Abstract
The importance of thyroid hormone receptors for isometric force, endurance and content of specific muscle enzymes was studied in isolated slow-twitch soleus and fast-twitch extensor digitorum longus (EDL) muscles in mice deficient in all known subtypes of thyroid hormone receptors (i.e. TR α1, β1, β2 and β3). The weights of soleus and EDL muscles were lower in TR-deficient (TRα1−/−β−/−) mice than in wild-type controls. The force per cross-sectional area was not significantly different between TRα1−/−β−/− and wild-type muscles. Soleus muscles of TRα1−/−β−/− mice showed increased contraction and relaxation times and the force-frequency relationship was shifted to the left. Soleus muscles of TRα1−/−β−/− mice were more fatigue resistant than wild-type controls. Protein analysis of TRα1−/−β−/− soleus muscles showed a marked increase in expression of the slow isoform of the sarcoplasmic reticulum Ca2+ pump (SERCa2), whilst expression of the fast type (SERCa1) was decreased. There was also a major decrease in the α2-subunit of the Na+−K+ pump in TRα1−/−β−/− soleus muscles. EDL muscles from TRα1−/−β−/− and wild-type mice showed no significant difference in contraction and relaxation times, fatigue resistance and protein expression. In conclusion, the present data show changes in contractile characteristics of skeletal muscles of TRα1−/−β−/− mice similar to those seen in hypothyroidism. We have previously shown that muscles of mice deficient in TRα1 or TRβ display modest changes in muscle function. Thus, in skeletal muscle there seems to be functional overlap between TRα1 and TRβ, so that the lack of one of the receptors to some extent can be compensated for by the presence of the other.
Thyroid hormone, by mediating its effect through thyroid hormone receptors (TRs), has a major role in skeletal muscle development and function. TRs belong to the superfamily of nuclear receptors. To date, there are five different subtypes of TRs (α1, α2, β1, β2 and β3) encoded by two different genes, TRα (NR1A1) and TRβ (NR1A2), which in the mouse are located on chromosomes 17 and 3, respectively (Evans, 1988; Williams, 2000). All subtypes except TRα2 bind triiodothyronine (T3). The exact physiological role of TRα2 remains uncertain (Glass & Holloway, 1990; Chen & Evans, 1995) and it belongs to the group of TRs due to its structural similarities.
TRs act to increase or decrease the rates of transcription of target genes by interacting with specific DNA sequences named thyroid hormone response elements (TRE). Even in the absence of T3, TRs may still bind to TRE to repress basal transcription in vivo. This suggests that TRs can regulate transcription by both T3-dependent and T3-independent mechanisms (Chen & Evans, 1995; Göthe et al. 1999). Several studies have shown that TRs are widely distributed, albeit at different levels in different tissues (Brent, 1994).
In order to find out the specific role of the TRs, mice deficient in one or several of the different subtypes of TR have been developed. Deletion of TRα1 (TRα1−/−) has rendered a phenotype that has a decreased heart rate and a lower body temperature (Johansson et al. 1998; Wikström et al. 1998). Mice deficient in β1, β2 and β3 (TRβ−/−) have high thyroid hormone and thyroid stimulating hormone (TSH) levels, indicating that TRβ has a specific role in the feedback control of the pituitary-thyroid axis. These mice also serve as a model for human resistance to thyroid hormone that is associated with TRβ mutation (Refetoff, 1993). Mice that lack all known subtypes of TRs (TRα1−/−β−/−) are growth retarded and have extremely high thyroid hormone and TSH levels, lower body temperature and a decreased heart rate (Johansson et al. 1999; Göthe et al. 1999).
Thyroid hormone has a fundamental role in skeletal muscle function and affects both physiological and biochemical properties in the young and adult vertebrate (Finkelstein et al. 1991) with a preferential influence on slow-twitch muscle (Nicol & Maybee, 1982; Caiozzo & Haddad, 1996). Results from studies of muscle mechanics show that alterations in thyroid hormone levels induce changes in both maximum shortening velocity (Vmax), determined from isotonic measurements, and contraction and relaxation times measured in the isometric twitch (Everts et al. 1983; Caiozzo & Haddad, 1996). While Vmax depends on the myosin heavy chain (MHC), isometric contraction and relaxation times depend on both the MHC and the sarcoplasmic reticulum (SR) Ca2+ pumps (SERCa). The relative proportions of the different isoforms of MHC and SERCa are affected by thyroid hormone (Fitts et al. 1980; Caiozzo et al. 1993; Muller et al. 1994).
