Abstract
Cardiac atrial cells lack a regular system of transverse tubules like that in cardiac ventricular cells. Nevertheless, many atrial cells do possess an irregular internal transverse-axial tubular system (TATS). To investigate the possible role of the TATS in excitation-contraction coupling in atrial myocytes, we visualized the TATS (labelled with the fluorescent indicator, Di-8-ANEPPS) simultaneously with Ca2+ transients and/or Ca2+ sparks (fluo-4). In confocal transverse linescan images of field-stimulated cells, whole-cell Ca2+ transients had two morphologies: ‘U-shaped’ transients and irregular or ‘W-shaped’ transients with a varying number of points of origin of the Ca2+ transient. About half (54 %, n=289 cells, 13 animals) of the cells had a TATS. Cells with TATS had a larger mean diameter (13.2 ± 2.8 μm) than cells without TATS (11.7 ± 2.0 μm) and were more common in the left atrium (n= 206 cells; left atrium: 76 with TATS, 30 without TATS; right atrium: 42 with TATS, 58 without TATS). Simultaneous measurement of Ca2+ sparks and sarcolemmal structures showed that cells without TATS had U-shaped transients that started at the cell periphery, and cells with TATS had W-shaped transients that began simultaneously at the cell periphery and the TATS. Most (82 out of 102 from 31 cells) ‘spontaneous’ (non-depolarized) Ca2+ sparks occurred within 1 μm of a sarcolemmal structure (cell periphery or TATS), and 33 % occurred within 1 pixel (0.125 μm). We conclude that the presence of a sarcolemmal membrane either at the cell periphery or in the TATS in close apposition to the sarcoplasmic reticulum is required for the initiation of an evoked Ca2+ transient and for spontaneous Ca2+ sparks.
In mammalian cardiac cells, the opening of voltage-gated L-type Ca2+ channels causes the opening of closely opposed sarcoplasmic reticulum (SR) Ca2+ release channels (ryanodine receptors; RyRs) through the process of Ca2+-induced Ca2+ release (CICR; Fabiato, 1983; Stern, 1992). In ventricular cells, the well-developed, homogenous network of transverse tubules (T-tubules) uniformly spreads depolarization into the cell, resulting in a nearly synchronous SR Ca2+ release throughout the entire cell (Cheng et al. 1994; Wier et al. 1995). Atrial cells lack T-tubules. Thus, depolarization occurs only at the outer cell membrane, resulting in a whole-cell Ca2+ transient that begins at the edges of the atrial cell and arrives at the centre of the cell after a significant delay (about 30–80 ms) (Berlin, 1995; Hüser et al. 1996; Mackenzie et al. 2001). Similarly, the loss of T-tubules in guinea-pig ventricular myocytes, which occurs over time in cell culture, is paralleled by the replacement of the spatially uniform whole-cell Ca2+ transient with a Ca2+ transient exhibiting marked spatial inhomogeneities (Lipp et al. 1996). Since the RyRs are uniformly distributed along the z-lines in both cell types (atrial and ventricular cells; Lewis Carl et al. 1995; Mackenzie et al. 2001), these differences in the spatial pattern of excitation result from the distribution of dihydropyridine receptors (DHPRs) in the sarcolemma and T-tubules.
Calcium sparks are localized, subcellular releases of calcium, which occur either spontaneously (Cheng et al. 1993), or can be evoked by membrane depolarization (López-López et al. 1994, 1995; Cannell et al. 1995). Regardless of their method of initiation, Ca2+ sparks represent the opening of a cluster of RyRs (Blatter et al. 1997; Cannell & Soeller, 1999; Bridge et al. 1999; Lukyanenko et al. 2000; Izu et al. 2001). ‘Spontaneous’ Ca2+ sparks, those that occur without cell depolarization, are thought to result from the random opening of a cluster of RyRs. In ventricular cells, spontaneous Ca2+ sparks occur at the z-line (Shacklock et al. 1995; Parker et al. 1996), where RyRs exist at the junctional SR (Lewis Carl et al. 1996). However, spontaneous Ca2+ sparks have been observed only rarely in the interior of cat atrial myocytes (Hüser et al. 1996), and only at the edges of canine Purkinje myocytes (Cordeiro et al. 2001), despite RyRs being distributed throughout the cell interior. Since these cell types lack T-tubules, these results suggest that spontaneous Ca2+ sparks may not be due to the opening of any cluster of RyRs but only clusters of RyRs that are in close apposition to the sarcolemmal membrane.
Rat atrial myocytes provide a useful system in which to study the role of sarcolemma-junctional SR interaction in excitation-contraction coupling. Most rat atrial myocytes have a sarcolemmal tubule system, which is intermediate between the mammalian ventricular cell (T-tubules at all the z-lines) and the typical mammalian atrial or Purkinje cell (no internal sarcolemmal system). This transverse-axial tubule system (TATS) has a predominantly longitudinal orientation, is poorly developed when compared to the T-tubules in ventricular cells, and has been observed in variable frequencies (Hibbs & Ferrans, 1969; Forssmann & Girardier, 1970; Leeson, 1980; Yamasaki et al. 1997).
In this study, we exploit the TATS to investigate the physiological significance of the sarcolemma in atrial excitation-contraction coupling and the generation of spontaneous Ca2+ sparks. We demonstrate that evoked Ca2+ transients and spontaneous Ca2+ sparks occur at sites where the SR is in close apposition to the sarcolemmal membrane, either at the cell surface or in the TATS.
METHODS
All rats used in the present study were maintained in accordance with the guidelines of the Institutional Animal Care and Use Committee of the University of Maryland School of Medicine and the Guide for the Care and Use of Laboratory Animals (Department of Health and Human Services Publication No. (NIH) 85–23, revised 1985).
