Abstract
Hibernation is characterised by a global reduction of metabolism, body temperature and blood flow, while arousal from hibernation is achieved by the reversal of these processes. Our experiments were performed on Syrian hamsters that had been chronically implanted with a cortical thermocouple and an optical fibre over the contralateral cortex, and acutely implanted with thermocouples in the rectal, cheek pouch and interscapular brown adipose tissue (BAT). Measurements revealed large thermal gradients in the body of the arousing animals. Maximum whole-body metabolic rate, which was 2.4 times normal cenothermic resting metabolic rate, coincided not with rectal temperature but more closely with respiratory rate (RR) or BAT temperature. Regional cortical blood flow (rCBF), as measured by laser-Doppler flowmetry, changed in parallel with whole-body metabolic rate, peaking at 3.8 times the normal cenothermic resting levels, when rectal temperature was 15 °C. When BAT temperature was less than 25 °C, RR, rCBF and heart rate (HR) were decreased by breathing hypercapnic gas, but these parameters were unresponsive to hyperoxic gases. At cenothermia the RR and rCBF of anaesthetised hamsters was increased by exposure to hypercapnic gases. Exposure to hyperoxic gas decreased RR but had no effect on rCBF. The mechanisms regulating rCBF, HR and RR exhibit state-dependent sensitivities to hypercapnic and hyperoxic stimuli. The large increase in rCBF observed during arousal implies that cerebral autoregulation is temporarily suspended and suggests that hamsters effectively use endogenous mechanisms to minimise the pathology normally associated with dramatic increases in rCBF.
Whole-animal mammalian hibernation can be generalised as a global reduction in metabolic rate, body temperature and blood flow in response to cold, dark environments (Hashimoto et al. 2002). At its simplest, a bout of hibernation can be divided into three periods with essentially separate physiologies: a cooling or entrance phase, a maintenance phase and a warming or arousing phase when body temperature rises to cenothermic levels (36–37 °C; cenothermia is the IUPS term replacing euthermy: IUPS, 2001). Despite the low body temperatures (2–5 °C) in the maintenance phase of hibernation, some species of hibernators are capable of regulation of respiratory rate (RR), heart rate (HR; Lyman, 1951) and body temperature via changes in metabolic rate (Lyman & O'Brien, 1972). RR and HR are probably modulated by changing the balance between parasympathetic and sympathetic tone (Harris & Milsom, 1995), while changes in body temperature occur by unknown complex mechanisms.
Syrian hamsters (Mesocricetus auratus) arouse from hibernation at regular intervals of approximately 90–100 h or in response to mechanical or vibrational stimuli. Arousal to cenothermia involves a dynamic transformation of biochemistry, and hence body state, via the coordinated recruitment of physiological sequences that generate, within a 2–3 h period, and then maintain a 30 °C increase in whole-body temperature. Arousal from hibernation can be likened to an accelerator period, in which sympathetic activity appears to dominate (Chatfield & Lyman, 1950; Harris & Milsom, 1995). During arousal, body temperature increases from slightly above ambient (4–6 °C) to cenothermic levels via the expenditure of energy, which, in other hibernating species, is recorded as peaking with the onset of shivering thermogenesis at approximately three times the resting cenothermic levels (Wang, 1978; Toien et al. 2001).
In hamsters arousing from hibernation, systemic blood pressure (BP) and HR are initially very low and increase to normal levels. BP increases faster than HR and attains near-normal pressure coincident with the onset of maximal metabolic rate (Chatfield & Lyman, 1950). Brain blood flow (BBF) during the maintenance phase of hibernation, as measured by radioisotope dilution, is approximately 10 % of resting cenothermic levels (Frericks et al. 1994). It is presumed to have returned to normal levels after arousal, although the temporal profile of this increase and the response to stimuli during this period of transition are unknown.
In cenothermic animals, provided BP is maintained above and below critical limits, BBF is regulated within a narrow range despite changes in cerebral perfusion pressure (Paulsen et al. 1990). Cerebral autoregulation is facilitated by stretch receptors and contraction of the smooth muscles lining cerebral blood vessels and by chemical receptivity to CO2, pH, nitric oxide (NO), O2, ion channel antagonists and agonists and a range of neuromodulators (Brian et al. 1996; Szabo, 1996; Gulbenkian et al. 2001). Inspiration of hypercapnic stimuli per se and/or decreases in pH, produces relaxation of smooth muscles and increased BBF via alteration of sympathetic tone with NO-dependent and NO-independent components (Iadecola & Zhang, 1994; Csete et al. 2001). Hypercapnia increases HR and RR (Shams, 1985; Melton et al. 1988; Bianchi et al. 1995) by direct action upon widely distributed chemosensitive cells in the ventral medulla that project to motor neurons driving respiratory muscles, or indirectly via the influence of peripheral chemoreceptors upon the nucleus tractus solitarius and ultimately the medullary respiratory group (Bianchi et al. 1995).
In animals arousing from hibernation, cerebral haemodynamic parameters are largely unknown. However, separate investigations have suggested that during arousal, BBF increases from very low levels to normal levels as temperature increases and metabolic rate and O2 consumption increase dramatically. At cenothermia, an analogous profile of changes occurs during recovery from reduced BBF and ischaemia; however, at cenothermic brain temperatures these changes are associated with oxidative stress via increased generation of reactive O2 species and damage to neural tissue (Kuroda & Siesjo, 1997). As an initial step towards understanding the mechanisms by which large changes in BBF occurring with high metabolic rates are routinely tolerated during arousal, we first determined whether changes in regional cortical blood flow (rCBF) parallel changes in whole-body metabolic rate or brain tissue temperature, and secondly determined whether the mechanisms regulating rCBF, HR and RR are conserved between cenothermia and arousal from hibernation.
