Abstract
Mast cells that are in close proximity to autonomic and enteric nerves release several mediators that cause neuronal hyperexcitability. This study examined whether mast cell tryptase evokes acute and long-term hyperexcitability in submucosal neurons from the guinea-pig ileum by activating proteinase-activated receptor 2 (PAR2) on these neurons. We detected the expression of PAR2 in the submucosal plexus using RT-PCR. Most submucosal neurons displayed PAR2 immunoreactivity, including those colocalizing VIP. Brief (minutes) application of selective PAR2 agonists, including trypsin, the activating peptide SL-NH2 and mast cell tryptase, evoked depolarizations of the submucosal neurons, as measured with intracellular recording techniques. The membrane potential returned to resting values following washout of agonists, but most neurons were hyperexcitable for the duration of recordings (> 30 min–hours) and exhibited an increased input resistance and amplitude of fast EPSPs. Trypsin, in the presence of soybean trypsin inhibitor, and the reverse sequence of the activating peptide (LR-NH2) had no effect on neuronal membrane potential or long-term excitability. Degranulation of mast cells in the presence of antagonists of established excitatory mast cell mediators (histamine, 5-HT, prostaglandins) also caused depolarization, and following washout of antigen, long-term excitation was observed. Mast cell degranulation resulted in the release of proteases, which desensitized neurons to other agonists of PAR2. Our results suggest that proteases from degranulated mast cells cleave PAR2 on submucosal neurons to cause acute and long-term hyperexcitability. This signalling pathway between immune cells and neurons is a previously unrecognized mechanism that could contribute to chronic alterations in visceral function.
In the digestive tract, enteric neurons control mucosal transport and motility, and extrinsic neurons permit communication between the enteric and central nervous systems (Furness et al. 1999, 2000). Persistent hyperexcitability of enteric and extrinsic neurons could cause long-lasting alterations in gastrointestinal secretion and motility (Giaroni et al. 1999; Furness et al. 2000) and induce hyperalgesia (Bueno et al. 1997), which are characteristics of functional bowel disorders such as irritable bowel syndrome (IBS). Mast cells, which play a prominent role in gut inflammation (Wershil, 1995; Wershil et al. 1998), release substances that cause profound increases in the excitability of enteric and autonomic neurons (Weinreich & Undem, 1987; Weinreich et al. 1992, 1995; Frieling et al. 1994a,b; Jiang et al. 2000) with ensuing changes in secretion, motility and visceral sensation (Weinreich & Undem, 1987; Weinreich et al. 1992, 1995; Frieling et al. 1994b; Vergnolle et al. 2001a). Moreover, studies that examined the effects of mast cell degranulation in autonomic ganglia demonstrate that this excitability persists long after the antigenic stimulus has ceased (> 30 min-hours: Weinreich & Undem, 1987; Weinreich et al. 1992, 1995; Frieling et al. 1994a,b). Histamine and inflammatory cytokines, such as PGE2 and leukotrienes, are potent mediators of acute increases in neuronal excitability (Cooke, 1992; Tamura & Wood, 1992; Dekkers et al. 1997), but their actions do not account for all excitation evoked by mast cell degranulation, including long-term excitation (Weinreich & Undem, 1987; Weinreich et al. 1992, 1995; Frieling et al. 1994a,b).
Tryptase is a major inflammatory mediator that is released from mast cells when they degranulate. It is found in almost all human mast cells, comprising up to 25 % of their total protein (Schwartz et al. 1981). Certain proteases, including tryptase, signal to cells through proteinase-activated receptors (PARs; Dery et al. 1998, 1999; Cocks & Moffatt, 2000; Vergnolle et al. 2001b). Proteases cleave within the extracellular N-terminal tails of PARs to expose tethered ligand domains that bind and activate cleaved receptors (Dery et al. 1998). Of the four cloned PARs, tryptase selectively activates PAR2 (Corvera et al. 1997; Molino et al. 1997).
PAR2 is highly expressed in the gastrointestinal tract, where it is localized to epithelial cells, myocytes and enteric neurons (Corvera et al. 1997, 1999; Kong et al. 1997). Mast cells are in close proximity to enteric and extrinsic neurons within the wall of the intestine (Stead et al. 1989; Bauer & Razin, 2000). Agonists of PAR2, including tryptase, can signal to spinal afferent neurons to cause persistent neurogenic inflammation and hyperalgesia, by unknown mechanisms (Steinhoff et al. 2000; Vergnolle et al. 2001a). Exogenous application of PAR2 agonists can acutely excite myenteric neurons (Linden et al. 2001), and although long-term changes in excitability following mast cell degranulation have not been studied systematically, both myenteric and submucosal neurons display an activity-dependent long-term hyperexcitability similar to that found in autonomic neurons (Clerc et al. 1999; Alex & Furness, 2002). The close proximity of mast cells containing tryptase to enteric neurons expressing PAR2 led us to hypothesize that tryptase cleaves PAR2 on submucosal neurons, resulting in acute and long-term excitation. Our aims were to determine whether (1) submucosal neurons express PAR2, (2) PAR2 agonists evoke acute and long-term excitation (> 30 min following stimulation) in these neurons, (3) mast cell degranulation has similar actions and (4) the effects of mast cell degranulation are mediated by mast cell proteases and PAR2.
METHODS
Animals
Adult Hartley or Simonsen guinea-pigs (150–450 g) were used. All procedures were approved by Queen's University and University of California Animal Care Committees. One group of animals was sensitized to the cow's milk protein β-lactoglobulin (β-LG; Atwood et al. 1998). Animals were anaesthetized with isofluorane or sodium pentobarbitone (200 mg kg−1i.p.) and killed by cervical transection. Submucosal preparations were dissected from the ileum (Vanner et al. 1990).
Reverse transcriptase-polymerase chain reaction
Total RNA was prepared from submucosal plexuses that were dissected from the guinea-pig ileum. Primers were chosen to amplify a 472 bp fragment of guinea-pig PAR2, as described elsewhere (Corvera et al. 1999). Controls included omission of reverse transcriptase, to exclude contamination with genomic DNA, and omission of cDNA.
Localization of immunoreactive PAR2
For sections, guinea-pigs were perfused transcardially with 4 % paraformaldehyde in 100 mm PBS, pH 7.4. The ileum was then dissected out and immersion fixed in the same fixative for 24 h, after which 10 μm frozen sections were prepared. For whole mounts, the ileum was placed in cold Ca2+- and Mg2+-free PBS containing 10−6m nicardipine. Segments were opened, placed in 4 % paraformaldehyde overnight at 4 °C, and whole mounts of the submucosal plexus were prepared. Tissues were incubated (16–64 h, 4 °C) with the following primary antibodies: anti-PAR2 (B5, raised in rabbits to the N-terminus of rat PAR2, diluted 1:250–1:800, Dr M. Hollenberg, University of Calgary, Canada; Kong et al. 1997); anti-PAR2 C17 (raised in goats to C-terminus of human PAR2, 1:100–1:400, Santa Cruz Biotechnology, CA, USA), anti-protein gene product (PGP)9.5 (raised in mice, diluted 1:100, Vector Laboratories, Burlingame, CA, USA) or anti-VIP (raised in mice, diluted 1:800, Dr John Walsh, UCLA, USA). Tissues were washed and incubated with secondary IgG conjugated to fluorescein isothiocyanate or Texas Red (1:200, 2 h, room temperature, Jackson ImmunoResearch, West Grove, PA, USA). In control sections, PAR2 antibodies were incubated with 10 μm of peptides used for immunization for 24–48 h before staining. Tissues were imaged as described previously (Grady et al. 1996).
