Abstract
Hyperpolarization-activated cyclic-nucleotide-gated (HCN) channels modulate the firing rates of neuronal and cardiac pacemaker cells. HCN channels resemble voltage-gated K+ channels structurally, but much less is known about their structure-function correlation. Although modulation of K+ channel gating by external K+ is a well-known phenomenon, such a link has not been established for HCN channels. Here we examined the effects of external permeant (K+, Na+ and Li+) and non-permeant (NMG+) ions on HCN1 and HCN2 gating. Substituting 64 of 96 mm external K+ with Na+, Li+ or NMG+ positively shifted steady-state activation (∼13 mV), and preferentially slowed activation of HCN1. Mutating the pore variant C-terminal to the GYG motif in HCN1, A352, to the analogous conserved Asp in K+ channels or Arg in HCN2 produced a significant hyperpolarizing activation shift (by 5–15 mV), slowed gating kinetics (up to 6-fold), and abolished or attenuated gating responses to external K+. Whereas Na+, Li+ and NMG+ substitutions produced depolarizing activation shifts of HCN2 similar to those of HCN1, deactivation but not activation of HCN2 was exclusively decelerated. We conclude that gating and permeation of HCN channels are coupled, and that modulation of this ‘pore-to-gate’ coupling by external K+ is isoform-specific.
The pacemaker current, If (or Ih), modulates cardiac and neuronal pacing by regulating the rate of cell depolarization. Despite the fact that If has been recognized for over 20 years (DiFrancesco, 1981), the encoding genes, collectively known as the hyperpolarization-activated cyclic-nucleotide-modulated (HCN) family, have only been identified relatively recently (Gauss et al. 1998; Ludwig et al. 1998; Santoro et al. 1998). To date, four HCN isoforms (1–4) have been found; they have distinct patterns of gene expression and tissue distribution (Ludwig et al. 1998; Santoro et al. 1998; Santoro & Tibbs, 1999; Santoro et al. 2000). For instance, HCN1 is most abundant in brain but it is also substantially expressed in the sino-atrial (SA) node of the heart. HCN2 is expressed in both the ventricles and atria, but only very low levels are found in the SA node. Different HCN isoforms may heteromultimerize to form the native currents (Chen et al. 2001; Ulens & Tytgat, 2001; Xue et al. 2002).
HCN channels resemble voltage-gated K+ (Kv) channels structurally (Xue et al. 2002; Henrikson et al. 2003), but much less is known about their structure-function correlation. Although HCN channels contain the glycine-tyrosine-glycine (GYG) motif found in K+-selective pores (Doyle et al. 1998) that is a prerequisite for ion conduction (Xue et al. 2002), they conduct K+, Na+ and Li+. The molecular basis of this non-selective profile is unknown. It has been speculated that variant residues flanking the GYG triplet may play a role in this difference (Santoro & Tibbs, 1999; Kaupp & Seifert, 2001; see also Supplementary material, Fig. 1). However, no direct experimental evidence is available. Further, HCN channels are activated by hyperpolarization rather than depolarization (Ludwig et al. 1998, 1999a; Santoro et al. 1998; Santoro & Tibbs, 1999; Kaupp & Seifert, 2001).
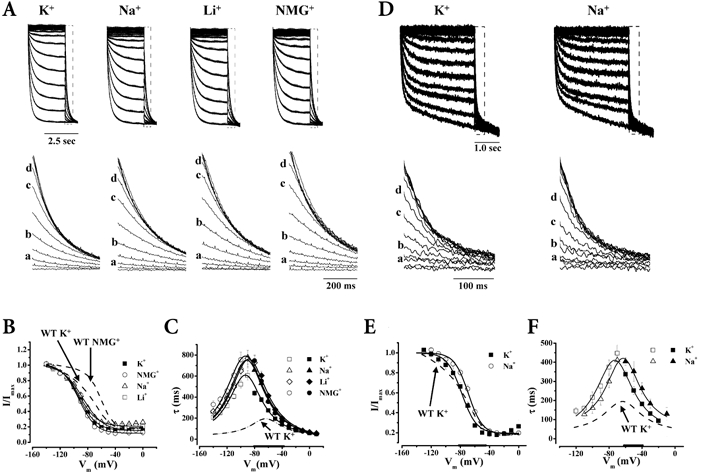
Figure 1. Effect of K+, Na+, Li+ and NMG+ on HCN1 gating.

