Abstract
The purpose of this study was to determine whether induced expression of the Ca2+ buffering protein parvalbumin (PV) in slow-twitch fibres would lead to alterations in physiological, biochemical and molecular properties reflective of a fast fibre phenotype. Transgenic (TG) mice were generated that overexpressed PV in slow (type I) muscle fibres. In soleus muscle (SOL; 58 % type I fibres) total PV expression was 2- to 6-fold higher in TG compared to wild-type (WT) mice. Maximum twitch and tetanic tensions were similar in WT and TG but force at subtetanic frequencies (30 and 50 Hz) was reduced in TG SOL. Twitch time-to-peak tension and half-relaxation time were significantly decreased in TG SOL (time-to-peak tension: 39.3 ± 2.6 vs. 55.1 ± 4.7 ms; half-relaxation time: 42.1 ± 3.5 vs. 68.1 ± 9.6 ms, P < 0.05 for TG vs. WT, respectively; n= 8–10). There was a significant increase in expression of type IIa myosin heavy chain (MHC) and ryanodine receptor at the mRNA level in TG SOL but there were no differences in MHC expression at the protein level and thus no difference in fibre type. Whole muscle succinate dehydrogenase activity was reduced by 12 ± 0.4 % in TG SOL and single fibre glycerol-3-phosphate dehydrogenase activity was decreased in a subset of type IIa fibres. These differences were associated with a 64 % reduction in calcineurin activity in TG SOL. These data show that overexpression of PV, resulting in decreased calcineurin activity, can alter the functional and metabolic profile of muscle and influence the expression of key marker genes in a predominantly slow-twitch muscle with minimal effects on the expression of muscle contractile proteins.
The ability of skeletal muscles to perform a wide range of functions is due to the diverse nature of the individual fibres within each muscle. This diversity is maintained by the expression of fibre type-specific proteins (Gunning & Hardeman, 1991; Schiaffino & Reggiani, 1996). The two major types of skeletal muscle fibres, slow (type I or slow oxidative) and fast (type II or glycolytic, with a varying range of oxidative potential), differ primarily in their contractile speed, metabolic profile and fatigue resistance. For fast fibres there are a number of subtypes based on their expression of the various myosin heavy chain (MHC) isoforms-type IIa, IIx and IIb which vary in their maximum contraction velocity, rate of ATP hydrolysis and fatigue resistance (Schiaffino & Reggiani, 1996; He et al. 2000). Myofibre subtypes are also distinguished by the expression of distinct fast and slow isoforms of other myofibrillar proteins such as the myosin light chains, tropomyosin and the troponin (Tn) subunits TnI, TnT and TnC. In addition, there are differences in the level of expression of enzymes of the glycolytic and oxidative pathways, in proteins involved in Ca2+ handling and in membrane bound receptors and signalling molecules which contribute to their slow or fast phenotype.
Muscle phenotype is established during embryonic and neonatal development by a complex pattern of gene expression (Buckingham, 1994). Differentiation of myogenic precursor cells into multinucleated myotubes and expression of muscle-specific genes in mature myofibres is regulated by a series of myogenic regulatory factors (MRFs) which include MyoD, myogenin, myf-5 and MRF4, as well as members of the myocyte enhancer factor 2 (MEF2) family, which form hetero-oligomeric complexes with other ubiquitous or muscle-specific co-activators (Molkentin et al. 1995). Although the transcription factors that regulate muscle determination have been identified, the transcriptional control mechanisms that lead to the differential expression of fast vs. slow fibre type-specific genes are not fully understood (Gunning & Hardeman, 1991; Buonanno & Rosenthal, 1996). Recent work implicates Ca2+-dependent regulation of nuclear factor of activated T cells (NFAT) and MEF2 proteins in determining fibre type specificity (Chin et al. 1998; Wu et al. 2000). NFAT proteins are well-characterised as Ca2+-sensitive transcription factors that regulate cytokine gene expression in T- and B-lymphocytes in response to Ca2+-dependent activation of the protein phosphatase calcineurin (Timmerman et al. 1996; Dolmetsch et al. 1997). NFAT4 and NFAT2 isoforms are abundant in skeletal muscle (Hoey et al. 1995) and a Ca2+-calcineurin-NFAT pathway has been implicated in the control of both skeletal muscle fibre type (Chin et al. 1998; Dunn et al. 1999; Wu et al. 2001), and myofibre hypertrophy (Dunn et al. 1999, 2000, 2001, 2002; Musaro et al. 1999; Semsarian et al. 1999). We have previously hypothesised that a Ca2+-calmodulin-dependent pathway exerts transcriptional control over fibre type-specific gene expression and that this is the major control point for alterations in gene expression and thus phenotype observed in different adult muscle fibre types (Chin et al. 1998; Wu et al. 2000).
In the present study we assessed whether overexpression of a major Ca2+ buffering protein, parvalbumin (PV), would influence the expression of fibre type-specific genes and the biochemical and physiological properties of skeletal muscle. Transgenic mice were generated that overexpressed PV in slow type I fibres. Parvalbumin is an EF-hand high affinity Ca2+-binding protein endogenously expressed only in fast fibre types (all type IIb but only some type IIa fibres; Fuchtbauer et al. 1991). We hypothesised that PV overexpression in type I fibres would buffer the endogenous [Ca2+]i and would attenuate any Ca2+-dependent transcriptional pathways that regulate the expression of slow fibre proteins. Our results indicate that PV overexpression in type I fibres attenuated calcineurin signalling and led to altered contractile properties of slow-twitch muscle, decreased oxidative and glycolytic capacities and increased expression of key genes, all towards a faster fibre phenotype, without altering MHC protein expression and thus fibre type per se. These findings support the general notion that Ca2+-dependent signalling pathways influence specialised properties of different myofibre subtypes but also suggest that a more complex signalling strategy exists for regulating the differences between muscle fibre types. The incomplete transformation from a slow to fast muscle phenotype in response to parvalbumin overexpression suggests that more pronounced perturbations of the Ca2+ regulatory system, or signalling inputs not altered in this model, are required to induce changes in the full complement of fibre type-specific genes.
METHODS
Generation of transgenic mice
Care and treatment of animals were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, approved by the Institutional Animal Care and Research Advisory Council (UT Southwestern), in accordance with the Canadian Council of Animal Care guidelines and approved by the Institutional Animal Care and Research Advisory Committee (Laurentian University). Transgenic mice were generated by the overexpression of a rat PV cDNA (Epstein et al. 1986) driven by the human troponin I slow (TnIs) promoter (Corin et al. 1994). A 0.8 kb PV cDNA, including an SV40 late gene intron and splice donor was excised by Xho I and Kpn I digests from the SV40-cPV plasmid (gift of Martin Berchtold; Castillo et al. 1995) and cloned into pGEM-3Zf vector (Promega). A haemaglutinin (HA) epitope tag was inserted at the 3′ end of the PV cDNA using a PCR sewing technique (Yang et al. 1997) and cloned into pGEM-7Zf vector (Promega) as a BamH I-Kpn I fragment. To direct expression to type I fibres, a 4.2 kb fragment from the 5′-flanking region of the human TnIs gene was used. The 4.2 kb TnIs Hind III fragment was excised from a −4200TnIsCAT vector (gift of Bob Wade; Corin et al. 1994) and cloned into pGEM-3Zf. The PV-HA cDNA was excised by BamH I/Kpn I digests and ligated to the −4.2 kb TnIs promoter in pGEM-3Zf. The 5 kb TnIsPV-HA transgene was released from the vector by partial Hind III and Kpn I digests and purified by electro-elution. Transgenic (TG) mice were generated using standard procedures (Hogan et al. 1994). Briefly, fertilised oocytes were obtained from super-ovulated and mated Institute of Cancer Research (ICR) females, linearised transgene was injected into the pronuclei of fertilised embryos and then transferred into pseudopregnant females. All surgical procedures were done under pentobarbital anaesthesia. Surrogate mothers gave birth to the first generation of transgenics (founders; F0). Founders were bred with wild-type (WT) littermates to generate F1 and F2 progeny and stable lines of mice were generated from offspring that showed transgene expression.
Genotyping
All mice were genotyped using genomic DNA obtained from tail samples. Transgenic F0 mice were identified by both PCR screening and Southern blot analyses. For Southern blot analysis, 15 μg of genomic DNA was digested with BstE II and separated on 0.8 % agarose gels in 40 mm Tris-acetate + 1 mm EDTA (TAE). The DNA was transferred to nylon membrane, and the membrane was probed with a 0.8 kb PV-HA fragment radiolabelled with α32P dATP and dCTP by random priming. For PCR screening, a 5′ forward primer from the PV cDNA and a 3′ reverse primer from the HA epitope were used to generate a 400 bp fragment (5′ forward: CGC GGA TCC CCC ACC AGC CCA GCT TTT CTA; 3′ reverse: TTA GGC GTA GTC GGG CAC GTC ATA TGG GTA GCT TTC GGC CAC CAG AGT GGA). Cycling conditions were: 94 °C 3 min; 94 °C 30 s, 65 °C 60 s, 72 °C 60 s for 25 cycles; 72 °C 3 min final extension. The integrity of the genomic DNA was confirmed using primers for an endogenous single copy thyroid stimulating hormone gene (TSHβ) as described by Wang et al. (1996). PCR genotyping was confirmed by Southern blot for F0 and F1 mice and then used exclusively for all subsequent generations.