In this study we utilised hamsters that had been chronically implanted with an optical fibre positioned above the cerebral cortex and a thermocouple into the contralateral cortex. During arousal from hibernation, we measured the profiles of rCBF, whole-body metabolic rate, the temperature of a variety of body tissues, and HR. We compared the responsiveness of these parameters from hamsters with different body temperatures during the arousal phase with the responses, in these same animals, at cenothermia to hypercapnic or hyperoxic respiratory challenges. Contrary to expectation, rCBF increased dramatically during arousal in parallel with metabolic rate (MR) rather than brain temperature, while the responses of rCBF and RR to hypercapnia were found to differ between arousing and cenothermic states.
METHODS
Animals and housing
The following experiments conformed to the ethical guidelines of the Japanese Physiological Society and Asahikawa Medical University (ethics approval no. 02166). Male hamsters were housed at 22 °C with a 12:12 h light:dark cycle and food and water available ad libitum, until 3 months of age, or when body weight (bwt) was greater than 120 g. Hamsters were then transferred to a refrigerated room maintained at 4 °C in constant darkness and housed individually in cages with woodchip nest-building material and access to hamster chow and water ad libitum. An infrared motion sensor was positioned above, and a copper/constantan thermocouple was positioned below, each animal's nest. The frequency of movement, time since last movement and nest temperature were monitored by computer using home-written software. This enabled the activity of each animal to be monitored and the duration of each hibernation bout to be measured. Regularly hibernating animals were aroused to cenothermia by handling, and subjected to surgery 12–14 h later.
Laser-Doppler guide construction and surgery
A 5 mm length of plastic optical fibre (0.5 mm diameter; Unique Medical, Tokyo, Japan) was glued inside a shortened guide tube (C311G; Plastics One, Roanoke, VA, USA) so that one end of the optical fibre protruded 1 mm from the base of the guide tube. A pre-calibrated, copper/constantan thermocouple (0.14 mm diameter, California Fine Wire, CA, USA) with an epoxy-coated 0.5-mm-long thermosensitive tip was glued to the guide tube containing the optical fibre. Under Nembutal anaesthesia (45 mg (kg bwt)−1, i.p.), supplemented with topical application of procaine (4 %) to the skin wound and ear canals, the hamster was placed in a stereotaxic frame (Narishige, Tokyo, Japan). Using a sterile surgical procedure, the skull was exposed and holes were drilled above the left and right cortex (AP =−2, L =± 3 mm). The guide cannula, containing the optical fibre and attached thermocouple, were soaked in 80 % ethanol in water for 30 min before implantation. The optical fibre was positioned with the stereotaxic equipment to touch the dura above the cortical surface, while the thermocouple was implanted about 1 mm into the contralateral cortex and secured permanently in position using dental cement and screws (0.8 mm diameter). When not in use, the optical fibre was covered with a dust cap. During surgery additional anaesthesia was given as necessary. After surgery, the skin wound was irrigated with xylocaine and the hamster was given a 5 ml (kg bwt)−1i.p. injection of sterile saline. The hamster was returned to its home cage after recovery from the effects of anaesthesia. All operated hamsters re-commenced regular hibernation within 10–15 days postsurgery.
Experimental procedure and measurement of biophysical parameters
After surgery, re-hibernating hamsters (bwt 105–85 g) were subject to experimental arousal 30–40 h after commencing a bout of hibernation. All arousal experiments were performed in an experimental room maintained at 4 °C. Hibernating animals were fitted with precalibrated rectal and cheek-pouch thermocouples. The skin above the interscapular brown adipose tissue (BAT) was anaesthetised with procaine, the interscapular BAT was acutely dissected through a 1 cm skin incision and a sterile, precalibrated thermocouple was secured between lobes of BAT. In some experiments, HR was recorded from stainless steel 27 gauge needle electrodes inserted acutely into the skin of the thorax on either side of the heart. All thermocouples were connected to a zero temperature reference refrigeration unit (Zero-con, Komatsu Electronics, Tokyo, Japan) and a thermocouple interface (IM-7, I-techno, Tokyo, Japan) and the data were stored electronically. HR was recorded on a biophysical amplifier (AVB-10, Nihon Kohden, Tokyo, Japan). The implanted optical fibre was connected via a homemade coupling adapter to a standard laser-Doppler flow probe (ALF probe, Advance, Tokyo, Japan). The optical fibre was connected to a laser-Doppler flow meter (ALF 21N, Advance). Outputs from the laser-Doppler flow meter, biophysical amplifier and expired gas analyser (1H21A, Sanei Instrument, Tokyo, Japan) were connected to computer interfaces (Mac Lab 2e, AD Instruments, Sydney, Australia) and the data were stored electronically. An observer used a video camera coupled to an infrared light source to remotely monitor and count the RR of hamsters. Respiratory volume was not measured.
In the first set of experiments, arousal from hibernation was monitored under freely moving conditions in a PVC metabolic chamber for the measurement of cortical, BAT, mouth and rectal temperatures, RR, O2 consumption and CO2 production. The metabolic chamber had a dead volume of 200 ml and gas flow through the metabolic chamber was 200 ml min−1.