Cell isolation and solutions
Two-month-old Sprague-Dawley rats (200–300 g body weight) were anaesthetized with sodium pentobarbitone (170 mg kg−1 injected i.p.). Single atrial cells were obtained using a modification of an enzymatic technique for obtaining isolated ventricular cells described in detail previously (López-López et al. 1995). Briefly, the hearts were removed from the animals via mid-line thoracotomy, and perfused with a nominally calcium-free physiological salt solution (PSS) containing (mm): NaCl 135, KCl 4, MgSO4 1, NaH2PO4 0.33, glucose 10, Hepes 10, pH 7.25, at room temperature (21–23 °C), for 3 min. The heart was then perfused with the same solution supplemented with 1 mg ml−1 collagenase (Boehringer Mannheim Type B, Indianapolis, IN, USA) and 0.02 mg ml−1 protease (Type XIV, Sigma, St Louis, MO, USA) for 3 min. The atria were removed, minced with microdissecting scissors and shaken in PSS supplemented with 5 mg ml−1 collagenase (Boehringer Mannheim Type B) and 1.5 mg ml−1 protease (Type XIV, Sigma) for 7–10 min. The tissue chunks were removed and gently triturated with a large bore Pasteur pipette. The supernatants from the enzyme digestion and from the trituration were then gently centrifuged at low speed for 5 min. The pellet was resuspended in PSS with 0.25 mm Ca2+. The cells were successively washed and resuspended in PSS containing 0.5 and 1.0 mm Ca2+. The only cells chosen for analysis were those that had the typical fusiform shape of isolated atrial cells, were relatively straight and qualitatively uniform in width, and had visible striations.
Fluorescence recording and analysis
Cells were loaded with the Ca2+ indicator, fluo-4, acetoxymethyl ester (fluo-4 AM, Molecular Probes, Eugene, OR, USA), by incubation for 30 min at 25 °C in PSS containing 1.0 mm Ca2+, fluo-4 AM (5 μm) and pluronic acid (2.5 μm). The sarcolemma and tubule membranes were labelled by incubation of the cells for 5 min in PSS containing 1.0 mm Ca2+ to which Di-8-ANEPPS (10 μm, Molecular Probes) had been added. All cells were studied within 1 h of loading. The leakage of Di-8-ANEPPS into intracellular structures was minimized by the short exposure times, as was observed in previous studies on ventricular cells (Shacklock et al. 1995).
The cells were studied using an Axiovert 100 inverted microscope (Carl Zeiss, Germany) equipped with a × 40 water immersion lens (numerical aperture = 1.2) and attached to a confocal laser scanning unit (LSM 410, Carl Zeiss). Fluo-4 fluorescence was excited by 488 nm light from a krypton/argon laser and emitted fluorescence was filtered with a 515 nm long-pass filter and collected with a Hammamatsu R3896 photomultiplier tube (Bridgewater, NJ, USA). For double-label experiments, a 560 nm dichroic mirror split the emitted light. The Di-8-ANEPPS signal was filtered with a 590 nm long-pass filter. The fluo-4 signal was passed through a 515–540 nm band-pass filter to a second photomultiplier tube. A 64 μm line was scanned and divided into 512 pixels of 0.125 μm each. This line was repeatedly scanned 512 times at a frequency of 0.717 or 0.478 lines per millisecond to make one linescan image. Two to sixteen consecutive images were collected at each scan location. The lateral resolution of the system is 0.26 μm and the theoretical z-resolution is 1.1 μm. Field stimulation was delivered between parallel platinum electrodes at the edges of the tissue bath, at a voltage at least twice the stimulation threshold. Cell width was measured on the screen of the Zeiss LSM 410 microscope using optical calipers, at the widest point of the cell in the x-y plane, excluding branch points. In order to separate Ca2+ release triggered by the TATS deep within the cell from that triggered by peripheral couplings at the cell periphery, the microscope's focal plane was placed at the central plane of the cell. To accomplish this, the bottom and top of the cell were identified and the focal plane chosen at the point equidistant from each edge. Although we did not routinely record the thickness of the cell, cells were rejected that were clearly flattened, i.e. cell thickness << cell width. We occasionally observed folds in the membrane (Fig. 2D, upper left, for example), which were distinguished from the TATS in z-sections by their much larger width and their pair-wise appearance in the x-t confocal linescans. Linescan positions were selected to not include these folds. Data were analysed using custom routines written in IDL (Research Systems, Inc., Boulder, CO, USA).
Figure 2. Relationship between TATS and the shape of the Ca2+ transient.
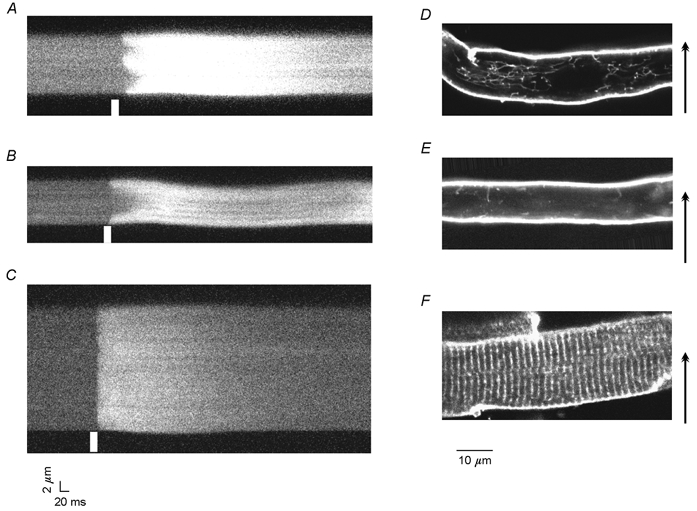
Linescan images of Ca2+ transients in atrial (A and B) and ventricular (C) cells were taken along a direction transverse to the longitudinal axis of the cells (arrows). Ca2+ transients in atrial cells fell into two categories: those with numerous initiation points termed W-shaped (A) and U-shaped transients (B). Ventricular cells (C) exhibited nearly simultaneous activation across the cell. White bars denote the time of the field stimulation pulse. D-F show confocal images of Di-8-ANEPPS labelling of sarcolemmal membranes of atrial cells (D and E) and a ventricular cell (F). D shows a cell with a TATS with a prominent longitudinal component while the cell in E is almost devoid of TATS. The ventricular cell (F) shows a regular pattern of Di-8-ANEPPS labelling of T-tubules that extend from the surface and project radially into the cell.
