Abstract
Sympathetic vasoconstriction is blunted in the vascular beds of contracting skeletal muscles. We sought to determine whether this blunted vasoconstriction is specific for post-junctional α1- or α2-adrenergic receptors. We measured forearm blood flow (Doppler ultrasound) and calculated the vascular conductance (FVC) responses to brachial artery infusions of tyramine (which evokes endogenous noradrenaline release), phenylephrine (an α1 agonist) and clonidine (an α2 agonist) in 10 healthy men during rhythmic handgrip exercise (10-15 % of maximum) and during a control non-exercise vasodilator condition (intra-arterial adenosine). Steady-state FVC during exercise and adenosine was similar in all trials (range: 243-272 and 234-263 ml min−1 (100 mmHg)−1, respectively; P > 0.5). During exercise the percentage reductions in FVC in response to tyramine (−24 ± 7 vs.−55 ± 6 %), phenylephrine (−12 ± 8 vs.−37 ± 8 %) and clonidine (−17 ± 6 vs.−49 ± 4 %) were significantly less compared with adenosine (all P < 0.05). The magnitude of the blunted vasoconstrictor responses was similar for both receptor subtypes. These findings are in contrast to those from studies in animals demonstrating that α2-adrenergic receptor-mediated vasoconstrictor responses are much more sensitive to contraction-induced inhibition than α1-mediated responses. We conclude that vasoconstrictor responses mediated via both post-junctional α1- and α2-adrenergic receptors are blunted in contracting human skeletal muscles.
Blood flow to exercising skeletal muscles increases and under certain circumstances there can be ‘competition’ between vasodilating metabolites from the active muscles and increased sympathetic vasoconstrictor nerve activity directed toward them (Strandell & Shepherd, 1967; Rowell, 1997). One concept, termed ‘functional sympatholysis’, is that muscle metabolites blunt sympathetic vasoconstriction so that blood flow and oxygen delivery to the exercising muscles is driven by metabolic demand (Remensnyder et al. 1962).
In this context, Thomas and colleagues (1994) have demonstrated that muscle contraction eliminates post-junctional α2 sympathetic vasoconstriction in rat muscle. However, post-junctional α1 vasoconstriction remained intact, indicating that functional sympatholysis mainly involves attenuation of post-junctional α2 receptor-mediated vasoconstriction. These observations are consistent with data from studies of the microcirculation in rat skeletal muscle showing that post-junctional vasoconstricting α2 receptors are much more sensitive to inhibition by acidosis and hypoxia than α1 receptors (McGillivray-Anderson & Faber, 1990; Anderson & Faber, 1991; Tateishi & Faber, 1995). Additionally, Buckwalter et al. (1998, 2001) studied dogs running on the treadmill and found that α2 vasoconstrictor responses in the hindlimbs were blunted throughout all exercise levels, but that α1-adrenergic vasoconstriction was only attenuated during heavy exercise.
With this information as a background, our laboratory recently demonstrated that exercise blunts (but does not eliminate) sympathetic vasoconstriction in active human muscles (Tschakovsky et al. 2002), and in this study we sought to determine which post-junctional α-adrenergic receptor subtype might contribute to functional sympatholysis in humans. Our hypothesis was that exercise would blunt α2 receptor-mediated vasoconstriction in the human forearm but have little impact on α1-mediated vasoconstriction.
METHODS
Subjects and instrumentation
After institutional review board approval and written informed consent, 10 healthy male subjects aged between 20 and 34 years participated in this study. All studies were performed according to the Declaration of Helsinki. The subjects were medication-free normotensive non-smokers. They had abstained from caffeine overnight and had not eaten for at least 3 h prior to the study. The studies were conducted in a laboratory maintained at 20 °C to minimise skin blood flow to the hand (Tschakovsky et al. 2002). Each subject had their forearm volume measured using water displacement. Subjects were studied in the supine position with their non-dominant arm extended laterally at heart level. A 5-cm 20-gauge brachial artery catheter was inserted into the non-dominant arm using aseptic technique after local anaesthesia (1.0 % lidocaine (lignocaine)). A stopcock and port system permitted simultaneous measurement of arterial pressure and infusion of study drugs (Dietz et al. 1994).