In a previous study, we compared soleus muscles from TRα1−/− mice or TRβ−/− mice with wild-type controls (Johansson et al. 2000). The results showed 20–60 % longer contraction and relaxation times of twitches and tetani in soleus muscles from TRα1−/− mice compared with those from the wild-type, whereas no such slowing was observed in soleus muscles from TRβ−/− mice. These changes were rather modest compared with those seen in hypothyroidism. This might indicate that TRα1 and TRβ are, to some extent, able to substitute for each other. We have now measured contractile properties, fatigue resistance and recovery in fast-twitch extensor digitorum longus (EDL) and slow-twitch soleus muscles of mice that lack all known subtypes of TRs, TRα1−/−β−/− mice. Measurements of the content of important muscle proteins known to be affected by thyroid hormones were also performed.
METHODS
Animals
Experiments were performed on male TRα1−/−β−/− and wild-type mice of the same age (12–14 weeks). These mice were generated by crossing TRβ−/− mice (on a hybrid background of 129/Sv and C57Bl/6J strains) (Forrest et al. 1996) and TRα1−/− mice (on a hybrid background of 129/OlaHsd and BALB/c) (Wikström et al. 1998) to generate both wild-type and TRα1−/−β−/− mice on the same mixed background. TRα1−/−β−/− mice were typically viable for at least 12 months, although they had a slightly increased mortality rate (∼30 %) as compared with wild-type littermates (0 %) (Göthe et al. 1999). All animals were housed under a cycle of 12 h light-12 h dark in a temperature- (21–23 °C) and humidity- (40–55 %) regulated room. Water and food were provided ad libitum. The experimental procedures were approved by the Stockholm North local ethical committee.
General
Animals were killed by cervical dislocation. Intact EDL and soleus muscles were dissected from both legs. Muscles were mounted in a stimulation chamber, which had a volume of 50 ml and was filled with continuously stirred Tyrode solution of the following composition (mm): 121 NaCl, 5 KCl, 0.5 MgCl2, 1.8 CaCl2, 0.4 NaH2PO4, 0.1 NaEDTA, 24 NaHCO3 and 5.5 glucose. Fetal calf serum (0.2 %) was added to the solution. The solution was continuously bubbled with 95 % O2−5 % CO2, which gives a bath pH of 7.4. All experiments were carried out at room temperature (24 °C).
Muscles were mounted between a fixed stainless steel hook and a hook attached to a lab-built force transducer. Muscles were stimulated with supramaximal electrical pulses (duration 0.5 ms; intensity about 150 % of that giving maximum contractile response). The stimulation pulses were applied via platinum plate electrodes placed on each side of the muscle and extending the whole length of the muscle. The resulting force was recorded and digitized (1 kHz; Axotape, Axon Instruments, Inc., CA, USA) and stored in a personal computer. After being mounted, a few tetanic contractions were produced to find the length that gave the maximum tetanic force response. This length was measured and muscles were kept at this length throughout the experiments. Muscles were then allowed to rest for at least 30 min in the oxygenated Tyrode solution before measurements were conducted. Whilst one muscle was being studied, the others were maintained in the oxygenated Tyrode solution. One EDL and one soleus muscle from each animal was used in the contractile studies. Muscles from both sides were pooled for protein content analysis.
Protocol
EDL muscles were stimulated to give a single twitch or 300 ms tetani at 10–150 Hz; soleus muscles were stimulated to give a single twitch or 1 s tetani at 10–100 Hz. These contractions were produced at 1 min intervals. Peak force in each contraction was measured. The specific force (i.e. force per cross-sectional area) produced by each muscle was calculated from the absolute force, muscle weight and muscle length, assuming a density of 1.056 g ml−1; note that this procedure does not correct for differences in muscle architecture and therefore direct comparisons of the specific force of EDL and soleus muscles cannot be made. Relative forces are presented as percentages of the maximal force (obtained at 150 Hz in EDL and at 100 Hz in soleus) in each muscle. Twitch kinetics were assessed by measuring the contraction time (i.e. from the onset of force production until peak force was produced) and the half-relaxation time (i.e. from peak force production until force was reduced to 50 % of the peak). Kinetics were also assessed in 150 and 100 Hz tetani in EDL and soleus, respectively, by measuring the half-contraction time (i.e. from the onset of contraction until 50 % of the maximum tetanic force was produced) and the half-relaxation time (i.e. from the last stimulation pulse until force was reduced to 50 % of the maximum).
After the force-frequency relationship had been established, the muscle was fatigued by tetani given at 3 s intervals. For EDL muscles we used 70 Hz, 300 ms tetani and the total number of contractions was 50. Soleus muscles were fatigued by 50 Hz, 600 ms tetani and a total of 100 tetani were produced. The recovery of force was studied by giving a tetanus at regular intervals up to 15 min after the end of fatiguing stimulation. Thereafter as much as possible of the tendons was cut off and the muscle was weighed before it was frozen in liquid nitrogen.