Measurement of cell width and thickness
Cells widths were determined with optical calipers that are part of the Zeiss software package. In double-labelled cells, the boundaries of the cell were determined from the Di-8-ANEPPS fluorescence or from the fluo-4 fluorescence. Cells chosen for study were relatively straight and qualitatively uniform in width as judged by eye. When there was some visible difference in width along the length of the cell, the average width was measured.
In some cells, both cell width and thickness were measured from y-z sections of Di-8-ANEPPS-labelled cells using optical cursors. The step size in the axial direction was 0.25 μm. Cells chosen for these measurements satisfied our criteria for analysis but otherwise were chosen at random. For this analysis, cells were not rejected if they were flattened.
Visualization of three-dimensional structure of TATS
The sarcolemma was labelled with Di-8-ANNEPS as above. After the cells had settled on the coverglass, the solution was carefully suctioned out and replaced with paraformaldehyde (4 %) to fix the cells. Fixation was needed to prevent cell movement while stacks of x-y confocal images of the cell were collected. For these experiments, we used a BioRad Radiance confocal microscope with × 60 Nikon 1.2 NA water immersion objective. The microscope was focused near the bottom of the cell and x-y confocal images were obtained at successively higher focal planes. The distance between focal planes was 0.25 μm, which was controlled by the LaserSharp software and the focus motor. The three-dimensional (3-D) structure of the TATS is visualized as surfaces of equal fluorescence (Fig. 1C). To obtain these isofluorescence surfaces, each x-y section was averaged using a 3 × 3 smoothing kernel, then all pixels with values less than 60 fluorescence units were eliminated. Deconvolution of the optical distortions of the microscope's objective was not carried out. Isofluorescence surfaces with values of 70 fluorescence units were found using IDL routines.
Figure 1. Three-dimensional structure of TATS.
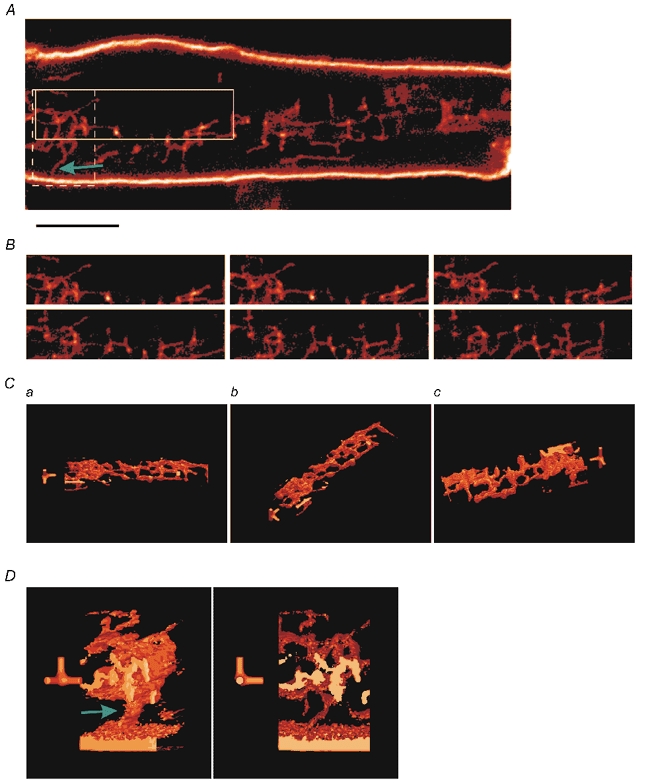
A shows an optical section of a Di-8-ANEPPS-labelled atrial cell. The surface sarcolemma is heavily labelled as are finer internal membrane structures that are consistent with the previously described transverse-axial tubular system (TATS). The focal plane lies 7.25 μm from the bottom of the cell. Scale bar is 5 μm. B shows the region enclosed by the continuous box in A at different focal planes. The focal plane is 7.25 μm from the bottom of the cell (top left) and increases by 0.25 μm increments (left to right, top to bottom). C shows a 3-D reconstruction of the 5.5 μm thick section of the region in B. D shows a reconstruction of the 4.25 μm thick region in the dashed box in A. The green arrows in A and D show the connection between the surface sarcolemma and the TATS. The full-axis is along the axial direction and the half-axes are along longitudinal and transverse axes of the cell. In both C and D,the short axes are 1 μm long; the full-axis is 5.5 μm long in C and 4.25 μm in D.
RESULTS
Three-dimensional organization of TATS
Figure 1A shows an x-y section of an atrial cell labelled with the membrane marker Di-8-ANEPPS. As expected the surface sarcolemma was heavily labelled. Additionally, what appear to be fine tube-like structures with a reticular pattern could be seen within the cell. These structures do not have a regular pattern but have strong longitudinal and transverse components. This reticular pattern is consistent with the transverse-axial tubule system, or TATS, previously described in rat atrial cells (Forssmann & Girardier, 1970; Leeson, 1980). Figure 1B shows the TATS at successive focal planes (top to bottom, left to right) of the region of Fig. 1A enclosed in the continuous box. The first image was taken at 7.25 μm from the cell bottom and the focal plane for each image was separated by 0.25 μm.
In order to obtain a clearer picture of the structure of the TATS, we made a 3-D reconstruction of the TATS from optical z-sections of the continuous box region that extended from 5.50 to 11 μm from the bottom of the atrial cell separated by 0.25 μm. In this section, the cell was 12.75 μm thick so the TATS is at least 1.75 μm from the surface. The 3-D reconstruction of the TATS viewed at different angles is shown in Fig. 1C. The axes in each of the views provide scale and orientation. The full-axis is along the microscope's axis and is 5.5 μm; the half-axes are along the x and y directions and are 1 μm long. Note the axes are near the compact TATS structure near the left-hand of the images in Fig. 1C. Figure 1Ca and b shows views from slightly off the microscope's axis. Figure 1Cc shows a similar view to that in Fig. 1Ca but viewed from the bottom. The reticulated pattern that is suggested in some of the single z-slices is clearer in the 3-D-reconstructed images. Volumes that extend beyond the region used in the reconstruction appear truncated and lightly coloured in Fig. 1C and 1D.