In the second set of experiments, which focussed primarily on the measurement of rCBF, hibernating hamsters were aroused under conditions that partially restricted movement of the hamster during the early semi-conscious stage of arousal. Laser-Doppler flowmetry measures the frequency of the Doppler-shifted signal reflected by red blood cells (RBC) moving within the field of incidence. An increase in the Doppler shift is proportional to an increase in the mean velocity of RBC in the field of incidence and is usually a reliable index of blood flow. Previous experiments have demonstrated that measurement of rCBF in conscious free-moving animals is feasible if movement-induced artefacts can be eliminated or minimised (Osborne, 1997). Hamsters arousing from hibernation are incapable of locomotion until rectal temperature exceeds 14–15 °C, however respiratory movements are large and restriction of head movement was necessary to prevent respiratory movements from influencing the laser-Doppler signal. In experiments in which the movement of the arousing animal was restricted, the instrumented, hibernating animal was placed into a stereotaxic holder at 4 °C, in a curled posture on cloth padding. Soft insulated ear bars were constructed to eliminate heat transfer between the stereotaxic instrument and the hibernating animal. The stereotaxic mouth bar was replaced with a mask (volume 25 ml) that was connected to the expired gas analyser by PVC tubing. This system enabled the measurement of O2 consumption and CO2 production and the periodic presentation of test gases directly to the arousing hamster without touching the animal. Thermocouples were connected as described previously, and HR electrodes were connected. The arousing hamster was kept in the stereotaxic apparatus until the animal demonstrated signs of consciousness or movement. This varied between animals, but usually occurred when the rectal temperature was about 16 °C.
As soon as the animal showed signs of movement, HR electrodes were removed and the instrumented animal was transferred to a circular cage mounted on a rotatable platform. Recordings ceased approximately 60 min after the rectal temperature had reached cenothermia when the animals were resting quietly. Hamsters were then disconnected from the thermocouples and optical fibre, wrapped in a soft towel and placed in a small metabolic chamber (dead volume 135 ml) to record basal cenothermic levels of O2 consumption and CO2 production.
Arousing hamsters in the stereotaxic frame were administered test gases for 2 min when the RR was between 50 and 100 breaths min−1. The gases were delivered through the mask at 300 ml min−1 and the effect on electronically measured parameters was recorded continuously while RR was recorded at 1–2 min intervals. The effect of the inspired gases on whole-body metabolism was calculated for the 2–3 min immediately after the test gas had been replaced with air. If CO2 gases were tested, O2 consumption was monitored; if O2 gases were given, CO2 production was monitored. At the end of each arousal experiment, the hamster was returned to his home cage. Five hamsters were tested during three or four consecutive arousals. During each arousal, each hamster received two test gases if gases had an effect or three test gases if gas had no measurable effect. After hamsters had attained cenothermic body temperatures (3–24 h after hibernating), they were anaesthetised with urethane (1.25–1.5 g (kg bwt)−1, i.p.), placed in the stereotaxic frame, rectal temperature was maintained at 37–38 °C by a thermostatically controlled heater (Paratherm S100, Tokyo, Japan) and the effects of 2-min presentation of test gases on RR, rCBF, BAT, rectal and cortical temperatures, O2 consumption and CO2 production were measured. In arousing and anaesthetised experiments, test gas mixtures were: (1) 100 % O2, (2) 5 % CO2:20 % O2:75 % N2, (3) 11 % CO2:19 % O2:70 % N2, (4) 24 % CO2:16 % O2:60 % N2, (5) 24 % CO2:24 % O2:52 % N2 and (6) 50 % N2O:50 % O2. Gases were supplied by Hokkaido Air and Water, Japan.
Postexperimentally, the urethane-anaesthetised hamsters were killed with an overdose of Nembutal. The brain was dissected and the positions of the optical fibre and thermocouple were determined. Results were not included in the analysis if the thermocouple was not in the cortex or if the cortical surface under the optical fibre showed signs of haemorrhage.
Analysis and presentation of data
The background laser-Doppler signal recorded from the dead hamster was deducted from the laser-Doppler signal during the experiment. Basal rCBF (in arbitrary units) was recorded when the animal was not moving, after the hamster had attained cenothermia. rCBF measurements from each hamster are presented as percentages of the basal signal to compensate for between-animal differences in coupling efficiencies between the laser probe and the chronically implanted cortical optical fibre. The rCBF signals recorded when the hamster was moving were deleted from the data set. In tests of the effect of spontaneously respired gas on rCBF or RR, the value 1 min preceding each application of the stimulus was compared with that 2 min after presentation of the stimulus. The magnitude of the effect was calculated as a percentage to account for interanimal differences in basal levels of rCBF and RR. Comparison of two groups of percentage data was carried out using Mann-Whitney U, non-parametric ranking tests (MW). Comparison of three or more groups of percentage data was by Kruskal-Wallis non-parametric ANOVA with Dunn's correction for multiple comparisons (KW). Temperature was analysed by the paired t test
RESULTS
The profiles of mean changes in temperature and metabolic parameters during the arousal from hibernation of four freely moving, instrumented hamsters is presented in Fig. 1. At the commencement of the experiment, the BAT temperature of hibernating animals was higher (6.57 ± 0.44 °C) than rectal temperature (5.58 ± 0.12 °C; t = 21.9, df = 9, P < 0.001). Over the arousal phase, tissues warmed at different rates. Brain and BAT reached maximum temperature at about the same time, approximately 30 min before rectal temperature. The rate of increase in cheek-pouch temperature was intermediate between brain and rectal temperatures. The maximum cortical temperature was 33.2 °C when ambient temperature was 4 °C and 36.5 °C when ambient temperature was 22 °C, indicating that heat loss across the dental cement to the environment was responsible for local cortical cooling. The maximum O2 consumption recorded during arousal was 6.9 ± 0.9 % (100 g bwt)−1, approximately 2.38 times basal metabolic rate and approximately 24.6 times the metabolic rate measured in the first 20 min of arousal. Similarly, maximum CO2 production recorded during arousal was 7.5 ± 0.7 % (100 g bwt)−1, approximately 2.42 times basal metabolic rate and approximately 25.9 times the metabolic rate measured in the first 20 min of arousal. During the period of maximum metabolic activity and highest RR, from −12 to +20 min, mean CO2 production (7.0 ± 0.08 % (100 g bwt)−1) was higher than mean O2 consumption (6.4 ± 0.08 % (100 g bwt)−1 (t = 7.84, df = 31, P < 0.001)).
