Abstract
Cyclic AMP-dependent protein kinase (PKA) targets contractile proteins, troponin-I (TnI) and myosin binding protein C (MyBP-C) in the heart and induces a decrease in myofilament Ca2+ sensitivity. Yet, the effect of sarcomere length (SL) change on Ca2+ sensitivity (length-dependent activation: LDA) following PKA-dependent phosphorylation is not clear. To clarify the role of PKA-dependent phosphorylation of TnI and MyBP-C on LDA in the heart, we examined LDA in skinned myocytes from a non-transgenic (NTG) and a transgenic murine model in which the native cardiac isoform (cTnI) was completely replaced by the slow skeletal isoform of TnI (ssTnI-TG) lacking the phosphorylation sites for PKA, while retaining PKA sites on MyBP-C. In NTG myocytes, PKA treatment decreased Ca2+ sensitivity at each SL, but enhanced the impact of SL change on Ca2+ sensitivity. Despite a greater sensitivity to Ca2+ and a reduction in LDA, neither Ca2+ responsiveness nor LDA was affected by PKA treatment in ssTnI-TG myocytes. To determine whether the above observations could be explained by the lateral separation between thick and thin filaments, as suggested by others, we measured interfilament spacing by X-ray diffraction as a function of SL in skinned cardiac trabeculae in the passive state from both NTG and ssTnI-TG models before and following treatment with PKA. Phosphorylation by PKA increased lattice spacing at every SL in NTG trabeculae. However, the relationship between SL and myofilament lattice spacing in ssTnI-TG was markedly shifted downward to an overall decreased myofilament lattice spacing following PKA treatment. We conclude: (1) PKA-dependent phosphorylation enhances length-dependent activation in NTG hearts; (2) replacement of native TnI with ssTnI increases Ca2+ sensitivity of tension but reduces length-dependent activation; (3) MyBP-C phosphorylation by PKA does not alter calcium responsiveness and induces a decrease in myofilament lattice spacing at all sarcomere lengths and (4) length-dependent activation in the heart cannot be entirely explained by alterations in myofilament lattice spacing.
A well-studied mechanism by which myocardial function is tuned to the haemodynamic load is through β-adrenoceptor stimulation and subsequent activation of cAMP-dependent protein kinase (PKA). Signalling through this pathway is associated with increased rates of relaxation and contraction in the myocardium (Ross & Sobel, 1972; Luo et al. 1994) and is believed to be predominantly due to phosphorylation of phospholamban by PKA leading to enhanced reuptake of Ca2+ by the sarcoplasmic reticulum (Kranias, 1985; Luo et al. 1994; Solaro & Van Eyk, 1996; Wolska et al. 1996). PKA additionally targets contractile proteins, specifically troponin I (TnI) and myosin binding protein C (MyBP-C), and results in a decrease in myofilament Ca2+ sensitivity (Solaro et al. 1976; Garvey et al. 1988). It has been suggested that phosphorylation of myofilament proteins may affect cardiac contractility independently of the alterations in the dynamics of Ca2+ handling (Kentish et al. 2001). Although a regulatory role for MyBP-C cannot be altogether eliminated in the light of an emerging literature (Kunst et al. 2000; Winegrad, 2000), there is good evidence to suggest that the PKA effect in the myocardium is due to phosphorylation of TnI and not MyBP-C (Fentzke et al. 1999; Kentish et al. 2001).
Other studies have shown that replacement of cardiac TnI (cTnI) with the slow skeletal isoform (ssTnI) in the heart can alter contractile properties of heart muscle by increasing Ca2+ sensitivity (Fentzke et al. 1999) and reducing the response to changes in muscle length (Arteaga et al. 2000). The TnI isoforms differ by the presence of a unique 32 amino acid extension at the N-terminus of cTnI. Since this extension contains the two targets for PKA, ssTnI cannot be phosphorylated by PKA (Fentzke et al. 1999; Kentish et al. 2001). Though the specific role of this N-terminus has not fully been elucidated, these latter studies implicate TnI as an important modulator of both Ca2+ sensitivity and length-dependent activation, a phenomenon believed to be the cellular mechanism for the Frank-Starling law of the heart (ter Keurs et al. 1980).
The increase in Ca2+ sensitivity upon an increase in sarcomere length may be due to a decrease in interfilament spacing with an increase in length (Rome, 1968) thereby influencing cross-bridge reactivity (Godt & Maughan, 1981; McDonald & Moss, 1995; Fuchs & Wang, 1996). That is, cross-bridges may be more likely to enter into a strong binding state at a given level of activator Ca2+ when interfilament spacing is reduced. The correlation between interfilament spacing, alterations in Ca2+ sensitivity, and length-dependent activation following TnI phosphorylation or TnI isoform composition are not known. Given the significance of sarcomere length, TnI isoform composition and TnI phosphorylation status on myocardial contraction, several questions arise. (1) Does PKA-mediated phosphorylation of cTnI affect the sarcomere length dependency of myofilament activation, and if so, is this effect due to the phosphorylation of cTnI or MyBP-C? (2) What is the role of interfilament spacing in determining myofilament Ca2+ sensitivity and length-dependent myofilament activation under these conditions?
In experiments reported here, we determined the effect of PKA-mediated phosphorylation on sarcomere length- (SL)-dependent activation in skinned single cardiac myocytes obtained from either non-transgenic mice or transgenic mice in which cTnI was completely replaced by ssTnI in the heart. In addition, interfilament spacing was measured over a wide range of sarcomere lengths in skinned cardiac trabeculae using synchrotron X-ray diffraction. We found that: (1) PKA-mediated phosphorylation induces an increase in SL-dependent activation; (2) this effect is due to phosphorylation of cTnI and not MyBP-C; and (3) alterations in Ca2+ sensitivity and SL-dependent activation upon PKA-mediated myofilament phosphorylation or TnI isoform composition cannot be predicted, solely, by alterations in interfilament spacing.
METHODS
Myocyte preparation
All experiments were performed according to institutional guidelines concerning the care and use of experimental animals. Mice (NTG and ssTnI-TG; 20–30 g) were anaesthetized (sodium pentobarbital 50 mg (kg body weight)−1i.p. injection) and the hearts were rapidly excised and retrogradely perfused with a modified Krebs-Henseleit (K-H) solution (de Windt et al. 1999) (mmol l−1): NaCl 118.5, KCl 5, MgSO4 1.2, NaH2PO4 2, ethylenediaminetetraacetic acid, EDTA 0.5, d(+)−glucose 10, NaHCO3 25, pyruvic acid 1.5, CaCl2 3.0. In addition, insulin (5 u l−1) and propranolol (5 μmol l−1), to block non-specific β-adrenergic activation, and carbamyl choline (10 μmol l−1), to enhance phosphatase activity (Gupta et al. 1994) were added to the perfusate. 3-Butanedione monoxime (20 mmol l−1) was also added to the K-H solution to inhibit contraction, presumably through the energetic stabilization of the unattached state of the myosin molecule (Backx et al. 1994). Skinned myocytes or myocyte fragments were obtained by mechanical isolation as described previously (Fan et al. 1997; Fentzke et al. 1999). Briefly, during perfusion with the K-H solution, a section of the left ventricle was minced into 1–2 mm pieces. Next, the pieces were mechanically disrupted at high speed for 15–20 s (Power Gen 700D; Fisher Scientific) in ice-cold standard relaxing solution (mmol l−1): Na+-ATP 4, MgCl2 1, ethylene glycol-bis(β-aminoethyl ether)-N, N, N′,N′-tetraacetic acid (EGTA) 2, KCl 100, imidazole 10. The homogenized tissue was then centrifuged at 800 r.p.m. (Marathon 22 Kbr; Fisher Scientific) for 1 min at 4 °C. The supernatant was discarded and the tissue was resuspended in standard relaxing solution containing 0.3 % Triton X-100 (Pierce Chemical) for 6 min to remove the sarcolemma and all remaining membranous structures. After washing twice with the relaxing solution, the cells and cell fragments were resuspended in standard relaxing solution and stored on ice for < 10 h before data collection.
