Abstract
The seven amino acid insert in the smooth muscle myosin heavy chain is thought to regulate the kinetics of contraction, contributing to the differences between fast and slow smooth muscle. The effects of this insert on force and stiffness were determined in bladder tissue of a transgenic mouse line expressing the insert SMB at one of three levels: an SMB wild type (+/+), an SMA homozygous type (−/−) and a heterozygous type (+/−). For skinned muscle, an increase in MgADP or inorganic phosphate (Pi) should shift the distribution of crossbridges in the actomyosin ATPase (AMATPase) to increase the relative population of the crossbridge state prior to ADP release and Pi release, respectively. Exogenous ADP increased force and stiffness in a manner consistent with increasing the Ca2+ concentration in both the +/+ and +/− mouse types. However, the −/− type showed a significantly greater increase in force than in stiffness suggesting that immediately prior to ADP release, the AMATPase either has an additional force producing isomerization state or a slower ADP dissociation rate for the −/− type compared to the +/+ or +/− types. Exogenous Pi led to a significantly greater decrease in stiffness than in force for all three mouse types suggesting that there is a force producing state prior to Pi release. In addition, the increase in Pi showed similar changes in the +/+ and −/− types whereas in the +/− type the decreases in both force and stiffness were greater than the other two mouse types indicating that the insert can affect the cooperativity between myosin heads. In conclusion, the seven amino acid insert modulates the kinetics and/or states of the AMATPase, which could lead to differences in the kinetics of contraction between fast and slow smooth muscle.
Smooth muscle has been dichotomized as fast and slow depending on the kinetics of muscle contraction. The fast and slow contractile properties of skinned, thiophosphorylated smooth muscle tissue are preserved using photolytic release of MgATP implicating that differences in the actomyosin ATPase (AMATPase) crossbridge cycle contribute to the differences in the contractile properties of smooth muscle (Horiuti et al. 1989). Although actin is not conserved among all smooth muscle tissue, the different isoforms have been shown to have negligible effects on the AMATPase activity in vitro (Mossakowska et al. 1985), implicating myosin as the cause of the differences in the contractile properties. The smooth muscle myosin molecule is composed of two heavy chains (MHC), two essential light chains (MLC17) and two regulatory light chains (MLC20). Several isoforms of each are expressed in smooth muscles.
The MLC17 has an alternatively spliced exon producing two isoforms, MLC17a and MLC17b, differing by their isoelectric points (Hasegawa & Morita, 1992). Faster smooth muscle generally expresses a higher level of MLC17a (Malmqvist & Arner, 1991), where changes in MLC17a/MLC17b between different smooth muscles correlate to differences in shortening velocity and ATPase rates (Helper et al. 1988; Malmqvist & Arner, 1991) but this finding is not universal (Meer & Eddinger, 1997; Sherwood & Eddinger, 2002). The MLC20 also expresses two isoforms separated by their acidity but only one isoform has been reported in postnatal tissue (Inoue et al. 1989). The MHC expresses two pairs of isoforms. At the tail region are SM1 and SM2 (Rovner et al. 1986; Nagai et al. 1989). Our interest is in the amino terminus MHC isoform where an alternatively spliced exon results in the presence (SMB) or absence (SMA) of seven amino acids near the nucleotide binding site (Hamada et al. 1990; White et al. 1993; Kelley et al. 1993). Since nucleotide attachment and detachment to the actomyosin complex plays an integral role in force development during the crossbridge cycle, changes in the myosin molecule near the nucleotide binding site may affect nucleotide affinity and thus change certain rate constants of the crossbridge cycle. Many studies have shown a correlation between the expression level of SMA/SMB and the speed of the crossbridge cycle in smooth muscle, finding the presence of the insert resulted in a shorter duty cycle (Lauzon et al. 1998), a faster motility velocity (Rovner et al. 1997) and a faster shortening velocity (Eddinger & Meer, 2001). A general pattern has emerged showing faster muscles express higher levels of both SMB and MLC17a making conclusions about the role of an individual isoform equivocal at best (Sjuve et al. 1996). Therefore any work that deals with intact tissue cannot isolate the cause to just one variable. On the other hand, experiments that are able to manipulate only the level of SMA/SMB have been performed using purified myosins and thus cannot mimic the environment of intact tissue.
We investigated whether changes in the SMA/SMB expression affects the crossbridge cycle in smooth muscle using a transgenic mouse line (Babu et al. 2001). This line is a knockout of SMB allowing for different expression levels of SMA/SMB in bladder without changing the expression levels of SM1/SM2 or MLC17a/MLC17b (Babu et al. 2001). This has allowed us to categorize three mouse types: predominantly expressing SMB (+/+); predominantly expressing SMA (−/−; SMB knockout); and a heterozygous expression (+/−).
Our work examines two steps in the crossbridge cycle and how changes in the expression level of SMA/SMB may influence crossbridge states. The crossbridge cycle describes the kinetics of muscle force generation through a series of states that involve the association and dissociation of actin (A) and myosin (M) along with MgATP and its hydrolysis products, ADP and Pi as shown in Scheme 1 and Fuglsang et al. (1993).
Scheme 1.

We probed two steps in the crossbridge cycle, the release of Pi and ADP. The addition of either exogenous inorganic Pi or MgADP shifts the equilibrium at their respective release points in the cycle, perturbing the crossbridge population by increasing the relative population of crossbridges in the state(s) immediately prior to either Pi or ADP release. Observing force and stiffness allows us to determine if the heavy chain insert adds or circumvents any of the states immediately preceding Pi or ADP release and also if the population of these state(s) varies due to the relative expression of SMA/SMB.
The kinetics of the bladder did differ depending on the expression level of the heavy chain NH2 insert. The −/− type showed that immediately prior to ADP release from the actomyosin complex, either an additional step in the crossbridge cycle is present or the single state has a slower ADP off-rate than either the +/+ or +/− types. In all three types, there was evidence of a force generating state immediately prior to the release of Pi from the actomyosin complex, however the population of this state is significantly greater in the two homozygous animals compared to the heterozygous one.
METHODS
Animals
The method for cloning and generation of transgenic mice expressing the different SMA/SMB levels has been published in detail (Babu et al. 2001). Bladder tissue from adult mice was used for all mechanical experiments. Mice were killed by exposure to a rising concentration of CO2 in accordance with the ethical treatment of animals using a protocol approved by the Case Western Reserve University Institutional Animal Care and Use Committee. Mice were categorized as homozygous positive (+/+; SMB homozygous), heterozygous (+/−) or homozygous negative (−/−; SMB knockout) for the heavy chain NH2-terminal insert.