Forearm blood flow and exercise
A 4 MHz pulsed Doppler probe (Model 500V, Multigon Industries, Mt Vernon, NY, USA) was used to measure brachial artery mean blood velocity (MBV) with the probe securely fixed to the skin over the brachial artery proximal to the catheter insertion site. The probe insonation angle was approximately 60 deg. A linear 7.0 MHz echo Doppler ultrasound probe (Acuson 128XP, Mountain View, CA, USA) was placed in a holder securely fixed to the skin immediately proximal to the velocity probe to measure brachial artery diameter. Forearm blood flow was calculated as:
where the FBF is forearm blood flow in ml min−1, the MBV is in cm s−1, the brachial diameter is in cm, and 60 is used to convert from ml s−1 to ml min−1.
Rhythmic forearm handgripping was performed using a 6.4 kg weight that was approximately 10-15 % of maximal voluntary contraction (MVC). The weight was lifted 4-5 cm over a pulley at a contraction-relaxation duty cycle of 1-2 Hz (20 contractions min−1) using a signal light to ensure the correct timing.
Drugs
The following drugs were infused via the brachial artery catheter: Tyramine was infused at 8 μg (dl forearm volume)−1 min−1. Tyramine evokes noradrenaline ‘leakage’ from neuronal vesicles and consequent diffusion of noradrenaline out of sympathetic nerves (Brandao et al. 1980) resulting in stimulation of both α1- and α2-adrenergic receptors (Jie et al. 1987; Dinenno et al. 2002b). This dose was based on the effects of forearm exercise on the tyramine vasoconstrictor dose-response curves reported by Tschakovsky et al. (2002). Phenylephrine (a selective α1 agonist) was infused at 0.0312 μg (dl forearm volume)−1 min−1 and clonidine (a selective α2 agonist) was infused at 0.15 μg (dl forearm volume)−1 min−1. The doses of phenylephrine and clonidine were based on our experience at rest (Dinenno et al. 2002a) and adjusted for hyperaemic conditions (see below).
Since exercise increases forearm blood flow, adenosine (6.25 μg (dl forearm volume)−1 min−1) was used to elevate resting forearm blood flow. We have previously shown that exercise blunts the vasoconstrictor responses to tyramine, whereas these constrictor responses are maintained when blood flow is elevated with adenosine and hence it was used to create a ‘high flow’ control state (Tschakovsky et al. 2002). In an effort to normalise the concentration of each vasoconstricting drug in the blood perfusing the forearm, the infusions were adjusted on the basis of brachial artery blood flow. Care was taken so that various concentrations of each compound were available and the absolute infusion rate was less than 4 ml min−1 in every trial.
Experimental protocol
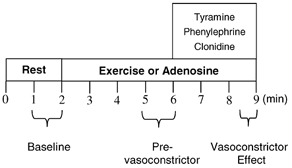
Figure 1 is a time-line of the study protocol. The subjects performed three bouts of forearm exercise and received three adenosine infusions; the total time for each trial was 9 min. After 2 min of baseline measurements, exercise or adenosine infusion was initiated and steady-state FBF was reached within 3 min. Between 3 and 4 min of hyperaemia the dose of adrenergic agonist was calculated on the basis of forearm volume and blood flow and vasoconstrictor infusion began at the 4-minute mark and lasted for 3 min. A 20 min break was allowed between each trial. Exercise bouts and adenosine infusions alternated, and the order of the tyramine, phenylephrine and clonidine infusions was randomised and counterbalanced across subjects.
Figure 1. Experimental protocol.
Data acquisition and analysis
Data were collected at 200 Hz and stored on computer to be analysed using Windaq signal processing software. Mean arterial pressure (MAP) was determined from the arterial pressure waveform. Resting FBF and MAP represent an average of the last minute of the baseline time period, the hyperaemic values represent an average of minutes 3-4 during adenosine or exercise, and the effects of the α agonists represent an average of the final 30 s drug infusion.
Forearm vascular conductance (FVC) was calculated as:
where FVC is in ml min−1 (100 mmHg)−1. Blood flow per 100 mmHg was used so that FVC was quantitatively similar to the standard units for FBF.
The percentage reduction in FVC during vasoconstrictor administration was calculated as:
We used the percentage reduction in FVC as our standard index to compare vasoconstriction across conditions when blood flow and conductance might differ. After much discussion this index has emerged as the favoured way to compare interventions that cause vasodilatation or vasoconstriction under conditions where there might be marked difference in baseline blood flow (Lautt, 1989; Thomas et al. 1994; Buckwalter & Clifford, 2001; Tschakovsky et al. 2002).
