, 1990; Partin et al. 1993; Wong & Mayer, 1993; Yue et al. 1995; Everts et al. 1997, 1999; Wilding & Huettner, 1997). As a result, it has been suggested that Con-A blocks kainate receptor desensitization (Mayer & Vyklicky, 1989; O'Dell & Christensen, 1989; Huettner, 1990; Partin et al. 1993; Wong & Mayer, 1993; Yue et al. 1995; Everts et al. 1997, 1999; Wilding & Huettner, 1997). Although the molecular basis of this mechanism is still not well understood, it has been assumed that Con-A slows the onset of desensitization and/or accelerates the recovery process. Consistent with this, Con-A crosslinks a number of physically separate N-glycosylated residues on the extracellular kainate receptor surface (Everts et al. 1997, 1999) and thus, may irreversibly affect the conformational states that lead into or out of desensitization. Paternain et al. (1998) have proposed an alternative and more complex mechanism based on the leftward shift of the agonist dose-response relationship that was observed following lectin treatment. To account for their observations, Paternain et al. (1998) proposed that Con-A converts high-affinity, non-conducting states of the receptor into conducting channels (Paternain et al. 1998).
More recently, the effects of postsynaptic density protein 95 (PSD-95), also known as synapse-associated protein 90, have been described and, like Con-A, PSD-95 has been shown to affect steady-state GluR6 desensitization (Garcia et al. 1998). In this case, PSD-95 binds to the cytoplasmic tail region of GluR6 receptor subunits with the first of three N-terminal PDZ domains (Garcia et al. 1998; Garner et al. 2000; Sheng & Pak, 2000; Sheng, 2001; Sheng & Sala, 2001). PSD-95 was the first PDZ-domain protein to be identified (Cho et al. 1992; Kistner et al. 1993) and since then has been shown to bind with high affinity to numerous acceptor proteins (Garner & Kindler, 1996; Saras & Heldin, 1996; Piserchio et al. 2002), accounting for the stability in shape, position and size of aggregates of ion channels clustered by PSD-95 (Burke et al. 1999; Okabe et al. 2001). Like Con-A, the mechanistic basis for the potentiation of steady-state GluR6 responses by PSD-95 is still not well understood.
We have tested the hypothesis that Con-A and PSD-95 regulate kainate receptors through a common allosteric mechanism. Contrary to previous work, Con-A does not regulate GluR6 receptors by blocking entry into or accelerating exit out of desensitization, or converting closed, desensitized channels into ion-conducting conformations of the receptor. Instead, we propose that Con-A affects macroscopic GluR6 responses by shifting the relative contribution of various open states of the channel. Although PSD-95 clusters GluR6 kainate receptors, as described previously (Garcia et al. 1998), our data in excised patches indicate that PSD-95 binding does not cause incomplete desensitization. Instead, we suggest that PSD-95 accelerates recovery from kainate receptor desensitization.
METHODS
Cell culture and transfections
Human embryonic kidney (HEK) 293 cells (CRL 1573; American Type Culture Collection, Manassas, VA, USA) and tsA201 cells (provided by Dr R. Horn, Jefferson Medical College, PA, USA) were maintained at a confluency of 70–80 % in minimal essential medium with Earle's salts, 2 mm glutamine and 10 % fetal bovine serum. After plating at low density on plastic dishes or glass coverslips, cells were transfected with cDNAs using the calcium phosphate technique, as described previously (Bowie et al. 1998). Wild-type GluR6 receptor subunits (supplied by Dr M.L. Mayer, National Institutes of Health, MD, USA) were used for electrophysiology experiments and N-terminal tagged green fluorescent protein (GFP)-GluR6 (supplied by Drs A. Ghetti and S. F. Heinemann, Salk Institute, CA, USA) was used to examine the spatial distribution and the surface expression of GluR6 receptors. The cDNA encoding PSD-95 (supplied by Dr M. Sheng, Harvard University, MA, USA) was transfected in excess of GluR6 cDNA (1:10 ratio) to ensure that the majority of kainate receptors were associated with PSD-95. Consistent with this, biochemical experiments shown in Fig. 8 demonstrate that the majority of plasma-membrane-bound GluR6 kainate receptors are tightly associated with PSD-95. To study heteromeric channels, HEK 293 cells were transfected with cDNAs for GluR6, kainate receptor subunit 2 (KA2) and PSD-95 at a ratio of 1:10:10, respectively. In electrophysiological experiments, the cDNA for GFP (GFP S65T mutant) was routinely co-transfected to help identify transfected cells.
Figure 8. PSD-95 and receptor activation do not affect the surface expression of GluR6 kainate receptors.
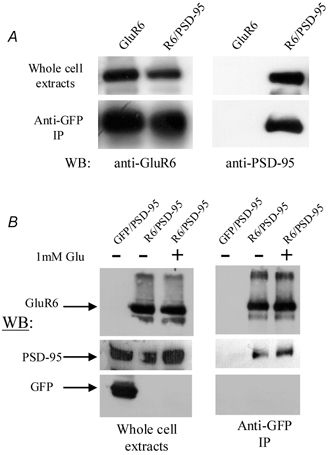
A, Western blots (WB) illustrating the effect of PSD-95 on the surface expression of GluR6 kainate receptors. Upper left panel: lysates of human embryonic kidney (HEK) 293T cells previously transfected with cDNA for GluR6 alone or with PSD-95 and probed with anti-GluR6 antibody. The immunoreactive bands were of similar intensity, demonstrating that the total amount of GluR6 extracted from each cell culture was similar. Lower left panel: bands immunoreactive to anti-GluR6 antibody obtained from co-immunoprecipitation experiments were also similar, suggesting that PSD-95 does not affect surface expression of GluR6. Upper right panel: lysates of HEK 293T cells probed with anti-PSD-95 antibody show that only cells transfected with PSD-95 exhibit immunoreactivity. Lower right panel: PSD-95 immunoreactive band co-immunoprecipitated with anti-GluR6 antibody reveals that the majority of plasma membrane bound GluR6 receptors are associated with PSD-95. In cells transfected with cDNA for GluR6 alone, immunoreactivity to anti-PSD-95 antibody is absent. B, Western blots showing the effect of kainate receptor activation on the surface expression of GluR6. Left panel: lysates of HEK 293T cells expressing GluR6 alone or in combination with PSD-95 probed with anti-GluR6, PSD-95 and GFP antibodies. The right-hand lane shows immunoreactivity of HEK 293T cells challenged with 1 mm l-glutamate for 5 min before whole-cell extraction. Right panel: immunoreactive bands to anti-GluR6 and PSD-95 antibodies obtained in a pull-down assay to determine the surface expression of GluR6 receptors. The right-hand lane shows immunoreactivity of HEK 293T cells challenged with 1 mm l-glutamate for 5 min before co-immunoprecipitation.
Spatial distribution of N-tagged GFP-GluR6 receptors
The spatial distribution of kainate receptors was determined 1–2 days after cells were transfected with cDNAs for GFP-GluR6 alone (2/3 of total coverslips) or in combination with PSD-95 (1/3 of total coverslips). In each experiment, transfected cells were first washed with divalent-ion-containing phosphate buffer solution (dPBS) to remove culture medium. Half of the coverslips containing cells transfected with GFP-GluR6 alone were treated with 10 μm Con-A at room temperature for 5 min and washed again with dPBS. All cells were then fixed for 20 min with 2 % paraformadehyde in PBS on ice, washed in dPBS and mounted onto glass slides in Vectashield (Vector Laboratories, CA, USA). GFP fluorescence, indicating the spatial distribution of GluR6 receptors, was visualized using a Zeiss LSM 510 confocal microscope. It is likely that GFP-GluR6 receptors are targeted to the plasma membrane, similar to wild-type GluR6 receptors, since the distribution of GFP-GluR6 reported in this study is comparable with antibody staining of GluR6 (Garcia et al. 1998). Moreover, preliminary electrophysiological experiments have revealed that GFP-GluR6 receptors exhibit gating and permeation properties similar to those of GluR6 receptors (data not shown).