A connection between the surface sarcolemma and the internal TATS is indicated by the green arrow in the region in the dashed box in Fig. 1A. A 3-D reconstruction of that region is shown at two viewing angles in Fig. 1D.
Relationship between ultrastructure and the spatial pattern of Ca2+ release
Excitation-contraction coupling was examined by obtaining linescan images oriented transversely to the long axis of field-stimulated atrial myocytes (Fig. 2A and B). Two types of Ca2+ transients were observed: (1) U-shaped transients (Fig. 2B) like those observed in cat atrial myocytes (Hüser et al. 1996), and (2) irregular or W-shaped transients (Fig. 2A) with varying numbers of points of origin. Both of these differed from Ca2+ transients recorded in rat ventricular cells (Fig. 2C), which appear as straight horizontal bands indicating near-simultaneous release of Ca2+ throughout the cell.
Sample full-frame (x -y) images from rat atrial cells treated with Di-8-ANEPPS to label sarcolemmal membrane structures are shown in Fig. 2D and E. Two hundred and eighty-nine cells from 13 animals were studied. Labelled cells were classified into three categories based on their appearance: those with TATS, those without TATS, and mixed. Cells with TATS had a fine reticular pattern of tubules throughout the cell (Fig. 2D). Cells classified as without TATS had no tubules or, more commonly, only a few short tubules (Fig. 2E). Cells were classified as mixed when they had a reticular network at one end of the cell but not the other, or when they had one or two isolated tubules running the length of the cell. Although there was a continuum of tubule density, about 90 % of cells could be classified as having either a TATS or no TATS by the aforementioned criteria. Of these cells, 54 % had a TATS. In all cases, the pattern of sarcolemmal labelling was in sharp contrast to the regular pattern (1.8 μm spacing) of transverse tubules observed in ventricular cells (Fig. 2F; and see Shacklock et al. 1995).
To evaluate whether the TATS seen in some rat atrial cells participates in excitation-contraction coupling, 34 cells were double-labelled with Di-8-ANEPPS and fluo-4 (Fig. 3). Linescan images oriented transverse to the long axis of the cell were collected during field stimulation (0.3–0.5 Hz). Cells without TATS had only U-shaped Ca2+ transients (18 cells; Fig. 3A). Cells with TATS (16 cells) usually had W-shaped Ca2+ transients with the points of the W corresponding to the location of the tubules as marked by the Di-8-ANEPPS signal (Fig. 3B and C; 14 cells). These results indicate that initiation of SR Ca2+ release in response to field stimulation is located at the TATS as well as the peripheral sarcolemmal membrane in rat atrial cells.
Figure 3. The presence and location of sarcolemmal tubules determines the shape of the whole-cell Ca2+ transient.
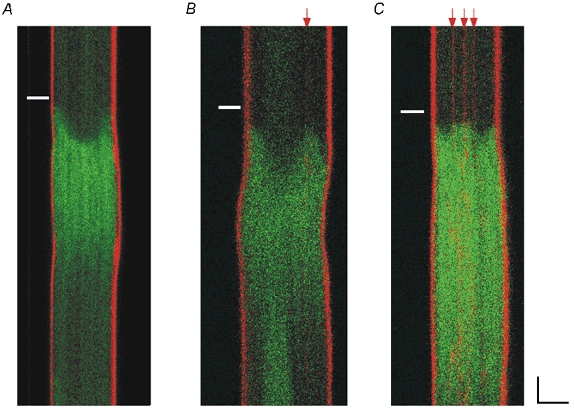
Cells were double-labelled with fluo-4 (green signal) and Di-8-ANEPPS (red signal) and scanned transversely. Red arrows highlight the positions of the sarcolemmal tubules. A, cell without TATS showing U-shaped transient. All cells without TATS had U-shaped transients (18 cells). B and C, cells with TATS usually (14 of 16 cells) had irregular, or W-shaped, transients and the points of the W corresponded to the locations of the tubules as marked by the Di-8-ANEPPS signal. White bars denote the time of the field stimulation pulse. Scale bars are 5 μm and 50 ms.
In mammalian ventricular cells, the T-tubule system serves to transmit sarcolemmal depolarization throughout the cell, ensuring synchronous contraction (Cheng et al. 1994; Shacklock et al. 1995). If the TATS serves a similar function in atrial cells, then there should be a correlation between the presence of a TATS and cell size. Although there was substantial overlap, cells with TATS had larger mean diameters than cells without TATS (13.2 ± 2.8 vs. 11.7 ± 2.0 μm, n= 289 cells from 13 animals, P < 0.0001, Mann-Whitney U test for comparison of non-normally distributed variable; Fig. 4A). We observed a TATS more frequently in left atrial cells than in right atrial cells (Fig. 4B; 72 % vs. 42 %; n= 206 cells from 4 animals; left atrium: 76 with TATS, 30 without TATS; right atrium: 42 with TATS and 58 without TATS). The Fisher exact test applied to this categorical data (presence vs. absence of TATS, left vs. right atrium) confirmed a statistically greater proportion of TATS in left atrial cells (P < 0.0001).
Figure 4. Cells with TATS are larger and the left atrium has more cells with TATS.
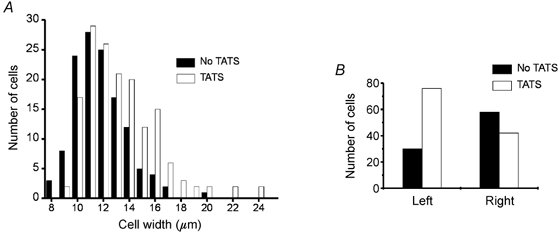
A, histogram of cell widths with (□) and without (▪) TATS (289 cells from 13 animals). Cells with TATS had a slightly larger mean width than cells without TATS (13.2 vs. 11.7 μm, P < 0.0001, Mann-Whitney U test). B, a large majority of left atrial cells had a TATS (76 of 106 cells (72 %) had TATS, 30 (28 %) did not have TATS) but there was a slight preponderance of cells without TATS in the right atrium (42 of 100 cells (42 %) had TATS, 58 (58 %) did not have TATS). There is a statistically greater proportion of TATS in left atrial cells than in right atrial cells (P < 0.0001 by the Fisher exact test).