Protein immunoblot analysis (Western blot)
Muscles were thawed and the two EDL and the two soleus muscles from each animal were pooled and muscle proteins were isolated as described previously (Ploug et al. 1993). Protein concentration was determined by the bicinchoninic acid assay (Pierce Biotechnology, Rockford, IL, USA) using bovine serum albumin as the standard. For semi-quantification of different proteins, 1, 2 and 5 μg of the protein preparation were loaded onto a polyvinylidene difluoride (PVDF) filter membrane by the use of a slot blot filtration manifold (Minifold II, Schleicher & Schuell GmbH, Dassel, Germany). PVDF membranes were blocked by incubation for 1 h at room temperature or overnight at 4 °C in 10 % non-fat dried milk in Tris-buffered saline, pH 7.5 with 0.1 % Tween-20 (TBS-T). The PVDF membranes were then incubated with primary antibodies diluted in 10 % dried milk in TBS-T, washed in five changes of TBS-T and then incubated for 1 h with either anti-mouse (NA931) or anti-rabbit (NA934) immunoglobulin G conjugated to horseradish peroxidase (Amersham, Oakland, Ontario, Canada). The PVDF membranes were washed in five changes of TBS-T and the immunoreactive bands were detected by the enhanced chemiluminescence method (Amersham). The membranes were exposed to Hyperfilm-ECL (Amersham) for various times and the signal intensity of the slots on the film was quantified with the ImageQuant software (Molecular Dynamics Pty Ltd, Queensland, Australia). The mean signal intensity of EDL and soleus muscles from wild-type muscles was set to 100 % and values are presented relative to this.
The primary antibodies used were 1:250 anti-Na- K-ATPase α1-subunit (MA3–929, Affinity Bioreagents), 1:1000 anti-Na-K-ATPase α2-subunit (no. 06–168, Upstate Biotechnology), 1:2500 anti-SERCa1 (MA3–912, Affinity Bioreagents), 1:1000 anti-SERCa2 (MA3–919, Affinity Bioreagents) and 1:750 anti-citrate synthase (MAB 3087, Chemicon). As controls we used rat kidney and rat brain microsomal preparation (Upstate Biotechnology, nos 12–146 and 12–144, respectively).
Statistics
All values were expressed as means ±s.e.m. Student's unpaired t tests were used to determine statistical significance between TRα1−/−β−/− and wild-type muscles. The level of significance was set at P < 0.05 or as indicated.
RESULTS
Contraction and relaxation times
Figure 1 shows typical examples of twitches as well as intermediate and maximal tetani produced in EDL (A-C) and soleus muscles (D-F) from TRα1−/−β−/− and wild-type mice. EDL muscles from TRα1−/−β−/− and wild-type mice showed no significant differences in contraction and half-relaxation times, neither in twitches nor 150 Hz tetani (Table 1). In soleus muscles from TRα1−/−β−/− mice, on the other hand, contraction and half-relaxation times were up to twofold longer than in wild-type controls.
Figure 1. Soleus muscles of TRα1−/−β−/− mice are markedly slower than controls, whereas the difference in EDL muscles is small.
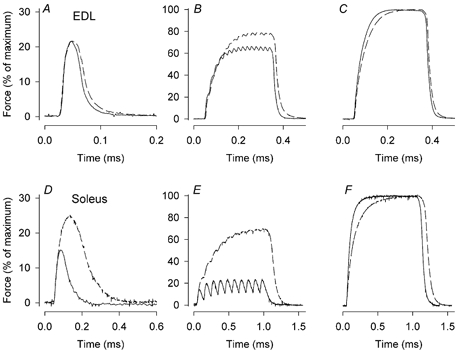
Typical force records from an EDL muscle (A-C) and a soleus muscle (D-E) of a wild-type (continuous lines) and a TRα1−/−β−/− (dashed lines) mouse. Records are from twitches (A and D), intermediate frequency tetani (50 Hz for EDL (B) and 10 Hz for soleus (E)) and maximal tetani (150 Hz for EDL (C) and 100 Hz for soleus (F)). Note that twitches are shown on expanded scales.
Table 1.