Representative currents (A) through HCN1 channels in 96 mm K+ (n = 9), 32 mm K+-64 mm Na+ (n = 10), 32 mm K+-64 mm Li+ (n = 6) and 32 mm K+-64 mm NMG+ (n = 14) (a, −120 mV; b, −100 mV; c, −80 mV; d, −70 mV) normalized to the maximum currents recorded, and the corresponding steady-state activation curves (B). Summary of τact (open) and τdeact (filled) (n= 10 (K+), 8 (Na+), 10 (NMG+), 6 (Li+)) (C), and normalized activation and deactivation tracings (D) recorded under different ionic conditions as noted. Thickened x-axes indicate voltage ranges over which all 3 ionic conditions differ significantly from 96 mm K+.
Whereas modulation of K+ channel gating (e.g. C-type inactivation that involves the pore) by external permeant ions (K+) is a well-known phenomenon (Yellen, 1998), such a link has not been established for HCN channels. This possibility is particularly intriguing given our recent demonstration that the outermost pore rim of HCN channels participates in gating (Xue & Li, 2002). Physiologically, K+ is known to modulate the natural pacemaking process, possibly through its effects on the HCN-encoded pacemaker currents. The molecular basis of this process, however, has not been studied.
In this study, we investigated whether permeation and gating of HCN channels are coupled by examining the effects of external permeant (K+, Na+ and Li+) and non-permeant (NMG+) ions on HCN gating; the molecular basis underlying such effects was further explored by introducing site-specific mutations into the pore. Our experiments reveal that HCN gating modulation by external permeant ions is isoform-specific. These results are discussed in the context of novel insights into the molecular coupling between the pore and the gate of HCN channels, and the mechanism by which native If (thus heart rate) may be modulated physiologically.
METHODS
Molecular biology and heterologous expression
mHCN1 and mHCN2 (provided by Dr Steven Siegelbaum, Columbia University) were subcloned into the pGH expression vector (Santoro et al. 1998). Site-directed mutagenesis was performed using PCR, and confirmed by sequencing (Li et al. 1999). cRNA was transcribed from NheI- and SphI-linearized DNA using T7 RNA polymerase (Promega, Madison, WI, USA) for HCN1 and HCN2, respectively. HCN conctructs were heterologously expressed and studied in Xenopus oocytes as described previously (Xue & Li, 2002; Xue et al. 2002).
Electrophysiology, protocols and data analysis
Two-electrode voltage-clamp recordings were performed at 23–25 °C as described previously (Xue & Li, 2002; Xue et al. 2002). The recording bath solution contained (mm): 96 KCl, 2 NaCl, 2 MgCl2 and 10 Hepes (pH 7.5). K+ was replaced with equimolar Na+, Li+ or NMG+ when needed.
Whole-cell currents were evoked by 3 s pulses from a holding potential of −30 mV to test voltages ranging from −140 to 0 mV. After each test voltage, tail currents were measured 3 ms after pulsing to −140 mV. Capacity transients were removed using the P/4 leak subtraction protocol. Tail current data for each individual oocyte was plotted as a function of the preceding test voltage and fitted to the Boltzman function using the Marquardt-Levenberg algorithm in a non-linear least-squares procedure:
where Vt is the test voltage, V1/2 is the mid-point and k=RT/zF is the slope factor of steady-state activation (m∞). The maximum current from each fit was used to normalize the tail current data of each oocyte.
For tail I-V, currents were recorded 3 ms after stepping to a family of test voltages ranging from −100 to +40 mV preceded by a 3 s prepulse to either −140 or −20 mV. The difference of tail currents resulting from the two prepulse potentials was plotted against the test voltages, and fitted with linear regression to obtain the reversal potential (Erev). Assuming independent interactions of ions and that PK was constant when external [K+] was kept constant, the permeability ratio (PK/PX) for a given cation (X) was calculated from the corresponding reversal potentials using the Goldman-Hodgkin-Katz equation (Hille, 2001).