Histology
Bladder tissues from wild type and SMB −/− null mice were fixed in 10 % neutral buffered formalin, dehydrated through a gradient of alcohols, embedded in paraffin, sectioned, and stained with haematoxylin and eosin.
Quantitative immunoblot analysis
Quantitative immunoblotting was carried out as described previously (Babu et al. 2001). Briefly, total proteins isolated from bladder and aorta were separated on a 12 % SDS-polyacrylamide gel (PAGE) and transferred to nitrocellulose membrane. Membranes were probed with the following primary antibodies: mouse mouse monoclonal anti-smooth muscle actin, mouse monoclonal anti-myosin light chain 20 (both from Sigma), mouse monoclonal anti- MLC17 antibodies. The signals were detected by Super signal West Dura substrate and quantified by densitometry scanning and analysed using IMAGE software (Version 6.1, National Institutes of Health).
Force
The bladder was removed and placed into Ca2+-free physiological saline solution (PSS) solution (mm): 140 NaCl, 4.7 KCl, 1.2 NaH2PO4.7H2O, 2.0 MOPS, 0.02 EDTA, 1.2 MgCl2.6H2O, 5.6 glucose and 0.5 EGTA, pH 7.0). Bladder strips were cleared of connective tissue and then cut into strips approximately 400–1000 μm long, 300–500 μm wide and 150–300 μm thick. As previously described (Rhee & Brozovich, 2000), aluminum foil T-clips were attached to each end of the bladder strips. The tissue was then immersed in pCa9 solution where one end was hooked to a force transducer (Akers AE 801 MEMSCAP, San Jose, CA, USA) and the other to a piezoelectric length driver (Physik Instrumente, Waldbron, Germany) or a servomotor (Aurora Scientific, Aurora, ON, Canada) on a mechanics workstation. The tissue was stretched to a level sufficient to just develop tension and then by an additional 30 %, defining the length Lo where force is a maximum (data not shown). Bladder strips were skinned in 1 % Triton solution at pCa4 similar to that described by Sward et al. (1998). Tissue was left in the skinning solution until force reached a steady state, approximately 20–40 min. Tissue was then returned to pCa9 solution where force relaxed to the baseline. Strips were activated with Ca2+. Upon reaching a steady state, the strip was transferred into 5 mm MgADP solution, 10 mm Pi solution or a different pCa level (5.8, 5.4, 5.0 or 4.0). The order of the pCa solutions varied between experiments.
Stiffness
After reaching a steady state force at any pCa level or in a nucleotide solution, the length driver oscillated with a peak-peak sine wave at an amplitude of 0.3 % Lo and at a frequency of 40 and 80 Hz. The length of the motor displacement and the corresponding tension of the tissue were individually transformed into a Fourier series (Shue & Brozovich, 1999). Stiffness was defined as the magnitude of the force transform divided by the length transform. Stiffness was normalized by cross-sectional area, such that the stiffness in pCa4 was defined as 100 % and in pCa9 was defined to be 0 %. Stiffness was the same at both 40 and 80 Hz, therefore only the data at 40 Hz is reported in the text.
Solutions
All solutions were prepared using a computer program that calculated the amount of stock solutions required for a set of free ion concentrations adjusting for temperature, pH and ionic strength (Brozovich & Yamakawa, 1995). Temperature was set at 25 °C. Solutions were prepared with a pH of 7.0. Ionic strength was set at 200 mm. Specific concentrations for each solution are provided in Table 1.
Table 1.
Solution constituents
| pCa | KMS (mm) | EGTA (mm) | BES (mm) | MgCl2 (mm) | CaCl2 (mm) | ATP (mm) | CP (mm) |
|---|---|---|---|---|---|---|---|
| 9.0 | 54.87 | 10 | 25 | 7.33 | 0.02 | 5.4 | 25 |
| 5.8 | 39.38 | 10 | 25 | 7.04 | 7.96 | 5.4 | 25 |
| 5.4 | 37.18 | 10 | 25 | 7.00 | 9.08 | 5.4 | 25 |
| 5.0 | 36.08 | 10 | 25 | 6.98 | 9.63 | 5.4 | 25 |
| 4.4 | 35.24 | 10 | 25 | 6.98 | 10.00 | 5.4 | 25 |
| 4.0 | 34.57 | 10 | 25 | 6.97 | 10.22 | 5.5 | 25 |
| 1% Triton | 25 | 5 | 5 | 6.9 | 0 | 5 | 25 |
| 4.0 + 5mM MgADP | 113.53 | 5.0 | 25 | 8.01 | 5.32 | 5.5 | 0 |
| 4.0 + 10 mM Pi | 107.94 | 5.0 | 25 | 7.58 | 5.2 | 5.3 | 0 |
KMS, potassium methane sulfonate; BES N,N-bis(2-hydroxyethyl)-2-aminoethanesulfonic acid. Triton solution includes an additional 5% (v/v) CaCl2. All solutions include calmodulin 250 u ml−1 and 20 u ml−1 creatine phosphokinase except the ADP solution which had no creatine phosphokinase.
Statistics
The Student's paired t test was used to compare the differences for the changes of force and stiffness with a level of significance P < 0.05. Data in the text are given as means ±s.e.m.
RESULTS
Tissue histology shows no significant differences between SMB wild type mice bladder strips and SMB knockout mice bladder strips (Fig. 1). The smooth muscle orientation was similar between the +/+ wild type mice and the −/− double knockout mice. Since the force measured is dependent on the cosine of the angle of the muscle fibres and the force transducer, only orientations with very large change in angles would affect the total forces measured. Therefore, orientation would not contribute to any significant differences in force.
Figure 1. Histological staining of wild-type and SMB −/− bladder.
Sections were stained with haematoxylin and eosin, × 40 magnification.
Changes in the SMB expression level did not affect the expression level of other myosin isoforms including MLC17 or MLC20 (Fig. 2). Only one band was seen for either the MLC17 or MLC20 in all three mouse types indicating no differences between the mice.
Figure 2. Western blot analysis of light chain isoform expression.

Three different concentrations of total proteins extracted from SMB+/+ (WT, wild-type), SMB+/−, and SMB−/− mouse bladder were resolved on a 12 % SDS-PAGE and immunoblotted with LC20, smooth muscle actin or LC17-specific antibody.