Detection of PV-HA transgene expression
For all subsequent phenotypic analyses, muscles were removed from TG and WT mice following anaesthetisation with sodium pentobarbital; animals were then killed by cervical dislocation following tissue removal. To confirm expression of the PV-HA transgene, both Western blot and immunohistochemical analyses were performed. For Western blot analysis, muscle homogenates were prepared from various tissues including skeletal muscles of varying fibre type composition. To confirm expression only in type I fibres, a muscle with a high percentage of type I fibres (soleus (SOL): 58 % type I and 42 % type IIa) was compared to muscles with little or no type I fibres (plantaris (PLT): 6 % type I, 59 % type IIa, 41 % type IIb; white gastrocnemius: 1 % type I, 30 % type IIa, 69 % type IIb (Burkholder et al. 1994); diaphragm (DIA): 7 % type I, 39 % type IIa, 40 % type IIx, 10 % type IIb (Seward et al. 2001)). For quantitative analyses, 2.5 μg of homogenate was loaded on a 15 % acrylamide gel and separated using SDS-PAGE; 2.5 μg was found to give a band in the linear range of detection (data not shown). Following separation, proteins were transferred to nitrocellulose membrane and probed with rabbit anti-PV antibody (Swant PV28) diluted 1:10 000 in TBS (20 mm Tris, 137 mm NaCl) + 0.25 % Uniblock (Analytical Genetic Testing Center, Inc., Denver, CO, USA). After several washes in TBS-T (TBS + 2 % Tween 20), membranes were incubated with secondary anti-rabbit IgG conjugated to horseradish peroxidase (1:10 000 in TBS + 0.125 % Uniblock). Bands were detected with autoradiography using ultra detection reagents (Pierce). The results were subsequently scanned and quantified using Imagequant analysis software (Amersham Biosciences Corp., Piscataway, NJ, USA).
For immunohistochemical analysis of PV, 8 μm frozen transverse sections were obtained from SOL muscles from WT and TG mice, post-fixed in chilled 4 % paraformaldehyde and permeabilised with 0.3 % Triton X-100 immediately prior to staining. Sections were incubated with primary antibody for PV (Swant PV28, rabbit anti-PV) diluted 1:200. Following overnight incubation at 4 °C, unbound primary antibody was removed and the sections incubated in peroxidase or fluorescently labelled secondary antibody conjugates of rabbit and mouse IgG. Peroxidase immunolabelling was visualised with diaminobenzidine (DAB) chromogen and viewed using bright-field optics while fluorescent immunolabelling was visualised directly using dark-field epifluorescence optics.
Calcineurin phosphatase activity measurements
Calcineurin activity was measured in SOL homogenates by Isotechnika Inc. (Edmonton, AB, Canada) as described previously (Dunn et al. 2000). For this assay, freshly frozen tissue samples were subjected to a 32P-based assay using the RII subunit of cAMP-dependent protein kinase as substrate in the presence or absence of cyclosporin A.
Physiological assessments
Extensor digitorum longus (EDL) and SOL muscles were isolated from anaesthetised mice (70 mg sodium pentobarbital (kg body weight)−1, i.p.) and suspended in jacketed organ baths (Radnoti, Monrovia, CA, USA). For each muscle, the proximal tendon was secured in a stationary clamp at the base of the bath and the distal tendon connected via 4-0 silk suture to a Grass FT03 force transducer (Grass Instruments). The muscles were immersed in a physiological salt solution containing (mm): 120.5 NaCl, 4.8 KCl, 1.2 MgSO4, 1.5 CaCl2, 1.2 Na2PO4, 20.4 NaHCO3, 10 dextrose and 1.0 pyruvate and gassed with 95 % O2/5 % CO2 to maintain pH 7.6. Experiments were carried out at 30 °C. Following a 10 min equilibration, muscle lengths were adjusted to elicit maximal twitch tension (Lo), and then muscles rested quietly for a further 10 min prior to pre-stimulation at 1 and 150 Hz. Lo was re-established and, following a subsequent 10 min equilibration period, muscles were stimulated (0.2 ms square pulse width) over a range of frequencies (1, 30, 50, 80, 120, 150 Hz), each for 500 ms. Force responses at each stimulation frequency were normalised to muscle cross-sectional area (mN cm−2) which was calculated from the muscle mass divided by the product of muscle length and a muscle density of 1.06 g cm−3 (Mendez & Keys, 1960). Relative force at each stimulation frequency was determined by dividing the tension response at a given frequency by the maximum tetanic force and expressed as a percentage of maximum force (%Fmax). Time-to-peak tension and half-relaxation time were assessed for at least two twitch responses per muscle, and expressed in milliseconds.
Analysis of muscle fibre type
Myosin ATPase activity
Muscle fibre type was determined in SOL and EDL muscles from WT and TG mice by myosin ATPase histochemistry and MHC immunolabelling. For myosin ATPase activity, muscles were removed and quick frozen in isopentane pre-cooled in liquid nitrogen and stored at −80 °C until analysed. Frozen transverse sections (8 μm) were serially cut and stained using pH 4.30, 4.54 and 10.4 pre-incubation methodologies (Brooke & Kaiser, 1970). Percentages of fast and slow twitch fibres were quantfied by counting dark (type I) and light (type II) fibres (pH 4.54) in a whole SOL muscle cross-section.
Immunohistochemical profile
In a separate set of mice, expression of MHCs, the fast isoform of myosin light chain 2 (MLC2f), the slow isoform TnIs, and the fast and slow isoforms of the sarcoplasmic/endoplasmic reticulum Ca2+ pumps, SERCA1 and SERCA2, respectively, were assessed immunohistochemically. SOL muscles from WT and TG mice were excised, quick frozen in isopentane precooled with liquid nitrogen, and subsequently stored at −80 °C until processing. Serial sections (10 μm) were cut at −20 °C using a cryostat (Reichart-Jung, Heidelberg, Germany), recovered onto superfrost slides and placed in a humidified chamber at room temperature (RT) to thaw. Tissue sections were incubated for 30 min in a blocking solution consisting of 25 mm phosphate buffered saline (PBS; pH 7.4) containing either goat serum or rabbit serum (Sigma, St Louis, MO, USA). Sections stained for TnIs required additional manipulation before the initial blocking step. Sections stained for TnIs were prefixed in 100 % methanol for 10 min at −20 °C and subsequently rinsed (3 × 5 min) with PBS. All sections were incubated for 4 h at RT in working dilutions of mouse monoclonal antibodies raised against type IIa MHC (SC-71;1:100) or adult isoforms of type I MHC (BA-F8; 1:100); goat polyclonal antibodies raised against TnIs (Santa Cruz; 1:550); mouse ascites fluid against SERCA2 or SERCA1 (Calbiochem; 1:50); and antiserum against MLC2f (MF-5, Hybridoma Bank, University of Iowa; 1:10). Sections were rinsed with PBS (3 × 10 min) and incubated at RT for 2 h in peroxidase-conjugated goat anti-mouse immunoglobin (Ig) G in the case of MHC I, MHC IIa, MLC2f, SERCA2 and SERCA1 (Sigma; 1:50 for MHC I and IIa, 1:25 for MLC and 1:75 for SERCA1/2), or peroxidase-conjugated rabbit anti-goat Ig G (Sigma; 1:250) in the case of TnIs. After another PBS rinse, the bound antibodies were visualised using DAB as a chromogen. Tissues were air-dried and mounted with glycerine jelly.
The MHC composition of each muscle was determined as described previously (Dunn et al. 1999). Briefly, three different images from the mid-belly cross-section of each muscle, each section containing approximately 150 fibres, were captured at a magnification of × 100. Since the mouse SOL muscle expresses type I and IIa MHC almost exclusively, we were able to determine the fibre type proportions by counting the number of stained fibres relative to unstained fibres in each area of a section probed for type I MHC. The fibre type proportions were calculated for each muscle and the average was taken for each treatment group.
MHC gel electrophoresis
To assess the relative amounts of different MHC isozymes in WT and TG mice, we analysed muscle extracts from SOL muscle by gel electrophoresis. MHC proteins were extracted according to the procedure described by Butler-Browne & Whalen, 1984. In brief, muscles were scissor minced in high salt buffer (mm: 300 NaCl, 100 NaH2PO4, 50 Na2HPO4, 1 MgCl2, 10 Na4P2O7 and 10 EDTA) and placed on ice for 20 min. Samples were centrifuged and then the supernatant was diluted 1:10 in low salt buffer (0.1 % β-mercaptoethanol and 0.04 % EDTA) and placed overnight at 4 °C. Samples were centrifuged once again and the supernatant was then discarded. The pellet was resuspended in myosin sample buffer (0.5 m NaCl and 10 mm NaH2PO4) and was then diluted in Laemmli buffer and separated by SDS-PAGE as described by Talmadge & Roy (1993). The separating gels were composed of 30 % glycerol, 8 % acrylamide-bis (50:1), 0.2 m Tris (pH 8.8), 0.1 m glycine and 0.4 % SDS. The stacking gels consisted of 30 % glycerol, 4 % acrylamide-bis (50:1), 70 mm Tris (pH 6.7), 4 mm EDTA and 0.4 % SDS.
Assessment of muscle enzyme activity
To assess changes in whole muscle oxidative and glycolytic capacities, SOL and EDL muscles were removed from WT and TG mice and homogenised in 100 × volume of homogenising medium (170 mm Na2HPO4, 170 mm KH2PO4, 0.02 % BSA, 5 mmβ-mercaptoethanol and 2.0 mm EDTA, pH 7.4) with protease inhibitors (Complete, Mini; Boehringer Mannhein, Germany). Whole muscle homogenates were assayed for succinate dehydrogenase (SDH) and lactate dehydrogenase (LDH) activity using standard enzymatic techniques (Bergmeyer, 1974). Homogenate protein concentration was determined using a bicinchoninic acid (BCA) method (Pierce) and activities expressed in μmole NADH (g protein)−1 min−1. Additional assessments of oxidative and glycolytic capacities were made using quantitative histochemical procedures in a separate set of mice.