Tryptase assay
Submucosal preparations from milk-sensitized guinea-pigs were placed in 3.0 ml physiological saline (mm): 126 NaCl, 2.5 NaH2PO4, 1.2 MgCl2, 2.5 CaCl2, 5 KCl, 25 NaHCO3, 11 glucose, 35–36 °C, 95 %O2-5 %CO2. Preparations were incubated with or without 10 μmβ-LG for 3 min and aliquots (1 ml) were frozen with 20 μg ml−1 heparin. Tryptase-like activity was assayed by the hydrolysis of tosyl-glycine-proline-arginine-p-nitroanilide in 0.1 m Tris/HCl pH 8.0, 1 m glycerol (McEuen, 1996). Samples (20 μl) were incubated in buffer (200 μl) containing 0.5 mm substrate with or without protease inhibitors for 17 h at room temperature. Absorbance was measured at 405 nm. Results are expressed as picomoles of p-nitroanilide generated per minute.
Electrophysiology
Submucosal preparations were pinned in organ baths (0.5–1.0 ml) and superfused with physiological saline (95 %O2-5 %CO2, 35–36 °C). Drugs were added to the bath by superfusion, except tryptase and 1,1-dimethyl-4-phenyl-piperazinium (DMPP), which were administered by pressure-pulse (‘puff’) application. Intracellular recordings from submucosal neurons were obtained using glass microelectrodes filled with 2 m KCl (70–110 MΩ tip resistance). Changes in membrane potential were recorded with an Axoclamp 2A amplifier and displayed on a Gould TA240 chart recorder (Axon Instruments). Fibre tracts were stimulated (single pulses and 20 Hz, 500 ms) with bipolar platinum electrodes placed on adjacent ganglia or fibre tracts.
Changes in excitability were assessed in each neuron by first establishing the rheobase (depolarizing pulses, 500 ms, eliciting a single action potential), the input resistance (hyperpolarizing pulses, 500 ms) and the amplitude of the fast EPSP elicited by a single pulse at time 0, prior to application of agonist or mast cell degranulation. During superfusion of agonists or mast-cell-degranulating agent, the amplitude of depolarization was measured at ∼1 min. Using the parameters applied at time 0, action potential discharge (number of action potentials elicited), amplitude of the fast EPSP and input resistance were also measured. Following washout of these agents and return to the resting membrane potential, measures of excitability were obtained at later time points (30 min, 1–5 h).
Drugs
SLIGRL-NH2 (SL-NH2; tethered ligand of rat PAR2), LRGILS-NH2 (LR-NH2; reverse of PAR2 tethered ligand) and human lung tryptase were prepared as described elsewhere (Steinhoff et al. 2000). β-Lactoglobulin (β-LG), cimetidine, di-isopropyl fluorophosphates (DIPF), trans-epoxysuccinyl-l-leucylamido-(4-guanidino)butane (E-64), EDTA, DMPP, indomethicin, leupeptin, nicardipine, pyrilimine, soybean trypsin inhibitor, tropisetron and tetrodotoxin were all obtained from Sigma-Aldrich. Trypsin was obtained from Boehringer Mannheim.
Statistics
Data are presented as means ±s.e.m., and were compared by Fisher's exact or two-tailed t test; P < 0.05 was considered significant.
RESULTS
Molecular and immunohistochemical localization of PAR2 on submucosal neurons
PAR2 mRNA
We examined the expression of PAR2 in the submucosal plexus of the guinea-pig ileum using RT-PCR (n= 4 animals). A PCR product of the predicted size of 472 bp was amplified from RNA prepared from the submucosal plexus (Fig. 1). No PCR product was amplified when reverse transcriptase or cDNA were omitted. These results indicate that PAR2 is expressed in the submucosal plexus. The submucosal plexus was dissected from myocytes and enterocytes, as assessed by microscopy. However, the preparations contained neurons, glial cells, connective tissue cells and perhaps contaminating myocytes and enterocytes. Thus, the source of the amplified mRNA could not be determined unequivocally.
Figure 1. Detection of proteinase-activated receptor 2 (PAR2) mRNA in the ileal submucosal plexuses by RT-PCR.
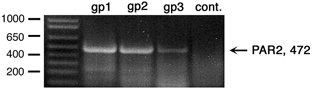
Lanes 1–3 correspond to three guinea-pigs (gp) and lane 4 is a control (no RNA).
PAR2 and VIP immunohistochemistry
To identify PAR2-expressing cells we used immunofluorescence with two different antibodies (n= 10 animals; multiple tissue sections from each animal). PAR2 was detected in enterocytes of the villi and crypts (Fig. 2Ai, ii) and in the muscularis externa (Fig. 2Ai, iv), as described previously (Corvera et al. 1997; Kong et al. 1997). Immunoreactive PAR2 was also detected prominently in the submucosal ganglia (Fig. 2Aii, iv, v, vii) and myenteric ganglia (Fig. 2Aiii). Inspection of whole mounts of submucosal ganglia revealed PAR2 staining of most cells (Fig. 2Biv), with prominent intracellular stores of variable intensity (Fig. 2Av). In the ganglia, PAR2 was detected in cells that were also stained for PGP9.5, and are thus neurons (not shown). Previous studies have shown that ∼50 % of submucosal neurons in a ganglion are non-cholinergic secretomotor neurons that express VIP (Bornstein & Furness, 1988). In the present study, VIP was found in submucosal neurons and PAR2 immunoreactivity was present in neurons that expressed VIP (Fig. 2Bi–iii), but the numbers were not quantitated. The possibility that calbindin-positive neurons were also immunostained with PAR2 was not examined, but electrophysiological studies in the myenteric plexus demonstrate that myenteric AH neurons respond to PAR2 agonists (Linden et al. 2001), suggesting that PARs are also found on these neurons. Staining of submucosal neurons was similar with both PAR2 antibodies (Fig. 2Av, vii). Preabsorption of PAR2 antibodies with peptides used for immunization abolished staining (Fig. 2Avi, viii). Thus, PAR2 is expressed by neurons of the submucosal plexus. Some of these neurons also express VIP and are secretomotor neurons.
Figure 2. Localization of immunoreactive PAR2.
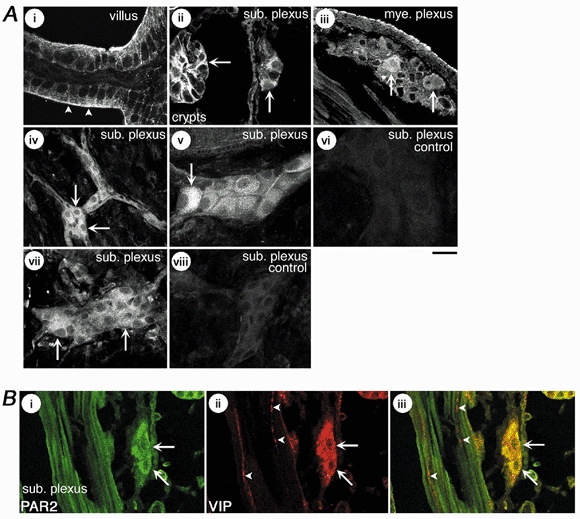
A, localization of PAR2 in sections (i-iii) and whole mounts (iv-viii) of guinea-pig ileum. Panels i-vi were stained with PAR2 antibody B5 and vii-viii with PAR2 antibody C17. Note PAR2 immunoreactivity at the apical membrane (i, arrow heads) and basolateral membranes (ii, arrow) of enterocytes of the villi and crypts, in the submucosal plexus (sub. plexus, ii, iv, v, vii, arrows) and myenteric plexus (mye. plexus, iii, arrows). Panels vi and vii are whole mounts of submucosal plexus incubated with preabsorbed PAR2 antibodies. Scale bar = 20 μm for i-iv, 30 μm for v-viii. B, localization of PAR2 (B5 antibody, i, iv) and VIP (ii, v) in sections of guinea-pig ileum. Note immunoreactive PAR2 in neurons of the submucosal plexus that were stained with the VIP antibody (arrows). VIP-positive fibres in the musculature were not stained for PAR2 (arrow heads). Scale bar = 25 μm.
