Abstract
Hypoglossal motoneurones (HMN) are selectively damaged in both human amyotrophic lateral sclerosis (ALS) and corresponding mouse models of this neurodegenerative disease, a process which has been linked to their low endogenous Ca2+ buffering capacity and an exceptional vulnerability to Ca2+-mediated excitotoxic events. In this report, we investigated local Ca2+ profiles in low buffered HMNs by utilizing multiphoton microscopy, CCD imaging and patch clamp recordings in slice preparations. Bath application of caffeine induced highly localized Ca2+ release events, which displayed an initial peak followed by a slow ‘shoulder’ lasting several seconds. Peak amplitudes were paralleled by Ca2+-activated, apamin-sensitive K+ currents (IKCa), demonstrating a functional link between Ca2+ stores and HMN excitability. The potential involvement of mitochondria was investigated by bath application of CCCP, which collapses the electrochemical potential across the inner mitochondrial membrane. CCCP reduced peak amplitudes of caffeine responses and consequently IKCa, indicating that functionally intact mitochondria were critical for store-dependent modulation of HMN excitability. Taken together, our results indicate localized Ca2+ release profiles in HMNs, where low buffering capacities enhance the role of Ca2+-regulating organelles as local determinants of [Ca2+]i. This might expose HMN to exceptional risks during pathophysiological organelle disruptions and other ALS-related, cellular disturbances.
Cytoplasmic free calcium concentrations ([Ca2+]i) are critical determinants of cell viability and contribute to a multitude of functions in neurones as well as other cell types (Friel & Tsien, 1992; Pozzan et al. 1994; Berridge et al. 1998). During neuronal activity, substantial fluctuations of [Ca2+]i with time constants from milliseconds to hundreds of milliseconds can be observed (Bacci et al. 1999; Frermann et al. 1999; Kato et al. 1999). These dynamics of [Ca2+]i reflect in part the spatio-temporal responses of postsynaptic signal cascades, including the regulation of Ca2+-dependent conductances and associated second messenger systems (Augustine et al. 1987; Zucker, 1999). For example, hypoglossal motoneurones discharge repetitive bursts of action potentials during rhythmic, respiratory-related activity (Smith et al. 1991; Mifflin, 1997; Lieske et al. 2000), and measurements in these motoneurones revealed large increases of [Ca2+]i during inspiratory-related bursts (Ladewig & Keller, 2000). Of considerable interest is the heterogeneity between somatic and dendritic Ca2+ processing that is reflected by differences in the kinetics and voltage dependence of [Ca2+]i.
The above findings raise the question: how are the dynamics of intracellular free Ca2+ concentrations regulated in motoneurones that are extremely active and whose burst-related Ca2+ oscillations have to follow maximum frequencies above 5 Hz. Earlier studies provided evidence that rapid Ca2+ oscillations in motoneurones are partially achieved by low endogenous Ca2+ buffering capacities (Lips & Keller, 1998), which enhance the efficiency of a given set of uptake and extrusion mechanisms. However, low buffering not only accelerates relaxation rates of Ca2+ transients, but also appears to increase the risk for Ca2+-mediated motoneurone damage during excitotoxic conditions and/or ALS-related pathophysiological states (DePaul et al. 1988; Alexianu et al. 1994; Reiner et al. 1995; Krieger et al. 1996; Vanselow & Keller, 2000). Previous studies therefore provided increasing evidence that cellular specializations that facilitate rapid dynamics of [Ca2+]i also enhance the vulnerability of motoneurones during human ALS and related animal models of this neurodegenerative disease.
Until recently, the analysis of Ca2+ dynamics in hypoglossal motoneurones has mainly been directed at the temporal change of average [Ca2+]i, in relatively large cellular compartments, such as the cell body (Lips & Keller, 1998; 1999). In the present investigation, we utilized a combination of multiphoton microscopy (MPM), CCD imaging and patch clamp recordings in slice preparations to examine Ca2+ signals in small compartments of hypoglossal motoneurones with high spatial and temporal resolution. More specifically, we evaluated the particular spatio-temporal pattern of store-operated Ca2+ release under different physiological and pharmacological conditions. Our measurements indicate that low endogenous Ca2+ buffering and a corresponding, low concentration of mobile Ca2+-binding proteins in the cytosol not only account for rapid relaxation times of Ca2+ transients, but also enhance the role of Ca2+-regulating organelles for localized control of [Ca2+]i. During ALS-related cellular disturbances, this might enhance the selective vulnerability of motoneurones to organelle disruption and associated excitotoxic disturbances. Parts of this study have been published in abstract form (Lalley et al. 1999; Ladewig et al. 2000).
METHODS
Preparation of slices
In vitro brain stem slice preparations were obtained from newborn mice at postnatal day (P)2–5. Animal experiments were carried out in accordance with the recommendations of the European Commission (no. L358, ISSN 0378–6978). Animals were decapitated by a small guillotine, brains were removed and subsequently cooled to 4 °C. Transverse slices of the brain stem with a thickness of 200 μm were prepared (Lips & Keller, 1998, 1999). For simultaneous imaging and patch clamp recordings, preservation of functionally intact neurones close to the slice surface was essential. This was achieved by minimizing mechanical disturbances of tissue during slice preparations, performing isolation of slices at low temperatures (4 °C) and optimizing metabolic conditions by maximum oxygen supply. Slices were maintained at room temperature in continuously bubbled (95 % O2, 5 % CO2) bicarbonate-buffered saline (mm):118 NaCl, 3 KCl, 1 MgCl2, 25 NaHCO3, 1 NaH2PO4, 1.5 CaCl2, 20 glucose) at pH 7.4 and investigated according to previously described methods (Lips & Keller, 1998). All experiments were performed at room temperature (21–23 °C).
Patch clamp recording
Prior to recording, the nucleus hypoglossus was visually identified by its location close to the nucleus vagus and the fourth ventricle. Somata and dendrites of hypoglossal motoneurones were discerned by infrared differential interference contrast (IR DIC) optics connected to video equipment (Dodt & Zieglgänsberger, 1994; Lips & Keller, 1998; Stuart et al. 1993). Hypoglossal motoneurones were visually identified by their somatic diameters of approximately 20 μm and extensive dendritic arborizations, and their location close to the central canal (Lips & Keller, 1998, 1999; Ladewig & Keller, 2000). To assure acceptable optical conditions, patch clamp experiments were performed on cells within 60 μm below the slice surface. Patch clamp recordings from slice preparations, as previously described (Hamill et al. 1981; Edwards et al. 1989; Titz & Keller, 1997), were made with intracellular pipette solutions containing (mm): 140 KCl or potassium gluconate, 10 Hepes, 2 MgCl2, 4 Na2-ATP, 0.4 Na2-GTP (adjusted to pH 7.3 with KOH or CsOH). Fura-2 and Calcium Green-1 were purchased from Molecular Probes (Eugene, OR, USA) and used in concentrations of 100 μm in the pipette solution. Patch pipettes were pulled from borosilicate glass tubing (Hilgenberg, Malsfeld, Germany) and heat polished before use. When filled they displayed resistances of 2.0–3.5 MΩ. Recordings were performed with an EPC-7 amplifier (HEKA Elektronik, Lambrecht, Germany) employing optimal series resistance compensation (Titz & Keller, 1997; Lips & Keller, 1998). Cells were selected for study if they had series resistances less than 15 MΩ. Whole-cell currents were recorded with sampling frequencies of 100 Hz to 5 kHz. Analysis of signals was performed off-line using Igor (Wavemetrics, Oregon, USA) plus Pulse and Pulse-Fit/v8.11 (HEKA) software.
Stimulus protocols
Unless otherwise stated, series of depolarizing voltage steps during voltage clamp from a holding potential of −60 mv (+10 mv depolarizing increments, 1 s−1, to reach a maximum of +20 mv) were applied to activate plasma membrane Ca2+ channels and increase cytosolic Ca2+.