Determining the surface expression of GluR6 receptors
Experiments to determine the effect of PSD-95 on GluR6 expression or agonist stimulation on GluR6/PSD-95 interactions were performed 48 h after transfection. In the experiments where the effect of agonist stimulation on GluR6 PSD-95 interactions was examined, cells were first washed and then placed in the incubator for 5 min in media +/− 1 mm l-glutamate. In both experiments, the media were then removed and the cells rinsed twice with pre-warmed Tris-buffered saline (TBS) (20 mm Tris, pH 7.5, 150 mm NaCl). Cells were then incubated for 1 h at 37 °C with 4 μg of anti-GFP antibody (QBiogen) before being washed to remove unbound antibody and solubilized in 150 mm NaCl/50 mm Tris, pH 8.0/1 % NP-40/0.5 % deoxycholate/0.1 % SDS/2 mm EDTA/1 mm phenylmethylsulphonyl fluoride/5 mm benzamidine/Sigma protease inhibitor cocktail. Protein G sepharose (AmershamPharmacia) was added to the extracts and washed in the same buffer containing 300 mm NaCl. Immunoprecipitated samples were separated by SDS-PAGE, transferred to nitrocellulose and Western blotted, and the relevant proteins were visualized by chemiluminescence. For the measurement of band intensity, each gel was scanned as a TIF file and densitometric readings for each band were determined using NIH Image software (version 1.62).
Electrophysiological solutions and techniques
The external solution for electrophysiological experiments contained 150 mm NaCl, 5 mm Hepes and 0.1 mm each of CaCl2 and MgCl2. In most cases, the internal solution was composed of 115 mm NaCl, 10 mm NaF, 5 mm Hepes, 5 mm Na4BAPTA, 0.5 mm CaCl2, 1 mm MgCl2 and 10 mm Na2ATP to chelate endogenous polyamines (Bähring et al. 1997; Bowie et al. 1998). Fluoride ions interfere with the modulatory effects of PSD-95 on GluR6 behaviour; therefore, NaCl replaced NaF in these experiments. We have not investigated the effect of fluoride ions systematically, although it may be related to documented effects of fluoride ions on the plasma membrane (Marty & Neher, 1995). The pH and osmotic pressure of internal and external solutions were adjusted to 7.3 and 295 mosmol l−1, respectively. Con-A and succinyl Con-A (sCon-A; Sigma, St Louis, USA) were prepared in glucose-free saline solution and filtered (0.2 μm filter, Corning) immediately before use.
All recordings were performed with an Axopatch 200B amplifier (Axon Instruments, CA, USA) using thin-walled borosilicate glass pipettes (2–5 MΩ) coated with dental wax to reduce electrical noise. Control and l-glutamate solutions were rapidly applied (10 mm, 50 ms duration) to outside-out patches excised from transfected HEK 293 or tsA201 cells, as described previously (Bowie et al. 1998; Bowie, 2002; Bowie & Lange, 2002). Solution exchange (10–90 %; rise time = 25–50 μs) was determined routinely at the end of the experiment by measuring the liquid junction current (or exchange current) between the solution containing 10 mm l-glutamate and the control solution, in which the total Na+ content was reduced by 5 % (Fig. 1A and B; Bowie et al. 1998; Bowie, 2002; Bowie & Lange, 2002). Current records were filtered at 5 kHz, digitized at 25–50 kHz and series resistances (3–10 MΩ) compensated by 95 %. Recordings were performed at −20 mV membrane potential to ensure adequate voltage-clamp control of peak currents (∼ 1–5 nA). Data acquisition was performed using pClamp8 software (Axon Instruments, CA, USA). All experiments were performed at room temperature.
Figure 1. Rapid perfusion of agonist solutions to excised membrane patches.
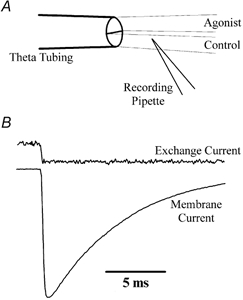
A, schematic diagram of a routine experiment showing the orientation of the theta tubing and recording pipette containing an outside-out membrane patch. To achieve rapid solution exchange of excised patches, both control and agonist solutions were fed continuously by gravity and the tip of the recording pipette was placed close to the control-agonist solution interface. The solution was rapidly exchanged by displacement of the theta tubing using a piezoelectric stack (Physik Instrumente). B, typical exchange and membrane currents recorded from the same patch recording (patch no. 010712p1). The solution exchange rate was determined routinely at the end of each experiment by measuring the current between the solution containing 10 mm l-glutamate and the control solution, in which the total Na+ content was reduced by 5 %.
To allow careful analysis of the effects of Con-A, recordings prior to and following treatment with Con-A were obtained in the same patch recording. The effect of Con-A on GluR6 receptors is irreversible and cannot be recovered by washing; therefore, care was taken to ensure that cells were not inadvertently pre-exposed to Con-A. To achieve this, the recording chamber, flowpipe and all glassware were washed thoroughly with saline before and after each recording. In addition, only one patch recording was permitted per culture dish of transfected cells. To verify that control responses reflected GluR6 responses prior to treatment with Con-A, only patches in which the steady-state responses observed with 10 mm glutamate were less than 0.5 % of the peak response were accepted for further analysis. Data were also rejected when Con-A formed a meshwork of long filaments around the electrode tip, since this process prevented rapid solution exchange. Irreversible modulators, such as Con-A or sCon-A, will saturate all binding sites on the target protein when present at any concentration exceeding or equivalent to the total number of binding sites. To ensure that all GluR6 channels in each recording were fully modified, the time course of increase in the agonist steady-state response, routinely observed during treatment with Con-A or sCon-A, was followed to completion. GluR6 receptors still exhibited lectin-induced effects even after the Con-A or sCon-A solution was removed, as expected for irreversible modulation. Moreover, a decline in the effects of Con-A or sCon-A on kainate receptors was not observed, even after 2–3 h of washout.
Experiments designed to map out recovery from and re-entry into GluR6 desensitization consisted of 33 paired agonist applications (10 mm l-glutamate, 50 ms duration each), each separated by varying time intervals. The first application, or conditioning response, was employed to accumulate receptors into the desensitized state(s). The second application, or test response, provided information on two quantities: (1) the amplitude reported the fraction of the response that had recovered from desensitization and (2) the decay kinetics reflected the rate at which resensitized channels re-enter desensitization (Bowie & Lange, 2002).
Data analysis
Model-independent analyses, such as measurement of response amplitudes or fitting of exponential functions, were performed using Clampfit 8.2 software (Axon Instruments, CA, USA). We have recently described a revised model of kainate receptor desensitization and the fitting methods used to account for the kinetics of both the onset of desensitization and the recovery process (Bowie & Lange, 2002). In this study, we report that neither Con-A nor PSD-95 affect the rate of onset of desensitization (see Fig. 2). Therefore, to simplify analysis, the molecular steps that describe onset kinetics have been omitted from our modelling and we have focussed exclusively on the recovery pathway. The text and mathematical expressions that follow were developed to describe recovery from GluR6 desensitization only.
Figure 2. Concanavalin-A (Con-A) but not postsynaptic density protein 95 (PSD-95) affects glutamate receptor (GluR)6 steady-state desensitization.
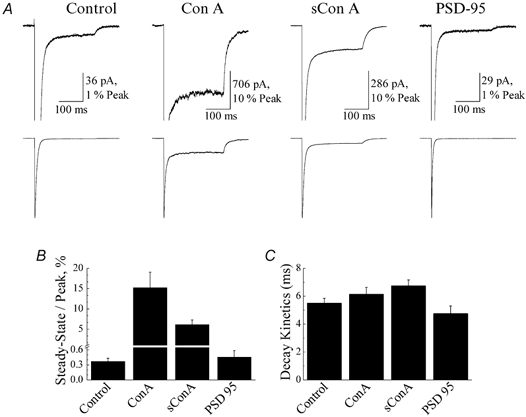
A, typical membrane currents evoked by 10 mm glutamate (250 ms duration, holding potential (VH) =−20 mV) on outside patches expressing GluR6 receptors alone (left panel, patch no. 010712p1), co-expressed with PSD-95 (right panel, patch no. 010619p1) or following treatment with 10 μm Con-A (patch no. 000327p2) and 10 μm succinyl Con-A (sCon-A; patch no. 010713p2). B and C, plots summarizing the effect of lectin treatment and co-expression of PSD-95 on GluR6 steady-state responses (B) and the fast kinetic component of desensitization (C). All data are expressed as the mean ±s.e.m.