Experimental apparatus and protocol
The experiments were performed on the stage of an inverted microscope (Olympus) that allowed visualization of the cell or cell fragments. The cell was attached at either end to a sensitive force transducer (Cambridge model 403A; ∼300 Hz resonant frequency) and a high-speed servomotor (Cambridge model 308; ∼1 ms 90 % step response) with stainless-steel minutien tips and silicone rubber. The sarcomere length was measured by video-microscopy; each horizontal pixel line was transformed by fast Fourier transformation (FFT) into a spatial frequency domain and converted into median sarcomere length across the region (Fan et al. 1997). Cell length was adjusted by using the servomotor, which was controlled via computer (Apple PowerPC) using custom-designed software (LabVIEW, National Instruments; Austin, TX, USA). Both minutien tips were connected to X-Y-Z manipulators (Newport) permitting accurate positioning of the attached cell. Cell length and width were measured by video-microscopy. Myocyte thickness was measured by placing a small mirror under a 45 deg angle close to the cell by using a three-dimensional manipulator. The myocyte was suspended above a movable stage that contained several solution wells (temperature controlled at 15°C). Rapid solution changes were made by translating the stage laterally via stage manipulators such that another solution-containing well was brought underneath the cell (Fan et al. 1997). The myofilament Ca2+ sensitivity of tension was characterized at two sarcomere lengths (2.25 and 1.95 μm) by measuring steady-state tension development followed by a rapid slackening to assess total tension. Active tension at each [Ca2+] was the difference between total tension and relaxed, passive tension (passive tension was not measurable at the short sarcomere length and was minimal at the long sarcomere length). Cells that did not maintain 80 % of initial maximal tension or a visible striation pattern were discarded. In addition, if the extent of internal shortening due to end-compliance in the preparation was extensive (<100 nm) the data were also discarded.
The ionic strength of the solutions was kept at 180 mmol l−1 by adding the appropriate amount of potassium propionate. In addition, all solutions contained the following (mmol l−1): phosphocreatine 10, N, N-bis[2-hydroxyethyl]-2-aminoethanesulphonic acid (BES) 100, leupeptin 0.1, phenylmethylsulphonyl fluoride (PMSF) 0.1, dithiothreitol (DTT) 1 and 4 U ml l−1 creatine phosphokinase. The free Mg2+ and Mg-ATP concentration was calculated at 1 and 5 mmol l−1, respectively. To achieve a range of free [Ca2+], activating and relaxing solutions were appropriately mixed with an apparent stability constant of the Ca2+-ethylene glycol-bis(β-aminoethyl ether)-N, N, N′,N′-tetraacetic acid (EGTA) complex of 106.39 assumed. The pH was adjusted to 7.0 at 15.0 °C with KOH. Force was stored on computer via an A/D converter using the custom software for off-line analysis (10 kHz per channel sampling frequency).
X-ray diffraction experiments
The overall experimental arrangement, sample preparation and protocol have been described in detail previously (Irving et al. 2000). Briefly, experiments were performed on the BioCAT undulator-based beamline at the Advanced Photon Source, Argonne National Laboratory. Following rapid excision, the heart was perfused with the identical K-H solution as described above. Trabeculae were dissected from the right ventricular free wall in both NTG and ssTnI-TG mice and demembranated overnight with 1 % Triton X-100 in standard relaxing solution. Preliminary experiments indicated robust PKA-dependent phosphorylation in cardiac trabeculae prepared in this manner (data not shown). For the X-ray studies, trabeculae were mounted between a force transducer and a servo-motor in a small trough, which allowed simultaneous collection of the X-ray patterns and viewing of the striation pattern using a long working distance objective (× 40) of an inverted microscope equipped with a CCD video camera. Low-angle X-ray diffraction patterns were collected on a CCD-based X-ray detector. Spacings between the 1,0 and 1,1 equatorial reflections in the diffraction pattern were converted to d10 lattice spacings using Bragg's law.
Individual trabeculae were stretched to arbitrary sarcomere lengths between 1.9 and 2.4 μm at the beginning of the experiment. Sarcomere lengths were determined from the video-image as described previously (Irving et al. 2000) and checked both before and immediately after the X-ray exposure. Fibre length was then systematically lengthened or shortened to collect at least 10–12 (at least every 50 nm) consecutive data points describing the relationship between sarcomere length and interfilament lattice spacing. Next, an additional 2–4 data points were collected at various sarcomere lengths back along the curve to check for reproducibility or hysteresis. The level of passive tension in the lattice was recorded when the X-ray exposure was taken.
Data analysis
Each individual Ca2+-force relationship was fitted to a modified Hill equation:
where Frel is the force relative to maximum Ca2+ saturated force, EC50 is the [Ca2+] at which force is half-maximal and nH is the slope of the Ca2+-force relationship (Hill coefficient). The ΔEC50 was calculated as the difference in EC50 at SL = 1.95 and 2.25 μm for each experiment. Likewise, the ΔLS was calculated as the difference in LS calculated at SL = 1.95 and 2.25 μm for each experiment. Separation between the symmetrical equatorial 1,0 and 1,1 X-ray diffraction reflections was measured directly from detector images using the program FIT2D (Hammersley, 1998) on a UNIX workstation and converted to d10 lattice spacing using Bragg's law, which can then, in turn, be converted to the inter-thick filament spacing by multiplying d10 by 2/□3. The differences among EC50, ΔEC50, LS and ΔLS for each group at each SL were analysed with a one-way ANOVA Student's t test with a post hoc Bonferroni correction to assess differences among mean values. All data are shown as means ±s.e.m.
RESULTS
Ca2+ sensitivity of tension
Figure 1 shows the relationship between steady-state force development and free [Ca2+] in skinned NTG and ssTnI-TG myocytes before (open symbols) and after (closed symbols) treatment with PKA. The Ca2+-force relationships were determined at SL = 1.95 μm (squares) and SL = 2.25 μm (triangles); the continuous lines indicate the best fit of the Ca2+-force co-ordinates to a modified Hill equation (see Methods). For both groups, the Ca2+-force relationship was shifted to the left at the greater sarcomere length indicating an increase in Ca2+ sensitivity (a decrease in EC50; [Ca2+] at which tension is half-maximal) consistent with SL-dependent myofilament activation (Kentish et al. 1986). The myofilaments in ssTnI-TG (Fig. 1B) were more sensitive to Ca2+ than NTG myocytes at both sarcomere lengths, as observed previously (Fentzke et al. 1999; Arteaga et al. 2000). In addition, the impact of a sarcomere length change on Ca2+ sensitivity, as calculated as the difference in the EC50 at each sarcomere length (ΔEC50), was significantly reduced in ssTnI-TG myocytes. Similar to results reported previously, there was no difference in maximum tension between NTG and TG preparations at either SL (Wolska et al. 2001). Earlier studies have employed pCa50 (−log[Ca2+]) to index Ca2+ sensitivity (Godt & Maughan, 1981; McDonald & Moss, 1995; Fuchs & Wang, 1996). Employing this method revealed statistically significant differences in Ca2+ sensitivity between NTG and ssTnI-TG myocytes. However, length-dependent activation determined as the difference in pCa50 at the two sarcomere lengths (ΔpCa50) indicated no significant differences in length-dependent activation between NTG and ssTnI-TG myocytes. The average parameters of the Hill fit from NTG and ssTnI-TG myocytes are summarized in Table 1.
Figure 1. Ca2+-dependent tension development in cardiac myocytes from NTG and ssTnI-TG hearts.