Contractile properties of EDL and soleus muscles of wild-type and TRα1−/−β−/− mice
| EDL | Soleus | |||
|---|---|---|---|---|
| Wt | TRα1−/−β−/− | Wt | TRα1−/−β−/− | |
| Twitch | ||||
| Contraction time (ms) | 23 ± 1.5 | 24 ± 1.2 | 34 ± 2.2 | 67 ± 6.9*** |
| Half relaxation time (ms) | 19 ± 2.0 | 29 ± 5.9 | 45 ± 2.2 | 90 ± 5.2*** |
| Absolute force (mN) | 89 ± 13.0 | 70 ± 2.2 | 22 ± 3.7 | 33 ± 4.7 |
| Tetanus | ||||
| Half contraction time (ms) | 32 ± 1.2 | 34 ± 2.4 | 60 ± 2.5 | 80 ± 2.7*** |
| Half relaxation time (ms) | 37 ± 3.0 | 39 ± 0.9 | 97 ± 7.8 | 144 ± 7.3** |
| Absolute force (mN) | 370 ± 17.0 | 296 ± 17.1* | 152 ± 24.5 | 135 ± 14.5 |
| Muscle weight (mg) | 14.2 ± 0.4 | 10.9 ± 0.7** | 11.9 ± 0.8 | 9.8 ±0.3* |
| Muscle length (mm) | 11.6 ± 0.3 | 10.7 ± 0.2* | 11.3 ± 0.3 | 10.5 ± 0.3 |
| Specific force (mN mm−2) | 320 ± 24.0 | 317 ± 26.4 | 159 ± 33.0 | 154 ± 18.3 |
Wt, wild-type. Values are presented as means ±s.e.m. for wild-type (Wt; n = 5) and TRα1−/−β−/− (n = 6) muscles.
P < 0.05
P < 0.01
P < 0.001 denote significant difference between TRα1−/−β−/− mice and their wild-type controls. Note that our estimate of the specific force does not correct for differences in muscle architecture between EDL and soleus muscles and therefore a direct comparison between specific forces of the two muscles cannot be performed.
Muscle weights were significantly lower and the absolute force in maximal tetani was lower (significant only in EDL) in TRα1−/−β−/− mice compared with controls (Table 1). However, the force per cross-sectional area (as estimated from the absolute force, muscle weight and muscle length) was not different in TRα1−/−β−/− and wild-type muscles.
Force-frequency relationship
Mean data on the force-frequency relationship are shown in Fig. 2. Soleus muscles from TRα1−/−β−/− mice developed a higher relative force at all frequencies up to 50 Hz compared with controls. That is, there was a clear leftward shift of the force-frequency relationship with a frequency giving 50 % force of 6.1 ± 0.6 Hz in TRα1−/−β−/− mice and 19.3± 3.3Hz in controls. There was a tendency towards a leftward shift of the force-frequency relationship in EDL muscles of TRα1−/−β−/− mice, but the mean relative force was only significantly higher than wild-type controls at 50 Hz.
Figure 2. Soleus muscles of TRα1−/−β−/− mice show a marked leftward shift of the force-frequency relationship.
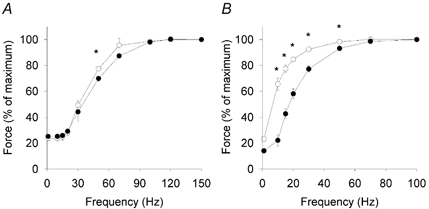
Force-frequency relationship of EDL (A) and soleus muscles (B) from TRα1−/−β−/− mice (○; n = 6 animals) and wild-type control mice (•; n = 5 animals). Values are presented as means ±s.e.m.*Significant difference (P < 0.05) between TRα1−/−β−/− mice and their wild-type controls.
Fatigue and recovery
Muscle weakness and reduced fatigue tolerance are prominent symptoms in hypothyroid patients (Wiles et al. 1979). We therefore studied force production during fatigue produced by intermittent tetanic stimulation. Mean data are shown in Fig. 3. There was no significant difference in the fatigue resistance in EDL muscles, although a tendency for a more rapid decline in force was seen in TRα1−/−β−/− mice compared with controls. On the other hand, soleus muscles from TRα1−/−β−/− mice were more fatigue resistant and produced significantly higher mean relative forces during the series of fatiguing tetani than did controls.
Figure 3. Soleus muscles of TRα1−/−β−/− are more fatigue resistant than wild-type controls.
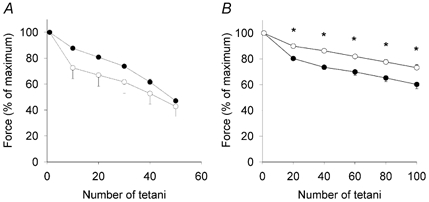
Mean data (±s.e.m.) from fatigue produced by repeated tetani. Data from TRα1−/−β−/− mice (○; n = 5 animals) and wild-type controls (•; n = 6 animals) from EDL (A) and soleus muscle (B) are presented. *Significant difference (P < 0.05) between TRα1−/−β−/− mice and their wild-type controls.
Recovery from fatigue was studied and 15 min after the end of fatiguing stimulation, tetanic force was fully restored in both TRα1−/−β−/− and wild-type soleus muscles. There was no significant difference between the recovery in TRα1−/−β−/− and wild-type EDL muscles and at 15 min they produced 83± 2 and 72 ± 6 % of the original force, respectively.