Overall forces in pCa4 are given in Table 2. Our results indicate that the insert has no role in total force generation, contradicting the findings from Babu et al. (2001). A possible explanation is the method of activation. Whereas we activated permeabilized tissue to high levels of Ca2+, the others used KCl activation on intact membranes. Also, laser trap studies suggest the insert does not affect unitary force or total average force (Lauzon et al. 1998), in accordance with our results.
Table 2.
Force and stiffness
| +/+ | +/− | −/− | |
|---|---|---|---|
| Absolute values in pCa4 | |||
| Force (mN mm−2) | 7.63 ± 1.54 | 7.47 ± 0.96 | 7.60 ± 0.96 |
| Stiffness at 40 Hz (mN mm−2) | 42.35 ± 7.71 | 36.23 ± 5.45 | 34.45 ± 4.48 |
| No. of muscle strips | 16 | 17 | 25 |
| No. of animals | 5 | 5 | 9 |
| Relative values in ADP | |||
| Force | 167 ± 15% | 152 ± 8% | 165 ± 10% |
| Stiffness | 163 ± 23% | 139 ± 12% | 136 ± 6% |
| No. of muscle strips | 9 | 7 | 14 |
| No. of animals | 3 | 2 | 5 |
| Relative values in Pi | |||
| Force | 67 ± 5% | 44 ± 5% | 62 ± 5% |
| Stiffness | 28 ± 8% | 11 ± 8% | 24 ± 6% |
| No. of muscle strips | 7 | 10 | 11 |
| No. of animals | 2 | 3 | 4 |
The +/+ condition represents SMB/SMB, −/− is the SMB knockout or the complete absence of the seven amino acid NH2 terminal MHC insert (Kelley et al. 1993) and +/− is the heterozygous state. Force and stiffness at pCa4 are defined as 100%. Percentages listed for ADP and Pi are relative to pCa4 values. The changes in force and stiffness for the +/− type in the Pi condition were statistically significant compared to the other two mouse types (P < 0.05). All values are given as means ±s.e.m.
Before studies comparing the effects of MgADP and Pi on the crossbridge kinetics of each mouse type, we first established a force-stiffness relationship at different Ca2+ levels to determine if any effect we observed was equivalent to changing the number of activated crossbridges. As the muscle was activated with Ca2+, both the steady state force and stiffness increased. The relationship between force and stiffness over various pCa levels (Fig. 3) showed the following linear relationship:
Figure 3. The force-stiffness relationship.
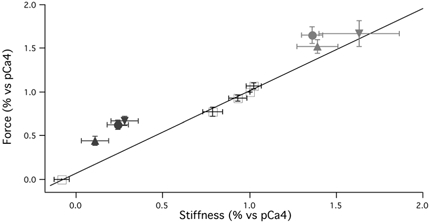
Five different force and corresponding stiffness values were observed at pCa values of 5.8, 5.4, 5.0, 4.4 and 4.0 (n= 10) and fit to a line, r2= 0.99 (black line, +). Force vs. stiffness changes: ▾=+/+, ▴=+/−, ×=−/−; Pi= black, ADP = grey. Force and stiffness in pCa4 were defined as 100 %. The relationship between steady state force and steady state stiffness during Ca2+ activation is linear. In the ADP condition, only the −/− mouse type significantly deviated from the force vs. stiffness regression line at various pCa concentrations (P < 0.05). All three mouse types significantly deviated from the line in the Pi condition (P < 0.05).
To investigate the state of the crossbridge cycle immediately before ADP release from the myosin head, the skinned bladder was transferred from pCa4 to pCa4 with 5 mm MgADP, which resulted in an increase in both force and stiffness for all three mouse types (Fig. 4A). For 5 mm MgADP in pCa4 vs. pCa4 alone, the +/+ type resulted in an increase in force of 67 % while the stiffness increased by 63 %, the +/− type had a force increase of 52 % and a stiffness increase of 39 % while the −/− type had a 65 % force increase and a 36 % stiffness increase (Table 2).
Figure 4. Force traces during Ca2+ activation.
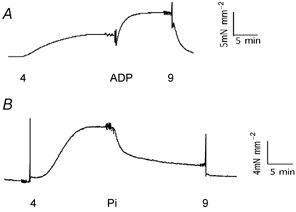
A, force trace during Ca2+ activation in 5 mm MgADP. Tissue was allowed to relax in pCa9 (9) before being shifted to pCa4 (4), pCa4 with 5 mm MgADP (ADP) and then back into pCa9. Ca2+ activation results in an increase in steady state force, which is further increased with MgADP. B, force trace during Ca2+ activation in 10 mm inorganic Pi. Tissue was allowed to relax in pCa9 before being shifted to pCa4 (4), pCa4 with 10 mm Pi (Pi), and then back into pCa9. Ca2+ activation results in an increase in steady state force, which is decreased by Pi.
In pCa4 with 5 mm MgADP vs. pCa4 alone, the changes in force and stiffness for each mouse type were compared to determine if results deviated from the force-stiffness relationship when the muscle was activated with only Ca2+ (Fig. 3). Only the −/− type showed a crossbridge behaviour that deviated from the Ca2+ force-stiffness relationship where the increase in force was significantly greater than the increase in stiffness, P < 0.05. On the other hand, for both the +/+ and +/− mouse types, the increases in force and stiffness were not statistically different.
To investigate the state of the crossbridge cycle immediately before Pi release from the myosin head, the skinned bladder was transferred from pCa4 to pCa4 with 10 mm Pi; both steady state force and stiffness decreased in all three mouse types (Fig. 4B). For 10 mm inorganic Pi in pCa4 vs. pCa4 alone, the +/+ type had force decreased by 33 % while stiffness decreased by 72 %, the +/− type had a force decrease of 56 % and a stiffness decrease of 89 % while the −/− type had a 38 % force decrease and a 76 % stiffness decrease (Table 2). Unlike for MgADP, comparing pCa4 with 10 mm Pivs. pCa4 showed all three mouse types had a significantly greater decrease in stiffness than in force (P < 0.05) and thus all three deviated from the Ca2+ force-stiffness relationship (Fig. 3).