Statistics
All values are reported as means ±s.e.m. Specific hypothesis testing within each of the exercise or adenosine trials with the three different drug infusions was performed using Student's paired t test. Significance was set at P < 0.05.
RESULTS
Tyramine infusion (α1 and α2 stimulation)
During exercise and adenosine infusion (i.e. control), absolute forearm blood flows (267 ± 19 and 243 ± 43 ml min−1) and vascular conductances (267 ± 19 vs. 264 ± 53 ml min−1 (100 mmHg)−1) were not different (P > 0.05). Tyramine caused a significantly greater percentage reduction in vascular conductance during adenosine infusion than during exercise (−58 ± 6 vs.−24 ± 7 %, P < 0.05). Absolute blood flow data are shown in Fig. 2.
Figure 2. Baseline forearm blood flow and the hyperaemic responses to adenosine infusion and rhythmic forearm handgripping were similar.
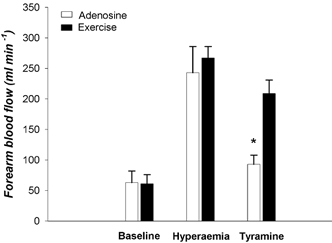
Tyramine infusion caused a robust vasoconstriction in the forearm pretreated with adenosine, but this response was reduced during exercise, indicating that vasoconstriction to endogenous noradrenaline release was blunted in contracting muscle. *P < 0.05 vs. exercise.
Phenylephrine infusion (α1 stimulation)
During exercise and adenosine infusion, absolute forearm blood flows (241 ± 16 and 222 ± 23 ml min−1) and vascular conductances (243 ± 19 and 238 ± 26 ml min−1 (100 mmHg)−1) were not different (P > 0.05). Phenylephrine caused a significantly greater percentage reduction in vascular conductance during adenosine infusion than during exercise (−37 ± 8 vs.−12 ± 8 %, P < 0.05). Absolute blood flow data are shown in Fig. 3.
Figure 3. Baseline forearm blood flow and the hyperaemic responses to adenosine infusion and rhythmic forearm handgripping were similar.
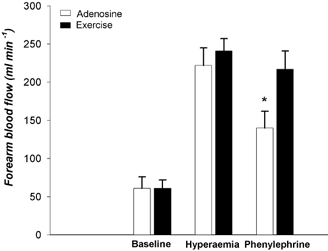
Phenylephrine infusion caused a robust vasoconstriction in the forearm pretreated with adenosine, but this response was reduced during exercise, indicating that post-junctional α1-adrenergic vasoconstriction was blunted in contracting muscle. *P < 0.05 vs. exercise.
Clonidine infusion
During exercise and adenosine infusion absolute forearm blood flows (258 ± 24 and 239 ± 27 ml min−1) and vascular conductances (272 ± 22 and 255 ± 28 ml min−1 (100 mmHg)−1) were not different (P > 0.05). Clonidine caused a significantly greater percentage reduction in vascular conductance during adenosine infusion than during exercise (−49 ± 5 vs.−17 ± 6 %, P < 0.05). Absolute blood flow data are shown in Fig. 4.
Figure 4. Baseline forearm blood flow and the hyperaemic responses to adenosine infusion and rhythmic forearm handgripping were similar.
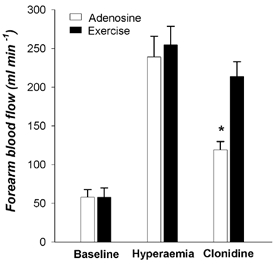
Clonidine infusion caused a robust vasoconstriction in the forearm pretreated with adenosine, but this response was reduced during exercise, indicating that post-junctional α2-adrenergic vasoconstriction was blunted in contracting muscle. *P < 0.05 vs. exercise.
Baseline MAP was ∼95 mmHg during all trials. MAP increased by ∼5 mmHg during exercise but was little influenced by adenosine. MAP was elevated ∼1-2 mmHg at the end of the adenosine trials.
Phenylephrine vs. clonidine
The effects of exercise on the vasoconstriction caused by phenylephrine and clonidine were similar (P > 0.05). This means that exercise (compared to adenosine) blunted both α1- and α2-adrenergic vasoconstrictor responses to a similar extent.