Western blot
Protein analyses showed that expression of the slow isoform of SR Ca2+ pumps (SERCa2) was markedly increased, whereas the level of the fast type (SERCa1) and the α2-subunit of the Na+−K+ pump were decreased in soleus muscles of TRα1−/−β−/− mice compared with those of controls (Table 2). There was no significant difference in the concentration of the α1-subunit of the Na+−K+ pump in soleus muscles, although it tended to be higher in TRα1−/−β−/− mice. In EDL muscles there were no significant differences between TRα1−/−β−/− and controls in any of the pump proteins analysed.
Table 2.
Western blot analysis of EDL and soleus muscles from wild-type and TRα1−/−β−/− mice
| EDL | Soleus | |||
|---|---|---|---|---|
| Wt | TRα1−/−β−/− | Wt | TRα1−/−β−/− | |
| SRCa2+ pumps | ||||
| SERCa1 | 100 ± 11.7 | 99.4 ± 17.4 | 100 ± 4.41 | 73.2 ± 7.77** |
| SERCa2 | — | — | 100 ± 35.5 | 536 ± 126*** |
| Na+-K+ pump subunits | ||||
| α1 | 100 ± 29.7 | 114 ± 66.9 | 100 ± 18.9 | 164 ± 76.3 |
| α2 | 100 ± 26.6 | 75 ± 21 | 100 ± 12.6 | 26.6 ± 10.4*** |
| Citrate synthase | 100 ± 16.0 | 104 ± 12.1 | 100 ± 14.8 | 105 ± 16.6 |
Wt, wild-type. Values are means ±s.e.m. (n = 5). The mean value in Wt is set to 100.
P < 0.01
P < 0.001.
Citrate synthase was measured to get an indication of changes in mitochondrial density in TRα1−/−β−/− muscles. The results of this analysis showed no significant differences between TR-deficient and control muscles (see Table 2).
DISCUSSION
The present study shows a reduced muscle weight and absolute force production in fast-twitch EDL muscles of mice devoid of all known TRs compared with those of wild-type controls. Except for these changes, EDL muscles of TRα1−/−β−/− mice display only subtle changes. Conversely, soleus muscles of TRα1−/−β−/− mice show several major changes: (i) markedly slower contraction and relaxation; (ii) a marked leftward shift of the force- frequency relationship; (iii) increased fatigue resistance; and (iv) marked alterations in protein composition. Thus, major changes were observed in the slow-twitch muscle, whereas only slight differences were seen in the fast-twitch muscle. This fits with earlier data showing that alteration in thyroid hormone status mainly affects slow-twitch fibres (Larsson et al. 1994; Caiozzo & Haddad, 1996). It is worth noting that the locomotor activity of TRα1−/−β−/− mice is not altered compared with that of wild-type controls (Johansson et al. 1999), which otherwise might have been a confounding factor.
The muscle weight is markedly reduced in TRα1−/−β−/− mice compared with that in wild-type controls (Yu et al. 2000; present results). We also found a reduction in the absolute tetanic force, although this difference was only significant in EDL muscles. However, the force per cross-sectional area, which takes into account the difference in muscle size, was not different between TR-deficient and wild-type muscles. This leads to two important conclusions. First, despite the marked switch from SERCa1 to SERCa2 in TRα1−/−β−/− soleus muscles compared with those of wild-type (Table 2), the SR in the TR-deficient muscles appears to take up and subsequently release a similar amount of Ca2+. Second, although there is a marked switch to the slow, type I myosin heavy chain (MHC) in soleus muscles of TRα1−/−β−/− mice and a corresponding reduction in fast, type IIa MHC (Yu et al. 2000), the tetanic force measured here per cross-sectional area was not different, which would indicate that there is no intrinsically lower force production by type I MHC than by type IIa MHC. It should be noted that our estimate of the force per cross-sectional area does not correct for possible changes in muscle architecture (see Methods) and any difference in this parameter between TRα1−/−β−/− and wild-type muscles might affect our results.
Soleus muscles of TRα1−/−β−/− mice display marked slowing of contraction and relaxation in twitches and tetani, whereas no significant changes of these parameters occur in EDL muscles (see Table 1). Slowed kinetics in isometric contractions can, in principle, be due to slowed cross-bridge kinetics or slower intracellular Ca2+ handling (Westerblad et al. 1997). Yu et al. (2000) have shown a major switch from fast to slow MHC isoforms in the slow-twitch soleus muscle of TRα1−/−β−/− mice, whereas only a minor transition was observed in the fast-twitch EDL muscle. We show here a marked switch from the fast isoform of the SR Ca2+ pump (SERCa1) to the slow isoform (SERCa2) in soleus muscles, which is in line with a fast-to-slow transition in these muscles. Furthermore, no change in the fast SERCa1 was observed in EDL muscles, supporting the idea that fast-twitch muscle is little affected.