DISCUSSION
Evidence indicates that varying the expression level of the myosin heavy chain seven amino acid insert (SMA/SMB) may play a significant role in the kinetics of smooth muscle contraction. Unfortunately, studies of smooth muscle strips are convoluted by several variations found between a fast smooth muscle and a slow smooth muscle that lie well beyond the expression level of SMA/SMB, including differences in expression levels of other myosin isoforms (MLC17, and SM1/SM2). The transgenic mice in our study are unique in that their bladder tissue expresses the SMA/SMB at three different levels without changes in the expression of SM1/SM2, MLC17a/MLC17b or MLC20. Thus in the bladder of this line of transgenic mice, any differences in the AMATPase cannot be attributable to changes in the expression or isoform ratios of SM1/SM2, MLC20 and MLC17 (Babu et al. 2001). This makes the bladder of these mice a preferable model to evaluate the effects of changes in the level of expression of SMA/SMB on the crossbridge cycle.
The crossbridge cycle describes the development of force through a series of complexes between actin (A), myosin (M), MgATP and its hydrolysis products, ADP and Pi (Scheme 1). Beginning in the rigor state (AM), ATP binding to AM results in rapid dissociation forming an A + M-ATP state followed by hydrolysis of ATP by myosin. Pi release from the A + M-ADP-Pi state is thought to result in crossbridge attachment, AM-ADP, beginning the duty cycle. The AM-ADP state then isomerizes to a high force generating state (AM-ADP′) followed by ADP release returning to the rigor state (AM). MgATP subsquently binds to the AM state causing detachment, ending the duty cycle and recommencing another crossbridge cycle. While the crossbridge cycle for all muscle is frequently described in a generic manner, differences do exist between the kinetics of skeletal, cardiac and smooth muscle and even within different smooth muscle tissues, requiring changes in the crossbridge cycle to explain the differences in AMATPase rates (Rosenfeld et al. 2000).
The points of ADP release and Pi release in the crossbridge cycle can be studied with the addition of excess ADP or inorganic Pi which results in shifting the equilibrium of the crossbridge cycle just before the ADP or Pi release and increasing the relative population of these crossbridge states. By measuring force and stiffness, one can observe the properties of the crossbridge immediately before the release of ADP or Pi to identify if the crossbridges are attached or detached, and should they be attached whether the state is a force generating state or a non-force generating state.
Elevation of exogenous MgADP is thought to promote a bound, force generating state (AM-ADP′ in Scheme 1). This was found in all three mouse types. Placing the tissue in 5 mm MgADP resulted in an increase in force and stiffness, however only the −/− form deviated from the Ca2+ force-stiffness relationship where force increased by 65 % but stiffness by only 36 %. The −/− results imply a change in the properties of the crossbridge state. The attached crossbridges exerted a greater force than had they been activated by simply increasing the Ca2+ concentration. In contrast, by not deviating from the Ca2+ force-stiffness relationship, the addition of exogenous MgADP for both the +/+ and +/− mouse types was equivalent to increasing the number of activated crossbridges attached to actin (67 % force and 63 % stiffness for +/+, 52 % force and 39 % stiffness for +/−). For the −/− type, significantly increasing the force per stiffness suggests either an additional step in the crossbridge cycle where the AM-ADP′ state isomerizes to produce a greater force (AM-ADP*), an isomerization not seen in either the +/+ or the +/− condition (Scheme 2) or simply a longer lived AM-ADP′ state due to a slower ADP off-rate in the −/− type resulting in a longer duration of a force generating state.
Scheme 2.
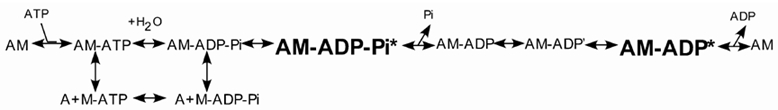
In skeletal muscle it has been suggested force can increase by having myosin heads bind in a stereospecific manner without increasing the number of crossbridges bound to the actin filament (Bershitsky et al. 1997). The absence of the NH2 terminal insert (−/−) may induce similar behaviour in smooth muscle. According to Scheme 1, crossbridges are already bound just prior to ADP release and realign to produce a force, but the absence of the insert could result in a stereospecific readjustment of these crossbridges immediately before ADP release to slow ADP release or cause crossbridges to exert more force without increasing the number of crossbridges attached. For the +/+ and +/− mouse types, force increases proportionally to the number of crossbridges, suggesting this reorientation of crossbridges does not occur when at least one head expresses the insert. Should the crossbridges exert more force at certain stages of the crossbridge cycle, the overall force per cross-sectional area should be highest in the −/− type. Forces, however, did not show a statistically significant difference between the three mouse types supporting the theory that the actomyosin interaction has the same unitary force independent of the heavy chain expression (Lauzon et al. 1998). There may in fact be a difference, but that it is not detectable at 5 mm ATP. Lauzon et al. (1998) showed that while the relative duty cycle between having the insert present and absent remains constant at approximately a twofold difference independent of ATP concentrations, the absolute values decrease significantly with increasing ATP. Our experiments were done using 5 mm ATP, five times greater than the maximal ATP concentration used in Lauzon et al.'s work. Therefore, while the difference may be significant, it may be so slight that it can only be seen if the bound state is stabilized with high ADP concentration.
Past studies have investigated why smooth muscle has a longer duty cycle than skeletal muscle, to try and explain smooth muscle's greater efficiency. Rosenfeld et al. (2000) suggested that an additional AM-ADP state exists in the smooth muscle AMATPase compared to the AMATPase in skeletal muscle, which would contribute to a longer duty cycle in smooth muscle. In addition, the extra rotation in the neck region at the end of the smooth muscle power stroke compared to skeletal muscle (Whittaker et al. 1995; Gollub et al. 1996) may also contribute to a longer duty cycle. Analogously, an extra AM-ADP state (Scheme 2) or a slower rate of ADP dissociation may be due to the absence of the heavy chain insert, which could result in an additional rotation of the myosin molecule while bound to the actin filament. If, however, it is not an additional state but rather the two S1 heads of a single myosin molecule realigning along the actin filament, additional time may be needed to achieve this realignment, slowing the ADP off-rate and resulting in a longer duty cycle.
Without knowledge of all the rate constants in the crossbridge cycle for fast and slow smooth muscle, one can only loosely connect our conclusions regarding ADP release with other studies. Khromov et al. (1995) have shown that fast smooth muscle has a faster off-rate for ADP than slow smooth muscle. However, the faster rate at this step may be compensated for in other steps in the crossbridge cycle. Laser trap work has shown a shorter duty cycle for myosin expressing the insert due to a change in the ADP off-rate (Lauzon et al. 1998). Assuming rates are approximately equal for the common steps in the cycle, an additional step similar to an additional isomerization (AM-ADP′⇌ AM-ADP*, Scheme 2) would result in a longer time to process through the states while actin and myosin are interacting. Alternatively, a slower transition between AM-ADP′⇌ AM could also produce a longer duty cycle, and thus, an overall slower crossbridge cycle in the −/− type.