Spontaneous Ca2+ sparks (i.e. those observed in quiescent cells) occur only in association with a closely apposed sarcolemmal membrane
If Ca2+ sparks in quiescent atrial cells are due to the opening of a random cluster of RyRs in the SR, then they should be distributed throughout the cell, as are the RyRs (Lewis Carl et al. 1995; Mackenzie et al. 2001). To test this hypothesis, 102 Ca2+ sparks (31 cells; 11 animals) were recorded from quiescent, double-labelled rat atrial cells. Linescans were obtained transverse to the long axis of the cell. Scan lines were chosen so as to cross the maximal number of defined tubules. Cells with Ca2+ sparks in the middle were selected for scanning, resulting in an enriched population of Ca2+ sparks occurring away from the edge of the cell and in a cell population consisting exclusively of cells with TATS. The scan line was positioned so as to intersect two or three discrete tubules if possible. In cells without TATS, Ca2+ sparks occurred only at the periphery of the cell. In cells with TATS, Ca2+ sparks arose at the periphery and at the tubules, as shown in Fig. 5A and B. The distribution of distances between the location of the peak of the Ca2+ spark to the nearest membrane structure (i.e. tubule system or sarcolemma) is shown in Fig. 5C. Thirty-three per cent of the Ca2+ sparks were within 1 pixel (0.125 μm) of the nearest sarcolemmal structure, and 80 % were within 1 μm. When a Ca2+ spark was not clearly associated with a tubule in the plane of the linescan, a manual search along the z-axis usually identified a tubule within 1 μm of the Ca2+ spark. In rare cases (2 cells), no tubules were visible in the plane of the scan. The mean cell width was 13.4 ± 2.7 μm (31 cells) and the mean number of tubules per scan line was 2.6 ± 1.4 (31 cells).
Figure 5. Spontaneous Ca2+ sparks occur on TATS.
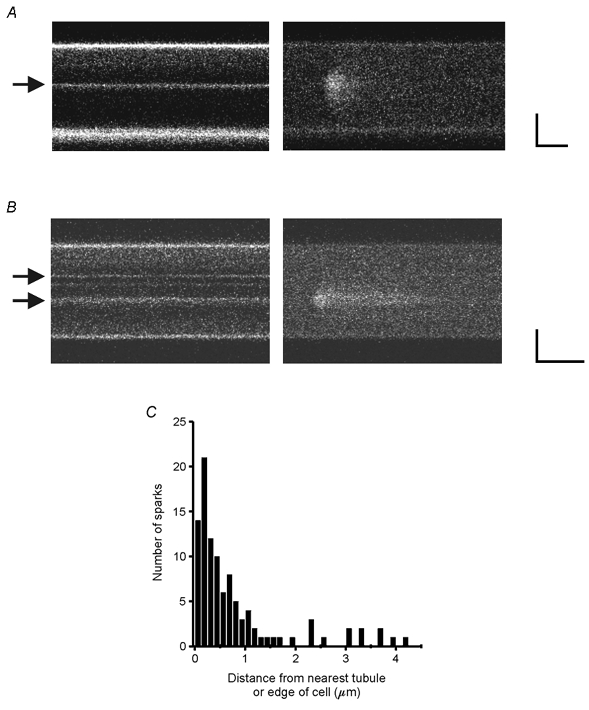
A and B show examples of spontaneous Ca2+ sparks in rat atrial cells double-labelled with Di-8-ANEPPS (left) and fluo-4 (right). Arrows highlight the location of the sarcolemmal tubules. Scale bars are 5 μm and 50 ms. C, histogram of distances between the position of the peak of the Ca2+ spark and the nearest membrane structure. Thirty-three per cent of the Ca2+ sparks were within 0.125 μm (1 pixel) of the nearest sarcolemmal structure and 80 % were within 1 μm.
Localized Ca2+ release vs. regenerative Ca2+ waves
Berlin (1995) and Hüser et al. (1996) both observed that, in some cells, the rise in Ca2+ concentration following electrical depolarization did not decrease from periphery to centre as would be expected if Ca2+ release were localized to only the cell periphery and Ca2+ passively diffused to the cell interior. Instead, the maximal Ca2+ concentration was similar at the periphery and centre of the cell suggesting regenerative SR Ca2+ release.
A method for distinguishing localized from regenerative Ca2+ release is to plot the time to reach a particular concentration vs. the distance from the cell edge. When Ca2+ release occurs only at the cell periphery and Ca2+ spreads by passive diffusion, the time-to-target curve is concave-up, indicating that it takes increasingly longer to reach the target value the greater the distance from the periphery. If Ca2+ release is triggered at the cell periphery and spreads as a regenerative Ca2+ wave to the centre of the cell, the time-to-target plot is initially linear and then becomes concave-down. Both types of time-to-target plots can be seen in field-stimulated rat atrial myocytes. Figure 6A shows a linescan image of a rat atrial cell during field stimulation. The corresponding time-to-target plot (Fig. 6B) is concave-up indicating that the myofibrils at the centre of the cell were activated by Ca2+ passively diffusing from release sites at the cell periphery. A linescan image from a different atrial cell during field stimulation is shown in Fig. 6C. The time-to-target plot from this cell (Fig. 6D) is linear, consistent with activation by a regenerative Ca2+ wave. Thus, both diffusion and regenerative Ca2+ waves are seen in these cells.
Figure 6. Time-to-target plots distinguish between non-propagating and propagating Ca2+ release.