Activation (τact) and deactivation (τdeact) time constants were estimated by fitting macroscopic and tail currents with a mono-exponential function. For estimating open and close rates, the bell-shaped distribution of averaged τact and τdeact was fitted to:
where α0 and β0 reflect the open and close rates at zero voltage, respectively, Vt is the test voltage, and V0 is the voltage at which the rate constants change by e-fold. All data reported are means ±s.e.m. Statistical significance was determined for all individual data points and fitting parameters using one-way ANOVA and Tukey's HSD post hoc test at the 5 % level.
RESULTS
Effects of K+, Na+, Li+ and NMG+ on HCN1 channels
Figures 1A and B shows that equimolar substitution of external K+ with Na+, Li+ or NMG+, while maintaining the osmotic and ionic strength constant, produced depolarizing steady-state activation shifts of HCN1 (P < 0.05), indicating that channels open at more positive potentials when external K+ is reduced. In contrast to the identical V1/2 shifts (ΔV1/2) (Fig. 4A; P > 0.05), the same ion substitutions had differential effects on gating kinetics. Replacing K+ with the non-permeant NMG+ induced a larger decelerating effect on τact and α0 than did replacement with the permeants Na+ and Li+; τdeact and β0 were more modestly affected by these ions (Figs 1C and D and 4B).
Figure 4. Summary of the effects on V1/2 (A), and open and close rates (B) of HCN1, HCN1-A352D, HCN1-A352R and HCN2 channels by ion substitutions.

Asterisks in A indicate significant V1/2 shifts from 96 mm K+ (P < 0.05). Filled and open symbols in B represent α0 and β0, respectively.
We next examined the permeation properties of WT HCN1. As anticipated, Erev became more negative when external K+ was substituted by NMG+ (Supplementary material, Table 1). Using 32 mm K+-64 mm NMG+ as the reference solution, we estimated the permeability ratios of WT HCN1 for K+, Na+ and Li+ from the corresponding reversal potentials when NMG+ was replaced. We found that the selectivity sequence of HCN1 estimated under these conditions was K+ > Na+ > Li+ (PK/PK= 1, PNa/PK= 0.12, PLi/PK= 0.02), similar to those reported previously for cloned HCN channels and native If (Zagotta & Siegelbaum, 1996; Ludwig et al. 1999a; Santoro & Tibbs, 1999). Interestingly, this selectivity sequence roughly parallels the accelerating effects of K+, Na+, Li+ and NMG+ on gating kinetics.
A352D abolished the gating effects of ion substitutions
The above observations hint that HCN1 gating and ion conduction are coupled. To seek experimental evidence, we converted the neutral HCN1 pore variant C-terminal to the GYG triplet, A352, to the analogous Asp conserved in K+-selective channels (i.e. A352D; see also Supplementary material, Fig. 1). This substitution was chosen for two reasons. First, if the pore and the gate of HCN1 channels are indeed coupled, pore mutations may alter gating properties and/or their coupling to permeation. Second, the K+ channel-inspired substitution provides an opportunity to test whether this charge variant in the pore is responsible for the non-selective profile of HCN1. In accordance with the first notion, A352D significantly altered HCN1 gating (Fig. 2A–C): (1) activation was negatively shifted (by 15 mV, P < 0.05); and (2) gating kinetics were decelerated (by up to 6-fold, P < 0.05). More interestingly, A352D almost completely abolished the modulatory effects of varying external K+ on gating (see also Fig. 4). Taken together, our observations suggest that residue 352 is a determinant for the gating effects of external permeant ions, and may be responsible for coupling the HCN1 pore to the gate. However, the permeability ratios of A352D channels for K+, Na+ and Li+ were not different from WT (PK/PK= 1, PNa/PK= 0.13, PLi/PK= 0.02), indicating that this single variant is insufficient to rationalize the non-selectivity of HCN1. The HCN2-inspired substitution A352R (GYGR in HCN2) produced similar changes in gating (i.e. 3-fold slowed kinetics and activation negatively shifted by 5 mV, P < 0.05). The gating responses of A352R channels to Na+ substitution were also significantly attenuated in comparison to WT HCN1 (Fig. 2D–F and Fig. 4). The gating effects of NMG+ and Li+ substitutions and selectivity of A352R channels, however, were not studied because of the small current amplitudes when K+ was replaced by these ions.