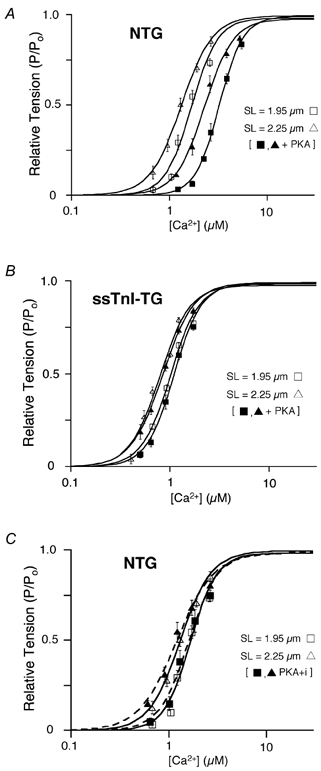
A and B, Ca2+-force relation of NTG (A) and ssTnI-TG (B) cardiac myocytes before (open symbols) and following (closed symbols) treatment with the catalytic subunit of PKA (3 μg protein (ml standard relaxing solution)−1) at SL = 2.25 μm (δ) and SL = 1.95 μm (□). C, Ca2+-force relation of NTG cardiac myocytes at two sarcomere lengths before (open symbols) and following (closed symbols) treatment with a PK inhibitor (PKI) added to PKA solution. Lines indicate the Hill fit to the data obtained at the indicated sarcomere length before and following PKA treatment.
Table 1.
Average values of Hill fit to Ca2+-force curves
| SL = 1.95μm | SL = 2.25μm | |||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | EC50 (μm) | pCa50(μm) | nH | EC50(μm) | pCa50(μm) | nH | ΔEC50 | ΔpCa50 |
| NTG | ||||||||
| −PKA(n = 7) | 1.72 ± 0.10 | 5.77 ± 0.03 | 3.74 ± 0.35 | 1.31 ± 0.09† | 5.89 ± 0.03 | 2.91 ± 0.23 | 0.41 ± 0.04 | 0.12 ± 0.01 |
| +PKA(n = 7) | 3.24 ± 0.14* | 5.49 ± 0.02 | 3.40 ± 0.11 | 2.45 ± 0.13*† | 5.61 ± 0.02 | 3.8 ± 0.16 | 0.79 ± 0.07* | 0.12 ± 0.01 |
| PKA + i(n = 7) | 1.67 ± 0.09 | 5.78 ± 0.02 | 3.02 ± 0.26 | 1.29 ± 0.08 | 5.89 ± 0.03 | 2.76 ± 0.25 | 0.38 ± 0.08 | 0.11 ± 0.03 |
| ssTnI-TG | ||||||||
| −PKA(n = 8) | 1.04 ± 0.05* | 5.99 ± 0.02 | 2.98 ± 0.14 | 0.78 ± 0.06*† | 6.11 ± 0.03 | 2.72 ± 0.23 | 0.26 ± 0.03* | 0.13 ± 0.02 |
| +PKA(n = 8) | 1.10 ± 0.04 | 5.96 ± 0.02 | 3.24 ± 0.17 | 0.84 ± 0.04† | 6.08 ± 0.02 | 2.83 ± 0.14 | 0.25 ± 0.02* | 0.11 ± 0.01 |
Average Hill fit parameters of the Ca2+-force relationships obtained from NTG and ssTn-ITG myocytes before and after treatment with PKA at the sarcomere lengths indicated. (PKA + I, PK inhibitor added to PKA solution). The final columns indicate the average length-dependent changes in EC50 (ΔEC50 or ΔpCa50) for each muscle type and treatment studied
(P < 0.01 from values obtained from untreated NTG controls
P < 0.01 obtained from respective values at the short SL indicating a significant length effect).
Figure 1 illustrates the effect of treatment of NTG and ssTnI-TG myocytes with the catalytic subunit of PKA (3 μg protein (ml standard relaxing solution)−1; Sigma Chemicals). As described in Methods, muscle preparation was designed to prevent additional, non-specific adrenergic phosphorylation or to enhance phosphatase activity resulting in minimal phosphorylation of TnI and MyBP-C. This was supported by robust 32P incorporation (data not shown) by TnI and MyBP-C in NTG myocardium and MyBP-C in ssTnI-TG preparations, as we have previously shown using this transgenic murine model (Fentzke et al. 1999; Kentish et al. 2001). Consistent with our previous studies (Solaro, 1995; Janssen & de Tombe, 1997), treatment of the NTG skinned myocardium with PKA induced a rightward shift of the Ca2+-force relationship at both sarcomere lengths, as indicated by an increase in the EC50 (Fig. 1A). In addition, the impact of a sarcomere length change (ΔEC50) on the Ca2+-force relationship was significantly enhanced following PKA treatment, indicating an increase in length-dependent activation properties following PKA treatment. Again, using the pCa50 parameter, there was a significant decrease in Ca2+ sensitivity following PKA treatment, yet the effect of length change on pCa50 (ΔpCa50) was the same before and following PKA treatment (Table 1). If a protein kinase inhibitor was added to the solution (63 μg protein (ml standard relaxing solution)−1; Sigma Chemicals), the effect of PKA was completely abolished independent of parameter choice (Fig. 1C). Furthermore, PKA did not alter maximum tension at either SL, as reported previously (Janssen & de Tombe, 1997).
PKA targets two serine residues (22, 23) at the N-terminus of cTnI, amino acid sites that are not found in ssTnI, and therefore, in ssTnI-TG hearts TnI is not phosphorylated by PKA (Fentzke et al. 1999; Kentish et al. 2001). However, PKA targets an additional thick filament protein, myosin-binding protein C (MyBP-C; Winegrad, 2000). Treatment of ssTnI-TG myocytes with PKA did not affect Ca2+ sensitivity of tension at either sarcomere length (Fig. 1B). Consequently, there was no impact on ΔEC50, or ΔpCa50, and thus no effect on length-dependent activation. These data show that the PKA effect on Ca2+ sensitivity and length-dependent activation in NTG hearts can be attributed, solely, to phosphorylation of TnI and not of MyBP-C. Again, the average parameters of the Hill fit from NTG and ssTnI-TG myocytes, both before and after PKA treatment, are summarized in Table 1.
Relationship between interfilament spacing and sarcomere length
To determine the influence of PKA-dependent phosphorylation of cTnI and MyBP-C in the heart on interfilament spacing, we simultaneously measured myofilament lattice spacing and sarcomere length in relaxed skinned cardiac trabeculae of NTG and ssTnI-TG mice before and after PKA treatment using synchrotron X-ray diffraction (Irving et al. 2000). Figure 2 illustrates typical X-ray diffraction patterns obtained in trabeculae at SL = 2.1 μm from NTG and ssTnI-TG hearts before and after treatment with PKA. These data show clearly resolved symmetrical pairs of 1,0 and 1,1 equatorial X-ray reflections. The adjacent panels illustrate line scans obtained from the 2-D diffraction patterns. Measurement of the distance between the symmetrical sets of reflections, indicated by the continuous vertical lines, allowed lattice spacing to be determined within 0.25 % accuracy, equivalent to a myofilament lattice spacing resolution of 0.1 nm (Irving et al. 2000). There was an evident inward displacement of the reflections following PKA treatment in NTG trabeculae, indicative of an increased myofilament lattice spacing. In contrast, in ssTnI-TG trabeculae, PKA treatment increased the distance between reflections, indicative of a reduction in myofilament lattice spacing.
Figure 2. Typical X-ray diffraction patterns obtained in skinned cardiac trabeculae from NTG and ssTnI-TG hearts before and following PKA treatment at SL = 2.10 μm.
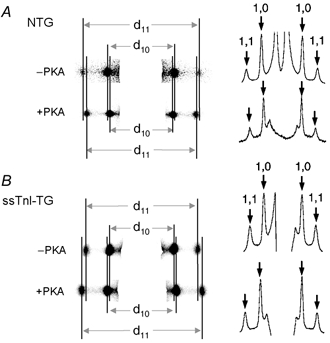
A, CCD images of the diffraction patterns. X-ray diffraction by the myofilament lattice gives rise to two sets of symmetrical, equatorial reflections, delineated by the continuous vertical lines. B, intensity profiles of the 2-dimensional CCD images shown in A. Arrows indicate the peak intensities, corresponding to the 1,0 and 1,1 reflections. These clearly resolved reflections or intensity peaks allowed lattice spacing to be determined within 0.25 % accuracy, equivalent to a myofilament lattice spacing resolution of 0.1 nm.