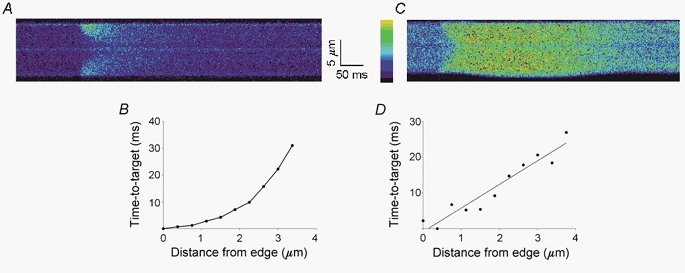
A, transverse linescan image of a rat atrial cell. B, corresponding plot of the time to reach a target fluorescence level as a function of distance from the cell's lower edge (time-to-target plot). The concave-up time-to-target plot is consistent with non-propagating Ca2+ release from the edge of the cell and the passive diffusion of Ca2+ to the centre of the cell. C shows a linescan image from another cell and D its corresponding time-to-target plot. The linear time-to-target plot suggests that Ca2+ spreads to the centre of the cell as a propagated Ca2+ wave. The target fluorescence value is usually chosen to be at the midpoint of the baseline and peak fluorescence values. In cases where the fluorescence decreases rapidly from the surface (e.g. A), a lower target fluorescence value (∼20 % of peak fluorescence) is used to obtain more data points allowing a better determination of the shape of the time-to-target plot. Using both simulated and experimental linescans, we found that the target value does not change either the shape of the time-to-target plot or the calculated Ca2+ wave velocity. Colour scale bar ranges from 0 to 255 fluorescence units from black to yellow.
Atrial cells are not flattened
If isolated atrial cells are very thin (in the z-direction) then Ca2+ released from SR near the cell periphery would be sufficient to fully activate myofibrils near the centre. To ascertain the thickness of the isolated cells, we measured the width and thickness in cells labelled with Di-8-ANEPPS. The mean cell width was only slightly larger than the thickness (14.02 ± 3.54 vs. 11.81 ± 2.47 μm, n= 20, P= 0.03, t test). Since the confocal microscope used here samples an area about 1 μm thick and the focal plane chosen for the experiments was at the centre of the cell, the linescan images were several microns away from either the top or bottom of the cell.
DISCUSSION
Contraction in cardiac cells requires the coordinated release of Ca2+ from the SR. In mammalian ventricular cells, the extensive T-tubular system conducts electrical depolarization rapidly into the cell allowing for near synchronous SR Ca2+ release throughout the cell. Atrial cells lack an extensive T-tubular network so coordinated SR Ca2+ release and contraction in atrial cells must depend on an entirely different cellular process(es). The data presented here demonstrate that in rat atrial cells a TATS exists and that it has a functional role in excitation- contraction coupling. In addition, the unique structural aspects of these cells allow us to demonstrate that spontaneous Ca2+ sparks do not occur uniformly throughout the cell as would be expected by the distribution of RyRs or Ca2+ release sites. Instead, spontaneous Ca2+ sparks require very specific conditions to exist. They occur almost exclusively where there is close apposition of the sarcolemma and the SR.
Calcium transient shape
In ventricular cells, the near synchronous SR Ca2+ release throughout the cell results in a horizontal band of fluorescence in a linescan image transverse to the longitudinal axis of the cell (Fig. 2C). In contrast, the rise in [Ca2+]i is asynchronous in atrial cells. In guinea-pig (Berlin, 1995) and cat (Hüser et al. 1996) atrial cells, Ca2+ release is initiated at the cell periphery and there is substantial delay in the rise of Ca2+ near the centre of the cell (about 30–80 ms). This asynchronous rise in [Ca2+]i results in a U-shaped Ca2+ transient in the confocal image. Our results confirm these findings in rat atrial cells that lack a TATS (Fig. 2B). However, Ca2+ release can also be initiated at the TATS giving rise to complex patterns of Ca2+ transients (Fig. 2A, and Fig. 3B and C).
Distribution of TATS in atrial cells and its functional implications
Previous studies of atrial ultrastructure have generated conflicting results. Several investigators have reported the presence of a TATS in rat atrial cells (Forssmann & Girardier, 1970; Leeson, 1980; Forbes et al. 1984). Others however, have not observed any intracellular structures consistent with these sarcolemmal invaginations (Hibbs & Ferrans, 1969; Yamasaki et al. 1997). It is interesting to note that in all studies where the sarcolemmal membranes were labelled or stained, a TATS was always identified. Our results confirm this observation. A TATS was observed in more than half of the rat atrial cells stained with the membrane indicator Di-8-ANEPPS.
In ventricular cells, the T-tubules ensure rapid and coordinated contraction throughout the cell. The TATS may serve a similar function in atrial cells, leading to a more rapid, coordinated, and forceful contraction. In support of this conclusion is the observation that atrial cells with TATS had a larger mean diameter than cells without TATS (13.2 ± 2.8 vs. 11.7 ± 2.0 μm, P < 0.0001), although there is substantial overlap between the groups (Fig. 4A). In addition, the TATS was more prevalent in cells from the left atria (Fig. 4B), which generate higher pressures than the right atria. Similar results are observed in mice where there is a higher incidence of interior junctional SR (Forbes et al. 1990) and TATS (Forbes et al. 1984) in left atrial cells than in right atrial cells.
Spontaneous Ca2+ sparks do not represent the random opening of Ca2+ release units (RyRs) in the sarcoplasmic reticulum
Despite the broad distribution of RyRs in atrial cells, principally on the z-lines (Lewis Carl et al. 1996), Ca2+ sparks that occur without cell depolarization occur predominantly where there is close apposition of RyRs with the sarcolemmal membrane. In cat atrial cells, which lack T-tubules, Hüser et al. (1996) found that spontaneous Ca2+ sparks occur mainly at the edge of cell at the surface sarcolemma. Similarly, spontaneous Ca2+ sparks occurred at the cell periphery in rabbit Purkinje and neonatal rat myocytes, both of which lack T-tubules (Löhn et al. 2000; Cordeiro et al. 2001). Our experiments extend these observations. In the vast majority of quiescent cells studied (31 of 33 from 11 animals), most Ca2+ sparks occurred within 1 μm of either the cell edge or a TATS. In fact, 33 % of the Ca2+ sparks occurred within 1 pixel (0.125 μm) of the cell edge or a TATS (Fig. 5).