Intracellular Ca2+ measurements
Two different methodologies for detecting intracellular levels of cytoplasmic Ca2+ in the soma and dendrites were utilized. In the first, Ca2+ concentrations were measured by loading the cells from patch pipettes with fura-2 and measuring Ca2+-induced changes of fluorescence by charge coupled device (CCD) camera imaging. In the second procedure, cells were loaded from patch pipettes with Calcium Green-1 or fura-2, and Ca2+-induced changes in fluorescence were measured by MPM.
CCD camera imaging
As previously described (Ladewig & Keller, 2000) a modified version of a CCD camera system (TILL Photonics, Planegg, Germany) was employed in experiments carried out in the Göttingen laboratory. Calculations and analysis of intracellular Ca2+ concentrations and kinetics were subsequently performed off-line with the Igor analysis software.
Calibration constants for fura-2 were determined according to the method of Grynkiewicz et al. (1985). Calibration constants were adjusted after several days of experiments to account for small fluorescence changes in the microfluorometric system.
We defined selected regions of interest (ROI) to measure the Ca2+ signals in the cell compartments. The ROIs were always of the same size including a larger amount of pixels in the soma than in the dendrites (nearly the same number in the dendritic compartments). Background fluorescence was measured in corresponding out-of-focus ROIs, containing no obvious cellular structures. Values beyond a certain threshold calculated from the out-of-focus ROI were set to zero while the other pixel values were summarized, averaged and divided through the number of non-zero pixels.
Simultaneous measurement of mitochondrial and cytoplasmic [Ca2+]
In the present study, the excitation wavelength for rhod-2 was 510 nm. In order to monitor intramitochondrial calcium concentration ([Ca2+]m), hypoglossal motoneurones were incubated with the Ca2+-sensitive fluorescent indicator rhod-2 AM (1–1.5 μm), for at least 1 h at room temperature. Rhod-2 has been used by several different laboratories to monitor changes in [Ca2+] within the mitochondrial matrix in different cell types (Babcock et al. 1997; Drummond & Tuft, 1999). Binding of Ca2+ to rhod-2 increases its fluorescence; however, since there is no significant shift in excitation or emission wavelengths upon Ca2+ binding, it cannot be used for ratio imaging. Rather, rhod-2 fluorescence is reported as 100 F/F0, where F is the measured fluorescence, and F0 is the fluorescence before application of the depolarizing stimulus.
Multiphoton excitation microscopy
Multiphoton imaging, originally described by Denk et al. (1990) was carried out in the Cornell laboratory with instrumentation and methodology previously described in detail (Kloppenburg et al. 2000). The multiphoton microscope consisted of a Spectra Physics Tsunami Ti:S laser with a 10 W Millenium (Spectra Physics, Mountain View, CA, USA), a retro-fitted Bio-Rad (Hercules, CA, USA) MRC-600 scanbox and a custom-made, fixed stage Olympus AX-70 upright microscope. A Hamamatsu (Bridgewater, NJ, USA) photomultiplier tube placed directly above the objective lens was used to collect the non-descanned emission (400–550 or 500–600 nm). The beam intensity was controlled using a ConOptics (Danbury, CT, USA) model 350–50 Pockels Cell, which also blanked the laser during fly-back (in between scan lines), eliminating unnecessary excitation of the preparation. Hypoglossal motoneurones loaded with Calcium Green-1 or fura-2 were imaged with 810 or 780 nm excitation respectively through a × 20/0.5 NA or a 40/0.8 NA water immersion objective lens. Ca2+ transients were acquired using scan lines at a rate of 2 or 4 ms line−1. Voltage clamp data were simultaneously recorded with an Axopatch 200 amplifier (Axon Instruments, Foster City, CA, USA) on the second channel of the Bio-Rad scanner during the line scans to synchronize the start of depolarizing voltage steps with the Ca2+ signal.
Data analysis was performed with laboratory-written software. Pixel values were extracted from the line scan images along the time axis in the area of interest (averaged across the spatial axis). The simultaneously acquired membrane potential was analysed by the software to determine the starting point of the voltage step, ensuring synchronization between the Ca2+ data and the voltage step. The fluorescence signal after the voltage pulse was fitted to a single exponential decay model using the Igor analysis software.
Drug solutions
Caffeine, carbonyl cyanide-m-chlorophenylhydrazine (CCCP), cyclopiazonic acid (CPA), and procaine hydrochloride were purchased from Sigma-Aldrich Chemie (Deisenhofen, Germany) and dissolved in bath solution just before use. Drug solutions were bubbled with 95 % O2, 5 % CO2 at room temperature immediately before application to brain slices.
Statistics
Summarized values related to [Ca2+]i are expressed as standard error of the mean (s.e.m.) unless otherwise stated. The statistical significance of differences between control and treatment groups was determined by Student's paired t test. Differences were taken to be significant for P < 0.05.
RESULTS
Voltage-dependent Ca2+ signals in hypoglossal motoneurones as revealed by CCD imaging and MPM
Highly dynamic Ca2+ signals in brain stem and spinal motoneurones are mediated by a specialized Ca2+ homeostasis, where low endogenous buffering capacities facilitate rapid relaxation rates of cytosolic Ca2+ transients and predict large domains of ‘unbuffered’ Ca2+ concentrations around open Ca2+ channel pores (Lips & Keller, 1998, 1999; Vanselow & Keller, 2000). In this report, we investigated local profiles of [Ca2+]i in hypoglossal motoneurones that are particularly vulnerable both during human amyotrophic lateral sclerosis (ALS) and in corresponding mouse models of this neurodegenerative disease, by utilizing whole-cell patch clamp recordings in slice preparations, CCD imaging and MPM. Figure 1A displays the anatomical landmarks of slice preparations utilized in our present study, where the arrow indicates the location of the nucleus hypoglossus in the brain stem. Figure 1B shows a multiphoton image of a hypoglossal motoneurone during whole-cell patch clamp recording, where the cell was pipette-filled with 100 μm fura-2 as a fluorescent marker of the soma and dendrites. Dendritic structures could be clearly imaged at distances larger than 100 μm from the soma and a notable absence of spines could be recognized in experiments similar to that pictured in Fig. 1B. During whole-cell patch clamp recordings, somatic depolarizations with voltage steps starting from a holding potential of −60 mV evoked rapid elevations of [Ca2+]i that were detected in the soma and dendrites with either CCD imaging or MPM (Fig. 2). The amplitude of voltage-evoked [Ca2+]i increases varied as a function of membrane depolarization as previously demonstrated (Ladewig & Keller, 2000).
Figure 1. Fura-2-loaded hypoglossal motoneurone and its location in the medulla oblongata.
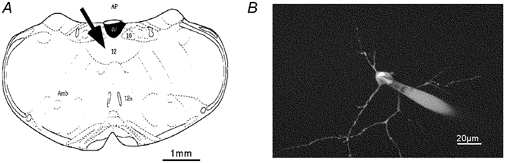
A, schematic drawing of a transverse section showing the slice preparation of the medulla oblongata including hypoglossal regions (arrow) and the important landmarks for orientation, such as the fourth ventricle (IV) and the dorsal nucleus of vagus (AP, area postrema; Amb, nucleus ambiguus; 12, nucleus hypoglossus; 12n, axons of 12; 10, nucleus vagus dorsalis). B, fura-2-loaded cell. The MPM image is a projection of 45 sections taken with the × 40 objective and a 1 μm step size. Excitation was at 780 nm.
Figure 2. Heterogeneous Ca2+ elevations in dendritic compartments of a hypoglossal motoneurone evoked by depolarization.