Figure 1. Time course of arousal from hibernation in four freely moving hamsters.
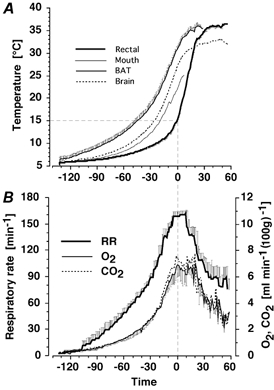
The profile has been presented so that for all simultaneously measured parameters, zero time on the x-axes coincides with rectal temperature reaching 15 °C. This is the approximate time of maximum metabolic rate in hamsters arousing in an ambient temperature of 4 °C. A, brown adipose tissue (BAT; left trace), cortex (Brain, dotted line), cheek pouch (Mouth, partial trace) and rectal (right trace) temperatures. B, respiratory rate (RR; upper trace), CO2 production (CO2; middle trace) and O2 consumption (O2; lower trace). Data are presented as means ±s.e.m.
Profile of rCBF during arousal
The profiles of mean changes in rCBF, RR, HR and BAT and rectal temperatures during the arousal phase from six movement-restricted hamsters is presented in Fig. 2. rCBF increased over the arousal period, peaking at 403 ± 21 % (n = 6) of basal cenothermic levels, coincident with maximal metabolic rate when rectal temperature was about 15 °C. Basal rCBF levels were measured once rectal temperature had reached cenothermia and the animal was sitting quietly in the recording chamber. All animals were still in the stereotaxic frame at t=−10 min and thus no component of the large increase in rCBF is derived from movement. Individual hamsters were transferred to the recording chamber between t=−9 and t=+10 min. Immediately before the transfer, O2 consumption was 226 ± 14 % (n = 6) of basal levels recorded at cenothermia. Blank periods on the rCBF trace represent time points when signals were not included because the animals were moving or grooming. Typically, movement induced a transient 500–2000 % increase in rCBF signal and was easily distinguished from the movement-free signal. At the beginning of recording of arousal, when BAT temperature was 6.5 ± 0.12 °C, hamster HR was 18.6 ± 1.3 beats min−1 (n = 5 hamsters). HR could only be recorded reliably to approximately 250 beats min−1, at which time the signal was compromised by muscular movements from respiration.
Figure 2. Time course of changes in regional cortical blood flow (rCBF, A), respiratory rate (RR) and heart rate (HR) (B), and BAT temperature (left trace, C) during arousal from hibernation in six movement-restricted hamsters.
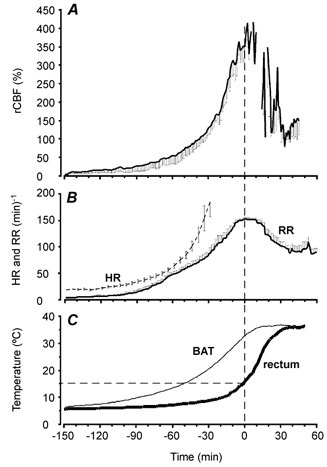
The profiles have been presented so that zero time on the x-axes coincides with rectal temperature reaching 15 °C. Periods in which the rCBF trace is absent from A are when all hamsters were moving and rCBF recordings were movement artefacts. All hamsters were in the stereotaxic frame until time =−10 min. Data are presented as means ±s.e.m.
Response of anaesthetised and arousing hamsters to respiratory gases
Anaesthetised cenothermic hamsters
Figure 3 shows the effects of spontaneous breathing of test gas on RR and rCBF in the same five anaesthetised and arousing hamsters. RR, but not respiratory volume, was measured. RR may change independently of respiratory volume. In anaesthetised hamsters 3–24 h after attainment of cenothermia, basal RR was 63 ± 3 breaths min−1. A 2-min presentation of 100 % O2 gas decreased RR by 26 ± 4 % (MW = 25; P < 0.01) but had no clear effect on rCBF. Spontaneous breathing of mildly hypercapnic (5 % CO2) gas had no effect on spontaneous RR, but increased rCBF by 7 ± 1 % (not significant, n.s.). Increasing the CO2 concentration of the inspired gas to 11 % or 24 % increased RR by 27 ± 7 % (n.s.) or 50 ± 16 % (P < 0.01), respectively (KW = 11.2; P < 0.004). In response to these same concentrations, rCBF was increased by 26 ± 6 % (P < 0.05) and 56 ± 14 % (P < 0.01), respectively (KW = 12.1; P < 0.001). Spontaneous breathing of 50 % N2O:50 % O2 gas decreased RR by 24 ± 6 % (MW = 20; P < 0.05) and increased rCBF by 24 ± 6 % (MW = 21; P < 0.05). Spontaneous breathing of 11 % or 24 % CO2 gas transiently and reversibly increased cortical temperature by 0.25 °C (P < 0.05), but had no effect on BAT or rectal temperatures (data not shown).