Ca2+ sparks distant from a sarcolemmal structure were very rare in quiescent cells. We observed spontaneous Ca2+ sparks away from a visible tubule or cell edge in only 2 of 33 quiescent cells. It is possible that spontaneous Ca2+ sparks never occur without an adjacent sarcolemmal membrane and that in these two cells a TATS was present near the observed Ca2+ spark but was out of the plane of the linescan image. Similarly, Hüser et al. (1996) saw Ca2+ sparks at the centre of cat atrial cells in only 2 of 12 cells even under conditions that cause Ca2+ overload of the SR (10 mm bath Ca2+).
In ventricular cells, Ca2+ sparks are observed even in the absence of extracellular Ca2+ (Cheng et al. 1993) and also in permeabilized cardiac cells (Lukyanenko & Györke, 1999) where voltage-dependent Ca2+ entry through surface membrane Ca2+ channels cannot occur. The fact that there is an association between Ca2+ sparks in quiescent atrial cells and the sarcolemma suggest several interesting possible mechanisms. There could be a direct coupling of the SR Ca2+ release elements to a surface membrane protein, involvement of a secondary protein that is only distributed in the SR at sites where there is close apposition to the sarcolemma (e.g. IP3 receptors; Mackenzie et al. 2002), an alteration in the microenvironment of RyRs when they are in apposition to the sarcolemmal membrane, a differential Ca2+ loading of the SR at these sites, or different properties of the RyRs at these sites. Distinguishing between these possibilities will require further studies.
Physiological significance of TATS and Ca2+ waves
When Ca2+ release is confined to the periphery of the cell, the time for the myofilaments to be activated increases disproportionately with distance from the surface. This slowing of activation with distance is seen in the concave-up shape of the time-to-target plots of Fig. 6B. A possible physiological function of regenerative Ca2+ waves and Ca2+ release from the TATS is to reduce the time lag between myofilament activation near the surface and centre of the cell. The concave-up time-to-target plot demonstrates that it takes a progressively longer time to increase the Ca2+ concentration the greater the distance from the edge of the cell or Ca2+ release site. When Ca2+ release at the edge of the cell initiates a Ca2+ wave, the Ca2+ concentration at the centre of the cell will rise more quickly. By allowing Ca2+ release at sites within the cell, the TATS also increases the speed at which the interior of the atrial cell is activated. The fact that the TATS is seen more frequently in larger cells is consistent with this concept.
Conversely, narrow or thin cells could be rapidly activated even when Ca2+ release is localized to the cell periphery. Two factors account for this. First, geometry demands that a large fraction of the cell's volume be located close to the cell periphery. Second, while diffusive transport is slow over large distances it is very rapid over short distances, as seen in the time-to-target plot in Fig. 6B. In thin or narrow cells, Ca2+ released from the SR close to the cell periphery would rapidly diffuse in a thin annular region that nevertheless activates a significant fraction of the myofibrils. Therefore, the TATS or Ca2+ waves may not play a significant role in hastening contraction in thin cells.
Acknowledgments
We gratefully acknowledge the advice of Dr Junichiro Miake on cell isolation, the help of Dr Shawn Robinson with the fixing of cells, and Dr Chris Ward for use of his BioRad confocal microscope. This study was supported by a Michael Bilitch Fellowship in Cardiac Pacing and Electrophysiology from the North American Society of Pacing & Electrophysiology (M.M.K.), a beginning-grant-in-aid from the American Heart Association (Mid-Atlantic Affiliate; L.T.I.), National Institutes of Health grants HL68704 (L.T.I.), HL55280 (W.G.W.) and HL50435 (C.W.B.), and a Department of Veterans Affairs Merit Review Award (S.R.S.). C.W.B. is an Established Investigator of the American Heart Association (National Center).
Supplementary material
The online version of this paper can be found at:
http://www.jphysiol.org/cgi/content/full/547/2/441 and contains supplementary material comprising a figure and a movie.
The figure shows an atrial cell labelled with Di-8-ANEPPS. The white box delineates the x-y region used for the 3-D reconstruction shown in the accompanying movie. The scale bar is 5 μm.
The movie shows a 3-D reconstruction of the Di-8-ANEPPS-labelled membrane; the rotation is around the longitudinal axis of the cell. The continuous walls are the surface sarcolemma. The fine reticular structures comprise the TATS. This 3-D image was created from 27 z-sections spaced 0.25 μm apart. The reconstruction encompasses the 6.75 μm thick volume 5.5 μm from the bottom of the cell to 0.5 μm to the top surface of the cell.