Quantitative histochemical determination of single fibre enzyme activity
Metabolic enzyme activity was assessed in both WT and TG mouse SOL using SDH as a marker for oxidative metabolism and FAD-linked glycerol 3-phosphate dehydrogenase (GPDH) as a marker of glycolytic activity as previously described (Dunn & Michel, 1997; Madison et al. 1998). Briefly, nitro blue tetrazolium (NBT) in the presence of succinic acid (SDH) or α-glycerophosphate (GPDH) is reduced to form an insoluble diformazan salt by dehydrogenase activity and the reduction rate is a proportional to enzyme activity. Rate measurements were made by finding the optical density of a tissue slice at timed intervals using a computer assisted image analysis system. The imaging apparatus and software are described in detail elsewhere (Jasmin et al. 1995). Images were filtered (540 nm), captured, saved and processed with an image-analysis software program (Media Cybernetics, Silver Spring, MD, USA).
For SDH activity measurements, serial cross-sections (10 μm) were cut (−20 °C) from the SOL midbelly, recovered on superfrost slides (Fisher Scientific), inverted and secured to a reaction chamber mounted on a temperature-controlled slide holder clamped to the microscope stage. All reactions were done in absence of light at 23 °C. A substrate-free blank solution containing 1 mm NaN3, 500 μm 1-methoxy-phenazine methosulfate (mPMS), 1.5 mm NBT and 5 mm EDTA in 100 mm sodium phosphate buffer (pH 7.6) was added to the chamber for 10 min to allow non-specific staining to plateau. The blank was subsequently removed and replaced with a substrate solution containing the same reagents as above plus 48 mm succinic acid. Upon addition of the substrate solution, images were taken every minute for 5 min. During this time, increases in optical density (OD) are linear (Madison, et al.1998).
For GPDH activity measurements, serial sections (14 μm) were cut, recovered on glass coverslips and distributed evenly between two coplin jars kept at −20 °C. A blank solution containing 1 mm NaN3, 1 mm mPMS, 1.2 mm NBT in 100 mm sodium phosphate buffer (pH 7.4, 37 °C) was added to one jar and an identical solution plus 9.3 mmα-glycerophosphate introduced to the other for the substrate reaction. Both jars were incubated in the dark for 23 min corresponding to the linear phase of the reaction (data not shown) at 37 °C. Both reactions were then stopped by extensive rinsing with distilled water (5 times plus 3 × 1 min). The sections were air dried and mounted onto slides with glycerine jelly (Sigma). Images of both the blank (considered as time 0) and end-point substrate reactions were captured and processed for OD analysis.
Analysis was carried out by tracing each selected individual cell using an interactive cursor, and storing the resulting area of interest onto a disk. The average greyness for all pixels within this traced area was determined across all saved sequential images and converted to an OD value. The enzyme-specific activities were expressed as the change of OD over time (OD min−1; average r2= 0.995). The cross-sectional area of each traced cell was also determined from these images.
Analysis of muscle gene expression
Changes in the expression of downstream target genes were assessed by reverse transcriptase polymerase chain reaction (RT-PCR) and, for selected genes, were confirmed by Northern blot analyses. For RT-PCR analysis, mRNA was obtained from whole SOL muscle, reverse-transcribed and amplified using PCR and final products run on ethidium-bromide stained gels (Gauthier et al. 1997). For the PCR reaction, the following protocol was used: 9 °C 3 min; 94 °C 30 s, 50 °C 60 s, 72 °C 60 s × 25 cycles; 72 °C 3 min final extension; the cycle number varied for the different primer pairs and was based on the previously determined linear range (Dunn et al. 1999). For each primer set, cycle number was adjusted to permit comparison of PCR products between treatments within the linear phase of amplification. Tubes that contained PCR reagents plus the RT-negative sample or ultrapure water served as controls. For quantification, product bands and representative background were excised from each lane and Cerenkov counted (cpm). The fibre type-specific mRNA assessed included: MHC I, IIa, IIx and IIb, slow type SERCA2, skeletal muscle ryanodine receptor (RYR1), myoglobin, phospholamban, actin, GAPDH and PV. The 28 S ribosomal RNA subunit served as an internal control and was not different between WT and TG.
Statistical analyses
Differences in muscle twitch contractile properties, twitch and tetanic force, the force-frequency relationship and whole muscle homogenate enzyme activities were assessed using one-way analysis of variance (ANOVA). Where necessary, post hoc analyses were completed using a Student-Newman-Keuls test. For single fibre enzyme activities, fibre cross-sectional area, MHC proportions, and RT-PCR products, values were compared among groups using an ANOVA and a Scheffépost hoc procedure. All groups had acceptable values for homogeneity of variance therefore parametric statistical tests were used. Significance was accepted at P < 0.05.
RESULTS
Establishment of transgenic lines
The TnIsPV-HA transgene was detected in 4 out of 25 pups born from two surrogate mothers. Founders were identified by Southern blot and PCR analysis (see Fig. 1). The four positive founders (nos 6404, 6412, 6418 and 6427) were all healthy and able to breed when crossed with wild-type mice to generate F1 progeny. Genotyping of F1 offspring revealed germline transmission in three lines (nos 6404, 6412 and 6418) with 53 % of F1 progeny being positive for the transgene. Western blot analyses of SOL muscle from F1 mice confirmed expression of the PV-HA protein in offspring from nos 6412 and 6418 (see Fig. 2). Independent lines were maintained from these two founders.
Figure 1. Genotyping of transgenic founders.
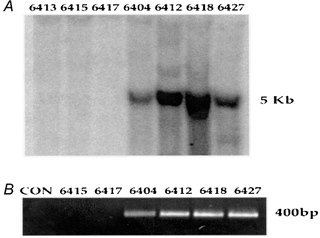
A, Southern blot analysis of the founder generation identified 4 out of 25 pups as being positive for the PV-HA transgene (see Methods for details). Mouse nos 6413, 6415 and 6417 were wild-type littermates and nos 6404, 6412, 6418 and 6427 were positive founders. B, these genotyping results were confirmed by PCR screening using a forward primer from the PV cDNA and a 3′ reverse primer from the HA epitope to generate a 400 bp product representing the PV-HA cDNA.
Figure 2. Expression of the PV-HA transgene.
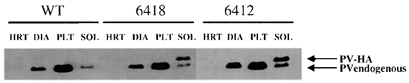
Western blot analysis of F1 offspring from two of the four founder lines (see Methods for details). Samples from the heart (HRT), diaphragm (DIA), plantaris (PLT) and soleus (SOL) muscles (2.5 μg total protein) were analysed using a rabbit anti-PV antibody. The endogenous PV (∼12 kDa) was detected as a strong band in PLT and DIA, a faint band in SOL and was not detected in HRT. The PV-HA transgene (∼14 kDa) was strongly expressed only in SOL muscle from TG mice.
Muscle fibre type specificity of transgene expression
To assess the fibre type-specific expression pattern of the PV-HA transgene, muscles of varying fibre type composition were examined. Figure 2 illustrates the specificity of PV-HA expression. Endogenous PV was detected in all skeletal muscles that have type IIa, IIx or IIb fibres, including SOL, PLT and DIA but not heart (HRT). The PV-HA transgene, however, was only detected in SOL muscle which has a high content of type I fibres. In WT SOL muscle, PV expression was 10–14 % of that in the fast PLT muscle. Endogenous PV expression was not changed in SOL from the 6418 line but was upregulated in the 6412 line (to 36 % of PLT level). As a result, total PV expression (endogenous + transgene) in TG SOL was 2- and 6-fold higher in the 6418 and 6412 lines, respectively, compared to WT levels. Analysis of non-muscle tissues including brain, liver, spleen and thymus confirmed there was no expression of the transgene in these non-muscle tissues (data not shown).
The fibre type specificity of transgene expression was further verified by immunohistochemical staining. It has previously been shown that PV is expressed in all type IIb but only in larger type IIa fibres (Fuchtbauer et al. 1991). We show that endogenous PV is only expressed in ∼13% of fibres in WT SOL muscle (see Fig. 3), which represent a subpopulation of type IIa fibres. In SOL muscle from TG mice the number of fibres expressing PV was dramatically increased to 74 % of total fibres with the unstained fibres representing type IIa fibres that have neither endogenous PV or transgene expression.
Figure 3. Immunohistochemical analysis of PV expression.
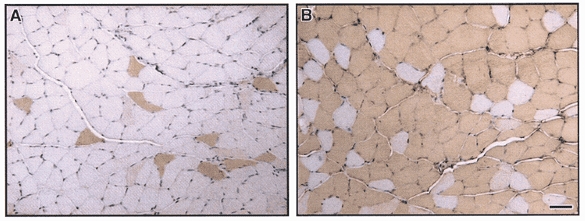
Immunohistochemical staining for PV in soleus (SOL) muscle from wild-type mice and transgenic mice overexpressing the PV-HA transgene. PV was expressed in only a subpopulation of fibres in SOL from wild-type mice (A) and in almost all fibres from TG mice (B). In SOL from TG mice, the positively staining fibres represent both endogenous PV and PV-HA transgene, resulting in ∼6-fold greater number of PV-expressing fibres in TG SOL compared to WT. Bar, 25 μm.
