Abstract
The purpose of this investigation was to examine the activation (phosphorylation) and total protein content of MAPK signalling cascade proteins (ERK 1/2, p90RSK, Mnk 1, eIF4E, p38 MAPK, JNK/SAPK, MKP 1) at rest and following exercise, in sedentary young and old men. Eight young (22 ± 1 years; YM) and eight old (79 ± 3 years; OM) men underwent a resting muscle biopsy of the vastus lateralis; they then performed a knee extensor resistance exercise session (29 contractions at ∼70 % of max), followed by a post-exercise biopsy. Western immunoblot analysis demonstrated that the OM had higher resting phosphorylation of ERK 1/2, p90RSK, Mnk 1, p38 MAPK and JNK/SAPK proteins versus YM (P < 0.05). The resistance exercise bout caused an increase in phosphorylation of the ERK 1/2, p90RSK and Mnk 1 proteins (P < 0.05) in the YM. Conversely, the OM had a decrease in ERK 1/2, p90RSK, Mnk 1, p38 MAPK and JNK/SAPK phosphorylation (P < 0.05) after the exercise bout. Neither group showed a change in eIF4E phosphorylation. The total amount of protein expression of the MAPK signalling proteins was not different between the YM and OM, except that there was a higher (P < 0.05) MKP 1 protein content in the OM. This investigation is the first to provide evidence that MAPK proteins are differentially activated at rest and in response to a bout of resistance exercise in skeletal muscle of young and old men. These findings may have implications for other processes (e.g. transcription and translation) involved in skeletal muscle type and growth, when examining the changes occurring with ageing muscle before and after resistance exercise/training.
Ageing is associated with a decline in skeletal muscle mass (Lexell et al. 1983) and function (Larsson et al. 1979). Progressive resistance training (PRT) has been used to increase the strength and size of skeletal muscle in older individuals (Frontera et al. 1988; Fiatarone et al. 1990), regardless of change in fibre type, thereby attenuating these age-associated decrements. Recently, our laboratory reported that single muscle fibre myosin heavy chain (MHC) distribution and function can be altered in older men with 12 weeks of PRT (Trappe et al. 2000; Williamson et al. 2000) in an age-dependent manner (Williamson et al. 2001). Therefore there appear to be different mechanisms among young and old adults that are underlying these skeletal muscle adaptations to resistance training.
Although it has not yet been determined how muscle contraction actually regulates signalling proteins and genes, several studies have characterized some of the signalling proteins that are associated with contractile activity and protein synthesis of skeletal muscle before, during and after exercise (Goodyear et al. 1996; Baar & Esser, 1999; Hernandez et al. 2000; Krook et al. 2000). More pertinent to exercise and skeletal muscle morphology are the mitogen-activated protein kinase (MAPK) pathways (Aronson et al. 1997; Widegren et al. 1998, 2000). More specifically, the p42/44 MAPK pathway, also known as extracellular signal-regulated kinase (ERK) 1/2, has a direct effect on muscle cells (Gredinger et al. 1998; Zester et al. 1999), activating several myogenic transcription factors. The ERK 1/2 pathway can activate several substrates, such as p90RSK (p90 ribsosomal S6 kinase), leading to the activation of transcription factors and the ribosomal subunit S6. ERK 1/2 can also activate kinases associated with protein translation such as Mnk 1 (MAPK-interacting kinase 1) and its downstream substrate, eukaryotic initiation factor 4E (eIF4E). Other neighbouring MAPKs, such as p38 MAPK and JNK/SAPK (c-Jun N-terminal and kinase/ stress-activated protein kinases, respectively), are also activated by contraction, exercise and stress, subsequently increasing c-Jun and c-fos activation. Acute bouts of cycle ergometry have been reported to cause increases in signalling intermediates from the ERK 1/2, p38 MAPK and JNK/SAPK pathways (Aronson et al. 1997; Widegren et al. 1998; Osman et al. 2000). Also, the ERK 1/2 pathway has been implicated in the differentiation of slow muscle fibres, similar to slow-type nerve activity (Murgia et al. 2000). Therefore, the interplay of these pathways during and after muscle contraction is important for muscle cell survival, proliferation, differentiation and/or adaptation. Furthermore, signalling pathways have not been investigated in ageing human skeletal muscle under resting conditions nor after a bout of resistance exercise.
The purpose of this investigation was to examine differences, under resting conditions and after a bout of resistance exercise, in the total amount and the activation (phosphorylation) of the MAPK signalling cascade proteins. We tested the following hypotheses in young (18–30 years) and old men (70–80 years) before and after one bout of resistance exercise: (1) the acute bout of resistance exercise would cause an increase in the phosphorylation of MAPK pathway proteins in the young and old over resting conditions; (2) the magnitude of change in phosphorylation of the MAPK pathway proteins would be greater in the young men compared to the old, following the bout of resistance exercise; (3) the protein content of the MAPK pathway proteins would be higher in the young men compared to the old. This study allowed us to quantify and qualify a well-characterized, ‘exercise’-activated signalling pathway in younger and older human skeletal muscle, using a resistance exercise stimulus for signalling protein activation.
METHODS
Subjects
Sixteen healthy, young (YM; n = 8) and old (OM; n = 8) men (see Table 1) were recruited from the community to serve as subjects in this investigation. The criteria for volunteers included non-exercising (aerobic, resistance or other within the last year), non-obese (body mass index (BMI) < 26 kg m−2), non-smoking, normotensive, healthy men. All potential subjects underwent a telephone interview, followed by a visit to the laboratory. This visit included a medical history and an interview with a physician, complete physical examination and a graded stress test (12-lead electrocardiogram) performed on a treadmill (OM only). Prior to the screening, all potential subjects were informed of all procedures and risks associated with the screening, testing and training. Informed consent was obtained from each volunteer, which was approved by the Institutional Review Boards of Ball State University and Ball Memorial Hospital. The study was in accordance with the Declaration of Helsinki.
Table 1.
Subject characteristics and whole muscle strength
| Young men | Old men | |
|---|---|---|
| Age (years) | 21.8 ± 0.9 | 79.1 ± 3.3 |
| Height (cm) | 183.6 ± 2.3 | 173.2 ± 2.6 |
| Weight (kg) | 82.9 ± 2.5 | 80.1 ± 5.6 |
| l-RM (kg) | 59.7 ± 2.0* | 29.8 ± 2.1 |
| Reps session−1 | 29 ± 1 | 29 ± 1 |
| %1-RM | 68 ± 1 | 67 ± 1 |
| Total work (kg) | 1187 ± 59* | 583 ± 48 |
Data are expressed as means ±s.e.m. 1-RM = one-repetition maximum; Reps session−1= repetitions per exercise bout; % 1-RM = percentage of 1-RM maintained throughout the 3 sets; Total work = amount lifted (kg) × repetitions performed.