Fits of recovery from GluR6 desensitization were performed using code written in Mathematica (Wolfram Research, IL, USA; Bowie & Lange, 2002). Recent experiments suggest that GluR6 kainate receptors recover from desensitization in several ordered transition steps (Bowie & Lange, 2002). The number of steps involved is dependent on the external ion concentration; however, in 150 mm NaCl, recovery can be approximated as a two-step process (Bowie & Lange, 2002), as illustrated below: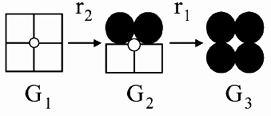
In this scheme, open squares denote ligand-bound subunits in the desensitized state, whereas filled circles reflect ligand-bound subunits in the open state. GluR6 receptors fully bound by agonist exist in three conducting states defined as G1, G2 and G3. In the continued presence of agonist, GluR6 receptors in the fully open state, G3, desensitize in two consecutive steps traversing state G2 before accumulating into state G1. Likewise, the recovery process reflects the transition of GluR6 tetramers from state G1 to state G3, traversing the intermediary state, G2. The transition rates between these states are denoted by the rate constants, r2 and r1. The dynamics of each of the states are expressed by a term that is a solution of a first-order differential equation proportional to the probability of being in that state multiplied by G, which is its conductance term. Therefore:
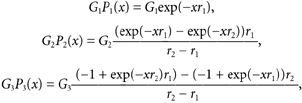 |
Evaluation of each of these terms produces graphs such as those seen in Fig. 6E and Fig. 7C. Recovery from desensitization at any interpulse interval, x, is then the sum of these three terms:
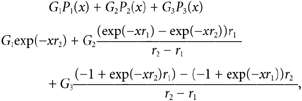 |
which simplifies to a form showing the fact that the model involves two exponential terms defined as:
Figure 6. Con-A shifts the relative contribution of conducting states.
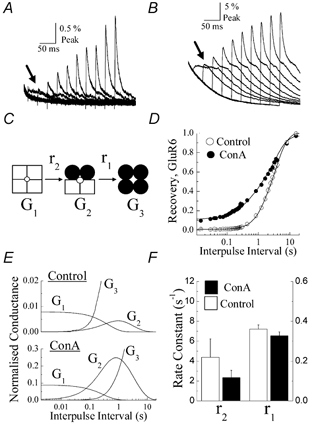
A and B, ten normalized test responses taken from Fig. 5A and detailed to show their temporal profile before (A) and after (B) Con-A treatment. Note that during the early phase of the recovery process in both cases, test responses re-enter desensitization with slower kinetics (see arrows). C, schematic showing only the recovery steps in the dimer model of GluR6 desensitization. G1, G2 and G3 are the macroscopic conductances associated with different states of the kinetic model and r2 and r1 are the rate constants that describe the rate of recovery from desensitization. The transition steps that lead from state G3 to state G1 have been omitted since Con-A treatment did not affect GluR6 desensitization kinetics (see Fig. 2). D, data from Fig. 5B re-fitted assuming that GluR6 receptors recover from desensitization in two sequential steps as shown in C. E, plots summarizing the contribution of conducting states G1-G3 to the macroscopic response at a range of interpulse intervals in control conditions (upper panel) and following Con-A treatment (lower panel). Note that the scale of the ordinate axis in the lower plot is an order of magnitude larger. F, summary plot showing the values of rate constants r1 and r2 estimated from fits of control and Con-A data shown in D.
Figure 7. PSD-95 accelerates recovery from GluR6 receptor desensitization.
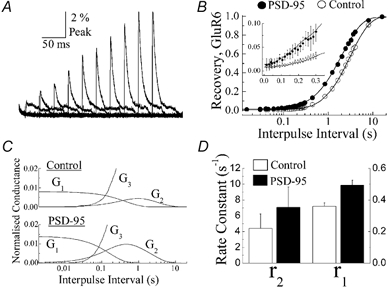
A, ten normalized test responses observed when GluR6 receptors were co-expressed with PSD-95 (1:10 ratio). As noted for control and Con-A conditions, test responses re-enter desensitization with slower kinetics during the early phase of recovery. B, summary plots comparing recovery of GluR6 responses from desensitization in control conditions (open circles) and with PSD-95 (filled circles). The time axis is plotted on a logarithmic scale to show the early phase of the recovery process. Insert: plot of the early phase of the recovery process showing that GluR6 channels recover faster from desensitization in the presence of PSD-95. The time axis is plotted on a linear scale. C, plots showing the contribution of conducting states G1-G3 to the macroscopic response for GluR6 receptors alone (upper panel) or when co-expressed with PSD-95 (lower panel). D, summary plot showing the values of rate constants r1 and r2 estimated from fits of data shown in B.
Code was written to fit recovery from GluR6 desensitization in control conditions, following Con-A treatment and in the presence of PSD-95. In each case, the time course of recovery was mapped out using paired conditioning and test agonist responses separated by varying interpulse intervals. A detailed treatise of this fitting method and assumptions therein is described elsewhere (Bowie & Lange, 2002; see also http://rsb.info.nih.gov/~gdl/supplement/).
RESULTS
Neither Con-A nor PSD-95 affect the development of GluR6 desensitization
Figure 2A compares the peak and steady-state membrane currents elicited by rapid application of 10 mm glutamate (250 ms duration, holding potential (VH) =−20 mV) in control conditions, following treatment (5 min) with Con-A (10 μm) or sCon-A (10 μm) and in patches co-expressing GluR6 and PSD-95 (1:10 ratio). As described in other studies (Partin et al. 1993; Patneau et al. 1994; Everts et al. 1997, 1999; Paternain et al. 1998), Con-A increased steady-state GluR6 responses. The steady-state response with respect to the peak was 15.2 ± 3.8 % (n= 5) following Con-A treatment compared to 0.37 ± 0.06 % (n= 13) prior to treatment. sCon-A also increased the steady-state response (steady-state/peak = 6.16 ± 1.11 %, n= 6) more than 15-fold (Fig. 2A and B), representing a more robust effect than that described previously at native AMPA receptors (Mayer & Vyklicky, 1989) and invertebrate glutamate receptors (Evans & Usherwood, 1985). Co-expression of PSD-95 with GluR6 receptors did not significantly affect the steady-state response amplitude (steady-state/peak = 0.46 ± 0.13 %, n= 10; Fig. 2A and B), suggesting that the association between PSD-95 and GluR6 receptors does not cause incomplete kainate receptor desensitization, as reported previously in whole-cell recordings (Garcia et al. 1998). Consistent with this, steady-state desensitization of heteromeric assemblies composed of GluR6 and KA2 receptor subunits was also unaffected by PSD-95, with control and PSD-95 patches displaying steady-state responses with respect to the peak of 0.40 ± 0.04 % (n= 10) and 0.43 ± 0.06 % (n= 12), respectively. As discussed later (see Discussion), slower solution exchange rates inherent with whole-cell recording conditions are likely to have overestimated the contribution of PSD-95 binding to the steady-state response in previous work (Garcia et al. 1998).