As is shown in Fig. 3, myofilament lattice spacing (LS) was highly dependent on SL, consistent with other and our previous results in mouse myocardium (Fig. 3) (Konhilas et al. 2000; Cazorla et al. 2001) and in striated muscle (Rome, 1968; Bagni et al. 1994; Irving et al. 2000). Furthermore, there was a parallel increase in LS at every SL in NTG trabeculae following treatment with PKA (Fig. 3A). Treatment of ssTnI-TG trabeculae, on the other hand, induced contraction of the myofilament lattice at all sarcomere lengths (Fig. 3B). It is also important to note that in both NTG and ssTnI-TG hearts, PKA-dependent phosphorylation greatly reduced the experimental variability among trabeculae, as indicated by a reduction in the standard error of each binned data point. The implication may be that, given the effect of phosphorylation status on LS, there existed a greater variation in basal phosphorylation levels under control conditions (untreated trabeculae). This variation was reduced by PKA treatment, presumably by maximally phosphorylating myofilament proteins. Indeed, preliminary back-phosphorylation experiments aimed at quantifying this variability in basal phosphorylation status revealed 27 % variation of 32P incorporation.
Figure 3. Average myofilament lattice spacing as a function of sarcomere length in skinned cardiac trabeculae from NTG and ssTnI-TG hearts (n = 8–10 for each group).
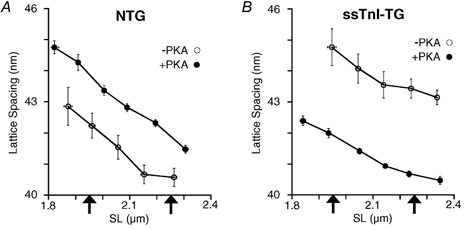
The relationships were obtained by averaging the data into 0.05 μm wide sarcomere length bins. A, relationship between lattice spacing and sarcomere length in NTG trabeculae prior to (○) and following (•) treatment with PKA. B, relationship between lattice spacing and sarcomere length in ssTnITG trabeculae prior to (○) and following (•) treatment with PKA. Each arrow indicates the sarcomere length at which the Ca2+-force relationships were determined.
To quantify the effects of SL on lattice spacing, lattice spacing was determined at precisely the same sarcomere lengths at which the Ca2+ sensitivity of tension was measured (SL = 1.95 and 2.25 μm). The magnitude of the effect of SL change on lattice spacing was defined as the difference in the lattice spacing at these two SLs: ΔLS. ΔLS was similar in untreated NTG (ΔLS = 1.57 ± 0.15 nm) and ssTnI-TG (ΔLS = 1.56 ± 0.24 nm) trabeculae. Furthermore, in NTG hearts, PKA treatment did not alter the effect of SL change on lattice spacing, i.e. ΔLS was similar before and following PKA treatment (ΔLS = 1.54 ± 0.12 nm). However, PKA treatment of ssTnI-TG trabeculae significantly reduced ΔLS (ΔLS = 0.87 ± 0.09 nm) relative to that of untreated ssTnI-TG hearts.
Relationship between Ca2+ sensitivity and interfilament spacing
In an attempt to correlate Ca2+ sensitivity and interfilament spacing (left panels, Fig. 4), Ca2+ sensitivity (bottom panel) and interfilament spacing (top panel) are illustrated in a bar graph at a common sarcomere length (SL = 2.1 μm; intermediate SL within the working range of the heart) in NTG and ssTnI-TG myocardium before and after PKA treatment. PKA treatment in NTG trabeculae increased LS at SL = 2.1 μm from 41.3 ± 0.40 to 42.8 ± 0.15 nm. On the other hand, in ssTnI-TG trabeculae PKA treatment induced a reduction in LS from 43.4 ± 0.49 to 41.1 ± 0.07 nm. Whereas PKA treatment significantly reduced Ca2+ sensitivity in NTG myocytes, PKA treatment did not affect Ca2+ sensitivity in ssTnI-TG cells. In addition, the right panels of Fig. 4 show the relative impact of a change in SL on Ca2+ sensitivity (bottom panel) and lattice spacing (top panel), as indexed by the ΔLS and ΔEC50 parameters before and after treatment with PKA. Treatment with PKA increased length-dependent activation in NTG trabeculae (increased ΔEC50), but did not affect the relative impact of SL on lattice spacing (ΔLS). Treatment of ssTnI-TG skinned trabeculae induced a reduction in ΔLS, but did not affect length-dependent activation (ΔEC50). If Ca2+ sensitivity and the length-dependent changes in Ca2+ sensitivity were directly influenced by changes in interfilament spacing, the relative changes in each Ca2+ sensitivity parameter should be in parallel with changes in interfilament spacing. From the bar graphs in each group, clearly, changes in interfilament spacing with PKA treatment or with length did not always correlate with changes in myofilament Ca2+ sensitivity, in either NTG or ssTnI-TG myocardium.
Figure 4. Correlation between length change, Ca2+ sensitivity and lattice spacing with and without PKA treatment.
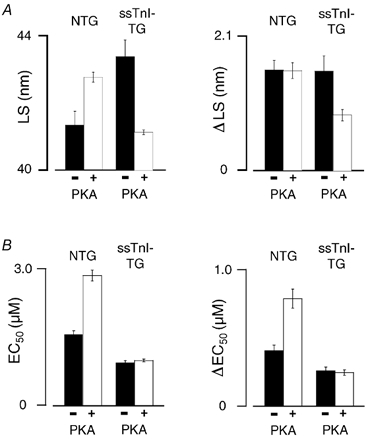
A, comparison of lattice spacing (LS) and the effect of length change on lattice spacing (ΔLS in nm). Left, LS at SL = 2.1 mm in NTG and ssTnI-TG trabeculae treated with or without PKA as indicated. Right, the effect of length change on LS (ΔLS) in NTG and ssTnI-TG trabeculae treated with or without PKA. Error bars are ±s.e.m.B, comparison of Ca2+ sensitivity (EC50 in μmol l−1) and the effect of length change on Ca2+ sensitivity (ΔEC50 in μmol l−1). Left, ΔEC50 at SL = 2.1 mm in NTG and ssTnI-TG trabeculae treated with or without PKA as indicated. Right, the effect of length change on Ca2+ sensitivity (ΔEC50) in NTG and ssTnI-TG trabeculae treated with or without PKA.
DISCUSSION
Myofilament activation requires co-operative and allosteric interactions among bound cross-bridges and thin filament proteins (Bremel & Weber, 1972; Swartz & Moss, 1992; Lehrer, 1994; Lehman et al. 2000). TnI has been shown be an important regulatory component during the macromolecular rearrangement of myofilament proteins that results in myofilament force development. Contractile activation is initiated by binding of Ca2+ to troponin C (Solaro & Rarick, 1998) inducing translocation of tropomyosin (Tm) such that potential cross-bridge binding sites on actin are revealed (Huxley, 1969; Huxley & Simmons, 1971). Strong cross-bridge binding further induces a conformational change in Tm into a third position on actin (Lehman et al. 2000; Tobacman & Butters, 2000). In this study, we provide further evidence that TnI plays an integral role in the signalling cascade during myofilament activation. Furthermore, the central mechanisms underlying TnI action on cardiac myofilament activation did not appear to involve interfilament spacing.
Impact of TnI composition or PKA treatment on Ca2+ sensitivity
It is well established that phosphorylation of the cardiac sarcomere mediated by protein kinase A (PKA) induces a reduction in myofilament Ca2+ sensitivity (Solaro et al. 1976; de Tombe & Stienen, 1995; Janssen & de Tombe, 1997), an observation that was confirmed in the present study. As for the impact of length change on Ca2+-dependent tension development, previous results have been quite variable. A study employing intact electrically stimulated isolated ferret papillary muscle found that treatment with isoprenaline (an agonist of the β-adrenergic pathway) induced an increase in the muscle length dependency of the intracellular [Ca2+]-tension relationship (Komukai & Kurihara, 1997). Others have found either no alteration (van der Velden et al. 2000) or a decrease (Kajiwara et al. 2000) in length-dependent activation following PKA treatment. In the present study, the impact of sarcomere length on myofilament Ca2+ sensitivity following treatment with PKA depended on the choice of indexing parameter. Using the EC50 as the measure of Ca2+ sensitivity, length-dependent activation, calculated as the difference in EC50 at the two sarcomere lengths, was enhanced following PKA treatment. However, length-dependent activation determined when Ca2+ sensitivity was indexed by pCa50 remained unaffected.