DISCUSSION
The main finding of this study is that both α1- and α2-adrenergic receptor-mediated vasoconstriction is blunted in contracting human skeletal muscle. Importantly, this attenuation of α1-mediated vasoconstriction was seen during moderate rhythmic handgripping and is in marked contrast to data from definitive animal studies (Anderson & Faber, 1991; Thomas et al. 1994; Buckwalter et al. 1998, 2001). Our findings also demonstrate that these conclusions are not artefacts related to elevations in muscle blood flow per se.
Functional sympatholysis: α1vs.α2 receptors
Previous investigations in experimental animals have demonstrated that functional sympatholysis is mainly due to a blunting of post-junctional α2-adrenergic receptor-mediated vasoconstriction (McGillivray-Anderson & Faber, 1990; Anderson & Faber, 1991; Thomas et al. 1994; Buckwalter et al. 1998, 2001). In contracting rat fast-twitch skeletal muscle the vasoconstrictor responses to α2-adrenergic stimulation were eliminated by contraction, but the α1-mediated responses were preserved (Thomas et al. 1994). These data are consistent with observations from the microcirculation demonstrating that the smallest resistance vessels in fast-twitch skeletal muscles contain a predominance of post-junctional α2 receptors and that these post-junctional α2 receptors are especially sensitive to acidosis and hypoxia (McGillivray-Anderson & Faber, 1990; Anderson & Faber, 1991; Tateishi & Faber, 1995). Consistent with these observations, exercise attenuated both α1- and α2-mediated vasoconstriction in the hindlimbs of dogs running on the treadmill, but α2 responsiveness was attenuated to a greater extent at much lower workloads while α1-mediated vasoconstriction was only blunted during heavy exercise (Buckwalter et al. 1998, 2001). Our observations contrast with these findings and demonstrate that in humans rhythmic handgripping at only 10-15 % of MVC blunts both α1- and α2-mediated vasoconstriction, and that the degree of blunting is similar between the two receptor subtypes.
A key question then is why are humans different from other mammals? One possible explanation is that human skeletal muscle (in contrast to that of rat) is typically ‘mixed’ and contains a mosaic of fast- and slow-twitch fibres. Additionally, in rat skeletal muscle post-junctional α2 receptors appear to be located mainly on the smallest resistance vessels, with α1 receptors predominating in the larger resistance vessels (Anderson & Faber, 1991; Ohyanagi et al. 1991). It is currently not known if this pattern also occurs in human skeletal muscle. Perhaps differences in the distribution of fibre types and α-adrenergic receptors between humans and rats could account for some of the differences in α1 and α2 vasoconstrictor responsiveness during exercise. Therefore, it will be important to determine how post-junctional α1 and α2 receptors are distributed in human skeletal muscle vascular beds, both in terms of their relationship to muscle fibre type and their location on the larger vs. smaller resistance vessels. However, we speculate that the distribution of post-junctional α1 and α2 receptors in resistance vessels might be more heterogeneous in human than animal skeletal muscle.
Experimental considerations
There are several experimental considerations that need to be addressed. First, the best way to compare vasomotor responsiveness under various conditions has led to a great deal of debate in the literature (Ruble et al. 2002; Tschakovsky et al. 2002). In our previous study, we used percentage reduction in FVC as an index of vasoconstrictor responsiveness (Buckwalter & Clifford, 2001; Tschakovsky et al. 2002). This choice was based on ideas from both Lautt (1989) and O'Leary (1991) who have pointed out that there is a linear relationship between changes in conductance and blood flow when perfusion pressure remains relatively constant. This approach has also been used in other key studies on functional sympatholysis (Thomas et al. 1994; Ruble et al. 2002) and, therefore, we adopted this approach in the present study.
Second, much of the debate with respect to data presentation/interpretation has been focused on comparing vasoconstrictor responses between resting (low blood flow) and exercising (high blood flow) conditions. Therefore, we used adenosine infusion as a non-exercising ‘high flow’ control condition and closely matched the adenosine blood flow values to our exercise values to minimise this potential limitation. Additionally, to insure the same arterial concentration of each vasoconstricting drug, infusion rates were based on calculations of individual forearm volume and adjusted according to the forearm blood flow achieved during the respective hyperaemic condition. In this context, all of the α agonists caused marked vasoconstriction during adenosine infusion that was similar to or greater than that caused by similar doses of the compounds given to the resting forearm (Tschakovsky et al. 2002; Dinenno et al. 2002a). It is also possible that the specific blood vessels dilated during adenosine infusion differed from those dilated during exercise, thus limiting the utility of our high flow control condition. However, in comparison to previous studies conducted in resting forearms (Tschakovsky et al. 2002; Dinenno et al. 2002a, b), the constriction evoked by comparable doses of tyramine, phenylephrine and clonidine were all blunted by exercise but not adenosine. If adenosine and exercise had differing effects on tyramine-evoked noradrenaline release this could explain our findings with tyramine but not with the infusions of the direct-acting agonists. Thus, it is unlikely that our findings of blunted constriction during exercise are artefacts of high blood flow states.