Figure 3. The effect of 2-min presentation of inspired gases on spontaneous RR (histogram) and rCBF (line graph) in urethane-anaesthetised hamsters (upper graph), and in the same five hamsters arousing from hibernation (lower graph).
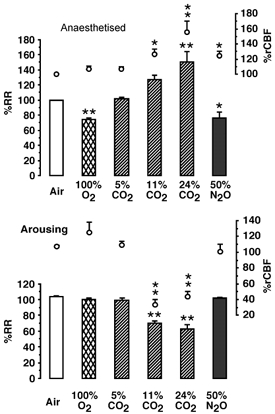
During arousal, gases were tested when BAT temperature was between 12 and 25 °C. The RR of anaesthetised hamsters was 63 ± 3 breaths min−1, while that of arousing hamsters was 71 ± 11 breaths min−1. Data are presented as means ±s.e.m.*P < 0.05; **P < 0.01. Statistical analysis is described in the text.
Hamsters arousing from hibernation
Hamsters arousing from hibernation have increasing body temperature and MR (see Fig. 1 and Fig. 2). For each animal, a 3-min air-breathing reference period was selected when BAT temperature was approximately 17.0 ± 0.4 °C and RR was 68 ± 4 breaths min−1 to assist in comparison between gas treatments of anaesthetised and non-anaesthetised animals. During this air breathing reference period, arousing hamster RR increased by 4 ± 0.5 %, rCBF increased by 7.5 ± 1.7 %, BAT temperature increased by 0.6 ± 0.03 °C, rectal temperature increased by 0.1 ± 0.01 °C, O2 consumption increased by 5.2 ± 0.4 % and CO2 production increased by 4.4 ± 0.7 % (n = 12 observations on six animals). The effect of spontaneous breathing of the test gases was determined in six animals during arousal from hibernation when BAT temperature was more than 12 °C and less than 25 °C and basal RR was 71 ± 11 breaths min−1. During this time interval, RR and MR were similar to those of cenothermic resting hamsters.
A 2-min presentation of 100 % O2 gas had no effect on RR or rCBF. The increases in BAT temperature, rectal temperature and CO2 production were not significantly different from air breathing controls. Spontaneous breathing of mildly hypercapnic 5 % CO2 gas had no effect on spontaneous increase of RR or rCBF. Changes in BAT temperature, rectal temperature and O2 consumption were not significantly different from air breathing controls. Increasing the CO2 concentration of the inspired gas to 11 or 24 % decreased RR by 30 ± 3 % (P < 0.01) or 36 ± 5 % (P < 0.01), respectively (KW = 23.68; P < 0.001). Tidal volume appeared to increase. In response to these same concentrations of CO2 gas, rCBF was decreased by 67 ± 6 % (P < 0.01) and 56 ± 6 % (P < 0.01), respectively (KW = 20.7; P < 0.001) and O2 consumption was decreased by 9 ± 6 % (n.s.) and 45 ± 11 % (P < 0.01), respectively (KW = 22.6; P < 0.001). Breathing 24 % CO2 gas decreased BAT temperature by −0.81 ± 0.4 °C (F(3, 27)= 14.1; P < 0.001), while rectal temperature was transiently increased by 0.3 ± 0.1 °C (t = 4.1, df = 18, P < 0.001). Lower concentrations of CO2 gas were without effect on these parameters. Spontaneous breathing of 50 % N2O:50 % O2 gas had no effect on RR, while rCBF was variably (1 ± 9 %) affected. rCBF and BAT temperature decreased slightly in some animals. Spontaneous breathing of hypercapnic gas for 2 min prevented the natural increase in HR during arousal. Once the gas had been returned to air, HR decreased briefly for 1–2 min (the duration depending upon the CO2 concentration of the gas) and then resumed the normal increase. The initiation of the inhibitory effect and the reversal of the inhibitory effect of breathing hypercapnic gas was most rapid on RR and rCBF, intermediate on BAT temperature and delayed on HR.
In five arousing hamsters 2–5 min spontaneous breathing of test gas containing 24 % CO2, 16 % O2, 60 % N2 when BAT temperature was 18–24 °C decreased the maximum increase in rCBF, relative to basal levels recorded during cenothermia, from 381 ± 36 % to 248 ± 28 % (MW = 23, P < 0.03), although peak O2 consumption at the time of release from the stereotaxic frame was not different in hamsters given a high concentration of CO2 to breathe and the same hamsters when not given a hypercapnic test.