Analysis of GluR6 decay kinetics suggests that neither Con-A nor PSD-95 affect the rate of kainate receptor desensitization (Fig. 2C). GluR6 desensitization was fitted well with a double-exponential function where the decay kinetics of the macroscopic response was dominated by the fast kinetic component of desensitization. In all experimental conditions, the fast component of desensitization was similar, with time constants of 5.5 ± 0.4 ms (% τfast= 97.1 ± 1.2 %, n= 13, mean ±s.e.m.), 6.1 ± 0.5 ms (% τfast= 87.9 ± 3.4 %, n= 5), 6.7 ± 1.1 ms (% τfast= 90.1 ± 2.3 %, n= 6) and 4.7 ± 0.6 ms (% τfast= 94.5 ± 0.9 %, n= 10) for control, Con-A, sCon-A and PSD-95 conditions, respectively (Fig. 2C). The slow component of desensitization contributed much less to the macroscopic response, with time constants of 25.7 ± 3.5 ms, 52.7 ± 10.4 ms, 41.6 ± 6.0 ms and 43.1 ± 27.4 ms for control, Con-A, sCon-A and PSD-95 conditions, respectively.
It was unexpected that PSD-95 did not affect kainate receptor behaviour in these initial experiments. One potential concern, therefore, was that PSD-95 might have failed to associate with GluR6 receptors under our experimental conditions. This is unlikely for two reasons. First, GFP-GluR6 receptors co-expressed with PSD-95 exhibited marked clustering in the plasma membrane (Fig. 3), consistent with previous work (Garcia et al. 1998). Second, the results of the electrophysiological experiments described below suggest that PSD-95 remains associated with GluR6 receptors even in outside-out patch recordings. Interestingly, by comparison with PSD-95, Con-A treatment did not affect GFP-GluR6 receptor distribution (Fig. 3), suggesting that lectin binding does not grossly disrupt or aggregate receptors in the membrane (Mayer & Vyklicky, 1989; Huettner, 1990; Everts et al. 1999; Lerma, 1999).
Figure 3. Con-A does not redistribute kainate receptors on the plasma membrane.
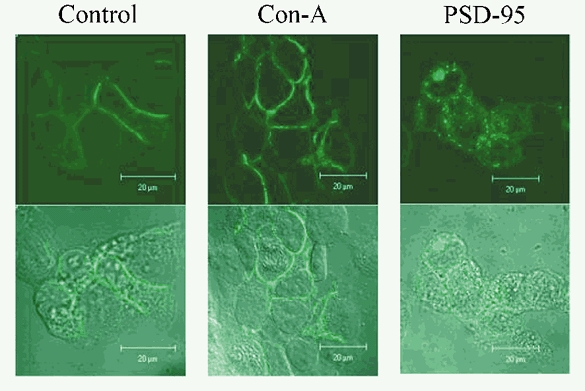
Spatial distribution of green fluorescent protein (GFP)-tagged GluR6 (GFP-GluR6) receptors visualized using fluorescence confocal microscopy in control conditions (left panels), following 5 min of treatment with 10 μm Con-A (middle panels) or in cells co-expressing PSD-95 (right panels). The upper row shows confocal images of GFP fluorescence and the lower row denotes the same cells in transmitted light, using digital interference contrast. The spatial distribution of GFP-GluR6 receptors was similar in control conditions and following Con-A treatment, but co-expression with PSD-95 induced a punctate appearance of the plasma membrane and cytoplasmic expression of kainate receptors.
Con-A does not affect GluR6 dose-response relationships
Paternain et al. (1998) have argued that the leftward shift in the dose-response curve observed following Con-A treatment reflects the conversion of closed, desensitized GluR6 receptors into ion-conducting channels. To examine this, dose-response curves to glutamate (5 μm-10 mm) were constructed prior to and following Con-A treatment in the same patch recording (Fig. 4). Figures 4A and C show glutamate-evoked membrane currents (VH=−20 mV, 250 ms duration) observed in a typical patch experiment before and after Con-A treatment, respectively. Fits of the logistic equation to peak and steady-state responses evoked by glutamate in control and Con-A-treated conditions revealed that Con-A did not shift the apparent agonist affinity (or EC50) for peak (Fig. 4B) or steady-state (Fig. 4D) responses, in contrast to previous reports (Jones et al. 1997; Paternain et al. 1998). EC50 values of peak responses in control and Con-A conditions were 564 ± 49 μm (Hill slope, nH= 0.84 ± 0.07, n= 6) and 513 ± 33 μm (nH= 0.73 ± 0.04, n= 5), respectively and EC50 values of steady-state responses were 30.6 ± 27.6 μm (nH= 0.49 ± 0.24, n= 6) and 45.3 ± 9.4 μm (nH= 0.83 ± 0.13, n= 5), respectively (Fig. 4B and D).
Figure 4. Con-A does not affect GluR6 dose-response relationships.
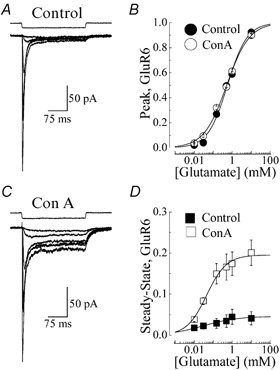
A and C, typical membrane currents elicited by l-glutamate (5 μm-10 mm, VH=−20 mV, 250 ms duration) in the same recording (patch no. 001002p1) before (A, peak EC50= 458 μm, nH= 0.91) and after (C, peak EC50= 512 μm, nH= 0.78) treatment with 10 μm Con-A. B and D, peak and steady-state GluR6 dose-response relationships observed prior to (filled circle or square, n= 6) and following (open circle or square, n= 5) Con-A treatment and fitted with the logistic equation (continuous lines): R=Imax/(1 + EC50/[agonist]nH) where R is the peak or steady-state response at a given agonist concentration, Imax is the maximum response and nH denotes the Hill coefficient. To allow comparison amongst different patches, peak and steady-state amplitudes were normalized with Imax values obtained from fits of individual dose-response relationships. Response rundown was not appreciable in control and Con-A-treated patches. However, to minimize this effect, all dose-response relationships were constructed by alternating between high and low agonist concentrations. All data are expressed as the mean ±s.e.m.
Recovery from GluR6 desensitization is a two-step process
To determine whether Con-A or PSD-95 influence the time course of recovery from GluR6 desensitization, we employed a series of paired conditioning and test agonist applications (10 mm glutamate, 50 ms duration, VH=−20 mV) separated by varying time intervals, to map out the recovery process. Figure 5A shows typical conditioning and test responses prior to (left panel) and following treatment (right panel) with 10 μm Con-A in the same patch recording. To estimate the recovery rates from desensitization in both cases, the amplitude of all the test responses were plotted with their respective interpulse intervals (Fig. 5B). Note that the time axis for the interpulse interval has been plotted on a logarithmic scale to permit examination of test responses during the early phase of recovery (Fig. 5B).
Figure 5. Recovery from GluR6 desensitization does not display first-order kinetics.
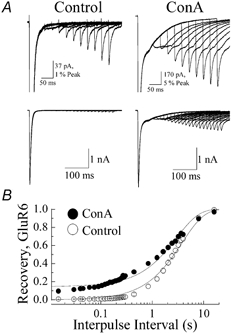
A, paired conditioning and test responses evoked by 10 mm l-glutamate (50 ms duration, VH=−20 mV, patch no. 010403p2) in the same recording before (left panels) and after (right panels) treatment with 10 μm Con-A. The lower row shows 20 paired conditioning and test responses in each condition separated by interpulse intervals with 15 ms increments. The upper row shows every alternate pair at higher gain to allow visual inspection of the amplitude and decay behaviour of individual test responses B, plot summarizing the effect of Con-A on recovery from GluR6 desensitization. Open and filled circles denote the test response amplitude at any given interpulse interval before and after Con-A treatment, respectively. To show the early phase of the recovery process, the time axis has been plotted on a logarithmic scale. In each case, the recovery process was fitted by the expression It=Ipeak× (1 - exp(-t/τrec)) +Iss, where It is the response amplitude at any time (t), Ipeak is the peak test response, Iss is the steady-state response (constrained to zero in control) and τrec is the time constant for recovery. All data are expressed as the mean ±s.e.m. Note that recovery behaviour in each condition was poorly fitted by a monoexponential function.