The NH2 insert is located in loop 1 near the ADP binding site of myosin. Studies have shown a positive relationship between a longer loop and a quicker release of ADP, although the physiological significance remains in question (Sweeney et al. 1998). These results would suggest that the absence of the seven amino acid insert (SMA) causes a slower release of ADP and a longer duty cycle. The observations made with the −/− bladder tissue support this conclusion; the notion a longer loop 1 releases ADP faster is consistent with our findings since the −/− type, having a shorter loop length, would have a longer duty cycle be it from an additional AMADP isomerization (Scheme 2) or a longer time constant in the AM-ADP′ state. However since neither the +/− nor +/+ types deviate from the Ca2+ force-stiffness line, it can be concluded that only one head needs to have a longer loop 1 in order to reduce the duty cycle.
It is thought that upon Pi release, actin and myosin bind to generate force (Scheme 1). This is seen by the fall in force and stiffness in pCa4 with 10 mm inorganic Pi compared to pCa4 alone. Additionally, because the drop in stiffness was greater than the drop in force, we can surmise that immediately prior to Pi release there exists a force generating state. This is seen in Fig. 3 where those crossbridges that are bound generate a force in a manner that deviates from the Ca2+ force-stiffness relationship. Force development before Pi release necessitates crossbridges to be bound to actin filaments, significantly modifying the model for the smooth muscle AMATPase. We propose that there exist two states between actin and myosin detachment and the Pi release, both involving crossbridge attachment. Following the A + M-ADP-Pi step, there is a low force producing step, AM-ADP-Pi, followed by an isomerization to a higher force state, AM-ADP-Pi* (Scheme 2). Skeletal and cardiac muscle studies have shown a similar transition from a non-force generating AM-ADP-Pi complex to a force generating AM-ADP-Pi* state prior to Pi release (Iwamoto, 1995; Dantzig et al. 1992; Ranatunga, 1999; Wang & Kawai, 1997). However, empirically this has not been seen. If more crossbridges were in a force generating state then one would expect to see an increase in the force per stiffness ratio as we see in smooth muscle. It may be that because smooth muscle is slower than striated muscle, we did see this effect where others could only deduce the existence of the higher force generating state. The data show the two homozygous conditions (+/+, −/−) have approximately equal populations of this state, and that it is higher than the population found in the heterozygous type (+/−). The falls in force and stiffness for the two homozygous cases were 38 % and 76 % for the +/+ and 33 % and 72 % for the −/− type, while the +/− type fell 56 % and 89 % for force and stiffness, respectively. That the fall in stiffness is greater than the fall in force when the tissue is immersed in high Pi solution indicates that the elevation of exogenous phosphate somehow favours the detachment of crossbridges in the low force generating state.
While all three mouse types show evidence for this state, the differences in populations suggest some sort of cooperative behaviour between myosin heads in order to bind to actin prior to Pi release in the crossbridge cycle. The cause of this cooperativity is unclear. As hypothesized for the AM states prior to MgADP release, crossbridges may reorient themselves in a stereospecific manner immediately before Pi release, but the number of crossbridges able to do this are far fewer than those detached from the actin filament since both force and stiffness decreased. The heterozygous condition (+/−) may not be as efficient in achieving this stereospecific orientation as the two homozygous conditions (+/+, −/−). Should the stereospecific orientation be more advantageous in homozygous mouse types, there should then exist a difference in the depression of force by Pi between different smooth muscle types. Löfgren et al. (2001) reported that the sensitivity of force to Pi was greater for tania coli than aorta. While their work dealt with the guinea-pig, it has been shown tania coli expresses 15–20 % SMB in mice (Siegman et al. 1997) while SMB is not expressed in chicken aorta (Fisher et al. 1997). It is possible that the asymmetrical heads are somehow disadvantaged to bind to actin in a Pi-bound state, but this describes not a kinetic role for the insert but rather a mechanical one, which is plausible and still describes a pertinent role for the insert.
Other factors beyond the SMA/SMB expression level could also be involved in the differences in the contractile properties between various smooth muscle tissues. SM1 and SM2 describe two heavy chain isoforms found at the carboxyl end of the myosin molecule (Rovner et al. 1986; Nagai et al. 1989). Data remain inconclusive regarding their roles. Some investigators have found the expression level of SM1/SM2 does affect the shortening velocity (Hewett et al. 1993). However, other investigators have not seen changes in shortening velocity (Meer & Eddinger, 1997; Sherwood & Eddinger, 2002) or in the motility assay (Kelley et al. 1992) and have attributed the carboxyl isoforms not to kinetics, but rather to filament assembly (Rovner et al. 2002). Similarly, the MLC17 has two isoforms thought to contribute to smooth muscle kinetics, MLC17a and MLC17b (Malmqvist & Arner, 1991; Matthew et al. 1998). Others, however, consider their role secondary having shown no correlation between the MLC17a/MLC17b expression levels and shortening velocity in single smooth muscle cells (Eddinger et al. 2000).
While most attention to smooth muscle kinetics is devoted to the activation of the actomyosin complex, differences in the dephosphorylation properties of the MLC20 between fast and slow muscle may also be responsible. It has been shown that different smooth muscle responds differently to phosphatase inhibitors influencing the rate of relaxation in a manner independent of the crossbridge cycle (Gong et al. 1992). It is not possible to rule this out as an explanation. However, that does not dismiss the role of the heavy chain. It is thought the heavy chain does interact with the myosin light chain phosphatase although not necessarily near loop 1 (Hartshorne, 1998). Nevertheless, isoforms in the heavy chain may affect this interaction and so would still have a kinetic influence on smooth muscle.