Significant age effect.
Study overview
The protocol for this investigation consisted of an assessment of one-repetition maximum (1-RM) and one resistance exercise session. We ensured that the subject was properly prepared for the exercise by implementing a warm-up of the legs on a cycle ergometer (Met 100, Cybex, NY, USA) for ∼5 min at a low level of intensity (25 W). This was followed by some light stretching of the lower limbs and back (∼5 min). Each subject, after being cleared by a physician to participate in the study, underwent: (1) an assessment of 1-RM (3–5 days prior to the exercise session); (2) rest and post-exercise biopsies of the exercise leg (same day as the exercise session); (3) a unilateral resistance exercise session. The muscle samples were then analysed for phosphorylation state and/or total protein content of MAPK pathway proteins by immunoblot analysis.
Resistance exercise protocol
The 1-RM session
Unilateral isotonic muscle strength was assessed using a modified bilateral knee extensor device (Cybex Eagle) 3–5 days prior to the exercise session. The 1-RM was assessed by increasing the weight by half (2.88 kg) or full plates (5.75 kg) with each successive full unilateral knee extension. The test continued until the subject was not able to maintain proper form and/or fully extend the exercise leg at the given weight. These procedures have been used by this laboratory in previous studies (Trappe et al. 2000, 2001; Williamson et al. 2000, 2001).
The resistance exercise session
Subjects performed 3 sets of 10 repetitions (unilateral knee extensions) at 70 % of 1-RM, with 3 min rest between each set. The exercise involved a concentric and an eccentric component, in which the subject was required to lift the weight to full knee extension (concentric) and lower the weight to the starting position (eccentric). Each component (concentric and eccentric) lasted ∼2 s. The goal of this protocol was to replicate an initial session that a subject would undergo during a 12 week PRT programme at our laboratory (Frontera et al. 1988; Fiatarone et al. 1990; Trappe et al. 2000, 2001; Williamson et al. 2000, 2001).
Muscle biopsy
Needle biopsy samples (∼100 mg) were taken from the vastus lateralis muscle (Bergstom, 1962) prior to and immediately after (within 10–20 s) the resistance exercise from the right leg (exercise), using separate incisions that were ∼5 cm apart (the post-exercise biopsy was proximal to the first biopsy) (Aronson et al. 1998). The muscle biopsies were preceded by a 12 h fast (typical start time was ∼08:00 h). Once the subjects arrived at the laboratory, they reclined for 30 min. The biopsy area was then anaesthetized with 1 % xylocaine (∼1 ml; without adrenaline; Aronson et al. 1998). Approximately 5 min later, an incision was made and a 5 mm biopsy needle was inserted into the muscle belly for sampling. The muscle sample was immediately divided into longitudinal sections, and then frozen in liquid nitrogen for future analysis. Immediately following the last contraction of the third set, a post-exercise biopsy sample was taken within 10–20 s of the last contraction, from a separate incision (described above).
Western blot analysis
Muscle from the biopsy sample was homogenized in a buffer (1:10) containing (mm): 10 MgCl2, 10 KH2PO4, 1 EDTA, 5 EGTA, 50 β-glycerolphosphate and 10 okadaic acid; (μg ml−1): 10 phenylmethylsulfonyl fluoride, 10 leupeptin, 10 aprotinin and 10 Na3VO4; and 1 % Nonidet NP-40. The homogenates were then gently rocked for 1 h at 4 °C, followed by clarification of the sample (centrifugation at 13 000 g, 4 °C for 30 min). The supernatant was recovered and stored at −80 °C for future analysis. Protein concentrations were determined on the homogenates by a protein assay kit (Sigma, St Louis, MO, USA), using a bovine serum albumin (BSA) standard in a 96-well plate and reader (Wallac Victor 2, Boston, MA, USA).
Immunoblot analysis was performed to determine p44/42 MAPK (ERK 1/2), p90RSK, Mnk 1, eIF4E, p38 MAPK, JNK/SAPK and MKP 1 (total protein content only) phosphorylation states and protein content. First, aliquots for ERK 1/2 (30 μg), p90RSK (100 μg), Mnk 1 (100 μg), eIF4E (30 μg), p38 MAPK (30 μg), JNK/SAPK (30 μg) and MKP 1 (30 μg), were boiled for 3 min in an equal amount of sample buffer (1 % SDS, 6 mg ml−1 EDTA, 0.06 m Tris (hydroxymethyl) aminomethane (pH 6.8), 2 mg ml−1 bromophenol blue, 15 % glycerol and 5 %β-mercaptoethanol), then resolved by 8 % (p90RSK only), 10 % or 15 % (eIF4E only) SDS-PAGE (Laemmli, 1970). Resting and post-exercise samples from each individual were loaded on the same gel to account for any possible variation between blots.
The electrophoretically separated proteins were then transferred (Idea Genie, Idea Scientific, Minneapolis, MN, USA) to a nitrocellulose membrane (Hybond ECL, Amersham Pharmacia Biotech, Piscatway, NJ, USA) over a 1 h period (12 V, ∼0.5 A, using the Towbin transfer buffer, Towbin et al. 1979), blocked with 5 % milk in Tris-buffered saline (pH 7.6) and 0.1 % Tween-20 (TBST) for 1 h (gently rocking at room temperature) and then probed with corresponding antibodies overnight (gently rocking in a heat-sealed bag at 4 °C). Phospho-specific antibodies for ERK 1/2 (1:1000; no. 9101), p90RSK (1:1000; no. 9346), Mnk 1 (1:1000; no. 2111), eIF4E (1:1000; no. 9741), p38 MAPK (1:1000; no. 9216) and JNK/SAPK (1:1000; no. 9251) were used to detect the phosphorylation state of each protein (New England Biolabs, Beverly, MA, USA). The primary antibodies were added to 30 ml of 5 % BSA or 5 % non-fat dry milk in TBST.