Electrical and synaptic properties of PAR2 effects on submucosal neurons
General properties of submucosal neurons
The mean resting membrane potential obtained with intracellular recordings from neurons in the submucosal ganglia was −53.9 ± 0.5 mV (n= 118 neurons). Previous studies have demonstrated that S- and AH-type submucosal neurons are organized in a unique topographical arrangement within the ganglia and this has important implications concerning selection bias during intracellular recordings. The neurons are predominantly comprised of two populations of S-type secretomotor neurons, a cholinergic (neuropeptide Y (NPY)-immunoreative) and a non-cholinergic (VIP-immunoreactive) group, as well as small numbers of vasodilator neurons, interneurons and AH sensory neurons (∼10 %). The VIP secretomotor neurons, which comprise ∼50 % of the neurons in the ganglia, occupy the central region of the ganglia. Given their location, these neurons are most likely to be impaled when intracellular recording techniques are employed (Reed & Vanner, 2001). These neurons can also be differentiated from NPY neurons electrophysiologcially because VIP-immunoreactive neurons receive IPSPs when fibre tracts are stimulated, whereas few if any NPY neurons do (Bornstein & Furness, 1988). In the current study, fibre tract stimulation (20 Hz, 500 ms) evoked inhibitory postsynaptic potentials in most cells (86 %, n= 102), as well as slow EPSPs (82 %, n= 97). All cells also exhibited fast EPSPs. Thus, given the selection bias of intracellular recording techniques in these ganglia and the preponderance of neurons receiving IPSPs in this study, all of the neurons examined were S-type and most were probably secretomotor neurons containing VIP (Bornstein & Furness, 1988).
Activation of PAR2 on these neurons was studied by examining the effects during application of PAR2 agonists or mast cell degranulation (minutes) followed by studies of neuronal excitability, 30 min to hours after washout of the agonist or degranulating agent (see Methods). Excitability was assessed by examining the number of action potentials discharged during intracellular depolarization, input resistance and fast synaptic transmission. All recordings were examined for 15 min prior to initial measurements to ensure stable impalements.
Trypsin and SL-NH2 depolarize submucosal neurons through PAR2
PAR2 agonists were added by superfusion, and depolarizations, input resistance and action potential discharge were measured after ∼1 min. Stimulus parameters established at time 0 (see Methods) were repeated to determine the number of action potentials discharged and the input resistance. Superfusion of 10 nm trypsin or 50 μm SL-NH2 for 3 min evoked a slow depolarization (5.4 ± 0.7 mV, range 2–8 mV and 7.4 ± 0.7 mV, range 6–10 mV, respectively; Fig. 3A and D). The mean input resistance increased (34.1 ± 20.1 and 11.7 ± 4.7 %, respectively) and the mean number of action potentials discharged following intracellular stimulation (3.0 ± 0.7 and 3.8 ± 0.9, respectively) increased compared to time 0. The peak depolarization to trypsin at ∼1 min was concentration dependent (EC50= 8 nm, Fig. 3E), consistent with PAR2 activation. The response to 10 nm trypsin was unaffected by 1 μm tetrodotoxin (trypsin: 5.4 ± 0.7 mV, n= 8; in separate neurons, trypsin and tetrodotoxin: 4.3 ± 0.5 mV, n= 3, P > 0.05). This result suggests that trypsin has a direct action on the neuron from which the intracellular recording was made.
Figure 3. Trypsin and the tethered ligand of rat PAR2 (SL-NH2) depolarize submucosal neurons through PAR2.
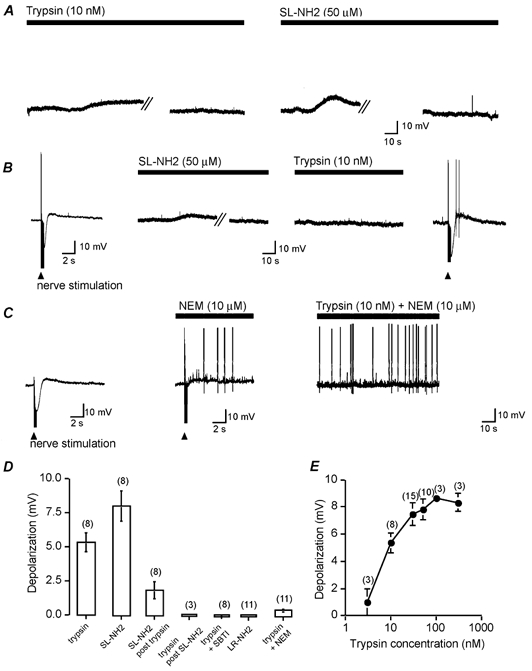
A, representative depolarizations to superfusion of trypsin (10 nm) or SL-NH2 (50 μm, solid bars), which desensitized during the 3 min application. The break lines refer to a break in the trace during which time the cell returned to the resting membrane potential, as indicated by the trace after the break lines. Resting membrane potentials were −61 mV for the neuron superfused with trypsin and −51 mV for the neuron superfused with SL-NH2. B, representative recording showing depolarization to SL-NH2 (50 μm). Superfusion for 3 min desensitized the response to trypsin (10 nm). The resting membrane potential was −52 mV. C, N-ethylmaleimide (NEM, 10 μm) suppressed the inhibitory and slow excitatory synaptic potentials evoked by electrical fibre-tract stimulation (triangle). When trypsin (10 nm) was applied with NEM it did not depolarize the membrane. The action of NEM was not reversible. The resting membrane potential was −58 mV. D, summary of mean depolarizations evoked by PAR2 agonists alone, SL-NH2 following trypsin desensitization, trypsin following SL-NH2 desensitization, trypsin incubated with soybean trypsin inhibitor (SBTI), the reverse sequence of the activating peptide (LR-NH2) and trypsin with NEM. E, concentration–response analysis of the magnitude of depolarization to graded concentrations of trypsin. n= number of neurons.
As there are no available PAR2 antagonists, we used several strategies to establish that these actions resulted from activation of PAR2. LR-NH2 (50 μm), which does not activate PAR2, did not depolarize the neurons (Fig. 3D) and had no appreciable effect on input resistance (mean increase = 3.7 ± 3.4 %) or the number of action potentials discharged (mean = 1.1 ± 0.4). Trypsin (10 nm), pretreated with soybean trypsin inhibitor (10 mg ml−1, 30 min), also failed to affect membrane potential (Fig. 3D), input resistance (mean increase = 4.8 ± 7.7 %) or the number of action potentials discharged (mean = 1.3 ± 0.2). In desensitization experiments, we examined whether both agonists activate the same receptor. Selective activation of PAR2 with SL-NH2 (50 μm) desensitized responses to trypsin (10 nm; Fig. 3B). Immediately following PAR2 desensitization, slow EPSPs and IPSPs could still be elicited, demonstrating that other G-protein-coupled responses were still active (Mihara, 1993; Surprenant, 1994; Bertrand et al. 2000). Similarly, trypsin (50 nm) almost completely blocked the depolarizations evoked by SL-NH2 (50 μm; Fig. 3D). Finally, N-ethylmaleimide (NEM, 10 μm), which blocks G-protein-coupled responses (Shapiro et al. 1994), abolished the trypsin (10 nm)-induced depolarization (Fig. 3C and D). Together, these results are consistent with trypsin and SL-NH2 inducing depolarization of submucosal neurons by activating PAR2.