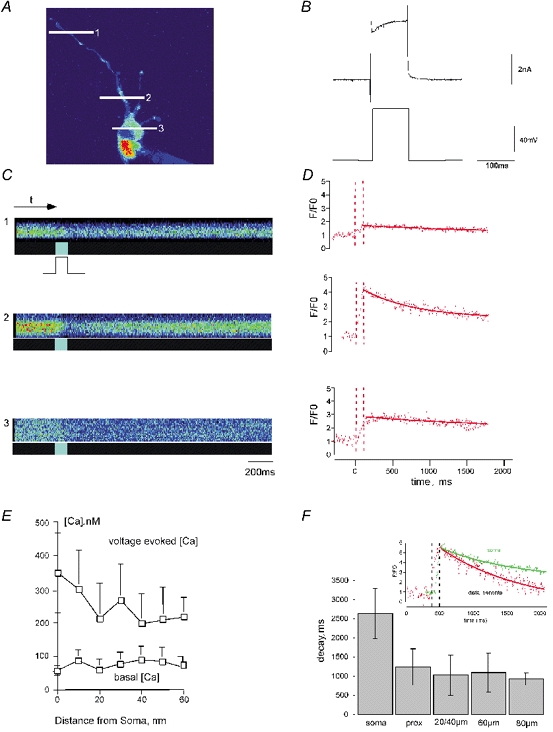
Multiphoton imaging (single optical section) showing voltage-evoked increases of intracellular calcium ([Ca2+]i) in a hypoglossal motoneurone loaded from a whole-cell patch pipette with fura-2. A, fluorescence imaging of a hypoglossal motoneurone. Line scan positions in the different cell compartments are marked with white lines (soma, 1; proximal dendrite, 2; and distal dendritic region, 3). B, electrophysiological response of a voltage-clamped hypoglossal motoneurone after somatic depolarization from −60 to +20 mV (100 ms). C, line scans taken at the points indicated in A. The temporal resolution was 4 ms per line. Line scan images reveal spatial (vertical) and temporal (horizontal) properties of Ca2+-sensitive fluorescence in the selected compartments. The brighter sections in the boxes below indicate the duration of the voltage pulse (100 ms), fura-2 fluorescence decreases while Ca2+ is binding, coinciding with soma step depolarization to +20 mV from a holding potential of −60 mV. D, data extracted from the images in C along the time axis. Decreasing fura-2 values were divided by the basal fluorsecence level (F0) and inversed. The dashed lines show the voltage pulse duration. The decay time constants were fitted to a single exponential function using the analysis software Igor. Note that Ca2+ transients are of dissimilar amplitude, indicative of heterogeneous Ca2+ elevations. E, graphical summary showing basal [Ca2+]i and [Ca2+]i signals evoked by depolarization to +20 mV at different distances from the soma of nine hypoglossal motoneurones detected by ratiometric measurements with the CCD system. Note that Ca2+ transients reveal a high variability in amplitude in the discrete compartments of the compared cells. F, mean decay time constants in the different cell compartments (soma, proximal dendrite at 10 μm, and dendritic compartments at 20/40, 60 and 80 μm from the centre of the soma) as revealed by multiphoton imaging. Inset, depolarizing pulses induce heterogeneous Ca2+ signals in somatic and dendritic compartments. Two compartments displaying the same amplitude are compared; Ca2+ elevations were evoked by a 100 ms voltage pulse. Ca2+ responses were notably different in the soma and the distal dendritic compartment (at 80 μm from the centre of the soma). The histogram shows a much faster decline to resting [Ca2+] in dendritic compartments compared with the soma.
Reliable Ca2+ responses in the soma and dendrites were detected when membrane potentials were depolarized to -50 mV and reached maximal levels at 0 to +20 mV. They exhibited different kinetics in the two cell compartments. A depolarizing voltage step with a step amplitude of +80 mV evoked transient, voltage-dependent increases in neuronal Ca2+ in the soma and dendritic compartments. CCD camera imaging revealed peak amplitudes with values of 362 ± 133 nm (+10 mV) in the soma and 312 ± 97 nm, 214 ± 59 nm (n = 9) for dendritic distances of 10 and 60 μm, respectively. Decay time constants of [Ca2+]i were faster in dendrites (τ= 1075 ± 113 ms; n = 34) compared to somata (τ= 2631 ± 649 ms) although the buffer capacities (κB= 42 ± 14 in dendrites, n = 5 cells; see also Lips & Keller, 1998) and the basal Ca2+ levels (Fig. 2E) were similar. Voltage-evoked Ca2+ signals were locally defined and exhibited a heterogeneous pattern in dendritic compartments as revealed by MPM (Fig. 2). Under physiological conditions, depolarization-induced Ca2+ responses are an important determinant of Ca2+-dependent afterhyperpolarizations that follow each action potential and control motoneurone firing rates during burst discharges (Lape & Nistri, 2000). In hypoglossal motoneurones, depolarization-evoked AHPs are due to activation of Ca2+-dependent, SK-type K+ channels, and could therefore be blocked by apamin (10 μm, Fig. 3).
Figure 3. Caffeine-induced intracellular release of Ca2+ and outward current in a hypoglossal motoneurone.
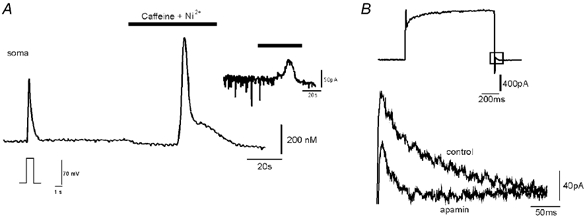
A, Ca2+ signals measured by CCD camera imaging of a fura-2-loaded, voltage-clamped hypoglossal motoneurone. Fura-2 Ca2+ signals evoked in the soma first (left) by a step depolarization from −60 to +20 mV, and second (right) by bath application of 5 mm caffeine after blocking high voltage-activated Ca2+ channels with bath-applied 2 mm nickel (Ni2+). Response to caffeine consists of fast and slow components. Inset (upper right), outward current measured in the soma of the motoneurone during caffeine and Ni2+ application. The caffeine-induced outward current in 19 cells displayed a mean amplitude of 85.6 ± 21.8 pA. B, superimposed tail currents (occurring at the end of a voltage step protocol) which could partially be blocked by apamin, showing the presence of SK channels in neonatal mouse hypoglossal motoneurones.
The heterogeneity of Ca2+ signals could be explained by the different surface-to-volume ratio in the soma and dendrites or by the variability of Ca2+ channel expression (Ladewig & Keller, 2000). Another possible explaination for this effect is the contribution of ryanodine receptor (RyR)-dependent Ca2+ release to Ca+2 signalling in motoneurones (Sah & McLaclan, 1991).
Store-operated Ca2+ release in hypoglossal motoneurones
To evaluate the contribution of store-operated Ca2+ release in hypoglossal motoneurones, we bath applied pharmacological agents which trigger or prevent release from intracellular storage sites (Kostyuk & Verhratsky, 1998). For the investigation of endoplasmic reticulum (ER)-dependent Ca2+ release, caffeine has been established as a specific activator of Ca2+ efflux from RyR-dependent stores (Kostyuk & Verhratsky, 1998). In a standard experiment, we transiently elevated [Ca2+]i in voltage clamp mode with a 1 s depolarization to 0 mV with the intention of refilling empty Ca2+ stores. Subsequently, RyR-dependent release was probed by 5 mm caffeine in the presence of 2 mm Ni3+ in the bath solution to block the potential entry of extracellular Ca2+ through voltage-activated Ca2+ channels (McFarlane & Gilly, 1998).