The blot was rinsed once in TBST, then incubated in the secondary antibody for 1 h at room temperature (40 ml of 5 % non-fat dry milk in TBST). Anti-rabbit-, anti-mouse-, anti-goat-conjugated horseradish peroxidase (HRP) secondary antibodies were used (1:2000; no. 7074, no. 7076 and no. sc-2020; New England Biolabs and Santa Cruz Biotechnology, Santa Cruz, CA, USA). The secondary antibody was removed and the blots were rinsed in TBST (4 × 10 min). Enhanced chemiluminescence (ECL) (ECL Western reagents and Amersham Pharmacia Biotech) was performed by adding equal amounts of the reagents, then incubating the blot in this mixture for 1 min. Excess reagent was removed and the blot was wrapped in plastic for exposure to X-ray film (Biomax Light-Kodak, Fisher Scientific). Multiple exposures were made to ensure the desired intensity was achieved. Sizes of the immunodetected proteins were verified by standard molecular weight and control proteins (New England Biolabs).
Following ECL, the membranes were stripped of antibodies by incubation in 1 × Re-Probe (Geno Tech, St Louis, MO, USA) for 30 min and then rinsed 3 × 5 min with TBST. The blots were blocked (see above), then hybridized with the following antibodies for the total amount of the respective protein: ERK 1/2 (1:1000; no. 9102), p90RSK (1:1000; no. 9342), Mnk 1 (1:1000; no. sc-6965), eIF4E (1:1000; no. 9201), p38 MAPK (1:1000; no. 9212), JNK/SAPK (1:1000; no. 9252) and MKP 1 (1:1000, no. sc-1102) (New England Biolabs and Santa Cruz Biotechnology; same procedure as above). Blots were also stained with Ponceau S (Sigma) to verify equal loading and transfer. X-ray film was quantified for the total and phosphorylated forms of the protein by a computer imaging system (Alpha Innotech, ChemImager 4000, San Leandro, CA, USA).
Statistics
Data are expressed as means ±s.e.m. Comparisons of resting versus post-exercise ERK 1/2, p90RSK, Mnk 1, eIF4E, p38 MAPK, JNK/SAPK and MKP 1 (total and phosphorylated), in the YM and OM at rest and post-exercise, were made using a general linear model (ANOVA), repeated measures design (Bonferroni post hoc analysis) (SPSS v.10.0.5). Whole muscle strength results were also analysed using Student's t test (SPSS v.10.0.5). The significance level was set at P < 0.05.
RESULTS
Rest
Under resting conditions, the skeletal muscle of older men (OM) displayed higher phosphorylation of several mitogen- (MAPK) and stress-activated protein kinase (p38 MAPK and JNK/SAPK) pathway proteins when compared to the young (YM). The ERK 1/2 pathway appeared to be more activated (OM versus YM), with higher (P < 0.05) phosphorylation of ERK 1/2 (131 %; Fig. 1) and its substrates, p90RSK (248 %; Fig. 2) and Mnk 1 (74 %; Fig. 3). The activation of the substrate of Mnk 1, eIF4E, was not different between the YM and OM (Fig. 4). Somewhat less than the activation of the ERK 1/2 pathway, p38 MAPK and JNK/SAPK were only 15 % (Fig. 5) and 25 % (Fig. 6) higher (P < 0.05) in the OM compared to the YM.
Figure 1. Effects of one bout of resistance exercise on ERK 1/2 phosphorylation in young (YM) and old (OM) men.
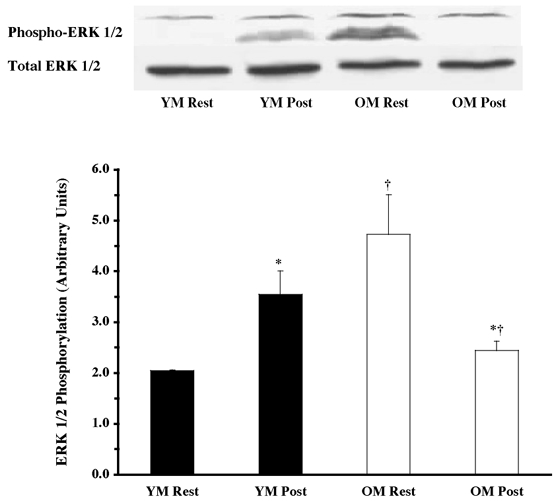
The ERK 1/2 phosphorylation state was determined from vastus lateralis samples (at rest and post-exercise) using Western blot analysis with antibodies specific for phosphorylation of ERK 1/2 (Thr202 and Tyr204). Equal loading was verified by Ponceau S staining. Data are expressed as arbitrary units (means ±s.e.m.). *P < 0.05 vs. resting. †P < 0.05 vs. age.
Figure 2. Effects of one bout of resistance exercise on p90RSK phosphorylation in YM and OM.
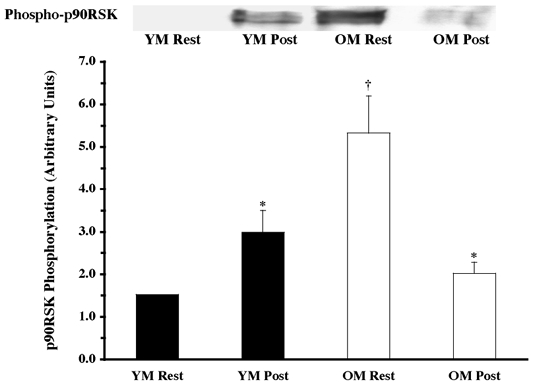
The p90RSK phosphorylation state was determined from vastus lateralis samples (at rest and post-exercise) using Western blot analysis with antibodies specific for p90RSK phosphorylation (Thr573). Equal loading was verified by Ponceau S staining. Data are expressed as arbitrary units (means ±s.e.m.). *P < 0.05 vs. resting. †P < 0.05 vs. age.
Figure 3. Effects of one bout of resistance exercise on Mnk 1 phosphorylation in YM and OM.
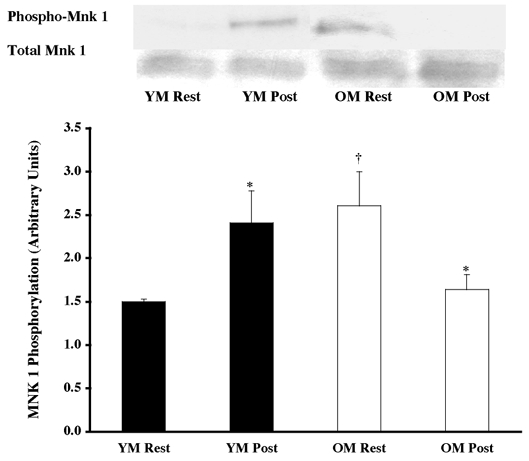
The Mnk 1 phosphorylation state was determined from vastus lateralis samples (at rest and post-exercise) using Western blot analysis with antibodies specific for Mnk 1 phosphorylation (Thr197 and Thr202). Equal loading was verified by Ponceau S staining. Data are expressed as arbitrary units (means ±s.e.m.). *P < 0.05 vs. resting. †P < 0.05 vs. age.