PAR2 agonists cause long-term excitation of submucosal neurons
We examined whether PAR2 agonists induce long-term excitation (> 30 min following washout of the agonist) by comparing action potential discharge, input resistance and magnitude of the fast EPSP in neurons at time 0 and at 0.5 h intervals after superfusion with 10 nm trypsin or 50 μm SL-NH2 for 3 min. Following PAR2 activation by both agonists, the number of action potentials discharged was increased approximately two- to threefold compared to control cells at 30 min and 1 h (Figs 4A and B and 5A). Remarkably, these findings persisted for the duration of impalements (up to 5 h). This increase in action potential discharge was associated with a > twofold increase in input resistance (Fig. 4E and Fig. 5B) and an increase in amplitude of the fast EPSP- (1/5) or fast EPSP-evoked action potential discharge (4/5) in all cells (Figs 4C and F and 5C). To establish that the increased amplitude results from changes in the postsynaptic membrane, depolarizations evoked by pressure-pulse application of the nicotinic agonist DMPP (100 μm, 5–10 ms) directly onto the ganglia were compared before and after the application of trypsin (30 nm, n= 3). The mean amplitude of the DMPP-evoked depolarization was increased by 51 % (range = 11–72 %) following trypsin application (10–30 min; Fig. 5D). In each cell, input resistance (mean = 47 %) and the fast EPSP (increased amplitude or action potential discharge, n= 1/3 and 2/3, respectively) were similarly increased (Fig. 5). Increased input resistance typically reflects closure of K+ channels in these neurons (Mihara, 1993; Surprenant, 1994) and accounts, at least in part, for the increased amplitude of the fast EPSP. We controlled for alterations in excitability of neurons with time by examining a series of neurons over 45 min that had not received PAR2 agonists. The excitability of these cells did not change significantly during this period (Fig. 4D, E and F). In addition, 10 nm trypsin pretreated with soybean trypsin inhibitor and 50 μm LR-NH2 failed to induce long-term excitation (Fig. 4D, E). Thus, transient activation of PAR2 causes persistent excitation of submucosal neurons.
Figure 4. PAR2 agonists evoke long-term excitation.
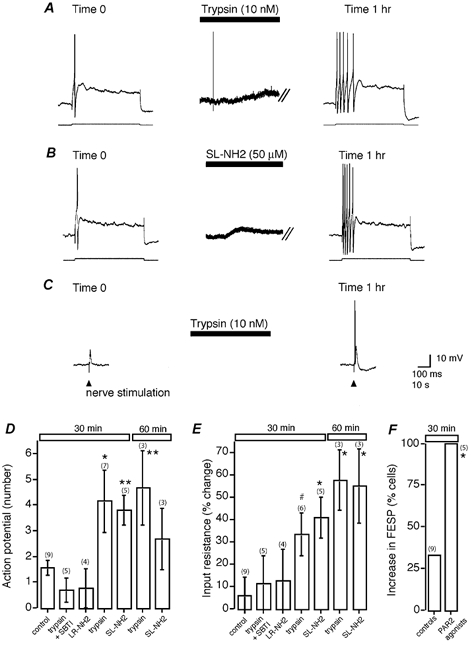
A, representative trace showing threshold current injection (lower trace) to elicit a single action potential (upper trace) at time 0. Superfusion of trypsin (10 nm) for 3 min evoked typical depolarization. One hour following the washout of trypsin, the intracellular current injection elicited five action potentials. The break lines refer to a brake in the trace during which time the cell returned to the resting membrane potential of −51 mV. B, representative trace showing that SL-NH2 (50 μm) had a similar action to trypsin, as shown in A. The resting membrane potential was −50 mV. C, one hour following superfusion of trypsin, as in (A), single pulse fibre-tract stimulation (triangle) evoked a fast EPSP that now triggered an action potential. D-F, summary of the mean number of action potentials (D), the percent change in input resistance (E) and the increase in fast EPSPs (increased amplitude of action potential discharge (F) under control conditions and following stimulation with PAR2 agonists. *P < 0.05, **P < 0.01, #P= 0.055.
Figure 5. PAR2-evoked changes in fast EPSPs correspond to alterations in the postsynaptic neuronal membrane.
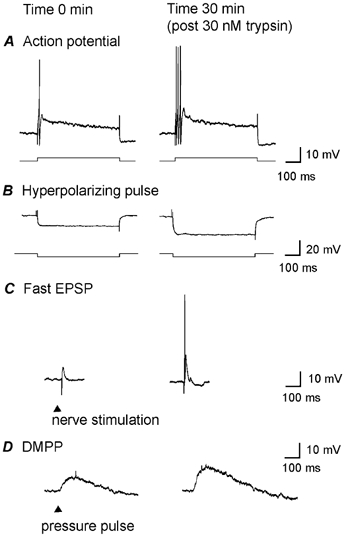
Control responses (A–D, left panels) were obtained from one neuron at time 0 and compared to responses from the same neuron 30 min after superfusion of trypsin (30 nm, 3 min; A–D, right panels). Exposure to trypsin depolarized the membrane potential by 7 mV; the membrane returned to resting potential after washout (not shown). A, threshold current injection (lower trace) elicited a single action potential (upper trace) at time 0 (left). Thirty minutes after trypsin, the same intracellular current injection elicited three action potentials (right). B, input resistance was measured from the electrotonic hyperpolarization (lower trace) evoked by a hyperpolarizing current injection (upper trace) at time 0 (right). Thirty minutes after trypsin, the same hyperpolarizing current evoked a much greater hyperpolarization, reflecting increased input resistance. C, the amplitude of the fast EPSP elicited by fibre-tract stimulation also increased after trypsin exposure and now triggered an action potential. D, the nicotinic agonist 1,1-dimethyl-4-phenyl-piperazinium (DMPP) was applied by pressure-pulse application (5–10 ms, 100 μm) at time 0 and at 30 min after trypsin to determine whether changes in the fast ‘nicotinic’ EPSPs were due to pre- or postsynaptic effects. The amplitude of the depolarization was markedly increased following application of trypsin, demonstrating a postsynaptic action. The resting membrane potential was −55 mV. Representative tracing of three experiments.
Mast cell tryptase and degranulation excite submucosal neurons via PAR2
To test the hypothesis that tryptase is the mast-cell mediator activating PAR2 in submucosal neurons, we first examined the actions of human tryptase, employing human purified tryptase (Steinhoff et al. 2000) because previous studies have shown that commercially available tryptase does not activate PAR2. We applied tryptase directly onto the soma by pressure-pulse application (3.5 μm tryptase, 50–200 ms pulse; there was insufficient quantity to allow tryptase to be applied by superfusion). In common with other PAR2 agonists, tryptase evoked depolarizations (Fig. 6A) in all neurons tested (10.6 ± 1.2 mV, range 6–14 mV; n= 5). Following application of tryptase, cells exhibited increased action potential discharge (3.7 ± 1.4), input resistance (3/4 cells) and amplitude of fast EPSPs (2/3 cells). The impalement was held in one neuron for > 30 min, and that particular cell displayed long-term excitation (Fig. 6A).