Caffeine evoked substantial increases of [Ca2+]i in the somatic compartment as illustrated in Fig. 3. The Ca2+ signal was biphasic, consisting of a relatively large and fast initial transient followed by a smaller and slower component (‘shoulder’). In contrast to depolarization-evoked Ca2+ responses, caffeine-induced Ca2+ transients were more restricted to the soma than to the proximal dendrites as revealed by CCD imaging (Fig. 4A). The initial fast responses were significantly larger in the soma (758 ± 263 nm, n = 13) compared to dendritic compartments at branching points (312 ± 98 nm, n = 13). Caffeine responses were absent after bath application of membrane-permeable compounds ryanodine (500 nm) or procaine (1 mm), supporting the notion that they resulted from RyR receptor-controlled Ca2+ stores.
Figure 4. Heterogeneous distribution of intracellular Ca2+ release sites in somatic and dendritic compartments of hypoglossal motoneurones.
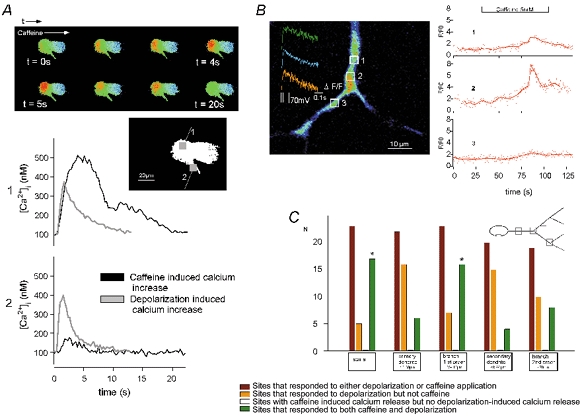
A, CCD imaging series showing the propagation of the caffeine-evoked response in a hypoglossal motoneurone. Time course of the different Ca2+ responses in the soma (1) and in the proximal dendrite (2) evoked by bath application of 5 mm caffeine (black traces) and depolarization to +20 mV from a holding potential of −60 mV (grey traces) are shown. Boxes in the inset indicate the selected regions in the peripheral soma and proximal dendrite at a distance of 20 μm. The caffeine response is reduced to a minimum in the proximal dendrite compared to the soma. B, multiphoton imaging (single optical section) showing non-uniformly distributed Ca2+‘hot spots’ in the more distal dendrites evoked by bath application of 5 mm caffeine and depolarization (inset). White squares identify regions of interest (ROIs) before and after the branching point where transients related to caffeine (5 mm)-induced intracellular Ca2+ release were measured. C, occurrence of both signal types in different compartments reveal their heterogeneous distribution. The graphical summary of 23 hypoglossal motoneurones shows the frequency of significant Ca2+ elevations in the defined compartments. Caffeine-evoked responses were selected by evaluating the distance from the initial release site and their typical time course. The total number (N) of hot spots that showed Ca2+ increases after either depolarization or caffeine application was: soma, 23; primary dendrite, 22; 1st order branches, 23; secondary dendrite, 20; and 2nd order branches, 19. The frequency (f=n/N) of the above hot spots that responded to depolarization but not caffeine in these compartments was 0.25, 0.73, 0.30, 0.75 and 0.58 and of those reacting to both caffeine and depolarization was 0.75, 0.27, 0.70, 0.25 and 0.42, respectively. Caffeine responses without the related depolarization-induced responses could not be observed. The frequency of a Ca2+ influx through voltage-gated Ca2+ channels without any inducible caffeine responses was very high for two compartments (0.73 for primary and 0.75 for secondary dendrites). The soma and the primary branching point both showed a high probability for the coexistence of voltage-gated Ca2+ channels and caffeine-induced responses (fdepol-caff»fdepol for the soma branching points, about 40 and 70 μm from the soma, and in dendritic regions > 30 μm from the soma (*).
Although caffeine was usually administered into the bath at a concentration of 5 mm, it was possible to evoke graded Ca2+ responses by varying the caffeine perfusion time. Exposure times of 30–45 s evoked submaximal increases in [Ca2+]i, on average 200 nm, whereas maximal increases (500–900 nm) occurred following exposure to caffeine for 60 s. Large caffeine-induced Ca2+ signals were accompanied by outward currents (85.6 ± 21.8 pA, n = 19) recorded at a holding potential of −60 mV. These outward currents coincided with Ca2+ signals and displayed a reversal potential close to the Nernst potential for K+, consistent with the idea that they were carried by Ca2+-dependent, apamin-sensitive K+ channels. Indeed, amplitudes and localization of Ca2+ signals were important determinants of outward current magnitudes, and Ca2+-dependent K+ currents were small or absent when Ca2+ increases were less than 200 nm (data not shown).
The high spatio-temporal resolution of MPM revealed that caffeine-induced release originated from defined sites in dendrites and somata. This is exemplified in Fig. 4B, where caffeine was bath applied at a concentration of 5 mm. This dose induced detectable Ca2+ release events in two locations labelled ‘1’ and ‘2’, but failed to evoke a response in a third location that was less than 15 μm away from ‘active’ release sites. This demonstrated that hypoglossal motoneurones: (i) displayed highly localized Ca2+ release events and (ii) were able to maintain substantial Ca2+ gradients over a distance of several micrometres for more than 20 s (see below), indicating a surprising functional separation of neighbouring dendritic compartments.
MPM was also used to investigate whether RyR-dependent Ca2+ release was located close to sites where voltage-dependent Ca2+ influx occurred. As shown in Fig. 4C, sites of caffeine-induced release could indeed be found in close vicinity of depolarization-activated Ca2+ influx pathways but only in distinct places like the somatic region and branching points. Such a co-localization could be observed in the soma and at branching points in 70 % of all cells investigated. On the other hand, a series of experiments (n = 5) with procaine (1 mm) showed no significant effect on kinetics of depolarization-evoked Ca2+ transients in the soma, dendrites or branching points of hypoglossal motoneurones (data not shown).
Involvement of ER and mitochondria in cytoplasmic Ca2+ regulation
To investigate the relative impact of endoplasmic reticulum and mitochondria, we tested pharmacological agents that selectively block Ca2+ uptake into different compartments. For example, CCCP was used as an uncoupler of the electrochemical potential across the inner mitochondrial membrane to abolish mitochondria-mediated regulation of [Ca2+]i (Hirsch et al. 1989). CPA was utilized as a sarcoplasmic/endoplasmic reticulum (SERCA) pump inhibitor.
As shown in Fig. 5A, Ca2+ stores were initially preloaded by voltage activation of plasma membrane Ca2+ channels. Subsequent bath application of 2 μm CCCP evoked a substantial, slow increase of cytoplasmic free [Ca2+]i, from 78 ± 28 to 256 ± 54 nm (n = 5). This increase could not be explained by a reduced level of ATP, as 4 mm ATP was continuously provided by pipette perfusion of the cytosol. As expected from the dominant role of KCa channels in hypoglossal neurones, CCCP-evoked [Ca2+]i increases were paralleled by outward currents with mean amplitudes of 39.8 ± 14.7 pA, n = 5 cells (Fig. 5B). Figure 5C shows a recording from a cell, where after a short depolarization to +20 mV to reload stores, initially CCCP and subsequently, within 3 min, caffeine were applied to the bath. A second application of CCCP without caffeine, within 3 min did not evoke a further basal increase in [Ca2+]i, indicating that the underlying mechanisms were still inactivated. Very important, however, was the finding that CCCP fully eliminated the fast component of the caffeine-evoked response (5/5 cells, Fig. 5C). In this case, the caffeine response was depressed to levels under 200 nm, and no outward current could be observed after caffeine application. Taken together, these results indicate a significant role of mitochondria for both caffeine-induced Ca2+ responses and the control of KCa-dependent motoneurone excitability.
Figure 5. Depression of caffeine-induced intracellular Ca2+ signals after depletion of mitochondrial Ca2+.