Figure 4. Effects of one bout of resistance exercise on eIF4E phosphorylation in YM and OM.
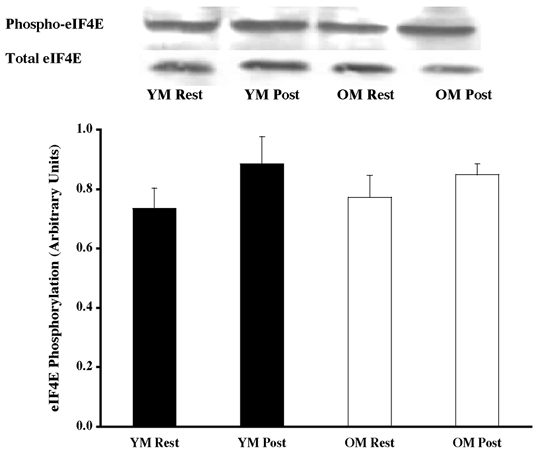
The eIF4E phosphorylation state was determined from vastus lateralis samples (at rest and post-exercise) using Western blot analysis with antibodies specific for eIF4E phosphorylation (Ser209). Equal loading was verified by Ponceau S staining. Data are expressed as arbitrary units (means ±s.e.m.).
Figure 5. Effects of one bout of resistance exercise on p38 MAPK phosphorylation in YM and OM.
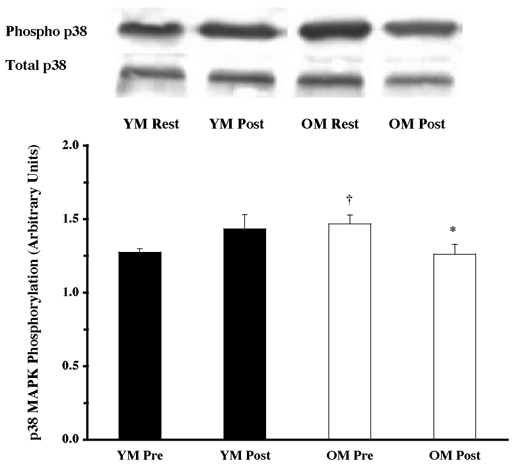
The p38 MAPK phosphorylation state was determined from vastus lateralis samples (at rest and post-exercise) using Western blot analysis with antibodies specific for p38 MAPK phosphorylation (Thr180 and Tyr182). Data are expressed as arbitrary units (means ±s.e.m.). *P < 0.05 vs. resting. †P < 0.05 vs. age.
Figure 6. Effects of one bout of resistance exercise on JNK/SAPK phosphorylation in YM and OM.
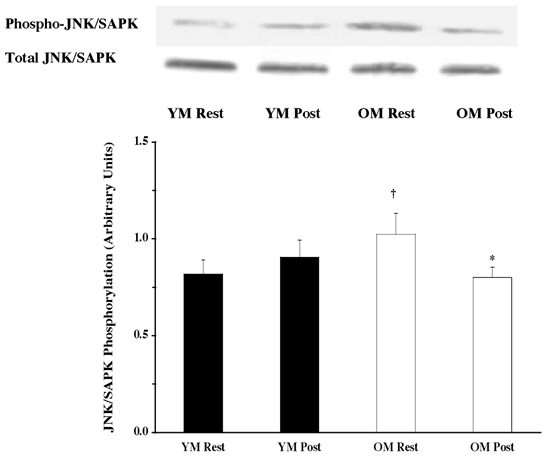
The JNK/SAPK phosphorylation state was determined from vastus lateralis (at rest and post-exercise) using Western blot analysis with antibodies specific for JNK/SAPK phosphorylation (Thr183 and Tyr185). Equal loading was verified by Ponceau S staining. Data are expressed as arbitrary units (means ±s.e.m.). *P < 0.05 vs. resting. †P < 0.05 vs. age.
Post-exercise
The resistance exercise bout caused a differential response of the MAPK pathway proteins, between the young and the old men, although this was most likely to be due to the apparent differences observed at rest. The phosphorylation of ERK 1/2 increased 73 % (Fig. 1; P < 0.05) in the young (versus YM resting), as a result of the exercise bout. ERK 1/2 phosphorylation declined by 49 % (Fig. 1; P < 0.05) in the OM following the resistance exercise (versus OM pre-exercise). Post-exercise ERK 1/2 phosphorylation was lower in the old compared to the young (−31 %; P < 0.05).
Downstream proteins of ERK 1/2, p90RSK, Mnk 1 and eIF4E were also analysed for activation following the resistance exercise bout. The exercise bout elicited a 95 % increase (P < 0.05) phosphorylation of p90RSK in the YM and a 62 % decrease (P < 0.05) in the OM (Fig. 2), although achieving the same level of post-exercise phosphorylated p90RSK. Following the pattern of upstream protein phosphorylation, the resistance exercise bout caused a 61 % increase (Fig. 3; P < 0.05) in phosphorylation of Mnk 1 in the YM, and a 37 % decline in the OM (P < 0.05). Levels of Mnk 1 phosphorylation showed a trend towards a difference (P = 0.084) between groups following the exercise session. Further downstream of Mnk 1, the translation initiation factor, eIF4E, did not show any change in phosphorylation following the exercise bout (Fig. 4).
Parallel to the ERK 1/2 pathway, the p38 MAPK and JNK/SAPK proteins did not appear to be as heavily relied upon following the resistance exercise. Phosphorylation did not change in either of the proteins for the YM (Fig. 5 and Fig. 6), unlike the OM, who decreased (P < 0.05) phosphorylation of both p38 MAPK (14 %; Fig. 5) and JNK/SAPK (22 %; Fig. 6) following the exercise. The amount of phosphorylation for both proteins was the same for the YM and OM when compared with the post-exercise amounts for the respective protein.
Total protein content
There were no age-related differences in the total protein content of the MAPK pathway proteins examined. ERK 1/2 (Fig. 1), p90RSK (Fig. 2), Mnk 1 (Fig. 3), eIF4E (Fig. 4), p38 MAPK (Fig. 5) and JNK/SAPK (Fig. 6) had the same protein content for the YM and OM, although the total protein content for the phosphatase, MKP 1, was ∼82 % greater (P < 0.05) in the OM (Fig. 7).