Figure 6. Mast cell tryptase and degranulation activate PAR2 on submucosal neurons.
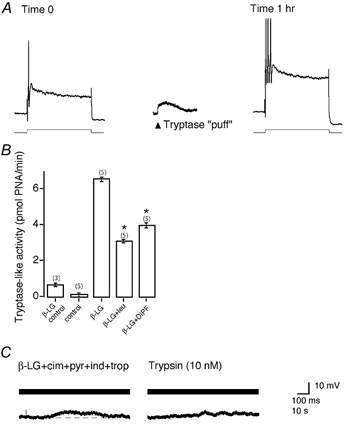
A, pressure-pulse application (‘puff’) of tryptase evoked a slow depolarization. Intracellular current injection (lower trace) sufficient to elicit a single action potential (upper trace) prior to tryptase stimulation, 1 h later evoked multiple action potentials. Resting membrane potential was −62 mV. B, tryptase-like activity was barely detectable in aliquots from unchallenged milk-sensitized tissue, but levels were significantly elevated in aliquots from β-lactoglobulin (β-LG)-treated preparations. Activity was significantly inhibited by leupeptin (leu, 1 mm) and di-isopropyl fluorophosphates (DIPF, 1 mm; P < 0.05). n= number of animals. Tryptase activity is expressed as picomoles of p-nitroanilide generated per minute (PNA min−1). C, representative trace showing β-LG-evoked depolarization desensitized responses to trypsin. The resting membrane potential was −56 mV.
Since tryptase is a prominent enzyme in guinea-pig mast cells (McEuen et al. 1996), we determined whether mast cell degranulation releases tryptase-like activity in the effluent from submucosal preparations (Fig. 6B). This was examined using tissue from guinea-pigs that had been sensitized to milk (Atwood et al. 1998), enabling selective degranulation of mast cells with the milk antigen β-LG (10 μm). Tryptase-like activity was minimally detectable in effluent from sensitized preparations that had not been challenged with milk antigen. Exposure to β-LG resulted in a > 100-fold increase in tryptase-like activity over control levels. This activity was significantly inhibited with the serine protease inhibitors leupeptin (1 mm) and DIPF (1 mm), both of which are known to inhibit guinea-pig tryptase (McEuen et al. 1996). Soybean trypsin inhibitor (50 μg ml−1), EDTA (1 mm) and E-64 (10 μm) failed to inhibit activity, thereby excluding the involvement of trypsin, metalloproteases, cysteine proteases and aspartyl proteases, respectively.
There are no known antagonists of PAR2. Although mice lacking PAR2 are available, technical limitations preclude intracellular recording from murine submucosal neurons. Thus, to determine whether the release of tryptase from mast cells activates PAR2, we used a desensitization strategy. We obtained intracellular recordings from submucosal neurons in milk-sensitized preparations in the presence of antagonists of established mediators from mast cells (histamine H1 receptors, pyrilamine, 1 μm; H2 receptors, cimetidine, 100 μm; 5-HT3 receptors, tropisetron, 1 μm; cyclooxygenase, indomethacin, 10 μm). β-LG induced depolarization (Fig. 6C), but subsequent challenge of neurons with the PAR2 agonist trypsin (10 nm) failed to elicit significant depolarizations (trypsin control mean = 5.4 ± 0.8 mV, n= 8versus trypsin-post β-LG mean = 1.4 ± 0.5 mV, n= 7; P= 0.001), indicating desensitization. Thus, mast cells release mediators that desensitize PAR2 on submucosal neurons.
β-LG-induced degranulation and long-term excitation
To determine whether mast cells release substances that cause sustained hyperexcitability of submucosal neurons, we obtained intracellular recordings from submucosal neurons in milk-sensitized preparations. β-LG (10 μm) induced slow depolarizations (mean = 6.2 ± 0.8 mV, range 2–10 mV) and increased neuronal input resistance (11/15 cells; mean = 20.3 ± 6.8). The amplitude of the fast EPSP was acutely (minutes) suppressed (mean = 42 ± 30.1 %) or unchanged in 3 out of 11 cells, as described previously (Frieling et al. 1994a), while 8 out of 11 cells had an increase in fast synaptic transmission (increased amplitude or action potential evoked by fast EPSP, 3/11 and 5/11, respectively). After 30 min and 1 h of washout of β-LG, long-term excitation was evident (Fig. 7A–D), as had been observed with PAR2 agonists (Fig. 4). Thus, mast cells release substances that depolarize and cause long-lasting excitation of submucosal neurons, which resembles responses to PAR2 agonists, including tryptase.
Figure 7. Mast cell degranulation evokes long-term excitation.
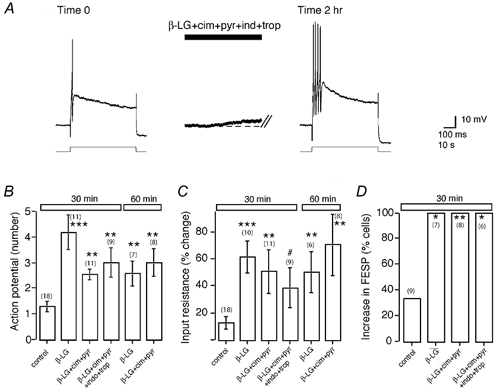
A, superfusion of β-LG (10 μm), which degranulates mast cells in milk-sensitized tissue, evoked a slow depolarization in the presence of cimetidine (cim; 100 μm), pyrilamine (pyr; 1 μm), tropisetron (trop; 1 μm) and indomethacin (ind; 10 μm). The intracellular current injection elicited multiple action potentials 2 h after washout of the β-LG, as seen with tryptase (Fig. 6A) and other PAR2 agonists (Fig. 4). The resting membrane potential was −61 mV. B–D, summary of mean action potential discharge (B), input resistance (C) and magnitude of the fast EPSP (increased amplitude or action potential discharge; D), for controls and following stimulation with β-LG alone or with antagonists. ***P < 0.001, **P < 0.01,*P < 0.05, #P= 0.053.
We studied the effects of β-LG on depolarization and long-term excitation in the presence of H1, H2, 5-HT3 antagonists and a cyclooxygenase inhibitor. β-LG still evoked slow depolarizations (Fig. 7A) in all cells (5.0 ± 0.8 mV, 3–6 mV), with an associated increase in input resistance (8/10 cells; mean = 20.3 ± 6.8 %). In four of the seven cells where fast EPSPs could be elicited at time 0 and not evoke action potential discharge, fast EPSPs evoked action potential discharge. Long-term excitation was evident at 30 min (Fig. 7). Thus, histamine, 5-HT and prostaglandins cannot fully account for the prolonged excitatory response to mast cell degranulation.
DISCUSSION
We found that submucosal secretomotor neurons, which mediate neurogenic secretion in the intestine, express PAR2, and that PAR2 agonists, including tryptase, evoke acute and long-term neuronal excitation. Moreover, nicotinic fast synaptic transmission, mediated by interneurons in the submucosal and myenteric plexus projecting to the secretomotor neurons (Bornstein & Furness, 1988; Moore & Vanner, 2000), was also enhanced during long-term excitation. As a result, fast EPSPs that were initially subthreshold were often sufficient to evoke action potential discharge in these motoneurons. These effects of PAR2 agonists were identical to those observed after mast cell degranulation, which resulted in release of tryptase-like activity and desensitization of PAR2. Our results suggest that proteases such as tryptase from degranulated mast cells acutely excite and induce persistent hyperexcitability of submucosal neurons. This process, by which a protease causes prolonged neuronal hyperexcitability, would greatly exacerbate neurally evoked mucosal secretion.