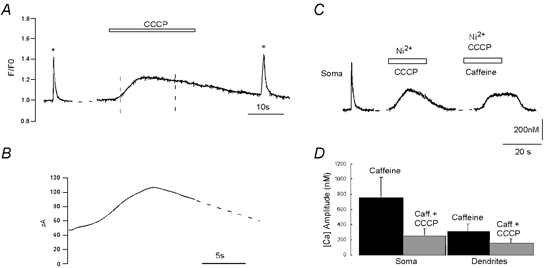
A, fura-2 CCD camera imaging of Ca2+ transients evoked in the soma of a hypoglossal motoneurone by a single step depolarization to +20 mV from a holding potential of −60 mV (1 s, *); by mitochondrial Ca2+ release evoked by 2 μm CCCP and finally by a second voltage pulse. B, CCCP evoked a small outward current (indicated by dashed lines in A), which displayed a mean amplitude of 39.8 ± 14.7 pA (n = 5). C, ER Ca2+ release triggered by 5 mm caffeine, 4 min after CCCP application and washout. The initial rapid [Ca2+]i transient typically evoked by caffeine is absent, while the slow transient is depressed (Ca2+ entry through voltage-gated Ca2+ channels was blocked by 2 mm Ni2+). D, graphical summary of results from 4 motoneurones showing the decrease of caffeine responses after CCCP application.
The role of mitochondria and ER for cytosolic Ca2+ sequestration is further illustrated in Fig. 6, where CCCP as well as CPA prolonged the decay of voltage-evoked Ca2+ signals in somatic and dendritic compartments. CCCP increased τ, the relaxation time constant of [Ca2+]i, from 3.2 ± 0.6 s to 6.3 ± 0.6 ms (P < 0.05 n = 6) in somatic and from 2.1 ± 0.4 to 6.3 ± 0.6 s in dendritic compartments (P < 0.05; n = 6). Again, this prolongation could not be explained by ATP depletion, as it was continuously supplied via the pipette. Also, the retardation of relaxation times was not explained by a potential ‘rundown’ of extrusion or uptake mechanisms, as previous studies have demonstrated a robust stability of Ca2+ recovery kinetics for up to 1 h during whole-cell recordings from hypoglossal motoneurones (Lips & Keller, 1998, 1999). As shown in the inset of Fig. 6A, the CCCP-mediated prolongation of Ca2+ transients was paralleled by a retardation of KCa-dependent tail currents measured after a standard depolarization protocol (500 ms, +20 mV). Interestingly, the effect of CCCP on decay times (approximately 2-fold prolongation) was significantly greater than that of CPA (increase of τ by 0.8 ± 0.1 s over control, P < 0.05, 1.2-fold prolongation; n = 4). Both drugs had only small effects on the amplitude of voltage-evoked Ca2+ signals (< 15 %).
Figure 6. Relative effectiveness of mitochondrial and ER uptake mechanisms in shaping Ca2+ responses to plasma membrane depolarization.
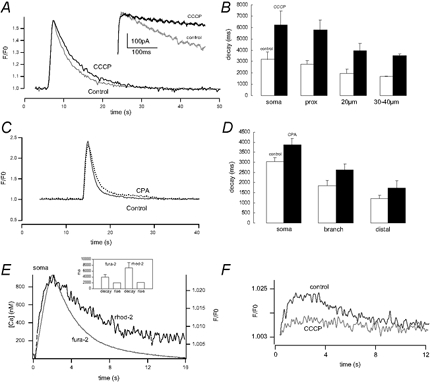
A, fura-2 CCD camera imaging of Ca2+ transients evoked in the soma by step depolarization to +20 mV from a holding potential of −60 mV, before (Control) and after bath application of 2 μm CCCP. Inset, prolongation of the tail current (depolarization to +20 mV) of the Ca2+-activated K+ conductance after CCCP application. B, graphical summary of results from 6 motoneurones showing effects of 2 μm CCCP on the decay time constants of Ca2+ signals (τ, ms) in different cell compartments (decay time constants were prolonged from 3.2 ± 0.6 to 6.2 ± 1.2 s for the soma; 2.7 ± 0.4 to 5.8 ± 0.8 s for the proximal dendrite; 1.9 ± 0.4 to 3.9 ± 0.7 s for distance from centre of soma (d) = 20 μm; and 1.7 ± 0.04 to 3.5 ± 0.1 s for d = 30–40 μm. Effective prolongation of decay time constants after CCCP: 2.1 ± 0.25 for somatic and dendritic sites). C, fura-2 CCD camera imaging of Ca2+ transients evoked in the soma by step depolarization to +20 mV from a holding potential of −60 mV, before (Control) and after blocking sarcoplasmic-endoplasmic reticulum (SERCA) pumps with bath application of cyclopiazonic acid (CPA), 50 μm. D, graphical summary of results from 4 motoneurones showing increased time constant of decay (P < 0.05) in different cell compartments (from 3 ± 0.2 to 3.9 ± 0.3 s in soma and proximal dendrite, 1.9 ± 0.24 to 2.6 ± 0.29 s for d = 20–30 μm, effective prolongation of decay time constants: 1.3 ± 0.2 for somatic and dendritic sites). E, kinetics of cytosolic and mitochondrial Ca2+ influx as revealed by the fluorescence of fura-2 and rhod-2. Hypoglossal motoneurones were loaded with 1–1.5 μm rhod-2 AM for 1 h to enable monitoring of [Ca2+]m and fura-2 was included in the patch pipette to allow simultaneous monitoring of [Ca2+]i. Representative recording showing overlay of [Ca2+]i and [Ca2+]m transients during a 2 s depolarizing pulse (from −60 to +30 mV) re-plotted on an expanded time scale ([Ca2+]i indicated by fura-2 label and F/F0 of calcium-dependent intramitochondrial fluorescence by rhod-2 label). F, mitochondrial depolarization (perfusion with 2 μm CCCP) partially (by 50–70 %) blocked Ca2+ accumulation by the mitochondria after depolarization (n = 2).
A further observation supporting the notion that mitochondrial Ca2+ uptake is an important regulator of cytosolic Ca2+ homeostasis results from experiments using the mitochondrial indicator dye rhod-2. After loading motoneurones with rhod-2 ester, its positive charge results in significant dye accumulation within the mitochondrial matrix. Following hydrolysis of the acetoxymethylester group, rhod-2 becomes trapped in the mitochondrial matrix. To wash out residual, cytosolic rhod-2 cells were then filled with fura-2 via the patch pipette. During a depolarizing step from −60 to +30 mV (duration of 2 s), [Ca2+]i increased to 814 ± 87 nm (n = 5; Fig. 6E). The total time to peak for the increase in [Ca2+]i was 2.0 ± 0.2 s and the decay (τ) for recovery following the end of the depolarizing pulse was 3.9 ± 0.8 s. The same stimulus also increased mitochondrial [Ca2+] as indicated by the increase in rhod-2 fluorescence. When the cytoplasmic and mitochondrial [Ca2+] records from the same cell were viewed on the same time scale, no significant difference in the rising phases of [Ca2+]i or intramitochondrial calcium dynamics could be detected. However, the recovery time constant τ of rhod-2 fluorescence was 7.1 ± 1.7 s which was significantly longer compared to the recovery of [Ca2+]i (P < 0.05).
Implications for quantitative models of Ca2+ homeostasis in hypoglossal motoneurones
For a more detailed understanding of cellular function, it is useful to develop quantitative models of Ca2+ profiles and their spatio-temporal dispersion in the cytosol. Earlier studies established a linear, quantitative model of Ca2+ homeostasis in hypoglossal motoneurones that, among others, identified a low endogenous buffering capacity, κS= 41, as a critical determinant of [Ca2+]i (Lips & Keller, 1998). Under physiological conditions, low κS presumably reflects low levels of mobile, Ca2+-binding proteins (CaBP), like calbindin or parvalbumin, and is thought to facilitate rapid relaxation times of global Ca2+ transients during high-frequency, rhythmic activity (Lips & Keller, 1999). As diffusible CaBPs also accelerate the dispersion of spatial gradients, low κS tends to stabilize localized Ca2+ signal domains in cytosolic compartments. This interpretation was underlined by observations using high-resolution MPM. As exemplified in Fig. 4, application of caffeine evoked detectable Ca2+ responses in two neighbouring locations that lasted many seconds, but failed to induce a response in a third location that was less than 15 μm away. Such results indeed illustrate a remarkable separation of Ca2+ profiles in neighbouring compartments.