Figure 7. MKP 1 total protein content in YM and OM.
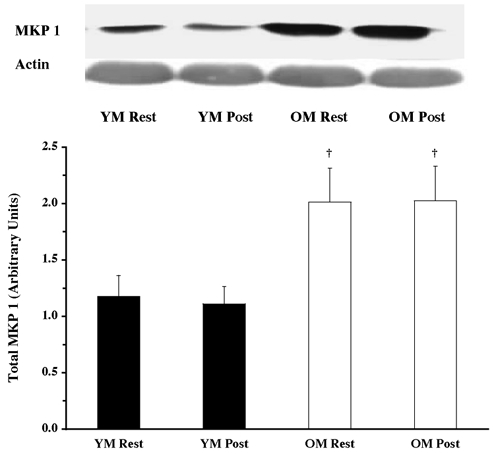
MKP 1 protein content was determined from vastus lateralis samples (at rest and post-exercise) using Western blot analysis with antibodies specific for MKP 1. Equal loading was verified by probing the same blot with antibodies for actin, as well staining the blot with Ponceau S. Data are expressed as arbitrary units (means ±s.e.m.). †P < 0.05 vs. age.
DISCUSSION
The purpose of this investigation was to examine the interaction of age and the activation of MAPK signalling proteins in response to resistance exercise. Before and after one bout of knee extensor resistance exercise, muscle biopsy samples from the vastus lateralis of young and old men were analysed for phosphorylation state and total protein expression of p44/42 MAPK (ERK 1/2) pathway proteins (ERK 1/2, p90RSK, Mnk 1 and eIF4E) and two parallel MAPK pathways (p38 MAPK and JNK/SAPK). Under resting conditions, the OM had higher levels of resting phosphorylation of ERK 1/2, p90RSK, Mnk 1, p38 MAPK and JNK/SAPK proteins (versus YM pre-exercise). The resistance exercise bout caused an increase in phosphorylation of the ERK 1/2 pathway proteins (ERK 1/2, p90RSK and Mnk 1) in the YM. Conversely, the OM showed a decrease in phosphorylation after the exercise bout (ERK 1/2, p90RSK, Mnk 1, p38 MAPK and JNK/SAPK), which may be due to the higher resting levels of phosphorylation in these proteins. Lastly, the OM had 82 % higher protein content of the protein phosphatase, MKP 1, compared to the YM. This investigation was the first to: (1) examine MAPKs before and after resistance exercise (isotonic) in humans; (2) examine MAPK activation in older adults before and after exercise; and (3) examine Mnk 1, eIF4E, MKP 1 activation and/or protein content in human muscle (rest or contraction).
Older men and MAPKs (rest and resistance exercise)
Contrary to our hypothesis, the older men had a decrease in ERK 1/2, p90RSK, Mnk 1, p38 MAPK and JNK/SAPK phosphorylation following the bout of resistance exercise. However, the OM had higher resting levels of phosphorylation compared to the YM, which could not be explained by a higher total protein content of the proteins analysed. Interestingly, post-exercise phosphorylation in the OM was comparable to the post-exercise phosphorylation state in the YM, with the exception of ERK 1/2. These data suggest that ageing skeletal muscle is functioning under ‘stress-like’ conditions at rest. There is an obvious decline in activation of the MAPKs with the addition of more stress (i.e. muscle contractions) in ageing skeletal muscle. This activation pattern in ageing skeletal muscle is in agreement with several studies examining ageing and the response to stress (rest and exercise) (Carlson & Faulkner 1989; Brooks & Faulkner 1990; Cannon et al. 1994; Jozsi et al. 2000), which appears to be associated with the age-related muscle loss.
Recently, a study examining skeletal muscle gene expression in young and old men reported higher baseline expression of stress/damage response genes (e.g. HSP27, NKEFB and MKK3) in older men, and a reduced activation of the stress/damage response genes with one bout of resistance exercise (Jozsi et al. 2000). Also, the IL-1β gene showed a 2-fold greater increase in transcripts in the young versus the old following exercise, thus demonstrating a reduced immune (acute phase) response. Levels of cytokines, such as TNFα and IL-1, are higher under resting conditions in older adults compared to young; these cytokines are associated with muscle wasting and insulin action (Paolisso et al. 1998; Kirwan et al. 2001). TNFα increases p38 MAPK, JNK/SAPK and ERK 1/2 phosphorylation (Raingeaud et al. 1995), which may partially explain the higher levels of phosphorylation in older men. However, progressive resistance training in older adults can reduce TNFα, which was reported to contribute to the gains in strength, size and protein synthesis (Greiwe et al. 2001).
MAPKs have been reported to exert control over muscle differentiation and proliferation (Bennett & Tonks 1997; Gredinger et al. 1998; Rommel et al. 1999), either through control of myogenic transcription factors or cell cycle control. Senescent rats have higher amounts of these myogenic transcription factors (e.g. Myo-D, myogenin) compared to young rats, which was suggested to be a compensatory mechanism by the senescent rats to maintain muscle mass and type (Musaro et al. 1995). This finding supports the higher baseline levels of ERK 1/2, p90RSK and Mnk 1 in the OM from the current study, possibly a compensation by the skeletal muscle with increasing age. Furthermore, when a group of older rats (2 years) underwent a resistance exercise bout, and were evaluated for regeneration of damaged muscle and myogenic-related genes (Myo-D), they had a limited myogenic response (compared to young rats; 3 months) (Tamaki et al. 2000). Similarly, we see that the OM had a lower amount of phosphorylation after exercise compared to their resting data, and similar or lower (ERK 1/2 only) post-exercise phosphorylation compared to the YM post-exercise. Once again, this lends evidence that skeletal muscle from older adults functions under a ‘stress-like’ condition, and that when additional stress is placed upon the muscle, there is a reduction in the response.
A recent study using senescent human fibroblasts (Tresini et al. 2001) provided evidence that upon activation by a mitogen, ERK 1/2 phosphorylation and nuclear accumulation is decreased (compared to young fibroblasts). The reduction of cellular signals (i.e. ERK 1/2) that exert their effects upon the nucleus, in senescent cells, can lead to a reduction in cell proliferation and DNA synthesis. Also, a recent study reported higher resting levels of ERK 1/2 phosphorylation in senescent versus young rats (Kostrominova et al. 2002). MAPK phosphatase (MKP) could contribute to the enhanced inhibition of ERK 1/2 phosphorylation once in the nucleus, effecting translocation of ERK 1/2 into and out of the nucleus (Sun et al. 1993). The OM in the current investigation had higher protein content of MKP 1 (compared to the YM), but we were unable to determine activity due to limited sample. If the OM had lower MKP 1 activity, this may help explain the resting differences, but not the post-exercise differences. MKP 1 is an immediate early gene product, paralleling ERK 1/2 activity and is transcribed and synthesized by ERK 1/2 phosphorylation in as little as 10 min (Sun et al. 1993). The older men in the current study had higher resting levels of ERK 1/2 phosphorylation compared to the young, thus MKP 1 protein content may be higher to counteract the constant high resting ERK 1/2 phosphorylation.