The bladder of this transgenic mouse line had an upregulation of calponin in both the hetereozygous mice (+/−) and the homozygous mice (−/−; SMB knockout). On the other hand, caldesmon was downregulated in the two (G. Babu and M. Periasamy, unpublished observations). Both caldesmon (Katsuyama et al. 1992; Malmqvist et al. 1996) and calponin (Winder & Walsh, 1993; Horowitz et al. 1996) have been suggested to modulate smooth muscle activation; however the role of the thin filament in smooth muscle activation is controversial (Adam et al. 1995; Brozovich & Yamakawa, 1995; Matthew et al. 2000). Calponin has been suggested to influence maximal muscle shortening velocity (Jaworoski et al. 1995; Matthew et al. 2000), but this is not a constant finding (Facemire et al. 2000). Furthermore, calponin has been reported to decrease on isometric force (Uyama et al. 1996) but others have not seen this effect (Jaworoski et al. 1995; Matthew et al. 2000). Differences in caldesmon content have been reported to affect force or stiffness of isometric contractions at submaximal Ca2+ activation but not at maximal levels (Szpacenko et al. 1985; Malmqvist et al. 1996) as employed in this study. These two studies were done by either adding exogenous caldesmon or by extracting it from the tissue. In contrast, one group even showed a decrease in caldesmon resulted in a decrease in isometric force using KCl activation (Earley et al. 1998). Whereas studies observing the insert have only shown faster kinetics in the presence of the insert, the work regarding calponin and caldesmon has not shown a consistent pattern regarding their effects on smooth muscle contractility. Consequently, while we cannot completely dismiss the influence of calponin or caldesmon on our results, we think their contribution, if any, is secondary to that of the insert.
Other contributors to variations in the cycle may be tissue architecture. The tissue itself produces an internal load slowing the shortening velocity (Harris & Warshaw, 1990; Ogut & Brozovich, 2000). Purified proteins or proteins expressed using a baculovirus system avoid problems of internal loads and tissue architecture; however, the folding of myosin, actin and any other relevant proteins under the aforementioned conditions is not necessarily comparable to the folding of these proteins in their endogenous environments (Li et al. 2000). The different folding properties may contribute to the effects seen when manipulating the expression level of SMA/SMB, effects that do not actually exist when the proteins are expressed naturally in their respective tissues. Because the bladder tissue of this mouse line is able to express different expression ratios of SMA/SMB without changing the expression levels of any other major myosin isoforms or proteins, differences found in the contractile kinetics of the bladder can be attributed to the SMA/SMB expression level more confidently than in other experiments.
In conclusion, exogenous ADP demonstrated either an additional state in the crossbridge cycle for the −/− type (SMB knockout) not found for either the +/+ or +/− cases or a longer ADP binding time. Either condition may contribute to a longer duty cycle consistently found for the −/− form in in vitro studies leading to a longer overall crossbridge cycle due to bound crossbridges reorienting themselves in a manner that increases the efficacy of force production.
Exogenous Pi studies showed that all three mouse types have two states between detached crossbridges and Pi release, the first being a low force producing state, the second a high force producing state. Once in the high force producing state, crossbridges continue on through the crossbridge cycle unlike the low force producing ones which can return to the detached state in high levels of Pi, explaining why stiffness dropped more than force. While all three mouse types showed evidence for these two additional states, the two homozygous cases had a higher population of the high force generating state than the heterozygous type. While this does not suggest any differences at this point in the crossbridge cycle between the three mouse types, it does imply a cooperative relationship between the two myosin heads suggesting a mechanical role for the heavy chain insert.
Acknowledgments
We would like to thank Dr Ozgur Ogut for his comments on the manuscript. This work was supported by NIH grants HL44181 & HL64137 (F.V.B.) and an AHA predoctoral fellowship 0110107B (P.K.).
REFERENCES
- Adam LP, Haeberle JR, Hathaway DR. Calponin is not phosphorylated during contractions of porcine carotid arteries. Am J Physiol. 1995;268:C903–909. doi: 10.1152/ajpcell.1995.268.4.C903. [DOI] [PubMed] [Google Scholar]
- Babu GJ, Loukianov E, Loukianova T, Pyne GJ, Huke S, Osol G, Low RB, Paul RJ, Periasamy M. Loss of SM-B myosin affects muscle shortening velocity and maximal force. Nat Cell Biol. 2001;3:1025–1029. doi: 10.1038/ncb1101-1025. [DOI] [PubMed] [Google Scholar]
- Bershitsky SY, Tsaturyan AK, Bershitskaya ON, Mashanov GI, Brown P, Burns R, Ferenczi MA. Muscle force is generated by myosin heads stereospecifically attached to actin. Nature. 1997;388:186–190. doi: 10.1038/40651. [DOI] [PubMed] [Google Scholar]
- Brozovich FV, Yamakawa M. Thin filament regulation of force activation is not essential in single α-toxin permeabilized vascular smooth muscle. Am J Physiol. 1995;268:C237–242. doi: 10.1152/ajpcell.1995.268.1.C237. [DOI] [PubMed] [Google Scholar]
- Dantzig JA, Goldman YE, Millar NC, Lacktis J, Homsher E. Reversal of the cross-bridge force-generating transition by photogeneration of phosphate in rabbit psoas muscle fibres. J Physiol. 1992;451:247–278. doi: 10.1113/jphysiol.1992.sp019163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley JJ, Su X, Moreland RS. Caldesmon inhibits active crossbridges in unstimulated vascular smooth muscle. Circ Res. 1998;83:661–667. doi: 10.1161/01.res.83.6.661. [DOI] [PubMed] [Google Scholar]