Earlier studies of expression of MHC isoforms in soleus muscle have shown that it is moderately altered in TRα1−/− mice, unaltered in TRβ−/− mice and dramatically changed in TRα1−/−β−/− mice (Yu et al. 2000). These observations suggest that TRα1 and TRβ are able to functionally substitute for each other. Our analyses of contractile speed are in line with this. Soleus muscles of TRα1−/− mice showed an increase in twitch contraction and relaxation times of about 45 %, whereas no significant changes were observed in TRβ−/− mice (Johansson et al. 2000), compared to an increase of about 100 % in TRα1−/−β−/− mice in the present study. The slowing of tetanic relaxation and the leftward shift of the force-frequency relationship were also substantially larger in TRα1−/−β−/− mice. Finally, changes in SERCa expression were more prominent in soleus muscles of TRα1−/−β−/− mice than in TRα1−/− or TRβ−/− mice.
During the repeated tetanic stimulation, soleus muscles of TRα1−/−β−/− mice were more fatigue resistant than their controls. At first glance, the increased fatigue resistance appears to be in conflict with the situation in humans with hypothyroidism, where decreased fatigue resistance is a frequently reported symptom (Wiles et al. 1979). It must be noted that the conditions are markedly different in the type of experiments performed in most animal studies, including the present study, from those in humans during exercise. In the present study, fatigue resistance was measured as the decline of force during a series of close-to-maximal tetani. In exercising humans, maximal force production is seldom required and fatigue is often defined as failure to maintain the required or expected force (Edwards & Wiles, 1981). The sensation of decreased fatigue resistance in patients with hypothyroidism may then be related to the reduced muscle mass and hence reduced absolute force production. This would mean that various types of physical activity must be performed at a higher relative muscle load, which would lead to premature fatigue. In addition, reduced thyroid hormone levels are generally associated with decreased oxidative capacity (Zoll et al. 2001), which would contribute to the reduced fatigue resistance in humans with hypothyroidism.
The Na+−K+ pump is a heterodimeric enzyme composed of two polypeptide subunits: an α subunit and a β subunit. Four α subunit types have been identified (α1–4) and α1 and α2 are the dominant isoforms in adult murine skeletal muscle (Hundal et al. 1994; He et al. 2001). We show a marked decrease in the expression of the α2 subunit in soleus muscles of TRα1−/−β−/− mice, which was not mirrored by a similar increase in the α1 subunit (Table 2). Thus, the total amount of Na+−K+ pumps would have been reduced in TRα1−/−β−/− soleus muscles. This agrees with the situation in hypothyroidism where the concentration of Na+−K+ pumps is decreased, mainly due to a reduced expression of the ouabain-sensitive α2 subunit (Everts, 1996). Reduced Na+−K+ pump capacity has been associated with reduced fatigue resistance, especially during continuous high-frequency stimulation (Clausen et al. 1998). However, TRα1−/−β−/− soleus muscles were more fatigue resistant than wild-type controls. This indicates that the rate of Na+−K+ pumping is not of major importance during the type of fatiguing stimulation (repeated tetani) used in the present study.
There was no difference in the expression of the mitochondrial enzyme citrate synthase in TRα1−/−β−/− and wild-type muscles. This suggests that there were no major changes in mitochondrial density in TRα1−/−β−/− muscles, which differs from the normal situation in hypothyroidism (Zoll et al. 2001). At any rate, the increased fatigue resistance in TRα1−/−β−/− soleus muscles cannot be explained by an increased capacity for oxidative ATP production. The increased fatigue resistance is therefore probably related to a switch to the slow isoforms of MHC (type I) and SERCa (SERCa2) and hence a reduced rate of ATP consumption during contractions. In line with this, the decline in force during fatiguing stimulation was not significantly different in TRα1−/−β−/− and wild-type EDL muscles and these muscles display little change in MHC composition (Yu et al. 2000), SERCa composition or force kinetics (present results).
Even though the TRα1−/−β−/− mice have extremely high thyroid hormone and TSH levels (Göthe et al. 1999), our findings mimic changes seen in hypothyroidism. This argues against the possibility that unidentified T3 receptors remain in TRα1−/−β−/− muscle. However, one might expect more dramatic phenotypic differences in TRα1−/−β−/− mice since congenital hypothyroidism results in irreversible mental and physical retardation, unless treatment is initiated within weeks after birth. In congenital hypothyroidism, muscle fibres may show swelling, loss of normal striations and separation by mucinous deposits (Vickery et al. 1966). Several reasons for the milder phenotype in TRα1−/−β−/− muscle can be suggested. Since the TRα1−/−β−/− mice have had their deficiency from the very beginning, more extensive compensatory effects might have occurred. Furthermore, an unoccupied TR (as in hypothyroid subjects) can strongly repress the basal expression level of target genes, whereas an absence of receptor (as in the case of the TR-deficient mice) would allow the genes to be expressed at a basal level but not to be regulated by thyroid hormone. The physiological consequences would therefore differ in that the hypothyroid animals would be likely to exhibit more severe symptoms of the hormonal disorder than receptor-deficient mice.