Figure 2. HCN1-A352D abolished the effects of permeant ions on gating.
Typical current tracings from the same protocol as shown in Fig. 1 (A), steady-state activation curves (n= 14 (K+), 7 (Na+), 11 (NMG+), 4 (Li+)) (B), and gating kinetics (n= 7 (K+), 8 (Na+), 7 (NMG+), 6 (Li+)) (C) of HCN1 A352D channels recorded under the same ionic conditions described in Fig. 1. D, E and F, same descriptions as A, B and C, respectively, but for A352R channels (n= 4 (K+), 4 (Na+)). Thickened x-axes indicate voltage ranges over which all 3 ionic conditions differ significantly from 96 mm K+.
Effects of external K+ on HCN2
Despite the fact that HCN1 and HCN2 share 85 % sequence homology, HCN2 has significantly slower gating kinetics (Ludwig et al. 1998, 1999a,b; Santoro et al. 1998; Santoro & Tibbs, 1999; Kaupp & Seifert, 2001). In addition, minK-related peptide 1 (MiRP1), a β-subunit for the rapid component of the cardiac delayed rectifier IKr, has been demonstrated to preferentially regulate HCN1 and HCN2 properties (Yu et al. 2001). Given these isoform differences, it is possible that external K+ also modulates HCN1 and HCN2 gating in an isoform-specific manner. Our results show that this is the case (Fig. 3 and Fig. 4). Although Na+, Li+ and NMG+ substitutions all positively shifted HCN2 activation in a manner similar to those observed with HCN1 (P < 0.05), τact and α0 were unaltered by these ions (P > 0.05). In contrast, τdeact was exclusively decelerated (P < 0.05). Interestingly, the effects of Na+, Li+ and NMG+ substitutions on τdeact and β0 also follow the HCN2 selectivity sequence K+ > Na+ > Li+ > NMG+ (PK/PK= 1, PNa/PK= 0.09, PLi/PK= 0.02).
Figure 3. Effect of K+, Na+, Li+ and NMG+ on HCN2.
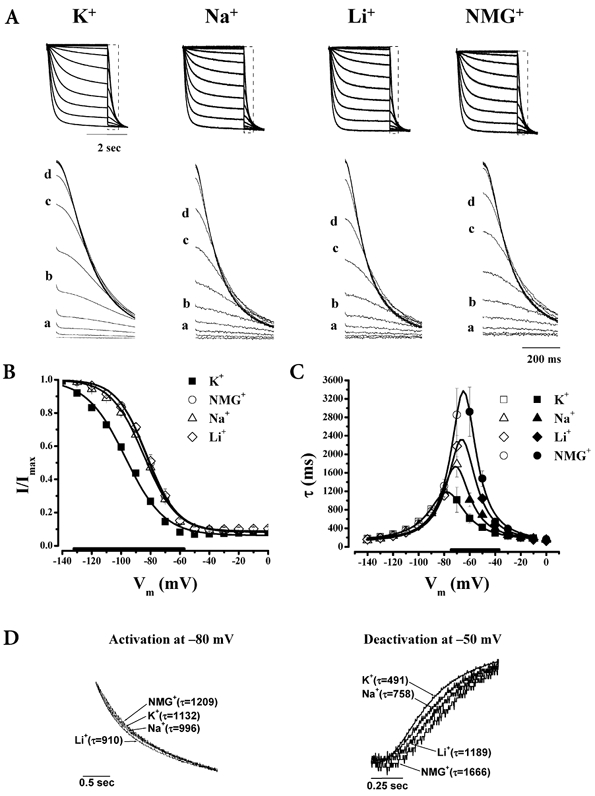
Same descriptions as Fig. 1 but for HCN2 channels. Unlike HCN1, Na+, Li+ and NMG+ substitutions decelerated deactivation without affecting activation. For activation curves, n = 14 (K+), 7 (Na+), 11 (NMG+), 4 (Li+). For kinetics, n = 7 (K+), 9 (Na+), 6 (NMG+), 7 (Li+).