As indicated by our study, the choice of parameter to index myofilament Ca2+ sensitivity could potentially affect data interpretation. As it stands, the extent of length dependency has a direct impact on the shape of the length-isometric tension relationship in skinned cardiac fibres and the shape of the end-systolic sarcomere length-tension relationship in intact cardiac fibres (Kentish et al. 1986). Thus, at a given inotropic state, i.e. a single Ca2+ concentration, the magnitude of tension development depends on sarcomere length. Similarly, at a given activation level (Ca2+ concentration), PKA treatment augments isometric tension development following a length change to a greater extent than in muscle fibres in the unphosphorylated state. Since tension is measured at a single concentration, this effect is independent of parameter choice. By reasoning, the use of EC50 more closely illustrates the physiological impact of PKA-dependent phosphorylation on length-dependent activation. It should be noted that the relation between pCa (-log[Ca2+]) and [Ca2+] is non-linear. For these reasons, we believe that the use of the EC50 parameter to index myofilament Ca2+ sensitivity is more appropriate than pCa50. Nevertheless, whether other factors, besides choice of Ca2+ sensitivity parameter, play a role in determining the impact of PKA on length dependency of myofilament activation awaits further study.
The length effect notwithstanding, our present study shows that replacement of cardiac TnI (cTnI) with the slow skeletal isoform (ssTnI) in the heart can impart contractile properties of slow skeletal muscle (Konhilas et al. 2002a), consistent with previous reports from our laboratory (Fentzke et al. 1999; Arteaga et al. 2000). The predominant difference between these two isoforms is the presence of a unique 32 amino acid N-terminus extension in cTnI that contains target sites for PKA-dependent phosphorylation (Solaro & Rarick, 1998). Given that PKA targets both cTnI and cardiac MyBP-C and that PKA treatment did not affect either Ca2+ sensitivity or sarcomere length dependency of Ca2+ in the ssTnI transgenic myocardium lacking the PKA phosphorylation sites on TnI, it is likely that the effects of PKA treatment on myofilament activation are due, solely, to phosphorylation of cTnI and not MyBP-C. Phosphorylation of TnI alters the Ca2+ binding characteristics of TnC (Robertson et al. 1982) and the structural interaction between TnI and TnC (Dong et al. 1997; Finley et al. 1999).
Thus, the changes in Ca2+ sensitivity and length-dependent activation upon structural modification on the N-terminus of TnI may be due to a change in the kinetics of thin filament protein-protein interactions in the molecular steps leading from Ca2+ binding to cross-bridge attachment. Although the impact of PKA-mediated phosphorylation in wild-type (non-transgenic) myocardium is well established, the importance of this N-terminus extension in regulation of the cardiac myofilament activation is not entirely clear. It is clear, however, that interfilament spacing plays, at best, only a minor role (see below). Similarly, the physiological function of MyBP-C in muscle contraction in not entirely clear based on these studies. Still, a role for MyBP-C as a regulator of cardiac contractility, in particular Ca2+ sensitivity, is emerging in the literature (Flavigny et al. 1999; Yang et al. 1999; Kunst et al. 2000; Winegrad, 2000). Finally, the generation of the ssTnI-TG mouse model (Fentzke et al. 1999) has provided an important tool to examine the mechanism of length-dependent activation, and to clarify the role of TnI protein isoform and PKA-dependent phosphorylation of cTnI in imparting the phenotypic differences between slow skeletal muscle and cardiac muscle.
Impact of TnI composition or PKA treatment on interfilament spacing
We also studied the impact of troponin isoform composition and PKA treatment on interfilament spacing by X-ray diffraction techniques. Several observations were apparent from these experiments. First, PKA treatment induced a parallel expansion of the myofilament lattice at all sarcomere lengths in wild-type relaxed skinned trabeculae. That is, phosphorylation by PKA of both cTnI and MyBP-C increased the separation between the thick filaments in equal proportion at each sarcomere length. Second, substitution of cTnI with ssTnI induced a parallel expansion of the myofilament lattice. Third, phosphorylation of MyBP-C, in ssTnI-TG trabeculae, induced a non-parallel contraction of the myofilament lattice such that interfilament spacing became less dependent on sarcomere length following PKA treatment.
Although the factors that determine the separation between thick filaments in the sarcomere are not completely understood, it is clear that electrostatic repulsion of the contractile filaments plays a prominent role in this process (Millman, 1998). It is conceivable that the addition of a negative charge at the N-terminus of cTnI upon PKA treatment induced the expansion of the cardiac myofilament. Alternatively, or in addition, lattice expansion may be due the molecular rearrangement of cTnI that has been observed previously upon phosphorylation by PKA (Chandra et al. 1997).
The molecular mechanism underlying the expansion in skinned ssTnI-TG trabeculae, however, is less clear. The structural differences between thin filaments containing either ssTnI or cTnI have not been defined. There are charge differences between ssTnI (pI = 9.61) and cTnI (pI = 9.87) making the slow skeletal variant a less positively charged protein than the cardiac variant. Cardiac cTnI has 14 positively charged residues in excess of the negatively charged residues, whereas ssTnI has 11. Moreover, there are charge differences in functionally important micro-domains, which we have proposed are important in the relative insensitivity of TG myofilaments containing ssTnI to deactivation by acidic pH (Li et al. 2001; Wolska et al. 2001). For example, one potentially significant amino acid difference in cTnI is at Gln157, which exists as Lys124 in ssTnI. These residues are in a region critical to interaction with TnC. Other significant charge differences exist at Ala164, Glu166 and His173 of cTnI, which are replaced by His131, Val133 and Asn140 in ssTnI. Since it is the outermost net negative charge on the thin filament that is most important in regard to lattice expansion (Millman & Nickel, 1980; Millman & Irving, 1988), removing a relatively small amount of positive charge could have a disproportionately large impact by unmasking negative charge at a relatively high radius on the thin filament. Interfilament spacing is much more sensitive to the charge radius than to the amount of charge (Millman & Nickel, 1980; Millman & Irving, 1988). Hence, without exact knowledge as to the location of the charge, it is difficult to make quantitative predictions regarding the impact on interfilament spacing. Future studies, using specific charge mutants of TnI, could provide insight into the role of specific charge distribution alterations on interfilament spacing.
Perhaps even more difficult to interpret is the non-parallel contraction of the myofilament lattice following PKA treatment of ssTnI-TG trabeculae. Since ssTnI lacks PKA phosphorylation sites, the lattice spacing changes must be attributed to phosphorylation of MyBP-C protein (Garvey et al. 1988; Fentzke et al. 1999). Despite an overall collapse of the myofilament lattice as observed in this study, it has been shown that phosphorylation of MyBP-C extends the myosin heads away from the backbone of isolated thick filaments and increases their apparent structural order (Weisberg & Winegrad, 1996, 1998). In contrast, preliminary analysis of the d10/d11 reflection intensity ratio from our data suggests a shift of mass away from the thin filament following phosphorylation of MyBP-C. Hence, complex thick filament structural changes may have occurred following phosphorylation of MyBP-C under our conditions. In any case, phosphorylation of MyBP-C in the presence of ssTnI on the thin filament appears to inhibit compression of the myofilament lattice upon an increase in sarcomere length. Whether this is purely the result of a structural change, a change in charge distribution, or a combination of both phenomena cannot be determined from the current study. Given the effect of phosphorylation on TnI (Chandra et al. 1997) coupled with the effect of net charge on lattice spacing, it is reasonable to propose that the structural and/or charge change of MyBP-C following PKA-mediated phosphorylation in the presence of a distinct TnI (ssTnI) could have a non-parallel consequence to the myofilament interfilament spacing-sarcomere length relationship.