Physiological effects of PV-HA transgene expression
The effects of PV-HA expression in type I fibres on muscle contractile responses were assessed by comparing differences in SOL muscles from WT and TG mice. The contractile function was also compared to the EDL, a fast muscle (51 % type IIa and 49 % type IIb fibres (Fuchtbauer et al. 1991)) of similar size and weight. For mice used in this study, body weights, muscle weights and lengths of SOL or EDL, and cross-sectional area of EDL were not different between conditions; SOL cross-sectional area was reduced in TG mice (see Table 1). In a separate set of mice it was determined that mean fibre cross-sectional area for type I or IIa fibres was not different between conditions (type I: 1891 ± 134 vs. 1614 ± 76 mm2 in WT vs. TG, respectively; type IIa: 1477 ± 166 vs. 1334 ± 87 mm2 in WT vs. TG, respectively) thus muscle fibre size was not altered.
Table 1.
Animal and muscle characteristics
| Wild-type (n) | Transgenic (n) | |
|---|---|---|
| Body weight (g) | 28.5 ± 7.4 (4) | 27.1 ± 0.6 (5) |
| Muscle weight (mg) | ||
| SOL | 8.66 ± 0.56 (8) | 7.54 ± 0.29(10) |
| EDL | 8.96 ± 0.(8) | 7.81 ± 0.38(10) |
| Muscle length (mm) | ||
| SOL | 11.61 ± 0.25(8) | 11.80 ± 0.19(10) |
| EDL | 13.32 ± 0.32(8) | 12.41 ± 0.14(10) |
| Cross-sectional area (cm2) | ||
| SOL | 0.702 ± 0.04 (8) | 0.604 ± 0.02* (10) |
| EDL | 0.635 ± 0.03(8) | 0.593 ± 0.03(10) |
Values are means ±s.e.m.
P < 0.05 for wild-type vs. transgenic. Number of muscle samples (n) shown in parentheses. Data are combined for the two transgenic lines 6412 (4 WT and 4 TG) and 6418 (4 WT and 6 TG).
Muscle twitch (1 Hz) and maximum tetanic (150 Hz) tensions, expressed per unit cross-sectional area, were not different in SOL from WT and TG mice; there were also no differences for EDL muscles (Fig. 4). There were, however, differences in the twitch contraction and relaxation properties of SOL muscle (Fig. 5). On average, twitch time-to-peak tension was reduced by 29 % in TG compared to WT SOL (39.3 ± 2.6 vs. 55.1 ± 4.7 ms, respectively) but was not altered in EDL (22.2 ± 0.6 vs. 22.7 ± 0.7 ms for TG vs. WT, respectively). Similarly, halfrelaxation time was reduced by 38 % in TG compared to WT SOL (42.1 ± 3.5 vs. 68.1 ± 9.6 ms, respectively) but was not different for EDL (18.6 ± 1.7 vs. 23.8 ± 3.4 ms for TG vs. WT, respectively). These data demonstrate a specificity of this phenotype in SOL muscle where the transgene was expressed and eliminate the possibility that all muscles were affected in a similar manner. The maximum rate of force decline (−dF/dt) was also faster in TG (−1.21 N s−1) compared to WT (−0.81 N s−1). The faster relaxation rate in TG SOL is consistent with PV-HA functioning as an effective buffer for Ca2+ during twitch contractions. Following the faster decline in force, there was a slower return to baseline, which may reflect a slower rate of Ca2+ uptake into the SR (Garcia & Schneider, 1983) or a decrease in the contribution of mitochondria to relaxation. The decrease in Ca2+ buffering by mitochondria would be predicted based on the increased rate of relaxation and faster removal of Ca2+ by mitochondria in fast twitch muscles from PV-/- mice (Chen et al. 2001) and are supported by the decrease in SDH activity observed in these muscles (see subsequent section). The differences in SOL muscle performance were similar for both lines of mice and the data are pooled (see footnote to Table 1).
Figure 4. Muscle twitch and maximum tetanic tension.
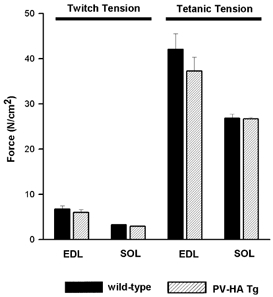
Twitch (1 Hz) and maximum tetanic (150 Hz) force were assessed in soleus (SOL) and extensor digitorum longus (EDL) muscles from wild-type (black bars) and transgenic (hatched bars) mice expressing the PV-HA transgene. Contractile force was normalised to cross-sectional area (see Methods). Values represent means ±s.e.m. for 10–12 muscles.
Figure 5. Muscle twitch contractile properties.
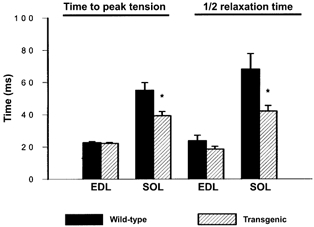
Muscle twitch contractile properties were assessed in SOL and EDL muscles from wild-type and transgenic mice. Summary data for time-to-peak tension and half-relaxation time for twitches analysed in SOL and EDL from WT and TG mice. Values represent means ±s.e.m. (n= 10 for WT and n= 12 for TG). *P < 0.05.
Force output was measured over a range of stimulation frequencies in SOL and EDL muscles and expressed as a percentage of maximum force. As illustrated in Fig. 6, force was reduced in SOL from TG compared to WT mice by 14 ± 0.8 % and 8 ± 0.5 % at 30 and 50 Hz, respectively. No differences were observed at higher frequencies or in EDL muscle at any of the frequencies tested. These data support the role of PV in buffering [Ca2+]i at sub-tetanic levels where there are non-saturating levels of [Ca2+]i (Westerblad & Allen, 1993).
Figure 6. Muscle force-frequency relationship.
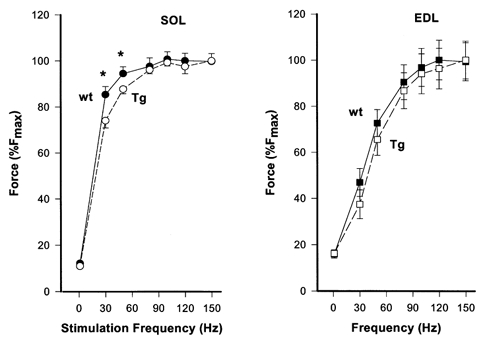
Force was measured over a range of stimulation frequencies in SOL and EDL muscles from wild-type (wt) and transgenic (Tg) mice. Force output is expressed as a percentage of the maximum (150 Hz) force for each muscle (%Fmax). Values plotted represent the means ±s.e.m. (n= 10 for WT and n= 12 for TG). *P < 0.05.
Effects of PV-HA expression on muscle fibre type in soleus muscle
Muscle fibre type was assessed by myosin ATPase activity, MHC immunohistochemistry and MHC isoform distribution by gel electrophoresis. Because fibre type distributions have been reported to vary between males and females (Ustunel & Demir, 1997) and transgene expression may have gender-specific effects, we assessed type I fibre content by myosin ATPase activity in SOL muscle from male and female TG mice and compared it to age and sex-matched WT mice. In males, type I fibre content in SOL was not different between WT (48 ± 4 %; n= 4) and TG (6412 line, 50 ± 3 %; n= 4). In females, type I fibre content in SOL was also similar between WT (54 ± 2 %; n= 3) and TG (6412 line, 60 ± 3 %; n= 5 and 6418 line, 54 ± 3 %; n= 4). Therefore, there was no difference in type I fibre content between females and males for either the WT or TG, nor was there a gender-specific effect of PV expression. Muscle fibre type was also assessed by the more sensitive immunohistochemical technique. Cross-sections of SOL muscle from female WT and TG mice were incubated with antibodies directed against type I or type IIa MHC. The results were consistent with the myosin ATPase results, showing no difference between WT (51 ± 4 % type I and 49 ± 4 % type IIa; n= 5) and TG (57 ± 2 % type I and 43 ± 2 % type IIa; n= 5) SOL, with both conditions displaying a similar number of cells (−5 %) decorating for both I and IIa MHC (data not shown). To further examine the myosin expression profile in TG vs. WT muscle, MHC composition of the SOL was analysed using gel electrophoresis. This method would also determine whether there were changes in the number of hybrid I/IIa fibres, which are a characteristic of isoform transitions. There were no differences in MHC distributions in TG compared to WT SOL (65.3 ± 2.7 % and 63.5 ± 1.4 % MHC I in WT and TG, respectively; see Fig. 7G). These data suggest there was no shift in MHC isoform or increase in the number of hybrid fibres. Higher type I MHC values as detected by SDS-PAGE compared to immunostaining fibre proportions may be related to the size of type I fibres, which in the SOL, are larger on average than type IIa fibres. Collectively, these data indicate there was no change in MHC expression at the protein level as a result of PV overexpression.
Figure 7. Immunohistochemical detection of myofibrillar and Ca2+ regulatory proteins in serial cross-sections of soleus muscle from transgenic mice.