DISCUSSION
This study demonstrates that during arousal from hibernation, large thermal gradients exist within the body of the hamster that probably result from coordinated, temporally specific restriction of blood to specific organs of the body. The finding that BAT is the most thermogenically reactive organ is consistent with the proposal that for Eutherians, BAT is the specialist thermogenic organ that provides the heat required to warm the vital organs to temperatures from which they can efficiently begin to function and produce heat (Haywood & Lyman, 1967; Hashimoto et al. 2002). The finding that cortical brain tissue is warmed before mouth or intestinal tissue is consistent with previous data (Chatfield & Lyman, 1950; Wang, 1978) and with the teleological proposal that warm, and hence functional, brain tissue is necessary to coordinate arousal physiology.
The mechanisms regulating respiration, HR and BBF at cenothermia, although not completely understood, are well characterised (Shams, 1985; Melton et al. 1988; Bianchi et al. 1995; Brian et al. 1996; Szabo, 1996; Gulbenkian et al. 2001). The results of this study demonstrate that when the hamster has warmed to cenothermia, control of RR and rCBF representative of normal physiology is exhibited. At cenothermia, challenge with increasingly hypercapnic gas produced a graded increase in RR and rCBF, while hyperoxia inhibited RR, and vasodilatory gas, such as N2O, increased rCBF whilst decreasing RR. These responses are consistent with previous reports from anaesthetised animals (Shams, 1985; Melton et al. 1988; Kumano et al. 1994; Dalkara et al. 1995; Osborne, 1997) and confirm that chronic implantation of the optical fibre over the cortex and the coupling with the laser-Doppler technique is a valid procedure for the measurement of rCBF in hamsters.
The principal finding of this study is that during arousal from hibernation, changes in rCBF more closely parallel whole-body metabolic rate or RR than brain or BAT temperature and that the magnitude of the changes in rCBF far exceed those encountered in cenothermic physiology. Temporally, the profile of change in rCBF appears to be correlated with whole-body O2 consumption or CO2 production. However, the relationship between rCBF and whole-body O2 consumption or CO2 production is probably indirect, since the relative contribution of individual organs to whole-body metabolic rate changes over the course of arousal. Early in arousal, the proportional contributions of BAT and brain to whole-body O2 consumption or CO2 production are substantial, but these are reduced once the bulk of the hind body becomes warm and begins to efficiently produce heat.
The unexpectedly large peak in rCBF during arousal occurs at the peak of whole-body metabolic activity while HR is increasing, and is probably coincident with the return of BP to normal levels (Chatfield & Lyman, 1950). Collectively this suggests that cerebral autoregulation is suspended during arousal. In addition, the mechanisms that regulate rCBF and the respiratory response to hypercapnic or hyperoxic stimuli are different in the arousing and cenothermic states. The spontaneous breathing of hypercapnic gases during arousal produced rapid and reversible decreases in RR, rCBF and MR, and a delayed and reversible decrease in BAT temperature and HR. This is in stark contrast to the graded increases in RR and rCBF observed in anaesthetised hamsters at cenothermia and the increase in RR and HR (Lyman, 1951; P. G. Osborne & M. Hashimoto, unpublished observations) recorded in hamsters during the maintenance phase of hibernation, and demonstrates state-dependent regulation of RR, HR and rCBF. It should be acknowledged that the respiratory response to hypercapnia during arousal probably involves changes in respiratory volume that may not be reflected in respiratory rate. In addition, it should be considered that, although unlikely, the profile of change in Doppler shift measured during arousal or challenge with hypercapnic gas could result from changes of the mean velocity of RBC in the cortex as opposed to changes in rCBF.
At cenothermic body temperatures, decreases in RR, HR and rCBF occur in response to hypoxia (Melton et al. 1988; Bianchi et al. 1995) and the induced metabolic depression. However, brief presentation of test gases is unlikely to induce hypoxia and metabolic depression. Consistent with this speculation is the finding that hyperoxic, hypercapnic gas (gas 5, see Methods) had the same inhibitory effect as hypercapnic gas (gas 4, see Methods). Normal regulation of respiration and rCBF to gas stimuli was demonstrated 3 h after arousal but probably occurs within a very short time after the hamster has achieved cenothermia. A small number of experiments was performed and it was found that normal cenothermic respiratory responses to breathing 11 % CO2 gas occurred 30 min after rectal temperature reached 36 °C. It is conceivable that normal increases of rCBF in response to hypercapnia are also evident as soon as cenothermic temperature is attained.
Some of the apparent anomalies in the response of rCBF and RR to low concentrations of CO2 and O2 gases during arousal from hibernation are probably related to the hamster's environmental adaptations. Wild hamsters are semi-fossorial rodents and tolerance to breathing 5 % CO2 gas is consistent with the animal's genetic history. The unresponsiveness of RR to breathing 100 % O2 during arousal may be influenced by enhanced oxygen- haemoglobin (Milsom et al. 1986) and oxygen-myoglobin (Postnikova et al. 1999) binding, which is evident in other fossorial hibernating species and may occur in hamsters. However, the inhibitory responses to 11 and 23 % CO2 gas during arousal are difficult to reconcile with the mode of action currently attributed to hypercapnia at cenothermia, while the distinct temporal profile of inhibition for each tissue indicates tissue-specific mechanisms of inhibition.