Interfilament spacing did not correlate with Ca2+ sensitivity (Fig. 4). This result suggests that the contribution of interfilament spacing to length-dependent activation of the cardiac sarcomere is negligible, consistent with our recent study in skinned rat cardiac trabeculae (Konhilas et al. 2002b). The increase in length-dependent activation following PKA treatment indicates that phosphorylation regulates the signalling between thin filament proteins such that transmission of the length signal is promoted, perhaps by a release of a prevailing limitation that may be imposed upon the co-operative influence of cross-bridge attachment. Likewise, the reverse of this same molecular mechanism may cause the reduction of length signal transmission in face of a lack of the N-terminus extension on troponin I.
Implications for the ventricular Frank-Starling law relation
Our results have implications with regard to the slope of the relation between end-systolic pressure (ESP) and end-systolic volume (ESV), which is rooted in length-dependent activation and is a manifestation of the Frank-Starling mechanism. We found that length dependence of activation is a variable function of the state of phosphorylation of cTnI. At the short SL, desensitization following PKA treatment was greater than at longer SL. As a result the ΔEC50 was greater after PKA phosphorylation than before. We (Arteaga et al. 2000) have predicted, on the basis of a simple model relating length-dependent activation to the slope of the ESP-ESV relation, that this ‘disproportional desensitization’ would result in a change in slope. In preliminary experiments (Nowak et al. 2001), we have tested this idea using a conductance catheter and comparing the ESP-ESV relation in ssTnI-TG hearts with NTG hearts beating in situ in the presence of isoprenaline stimulation. In this case, there was a correlation between the changes in length-dependent activation and the ESP-ESV relation.
Limitations
In this study we determined active Ca2+-dependent force development in single skinned myocytes, while interfilament spacing was measured in relaxed skinned cardiac trabeculae. It has been illustrated in skeletal muscle that skinned fibres in a rigor state possess an interfilament spacing that is smaller than that of the same muscle fibre under relaxed conditions (Brenner & Yu, 1985; Kawai et al. 1993). The suggestion is that the measured lattice spacing in relaxed muscle at a particular SL may not functionally be equivalent to that of activated fibres at the same SL. Thus, although a role for interfilament spacing in the regulation of cardiac muscle contraction appears unlikely, it cannot altogether be excluded based on the present study. Differences in Ca2+ activation properties between skinned myocytes and skinned cardiac trabeculae are not likely to have affected our data; we found similar values for Ca2+ sensitivity and length-dependent activation in either skinned papillary or RV trabeculae preparations from either NTG or ssTnI-TG mice (Konhilas et al. 1998; Arteaga et al. 2000). Also, our study employed skinned muscle preparations in all protocols. It has been suggested that length-dependent activation is less pronounced in intact myocardium as compared with skinned myocardium (Komukai & Kurihara, 1997), despite a similar impact of sarcomere length on interfilament spacing (Irving et al. 2000). Hence, there appear to be differences between intact and skinned muscle, such that our results on skinned muscle preparations may not be applicable to intact muscle.
Finally, our data suggest that phosphorylation of either cardiac TnI or MyBP-C may have a large, and possibly opposite, impact on interfilament. Although preliminary studies indicate similar phosphorylation levels of C-protein in NTG and ssTnI-TG animals, we cannot exclude other, compensatory phosphorylation-induced myofilament lattice expansion, as opposed to the lack of the N-terminus extension in ssTnI-TG myocardium. In addition, the small, but variable, level of basal phosphorylation in NTG myocardium may have caused us to overestimate length-dependent activation and Ca2+ sensitivity in NTG myocardium that would have existed in the absence of basal phosphorylation.
However, it is unlikely that this phenomenon would hamper the comparison between NTG and ssTnI-TG Ca2+ sensitivity and length-dependent activation. We have recently observed in preliminary experiments (Smith et al. 2002) that extraction-reconstitution of either cTnI or ssTnI in skinned rat myocardium resulted in differences in myofilament Ca2+ sensitivity and length-dependent activation similar to those reported here and in our previous study (Fentzke et al. 1999). Finally, recent studies indicate additional myofilament targets (titin and myomesin) for PKA-dependent phosphorylation (Obermann et al. 1998; Yamasaki et al. 2002). The studies described in this paper have not addressed the role that these myofilament proteins may play and thus cannot be excluded as potential regulators of the PKA effect.
Conclusions
In the present study, we found that treatment of skinned myocardium with PKA induced a reduction in Ca2+ sensitivity and an increase in sarcomere length dependency of contractile activation. This effect was due, solely, to phosphorylation of cardiac troponin I (cTnI), not cardiac MyBP-C. In addition, complete substitution of cTnI by ssTnI in the cardiac sarcomere induced an increase in Ca2+ sensitivity concomitant with a decrease in sarcomere-length dependency of myofilament activation. Both PKA treatment and TnI isoform composition had a profound and opposite impact on interfilament spacing. Interfilament spacing, however, did not appear to mediate alterations in either myofilament Ca2+ sensitivity or the sarcomere length dependency of myofilament activation.
Acknowledgments
This work was supported in part by the Cardiovascular Sciences Program Training Grant T32 07692 (J. P. Konhilas), a national Grant In Aid from the American Heart Association 9950459N (T. C. Irving), NIH R37 HL 22231 and NIH PO1 HL 62426 (Project 1, R. J. Solaro and Project 4, P. P. de Tombe) and NIH HL 52322 and HL 63704 (P. P. de Tombe). We would like to thank Dr Karyn Bischoff for help with data analysis, and Ron McKinney for assistance with autoradiography. Use of the Advanced Photon Source was supported by the US Department of Energy, Basic Energy Sciences, Office of Energy Research, under Contract No. W-31-109-ENG-38. BioCAT is a US National Institutes of Health-supported Research Center RR08630. Dr de Tombe was an established investigator of the American Heart Association during the time these studies were performed.