Finally, while it is now known that there are significant numbers of post-junctional vasoconstricting α2 receptors, intra-arterial infusions of selective α2 agonists such as clonidine might also have presynaptic effects and inhibit noradrenaline release (Jie et al. 1987; Buckwalter et al. 2001; Dinenno et al. 2002a). However, the high flow adenosine control studies demonstrate that the net effect of clonidine is post-junctional α-adrenergic vasoconstriction (a 49 % reduction in FVC) which overrides any pre-junctional inhibition of noradrenaline release.
Summary and perspectives
In summary, we have demonstrated that both α1 and α2 vasoconstrictor responsiveness is blunted in human skeletal muscle performing moderate rhythmic contractions. In a variety of skeletal muscle preparations acidosis and hypoxia are seen as essential for the blunting of α-mediated vasoconstrictor responsiveness (McGillivray-Anderson & Faber, 1990; Tateishi & Faber, 1995; Hansen et al. 2000). However, it is unlikely that there was overwhelming acidosis and/or tissue hypoxia during the moderate levels of rhythmic exercise we used (Strandell & Shepherd, 1967; Joyner & Wieling, 1993). Therefore, our data suggest that the relative contribution of postjunctional α1- and α2-adrenergic receptors to functional sympatholysis may differ in humans and animals. The data also raise questions about the mechanisms responsible for functional sympatholysis in humans and the distribution of post-junctional α1- and α2-adrenergic receptors in human skeletal muscle resistance vessels.
Acknowledgments
J.B.R. was a visiting fellow from the Copenhagen Muscle Research Centre and her research was supported by the Danish Heart Foundation grant 136022812, Astra Zeneca, Novo Nordisk Foundation, The Fulbright Commission, Denmark; this study was also supported by the National Institutes of Health (NIH) grants HL-46493 and NS-32353, USA to M.J.J.; NIH grant NIH F32 AG-05912, USA to F.A.D.; and by NIH General Clinical Research Center grant RR-00585 (Mayo Clinic), USA.
REFERENCES
- Anderson KM, Faber JE. Differential sensitivity of arteriolar alpha 1- and alpha 2-adrenoceptor constriction to metabolic inhibition during rat skeletal muscle contraction. Circ Res. 1991;69:174–184. doi: 10.1161/01.res.69.1.174. [DOI] [PubMed] [Google Scholar]
- Brandao F, Rodrigues-Periera E, Guilherme MJ, Osswald W. Characteristics of tyramine induced release of noradrenaline: mode of action of tyramine and metabolic fate of the transmitter. N-S Arch Pharmacol. 1980;311:9–15. doi: 10.1007/BF00500297. [DOI] [PubMed] [Google Scholar]
- Buckwalter JB, Clifford PS. The paradox of sympathetic vasoconstriction in exercising skeletal muscle. Exerc Sports Sci Rev. 2001;29:159–163. doi: 10.1097/00003677-200110000-00005. [DOI] [PubMed] [Google Scholar]
- Buckwalter JB, Mueller PJ, Clifford PS. α1-Adrenergic-receptor responsiveness in skeletal muscle during dynamic exercise. J Appl Physiol. 1998;85:2277–2283. doi: 10.1152/jappl.1998.85.6.2277. [DOI] [PubMed] [Google Scholar]
- Buckwalter JB, Naik JS, Valic Z, Clifford PS. Exercise attenuates α-adrenergic-receptor responsiveness in skeletal muscle vasculature. J Appl Physiol. 2001;90:172–178. doi: 10.1152/jappl.2001.90.1.172. [DOI] [PubMed] [Google Scholar]