DISCUSSION
In Kv channels, C-type inactivation is known to involve dynamic rearrangements of the outer pore (see Yellen, 1998 for review). It is generally accepted that the pore narrows when sufficiently depolarized and becomes dilated when hyperpolarized (recovery from inactivation). Such pore constriction is inhibited by the presence of permeant ions (such as K+ and Rb+) or pore blockers (e.g. TEA+ and pore-blocking toxins) but not non-permeants (like Na+ and NMG+), presumably via a ‘foot-in-the-door’ mechanism (Yeh & Armstrong, 1978). In fact, Na+ channels slow-inactivate in a very similar fashion (Townsend et al. 1997). Our finding that increasing the external permeant accelerated HCN1 activation and opening rates is consistent with the notion that the HCN pore is also involved in gating. Interestingly, the modulatory effects of external ions also appear to follow the selectivity sequence. Therefore, it is possible that HCN activation involves a gating process that is analogous to C-type and slow-inactivation of K+ and Na+ channels. The voltage dependence of the associated gating-induced conformational changes of the HCN pore may be similar such that it is constricted and dilated when depolarized and hyperpolarized, respectively. However, the transition during hyperpolarization is from closed to open in the case of HCN channels, but from C-type or slow-inactivated to closed (or N-type inactivated or open depending on the exact voltage, but the pore is dilated in all these states; cf. Fig. 8 of Xue & Li, 2002) for K+ and Na+ channels, and vice versa during depolarization. The transitions between these pore conformations of HCN channels, like K+ and Na+ channels, may be modulated by external permeants as we have demonstrated experimentally.
In support of this pore-to-gate coupling model of HCN channels, covalent attachment of a bulky moiety to the extra-pore S5-P linker of HCN1 significantly slowed gating kinetics (Xue & Li, 2002). In addition, the HCN1 pore mutations A352D and A352R significantly altered gating and attenuated its responses to external ions, presumably by disrupting the associated pore motions and/or their interactions with permeant ions. These results further suggest that the lack of effect of ion substitutions on τact of HCN2 could be attributed, at least partially, to the A-to-R variant in its pore. Interestingly, reducing K+ decelerated deactivation and destabilized closed channels (as reflected by the positive V1/2 shifts) of both HCN1 and HCN2. At first glance, these results were inconsistent with the foot-in-the-door hypothesis. However, the channel close rate of HCN2 at zero voltage indeed increased with decreasing permeants. Furthermore, given that multiple processes determine the gating properties of voltage-gated ion channels (e.g. C-type inactivation is also coupled to activation), external permeants may influence mechanisms other than HCN pore motions. Indeed, pore motions may also be coupled to the opening of the cytoplasmic gate at the inner pore (Rothberg et al. 2002), both contributing to HCN channel activation. Clearly, additional experiments are needed to prove this concept. Irregardless, it is becoming increasingly apparent that HCN and K+ channels share a number of key structural and functional features.
Our observations may have pathophysiological relevance. For instance, accumulation of extracellular K+ during ischaemia, which already leads to membrane depolarization unfavourable for If activation, would further shift its activation range negatively, thereby slowing cardiac pacing (i.e. heart rate) to conserve metabolic energy. Similarly, extracellular Na+ and K+ in the central nervous system are known to vary significantly during normal and pathological (e.g. epileptic events) neuronal activity. These changes in K+/Na+ can subsequently alter neuronal excitability by modulating the hyperpolarization-activated currents in these tissues. However, it should be noted that K+ accumulation may also augment currents simply by facilitating ion conduction, implying that pacing modulation via alterations of If is a balance between these permeation and gating processes. Further, the K+ concentrations used in our experiments were higher than the physiological levels. Further experiments are needed to verify that our present results can be extrapolated to the physiological range.