To analyse the impact of ‘buffered diffusion’ in motoneurones in a more quantitative way, it is useful to consider a patch-clamped cell where endogenous buffers are either fixed or mobile. In this case, the ‘effective’ diffusion constant for Ca2+ can be estimated from elementary parameters of the underlying buffers by using the equation (Zhou & Neher, 1993; Klingauf & Neher, 1997):
where DCa= 220 μm2 s−1 and Dfura-2= 170 μm2 s−1 represent values from the literature for diffusion constants of Ca2+ and fura-2; and κB′, κmob and κS denote Ca2+-binding capacities of fura-2, mobile and fixed buffers, respectively (Zhou & Neher, 1993; Klingauf & Neher, 1997). A direct implication of this relation is that highly mobile buffers such as fura-2 account for a large product Dfura-2κB′′ and thus notably accelerate the diffusion of Ca2+. Accordingly, caffeine-induced transients are expected to be substantially more localized under undisturbed conditions compared to the whole-cell recording situation employed in this report. Moreover, earlier studies established that mobile endogenous buffers were either absent or reduced to minimal levels in low buffered motoneurones (Lips & Keller, 1999; Palecek et al. 1999), suggesting that κmob«κB′. For the experimental conditions in patch clamped hypoglossal motoneurones (κS= 41, [fura-2]= 100 μm), we therefore calculate an expected value Deff= 158 μm2 s−1 based on the motoneurone-specific profile of buffers.
This value can be directly compared to actual experimental results from CCD imaging and MPM. For example, the effective diffusion constant can be measured by observing the distance r that Ca2+ diffuses in a time interval t by using the equation (Neher, 1995):
To illustrate this relation, we consider experimental conditions similar to those presented in Fig. 4, where a notable gradient occurs over a distance r = 15 μm for t > 20 s. Although several distinct molecular mechanisms might account for this spatial heterogeneity, it is still useful to consider the elementary diffusion model. By using the above equation, we obtain Deff < 5.4 μm2 s−1, which is a factor of 30 smaller compared to the diffusion constant of 158 μm2 s−1 expected from theoretical considerations. Such results have two interesting implications. First, low levels of endogenous, mobile buffers in hypoglossal motoneurones presumably retard the dispersion of localized Ca2+ gradients around open membrane channels and release sites. Second, this effect is apparently enhanced by an additional, functional separation of dendritic compartments that further prevents the rapid dispersion of Ca2+ gradients in the cytosol.
In this context, it is interesting to note that multiphoton images of hypoglossal motoneurones such as those exemplified in Fig. 1 demonstrated a remarkable absence of dendritic spines, which are thought to restrict the dispersion of Ca2+ gradients in other cells like hippocampal neurones or cerebellar Purkinje cells. As these neurones are known to display substantially higher Ca2+ buffering capacities with notably increased concentrations of diffusable CaBP in the cytosol, it is reasonable to interpret low buffering capacities and functional separations of dendritic compartments in motoneurones as two complementary, cellular adaptations that facilitate localized Ca2+ signal domains in the absence of specific anatomical formations (e.g. dendritic spines).
DISCUSSION
Ca2+ signalling in motoneurones is a critical determinant of synaptic and electrical properties and has been associated with many aspects of neuronal plasticity (Rekling et al. 2000). Failure of precise regulation of free intracellular Ca2+ levels may lead to severe pathophysiological conditions and/or cell death (Krieger et al. 1996). In the present study, we investigated the local profile of Ca2+ signals in hypoglossal motoneurones of newborn mice by employing MPM, CCD imaging and whole-cell patch clamp recording in brain stem slices (Ladewig & Keller, 2000). We show the existence of dynamic intracellular Ca2+ storage and release mechanisms, and demonstrate physiological interaction between RyR-dependent Ca2+ release, mitochondrial processes and KCa-dependent control of electrical activity. The high spatio-temporal resolution of MPM offered the unique advantage to analyse highly localized Ca2+ signals in somatic and dendritic compartments.
Functional interaction of Ca2+ storage and release sites
During bath application, caffeine evoked localized RyR-mediated increases in [Ca2+]i which varied substantially between different locations within a given cell. As our indicator dye (50–100 μm fura-2 in standard experiments) is thought to substantially accelerate local dispersion of [Ca2+]i, Ca2+ profiles are expected to be even more localized under physiological, undisturbed conditions. A close interaction between Ca2+ release and mitochondrial Ca2+ homeostasis was suggested by our observation that caffeine-induced responses were significantly suppressed after disruption of the mitochondrial electrochemical potential by CCCP. As mitochondria are not thought to contain RyR-channels (Szalai et al. 2000), the most likely interpretation is that RyR-induced Ca2+ release is modulated by mitochondrial processes. It is interesting to note that studies on the interaction of RyR and mitochondrial homeostasis performed on smooth muscle cells (Nassar & Simpson, 2000), cardiac cells (Szalai et al. 2000) and HeLa cells (Rizzuto et al. 2000) displayed results that were similar to those observed in hypoglossal neurones. This indicates that a tight coupling between Ca2+ regulation in mitochondria and RyR-sensitive Ca2+ storage sites represents a more general molecular scheme. Our interpretation is clearly different from that developed for dissociated basal forebrain neurones of rats (Murchison & Griffith, 2000), where a similar, biphasic caffeine-induced signal was transformed by co-application of CCCP into a much larger monophasic Ca2+ signal lacking a late, slower ‘shoulder’. In this case, the rapid initial decline of the caffeine-induced signal in forebrain neurones was rather associated with avid mitochondrial uptake of cytoplasmic Ca2+.
In addition to RyR-mediated Ca2+ responses, caffeine evoked outward currents that were strongly correlated with caffeine-evoked Ca2+ signals. This indicated that they were primarily mediated by apamin-sensitive, Ca2+-dependent K+ channels known to be present in hypoglossal motoneurones of that age (Lape & Nistri, 2000). Similarly, in hippocampal pyramidal neurones, caffeine activates KCa currents that hyperpolarize membrane potentials (Uneyama et al. 1993) and induces spontaneous hyperpolarizations in dopaminergic neurones of neonatal rats (Seutin et al. 2000). In a corresponding scheme, Ca2+-induced membrane hyperpolarization might reduce build-up of [Ca2+]i in hypoglossal motoneurones during hypoxia or ischaemia (Zhang & Lipton, 1999; Semenov et al. 2000), and thus avoid excess Ca2+ influx through voltage-activated Ca2+ channels during persistent depolarization.
Functional significance of localized Ca2+ profiles during physiological motoneurone activity
Our findings clearly demonstrate the presence of efficient Ca2+ stores in the ER of hypoglossal motoneurones, which can be readily activated by established pharmacological tools. Together with the strong impact on the decay of voltage-activated Ca2+ signals, these observations indicate that Ca2+-storing organelles may play a significant role in physiological Ca2+ regulation, and as the mitochondrial Ca2+ clearance is highly temperature sensitive the effect will be much higher at body temperature (David & Barrett, 2000). It is interesting to note that in low buffered cells the presence of dynamic, Ca2+-sequestering organelles could have several advantages. First, this arrangement offers a mechanism for offsetting the potentially dangerous consequences of low endogenous Ca2+ buffering resulting from small concentrations of Ca2+-binding proteins in the cytosol. A second advantage might be related to the fact that hypoglossal motoneurones exhibit highly variable and rapidly changing discharge patterns which control the genioglossus muscles during mastication, deglutition, phonation, vomiting and ventilation. In the latter case, precise postural control of the tongue must be quickly adjusted to optimize upper airway resistance and adjust ventilation across a wide range of breathing frequencies. In rodents, for example, frequencies may be as high as 5 breaths s−1 (Jaquin et al. 1996) and rapid adjustments in [Ca2+]i are necessary to fine-tune activity-dependent changes in muscle force and corresponding tongue position.