REFERENCES
- Berlin J. Spatiotemporal changes of Ca2+ during electrically evoked contractions in atrial and ventricular cells. Am J Physiol. 1995;269:H1165–1170. doi: 10.1152/ajpheart.1995.269.3.H1165. [DOI] [PubMed] [Google Scholar]
- Blatter LA, Hüser J, Rios E. Sarcoplasmic reticulum Ca2+ release flux underlying Ca2+ sparks in cardiac muscle. Proc Natl Acad Sci U S A. 1997;94:4176–4181. doi: 10.1073/pnas.94.8.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge JH, Ershler PR, Cannell MB. Properties of Ca2+ sparks evoked by action potentials in mouse ventricular myocytes. J Physiol. 1999;518:469–478. doi: 10.1111/j.1469-7793.1999.0469p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell MB, Cheng H, Lederer WJ. The control of calcium release in heart muscle. Science. 1995;268:1045–1049. doi: 10.1126/science.7754384. [DOI] [PubMed] [Google Scholar]
- Cannell MB, Soeller C. Mechanisms underlying calcium sparks in cardiac muscle. J Gen Physiol. 1999;113:373–376. doi: 10.1085/jgp.113.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Cannell MB, Lederer WJ. Propagation of excitation-contraction coupling into ventricular myocytes. Pflugers Arch. 1994;428:415–417. doi: 10.1007/BF00724526. [DOI] [PubMed] [Google Scholar]
- Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Cordeiro JM, Spitzer KW, Giles WR, Ershler PE, Cannell MB, Bridge JHB. Location of the initiation site of calcium transients and sparks in rabbit heart Purkinje cells. J Physiol. 2001;531:301–314. doi: 10.1111/j.1469-7793.2001.0301i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Calcim-induced release of calcium from the sarcoplasmic reticulum. Am J Physiol. 1983;245:C1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Forbes MS, Hawkey LA, Sperelakis N. The transverse axial tubular system (TATS) of mouse myocardium: its morphology in the developing and adult animal. Am J Anat. 1984;170:143–162. doi: 10.1002/aja.1001700203. [DOI] [PubMed] [Google Scholar]
- Forbes MS, Van Niel EE, Purdy-Ramos SI. The atrial myocardial cells of mouse heart: a structural and stereological study. J Struct Biol. 1990;103:266–279. doi: 10.1016/1047-8477(90)90045-e. [DOI] [PubMed] [Google Scholar]
- Forssmann WG, Girardier L. A study of the T system in rat heart. J Cell Biol. 1970;44:1–19. doi: 10.1083/jcb.44.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzini-Armstrong C, Protasi F, Ramesh V. Shape, size and distribution of Ca2+ release units and couplings in skeletal and cardiac muscles. Biophys J. 1999;77:1528–1539. doi: 10.1016/S0006-3495(99)77000-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs RG, Ferrans VJ. An ultrastructural and histochemical study of rat atrial myocardium. Am J Anat. 1969;124:251–270. doi: 10.1002/aja.1001240302. [DOI] [PubMed] [Google Scholar]
- Hüser J, Lipsius SL, Blatter LA. Calcium gradients during excitation-contraction coupling in cat atrial myocytes. J Physiol. 1996;494:641–651. doi: 10.1113/jphysiol.1996.sp021521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izu LT, Marban JRH, Balke CW, Wier WG. Large currents generate large cardiac Ca2+ sparks. Biophys J. 2001;80:88–102. doi: 10.1016/S0006-3495(01)75997-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeson TS. T-tubules, couplings and myofibrillar arrangements in rat atrial myocardium. Acta Anat. 1980;108:374–388. doi: 10.1159/000145320. [DOI] [PubMed] [Google Scholar]
- Lewis Carl S, Felix K, Caswell AH, Brandt NR, Ball WJ, Jr, Vaghy PL, Meissner G, Ferguson DG. Immunolocalization of sarcolemmal dihydropyridine receptor and sarcoplasmic reticular triadin and ryanodine receptor in rabbit ventricle and atrium. J Cell Biol. 1995;129:673–682. doi: 10.1083/jcb.129.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp P, Hüser J, Pott L, Niggli E. Spatially non-uniform Ca2+ signals induced by the reduction of transverse tubules in citrate-loaded guinea-pig ventricular myocytes in culture. J Physiol. 1996;497:589–597. doi: 10.1113/jphysiol.1996.sp021792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löhn M, Furstenau M, Sagach V, Elger M, Schulze W, Luft FC, Haller H, Gollash M. Ignition of calcium sparks in arterial and cardiac muscle through caveolae. Circ Res. 2000;87:1034–1039. doi: 10.1161/01.res.87.11.1034. [DOI] [PubMed] [Google Scholar]
- López-López JR, Shacklock PS, Balke CW, Wier WG. Local, stochastic release of Ca2+ in voltage-clamped rat heart cells: visualization with confocal microscopy. J Physiol. 1994;480:21–29. doi: 10.1113/jphysiol.1994.sp020337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-López JR, Shacklock PS, Balke CW, Wier WG. Local calcium transients triggered by single L-type calcium channel currents in cardiac cells. Science. 1995;268:1042–1045. doi: 10.1126/science.7754383. [DOI] [PubMed] [Google Scholar]
- Lukyanenko V, Györke S. Ca2+ sparks and Ca2+ waves in saponin-permeabilized rat ventricular myocytes. J Physiol. 1999;521:575–585. doi: 10.1111/j.1469-7793.1999.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukyanenko V, Györke I, Subramanian S, Smirnov A, Wiesner TF, Györke S. Inhibition of Ca2+ sparks by ruthenium red in permeabilized ventricular myocytes. Biophys J. 2000;79:1273–1284. doi: 10.1016/S0006-3495(00)76381-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie L, Bootman MD, Berridge MJ, Lipp P. Predetermined recruitment of calcium release sites underlies excitation-contraction coupling in rat atrial myocytes. J Physiol. 2001;530:417–429. doi: 10.1111/j.1469-7793.2001.0417k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie L, Bootman MD, Laine M, Berridge MJ, Thuring J, Holmes A, Li W-H, Lipp P. The role of inositol 1,4,5-trisphosphate receptors in Ca2+ signalling and the generation of arrhythmias in rat atrial myocytes. J Physiol. 2002;541:395–409. doi: 10.1113/jphysiol.2001.013411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I, Zang W-J, Wier WG. Ca2+ sparks involving multiple Ca2+ release sites along Z-lines in rat heart cells. J Physiol. 1996;497:31–38. doi: 10.1113/jphysiol.1996.sp021747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shacklock PS, Wier WG, Balke CW. Local Ca2+ transients (Ca2+ sparks) originate at transverse tubules in rat heart cells. J Physiol. 1995;487:601–608. doi: 10.1113/jphysiol.1995.sp020903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern MD. Theory of excitation-contraction coupling in cardiac muscle. Biophys J. 1992;67:447–456. doi: 10.1016/S0006-3495(92)81615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wier WG, López-López JR, Shacklock PS, Balke CW. Calcium signalling in cardiac muscle cells. Ciba Found Symp. 1995;188:146–160. doi: 10.1002/9780470514696.ch9. [DOI] [PubMed] [Google Scholar]
- Yamasaki Y, Furuya Y, Araki K, Matsuura K, Kobayashi M, Ogata T. Ultra-high-resolution scanning electron microscopy of the sarcoplasmic reticulum of the rat atrial myocardial cells. Anat Rec. 1997;248:70–75. doi: 10.1002/(SICI)1097-0185(199705)248:1<70::AID-AR8>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]