The recovery process was initially fitted with a first-order exponential function, as reported in other studies (Heckmann et al. 1996; Traynelis & Wahl, 1997; Paternain et al. 1998), where the time constant of recovery prior to and after Con-A treatment was 2.99 ± 0.11 s and 3.83 ± 0.11 s, respectively (n= 9; Fig. 5B). The plateau amplitude observed following Con-A treatment was 0.15 ± 0.007 (n= 9). Assuming that GluR6 kainate receptors recover from desensitization in single conformational steps, fits of experimental data suggest that Con-A slows recovery from desensitization. However, close inspection of the fits shown in Fig. 5B reveal that GluR6 recovery behaviour either before or after Con-A treatment is not well described as a single exponential function (continuous line, Fig. 5B). Consistent with this, recent experiments suggest that kainate receptors enter and recover from desensitization in several conformational steps (Bowie & Lange, 2002). The number of steps involved is variable and ion-dependent, and at physiological ion levels (i.e. 150 mm NaCl), GluR6 channels operate as dimers, where entry into and exit from desensitization is a two-step process (Bowie & Lange, 2002; Fig. 6C; see Methods). Moreover, the transition rates between steps are dependent on the number of desensitized subunits per GluR6 receptor tetramer (Bowie & Lange, 2002). In agreement with this, the desensitization kinetics of test responses were slowed at the briefest interpulse intervals (Fig. 5A and arrows in Fig. 6A and B) and similar to observations described in more detail elsewhere (Bowie & Lange, 2002). However, the kinetic behaviour of an individual test response was similar before and after Con-A treatment (Fig. 6A and B), consistent with the previous observation, described in Fig. 2C, that Con-A does not significantly affect the onset of kainate receptor desensitization.
Con-A shifts the relative contributions of kinetically distinct open states to the macroscopic response
The recovery behaviour of GluR6 channels prior to and following Con-A treatment was fitted again, this time assuming a two-step process (Fig. 6C). Figure 6D shows that recovery from GluR6 receptor desensitization was best fitted in both the control and Con-A-treated patches when assuming a dimer model of desensitization (Bowie & Lange, 2002). The fits under the two conditions were different with a confidence level of at least 99 % (i.e. P < 0.01) using an F-ratio test on the sum of squares. Recovery rate constants were similar in both conditions, where r2 and r1 (Fig. 6C) were 2.34 ± 0.74 s−1 and 0.33 ± 0.02 s−1 following Con-A treatment and 4.39 ± 1.84 s−1 and 0.36 ± 0.02 s−1 in control patches, respectively (n= 9; Fig. 6F). This finding suggests that modulation by Con-A does not affect the rate at which GluR6 receptors recover from desensitization. The increase in the agonist steady-state response observed following Con-A treatment was due mainly to a greater contribution of states G1 and G2 to the overall macroscopic response (Fig. 6C and E). From the fits shown in Fig. 6D, the conductance of states G1 and G2 were 0.008 ± 0.007 and 0.006 ± 0.024, respectively, in control conditions, which increased more than 10-fold to 0.090 ± 0.008 (G1) and 0.314 ± 0.033 (G2) after treatment with 10 μm Con-A (n= 9). Since peak response amplitudes were unchanged following Con-A treatment (peak Con-A = 102 ± 17 % of peak control, n= 13), we have constrained the macroscopic conductance of state, G3, to a value of 1 before and after Con-A treatment. Using the mean macroscopic conductance values for states G1-G3 as well as the mean values for rate constants r1 and r2, we were able to calculate the contribution of each state to the overall macroscopic conductance at different time points during recovery from desensitization. The distribution of states G1-G3 at different interpulse intervals is shown for control and Con-A conditions in Fig. 6E. Note that the axes for the normalized conductance (i.e. ordinate axes) are plotted on different scales to allow visual inspection of the distribution of states G1 and G2 in both control and Con-A conditions (Fig. 6E).
PSD-95 accelerates recovery from GluR6 desensitization
A similar experimental approach was used to examine whether PSD-95 affects recovery from kainate receptor desensitization (Fig. 7). Figure 7A shows typical test GluR6 responses observed in a patch co-expressing PSD-95 (1:10 ratio). The association between PSD-95 and GluR6 receptors was confirmed for each transfection by observing GFP-GluR6 clustering on each day of recording. As described for Con-A-treated patches, test responses decayed similarly in control and PSD-95 conditions, suggesting that the association between kainate receptors and PSD-95 did not affect entry rates into desensitization, as described previously in Fig. 2C. Unlike Con-A-treated patches, however, GluR6 receptors recovered faster from desensitization when associated with PSD-95 (Fig. 7B). Differences in recovery rates from desensitization in control and PSD-95 conditions were observed even during the early phase of the recovery process (Fig. 7B inset). The fits under the two conditions were different, with a confidence level of at least 99 % (i.e. P < 0.01) using an F-ratio test on the sum of squares. Fits of the recovery plots further revealed that differences between control and PSD-95 patches reflected changes in the rate-limiting step defined by the rate constant r1, which increased to 0.49 ± 0.03 s−1 compared to 0.36 ± 0.02 s−1 observed in the control (Fig. 7D, n= 8, t test, P < 0.05). The rate constant r2, estimated from fits, was similar in both control (r2= 4.39 ± 1.84 s−1) and PSD-95 (r2= 7.07 ± 2.58 s−1) conditions. Unlike Con-A treatment, the binding of PSD-95 did not affect the relative contribution of states G1-G3 to the overall macroscopic conductance. From fits shown in Fig. 7B, G1 and G2 were estimated to be 0.014 ± 0.010 and 0.012 ± 0.011, respectively, in patches co-expressing PSD-95 and GluR6 receptors, compared to 0.008 ± 0.007 (G1) and 0.006 ± 0.024 (G2) in GluR6 control patches. We have assumed that the conductance of open state G3 was unchanged by PSD-95 for two reasons. First, peak macroscopic responses excised in outside-out patches expressing GluR6 receptors alone or in combination with PSD-95 were similar (peak control = 2.0 ± 0.5 nA vs peak PSD-95 = 2.6 ± 0.8 nA, n= 12). Secondly, as described below, the surface expression of GluR6 receptors was unaffected by co-expression with PSD-95. Using the mean macroscopic conductance values for each state and the mean values for each rate constants, we calculated the contribution of each state to the overall macroscopic conductance at different time points during recovery from desensitization. The distribution of states G1-G3 at different interpulse intervals is shown for control and PSD-95 conditions in Fig. 7C.
Effect of PSD-95 and agonist stimulation on GluR6 receptor surface expression
Although some PDZ-domain-containing proteins affect the surface expression of a number of voltage-gated ion channels (Horio et al. 1997; Burke et al. 1999; Nehring et al. 2001), this possibility has not been explored for ligand-gated ion channels such as GluR6 receptors. Likewise, the enhanced association of some PDZ-domain proteins (Hall et al. 1998; Xu et al. 2001), with their targets following agonist stimulation, has not been investigated for GluR6 and PSD-95. To examine both of these issues, the effect of PSD-95 and/or receptor activation on the total surface expression of GluR6 was examined.
The effect of PSD-95 on the total number of plasma-membrane-bound GluR6 receptors is summarized in Fig. 8A. As a control, whole-cell extracts from HEK 293T cells expressing GluR6 receptors alone or co-expressed with PSD-95 were initially probed with anti-GluR6 antibody to ensure that similar amounts of total GluR6 protein were extracted from both cell cultures (Fig. 8A, upper left panel). The total amount of GluR6 on the plasma membrane surface was then determined by immunoprecipitating surface GluR6 receptors previously bound to anti-GFP antibody in intact cells (see Methods). As illustrated in Fig. 8A (lower left panel), the immunoreactive bands to anti-GluR6 antibody were of similar intensity in the presence and absence of PSD-95 (PSD-95/control ratio = 1.18 ± 0.32, n= 4). In cells co-expressing PSD-95 and GluR6, the anti-GFP antibody also strongly co-immunoprecipitated PSD-95 (Fig. 8A, lower right panel) demonstrating that almost all surface kainate receptors were tightly bound to PSD-95 in our experimental conditions. Taken together, these results suggest that the association between PSD-95 and GluR6 receptors does not affect total surface expression of kainate receptors.