The thermal, and hence cellular biochemical, environment that exists during arousal is vastly different from that at cenothermia. Although the nature of these differences is not understood, an outline of the putative mechanism by which the functional circuitry of the CNS is altered between the two states may prove useful when considering the inhibitory effect of hypercapnic gas. The maintenance of large thermal gradients within the body, and possibly to a lesser extent in the brain, demonstrates regulation of regional blood flow that is probably maintained by active control of vascular resistance. These thermal, blood flow and nutrient gradients may provide the basis of a mechanism that enables warmer central neurons to function when peripheral feedback neurons remain cooler and have attenuated function. With regard to the regulation of rCBF, this produces the possibility of transient separation between neurogenic and local metabolic regulation. The second difference to be considered is that during the accelerator cycle of arousal, sympathetic stimulation overrides parasympathetic input in the regulation of RR and HR (Chatfield & Lyman, 1950; Harris & Milsom, 1995). This inactivation of tonic control may also occur in other systems, and, as such, a component of the atypical response to a hypercapnic stimulus exhibited by RR, HR, rCBF and BAT temperature may result from the attenuation of parasympathetic feedback.
In addition to the above, the inhibitory effect of acidosis should be considered. Tests of inspired gases were performed in the period shortly before the maximum metabolic output, when CO2 was produced in excess of O2 consumed, coincident with hyperventilation, and is suggestive of a physiological need for alkalosis at this time. Increasing the CO2 concentration of the inspired air to 11 % and above may add to the existing thermodynamic (Reaves, 1977) and metabolic factors promoting acidosis. In vitro BAT thermogenesis is inhibited by decreasing pH (Malan & Mioskowski, 1988), and although the in vivo inhibitory effect on BAT blood flow or thermogenesis has not been documented, it has been postulated that metabolic suppression induced by acidosis is one of the mechanisms by which metabolic rate is actively reduced during entrance to hibernation (Malan et al. 1988). Collectively these data suggest that regulation of pH within critical limits is a physiologically fundamental parameter in arousal from hibernation in general, and in the regulation of rCBF and cardiac and respiratory systems in particular.
In conclusion, these results demonstrate the maintenance of large thermal gradients in body tissue during arousal from hibernation that probably reflect regional metabolism and result from the coordinated, temporally specific restriction of blood to specific organs of the body. The large increase in rCBF recorded during arousal suggests that during this transitional phase, cerebral autoregulation is temporarily suspended. The mechanisms regulating rCBF exhibit state-dependent sensitivities to hypercapnic and hyperoxic stimuli. These results demonstrate that when warming towards cenothermia, some regulatory systems, such as those responsible for increasing RR, rCBF, HR and regional metabolism, are functional, but others, such as those responsible for the regulation of RR, rCBF and HR responses to hypercapnia, require higher temperatures to attain function or have an appropriate alternative function at intermediate body temperatures.
It is not known how arousing hamsters routinely tolerate the extremely large increases in rCBF recorded at the peak of metabolic activity. It may be that the state-dependent response to hypercapnia or acidosis, along with temporally specific oxidation of circulating plasma ascorbic acid (Toien et al. 2001), are fundamental to the endogenous mechanisms utilised by the arousing hamster to minimise the neuropathology that would normally be associated with large increases in rCBF and the associated production of reactive O2 species. Clearly further experimentation is required to determine the mechanisms by which large increases in rCBF coincident with high metabolic rate can be tolerated without pathology.
Acknowledgments
This work was funded by JSPS Grant-in-Aid for Scientific Research (B) no. 14370018 and partially by Mitsubishi Rayon Medical Research Grant and an International Fellowship grant to P.G.O. from the Takeda Science Foundation.
REFERENCES
- Bianchi AL, Denavit-Saubie M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties and neurotransmitters. Physiol Rev. 1995;75:1–46. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- Brian JE, Faraci FM, Heistad DD. Recent insights into the regulation of cerebral circulation. Clinic Exp Pharm Physiol. 1996;23:449–457. doi: 10.1111/j.1440-1681.1996.tb02760.x. [DOI] [PubMed] [Google Scholar]
- Chatfield PO, Lyman CP. Circulatory changes during the process of arousal in the hibernating hamster. Am J Physiol. 1950;163:566–574. doi: 10.1152/ajplegacy.1950.163.3.566. [DOI] [PubMed] [Google Scholar]
- Csete K, Barzo P, Bodosi M, Papp JG. Influence of nitrovasodilators and cyclooxygenase inhibitors on cerebral vasoreactivity in conscious rabbits. Eur J Pharmacol. 2001;412:301–309. doi: 10.1016/s0014-2999(01)00725-7. [DOI] [PubMed] [Google Scholar]
- Dalkara T, Irikura K, Huang Z, Panahian N, Moskowitz MA. Cerebrovascular responses under controlled and monitored physiological conditions in the anesthetized mouse. J Cereb Blood Flow Metab. 1995;15:631–638. doi: 10.1038/jcbfm.1995.78. [DOI] [PubMed] [Google Scholar]
- Frerichs KU, Kennedy C, Sokoloff L, Hallenbeck JM. Local cerebral blood flow during hibernation, a model of natural tolerance to cerebral ischemia. J Cereb Blood Flow Metab. 1994;14:193–205. doi: 10.1038/jcbfm.1994.26. [DOI] [PubMed] [Google Scholar]
- Gulbenkian S, Uddman R, Edvinsson L. Neuronal messengers in the human cerebral circulation. Peptides. 2001;22:995–1007. doi: 10.1016/s0196-9781(01)00408-9. [DOI] [PubMed] [Google Scholar]
- Harris MB, Milsom WK. Parasympathetic influence on heart rate in euthermic and hibernating ground squirrels. J Exp Biol. 1995;198:931–937. doi: 10.1242/jeb.198.4.931. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Gao B, Kikuchi-Utsumi K, Ohinata H, Osborne PG. Arousal from hibernation and BAT thermogenesis against cold. Central and molecular basis. J Thermal Biol. 2002;27:55–67. [Google Scholar]
- Haywood JS, Lyman CP. Nonshivering heat production during arousal from hibernation and evidence for the contribution of brown fat. In: Fisher KC, Dawe AR, Lyman CP, Schonbaum E, South FE, editors. Mammalian Hibernation 3. New York: Elsevier; 1967. pp. 346–355. [Google Scholar]
- Iadecola C, Zhang F. Nitric oxide-dependent and -independent components of cerebrovasodilation elicited by hypercapnia. Am J Physiol. 1994;266:R546–552. doi: 10.1152/ajpregu.1994.266.2.R546. [DOI] [PubMed] [Google Scholar]
- International Union of Physiological Sciences. IUPS Thermal Commission. Glossary of terms for thermal physiology. Jpn J Physiol. 2001. pp. 245–280.