Our present results with cloned channels differ from those previously reported for native currents (DiFrancesco et al. 1986; McCormick & Pape, 1990; Wollmuth & Hille, 1992; Ho et al. 1993, 1994; Mangoni & Nargeot, 2001). For instance, gating properties of If measured from rod photoreceptors were not dependent on external permeant ions (Wollmuth & Hille, 1992). Such discrepancies may be attributable to the different molecular composition of these native channels. Given that different HCN isoforms exhibit distinct sensitivity to modulations by ions, cAMP, etc., and can co-assemble to form heteromultimers (Chen et al. 2001; Ulens & Tytgat, 2001; Xue et al. 2002), our results suggest that native If, depending on the tissues and thus isoform compositions, may have a diverse range of sensitivities to different regulatory mechanisms.
In summary, we conclude that external permeants modulate HCN gating in an isoform-specific manner. This phenomenon may come into play physiologically in the modulation of cardiac and neuronal pacing. Furthermore, our findings provide evidence that gating and permeation of HCN channels are coupled, and that residue 352 is probably a determinant of this coupling.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (R01 HL-52768) and a Research Career Development Award from the Cardiac Arrhythmias Research & Education Foundation (to R.A.L.).
Supplementary material
The online version of this paper can be found at:
http://www.jphysiol.org/cgi/content/full547/2/349and contains material entitled
Table of reversal potentials and schematic diagram of HCN channel pore region
REFERENCES
- Chen S, Wang J, Siegelbaum SA. Properties of hyperpolarization-activated pacemaker current defined by coassembly of HCN1 and HCN2 subunits and basal modulation by cyclic nucleotide. J Gen Physiol. 2001;117:491–504. doi: 10.1085/jgp.117.5.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Difrancesco D. A study of the ionic nature of the pace-maker current in calf Purkinje fibres. J Physiol. 1981;314:377–393. doi: 10.1113/jphysiol.1981.sp013714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Difrancesco D, Ferroni A, Mazzanti M, Tromba C. Properties of the hyperpolarizing-activated current (if) in cells isolated from the rabbit sino-atrial node. J Physiol. 1986;377:61–88. doi: 10.1113/jphysiol.1986.sp016177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- Gauss R, Seifert R, Kaupp UB. Molecular identification of a hyperpolarization-activated channel in sea urchin sperm. Nature. 1998;393:583–587. doi: 10.1038/31248. [DOI] [PubMed] [Google Scholar]
- Henrikson CA, Xue T, Dong P, Sang D, Marban E, Li RA. The S3–S4 linker is a determinant of the gating phenotype of pacemaker (HCN) channels. Biophys J. 2003 doi: 10.1074/jbc.M211025200. (in the Press; abstract) [DOI] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. Sunderland, MA, USA: Sinauer Associates, Inc.; 2001. [Google Scholar]
- Ho WK, Brown HF, Noble D. Internal K ions modulate the action of external cations on hyperpolarization-activated inward current in rabbit isolated sinoatrial node cells. Pflugers Archiv. 1993;424:308–314. doi: 10.1007/BF00384357. [DOI] [PubMed] [Google Scholar]
- Ho WK, Brown HF, Noble D. High selectivity of the i(f). channel to Na+ and K+ in rabbit isolated sinoatrial node cells. Pflugers Archiv. 1994;426:68–74. doi: 10.1007/BF00374672. [DOI] [PubMed] [Google Scholar]
- Kaupp UB, Seifert R. Molecular diversity of pacemaker ion channels. Annu Rev Physiol. 2001;63:235–257. doi: 10.1146/annurev.physiol.63.1.235. [DOI] [PubMed] [Google Scholar]