A similar strategy was employed to determine whether the association between GluR6 receptors and PSD-95 is altered by agonist stimulation (Fig. 8B). As before, we first verified that the total amount of GluR6 (Fig. 8B, upper left panel) and PSD-95 (Fig. 8B, middle left panel) expressed by HEK 293T cells in each transfection was similar using anti-GluR6 and PSD-95 antibodies, respectively. To examine the effect of receptor activation on the association of GluR6 with PSD-95, HEK 293T cells expressing both proteins were incubated for 5 min in either 1 mm l-glutamate or control medium (Fig. 8B, right panels). In each case, GluR6 and PSD-95 co-immunoprecipitated to a similar extent (+agonist/-agonist ratio = 0.96 ± 0.13, n= 4, mean ±s.e.m.) in the presence and absence of agonist (Fig. 8B, upper and middle panels), suggesting that kainate receptor activation does not affect GluR6/PSD-95 association.
DISCUSSION
Our recent work suggests that kainate receptors enter and exit desensitization in several sequential conformational steps that are regulated by the external ion composition (Bowie, 2002; Bowie & Lange, 2002). In view of this revised model of desensitization, we have designed experiments to re-examine the modulatory behaviour of Con-A and PSD-95 on GluR6 kainate receptors. Our results show that both Con-A and PSD-95 regulate kainate receptors through separate allosteric mechanisms that are distinct from that proposed in previous work. Con-A shifts the relative contribution of kinetically distinct open states, whereas PSD-95 accelerates recovery from desensitization. Con-A has been used by numerous investigators as a pharmacological tool to block desensitization of recombinant and native kainate receptors. Our findings suggest that further use of Con-A to provide insight into the behaviour of kainate receptors requires careful consideration of its mechanism of action. Although PSD-95 has a well-recognized role as a scaffolding protein in vertebrate brains, our results suggest that the direct association between PSD-95 and GluR6-containing kainate receptors also modify information flow through glutamatergic synapses.
Comparison with previous studies
Previous studies have proposed that Con-A may modulate kainate receptors by one of two mechanisms. Most investigations have assumed that Con-A blocks or reduces significantly kainate receptor desensitization (Mayer & Vyklicky, 1989; O'Dell & Christensen, 1989; Huettner, 1990; Partin et al. 1993; Wong & Mayer, 1993; Yue et al. 1995; Everts et al. 1997, 1999; Wilding & Huettner, 1997), presumably by sterically hindering protein conformations that bring about desensitization or by facilitating the recovery process. The most convincing evidence in favour of this mechanism is supported by several studies that show that the rapid onset of kainate receptor desensitization observed in control conditions is almost completely abolished following Con-A treatment (Huettner, 1990; Partin et al. 1993; Patneau et al. 1994; Wilding & Huettner, 1997; Everts et al. 1999). Our results show that although Con-A increases the agonist steady-state response, peak GluR6 responses still desensitize (and recover) with kinetics comparable to those of unmodified receptors. It is unlikely that our observations reflect the behaviour of two independent populations of channels, one that is modified by Con-A and another population resistant to Con-A treatment since, as described in Results, lectin binding is irreversible. Although we cannot negate the possibility that some GluR6 channels remain inaccessible to Con-A due to patch deformation, this effect would require that the same GluR6 channels are fully accessible to agonist molecules.
The most straightforward explanation for differences between the present study and previous work is the exchange rate of agonist application. In previous work, kainate receptor responses were recorded in the whole-cell configuration, where the rate of agonist solution exchange was markedly slower than channel gating kinetics (Huettner, 1990; Partin et al. 1993; Patneau et al. 1994; Wilding & Huettner, 1997; Everts et al. 1999). This phenomenon could account for the apparent increase in maximum current amplitude elicited by Con-A in whole-cell recordings (Huettner, 1990; Partin et al. 1993; Wong & Mayer, 1993) and the absence of this effect with the faster perfusion used in this study (although see Wilding & Huettner, 1997). Taken together, although rapidly desensitizing peak GluR6 responses are still observed in this study following Con-A treatment, the absence of similar responses in whole-cell experiments most likely reflects the inherent limitation of slower solution exchange rates.
Paternain et al. (1998) have suggested an alternative proposal whereby Con-A promotes the conversion of non-conducting GluR6 channels into the open state. In agreement with this, the authors observed the leftward shift in dose-response relationships when GluR6 receptors were treated with Con-A. Moreover, Jones et al. (1997) have also reported a similar effect with high-affinity agonists at GluR6 receptors. Using a revised gating scheme for kainate receptors (Bowie & Lange, 2002), we propose a different mechanism, whereby Con-A promotes a shift in the relative contribution of kinetically distinct open states of the channel (see below). This mechanism accounts fully for the increase in the agonist steady-state response observed in this study as well as the lack of effect of Con-A on the peak response amplitude, dose-response relationships and kinetics into and out of desensitization. Whether a similar shift in the various open states of the channel can also account for the effect of Con-A on responses evoked by other receptor agonists, such as domoate or kainic acid, awaits further investigation. Likewise, the molecular basis of the effects of Con-A on kainate-receptor-containing subunits other than GluR6 (e.g. sensory neurons: Huettner, 1990; Partin et al. 1993) requires further experimentation.
For PSD-95, previous work from whole-cell recordings has proposed that binding of this scaffolding protein elicits incomplete desensitization of homomeric GluR6 receptors and GluR6/KA2 heteromeric assemblies (Garcia et al. 1998). We have repeated these experiments in excised patches but did not observe an effect of PSD-95 on steady-state desensitization of either homo- or heteromeric kainate receptors. In this study and previous work, steady-state desensitization was estimated from a ratio of the amplitude of peak and steady-state agonist responses. Given the rapid activation and desensitization kinetics of kainate receptors, an evaluation of steady-state desensitization amongst a population of cells is largely dependent on an accurate measurement of the peak response amplitude. In ideal conditions, the amplitude of peak responses indicates the number of functional channels in an individual recording and permits comparison amongst different recordings. Since Garcia et al. (1998) examined kainate receptor responses in the whole-cell recording mode, it is likely, as discussed above for Con-A, that the actual amplitude of peak kainate receptor responses was underestimated. Moreover, due to the variable geometry of transfected cells, estimates of the peak response are likely to have been further compromised. These factors would favour the larger steady-state/peak ratios (i.e. 10–40 % peak) observed by Garcia et al. (1998) as opposed to the significantly smaller values (i.e. 0.4–0.5 % peak) reported in this study. Despite this, it is possible that in intact cells, PSD-95 exerts additional effects on kainate receptors that are lost in excised patches, including the regulation of steady-state desensitization or even shifts in the apparent agonist affinity. However, the effect of PSD-95 on recovery kinetics in this study suggests that PSD-95 and GluR6 receptors remain tightly associated in excised patches, consistent with the high binding affinity reported for PDZ-domain proteins (Garner & Kindler, 1996; Saras & Heldin, 1996; Piserchio et al. 2002).
How does Con-A regulate kainate receptors?
Con-A affects GluR6 receptors in an irreversible manner, which probably reflects the crosslinking of a number of physically separate N-glycosylated residues on the extracellular kainate receptor surface (Everts et al. 1997, 1999). In a structural sense, however, it is not clear how the binding and crosslinking elicited by Con-A promotes a shift in the contribution of open states, G1-G3, to the overall macroscopic response. An attractive possibility is that Con-A restricts conformations within the agonist-binding domain affecting downstream transduction events that control the channel gate(s). An analogous situation has recently been reported for AMPA receptors, where X-ray crystallography has shown that agonists with different efficacies promote the closure of the agonist-binding domain to different degrees (Armstrong et al. 1998; Armstrong & Gouaux, 2000). Although the crystal structure of kainate receptors is not yet available, their high homology to AMPA receptors (Stern-Bach et al. 1994) suggests that the agonist-binding domain behaves similarly. Consistent with this, all nine N-glycosylated amino acid residues that bind Con-A are located within the vicinity of the proposed GluR6 agonist-binding domain (Everts et al. 1999), suggesting that the plant lectin may restrict protein movements initiated by agonist binding. In agreement with this, the Con-A tetramer is more effective on GluR6 receptors than the dimer, succinyl Con-A, suggesting that lectins with a greater number of carbohydrate binding sites restrict conformational events to a greater extent.