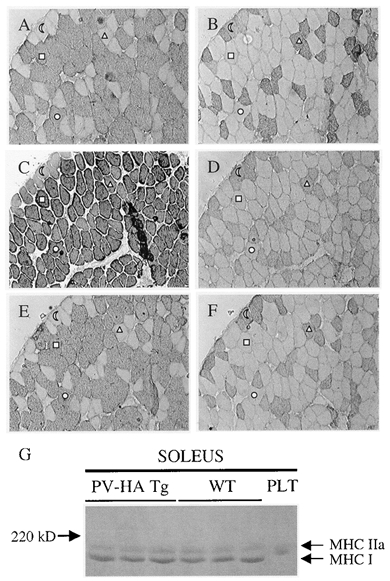
Serial sections of SOL from a transgenic mouse (A–F) were stained with antibodies raised against MHC types I (A) and IIa (B) as well as troponin I slow (C), the regulatory MLC2f (D), SERCA2 (E) and SERCA1 (F) viewed at w 100 magnification. Fibres labelled with (half moon) and (triangle) express type I MHC and the ones labelled with (square) and (circle) express type IIa MHC in the different cross-sections. This pattern was consistent in all muscle sections analysed. G, MHCs from soleus muscles of WT (n = 3) and Tg (n = 3) mice were extracted and separated by SDS-PAGE. The PLT serves as an example of IIx/IIb MHC migration. Note that in the soleus, only type I and IIa MHC were detected.
Effect of PV-HA overexpression on other muscle regulatory proteins and calcineurin activity
The observation of a faster time-to-peak tension despite a similar MHC profile in the TG SOL suggested there may have been adaptations in other myofibrillar or regulatory proteins that would explain the faster contractile speed. To directly assess this possibility, serial cross-sections were analysed for expression of marker proteins of slow (TnIs, SERCA2 and MHC I) and fast (MLC2f, SERCA1 and MHC IIa) fibres. Although non-quantitative, this analysis provides information on colocalisation of fibre type-specific gene products. Figure 7 shows representative sections of SOL muscle from TG mice (6412 line). All fibres showed the expected co-expression pattern of the various fibre type-specific proteins. Thus, TnIs and SERCA2 were only expressed in type I fibres and MLC2f and SERCA1 were only expressed in type IIa fibres. This was consistent in all muscle sections analysed (n= 5 each for WT and TG). Thus, mismatches in the regulatory proteins expressed in slow and fast fibres cannot account for the contractile differences observed between WT and TG mice.
Alterations in gene expression at the mRNA level were assessed by both RT-PCR (Fig. 8) and Northern blot (data not shown). As shown in Fig. 8, in TG compared to WT SOL, there were increases in expression of type IIa fast MHC and in the ryanodine receptor (RYR1), a gene that is expressed in higher abundance in fast fibres. As expected, PV was also increased in TG SOL. Expression of the slow MHC I or SERCA2 or of the fast type IIx MHC were not changed. There were also no changes for other genes regulated in a fibre type-specific fashion including phospholamban, myoglobin or GAPDH (data not shown). Interestingly, the level of PV expression was highly correlated (r= 0.759) to MHC IIa expression and displayed a distinct transgene effect in that mRNA levels for PV and MHC IIa were clearly higher in the muscles of TG mice. To verify if changes in mRNA levels for these proteins were related to changes in calcineurin activity, we measured the latter in homogenates of SOL muscles from WT and TG mice. Transgenic mice expressing the PV-HA transgene displayed calcineurin activities that were 36 % of WT counterparts (Fig. 8D). These data indicate that overexpression of PV, and the resultant decreased signalling through calcineurin, results in persistent changes in the expression of some, but not all, representative fast fibre genes. However, the change in type IIa MHC expression was not manifest at the protein level. This suggests that a more complex strategy (i.e. post-translational modification or protein turnover) exists to regulate steady state MHC protein levels and therefore contractile speed in SOL muscles.
Figure 8. Downstream target gene expression in soleus muscle assessed by RT-PCR.
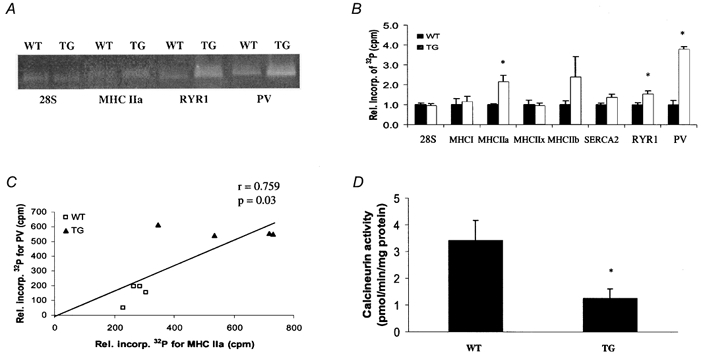
The effects of PV overexpression on the expression of fibre type-specific genes that may be downstream of a Ca2+-regulated transcriptional pathway were assessed by RT-PCR. The 28S ribosomal RNA served as an internal control and was not different between groups. A, ethidium bromide-stained gels of RT-PCR products for 28S, MHC IIa, RYR1 and PV transcripts from wild-type (WT) and transgenic (TG) soleus muscle. B, abundance of RT-PCR products expressed relative to WT control. Values represent means ±s.e.m. counts min−1 (cpm) of RT-PCR products derived from individual muscle RNA samples (n= 4 per group). There was a significant increase in MHC IIa, RYR and PV expression in TG compared to WT SOL. C, linear correlation between PV and MHC IIa expression. D, calcineurin phosphatase activity from WT and TG soleus muscle. Values represent means ±s.e.m. (n= 3 per group). *P < 0.05vs. WT.
Muscle oxidative and glycolytic capacity
The oxidative and glycolytic capacities for SOL and EDL muscles from WT and TG mice were assessed in whole muscle homogenates. Subsequent analyses of the SOL muscle only were completed at the single fibre level. SDH activity, an index of muscle oxidative capacity, was significantly reduced in homogenates from TG SOL (7.5 ± 0.4 vs. 6.6 ± 0.3 μmole NADH (g protein)−1 min−1 for WT vs. TG SOL, respectively) but was not different in TG EDL (3.8 ± 0.2 vs. 3.5 ± 0.2 μmol NADH (g protein)−1 min−1 for WT vs. TG EDL, respectively). These data are consistent with the observed increase in mitochondrial volume and cytochrome c oxidase activity in PV-deficient mice (Chen et al. 2001). Since the 6412 line had a higher level of PV protein expression (endogenous + PV-HA transgene), we tested whether there was a dose-response relationship between muscle PV content and oxidative capacity by measuring SDH activity in SOL muscle from each line independently and from TG mice obtained from a 6412 × 6418 cross with both insertional sites. As illustrated in Fig. 9, for both the single and the combined TG lines, there was a significant decrease in SDH activity compared to WT response (Fig. 9A). This decreased activity was consistent with the higher levels of PV total protein in TG SOL (Fig. 9B). As a result there was a strong inverse correlation (r=−0.975) between PV protein content and SDH activity (Fig. 9C). In an attempt to determine whether metabolic capacity was reduced in type I or type IIa fibres or both, SDH activity was measured in serial cross-sections from WT and TG SOL muscles. Densitometric analyses of SDH activities for five WT and five TG SOL (6412 line n= 2; 6418 line n= 3) showed small, but non-significant decreases in SDH activity in both type I and IIa fibres (see Fig. 10B and D), consistent with the small (12 %) decrease in whole muscle homogenate SDH activity.
Figure 9. Biochemical determination of muscle oxidative capacity.
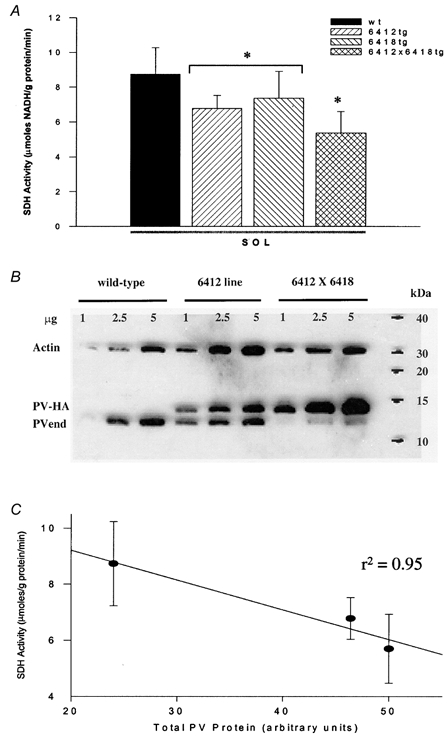
Whole muscle succinate dehydrogenase (SDH) activity was measured in SOL muscle from transgenic (TG) mice from the 6412 and 6418 lines and the 6412 × 6418 cross compared to wild-type (WT) mice to determine whether there was a dose-response relationship with PV expression. A, average data for SDH activity for each TG line. B, representative Western blot of SOL muscle from the 6412 and 6412 × 6418 TG lines shows the level of PV expression (endogenous PV (PVend) and PV transgene (PV-HA)) relative to actin expression. C, there was a strong negative correlation (r=−0.975) between total PV (endogenous +PV-HA) and whole muscle SDH activity. Values shown in bar graphs are means ±s.e.m.*P < 0.05vs. WT.
Figure 10. Scattergrams of FAD-linked GPDH activity, SDH activity and cross-sectional area of soleus single muscle fibres.