Potential implication of localized Ca2+ profiles for pathophysiological conditions and ALS-related motoneurone disease
Previous investigations indicated that motoneurones which are particularly vulnerable during ALS are also characterized by exceptionally low endogenous buffering capacities and relatively large microdomains around open Ca2+ channels (Lips & Keller, 1999). By using MPM and CCD imaging, we now present strong evidence that this cell-specific specialization of Ca2+ homeostasis is accompanied by efficient control of local [Ca2+]i by Ca2+-regulating organelles. Although major changes in morphological and electrophysiological properties have been documented during the first 2 postnatal weeks in hypoglossal motoneurones (Viana et al. 1994), our findings have several functional implications for motoneurones during human ALS and related animal models of this neurodegenerative disease. For example, localized Ca2+ regulation becomes particularly vulnerable to disruptions of mitochondrial function, a phenomenon that is a well-known symptom of motoneurone degeneration during ALS. In such pathophysiological situations, bursts of action potentials might increase local Ca2+ amplitudes to high concentrations above the micromolar domain, due to the combination of massive Ca2+ influx and low buffering (Lips & Keller, 1999; Herrington et al. 1996). Without efficient Ca2+ sequestration machinery, the local Ca2+ concentration might easily reach levels that are sufficient to activate mitochondria-related apoptotic mechanisms and associated signal cascades (Jacotot et al. 1999). Taken together, our measurements therefore indicate that cellular specializations, which facilitate highly dynamic and localized Ca2+ profiles in motoneurones under physiological conditions, might also enhance their vulnerability during ALS-related motoneurone disease and associated cellular disturbances.
Acknowledgments
We thank D. Crzan for support with slice preparations and excellent technical assistance. This research was supported by DFG grants Ke 403/6–1, the Schwerpunktprogramm ‘Definierte Mutanten’ and the Sonderforschungsbereich 406, and further by the National Institute of Health/National Centre for Research Resources (P41-RR0422) and the National Science Foundation (DIR 88002787).
REFERENCES
- Alexianu ME, Ho B-K, Mohamed AH, La Bella V, Smith RG, Appel SH. The role of calcium-binding proteins in selective motoneurone vulnerability in amyotrophic lateral sclerosis. Ann Neurol. 1994;36:846–858. doi: 10.1002/ana.410360608. [DOI] [PubMed] [Google Scholar]
- Augustine GJ, Charlton MP, Smith SJ. Calcium action in synaptic transmitter release. Ann Rev Neurosci. 1987;10:633–693. doi: 10.1146/annurev.ne.10.030187.003221. [DOI] [PubMed] [Google Scholar]
- Babcock DF, Herrington J, Goodwin PC, Park YB, Hille B. Mitochondrial participation in the intracellular Ca2+ network. J Cell Biol. 1997;136:833–844. doi: 10.1083/jcb.136.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacci A, Verderio C, Pravettoni E, Matteoli M. Synaptic and intrinsic mechanisms shape synchronous oscillations in hippocampal neurones in culture. Eur J Neurosci. 1999;11:389–397. doi: 10.1046/j.1460-9568.1999.00440.x. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Lipp P. Calcium – A life and death signal. Nature. 1998;395:645–648. doi: 10.1038/27094. [DOI] [PubMed] [Google Scholar]
- David G, Barrett EF. Stimulation-evoked increases in cytosolic [Ca2+] in mouse motor nerve terminals are limited by mitochondrial uptake and are temperature-dependent. J Neurosci. 2000;20:7290–7296. doi: 10.1523/JNEUROSCI.20-19-07290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk W, Strickler JH, Webb WW. Two-photon laser scanning fluorescence microscopy. Science. 1990;248:73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- DePaul R, Abbs JH, Caliguiri M, Gracco VL, Brooks BR. Hypoglossal, trigeminal and facial motoneurone involvement in amyotrophic lateral sclerosis. Neurology. 1988;38:281–283. doi: 10.1212/wnl.38.2.281. [DOI] [PubMed] [Google Scholar]
- Dodt HU, Zieglgänsberger W. Infrared videomicroscopy: a new look at neuronal structure and function. Trends Neurosci. 1994;17:453–458. doi: 10.1016/0166-2236(94)90130-9. [DOI] [PubMed] [Google Scholar]
- Drummond RM, Tuft RA. Release of Ca2+ from the sarcoplasmic reticulum increases mitochondrial [Ca2+] in rat pulmonary artery smooth muscle cells. J Physiol. 1999;516:139–147. doi: 10.1111/j.1469-7793.1999.139aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards FA, Konnerth A, Sakmann B, Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflugers Arch. 1989;414:600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Frermann D, Keller BU, Richter DW. Calcium oscillations in rhythmically active respiratory neurones in the brain-stem of the mouse. J Physiol. 1999;515:119–131. doi: 10.1111/j.1469-7793.1999.119ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel DD, Tsien RW. A caffeine- and ryanodine-sensitive Ca2+ store in bullfrog sympathetic neurones modulates effects of Ca2+ entry on [Ca2+]i. J Physiol. 1992;450:217–246. doi: 10.1113/jphysiol.1992.sp019125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of calcium indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hamill O, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Herrington J, Park YB, Babcock DF, Hille B. Dominant role of mitochondria in clearance of large Ca2+ loads from rat adrenal chromaffin cells. Neuron. 1996;16:219–228. doi: 10.1016/s0896-6273(00)80038-0. [DOI] [PubMed] [Google Scholar]
- Hirsch JD, Beyer CF, Malkowitz L, Beer B, Blume AJ. Mitochondrial benzodiazepine receptors mediate inhibition of mitochondrial respiratory control. Mol Pharmacol. 1989;35:157–163. [PubMed] [Google Scholar]
- Jacotot E, Costantini P, Laboureau E, Zamzami N, Susin SA, Kroemer G. Mitochondrial membrane permeabilization during the apoptotic process. Ann NY Acad Sci. 1999;887:18–30. doi: 10.1111/j.1749-6632.1999.tb07919.x. [DOI] [PubMed] [Google Scholar]
- Jaquin TD, Borday V, Schneider-Maunoury S, Topilko P, Ghilini G, Kato F, Charnay P, Champagnat J. Reorganization of pontine rhythmogenetic neuronal networks in Krox-20 knockout mice. Neuron. 1996;17:747–758. doi: 10.1016/s0896-6273(00)80206-8. [DOI] [PubMed] [Google Scholar]
- Kato N, Tanaka T, Yamamoto K, Isomura Y. Distinct temporal profiles of activity-dependent calcium increase in pyramidal neurones of the rat visual cortex. J Physiol. 1999;519:467–479. doi: 10.1111/j.1469-7793.1999.0467m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingauf J, Neher E. Modelling buffered Ca++ diffusion near the membrane: implications for secretion in neuroendocrine cells. Biophys J. 1997;72:674–691. doi: 10.1016/s0006-3495(97)78704-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloppenburg P, Zipfel WR, Webb WW, Harris-Warrick RM. Highly localized Ca2+ accumulation revealed by multiphoton microscopy in an identified motoneurone and its modulation by dopamine. J Neurosci. 2000;20:2523–2533. doi: 10.1523/JNEUROSCI.20-07-02523.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk P, Verhratsky A. Basic properties of calcium release channels in neural cells. In: Soria B, Cena V, editors. Ion Channel Pharmacology. Oxford: Oxford University Press; 1998. [Google Scholar]