- Dietz NM, Rivera JM, Eggener SE, Fix RT, Warner DO, Joyner MJ. Nitric oxide contributes to the rise in forearm blood flow during mental stress in humans. J Physiol. 1994;480:361–368. doi: 10.1113/jphysiol.1994.sp020366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinenno FA, Dietz NM, Joyner MJ. Aging and forearm postjunctional α-adrenergic vasoconstriction in healthy men Circulation. 2002a;106:1349–1354. doi: 10.1161/01.cir.0000028819.64790.be. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Eisenach JH, Dietz NM, Joyner MJ. Postjunctional a-adrenoceptors and basal limb vascular tone in healthy men. J Physiol. 2002b;540:1110. doi: 10.1113/jphysiol.2001.015297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J, Sander M, Hald CF, Victor RG, Thomas GD. Metabolic modulation of sympathetic vasoconstriction in human skeletal muscle: role of tissue hypoxia. J Physiol. 2000;527:387–396. doi: 10.1111/j.1469-7793.2000.00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jie K, van Brummelen P, Vermey P, Timmermans PB, van Zwieten PA. Postsynaptic α1- and α2-adrenoceptors in human blood vessels: interactions with exogenous and endogenous catecholamines. Eur J Clin Invest. 1987;17:174–181. doi: 10.1111/j.1365-2362.1987.tb02397.x. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Wieling W. Increased muscle perfusion reduces muscle sympathetic nerve activity during handgripping. J Appl Physiol. 1993;75:2450–2455. doi: 10.1152/jappl.1993.75.6.2450. [DOI] [PubMed] [Google Scholar]
- Lautt WW. Resistance or conductance for expression of arterial vascular tone. Microvasc Res. 1989;37:230–236. doi: 10.1016/0026-2862(89)90040-x. [DOI] [PubMed] [Google Scholar]
- McGillivray-Anderson KM, Faber JE. Effect of acidosis on contraction of microvascular smooth muscle by alpha 1- and alpha 2-adrenoceptors. Implications for neural and metabolic regulation. Circ Res. 1990;66:1643–1657. doi: 10.1161/01.res.66.6.1643. [DOI] [PubMed] [Google Scholar]
- Ohyanagi M, Faber JE, Nishigaki K. Differential activation of alpha 1- and alpha 2-adrenoceptors on microvascular smooth muscle during sympathetic nerve stimulation. Circ Res. 1991;68:232–244. doi: 10.1161/01.res.68.1.232. [DOI] [PubMed] [Google Scholar]
- O'Leary DS. Regional vascular resistance vs. conductance: which index for baroreflex responses? Am J Physiol. 1991;260:H632–637. doi: 10.1152/ajpheart.1991.260.2.H632. [DOI] [PubMed] [Google Scholar]
- Remensnyder JP, Mitchell JH, Sarnoff SJ. Functional sympatholysis during muscular activity. Circ Res. 1962;11:370–380. doi: 10.1161/01.res.11.3.370. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Neural control of muscle blood flow: importance during dynamic exercise. Clin Exp Pharmacol Physiol. 1997;24:117–125. doi: 10.1111/j.1440-1681.1997.tb01793.x. [DOI] [PubMed] [Google Scholar]
- Ruble SB, Valic Z, Buckwalter JB, Tschakovsky ME, Clifford PS. Attenuated vascular responsiveness to noradrenaline release during dynamic exercise in dogs. J Physiol. 2002;541:637–644. doi: 10.1113/jphysiol.2001.014738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandell T, Shepherd JT. The effect in humans of increased sympathetic activity on the blood flow to active muscles. Acta Med Scand. 1967;472:146–167. doi: 10.1111/j.0954-6820.1967.tb12622.x. [DOI] [PubMed] [Google Scholar]
- Tateishi J, Faber JE. Inhibition of arteriole alpha 2- but not alpha 1-adrenoceptor constriction by acidosis and hypoxia in vitro. Am J Physiol. 1995;268:H2068–2076. doi: 10.1152/ajpheart.1995.268.5.H2068. [DOI] [PubMed] [Google Scholar]
- Thomas GD, Hansen J, Victor RG. Inhibition of alpha 2-adrenergic vasoconstriction during contraction of glycolytic, not oxidative, rat hindlimb muscle. Am J Physiol. 1994;266:H920–929. doi: 10.1152/ajpheart.1994.266.3.H920. [DOI] [PubMed] [Google Scholar]
- Tschakovsky ME, Sujirattanawimol K, Ruble SB, Valic Z, Joyner MJ. Is sympathetic neural vasoconstriction blunted in the vascular bed of exercising human muscle? J Physiol. 2002;541:635. doi: 10.1113/jphysiol.2001.014431. [DOI] [PMC free article] [PubMed] [Google Scholar]