In conclusion, skeletal muscles of TRα1−/−β−/− mice show changes in muscle function and protein content similar to those observed in hypothyroidism. The changes in TRα1−/−β−/− muscles were clearly larger than those previously observed in muscles from mice deficient in either TRα1 or TRβ. This suggests some functional overlap between TRα1 and TRβ in skeletal muscle. This finding might be of great importance in the future when specific agonists and antagonists are developed, because treatment with a TR-specific agent will have limited side effects on skeletal muscle function.
Acknowledgments
We thank Ingvild B. Hoen for technical assistance with Western blot analyses. This study was supported by grants from the Swedish Research Council (Nos 4764 & 10842), Human Frontier Science program (RG0 318/1997), Cancerfonden, Swedish Heart and Lung Foundation, the Swedish National Centre for Sports Research and Funds at the Karolinska Institutet.
REFERENCES
- Brent GA. The molecular basis of thyroid hormone action. N Engl J Med. 1994;331:847–853. doi: 10.1056/NEJM199409293311306. [DOI] [PubMed] [Google Scholar]
- Caiozzo VJ, Haddad F. Thyroid hormone: modulation of muscle structure, function, and adaptive responses to mechanical loading. Exerc Sport Sci Rev. 1996;24:321–361. [PubMed] [Google Scholar]
- Caiozzo VJ, Swoap S, Tao M, Menzel D, Baldwin KM. Single fiber analyses of type IIA myosin heavy chain distribution in hyper- and hypothyroid soleus. Am J Physiol. 1993;265:C842–850. doi: 10.1152/ajpcell.1993.265.3.C842. [DOI] [PubMed] [Google Scholar]
- Chen JD, Evans RM. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature. 1995;377:454–457. doi: 10.1038/377454a0. [DOI] [PubMed] [Google Scholar]
- Clausen T, Nielsen OB, Harrison AP, Flatman JA, Overgaard K. The Na+, K+ pump and muscle excitability. Acta Physiol Scand. 1998;162:183–190. doi: 10.1046/j.1365-201X.1998.0295e.x. [DOI] [PubMed] [Google Scholar]
- Edwards RH, Wiles CM. Energy exchange in human skeletal muscle during isometric contraction. Circ Res. 1981;48:I11–17. [PubMed] [Google Scholar]
- Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts ME. Effects of thyroid hormones on contractility and cation transport in skeletal muscle. Acta Physiol Scand. 1996;156:325–333. doi: 10.1046/j.1365-201X.1996.203000.x. [DOI] [PubMed] [Google Scholar]
- Everts ME, van Hardeveld C, Ter Keurs HE, Kassenaar AA. Force development and metabolism in perfused skeletal muscle of euthyroid and hyperthyroid rats. Horm Metab Res. 1983;15:388–393. doi: 10.1055/s-2007-1018732. [DOI] [PubMed] [Google Scholar]
- Finkelstein DI, Andrianakis P, Luff AR, Walker D. Effects of thyroidectomy on development of skeletal muscle in fetal sheep. Am J Physiol. 1991;261:R1300–1306. doi: 10.1152/ajpregu.1991.261.5.R1300. [DOI] [PubMed] [Google Scholar]
- Fitts RH, Winder WW, Brooke MH, Kaiser KK, Holloszy JO. Contractile, biochemical, and histochemical properties of thyrotoxic rat soleus muscle. Am J Physiol. 1980;238:C14–20. doi: 10.1152/ajpcell.1980.238.1.C15. [DOI] [PubMed] [Google Scholar]
- Forrest D, Hanebuth E, Smeyne RJ, Everds N, Stewart CL, Wehner JM, Curran T. Recessive resistance to thyroid hormone in mice lacking thyroid hormone receptor beta: evidence for tissue-specific modulation of receptor function. EMBO J. 1996;15:3006–3015. [PMC free article] [PubMed] [Google Scholar]
- Glass CK, Holloway JM. Regulation of gene expression by the thyroid hormone receptor. Biochim Biophys Acta. 1990;1032:157–176. doi: 10.1016/0304-419x(90)90002-i. [DOI] [PubMed] [Google Scholar]
- Göthe S, Wang Z, Ng L, Kindblom JM, Barros AC, Ohlsson C, Vennström B, Forrest D. Mice devoid of all known thyroid hormone receptors are viable but exhibit disorders of the pituitary-thyroid axis, growth, and bone maturation. Genes Dev. 1999;13:1329–1341. doi: 10.1101/gad.13.10.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Shelly DA, Moseley AE, James PF, James JH, Paul RJ, Lingrel JB. The α1- and α2-isoforms of Na-K-ATPase play different roles in skeletal muscle contractility. Am J Physiol Regul Integr Comp Physiol. 2001;281:R917–925. doi: 10.1152/ajpregu.2001.281.3.R917. [DOI] [PubMed] [Google Scholar]