Younger men and MAPKs (rest and resistance exercise)
The current investigation shows that one bout of resistance exercise causes an activation of the ERK 1/2 pathway in the skeletal muscle of young men, which supports our hypothesis. The increase in ERK 1/2 phosphorylation was less than the changes reported from 60 min of cycle ergometry (1.7 vs.∼10- to 30-fold, respectively) (Widegren et al. 1998, 2000). However, the number of contractions performed during the exercise bout may account for some of these differences. Subjects in the current study performed ∼29 muscle contractions at 70 % of 1-RM (and ∼250 contractions at 25 W during the cycling warm-up), whereas 60 min of cycling (60 r.p.m.) will require ∼3600 contractions (70 % of maximum). If the subjects had performed more contractions over a longer period of time, the increase in ERK 1/2 activation may have reached the same magnitude. Although the activation of ERK 1/2 appears to increase through 30 min of cycling, then plateau during a 60 min cycle (Widegren et al. 1998), this suggests that activation may be complete after a certain amount of time or number of contractions. Also, as exercise intensity increases (40 vs. 75 %V̇O2,max) the phosphorylation of ERK 1/2 increases (Widegren et al. 2000). Cycle ergometry requires concentric contractions only, unlike the isotonic resistance exercise performed in the current study, which requires both the concentric and eccentric movements. The eccentric component appears to increase the MAPK activation more than concentric exercise (i.e. isokinetic resistance exercise; Boppart et al. 1999). Thus, it appears that contraction frequency, type and intensity influence ERK 1/2 activation. Downstream of ERK 1/2, the ribosomal protein p90RSK phosphorylates transcription factors and other intranuclear events (Chen et al. 1992; Dufresne et al. 2001). Contraction-mediated activation of p90RSK causes upregulation of immediate early genes and myogenic transcription factors related to cellular proliferation. Interestingly, the activation of p90RSK paralleled ERK 1/2 activation in the current investigation, and the increase in phosphorylation of p90RSK in the YM was similar to that reported in the literature (2- to 5-fold using cycle exercise; (Aronson et al. 1997; Krook et al. 2000).
This is the first study to report Mnk 1 or eIF4E activation at rest or following skeletal muscle contractions in humans. The current investigation found that contraction of human skeletal muscle phosphorylates Mnk 1 (∼61 %), but not eIF4E. These data show an immediate activation of a translation-related protein (Mnk 1 only) by muscle contraction (within ∼10 min). Activation of Mnk 1 by contraction may have implications for immediate control of protein translation and synthesis (Waskiewicz et al. 1997, 1999; Pyronnet et al. 1999); however, this was not supported by an increase in eIF4E activation, although increases in eIF4E phosphorylation do not always correlate with increases in protein translation and synthesis (Kimball et al. 1998; Raught & Gingras 1999; Scheper et al. 2002). Farrell et al. (2000) support these findings that eIF4E did not increase with exercise in rats, although these data were collected 16 h after the exercise bout.
Practical implications
Ageing skeletal muscle declines in size and strength (i.e. sarcopenia), but this decline can be partially attenuated with resistance exercise. Older adults achieve the same magnitude of change in size and strength with resistance training as the young, but do so with greater changes in the MHC I (slow fibre) population (versus MHC IIa in the young). There are several factors contributing to sarcopenia and changes with resistance exercise, such as neural, hormonal, physical activity and the activating signals that (i.e. MAPKs) ‘turn on’ genes regulating skeletal muscle proliferation and development. Any one of these factors may equally contribute to the decline of muscle size and strength with age, but the activation of the MAPK pathways is one of the first means of communication from the cell surface to the nucleus. Alterations this early in the process (cell signalling) could contribute to the age-related changes in skeletal muscle at rest and after one or more bouts of exercise.
Conclusions
This investigation is the first to provide evidence that MAPK signalling pathways are differentially activated under resting and exercise conditions in skeletal muscle from young and old men. The OM had higher resting levels of phosphorylation of the MAPK pathway proteins (ERK 1/2, p90RSK, Mnk 1, p38 MAPK and JNK/SAPK) compared to the YM. Following a bout of resistance exercise, the YM increased phosphorylation of the MAPK proteins, whereas the OM decreased phosphorylation following the resistance exercise. The amount of post-exercise protein phosphorylation in the OM was not different from the young post-exercise (except ERK 1/2), and may have been due to the higher resting phosphorylation in the old. Total protein content was not different between the groups (except for MKP 1), therefore not contributing to the differences in phosphorylation. Thus, resting skeletal muscle of older men appears to be under a state of ‘stress’. These findings may have implications for other processes (e.g. transcription and translation) involved in skeletal muscle type and growth, when examining the changes occurring with ageing muscle before and after resistance exercise/training.
Acknowledgments
The authors would like to thank the volunteers for their time and effort, Scott Mazzetti and Andy Creer for their assistance throughout the project and Gustavo Nader for his technical assistance. This project was sponsored by NIH grant (no. AG18409; to S.T.), and graduate student awards from Ball State University (D.W.) and Gatorade Sport Science Institute (D.W.).