- Li RA, Velez P, Chiamvimonvat N, Tomaselli GF, Marban E. Charged residues between the selectivity filter and S6 segments contribute to the permeation phenotype of the sodium channel. J Gen Physiol. 1999;115:81–92. doi: 10.1085/jgp.115.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig A, Zong X, Hofmann F, Biel M. Structure and function of cardiac pacemaker channels. Cell Physiol Biochem. 1999a;9:179–186. doi: 10.1159/000016315. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Zong X, Jeglitsch M, Hofmann F, Biel M. A family of hyperpolarization-activated mammalian cation channels. Nature. 1998;393:587–591. doi: 10.1038/31255. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Zong X, Stieber J, Hullin R, Hofmann F, Biel M. Two pacemaker channels from human heart with profoundly different activation kinetics. Embo J. 1999b;18:2323–2329. doi: 10.1093/emboj/18.9.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangoni ME, Nargeot J. Properties of the hyperpolarization-activated current (I(f)) in isolated mouse sino-atrial cells. Cardiovasc Res. 2001;52:51–64. doi: 10.1016/s0008-6363(01)00370-4. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Pape HC. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol. 1990;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg BS, Shin KS, Phale PS, Yellen G. Voltage-controlled gating at the intracellular entrance to a hyperpolarization-activated cation channel. J Gen Physiol. 2002;119:83–91. doi: 10.1085/jgp.119.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro B, Chen S, Luthi A, Pavlidis P, Shumyatsky GP, Tibbs GR, Siegelbaum SA. Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS. J Neurosci. 2000;20:5264–5275. doi: 10.1523/JNEUROSCI.20-14-05264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro B, Liu DT, Yao H, Bartsch D, Kandel ER, Siegelbaum SA, Tibbs GR. Identification of a gene encoding a hyperpolarization-activated pacemaker channel of brain. Cell. 1998;93:717–729. doi: 10.1016/s0092-8674(00)81434-8. [DOI] [PubMed] [Google Scholar]
- Santoro B, Tibbs GR. The HCN gene family: molecular basis of the hyperpolarization-activated pacemaker channels. Ann NY Acad Sci. 1999;868:741–764. doi: 10.1111/j.1749-6632.1999.tb11353.x. [DOI] [PubMed] [Google Scholar]
- Townsend C, Hartmann HA, Horn R. Anomalous effect of permeant ion concentration on peak open probability of cardiac Na+ channels. J Gen Physiol. 1997;110:11–21. doi: 10.1085/jgp.110.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulens C, Tytgat J. Functional heteromerization of HCN1 and HCN2 pacemaker channels. J Biol Chem. 2001;276:6069–6072. doi: 10.1074/jbc.C000738200. [DOI] [PubMed] [Google Scholar]
- Wollmuth LP, Hille B. Ionic selectivity of Ih channels of rod photoreceptors in tiger salamanders. J Gen Physiol. 1992;100:749–765. doi: 10.1085/jgp.100.5.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue T, Li RA. An external determinant in the S5-P linker of the pacemaker (HCN) channel identified by sulfhydryl modification. J Biol Chem. 2002;277:46233–46242. doi: 10.1074/jbc.M204915200. [DOI] [PubMed] [Google Scholar]
- Xue T, Marban E, Li RA. Dominant-negative suppression of HCN1- and HCN2-encoded pacemaker currents by an engineered HCN1 construct: insights into structure-function relationships and multimerization. Circ Res. 2002;90:1267–1273. doi: 10.1161/01.res.0000024390.97889.c6. [DOI] [PubMed] [Google Scholar]
- Yeh JZ, Armstrong CM. Immobilisation of gating charge by a substance that simulates inactivation. Nature. 1978;273:387–389. doi: 10.1038/273387a0. [DOI] [PubMed] [Google Scholar]
- Yellen G. The moving parts of voltage-gated ion channels. Q Rev Biophys. 1998;31:239–295. doi: 10.1017/s0033583598003448. [DOI] [PubMed] [Google Scholar]
- Yu H, Wu J, Potapova I, Wymore RT, Holmes B, Zuckerman J, Pan Z, Wang H, Shi W, Robinson RB, El-Maghrabi MR, Benjamin W, Dixon J, McKinnon D, Cohen IS, Wymore R. MinK-related peptide 1: A beta subunit for the HCN ion channel subunit family enhances expression and speeds activation. Circ Res. 2001;88:E84–87. doi: 10.1161/hh1201.093511. [DOI] [PubMed] [Google Scholar]
- Zagotta WN, Siegelbaum SA. Structure and function of cyclic nucleotide-gated channels. Annu Rev Neurosci. 1996;19:235–263. doi: 10.1146/annurev.ne.19.030196.001315. [DOI] [PubMed] [Google Scholar]