- Hundal HS, Maxwell DL, Ahmed A, Darakhshan F, Mitsumoto Y, Klip A. Subcellular distribution and immunocytochemical localization of Na, K-ATPase subunit isoforms in human skeletal muscle. Mol Membr Biol. 1994;11:255–262. doi: 10.3109/09687689409160435. [DOI] [PubMed] [Google Scholar]
- Johansson C, Göthe S, Forrest D, Vennström B, Thorén P. Cardiovascular phenotype and temperature control in mice lacking thyroid hormone receptor-β or both α1 and β. Am J Physiol. 1999;276:H2006–2012. doi: 10.1152/ajpheart.1999.276.6.H2006. [DOI] [PubMed] [Google Scholar]
- Johansson C, Lännergren J, Lunde PK, Vennström B, Thorén P, Westerblad H. Isometric force and endurance in soleus muscle of thyroid hormone receptor-α1- or -β-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2000;278:R598–603. doi: 10.1152/ajpregu.2000.278.3.R598. [DOI] [PubMed] [Google Scholar]
- Johansson C, Vennström B, Thorén P. Evidence that decreased heart rate in thyroid hormone receptor-α1-deficient mice is an intrinsic defect. Am J Physiol. 1998;275:R640–646. doi: 10.1152/ajpregu.1998.275.2.R640. [DOI] [PubMed] [Google Scholar]
- Larsson L, Li X, Teresi A, Salviati G. Effects of thyroid hormone on fast- and slow-twitch skeletal muscles in young and old rats. J Physiol. 1994;481:149–161. doi: 10.1113/jphysiol.1994.sp020426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller A, van der Linden GC, Zuidwijk MJ, Simonides WS, van der Laarse WJ, van Hardeveld C. Differential effects of thyroid hormone on the expression of sarcoplasmic reticulum Ca2+-ATPase isoforms in rat skeletal muscle fibers. Biochem Biophys Res Commun. 1994;203:1035–1042. doi: 10.1006/bbrc.1994.2286. [DOI] [PubMed] [Google Scholar]
- Nicol CJ, Maybee SH. Contractile properties and fibre composition of rat skeletal muscle: effect of mild hyperthyroidism. J Exp Physiol. 1982;67:467–472. doi: 10.1113/expphysiol.1982.sp002662. [DOI] [PubMed] [Google Scholar]
- Ploug T, Wojtaszewski J, Kristiansen S, Hespel P, Galbo H, Richter EA. Glucose transport and transporters in muscle giant vesicles: differential effects of insulin and contractions. Am J Physiol. 1993;264:E270–278. doi: 10.1152/ajpendo.1993.264.2.E270. [DOI] [PubMed] [Google Scholar]
- Refetoff S. Resistance to thyroid hormone. Clin Lab Med. 1993;13:563–581. [PubMed] [Google Scholar]
- Vickery AL, Fierro-Benitez R, Kakulas BA. Skeletal muscle structure in endemic cretinism. Am J Pathol. 1966;49:193–201. [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Lännergren J, Allen DG. Slowed relaxation in fatigued skeletal muscle fibers of Xenopus and mouse. Contribution of [Ca2+]i and cross-bridges. J Gen Physiol. 1997;109:385–399. doi: 10.1085/jgp.109.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abnormal heart rate and body temperature in mice lacking thyroid hormone receptor α1. EMBO J. 1998;17:455–461. doi: 10.1093/emboj/17.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiles CM, Young A, Jones DA, Edwards RH. Muscle relaxation rate, fibre-type composition and energy turnover in hyper- and hypo-thyroid patients. Clin Sci. 1979;57:375–384. doi: 10.1042/cs0570375. [DOI] [PubMed] [Google Scholar]
- Williams GR. Cloning and characterization of two novel thyroid hormone receptor β isoforms. Mol Cell Biol. 2000;20:8329–8342. doi: 10.1128/mcb.20.22.8329-8342.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Göthe S, Wikström L, Forrest D, Vennström B, Larsson L. Effects of thyroid hormone receptor gene disruption on myosin isoform expression in mouse skeletal muscles. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1545–1554. doi: 10.1152/ajpregu.2000.278.6.R1545. [DOI] [PubMed] [Google Scholar]
- Zoll J, Ventura-Clapier R, Serrurier B, Bigard AX. Response of mitochondrial function to hypothyroidism in normal and regenerated rat skeletal muscle. J Muscle Res Cell Motil. 2001;22:141–147. doi: 10.1023/a:1010521108884. [DOI] [PubMed] [Google Scholar]