The microscopic details of Con-A modulation of GluR6 kainate receptors await future work on the behaviour of single-channel events. However, since the conductance of each open state in our models is determined by two factors, the unitary conductance and open-channel probability (see also Bowie & Lange, 2002), a number of possible outcomes can be expected. The simplest possibility is that Con-A exerts a preferential effect on open-channel probability or the unitary conductance. A more complicated, and perhaps more likely, scenario is that Con-A imposes a combined effect on both the open-channel probability and unitary conductance. Dissection of the microscopic details in each of these cases will be further complicated by the fact that each open state in our models probably represents several sublevels, each of which may exhibit different open-channel probabilities, as discussed in detail previously (Bowie & Lange, 2002). Moreover, each of the open states, G1-G3, is likely to exhibit different apparent affinities for the agonist. Since peak and steady-state responses reflect mainly membrane conductance through states G3 and G1, respectively, the dose-response curves shown in Fig. 4 imply that state G3 has a lower apparent agonist affinity (e.g. EC50∼ 500 μm) than state G1 (e.g. EC50= 30–45 μm). Consequently, success in dissecting apart these possibilities will depend critically on achieving high-quality single-channel recordings that provide insight into the conductance levels of the various open states and their respective channel open times.
Physiological role of PSD-95 at synapses containing kainate receptors
Although most studies indicate that PSD-95 operates mainly as a scaffolding protein, experiments described here for GluR6 kainate receptors and elsewhere for potassium channels (Cohen et al. 1996; Nehring et al. 2001) suggest that it also fulfils a more dynamic role in cell signalling. PSD-95 downregulates the single-channel conductance of inwardly rectifying K+ channels composed of Kir2.3 subunits (Nehring et al. 2001); although this modulatory effect is unlikely to be conferred on all inwardly rectifying K+ channels, since Kir4.1 unitary events are not affected (Horio et al. 1997). The molecular basis by which PSD-95 regulates Kir2.3 channels is, as yet, unresolved. However, protein-protein interactions of this nature are likely to be regulated dynamically, perhaps by elevations in cytoplasmic cAMP, since protein kinase A controls the binding of Kir2.3 subunits with PSD-95 (Cohen et al. 1996). Whether the interaction between GluR6 receptor subunits and PSD-95 is similarly modulated by kinase activity requires further study. We show that GluR6 receptor subunits associated with PSD-95 recover from desensitization almost twofold faster than GluR6 receptors alone. Consequently, if GluR6/PSD-95 interactions are regulated dynamically, PSD-95 may fine-tune information flow through excitatory synapses by regulating the time course of recovery from desensitization. The structural determinants of the effects of PSD-95 on GluR6 desensitization have not yet been identified, although, Garcia et al. (1998) have reported that GluR6 receptor clustering is dependent on the PDZ1 domain. Whether the PDZ1 domain also confers allosteric effects on GluR6 receptor recovery behaviour requires further investigation. Taken together, our results identify a dual role for PSD-95 at postsynaptic densities consisting of the clustering of GluR6-containing kainate receptors in the plasma membrane and subsequent regulation of desensitization behaviour.
Acknowledgments
We thank Dr P. Seeburg for permission to use the cDNA for GluR6 and Drs M.L. Mayer (N.I.H., Maryland, USA), A. Ghetti and S.F. Heinemann (Salk Institute, CA, USA) as well as M. Sheng (Harvard University, MA, USA) for kindly providing cDNAs for GluR6, GFP-GluR6 and PSD-95, respectively. We also thank Dr R. Horn for the tsA201 cell line. We are indebted to Drs H. Rees and A. Levy for providing access to their confocal microscope and instruction on its use. This work was supported by the National Institutes of Mental Health (R01 MH62144, D. Bowie) and Neurological Disorders and Stroke (R01 NS36654, S.F. Traynelis; R01 NS39309, John Marshall) as well as the National Institutes of Health intramural research program (G. D. Lange). D. Bowie is a recipient of a Canada Research Chair award.
REFERENCES
- Armstrong N, Gouaux E. Mechanisms for activation and antagonism of an AMPA-sensitive glutamate receptor: crystal structures of the GluR2 ligand binding core. Neuron. 2000;28:165–181. doi: 10.1016/s0896-6273(00)00094-5. [DOI] [PubMed] [Google Scholar]
- Armstrong N, Sun Y, Chen G-Q, Gouaux E. Structure of a glutamate-receptor ligand-binding core in complex with kainate. Nature. 1998;395:913–917. doi: 10.1038/27692. [DOI] [PubMed] [Google Scholar]
- Bähring R, Bowie D, Benveniste M, Mayer ML. Permeation and block of rat GluR6 glutamate receptor channels by internal and external polyamines. J Physiol. 1997;502:575–589. doi: 10.1111/j.1469-7793.1997.575bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie D. External anions and cations distinguish between AMPA and kainate receptor gating mechanisms. J Physiol. 2002;539:725–733. doi: 10.1113/jphysiol.2001.013407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie D, Lange GD. Functional stoichiometry of glutamate receptor desensitization. J Neurosci. 2002;22:3392–3403. doi: 10.1523/JNEUROSCI.22-09-03392.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie D, Lange GD, Mayer ML. Activity-dependent modulation of glutamate receptors by polyamines. J Neurosci. 1998;18:8175–8185. doi: 10.1523/JNEUROSCI.18-20-08175.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke NA, Takimoto K, Li D, Han W, Watkins SC, Levitan ES. Distinct structural requirements for clustering and immobilization of K+ channels by PSD-95. J Gen Physiol. 1999;113:71–80. doi: 10.1085/jgp.113.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KO, Hunt CA, Kennedy MB. The rat brain postsynaptic density fraction contains a homolog of the drosophila discs-large tumor suppressor protein. Neuron. 1992;9:929–942. doi: 10.1016/0896-6273(92)90245-9. [DOI] [PubMed] [Google Scholar]
- Cohen NA, Brenman JE, Snyder SH, Bredt DS. Binding of the inward rectifier K+ channel Kir 2. 3 to PSD-95 is regulated by protein kinase A phosphorylation. Neuron. 1996;17:759–767. doi: 10.1016/s0896-6273(00)80207-x. [DOI] [PubMed] [Google Scholar]
- Evans ML, Usherwood PNR. The effect of lectins on desensitization of locust muscle glutamate receptors. Brain Res. 1985;358:34–39. doi: 10.1016/0006-8993(85)90945-x. [DOI] [PubMed] [Google Scholar]
- Everts I, Petroski R, Kizelstein P, Teichberg VI, Heinemann S, Hollmann M. Lectin-induced inhibition of desensitization of the kainate receptor GluR6 depends on the activation state and can be mediated by a single native or ectopic N-linked carbohydrate side chain. J Neurosci. 1999;19:916–927. doi: 10.1523/JNEUROSCI.19-03-00916.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts I, Villmann C, Hollmann M. N-glycosylation is not a prerequisite for glutamate receptor function but is essential for lectin modulation. Mol Pharmacol. 1997;52:861–873. doi: 10.1124/mol.52.5.861. [DOI] [PubMed] [Google Scholar]