REFERENCES
- Aronson D, Violan M, Dufresne S, Zangen D, Fielding R, Goodyear L. Exercise stimulates the mitogen-activated protein kinase pathway in human skeletal muscle. J Clin Invest. 1997;99:1251–1257. doi: 10.1172/JCI119282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson D, Wojtaszewski JFP, Thorell A, Nygren J, Zangen D, Richter EA, Ljungqvist O, Fielding RA, Goodyear LJ. Extracellular-regulated protein kinase cascades are activated in response to injury in human skeletal muscle. Am J Physiol. 1998;275:C555–561. doi: 10.1152/ajpcell.1998.275.2.C555. [DOI] [PubMed] [Google Scholar]
- Baar K, Esser K. Phosphorylation of p70s6k correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol. 1999;276:C120–127. doi: 10.1152/ajpcell.1999.276.1.C120. [DOI] [PubMed] [Google Scholar]
- Bennett AM, Tonks NK. Regulation of distinct stages of skeletal muscle differentiation by mitogen-activated protein kinases. Science. 1997;278:1288–1291. doi: 10.1126/science.278.5341.1288. [DOI] [PubMed] [Google Scholar]
- Bergstom J. Muscle electrolytes in man. Scand J Clin Lab Invest. 1962;68:1–110. [Google Scholar]
- Boppart MD, Aronson D, Gibson L, Roubenoff R, Abad LW, Bean J, Goodyear LJ, Fielding RA. Eccentric exercise markedly increases c-Jun NH2-terminal kinase acitivty in human skeletal muscle. J Appl Physiol. 1999;87:1668–1673. doi: 10.1152/jappl.1999.87.5.1668. [DOI] [PubMed] [Google Scholar]
- Brooks SV, Faulkner JA. Contraction-induced injury: recovery of skeletal muscles in young and old mice. Am J Physiol. 1990;258:C436–442. doi: 10.1152/ajpcell.1990.258.3.C436. [DOI] [PubMed] [Google Scholar]
- Cannon JG, Fiatarone MA, Fielding RA, Evans WJ. Aging and stress-induced changes in complement activation and neutrophil mobilization. J Appl Physiol. 1994;76:2616–2620. doi: 10.1152/jappl.1994.76.6.2616. [DOI] [PubMed] [Google Scholar]
- Carlson BM, Faulkner JA. Muscle transplantation between young and old rats: age of host determines recovery. Am J Physiol. 1989;256:C1262–1266. doi: 10.1152/ajpcell.1989.256.6.C1262. [DOI] [PubMed] [Google Scholar]
- Chen RH, Sarnecki C, Blenis J. Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Mol Cell Biol. 1992;12:915–927. doi: 10.1128/mcb.12.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufresne SD, Bjorbaek C, El-Haschimi K, Zhao Y, Aschenbach WG, Moller DE, Goodyear LJ. Altered extracellular signal-related kinase signaling and glycogen metabolism in skeletal muscle from p90 ribosomal S6 kinase knockout mice. Mol Cell Biol. 2001;21:81–87. doi: 10.1128/MCB.21.1.81-87.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell P, Hernandez J, Fedele M, Vary T, Kimball S, Jefferson L. Eukaryotic initiation factors and protein synthesis after resistance exercise in rats. J Appl Physiol. 2000;88:1036–1042. doi: 10.1152/jappl.2000.88.3.1036. [DOI] [PubMed] [Google Scholar]
- Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians: effects on skeletal muscle. J Am Med Assoc. 1990;263:3029–3034. [PubMed] [Google Scholar]
- Frontera WR, Meredith CN, O'Reilly KP, Knuttgen HG, Evans WJ. Strength conditioning in older men: skeletal muscle hypertrophy and improved function. J Appl Physiol. 1988;64:1038–1044. doi: 10.1152/jappl.1988.64.3.1038. [DOI] [PubMed] [Google Scholar]
- Goodyear L, Chang P, Sherwood D, Dufresne S, Moller D. Effects of exercise and insulin on mitogen-activated protein kinase signaling pathways in rat skeletal muscle. Am J Physiol. 1996;271:E403–408. doi: 10.1152/ajpendo.1996.271.2.E403. [DOI] [PubMed] [Google Scholar]
- Gredinger E, Gerber A, Tamir Y, Tapscott S, Bengal E. Mitogen-activated protein kinase pathway is involved in the differentiation of muscle cells. J Biol Chem. 1998;273:10436–10444. doi: 10.1074/jbc.273.17.10436. [DOI] [PubMed] [Google Scholar]
- Greiwe JS, Cheng B, Rubin DC, Yarasheski KE, Semenkovich CF. Resistance exercise decreases skeletal muscle tumor necrosis factor alpha in frail elderly humans. FASEB J. 2001;15:475–482. doi: 10.1096/fj.00-0274com. [DOI] [PubMed] [Google Scholar]
- Hernandez J, Fedele M, Farrell P. Time course evaluation of protein synthesis and glucose uptake after acute resistance exercise in rats. J Appl Physiol. 2000;88:1142–1149. doi: 10.1152/jappl.2000.88.3.1142. [DOI] [PubMed] [Google Scholar]
- Jozsi A, Dupont-Versteegden E, Taylor-Jones J, Evans W, Trappe T, Campbell W, Peterson C. Aged human muscle demonstrates an altered gene expression profile consistent with an impaired response to exercise. Mech Ageing Dev. 2000;120:45–56. doi: 10.1016/s0047-6374(00)00178-0. [DOI] [PubMed] [Google Scholar]
- Kimball SR, Horetsky RL, Jefferson LS. Signal transduction pathways involved in the regulation of protein synthesis by insulin in L6 myoblasts. Am J Physiol. 1998;274:C221–228. doi: 10.1152/ajpcell.1998.274.1.C221. [DOI] [PubMed] [Google Scholar]
- Kirwan JP, Krishnan RK, Weaver JA, del Aguila LF, Evans WJ. Human aging is associated with altered TNF-alpha production during hyperglycemia and hyperinsulinemia. Am J Physiol Endocrinol Metab. 2001;281:E1137–1143. doi: 10.1152/ajpendo.2001.281.6.E1137. [DOI] [PubMed] [Google Scholar]
- Kostrominova TY, MacPherson PCD, Dedkov EI, Carlson BM, Goldman D. Increased accumulation of phosphorylated extracellular signal-regulated protein kinases 1/2 in skeletal muscle after denervation and during aging. FASEB J. 2002;16 A390 (abstract) [Google Scholar]
- Krook A, Widegren U, Jiang X, Henriksson J, Wallberg-Henrikssen H, Alessi D, Zierath J. Effects of exercise on mitogen- and stress-activated kinase signal transduction in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1716–1721. doi: 10.1152/ajpregu.2000.279.5.R1716. [DOI] [PubMed] [Google Scholar]