REFERENCES
- Arteaga GM, Palmiter KA, Leiden JM, Solaro RJ. Attenuation of length dependence of calcium activation in myofilaments of transgenic mouse hearts expressing slow skeletal troponin I. J Physiol. 2000;526:541–549. doi: 10.1111/j.1469-7793.2000.t01-1-00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backx PH, Gao WD, Azan-Backx MD, Marban E. Mechanism of force inhibition by 2,3-butanedione monoxime in rat cardiac muscle: roles of [Ca2+]i and cross-bridge kinetics. J Physiol. 1994;476:487–500. doi: 10.1113/jphysiol.1994.sp020149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagni MA, Cecchi G, Griffiths PJ, Maeda Y, Rapp G, Ashley CC. Lattice spacing changes accompanying isometric tension development in intact single muscle fibers. Biophys J. 1994;67:1965–1975. doi: 10.1016/S0006-3495(94)80679-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremel RD, Weber A. Cooperation with actin filament in vertebrate skeletal muscle. Nat New Biol. 1972;238:97–101. doi: 10.1038/newbio238097a0. [DOI] [PubMed] [Google Scholar]
- Brenner B, Yu LC. Equatorial x-ray diffraction from single skinned rabbit psoas fibers at various degrees of activation. Changes in intensities and lattice spacing. Biophys J. 1985;48:829–834. doi: 10.1016/S0006-3495(85)83841-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazorla O, Wu Y, Irving TC, Granzier H. Titin-based modulation of calcium sensitivity of active tension in mouse skinned cardiac myocytes. Circ Res. 2001;88:1028–1035. doi: 10.1161/hh1001.090876. [DOI] [PubMed] [Google Scholar]
- Chandra M, Dong W, Pan B, Cheung HC, Solaro RJ. Effects of protein kinase A phosphorylation on signaling between cardiac troponin I and the N-terminal domain of cardiac troponin C. Biochemistry. 1997;36:13305–13311. doi: 10.1021/bi9710129. [DOI] [PubMed] [Google Scholar]
- de Tombe PP, Stienen GJM. Protein kinase A does not alter economy of force maintenance in skinned cardiac trabeculae. Circ Res. 1995;76:734–741. doi: 10.1161/01.res.76.5.734. [DOI] [PubMed] [Google Scholar]
- de Windt LJ, Willems J, Reneman RS, Van der Vusse GJ, Arts T, Van Bilsen M. An improved isolated, left ventricular ejecting, murine heart model. Functional and metabolic evaluation. Pflugers Arch. 1999;437:182–190. doi: 10.1007/s004240050767. [DOI] [PubMed] [Google Scholar]
- Dong W, Chandra M, Xing J, She M, Solaro RJ, Cheung HC. Phosphorylation-induced distance change in a cardiac muscle troponin I mutant. Biochemistry. 1997;36:6754–6761. doi: 10.1021/bi9622276. [DOI] [PubMed] [Google Scholar]
- Fan D, Wannenburg T, De Tombe PP. Decreased myocyte tension development and calcium responsiveness in rat right ventricular pressure overload. Circulation. 1997;95:2312–2317. doi: 10.1161/01.cir.95.9.2312. [DOI] [PubMed] [Google Scholar]
- Fentzke RC, Buck SH, Patel JR, Lin H, Wolska BM, Stojanovic MO, Martin AF, Solaro RJ, Moss RL, Leiden JM. Impaired cardiomyocyte relaxation and diastolic function in transgenic mice expressing slow skeletal troponin I in the heart. J Physiol. 1999;517:143–157. doi: 10.1111/j.1469-7793.1999.0143z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley N, Abbott MB, Abusamhadneh E, Gaponenko V, Dong W, Gasmi-Seabrook G, Howarth JW, Rance M, Solaro RJ, Cheung HC, Rosevear PR. NMR analysis of cardiac troponin C- troponin I complexes: effects of phosphorylation. FEBS Lett. 1999;453:107–112. doi: 10.1016/s0014-5793(99)00693-6. [DOI] [PubMed] [Google Scholar]
- Flavigny J, Souchet M, Sebillon P, Berrebi-Bertrand I, Hainque B, Mallet A, Bril A, Schwartz K, Carrier L. COOH-terminal truncated cardiac myosin-binding protein C mutants resulting from familial hypertrophic cardiomyopathy mutations exhibit altered expression and/or incorporation in fetal rat cardiomyocytes. J Mol Biol. 1999;294:443–456. doi: 10.1006/jmbi.1999.3276. [DOI] [PubMed] [Google Scholar]
- Fuchs F, Wang YP. Sarcomere length versus interfilament spacing as determinants of cardiac myofilament Ca2+ sensitivity and Ca2+ binding. J Mol Cell Cardiol. 1996;28:1375–1383. doi: 10.1006/jmcc.1996.0129. [DOI] [PubMed] [Google Scholar]
- Garvey JL, Kranias EG, Solaro RJ. Phosphorylation of C-protein, troponin I and phospholamban in isolated rabbit hearts. Biochem J. 1988;249:709–714. doi: 10.1042/bj2490709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt RE, Maughan DW. Influence of osmotic compression on calcium activation and tension in skinned muscle fibers of the rabbit. Pflugers Arch. 1981;391:334–337. doi: 10.1007/BF00581519. [DOI] [PubMed] [Google Scholar]
- Gupta RC, Neumann J, Boknik P, Watanabe AM. M2-specific muscarinic cholinergic receptor-mediated inhibition of cardiac regulatory protein phosphorylation. Am J Physiol. 1994;266:H1138–1144. doi: 10.1152/ajpheart.1994.266.3.H1138. [DOI] [PubMed] [Google Scholar]
- Hammersley AP. FIT2DV9.129 Reference Manual Version 3.1. Grenoble, France: European Synchrotron Radiation Facility; 1998. [Google Scholar]
- Huxley AF, Simmons RM. Proposed mechanism of force generation in striated muscle. Nature. 1971;233:533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Huxley HE. The mechanism of muscular contraction. Science. 1969;164:1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- Irving TC, Konhilas JP, Perry D, Fischetti R, de Tombe PP. Myofilament lattice spacing as a function of sarcomere length in isolated rat myocardium. Am J Physiol Heart Circ Physiol. 2000;279:H2568–2573. doi: 10.1152/ajpheart.2000.279.5.H2568. [DOI] [PubMed] [Google Scholar]
- Janssen PML, de Tombe PP. Protein kinase A does not alter unloaded velocity of sarcomere shortening in skinned rat cardiac trabeculae. Am J Physiol. 1997;273:H2415–2422. doi: 10.1152/ajpheart.1997.273.5.H2415. [DOI] [PubMed] [Google Scholar]
- Kajiwara H, Morimoto S, Fukuda N, Ohtsuki I, Kurihara S. Effect of troponin I phosphorylation by protein kinase A on length-dependence of tension activation in skinned cardiac muscle fibers. Biochem Biophys Res Commun. 2000;272:104–110. doi: 10.1006/bbrc.2000.2741. [DOI] [PubMed] [Google Scholar]
- Kawai M, Wray JS, Zhao Y. The effect of lattice spacing change on cross-bridge kinetics in chemically skinned rabbit psoas muscle fibers: I. Proportionality between the lattice spacing and the fiber width. Biophys J. 1993;64:187–196. doi: 10.1016/S0006-3495(93)81356-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentish JC, McCloskey DT, Layland J, Palmer S, Leiden JM, Martin AF, Solaro RJ. Phosphorylation of troponin i by protein kinase a accelerates relaxation and crossbridge cycle kinetics in mouse ventricular muscle. Circ Res. 2001;88:1059–1065. doi: 10.1161/hh1001.091640. [DOI] [PubMed] [Google Scholar]
- Kentish JC, ter Keurs HE, Ricciardi L, Bucx JJ, Noble MI. Comparison between the sarcomere length-force relations of intact and skinned trabeculae from rat right ventricle. Influence of calcium concentrations on these relations. Circ Res. 1986;58:755–768. doi: 10.1161/01.res.58.6.755. [DOI] [PubMed] [Google Scholar]
- Komukai K, Kurihara S. Length dependence of Ca(2+)-tension relationship in aequorin-injected ferret papillary muscles. Am J Physiol. 1997;273:H1068–1074. doi: 10.1152/ajpheart.1997.273.3.H1068. [DOI] [PubMed] [Google Scholar]
- Konhilas JP, Irving TC, de Tombe PP. Length-dependent activation in three striated muscle types of the rat. J Physiol. 2002a;544:225–236. doi: 10.1113/jphysiol.2002.024505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konhilas JP, Irving TC, de Tombe PP. Myofilament calcium sensitivity in skinned rat cardiac trabeculae: role of interfilament spacing. Circ Res. 2002b;90:59–65. doi: 10.1161/hh0102.102269. [DOI] [PubMed] [Google Scholar]