- Garcia EP, Mehta S, Blair LAC, Wells DG, Shang J, Fukushima T, Fallon JR, Garner CC, Marshall J. SAP90 binds and clusters kainate receptors causing incomplete desensitization. Neuron. 1998;21:727–739. doi: 10.1016/s0896-6273(00)80590-5. [DOI] [PubMed] [Google Scholar]
- Garner CC, Kindler S. Synaptic proteins and the assembly of synaptic junctions. Trends Cell Biol. 1996;6:429–433. doi: 10.1016/s0962-8924(96)10036-2. [DOI] [PubMed] [Google Scholar]
- Garner CC, Nash J, Huganir RL. PDZ domains in synapse assembly and signaling. Trends Cell Biol. 2000;10:274–280. doi: 10.1016/s0962-8924(00)01783-9. [DOI] [PubMed] [Google Scholar]
- Hall RA, Premont RT, Chow C-W, Blitzer JT, Pitcher JA, Claing A, Stoffel RH, Barak LS, Shenolikar S, Weinman EJ, Grinstein S, Lefkowitz RJ. The β2-adrenergic receptor interacts with the Na+/H+-exchanger regulatory factor to control Na+/H+ exchange. Nature. 1998;392:626–630. doi: 10.1038/33458. [DOI] [PubMed] [Google Scholar]
- Heckmann M, Bufler J, Franke C, Dudel J. Kinetics of homomeric GluR6 glutamate receptor channels. Biophys J. 1996;71:1743–1750. doi: 10.1016/S0006-3495(96)79375-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horio Y, Hibino H, Inanobe A, Yamada M, Ishii T, Tada Y, Satoh E, Hata Y, Takai Y, Kurachi Y. Clustering and enhanced activity of an inwardly rectifying potassium channel, Kir4. 1, by an anchoring protein, PSD-95/SAP90. J Biol Chem. 1997;272:12885–12888. doi: 10.1074/jbc.272.20.12885. [DOI] [PubMed] [Google Scholar]
- Huettner JE. Glutamate receptor channels in rat dorsal root ganglion neurons: activation by kainate and quisqualate, and blockade of desensitization by concanavalin A. Neuron. 1990;5:255–266. doi: 10.1016/0896-6273(90)90163-a. [DOI] [PubMed] [Google Scholar]
- Jones KA, Wilding TJ, Huettner JE, Costa AC. Desensitization of kainate receptors by kainate, glutamate and diasteromers of 4-methylglutamate. Neuropharmacology. 1997;36:853–863. doi: 10.1016/s0028-3908(97)00066-x. [DOI] [PubMed] [Google Scholar]
- Kistner U, Wenzel BM, Veh RW, Cases-Langhoff C, Garner AM, Appeltauer U, Voss B, Gundelfinger ED, Garner CC. SAP90, a rat presynaptic protein related to the product of the Drosophila tumor suppressor gene dlg-A. J Biol Chem. 1993;268:4580–4583. [PubMed] [Google Scholar]
- Lerma J. Kainate receptors. In: Jonas P, Monyer H, editors. Ionotropic Glutamate Receptors in the CNS. Berlin: Springer; 1999. pp. 275–307. [Google Scholar]
- Marty A, Neher E. Tight-seal whole-cell recording. In: Sakmann B, Neher E, editors. Single-Channel Recording. New York: Plenum; 1995. pp. 31–52. [Google Scholar]
- Mayer ML, Vyklicky L. Concanavalin A selectively reduces desensitization of mammalian neuronal quisqualate receptors. Proc Natl Acad Sci U S A. 1989;86:1411–1415. doi: 10.1073/pnas.86.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehring RB, Wischmeyer E, Veh RW, Sheng M, Karschin A. Neuronal inwardly rectifying K(+) channels differentially couple to PDZ proteins of the PSD-95/SAP90 family. J Neurosci. 2001;20:156–162. doi: 10.1523/JNEUROSCI.20-01-00156.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell TJ, Christensen BN. A voltage-clamp study of isolated stingray horizontal cell non-NMDA excitatory amino acid receptors. J Neurophysiol. 1989;61:162–172. doi: 10.1152/jn.1989.61.1.162. [DOI] [PubMed] [Google Scholar]
- Okabe S, Urushido T, Konno D, Okado H, Sobue K. Rapid redistribution of the postsynaptic density protein PSD-Zip45 (Homer 1c) and its differential regulation by NMDA receptors and calcium channels. J Neurosci. 2001;21:9561–9571. doi: 10.1523/JNEUROSCI.21-24-09561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partin KM, Patneau DK, Winters CA, Mayer ML, Buonanno A. Selective modulation of desensitization at AMPA versus kainate receptors by cyclothiazide and concanavalin A. Neuron. 1993;11:1069–1082. doi: 10.1016/0896-6273(93)90220-l. [DOI] [PubMed] [Google Scholar]
- Paternain AV, Rodriguez-Moreno A, Villarroel A, Lerma J. Activation and desensitization properties of native and recombinant kainate receptors. Neuropharmacology. 1998;37:1249–1259. doi: 10.1016/s0028-3908(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Patneau DK, Wright PW, Winters C, Mayer ML, Gallo V. Glial cells of the oligodendrocyte lineage express both kainate- and AMPA-preferring subtypes of glutamate receptor. Neuron. 1994;12:357–371. doi: 10.1016/0896-6273(94)90277-1. [DOI] [PubMed] [Google Scholar]
- Piserchio A, Pellegrini M, Mehta S, Blackman SM, Garcia EP, Marshall J, Mierke DF. The PDZ1 domain of SAP90: characterization of structure and binding. J Biol Chem. 2002;277:6967–6973. doi: 10.1074/jbc.M109453200. [DOI] [PubMed] [Google Scholar]
- Saras J, Heldin CH. PDZ domains bind carboxy-terminal sequences of target proteins. Trends Biochem Sci. 1996;21:455–458. doi: 10.1016/s0968-0004(96)30044-3. [DOI] [PubMed] [Google Scholar]
- Sheng M. Molecular organization of the postsynaptic specialization. Proc Natl Acad Sci U S A. 2001;98:7058–7061. doi: 10.1073/pnas.111146298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Pak DTS. Ligand-gated ion channel interactions with cytoskeletal and signaling proteins. Ann Rev Physiol. 2000;62:755–778. doi: 10.1146/annurev.physiol.62.1.755. [DOI] [PubMed] [Google Scholar]
- Sheng M, Sala C. PDZ domains and the organization of supramolecular complexes. Ann Rev Neurosci. 2001;24:1–29. doi: 10.1146/annurev.neuro.24.1.1. [DOI] [PubMed] [Google Scholar]
- Stern-Bach Y, Bettler B, Hartley M, Sheppard PO, O'Hara PJ, Heinemann SF. Agonist-selectivity of glutamate receptors is specified by two domains structurally related to bacterial amino acid binding proteins. Neuron. 1994;13:1345–1357. doi: 10.1016/0896-6273(94)90420-0. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wahl P. Control of rat GluR6 glutamate receptor open probability by protein kinase A and calcineurin. J Physiol. 1997;503:513–531. doi: 10.1111/j.1469-7793.1997.513bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding TJ, Huettner JE. Activation and desensitization of hippocampal kainate receptors. J Neurosci. 1997;17:2713–2721. doi: 10.1523/JNEUROSCI.17-08-02713.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong LA, Mayer ML. Differential modulation by cyclothiazide and concanavalin A of desensitization at native alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid- and kainate-preferring glutamate receptors. Mol Pharmacol. 1993;44:504–510. [PubMed] [Google Scholar]
- Xu J, Paquet M, Lau AG, Wood JD, Ross CA, Hall RA. β1-Adrenergic receptor association with the synaptic scaffolding protein membrane-associated guanylate kinase inverted-2 (MAGI-2) J Biol Chem. 2001;276:41310–41317. doi: 10.1074/jbc.M107480200. [DOI] [PubMed] [Google Scholar]
- Yue K-T, MacDonald JF, Pekhletski R, Hampson DR. Differential effects of lectins on recombinant glutamate receptors. Eur J Pharmacol. 1995;291:229–235. doi: 10.1016/0922-4106(95)90062-4. [DOI] [PubMed] [Google Scholar]