- Laemmli U. Cleavage of structural proteins during the assembly of the head of bacteriophage. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larsson L, Grimby G, Karlsson J. Muscle strength and speed of movement in relation to age and muscle morphology. J Appl Physiol. 1979;46:451–456. doi: 10.1152/jappl.1979.46.3.451. [DOI] [PubMed] [Google Scholar]
- Lexell J, Hendriksson-Larsen K, Winblad B, Sjostrom M. Distribution of different fibre types in human skeletal muscles: effects of aging studies in whole muscle cross section. Muscle Nerve. 1983;6:588–595. doi: 10.1002/mus.880060809. [DOI] [PubMed] [Google Scholar]
- Murgia M, Serrano A, Calabria E, Pallafacchina G, Lomo T, Schiaffino S. Ras is involved in nerve-activity-dependent regulation of muscle genes. Nat Cell Biol. 2000;2:142–147. doi: 10.1038/35004013. [DOI] [PubMed] [Google Scholar]
- Musaro A, De Angelis C, Germani A, Ciccarelli C, Molinaro M, Zani B. Enhanced expression of myogenic regulatory genes in aging skeletal muscle. Exp Cell Res. 1995;221:241–248. doi: 10.1006/excr.1995.1372. [DOI] [PubMed] [Google Scholar]
- Osman A, Pendergrass M, Koval J, Maezono K, Cusi K, Pratipanawater T, Mandarino L. Regulation of MAP kinase pathway activity in vivo in human muscle. Am J Physiol Endocrinol Metab. 2000;278:E992–999. doi: 10.1152/ajpendo.2000.278.6.E992. [DOI] [PubMed] [Google Scholar]
- Paolisso G, Rizzo M, Mazziotti G, Tagliamonte M, Gambardella A, Rotondi M, Carella C, Giugliano D, Varricchio M, D'Onofrio F. Advancing age and insulin resistance: role of plasma tumor necrosis factor-a. Am J Physiol. 1998;275:E294–299. doi: 10.1152/ajpendo.1998.275.2.E294. [DOI] [PubMed] [Google Scholar]
- Pyronnet S, Imataka H, Gingras A, Fukunaga R, Hunter T, Sonenberg N. Human eukaryotic translation factor 4G (eIF4G) recruits Mnk1 to phosphorylate eIF4E. EMBO J. 1999;18:270–279. doi: 10.1093/emboj/18.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raingeaud J, Gupta S, Rogers J, Dickens M, Han J, Ulevitch RJ, Davis RJ. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- Raught B, Gingras A. eIF-4E activity is regulated at multiple levels. Int J Biochem Cell Biol. 1999;31:43–57. doi: 10.1016/s1357-2725(98)00131-9. [DOI] [PubMed] [Google Scholar]
- Rommel C, Clarke BA, Zimmermann S, Nunez L, Rossman R, Reid K, Moelling K, Yancopoulos GD, Glass DJ. Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science. 1999;286:1738–1741. doi: 10.1126/science.286.5445.1738. [DOI] [PubMed] [Google Scholar]
- Scheper GC, van Kollenburg B, Hu J, Lou Y, Goss DJ, Proud CG. Phosphorylation of eukaryotic initiation factor 4E markedly reduces its affinity for capped mRNA. J Biol Chem. 2002;277:3303–3309. doi: 10.1074/jbc.M103607200. [DOI] [PubMed] [Google Scholar]
- Sun H, Charles CH, Lau LF, Tonks NK. MKP-1 (3CH134), and immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell. 1993;75:487–493. doi: 10.1016/0092-8674(93)90383-2. [DOI] [PubMed] [Google Scholar]
- Tamaki T, Uchiyama S, Uchiyama Y, Akatsuka A, Yoshimura S, Roy RR, Edgerton VR. Limited myogneic response to a single bout of weight-lifting exercise in old rats. Am J Physiol Cell Physiol. 2000;278:C1143–1152. doi: 10.1152/ajpcell.2000.278.6.C1143. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trappe S, Williamson D, Godard M, Porter D, Rowden G, Costill D. Effect of resistance training on single muscle fiber contractile properties in older men. J Appl Physiol. 2000;89:143–152. doi: 10.1152/jappl.2000.89.1.143. [DOI] [PubMed] [Google Scholar]
- Trappe SW, Godard MP, Gallagher PM, Carroll CC, Rowden G, Porter D. Resistance training improves single muscle fiber contractile function in older women. Am J Physiol Cell Physiol. 2001;281:C398–406. doi: 10.1152/ajpcell.2001.281.2.C398. [DOI] [PubMed] [Google Scholar]
- Tresini M, Lorenzini A, Frisoni L, Allen RG, Cristofalo VJ. Lack of Elk-1 phosphorylation and dysregulation of the extracellular regulated kinase signaling pathway in senescent human fibroblast. Exp Cell Res. 2001;269:287–300. doi: 10.1006/excr.2001.5334. [DOI] [PubMed] [Google Scholar]
- Waskiewicz AJ, Flynn A, Proud CG, Cooper JA. Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J. 1997;16:1909–1920. doi: 10.1093/emboj/16.8.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waskiewicz AJ, Johnson JC, Penn B, Mahalingam M, Kimball SR, Cooper JA. Phosphorylation of the cap-binding protein eukaryotic translation factor 4E by protein kinase Mnk1 in vivo. Mol Cell Biol. 1999;19:1871–1880. doi: 10.1128/mcb.19.3.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widegren U, Jiang X, Krook A, Chibalin A, Bjornholm M, Tally M, Roth R, Henrikssen J, Wallberg-Henrikssen H, Zierath J. Divergent pathways of exercise on metabolic and mitogenic signaling pathways in human skeletal muscle. FASEB J. 1998;12:1379–1389. doi: 10.1096/fasebj.12.13.1379. [DOI] [PubMed] [Google Scholar]
- Widegren U, Wretman C, Lionikas A, Hedin G, Henriksson J. Influence of exercise intensity on ERK/MAP kinase signaling in human skeletal muscle. Pflugers Arch. 2000;441:317–322. doi: 10.1007/s004240000417. [DOI] [PubMed] [Google Scholar]
- Williamson D, Gallagher P, Carroll C, Raue U, Trappe S. Reduction in hybrid single muscle fiber proportions with resistance training in humans. J Appl Physiol. 2001;91:1955–1961. doi: 10.1152/jappl.2001.91.5.1955. [DOI] [PubMed] [Google Scholar]
- Williamson DL, Godard MP, Porter D, Costill DL, Trappe SW. Progressive resistance training reduces myosin heavy chain co-expression in single muscle fibers from older men. J Appl Physiol. 2000;88:627–633. doi: 10.1152/jappl.2000.88.2.627. [DOI] [PubMed] [Google Scholar]
- Zester A, Gredinger E, Bengal E. p38 Mitogen-activated protein kinase pathway promotes skeletal muscle differentiation. Participation of the Mef2c transcription factor. J Biol Chem. 1999;274:5193–5200. doi: 10.1074/jbc.274.8.5193. [DOI] [PubMed] [Google Scholar]