PAR2 is expressed by submucosal secretomotor neurons
Trypsin and tryptase cleave PAR2 at the Arg34-Ser35 bond to expose the tethered ligand domain SLIGRL, which binds and activates the cleaved receptor (Nystedt et al. 1994; Molino et al. 1997). Several observations suggest that proteases signal to submucosal neurons by activating PAR2 in this manner. Firstly, we detected immunoreactive PAR2 on the soma of submucosal neurons and confirmed the expression of PAR2 mRNA in the submucosal plexus. Secondly, trypsin and tryptase caused short- and long-term excitation of submucosal neurons. This excitation required the enzymatic activity of trypsin, since it was abolished by soybean trypsin inhibitor. Thirdly, the actions of trypsin and tryptase were mimicked by SL-NH2, which corresponds to the tethered ligand domain of PAR2 that would be revealed by tryptic cleavage. SL-NH2 is a selective agonist of PAR2 (Nystedt et al. 1994), and the reverse sequence was inactive. Finally, NEM inhibited the effect of trypsin, consistent with activation of a G-protein-coupled receptor such as PAR2 (Shapiro et al. 1994; Dery et al. 1998). Together, our data strongly imply that proteases can signal to submucosal neurons by cleaving and triggering PAR2. In support of our results, PAR2 has been detected in myenteric neurons (Corvera et al. 1999), and trypsin and SL-NH2 can regulate intestinal secretion by neurogenic mechanisms (Green et al. 2000).
PAR2 agonists evoke acute and long-term excitation of submucosal neurons
PAR2 agonists evoked a slow depolarization of all of the submucosal neurons examined, an effect that was associated with enhanced excitability. These responses were not as large as with other mast cell mediators, such as PGE2 (Dekkers et al. 1997) and histamine (Tamura & Wood, 1992). However, the effects are functionally important because PAR2 agonists evoke a large increase in chloride secretion in the porcine intestine by neurogenic and prostaglandin-dependent mechanisms (Green et al. 2000). The contribution of cytokines to the neural response is unclear, as some of the prostaglandin effect could result from stimulation of the submucosal secretomotor neurons. The present study provides direct evidence that PAR2 agonists depolarize submucosal neurons. In contrast, a neural component has not been observed in the rat, possibly due to activation of a different receptor (Vergnolle et al. 1998).
A major finding of the current investigation is that transient exposure of submucosal neurons to PAR2 agonists and mast-cell mediators evokes a long-term hyperexcitability of these neurons. In support of our findings, others have shown that mast cell degranulation in sympathetic ganglia (Weinreich & Undem, 1987; Weinreich et al. 1992, 1995) not only evokes an acute excitation of neurons, but also causes a persistent excitation of many neurons. While long-term excitation has not been studied systematically in previous studies of enteric neurons, these neurons do demonstrate a similar activity-dependent long-term hyperexcitability to that found in sympathetic neurons (Weinreich et al. 1995; Alex & Furness, 2002). The persistent hyperexcitability in sympathetic neurons that follows mast cell degranulation is not blocked by antagonists of non-protein inflammatory mediators (Weinreich & Undem, 1987; Weinreich et al. 1995), and cannot be mimicked by histamine in enteric neurons (Tamura & Wood, 1992). These findings suggest that other agents, such as tryptase, mediate long-term excitation by cleaving neuronal PAR2. Consistent with this suggestion, we found that brief (minutes) application of PAR2 agonists caused prolonged neuronal excitation, as evidenced by increased action potential discharge and enhanced nicotinic synaptic neurotransmission, which persisted for at least 5 h, the longest time that a neuron could be studied. In support of the suggestion that PAR2 agonists cause long-term neuronal excitability are recent reports that PAR2 agonists signal to primary spinal afferent neurons to induce a long-lasting neurogenic inflammation (> 6 h) and hyperalgesia (> 24 h; Steinhoff et al. 2000; Vergnolle et al. 2001a).
The molecular mechanism that underlies the long-term neuronal excitation in response to PAR2 agonists is unknown. The irreversible nature of PAR activation by proteolysis means that the tethered ligand is always available to bind a cleaved receptor, which presents unique problems for terminating the signal (Dery et al. 1998, 1999). However, PAR2 signalling is rapidly attenuated by receptor phosphorylation and association with β-arrestins, so the mechanism of irreversible activation is unlikely to account for the prolonged excitation of neurons. An alternative mechanism could be regulation of channel activity by PAR2. Such mechanisms have been proposed to explain how bradykinin, another inflammatory mediator, sensitizes spinal afferent neurons by phosphorylating the VR1 channel (Premkumar & Ahern, 2000). In addition, the sustained hyperexcitability induced in myenteric (Clerc et al. 1999) and submucosal (Alex & Furness, 2002) neurons by repetitive stimulation of slow EPSPs has been suggested to result from phosphorylation of an ion channel or a regulatory protein (Clerc et al. 1999; Furness et al. 2000). Further studies are needed to determine whether activation of PAR2 can irreversibly alter the properties of membrane channels in spinal afferent and enteric neurons.
PAR2 contributes to the prolonged excitability of submucosal neurons following mast cell degranulation
Tryptase is a prominent protease of human and guinea-pig mast cells (McEuen et al. 1996) that can activate PAR2 (Corvera et al. 1997; Molino et al. 1997). In the current study, several lines of evidence from milk-sensitized guinea-pig experiments suggest that proteases and PAR2 mediate the acute and long-term excitation of submucosal neurons that results from degranulation of mast cells. Previous studies (Frieling et al. 1994a; Atwood et al. 1998) have shown that β-LG acts selectively on sensitized mast cells in the submucosal plexus to induce degranulation. We found that degranulation of mast cells with β-LG evoked both acute and long-term excitation of the submucosal neurons, which persisted for hours. These responses were typical of those evoked by exogenous application of the PAR2 agonists trypsin, tryptase and SL-NH2. Moreover, hyperexcitability caused by β-LG was evoked in the presence of antagonists to histamine, 5-HT and prostaglandins, implicating other mast cell products, such as tryptase. In support of this possibility, β-LG caused a significant increase in tryptase-like activity in the medium, demonstrating that mast cell degranulation results in tryptase release. Importantly, mast cell degranulation desensitized responses to agonists of PAR2, suggesting that PAR2 mediates the response to mast cell degranulation when combined with the antagonists. Together, these findings provide strong evidence that mast cell degranulation induces the release of proteases (e.g. tryptase), which activates PAR2 on submucosal neurons, resulting in both acute and long-term excitation. Unequivocal proof of this hypothesis will require the development of antagonists to guinea-pig tryptase and PAR2.
Functional significance of PAR2-activated acute and long-term excitation of submucosal neurons
Mast cells play a prominent role in intestinal inflammation (Wershil, 1995; Wershil et al. 1998). They are found in close proximity to submucosal secretomotor neurons innervating mucosal enterocytes (Stead et al. 1989; Bauer & Razin, 2000). The current study demonstrates that proteases from degranulated mast cells can cleave neuronal PAR2 and induce excitability, which would be expected to stimulate mucosal secretion. Indeed, PAR2 agonists stimulate chloride secretion in the intestine by a neurogenic process (Green et al. 2000). Importantly, the excitation that we observed following mast cell degranulation persisted long after the antigenic stimulus was removed. Although such a mechanism could be protective, prolonged hyperexcitability of submucosal neurons may contribute to detrimental symptoms, like those often seen in post-infectious IBS. Recent studies of patients with IBS have demonstrated that a proportion of them display increased numbers of colonic mucosal mast cells (Chadwick et al. 2002). In preliminary studies, we have found increased spontaneous release of mast cell tryptase from colonic tissue obtained from patients with IBS (Barbara et al. 2002). Moreover, there is evidence to suggest that repeated activation of immune cells can occur, providing a mechanism to perpetuate the hyperexcitability (Collins et al. 1999). Similar hyperexcitability of myenteric neurons could contribute to motor disorders, whereas hyperexcitability of extrinsic afferent neurons might cause hyperalgesia.