- Kumano H, Furuya H, Yomosa H, Nagahata T, Okuda T, Sakaki T. Response of pial vessel diameter and regional cerebral blood flow to CO2 during midazolam administration in cats. Acta Anaesthesiol Scand. 1994;38:729–733. doi: 10.1111/j.1399-6576.1994.tb03986.x. [DOI] [PubMed] [Google Scholar]
- Kuroda S, Siesjo BK. Reperfusion damage following focal ischemia: pathophysiology and therapeutic windows. Clin Neurosci. 1997;4:199–212. [PubMed] [Google Scholar]
- Lyman CP. Effect of increasing CO2 on respiration and heart rate of hibernating hamsters and ground squirrels. Am J Physiol. 1951;167:638–643. doi: 10.1152/ajplegacy.1951.167.3.638. [DOI] [PubMed] [Google Scholar]
- Lyman CP, O'Brien RC. Sensitivity to low temperature in hibernating rodents. Am J Physiol. 1972;222:864–869. doi: 10.1152/ajplegacy.1972.222.4.864. [DOI] [PubMed] [Google Scholar]
- Malan A, Mioskowski E. pH temperature interactions on protein function and hibernation: GDP binding to brown adipose tissue mitochondria. J Comp Physiol B. 1988;158:487–493. doi: 10.1007/BF00691146. [DOI] [PubMed] [Google Scholar]
- Malan A, Mioskowski E, Calgari C. Time course of acid blood state during arousal from hibernation in the European hamster. J Comp Physiol B. 1988;158:495–500. doi: 10.1007/BF00691147. [DOI] [PubMed] [Google Scholar]
- Melton JE, Neubauer JA, Edelman N. CO2 sensitivity of cat phrenic neurogram during hypoxic respiratory depression. J App Physiol. 1988;65:736–743. doi: 10.1152/jappl.1988.65.2.736. [DOI] [PubMed] [Google Scholar]
- Milsom WK, McArthur MD, Webb CL. Control of breathing in hibernating ground squirrels) In: Heller HC, Musacchia XJ, Wang LC, editors. Living in the Cold. Physiological and Biochemical Adaptations. New York: Elsevier; 1986. pp. 469–475. [Google Scholar]
- Osborne PG. fhippocampal and striatal blood flow during behavior in rats. Acute and chronic laser Doppler flowmetry study. Physiol Behav. 1997;61:485–492. doi: 10.1016/s0031-9384(96)00460-x. [DOI] [PubMed] [Google Scholar]
- Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev. 1990;2:161–192. [PubMed] [Google Scholar]
- Postnikova GB, Tselikova SV, Kolaeva SG, Solomonov NG. Myoglobin content in skeletal muscles of hibernating ground squirrels rises in autumn and winter) Comp Biochem Physiol A Mol Integr Physiol. 1999;124:35–37. doi: 10.1016/s1095-6433(99)00085-9. [DOI] [PubMed] [Google Scholar]
- Reaves KB. The interaction of body temperature and acid-base balance in ectothermic vertebrates. Annu Rev Physiol. 1977;39:559–586. doi: 10.1146/annurev.ph.39.030177.003015. [DOI] [PubMed] [Google Scholar]
- Shams H. Differential effects of CO2 and H+ as central stimuli of respiration in the cat. J Applied Physiol. 1985;58:357–364. doi: 10.1152/jappl.1985.58.2.357. [DOI] [PubMed] [Google Scholar]
- Szabo C. Physiological and pathophysiological roles of nitric oxide in the central nervous system. Brain Res Bull. 1996;41:131–134. doi: 10.1016/0361-9230(96)00159-1. [DOI] [PubMed] [Google Scholar]
- Toien O, Drew KL, Chao ML, Rice ME. Ascorbate dynamics and oxygen consumption during arousal from hibernation in Arctic ground squirrels. Am J Physiol. 2001;1:R572–583. doi: 10.1152/ajpregu.2001.281.2.R572. [DOI] [PubMed] [Google Scholar]
- Wang LC. Energetic and field aspects of mammalian torpor: The Richardson's ground squirrel) In: Wang LC, Hudson JW, editors. Strategies in Cold: Natural Torpidity and Thermogenesis. New York: Academic; 1978. pp. 109–145. [Google Scholar]