- Eddinger TJ, Korwek AA, Meer DP, Sherwood JJ. Expression of smooth muscle myosin light chain 17 and unloaded shortening velocity in single smooth muscle cells. Am J Physiol Cell Physiol. 2000;278:C1133–1142. doi: 10.1152/ajpcell.2000.278.6.C1133. [DOI] [PubMed] [Google Scholar]
- Eddinger TJ, Meer DP. Single rabbit stomach smooth muscle cell myosin heavy chain SMB expression and shortening velocity. Am J Physiol Cell Physiol. 2001;280:C309–316. doi: 10.1152/ajpcell.2001.280.2.C309. [DOI] [PubMed] [Google Scholar]
- Facemire C, Brozovich FV, Jin JP. The maximal velocity of vascular smooth muscle shortening is independent of the expression of calponin. J Muscle Res Cell Motil. 2000;21:367–373. doi: 10.1023/a:1005680614296. [DOI] [PubMed] [Google Scholar]
- Fisher SA, Ikebe M, Brozovich FV. Endothelial-1 alters the contractile phenotype of cultured embryonic smooth muscle cells. Circ Res. 1997;80:885–893. doi: 10.1161/01.res.80.6.885. [DOI] [PubMed] [Google Scholar]
- Fuglsang A, Khromov A, Torok K, Somlyo AV, Somlyo AP. Flash photolysis studies of relaxation and cross-bridge detachment: higher sensitivity of tonic than phasic smooth muscle to MgADP. J Muscle Res Cell Motil. 1993;14:666–673. doi: 10.1007/BF00141563. [DOI] [PubMed] [Google Scholar]
- Gollub J, Cremo CR, Cooke R. ADP release produces a rotation of the neck region of smooth myosin but not skeletal myosin. Nat Struct Biol. 1996;3:737–739. doi: 10.1038/nsb0996-796. [DOI] [PubMed] [Google Scholar]
- Gong MC, Cohen P, Kitazawa T, Ikebe M, Masuo M, Somlyo AP, Somlyo AV. Myosin light chain phosphatase activities and the effects of phosphatase inhibitors in tonic and phasic smooth muscle. J Biol Chem. 1992;267:14662–14668. [PubMed] [Google Scholar]
- Hamada Y, Yanagisawa M, Katsuragawa Y, Coleman JR, Nagata S, Matsuda G, Masaki T. Distinct vascular and intestinal smooth muscle myosin heavy chain mRNAs are encoded by a single-copy gene in the chicken. Biochem Biophys Res Commun. 1990;170:53–58. doi: 10.1016/0006-291x(90)91239-o. [DOI] [PubMed] [Google Scholar]
- Harris DE, Warshaw DM. Slowing of velocity during isotonic shortening in single isolated smooth muscle cells. J Gen Physiol. 1990;96:581–601. doi: 10.1085/jgp.96.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorne D. Myosin phosphatase: subunits and interactions. Acta Physiol Scand. 1998;164:483–493. doi: 10.1046/j.1365-201X.1998.00447.x. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Morita F. Role of 17-kDa essential light chain isoforms of aorta smooth muscle myosin. J Biochem (Tokyo) 1992;111:804–809. doi: 10.1093/oxfordjournals.jbchem.a123840. [DOI] [PubMed] [Google Scholar]
- Helper DJ, Lash JA, Hathaway DR. Distribution of isoelectric variants of the 17000-dalton myosin light chain in mammalian smooth muscle. J Biol Chem. 1988;263:15748–15753. [PubMed] [Google Scholar]
- Hewett TE, Martin AF, Paul RJ. Correlations between myosin heavy chain isoforms and mechanical parameters in rat myocardium. J Physiol. 1993;460:351–364. doi: 10.1113/jphysiol.1993.sp019475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuti K, Somlyo AV, Goldman YE, Somlyo AP. Kinetics of contraction initiated by flash photolysis of caged adenosine triphosphate in tonic and phasic smooth muscles. J Gen Physiol. 1989;94:769–781. doi: 10.1085/jgp.94.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz A, Clement-Chomienne O, Walsh MP, Tao T, Katsuyama H, Morgan KG. Effects of calponin on force generation by single smooth muscle cells. Am J Physiol. 1996;270:H1858–1863. doi: 10.1152/ajpheart.1996.270.5.H1858. [DOI] [PubMed] [Google Scholar]
- Inoue A, Yanagisawa M, Takano-Ohmuro H, Masaki T. Two isoforms of smooth muscle myosin regulatory light chain in chicken gizzard. Eur J Biochem. 1989;183:645–651. doi: 10.1111/j.1432-1033.1989.tb21094.x. [DOI] [PubMed] [Google Scholar]
- Iwamoto H. Evidence for increased low force crossbridge population in shortening skinned skeletal muscle fibers: implications for actomyosin kinetics. Biophys J. 1995;69:1022–1035. doi: 10.1016/S0006-3495(95)79977-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworowski A, Anderson KI, Arner A, Engstroem M, Gimona M, Strasser P, Small JV. Calponin reduces shortening velocity in skinned taenia coli smooth muscle fibres. FEBS Lett. 1995;365:167–171. doi: 10.1016/0014-5793(95)00451-e. [DOI] [PubMed] [Google Scholar]
- Katsuyama H, Wang CLA, Morgan KG. Regulation of vascular smooth muscle tone by caldesmon. J Biol Chem. 1992;267:14555–14558. [PubMed] [Google Scholar]
- Kelley CA, Sellers JR, Goldsmith PK, Adelstein RS. Smooth muscle myosin is composed of homodimeric heavy chains. J Biol Chem. 1992;267:2127–2130. [PubMed] [Google Scholar]
- Kelley CA, Takahashi M, Yu JH, Adelstein RS. An insert of seven amino acids confers functional differences between smooth muscle myosins from the intestines and vasculature. J Biol Chem. 1993;17:12848–12854. [PubMed] [Google Scholar]
- Khromov A, Somlyo AV, Trentham DR, Zimmerman B, Somlyo AP. The role of MgADP in force maintenance by dephosphorylated cross-bridges in smooth muscle: a flash photolysis study. Biophys J. 1995;69:2611–2622. doi: 10.1016/S0006-3495(95)80132-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauzon AM, Tyska MJ, Rovner AS, Freyzon Y, Warshaw DM, Trybus KM. A 7 amino acid insert in the heavy chain nucleotide binding loop alters the kinetics of smooth muscle myosin in the laser trap. J Muscle Res Cell Motil. 1998;19:825–837. doi: 10.1023/a:1005489501357. [DOI] [PubMed] [Google Scholar]
- Li XD, Saito J, Ikebe R, Mabuchi K, Ikebe M. The interaction between the regulatory light chain domains on two heads is critical for regulation of smooth muscle myosin. Biochem. 2000;39:2254–2260. doi: 10.1021/bi9924617. [DOI] [PubMed] [Google Scholar]
- Löfgren M, Malmqist U, Arners A. Substrate and product dependence of force and shortening in fast and slow smooth muscle. J Gen Physiol. 2001;117:407–417. doi: 10.1085/jgp.117.5.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmqvist U, Arner A. Correlation between isoform composition of the 17 kDa myosin light chain and maximal shortening velocity in smooth muscle. Pflugers Arch. 1991;418:523–530. doi: 10.1007/BF00370566. [DOI] [PubMed] [Google Scholar]