Acknowledgments
This work was supported by NIH DK57840, DK43207, R.W. Johnson Focussed Giving Grant (N.W.B.) and CIHR (S.V., C.B.-L.). We thank Margaret O'Reilly, Rosa Espinosa-Luna and William Raymond for technical support, and George Caughey for providing the tryptase.
REFERENCES
- Alex G, Furness JB. Sustained post-synaptic excitation in submucosal neurons. Gastroenterology. 2002;122:A38. [Google Scholar]
- Atwood L, James C, Morris GP, Vanner S. Cellular pathways of mast cell- and capsaicin-sensitive nerve-evoked ileal submucosal arteriolar dilations. Am J Physiol. 1998;275:G1063–1072. doi: 10.1152/ajpgi.1998.275.5.G1063. [DOI] [PubMed] [Google Scholar]
- Barbara G, Cottrell G, Grady E, Cremon C, Di Giogio R, Stanghellini V, Corinaldesi R, Andrade-Gordon P, Bunnett NW. Expression and release of mast cell tryptase in irritable bowel syndrome (IBS) Gastroenterology. 2002;122:A276. [Google Scholar]
- Bauer O, Razin E. Mast cell-nerve interactions. News Physiol Sci. 2000;15:213–218. doi: 10.1152/physiologyonline.2000.15.5.213. [DOI] [PubMed] [Google Scholar]
- Bertrand PP, Thomas EA, Kunze WA, Bornstein JC. A simple mathematical model of second-messenger mediated slow excitatory postsynaptic potentials. J Comput Neurosci. 2000;8:127–142. doi: 10.1023/a:1008969115017. [DOI] [PubMed] [Google Scholar]
- Bornstein JC, Furness JB. Correlated electrophysiological and histochemical studies of submucous neurons and their contribution to understanding enteric neural circuits. J Auton Nerv Syst. 1988;25:1–13. doi: 10.1016/0165-1838(88)90002-1. [DOI] [PubMed] [Google Scholar]
- Bueno L, Fioramonti J, Delvaux M, Frexinos J. Mediators and pharmacology of visceral sensitivity: from basic to clinical investigations. Gastroenterology. 1997;112:1714–1743. doi: 10.1016/s0016-5085(97)70056-8. [DOI] [PubMed] [Google Scholar]
- Chadwick VS, Chen W, Shu D, Paulus B, Bethwaite P, Tie A, Wilson I. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122:1778–1783. doi: 10.1053/gast.2002.33579. [DOI] [PubMed] [Google Scholar]
- Clerc N, Furness JB, Kunze WA, Thomas EA, Bertrand PP. Long-term effects of synaptic activation at low frequency on excitability of myenteric AH neurons. Neuroscience. 1999;90:279–289. doi: 10.1016/s0306-4522(98)00431-x. [DOI] [PubMed] [Google Scholar]
- Cocks TM, Moffatt JD. Protease-activated receptors: sentries for inflammation? Trends Pharmacol Sci. 2000;21:103–108. doi: 10.1016/s0165-6147(99)01440-6. [DOI] [PubMed] [Google Scholar]
- Collins SM, Barbara G, Vallance B. Stress, inflammation and the irritable bowel syndrome. Can J Gastroenterol. 1999;13:47A–49A. doi: 10.1155/1999/916075. [DOI] [PubMed] [Google Scholar]
- Cooke HJ. Neuro-modulation of ion secretion by inflammatory mediators. Ann N Y Acad Sci. 1992;664:346–352. doi: 10.1111/j.1749-6632.1992.tb39773.x. [DOI] [PubMed] [Google Scholar]
- Corvera CU, Dery O, McConalogue K, Bohm SK, Khitin LM, Caughey GH, Payan DG, Bunnett NW. Mast cell tryptase regulates rat colonic myocytes through proteinase- activated receptor 2. J Clin Invest. 1997;100:1383–1393. doi: 10.1172/JCI119658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvera CU, Dery O, McConalogue K, Gamp P, Thoma M, Al Ani B, Caughey GH, Hollenberg MD, Bunnett NW. Thrombin and mast cell tryptase regulate guinea-pig myenteric neurons through proteinase-activated receptors-1 and -2. J Physiol. 1999;517:741–756. doi: 10.1111/j.1469-7793.1999.0741s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekkers JA, Akkermans LM, Kroese AB. Effects of the inflammatory mediator prostaglandin E2 on myenteric neurons in guinea-pig ileum. Am J Physiol. 1997;272:G1451–1456. doi: 10.1152/ajpgi.1997.272.6.G1451. [DOI] [PubMed] [Google Scholar]
- Dery O, Corvera CU, Steinhoff M, Bunnett NW. Proteinase-activated receptors: novel mechanisms of signaling by serine proteases. Am J Physiol. 1998;274:C1429–1452. doi: 10.1152/ajpcell.1998.274.6.C1429. [DOI] [PubMed] [Google Scholar]
- Dery O, Thoma MS, Wong H, Grady EF, Bunnett NW. Trafficking of proteinase-activated receptor-2 and beta-arrestin-1 tagged with green fluorescent protein. beta-Arrestin-dependent endocytosis of a proteinase receptor. J Biol Chem. 1999;274:18524–18535. doi: 10.1074/jbc.274.26.18524. [DOI] [PubMed] [Google Scholar]
- Frieling T, Cooke HJ, Wood JD. Neuroimmune communication in the submucous plexus of guinea-pig colon after sensitization to milk antigen. Am J Physiol. 1994a;267:G1087–1093. doi: 10.1152/ajpgi.1994.267.6.G1087. [DOI] [PubMed] [Google Scholar]
- Frieling T, Palmer JM, Cooke HJ, Wood JD. Neuroimmune communication in the submucous plexus of guinea-pig colon after infection with Trichinella spiralis. Gastroenterology. 1994b;107:1602–1609. doi: 10.1016/0016-5085(94)90798-6. [DOI] [PubMed] [Google Scholar]
- Furness JB, Clerc N, Kunze WA. Memory in the enteric nervous system. Gut. 2000;47(suppl. 4):iv60–iv62. doi: 10.1136/gut.47.suppl_4.iv60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness JB, Kunze WA, Bornstein JC, Clerc N. The enteric nervous system and its extrinsic connections. In: Yamada T, editor. Textbook of Gastroenterology. Philadelphia, PA, USA: Lippincott; 1999. pp. 11–35. [Google Scholar]
- Giaroni C, De Ponti F, Cosentino M, Lecchini S, Frigo G. Plasticity in the enteric nervous system. Gastroenterology. 1999;117:1438–1458. doi: 10.1016/s0016-5085(99)70295-7. [DOI] [PubMed] [Google Scholar]
- Grady EF, Gamp PD, Jones E, Baluk P, McDonald DM, Payan DG, Bunnett NW. Endocytosis and recycling of neurokinin 1 receptors in enteric neurons. Neuroscience. 1996;75:1239–1254. doi: 10.1016/0306-4522(96)00357-0. [DOI] [PubMed] [Google Scholar]
- Green BT, Bunnett NW, Kulkarni-Narla A, Steinhoff M, Brown DR. Intestinal type 2 proteinase-activated receptors: expression in opioid- sensitive secretomotor neural circuits that mediate epithelial ion transport. J Pharmacol Exp Ther. 2000;295:410–416. [PubMed] [Google Scholar]