- Krieger C, Lanius RA, Pelech SL, Shaw CA. Amyotrophic lateral sclerosis: the involvement of intracellular Ca2+ and protein. kinase C. Trends Pharmacol Sci. 1996;17 doi: 10.1016/0165-6147(96)10004-3. [DOI] [PubMed] [Google Scholar]
- Ladewig T, Keller BU. Simultaneous patch clamp recording and calcium imaging in a rhythmically active neuronal network in the brainstem slice preparation from mouse. Pflugers Arch. 2000;440:322–332. doi: 10.1007/s004240000277. [DOI] [PubMed] [Google Scholar]
- Ladewig T, Kloppenburg P, Zipfel WR, Webb WW, Keller BU. Intracellular calcium release in hypoglossal motoneurones monitored by multiphoton microscopy. Soc Neurosci Abstr. 2000;26 182.18. [Google Scholar]
- Lalley PM, Ladewig T, Keller BU. Serotonergic modulation of intracellular calcium profiles in neonatal hypoglossal motoneurones from mouse. Soc Neurosci Abstr. 1999;26 doi: 10.1016/j.brainres.2003.10.033. 721.5. [DOI] [PubMed] [Google Scholar]
- Lape R, Nistri A. Current and voltage clamp studies of the spike medium afterhyperpolarization of hypoglossal motoneurones in a rat brain stem slice preparation. J Neurophysiol. 2000;83:2987–2995. doi: 10.1152/jn.2000.83.5.2987. [DOI] [PubMed] [Google Scholar]
- Lieske SP, Thoby-Brisson M, Telgkamp P, Ramirez JM. Reconfiguration of the neural network controlling multiple breathing patterns: eupnea, sighs and gasps. Nat Neurosci. 2000;3:600–607. doi: 10.1038/75776. [DOI] [PubMed] [Google Scholar]
- Lips MB, Keller BU. Endogenous calcium buffering in motoneurones of the nucleus hypoglossus from mouse. J Physiol. 1998;511:105–117. doi: 10.1111/j.1469-7793.1998.105bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips MB, Keller BU. Activity-related calcium dynamics in motoneurones of the nucleus hypoglossus from mouse. J Neurophysiol. 1999;82:2936–2946. doi: 10.1152/jn.1999.82.6.2936. [DOI] [PubMed] [Google Scholar]
- McFarlane MB, Gilly WF. State-dependent nickel block of a high-voltage-activated neuronal calcium channel. J Neurophysiol. 1998;80:1678–1685. doi: 10.1152/jn.1998.80.4.1678. [DOI] [PubMed] [Google Scholar]
- Mifflin SW. Intensity and frequency dependence of laryngal afferent inputs to respiratory hypoglossal motoneurones. J Appl Physiol. 1997;83:1890–1899. doi: 10.1152/jappl.1997.83.6.1890. [DOI] [PubMed] [Google Scholar]
- Murchison D, Griffith WH. Mitochondria buffer non-toxic calcium loads and release calcium through the permeability transition pore and sodium/calcium exchanger in rat basal forebrain neurones. Brain Res. 2000;854:139–151. doi: 10.1016/s0006-8993(99)02297-0. [DOI] [PubMed] [Google Scholar]
- Nassar A, Simpson AW. Elevation of mitochondrial calcium by ryanodine-sensitive calcium-induced calcium release. J Biol Chem. 2000;275:23661–23665. doi: 10.1074/jbc.M000457200. [DOI] [PubMed] [Google Scholar]
- Neher E. The use of fura-2 for estimating Ca buffers and Ca fluxes. Neuropharmacology. 1995;34:1423–1442. doi: 10.1016/0028-3908(95)00144-u. [DOI] [PubMed] [Google Scholar]
- Pozzan T, Rizzuto R, Volpe P, Meldolesi J. Molecular and cellular physiology of intacellular calcium stores. Physiol Rev. 1994;74:505–636. doi: 10.1152/physrev.1994.74.3.595. [DOI] [PubMed] [Google Scholar]
- Reiner A, Medina L, Figueredo CG, Anfinson S. Brainstem motor pools that are selectively resistant in amyotrophic lateral sclerosis are preferentially enriched in parvalbumin: evidence from monkey brainstem for a calcium-mediated mechanism in sporadic ALS. Exp Neurol. 1995;131:239–255. doi: 10.1016/0014-4886(95)90046-2. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic control of motoneuronal excitability. Physiol Rev. 2000;80:767–852. doi: 10.1152/physrev.2000.80.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 2000;280:1783–1787. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- Sah P, McLachlan EM. Ca2+-activated K+ currents underlying the afterhyperpolarization in guinea pig vagal neurones: a role for Ca2+-activated Ca2+ release. Neuron. 1991;7:257–264. doi: 10.1016/0896-6273(91)90264-z. [DOI] [PubMed] [Google Scholar]
- Semenov DG, Samoilov MO, Zielonka P, Lazarewicz JW. Responses to reversible anoxia of intracellular free and bound Ca2+ in rat cortical slices. Resuscitation. 2000;44:207–214. doi: 10.1016/s0300-9572(00)00136-2. [DOI] [PubMed] [Google Scholar]
- Seutin V, Mkahli F, Massotte L, Dresse A. Calcium release from internal stores is required for the generation of spontaneous hyperpolarizations in dopaminergic neurones of neonatal rats. J Neurophysiol. 2000;83:192–197. doi: 10.1152/jn.2000.83.1.192. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart GJ, Dodt HU, Sakmann B. Patch clamp recordings from the soma and dendrites of neurones in brain slices using infrared video microscopy. Pflugers Arch. 1993;423:511–518. doi: 10.1007/BF00374949. [DOI] [PubMed] [Google Scholar]
- Szalai G, Csordas G, Hantash BM, Thomas AP, Hajnoczy G. Calcium signal transmission between ryanodine receptors and mitochondria. J Biol Chem. 2000;275:15305–15313. doi: 10.1074/jbc.275.20.15305. [DOI] [PubMed] [Google Scholar]
- Titz S, Keller BU. Rapidly deactivating AMPA receptors determine excitatory synaptic transmission to interneurones in the nucleus tractus solitarius from rat. J Neurophysiol. 1997;78:82–91. doi: 10.1152/jn.1997.78.1.82. [DOI] [PubMed] [Google Scholar]
- Uneyama H, Munakata M, Akaike N. Caffeine response in pyramidal neurones freshly dissociated from rat hippocampus. Brain Res. 1993;604:24–31. doi: 10.1016/0006-8993(93)90348-q. [DOI] [PubMed] [Google Scholar]
- Vanselow BK, Keller BU. Calcium dynamics and buffering in oculomotor neurones from mouse that are particularly resistant during amyotrophic lateral sclerosis (ALS)-related motoneurone disease. J Physiol. 2000;525:433–445. doi: 10.1111/j.1469-7793.2000.t01-1-00433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana F, Bayliss DA, Berger AJ. Postnatal changes in rat hypoglossal motoneurone membrane properties. Neuroscience. 1994;59:131–148. doi: 10.1016/0306-4522(94)90105-8. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lipton P. Cytosolic Ca2+ changes during in vitro ischemia in rat hippocampal slices: major roles for glutamate and Na+-dependent calcium release from mitochondria. J Neurosci. 1999;19:3307–3315. doi: 10.1523/JNEUROSCI.19-09-03307.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Neher E. Mobile and immobile calcium buffers in bovine adrenal chromaffin cells. J Physiol. 1993;469:245–273. doi: 10.1113/jphysiol.1993.sp019813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS. Calcium- and activity-dependent synaptic plasticity. Curr Opin Neurobiol. 1999;9:305–313. doi: 10.1016/s0959-4388(99)80045-2. [DOI] [PubMed] [Google Scholar]