- Malmqvist U, Arner A, Makuch R, Dabroska R. The effects of caldesmon extraction on mechanical properties of skinned smooth muscle fibre preparations. Pflugers Arch. 1996;432:241–247. doi: 10.1007/s004240050130. [DOI] [PubMed] [Google Scholar]
- Matthew JD, Khromov AS, McDuffie MJ, Somlyo AV, Somlyo AP, Taniguchi S, Takahashi K. Contractile properties of smooth muscles of a calponin knockout mouse. J Physiol. 2000;529:811–824. doi: 10.1111/j.1469-7793.2000.00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthew JD, Khromov AS, Trybus KM, Somlyo AP, Somlyo AV. Myosin essential light chain isoforms modulate the velocity of shortening propelled by nonphosphorylated cross-bridges. J Biol Chem. 1998;47:31289–31296. doi: 10.1074/jbc.273.47.31289. [DOI] [PubMed] [Google Scholar]
- Meer DP, Eddinger TJ. Expression of smooth muscle myosin heavy chains and unloaded shortening in single smooth muscle cells. Am J Physiol. 1997;273:C1259–1266. doi: 10.1152/ajpcell.1997.273.4.C1259. [DOI] [PubMed] [Google Scholar]
- Mossakowska M, Strzelecka-Golaszewska H. Identification of amino acid substitution differentiating actin isoforms in their interaction with myosin. Eur J Chem. 1985;153:373–381. doi: 10.1111/j.1432-1033.1985.tb09313.x. [DOI] [PubMed] [Google Scholar]
- Nagai R, Kuro-o M, Babij P, Periasamy M. Identification of two types of smooth muscle myosin heavy chain isoforms by cDNA cloning and immunoblot analysis. J Biol Chem. 1989;264:9734–9737. [PubMed] [Google Scholar]
- Ogut O, Brozovich FV. Determinants of the contractile properties in the embryonic chicken gizzard and aorta. Am J Physiol Cell Physiol. 2000;279:C1722–1732. doi: 10.1152/ajpcell.2000.279.6.C1722. [DOI] [PubMed] [Google Scholar]
- Ranatunga KW. Effects of inorganic phosphate on endothermic force generation in muscle. Proc R Soc London B Biol Sci. 1999;266:138–1385. doi: 10.1098/rspb.1999.0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee AY, Brozovich FV. The smooth muscle cross-bridge cycle studied using sinusoidal length perturbations. Biophys J. 2000;79:1511–1523. doi: 10.1016/S0006-3495(00)76402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld SS, Xing J, Whitaker M, Cheung HC, Brown F, Wells A, Milligan RA, Sweeney HL. Kinetic and spectroscopic evidence for three actomyosin: ADP states in smooth muscle. J Biol Chem. 2000;275:25418–25426. doi: 10.1074/jbc.M002685200. [DOI] [PubMed] [Google Scholar]
- Rovner AS, Fagnant PM, Lowey S, Trybus KM. The carboxyl-terminal isoforms of smooth muscle myosin heavy chain determine thick filament assembly properties. J Cell Biol. 2002;156:113–123. doi: 10.1083/jcb.200107131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovner AS, Freyzon Y, Trybus KM. An insert in the motor domain determines the functional properties of expressed smooth muscle myosin isoforms. J Muscle Res Cell Motil. 1997;18:103–110. doi: 10.1023/a:1018689102122. [DOI] [PubMed] [Google Scholar]
- Rovner AS, Thompson MM, Murphy RA. Two different heavy chains are found in smooth muscle myosin. Am J Physiol. 1986;250:C861–870. doi: 10.1152/ajpcell.1986.250.6.C861. [DOI] [PubMed] [Google Scholar]
- Sherwood JJ, Eddinger TJ. Shortening velocity and myosin heavy- and light-chain isoform mRNA in rabbit arterial smooth muscle cells. Am J Physiol Cell Physiol. 2002;282:C1093–1102. doi: 10.1152/ajpcell.00307.2001. [DOI] [PubMed] [Google Scholar]
- Shue G, Brozovich FV. The frequency response of smooth muscle stiffness during Ca2+-activated contraction. Biophys J. 1999;76:2361–2369. doi: 10.1016/S0006-3495(99)77393-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegman MJ, Butler TM, Mooers SU, Trinkle-Mulcahy L, Narayan S, Adam L, Chacko S, Haase H, Morano I. Hypertrophy of colonic smooth muscle: contractile proteins, shortening velocity, and regulation. Am J Physiol. 1997;272:G1571–1580. doi: 10.1152/ajpgi.1997.272.6.G1571. [DOI] [PubMed] [Google Scholar]
- Sjuve R, Haase H, Morano I, Uvelius B, Arner A. Contraction kinetics and myosin isoform composition in smooth muscle from hypertrophied rat urinary bladder. J Cell Biochem. 1996;63:86–93. doi: 10.1002/(SICI)1097-4644(199610)63:1%3C86::AID-JCB7%3E3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Sward K, Dreja K, Hellstrand P. Contractile effects of polycations in permeabilized smooth muscle. J Muscle Res Cell Motil. 1998;19:463–472. doi: 10.1023/a:1005368728376. [DOI] [PubMed] [Google Scholar]
- Sweeney HL, Rosenfeld SS, Brown F, Faust L, Smith J, Xing J, Stein LA, Sellers JR. Kinetic tuning of myosin via a flexible loop adjacent to the nucleotide binding pocket. J Biol Chem. 1998;273:6262–6270. doi: 10.1074/jbc.273.11.6262. [DOI] [PubMed] [Google Scholar]
- Szpacenko A, Wagner J, Dabrowska R, Rüegg JC. Caldesmon-induced inhibition of ATPase activity of actomyosin and contraction of skinned fibres of chicken gizzard smooth muscle. FEBS Lett. 1985;192:9–12. doi: 10.1016/0014-5793(85)80032-6. [DOI] [PubMed] [Google Scholar]
- Uyama Y, Imaizumi Y, Watanabe M, Walsh MP. Inhibition by calponin of isometric force in demembranated vascular smooth muscle strips: the critical role of serine-175. Biochem J. 1996;319:551–558. doi: 10.1042/bj3190551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Kawai M. Force generation and phsophate release steps in skinned rabbit soleus slow-twitch muscle fibers. Biophys J. 1997;73:878–894. doi: 10.1016/S0006-3495(97)78121-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker M, Wilson-Kubalek EM, Smith JE, Faust L, Milligan RA, Sweeney HL. A 35-Å movement of smooth muscle myosin on ADP release. Nature. 1995;378:748–751. doi: 10.1038/378748a0. [DOI] [PubMed] [Google Scholar]
- White S, Martin AF, Periasamy M. Identification of a novel smooth muscle heavy chain cDNA: isoform diversity in the S1 head region. Am J Physiol. 1993;264:C1252–1258. doi: 10.1152/ajpcell.1993.264.5.C1252. [DOI] [PubMed] [Google Scholar]
- Winder SJ, Walsh MP. Calponin: Thin filament-linked regulation of smooth muscle contraction. Cell Signal. 1993;5:677–686. doi: 10.1016/0898-6568(93)90029-l. [DOI] [PubMed] [Google Scholar]