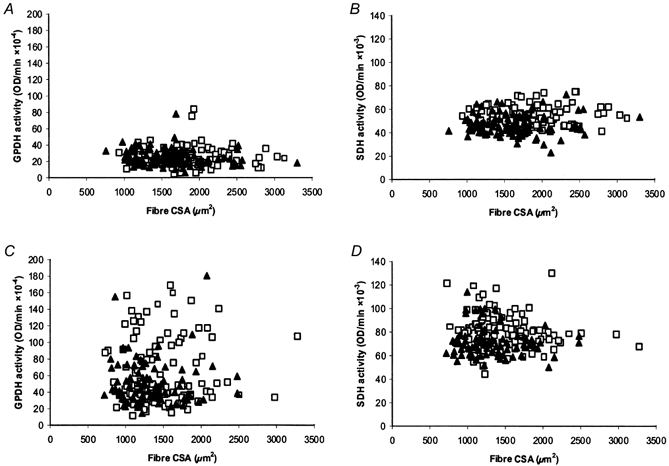
Single muscle fibres expressing MHC types I (A and B) and IIa (C and D) were analysed in serial cross-sections for fibre size (cross-sectional area, CSA), FAD-linked GPDH activity and SDH activity. Fibres for wild-type (□) and PV transgenic animals (▴) are shown. Each graph is a composite of five animals from each group.
Muscle homogenate LDH activity, a marker for muscle glycolytic capacity, was not different between TG and WT SOL. GPDH, a glycolytic marker which has been shown to be more sensitive to, and to precede, changes in muscle fibre type transitions (Dunn & Michel, 1997) was also analysed at the single fibre level using FAD-linked GPDH activity. This analysis revealed a subtle shift to lower GPDH activity in type IIa fibres due in large part to a population of IIa fibres displaying a lower level of GPDH (i.e. activities between those observed in type I and IIa fibres) (Fig. 10A and C). On average, GPDH activity in type IIa fibres from WT SOL ranged from 20 to 180 optical density units (ODU) min−1 with fibres clustered between 20–50 and 60–100 ODU min−1. The overall range for TG fibres was similar (10-170 ODU min−1) but in contrast to WT, most fibres were 20–50 ODU min−1 and only a few outliers were at the extreme range of > 100 ODU min−1. This decrease in GPDH activity suggests that a greater number of fibres are in a transitional metabolic state from type IIa to type I in TG SOL. It is tempting to speculate that this subset of type IIa fibres with lower GPDH levels in TG mice are cells that are in a perpetual state of IIa to I to IIa transitions as a result of the TnIs-linked PV transgene, though these transitions were apparently limited to metabolic enzymes since we did not observe a higher number of fibres co-immunostaining for type I and IIa MHCs under these conditions. Overall, these data indicate that subtle alterations in muscle oxidative and glycolytic capacities occurred without any alteration in the major muscle fibre type.
DISCUSSION
In this study we examined the physiological, biochemical and molecular alterations in slow-twitch skeletal muscles from transgenic mice in which the fast fibre-specific Ca2+-buffering protein parvalbumin was overexpressed in slow fibres. In the predominantly slow soleus muscle, twitch and tetanic force were not altered but force at subtetanic frequencies (30 and 50 Hz) was attenuated, and twitch contraction and half-relaxation times were reduced. These physiological alterations, along with the decreased level of calcineurin activity, are consistent with the PV-HA protein functioning as an effective Ca2+ buffer and resulting in decreased signalling via the calcineurin pathway. Overexpression of PV also resulted in decreased activities of enzymes representative of muscle oxidative and glycolytic capacities. Myosin heavy chain type IIa and RYR1 expression were altered at the mRNA level but at the protein level, MHC, and thus muscle fibre type, was not altered. These results indicate that subtle fibre type differences persist in muscle in vivo following increased expression of a Ca2+ buffer and suggest that metabolic capacities are regulated differently from the major myofibrillar proteins. Furthermore, these data are consistent with the hypothesis that the endogenous level of intracellular Ca2+ in a muscle fibre plays an important role in regulating its gene expression pattern but also suggest that counter regulatory strategies exist to maintain the native myofibrillar protein content.
Effects of PV overexpression on muscle mechanical function
In the present study, overexpression of PV in type I muscle fibres resulted in a decrease in twitch time-to-peak tension, a decrease in twitch half-relaxation time and reduction in force at intermediate stimulation frequencies. Since PV is a slow Ca2+ buffer, the rise time of free Ca2+ would be reduced and the amount of Ca2+ bound to TnC reduced at any given time during a semi-fused tetanus. This would explain why force output at sub-tetanic frequencies was lower in the PV-HA TG mice. The alterations in relaxation time and sub-tetanic force were probably a direct consequence of the increased buffering of cytosolic Ca2+ by PV. These data are consistent with observations in PV deficient mice where there was a prolongation of relaxation in the absence of PV (Schwaller et al. 1999) and with observations of a faster rate of relaxation in muscles injected with the Ca2+ chelator EDTA (Johnson et al. 1999). The shortened contraction time cannot, however, be explained by Ca2+ buffering since increased buffering of Ca2+ upon release from the SR would slow the increase in force. Thus, the faster time-to-peak tension suggests that alterations in other contractile proteins may have occurred. We have ruled out changes at the protein level of the major myofibrillar proteins including the MHCs, TnIs and MLC2f as well the Ca2+ regulatory proteins SERCA1 and SERCA2, data which is consistent with those in PV deficient mice where no changes in MHC isoform expression or SR Ca2+ ATPase activity were observed (Raymackers et al. 2000). Increased expression of RYR1 mRNA, if it were to proceed to the protein level, would account for the faster contraction rate. Alternatively, changes in other troponin subunits or in tropomyosin could explain the decrease in time-to-peak tension observed in soleus muscle from TG mice.
Transgenic approach to understanding muscle phenotype
Our current understanding of the transcriptional mechanisms that regulate skeletal muscle fibre type differences has primarily been based upon findings from studies using reporter genes in cultured myotubes. It is difficult to investigate the regulation of muscle fibre type-specific genes in cultured myocytes due to the absence of neural input and the lack of cell lines that accurately reflect fast and slow muscle phenotypes. In the present study a transgenic mouse model was used to assess differences in the expression of fibre type-specific genes between muscles of varying fibre type composition in response to the overexpression of a Ca2+-buffering protein. In our model, the transgene was regulated by the TnIs promoter and therefore expressed during primary myofibre formation, throughout embryonic development and then postnatally restricted to slow-twitch skeletal muscle fibres (Sutherland et al. 1993; Levitt et al. 1995). Thus, it was expected that [Ca2+]i would be modulated by the PV-HA protein in both fetal and adult muscle fibres, although we cannot distinguish the effects of PV expression during embryogenesis from those occurring during the adult state. We were unable to determine the changes in [Ca2+]i in response to stimulation in vitro in myofibres from these transgenic mice due to technical limitations related to obtaining single fibres for Ca2+ imaging from SOL muscle. While it is possible that the PV-HA protein had altered Ca2+-binding characteristics compared to endogenous PV and could not effectively buffer [Ca2+]i, the effects on relaxation and downstream signalling were still manifest by expression of the transgene. Furthermore, the attenuation of sub-tetanic force (30 and 50 Hz) in transgenic SOL muscles in vitro provides indirect evidence that the PV-HA protein was an effective Ca2+ buffer at lower frequencies of stimulation. This further implies that [Ca2+]i would be attenuated and downstream signalling blunted during normal locomotor cage activity in vivo (at 10–15 Hz; Hennig & Lomo, 1985). Thus, one would predict that the PV-HA protein could decrease [Ca2+]i and force during normal tonic activity (i.e. standing, ambulation) and affect gene expression patterns throughout the adult life of the mice.
Previous studies have shown that direct injection of a PV cDNA into regenerating skeletal muscle results in shortening of half-relaxation time and a shift in muscle fibre type to increased type I fibres (Muntener et al. 1995). Our study has confirmed the role of PV as a relaxing factor in skeletal muscle. However, unlike the previous study we did not observe alterations in MHC expression at the protein level. This discrepancy may reflect different levels of transgene expression or differences in the timing of PV expression, the latter being a direct consequence of differences in the two models used. In the present study we used germline transmission whereas the previous study used somatic gene transfer during muscle regeneration. With somatic gene transfer, the cDNA can be incorporated into satellite cells that are recruited in the regeneration process and transgene expression is transient. This would not necessarily induce the same changes in muscle phenotype as germline transmission where transgene expression is ongoing and may invoke different compensatory mechanisms. A further advantage of the transgenic approach is the ability to investigate normal physiological mechanisms involved in modulating gene expression such as those initiated by the nerve, without initiating injury-response mechanisms of the type related to regeneration.
Regulation of fibre type-specific gene expression in skeletal muscle
The results of this study indicate that selected oxidative and glycolytic enzyme levels were decreased and the expression of some fibre type-specific proteins altered due to the increase in PV expression and decreased signalling via the calcineurin pathway. It is known that during differentiation of myoblasts into elongated myotubes, the myogenic regulatory factors direct expression of a range of muscle-specific genes that mimic those of mature adult muscle fibres including myosin heavy chains, oxidative and glycolytic enzymes and proteins involved in excitation-contraction coupling. Various transcription factors regulated by Ca2+-dependent signalling pathways may be involved in the neurally mediated slow fibre gene expression programme. We previously postulated that a sustained low amplitude intracellular Ca2+ level could activate the Ca2+-dependent phosphatase calcineurin and downstream transcription factors to selectively activate slow fibre-specific genes (Chin et al. 1998). The interaction of the Ca2+-dependent transcription factors (NFATs) with muscle-specific transcription factors such as MEF2 are thought to activate slow fibre gene expression by binding to consensus sequences on slow fibre-specific promoters. MEF2 is known to be regulated by both the Ca2+-calmodulin-dependent kinase CamKIV (Wu et al. 2000, 2002) and calcineurin (Dunn et al. 2001; Wu et al. 2001) pathways. Others have shown a Ca2+ dependence of slow fibre-specific gene expression through a Ca2+-sensitive protein kinase C pathway (Freyssenet et al. 1999), although the downstream transcription factors have not been identified. Myogenin itself may play a role in the neural or Ca2+-regulated transcriptional pathway since it is expressed at higher levels in slow-twitch fibres, is controlled by innervation (Hughes et al. 1993) and its expression is upregulated by increased intracellular Ca2+ levels (Thelen et al. 1998). Myogenin appears to play a greater role in regulating metabolic capacity than in determining myofibrillar protein expression since transgenic mice overexpressing myogenin in fast skeletal muscle fibres have decreased glycolytic and increased oxidative capacities in the absence of any changes in myosin heavy chain profile (Hughes et al. 1999). In the present study we showed that PV overexpression and decreased calcineurin activity resulted in alterations in the metabolic profile but not in myosin heavy chain expression at the protein level although it did result in significant upregulation of MHCIIa at the mRNA level. Collectively these data suggest that there are multiple Ca2+-dependent transcriptional pathways that may act synergistically through post-translational modification of different transcription factors to selectively activate a slow fibre programme. Our current data also suggests that transcriptional changes may or may not translate to stable changes at the protein level.