- Konhilas JP, Wolska B, Martin AF, Solaro RJ, de Tombe PP. PKA modulates length-dependent activation in murine myocardium. Biophys J. 2000;78:108A. doi: 10.1113/jphysiol.2002.038117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konhilas JP, Wolska BM, Martin AF, Palmiter KA, Leiden JM, Fentzke R, Solaro RJ, de Tombe PP. Troponin I composition modulates length-dependent activation in murine myocardium. Circulation. 1998;98:I-836. [Google Scholar]
- Kranias EG. Regulation of calcium transport by protein phosphatase activity associated with cardiac sarcoplasmic reticulum. J Biol Chem. 1985;260:11006–11010. [PubMed] [Google Scholar]
- Kunst G, Kress KR, Gruen M, Uttenweiler D, Gautel M, Fink RH. Myosin binding protein C, a phosphorylation-dependent force regulator in muscle that controls the attachment of myosin heads by its interaction with myosin S2. Circ Res. 2000;86:51–58. doi: 10.1161/01.res.86.1.51. [DOI] [PubMed] [Google Scholar]
- Lehman W, Hatch V, Korman V, Rosol M, Thomas L, Maytum R, Geeves MA, Van Eyk JE, Tobacman LS, Craig R. Tropomyosin and actin isoforms modulate the localization of tropomyosin strands on actin filaments. J Mol Biol. 2000;302:593–606. doi: 10.1006/jmbi.2000.4080. [DOI] [PubMed] [Google Scholar]
- Lehrer SS. The regulatory switch of the muscle thin filament: Ca2+ or myosin heads? J Muscle Res Cell Motil. 1994;15:232–236. doi: 10.1007/BF00123476. [DOI] [PubMed] [Google Scholar]
- Luo W, Grupp IL, Harrer J, Ponniah S, Grupp G, Duffy JJ, Doetschman T, Kranias EG. Targeted ablation of the phospholamban gene is associated with markedly enhanced myocardial contractility and loss of beta-agonist stimulation. Circ Res. 1994;75:401–409. doi: 10.1161/01.res.75.3.401. [DOI] [PubMed] [Google Scholar]
- McDonald KS, Moss RL. Osmotic compression of single cardiac myocytes eliminates the reduction in Ca2+ sensitivity of tension at short sarcomere length. Circ Res. 1995;77:199–205. doi: 10.1161/01.res.77.1.199. [DOI] [PubMed] [Google Scholar]
- Millman BM. The filament lattice of striated muscle. Physiol Rev. 1998;78:359–391. doi: 10.1152/physrev.1998.78.2.359. [DOI] [PubMed] [Google Scholar]
- Millman BM, Irving TC. Filament lattice of frog striated muscle. Radial forces, lattice stability, and filament compression in the A-band of relaxed and rigor muscle. Biophys J. 1988;54:437–447. doi: 10.1016/S0006-3495(88)82977-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millman BM, Nickel BG. Electrostatic forces in muscle and cylindrical gel systems. Biophys J. 1980;32:49–63. doi: 10.1016/S0006-3495(80)84915-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak G, Pena JR, Arteaga GM, Geenen DL, Pieples K, Wieczorek DF, Solaro RJ, Wolska BM. Correlations between alterations in length-dependent Ca2+ activation of cardiac myofilaments and the end systolic pressure-volume relation. Circulation. 2001;104:1507. doi: 10.1007/s10974-008-9136-y. [DOI] [PubMed] [Google Scholar]
- Obermann WM, van der Ven PF, Steiner F, Weber K, Furst DO. Mapping of a myosin-binding domain and a regulatory phosphorylation site in M-protein, a structural protein of the sarcomeric M band. Mol Biol Cell. 1998;9:829–840. doi: 10.1091/mbc.9.4.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SP, Johnson JD, Holroyde MJ, Kranias EG, Potter JD, Solaro RJ. The effect of troponin I phosphorylation the Ca2+-binding properties of the Ca2+-regulatory site of bovine cardiac troponin. J Biol Chem. 1982;257:260–263. [PubMed] [Google Scholar]
- Rome E. X-ray diffraction studies of the filament lattice of striated muscle in various bathing media. J Mol Biol. 1968;37:331–344. doi: 10.1016/0022-2836(68)90272-6. [DOI] [PubMed] [Google Scholar]
- Ross J, Jr, Sobel BE. Regulation of cardiac contraction. Ann Rev Physiol. 1972;34:47–90. doi: 10.1146/annurev.ph.34.030172.000403. [DOI] [PubMed] [Google Scholar]
- Smith SH, Versluis JP, Martin AF, Solaro RJ, de Tombe PP. Role of troponin I in the sarcomere length dependence of calcium sensitivity in skinned rat trabeculae. Circulation. 2002;106:101–102. [Google Scholar]
- Solaro RJ. Control mechanisms regulating contractile activity of cardiac myofilaments. In: Sperelakis N, editor. Physiology and Pathophysiology of the Heart. Boston: Kluwer Publishers; 1995. pp. 355–365. [Google Scholar]
- Solaro RJ, Moir AJG, Perry SV. Phosphorylation of troponin I and the inotropic effect of adrenaline in the perfused rabbit heart. Nature. 1976;262:615–617. doi: 10.1038/262615a0. [DOI] [PubMed] [Google Scholar]
- Solaro RJ, Rarick HM. Troponin and tropomyosin: proteins that switch on and tune in the activity of cardiac myofilaments. Circ Res. 1998;83:471–480. doi: 10.1161/01.res.83.5.471. [DOI] [PubMed] [Google Scholar]
- Solaro RJ, Van Eyk J. Altered interactions among thin filament proteins modulate cardiac function. J Mol Cell Cardiol. 1996;28:217–230. doi: 10.1006/jmcc.1996.0021. [DOI] [PubMed] [Google Scholar]
- Swartz DR, Moss RL. Influence of a strong-binding myosin analogue on calcium-sensitive mechanical properties of skinned skeletal muscle fibers. J Biol Chem. 1992;267:20497–20506. [PubMed] [Google Scholar]
- ter Keurs HE, Rijnsburger WH, van Heuningen R, Nagelsmit MJ. Tension development and sarcomere length in rat cardiac trabeculae. Evidence of length-dependent activation. Circ Res. 1980;46:703–714. doi: 10.1161/01.res.46.5.703. [DOI] [PubMed] [Google Scholar]
- Tobacman LS, Butters CA. A new model of cooperative myosin-thin filament binding. J Biol Chem. 2000;275:27587–27593. doi: 10.1074/jbc.M003648200. [DOI] [PubMed] [Google Scholar]
- van der Velden J, de Jong JW, Owen VJ, Burton PB, Stienen GJ. Effect of protein kinase A on calcium sensitivity of force and its sarcomere length dependence in human cardiomyocytes. Cardiovasc Res. 2000;46:487–495. doi: 10.1016/s0008-6363(00)00050-x. [DOI] [PubMed] [Google Scholar]
- Weisberg A, Winegrad S. Alteration of myosin cross bridges by phosphorylation of myosin-binding protein C in cardiac muscle. Proc Natl Acad Sci U S A. 1996;93:8999–9003. doi: 10.1073/pnas.93.17.8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg A, Winegrad S. Relation between crossbridge structure and actomyosin ATPase activity in rat heart. Circ Res. 1998;83:60–72. doi: 10.1161/01.res.83.1.60. [DOI] [PubMed] [Google Scholar]
- Winegrad S. Myosin binding protein C, a potential regulator of cardiac contractility. Circ Res. 2000;86:6–7. doi: 10.1161/01.res.86.1.6. [DOI] [PubMed] [Google Scholar]
- Wolska BM, Stojanovic MO, Luo W, Kranias EG, Solaro RJ. Effect of ablation of phospholamban on dynamics of cardiac myocyte contraction and intracellular Ca2+ Am J Physiol. 1996;271:C391–397. doi: 10.1152/ajpcell.1996.271.1.C391. [DOI] [PubMed] [Google Scholar]
- Wolska BM, Vijayan K, Arteaga GM, Konhilas JP, Phillips RM, Kim R, Naya T, Leiden JM, Martin AF, de Tombe PP, Solaro RJ. Expression of slow skeletal troponin I in adult transgenic mouse heart muscle reduces the force decline observed during acidic conditions. J Physiol. 2001;536:863–870. doi: 10.1111/j.1469-7793.2001.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki R, Wu Y, McNabb M, Greaser M, Labeit S, Granzier H. Protein kinase A phosphorylates titin's cardiac-specific N2B domain and reduces passive tension in rat cardiac myocytes. Circ Res. 2002;90:1181–1188. doi: 10.1161/01.res.0000021115.24712.99. [DOI] [PubMed] [Google Scholar]
- Yang Q, Sanbe A, Osinska H, Hewett TE, Klevitsky R, Robbins J. In vivo modeling of myosin binding protein C familial hypertrophic cardiomyopathy. Circ Res. 1999;85:841–847. doi: 10.1161/01.res.85.9.841. [DOI] [PubMed] [Google Scholar]