- Jiang W, Kreis ME, Eastwood C, Kirkup AJ, Humphrey PP, Grundy D. 5-HT(3) and histamine H(1) receptors mediate afferent nerve sensitivity to intestinal anaphylaxis in rats. Gastroenterology. 2000;119:1267–1275. doi: 10.1053/gast.2000.19461. [DOI] [PubMed] [Google Scholar]
- Kong W, McConalogue K, Khitin LM, Hollenberg MD, Payan DG, Bohm SK, Bunnett NW. Luminal trypsin may regulate enterocytes through proteinase-activated receptor 2. Proc Natl Acad Sci U S A. 1997;94:8884–8889. doi: 10.1073/pnas.94.16.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DR, Manning BP, Bunnett NW, Mawe GM. Agonists of proteinase-activated receptor 2 excite guinea-pig ileal myenteric neurons. Eur J Pharmacol. 2001;431:311–314. doi: 10.1016/s0014-2999(01)01447-9. [DOI] [PubMed] [Google Scholar]
- McEuen AR, He S, Brander ML, Walls AF. guinea-pig lung tryptase. Localisation to mast cells and characterisation of the partially purified enzyme. Biochem Pharmacol. 1996;52:331–340. doi: 10.1016/0006-2952(96)00211-0. [DOI] [PubMed] [Google Scholar]
- Mihara S. Intracellular recordings from neurones of the submucous plexus. Prog Neurobiol. 1993;40:529–572. doi: 10.1016/0301-0082(93)90034-p. [DOI] [PubMed] [Google Scholar]
- Molino M, Barnathan ES, Numerof R, Clark J, Dreyer M, Cumashi A, Hoxie JA, Schechter N, Woolkalis M, Brass LF. Interactions of mast cell tryptase with thrombin receptors and PAR-2. J Biol Chem. 1997;272:4043–4049. doi: 10.1074/jbc.272.7.4043. [DOI] [PubMed] [Google Scholar]
- Moore BA, Vanner S. Properties of synaptic inputs from myenteric neurons innervating submucosal S neurons in guinea-pig ileum. Am J Physiol Gastrointest Liver Physiol. 2000;278:G273–280. doi: 10.1152/ajpgi.2000.278.2.G273. [DOI] [PubMed] [Google Scholar]
- Nystedt S, Emilsson K, Wahlestedt C, Sundelin J. Molecular cloning of a potential proteinase activated receptor. Proc Natl Acad Sci U S A. 1994;91:9208–9212. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premkumar LS, Ahern GP. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985–990. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- Reed DE, Vanner SJ. Converging and diverging cholinergic inputs from submucosal neurons amplify activity of secretomotor neurons in guinea-pig ileal submucosa. Neuroscience. 2001;107:685–696. doi: 10.1016/s0306-4522(01)00392-x. [DOI] [PubMed] [Google Scholar]
- Schwartz LB, Lewis RA, Austen KF. Tryptase from human pulmonary mast cells. Purification and characterization. J Biol Chem. 1981;256:11939–11943. [PubMed] [Google Scholar]
- Shapiro MS, Wollmuth LP, Hille B. Modulation of Ca2+ channels by PTX-sensitive G-proteins is blocked by N-ethylmaleimide in rat sympathetic neurons. J Neurosci. 1994;14:7109–7116. doi: 10.1523/JNEUROSCI.14-11-07109.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead RH, Dixon MF, Bramwell NH, Riddell RH, Bienenstock J. Mast cells are closely apposed to nerves in the human gastrointestinal mucosa. Gastroenterology. 1989;97:575–585. doi: 10.1016/0016-5085(89)90627-6. [DOI] [PubMed] [Google Scholar]
- Steinhoff M, Vergnolle N, Young SH, Tognetto M, Amadesi S, Ennes HS, Trevisani M, Hollenberg MD, Wallace JL, Caughey GH, Mitchell SE, Williams LM, Geppetti P, Mayer EA, Bunnett NW. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat Med. 2000;6:151–158. doi: 10.1038/72247. [DOI] [PubMed] [Google Scholar]
- Surprenant A. Control of the gastrointestinal tract by enteric neurons. Annu Rev Physiol. 1994;56:117–140. doi: 10.1146/annurev.ph.56.030194.001001. [DOI] [PubMed] [Google Scholar]
- Tamura K, Wood JD. Effects of prolonged exposure to histamine on guinea-pig intestinal neurons. Dig Dis Sci. 1992;37:1084–1088. doi: 10.1007/BF01300291. [DOI] [PubMed] [Google Scholar]
- Vanner S, Jiang MM, Brooks VL, Surprenant A. Characterization of vasopressin actions in isolated submucosal arterioles of the intestinal microcirculation. Circ Res. 1990;67:1017–1026. doi: 10.1161/01.res.67.4.1017. [DOI] [PubMed] [Google Scholar]
- Vergnolle N, Bunnett NW, Sharkey KA, Brussee V, Compton SJ, Grady EF, Cirino G, Gerard N, Basbaum AI, Andrade-Gordon P, Hollenberg MD, Wallace JL. Proteinase-activated receptor-2 and hyperalgesia: a novel pain pathway. Nat Med. 2001a;7:821–826. doi: 10.1038/89945. [DOI] [PubMed] [Google Scholar]
- Vergnolle N, MacNaughton WK, Al Ani B, Saifeddine M, Wallace JL, Hollenberg MD. Proteinase-activated receptor 2 (PAR2)-activating peptides: identification of a receptor distinct from PAR2 that regulates intestinal transport. Proc Natl Acad Sci U S A. 1998;95:7766–7771. doi: 10.1073/pnas.95.13.7766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergnolle N, Wallace JL, Bunnett NW, Hollenberg MD. Protease-activated receptors in inflammation, neuronal signaling and pain. Trends Pharmacol Sci. 2001b;22:146–152. doi: 10.1016/s0165-6147(00)01634-5. [DOI] [PubMed] [Google Scholar]
- Weinreich D, Undem BJ. Immunological regulation of synaptic transmission in isolated guinea-pig autonomic ganglia. J Clin Invest. 1987;79:1529–1532. doi: 10.1172/JCI112984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreich D, Undem BJ, Leal-Cardoso JH. Functional effects of mast cell activation in sympathetic ganglia. Ann N Y Acad Sci. 1992;664:293–308. doi: 10.1111/j.1749-6632.1992.tb39769.x. [DOI] [PubMed] [Google Scholar]
- Weinreich D, Undem BJ, Taylor G, Barry MF. Antigen-induced long-term potentiation of nicotinic synaptic transmission in the superior cervical ganglion of the guinea-pig. J Neurophysiol. 1995;73:2004–2016. doi: 10.1152/jn.1995.73.5.2004. [DOI] [PubMed] [Google Scholar]
- Wershil BK. Role of mast cells and basophils in gastrointestinal inflammation. Chem Immunol. 1995;62:187–203. [PubMed] [Google Scholar]
- Wershil BK, Castagliuolo I, Pothoulakis C. Direct evidence of mast cell involvement in Clostridium difficile toxin A-induced enteritis in mice. Gastroenterology. 1998;114:956–964. doi: 10.1016/s0016-5085(98)70315-4. [DOI] [PubMed] [Google Scholar]