The role of Ca2+ in regulating muscle gene expression
There is increasing evidence that intracellular Ca2+ plays an important role in regulating muscle gene expression. In addition to regulating fibre type-specific gene expression, Ca2+-dependent transcriptional pathways have been implicated in muscle hypertrophic growth. A calcineurin-dependent pathway has been implicated in both cardiac (Molkentin & Olson, 1997; Molkentin et al. 1998) and skeletal (Musaro et al. 1999; Semsarian et al. 1999; Dunn et al. 1999, 2000, 2001, 2002) muscle hypertrophy. In the present study we did not see a change in fibre size due to PV overexpression. This is not surprising since PV would putatively buffer additional Ca2+ in non-stressed fibres. It is expected that the amplitude and duration of the Ca2+ transients were altered by the PV-HA protein but the precise mechanism by which these variables regulate fibre size, metabolic enzymes or contractile proteins is not known. In addition to buffering Ca2+, the occupancy of PV by Ca2+ could result in a redistribution of intracellular Ca2+ stores and possibly the depletion of SR Ca2+ stores. It is postulated that both a decrease in cytosolic free Ca2+ concentration and an emptying of Ca2+ stores may have contributed to the observed changes in phenotype since both of these mechanisms have been implicated in transcriptional regulation (Drummond et al. 1987; Huang et al. 1994).
In summary we have generated transgenic mice that overexpress the Ca2+-buffering protein PV in slow-twitch muscle fibres. The resultant alterations in contractile and biochemical characteristics in SOL muscles suggest that this transgene altered both the acute contractile protein function and the long-term gene expression pathways, the latter most likely due to the associated changes in calcineurin activity and possibly other Ca2+-regulated transcriptional pathways. Alterations in oxidative and glycolytic enzyme activities in the absence of changes in expression of myosin heavy chain or other markers of fast vs. slow fibre phenotype, however, suggests a selective role for Ca2+-dependent transcriptional pathways in regulating muscle energetics but not all fibre type-related muscle characteristics. Our findings also indicate that while calcineurin and other transcriptional pathways may be important regulators of cellular change, other post-transcriptional mechanisms appear to counter these changes to maintain a native myofibrillar protein profile. A complete transformation from a slow to fast muscle phenotype may require more pronounced perturbations of [Ca2+]i or other signalling inputs not altered in this model.
Acknowledgments
We thank Kathy Graves and the transgenic core facility at UT Southwestern Medical Center for expertise in generating the transgenic founders. We also thank Zhao-Hui Xiong (UTSWMC) and Shannon Dunn (LU) for technical assistance and Maggy Fina, Annette Meeson (UTSWMC), Chris Blomme and Lorraine Brosseau (LU), for expert advice on breeding and maintaining the transgenic lines. A special thanks to Professor David Allen for his insightful comments in preparation of this manuscript. This work was supported by grants from the National Institutes of Health (NIH) (R.S.W.) and the Natural Sciences and Engineering Research Council (NSERC) of Canada (R.N.M.). A. R. Simard (LU) is supported by a NSERC graduate scholarship.
REFERENCES
- Bergmeyer HU. Methods of Enzymatic Analysis. New York: Academic Press; 1974. [Google Scholar]
- Brooke MH, Kaiser KK. Muscle fiber types: how many and what kind? Archiv Neurol. 1970;23:369–379. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
- Buckingham B. Muscle differentiation. Which myogenic factors make muscle? Curr Biol. 1994;4:61–63. doi: 10.1016/s0960-9822(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Buonanno A, Rosenthal N. Molecular control of muscle diversity and plasticity. Dev Gen. 1996;19:95–107. doi: 10.1002/(SICI)1520-6408(1996)19:2<95::AID-DVG1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Burkholder TJ, Fingado B, Baron S, Lieber RL. Relationship between muscle fibre type and sizes and muscle architectural properties in the mouse hindlimb. J Morphol. 1994;221:177–190. doi: 10.1002/jmor.1052210207. [DOI] [PubMed] [Google Scholar]
- Butler-Browne GS, Whalen RG. Myosin isozyme transitions occurring during the postnatal development of the rat soleus muscle. Dev Biol. 1984;102:324–334. doi: 10.1016/0012-1606(84)90197-0. [DOI] [PubMed] [Google Scholar]
- Castillo MB, Celio MR, Andressen C, Gotzos V, Rulicke T, Berger MC, Weber J, Berchtold MW. Production and analysis of transgenic mice with ectopic expression of parvalbumin. Arch Biochem Biophys. 1995;317:292–298. doi: 10.1006/abbi.1995.1165. [DOI] [PubMed] [Google Scholar]
- Chen G, Carroll S, Racay P, Dick J, Pette D, Traub I, Vrbova G, Eggli P, Celio M, Schwaller B. Deficiency in parvalbumin increases fatigue resistance in fast-twitch muscle and upregulates mitochondria. Am J Physiol Cell Physiol. 2001;281:C114–122. doi: 10.1152/ajpcell.2001.281.1.C114. [DOI] [PubMed] [Google Scholar]
- Chin ER, Olson EN, Richardson JA, Yang Q, Humphries C, Shelton JM, Wu H, Zhu W, Bassel-Duby R, Williams RS. A calcineurin-dependent transcriptional pathway controls skeletal muscle fibre type. Genes Dev. 1998;12:2499–2509. doi: 10.1101/gad.12.16.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corin SJ, Juhasz O, Zhu L, Conley P, Kedes L, Wade R. Structure and expression of the human slow twitch skeletal muscle troponin I gene. J Biol Chem. 1994;269:10651–10659. [PubMed] [Google Scholar]
- Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- Drummond IA, Lee AS, Resendez E, Steinhardt RA. Depletion of intracellular calcium stores by calcium ionophore A23187 induces the genes for glucose-regulated proteins in hamster fibroblasts. J Biol Chem. 1987;262:12801–12805. [PubMed] [Google Scholar]
- Dunn SE, Burns JL, Michel RN. Calcineurin is required for skeletal muscle hypertrophy. J Biol Chem. 1999;274:21908–21912. doi: 10.1074/jbc.274.31.21908. [DOI] [PubMed] [Google Scholar]
- Dunn SE, Chin ER, Michel RN. Matching of calcineurin activity to upstream effectors is critical for skeletal muscle fiber growth. J Cell Biol. 2000;151:663–672. doi: 10.1083/jcb.151.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn SE, Michel RN. Co-ordinated expression of myosin heavy chain isoforms and metabolic enzymes within overloaded rat muscle fibers. Am J Physiol Cell Physiol. 1997;273:C371–383. doi: 10.1152/ajpcell.1997.273.2.C371. [DOI] [PubMed] [Google Scholar]
- Dunn SE, Simard AR, Bassel-Duby R, Williams RS, Michel RN. Nerve activity-dependent modulation of calcineurin signaling in adult fast and slow skeletal muscle fibers. J Biol Chem. 2001;276:45243–45254. doi: 10.1074/jbc.M105445200. [DOI] [PubMed] [Google Scholar]
- Dunn SE, Simard AR, Prud'homme R, Michel RN. Calcineurin and muscle growth. Nature Cell Biol. 2002;4:E46–47. doi: 10.1038/ncb0302-e46a. [DOI] [PubMed] [Google Scholar]
- Epstein P, Means AR, Berchtold MW. Isolation of a rat parvalbumin gene and full length cDNA. J Biol Chem. 1986;261:5886–5891. [PubMed] [Google Scholar]
- Freyssenet D, Di Carlo M, Hood DA. Calcium-dependent regulation of cytochrome c gene expression in skeletal muscle cells. Identification of a protein kinase c-dependent pathway. J Biol Chem. 1999;274:9305–9311. doi: 10.1074/jbc.274.14.9305. [DOI] [PubMed] [Google Scholar]
- Fuchtbauer EM, Rowlerson AM, Gotz K, Friedrich G, Mabuchi K, Gergely J, Jockusch H. Direct correlation of parvalbumin levels with myosin isoforms and succinate dehydrogenase activity on frozen sections of rodent muscle. J Histochem Cytochem. 1991;39:355–361. doi: 10.1177/39.3.1825216. [DOI] [PubMed] [Google Scholar]
- Garcia J, Schneider MF. Calcium transients and calcium release in rat fast-twitch skeletal muscle fibres. J Physiol. 1983;463:709–728. doi: 10.1113/jphysiol.1993.sp019618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier ER, Madison SD, Michel RN. Rapid RNA isolation without the use of commercial kits: application to small tissue samples. Pflugers Arch. 1997;433:664–668. doi: 10.1007/s004240050328. [DOI] [PubMed] [Google Scholar]
- Gunning P, Hardeman E. Multiple mechanisms regulate muscle fibre diversity. FASEB J. 1991;5:3064–3070. doi: 10.1096/fasebj.5.15.1835946. [DOI] [PubMed] [Google Scholar]
- He ZH, Bottinelli R, Pellegrino MA, Ferenczi MA, Reggiani C. ATP consumption and efficiency of human single fibers with different myosin isoform composition. Biophys J. 2000;79:945–961. doi: 10.1016/S0006-3495(00)76349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig R, Lomo T. Firing patterns of motor units in normal rats. Nature. 1985;314:164–166. doi: 10.1038/314164a0. [DOI] [PubMed] [Google Scholar]
- Hoey T, Sun YL, Williamson K, Xu X. Isolation of two new members of the NF-AT gene family and functional characterization of the NF-AT proteins. Immunity. 1995;2:461–472. doi: 10.1016/1074-7613(95)90027-6. [DOI] [PubMed] [Google Scholar]
- Hogan BR, Beddington R, Constantini F, Lacey E. Manipulating the Mouse Embryo. NY, USA: Cold Spring Harbor; 1994. pp. 79–203. [Google Scholar]
- Huang CF, Flucher BE, Schmidt MM, Stroud SK, Schmidt J. Depolarization-transcription signals in skeletal muscle use calcium flux through L channels but bypass the sarcoplasmic reticulum. Neuron. 1994;13:167–177. doi: 10.1016/0896-6273(94)90467-7. [DOI] [PubMed] [Google Scholar]
- Hughes SM, Chi MM, Lowry OH, Gundersen K. Myogenin induces a shift of enzyme activity from glycolytic to oxidative metabolism in muscles of transgenic mice. J Cell Biol. 1999;145:633–642. doi: 10.1083/jcb.145.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes SM, Taylor JM, Tapscott SJ, Gurley CM, Carter WJ, Peterson CA. Selective accumulation of of myoD and myogenin mRNA s in fast and slow adult skeletal muscle is controlled by innervation and hormones. Development. 1993;118:1137–1147. doi: 10.1242/dev.118.4.1137. [DOI] [PubMed] [Google Scholar]
- Jasmin BJ, Campbell RJ, Michel RN. Nerve-dependent regulation of succinate dehydrogenase in junctional and extrajunctional compartments of rat muscle fibres. J Physiol. 1995;484:155–164. doi: 10.1113/jphysiol.1995.sp020654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DJ, Jiang Y, Rall JA. Intracellular EDTA mimics parvalbumin in the promotion of skeletal muscle relaxation. Biophys J. 1999;76:1514–1522. doi: 10.1016/S0006-3495(99)77310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt LK, O'Mahoney JV, Brennan KJ, Joya JE, Zhu L, Wade RP, Hardeman EC. The human troponin I slow promoter directs slow fibre-specific expression in transgenic mice. DNA Cell Biol. 1995;14:599–607. doi: 10.1089/dna.1995.14.599. [DOI] [PubMed] [Google Scholar]
- Madison SD, Dunn SE, Michel RN. Quantitative microphotometric assessment of membrane-bound dehydrogenase activities in excitable cells: obtaining linear and slowly progressing histochemical reactions. J Histochem Cytochem. 1998;46:1211–1212. doi: 10.1177/002215549804601015. [DOI] [PubMed] [Google Scholar]
- Mendez J, Keys A. Density and composition of mammalian muscle. Metabolism. 1960;9:184–188. [Google Scholar]
- Molkentin JD, Black BL, Martin JF, Olson EN. Cooperative activation of muscle gene expression by MEF2 and myogenic bHLH proteins. Cell. 1995;83:1125–1136. doi: 10.1016/0092-8674(95)90139-6. [DOI] [PubMed] [Google Scholar]
- Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molkentin JD, Olson EN. GATA4: a novel transcriptional regulator of cardiac hypertrophy? Circulation. 1997;96:3833–3835. [PubMed] [Google Scholar]
- Muntener M, Kaser L, Weber J, Berchtold MW. Increase of skeletal muscle relaxation speed by direct injection of parvalbumin cDNA. Proc Nat Acad Sci U S A. 1995;92:6504–6508. doi: 10.1073/pnas.92.14.6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musaro A, McCullagh KJ, Naya FJ, Olson EN, Rosenthal N. IGF-1 induces skeletal myocyte hypertrophy through calcineurin in association with GATA-2 and NF-ATc1. Nature. 1999;400:581–585. doi: 10.1038/23060. [DOI] [PubMed] [Google Scholar]
- Raymackers JM, Gailly P, Colson-VanSchoor M, Pette D, Schwaller B, Hunziker W, Celio MR, Gillis JM. Tetanus relaxation of fast skeletal muscles of the mouse made parvalbumin deficient by gene inactivation. J Physiol. 2000;527:355–364. doi: 10.1111/j.1469-7793.2000.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: Gene regulation and functional significance. Physiol Rev. 1996;76:371–423. doi: 10.1152/physrev.1996.76.2.371. [DOI] [PubMed] [Google Scholar]
- Schwaller B, Dick J, Dhoot G, Carroll S, Vrbova G, Nicotera P, Pette D, Wyss A, Bluethmann H, Hunziker W, Celio MR. Prolonged contraction-relaxation cycle of fast-twitch muscles in parvalbumin knockout mice. Am J Physiol Cell Physiol. 1999;276:C395–403. doi: 10.1152/ajpcell.1999.276.2.C395. [DOI] [PubMed] [Google Scholar]
- Semsarian C, Sutrave P, Richmond DR, Graham RM. Insulin-like growth factor (IGF-I). induces myotube hypertrophy associated with an increase in anaerobic glycolysis in a clonal skeletal-muscle cell model. Biochem J. 1999;339:443–451. [PMC free article] [PubMed] [Google Scholar]
- Seward DJ, Haney JC, Rudnicki MA, Swoap SJ. BHLH transcription factor MyoD affects myosin heavy chain expression pattern in a muscle-specific fashion. Am J Physiol Cell Physiol. 2001;85:C408–413. doi: 10.1152/ajpcell.2001.280.2.C408. [DOI] [PubMed] [Google Scholar]
- Sutherland CJ, Esser KA, Elsom VL, Gordon ML, Hardeman EC. Identification of a program of contractile protein gene expression initiated upon skeletal muscle differentiation. Dev Dyn. 1993;196:25–36. doi: 10.1002/aja.1001960104. [DOI] [PubMed] [Google Scholar]
- Talmadge RJ, Roy RR. Electrophoretic separation of rat skeletal muscle myosin heavy-chain isoforms. J Appl Physiol. 1993;75:2337–2340. doi: 10.1152/jappl.1993.75.5.2337. [DOI] [PubMed] [Google Scholar]
- Thelen MH, Simonides WS, Muller A, vanHardeveld C. Cross-talk between transcriptional regulation by thyroid hormone and myogenin: new aspects of the Ca2+-dependent expression of the fast-type sarcoplasmic reticulum Ca2+ ATPase. Biochem J. 1998;329:131–136. doi: 10.1042/bj3290131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman LA, Clipstone NA, Ho SN, Northrup JP, Crabtree GR. Rapid shuttling of NF-AT in discrimination of Ca2+ signals and immunosuppression. Nature. 1996;383:837–840. doi: 10.1038/383837a0. [DOI] [PubMed] [Google Scholar]
- Ustunel I, Demir R. A histochemical morphometric and ultrastructural study of gastrocnemius and soleus muscle fibre type composition in male and female rats. Acta Anatom. 1997;158:279–286. doi: 10.1159/000147941. [DOI] [PubMed] [Google Scholar]
- Wang J, Moreira KM, Campos B, Kaetzel MA, Dedman JR. Targeted neutralization of calmodulin in the nucleus blocks DNA synthesis and cell cycle progression. Biochem Biophys Acta. 1996;1313:223–228. doi: 10.1016/0167-4889(96)00093-6. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. The contribution of [Ca2+]i to the slowing of relaxation in fatigued single fibres from mouse skeletal muscle. J Physiol. 1993;468:729–740. doi: 10.1113/jphysiol.1993.sp019797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Kanatous SB, Thurmond FA, Gallardo T, Isotani E, Bassel-Duby R, Williams RS. Regulation of mitochondrial biogenesis in skeletal muscle by CaMK. Science. 2002;296:349–352. doi: 10.1126/science.1071163. [DOI] [PubMed] [Google Scholar]
- Wu H, Naya FJ, McKinsey TA, Mercer B, Shelton J, Chin ER, Simard AN, Michel RN, Bassel-Duby R, Olson EN, Williams RS. MEF2 responds to multiple calcium-regulated signals in the control of skeletal muscle fibre type. EMBO J. 2000;19:1–11. doi: 10.1093/emboj/19.9.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Rothermel B, Kanatous S, Rosenberg P, Naya FJ, Shelton JM, Hutcheson KA, Dimaio JM, Olson EN, Bassel-Duby R, Williams RS. Activation of MEF2 by muscle activity is mediated through a calcineurin-dependent pathway. EMBO J. 2001;20:6414–6423. doi: 10.1093/emboj/20.22.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Bassel-Duby R, Williams RS. Transient expression of a winged-helix protein MNF-beta during myogenesis. Mol Cell Biol. 1997;17:5236–5243. doi: 10.1128/mcb.17.9.5236. [DOI] [PMC free article] [PubMed] [Google Scholar]


