Abstract
Despite the prominent role played by intracellular Ca2+ stores in the regulation of neuronal Ca2+ homeostasis and in invertebrate photoreception, little is known about their contribution to the control of free Ca2+ concentration ([Ca2+]i) in the inner segments of vertebrate photoreceptors. Previously, caffeine-sensitive intracellular Ca2+ stores were shown to play a role in regulating glutamate release from photoreceptors. To understand the properties of these intracellular stores better we used pharmacological approaches that alter the dynamics of storage and release of Ca2+ from intracellular compartments. Caffeine evoked readily discernible changes in [Ca2+]i in the inner segments of rods, but not cones. Caffeine-evoked Ca2+ responses in cone inner segments were unmasked in the presence of inhibitors of the plasma membrane Ca2+ ATPases (PMCAs) and mitochondrial Ca2+ sequestration. Caffeine-evoked responses were blocked by ryanodine, a selective blocker of Ca2+ release and by cyclopiazonic acid, a blocker of Ca2+ sequestration into the endoplasmic reticulum. These two inhibitors also substantially reduced the amplitude of depolarization-evoked [Ca2+]i increases, providing evidence for Ca2+-induced Ca2+ release (CICR) in rods and cones. The magnitude and kinetics of caffeine-evoked Ca2+ elevation depended on the basal [Ca2+]i, PMCA activity and on mitochondrial function. These results reveal an intimate interaction between the endoplasmic reticulum, voltage-gated Ca2+ channels, PMCAs and mitochondrial Ca2+ stores in photoreceptor inner segments, and suggest a role for CICR in the regulation of synaptic transmission.
Cellular Ca2+ homeostasis is a dynamic process that relies on a precise balance between Ca2+ influx, sequestration, buffering and extrusion. Maintenance of steady-state [Ca2+]i therefore requires concerted co-ordination of Ca2+ channels, transporters and soluble Ca2+-buffering proteins (Pozzan et al. 1994; Berridge et al. 2000; Delmas & Brown, 2002). Most neurones utilize two main sources of Ca2+ for initiating Ca2+-dependent processes within the cytoplasm: Ca2+ entry across the plasma membrane via voltage- and/or ligand-gated Ca2+ channels and Ca2+ release from internal stores. One important class of Ca2+ stores belongs to the ryanodine receptor (RyR) family localized to the endoplasmic reticulum (ER). Ca2+ release from these stores is often studied by using the methylxanthine compound caffeine (Neering & McBurney, 1984; Akaike & Sadoshima, 1989; Sitsapesan & Williams, 1990). Ca2+ released from caffeine-sensitive stores has been shown to control a wide variety of neuronal processes, including development (Hong et al. 2000), exocytosis (Smith & Cunnane, 1996; Narita et al. 2000) and both short- and long-term synaptic plasticity (Llano et al. 2000; Emptage et al. 2001; Sabatini et al. 2001). Ca2+ release from caffeine- and ryanodine-sensitive stores typically amplifies the [Ca2+]i changes induced by influx of Ca2+ through voltage-sensitive Ca2+ channels through the process of Ca2+-induced Ca2+ release (CICR; McPherson et al. 1991; Friel & Tsien, 1992; Hernandez-Cruz et al. 1995; Verkhratsky & Shmigol, 1996).
Photoreceptors can be divided into two general classes: (1) rods, which reliably signal single-photon absorptions, are relatively slow in response to light stimulation and exhibit a high sensitivity to light, and (2) cones, which are less sensitive to light, faster, more noisy and operate optimally in bright daylight. It has been suggested that many key differences in rod and cone function can be accounted for by different features of Ca2+ homeostasis in these two classes of photoreceptor (Korenbrot, 1995; Fain et al. 2001; Krizaj & Copenhagen, 2002). For example, the dynamic range of Ca2+ homeostasis in cone outer segments (OSs) may be more than three times greater than in the rods (Sampath et al. 1999). Rods and cones differ in the fraction of Ca2+ in the current through cyclic nucleotide-gated (CNG) channels, Ca2+ sensitivity of CNG channels, the rates of Ca2+ clearance from the OS and the inner segment (IS) and the kinetics of synaptic transmission at rod and cone synapses (Schnapf & Copenhagen, 1982; Korenbrot, 1995; Krizaj & Copenhagen, 1998; Sampath et al. 1999; Ohyama et al. 2002).
There are, however, significant gaps in our understanding of light-dependent changes in free Ca2+ in rods and cones. Little, for example, is known about the contribution of intracellular compartmentalization and Ca2+ buffering to [Ca2+] homeostasis in photoreceptor ISs and OSs. Previously we have shown that caffeine-induced Ca2+ release from internal stores regulates glutamate release from rod photoreceptor synaptic terminals (Krizaj et al. 1999). However, Ca2+ release from intracellular stores in the rod OS was not addressed in that study, nor did we investigate internal Ca2+ stores in cone ISs. Moreover, the mechanisms of Ca2+ release from the ER and the mitochondria in the IS are not yet understood. This present study was designed to characterize the mechanisms underlying the release of Ca2+ from internal compartments in both rods and cones. Our results suggest that Ca2+ release via RyR-gated channels is tightly controlled by the uptake into the ER (via the sarcoplasmic-endoplasmic Ca2+ ATPases, SERCAs), by sequestration into mitochondrial Ca2+ stores and by Ca2+ extrusion across the plasma membrane via plasma membrane Ca2+ ATPases (PMCAs). We also show that rods and cones exhibit marked differences in their responses to Ca2+-releasing agents such as caffeine, and provide evidence suggesting that signalling domains that link the plasma membrane to the ER differ between rods and cones.
METHODS
Preparation of isolated cells
Larval-stage tiger salamanders (Ambystoma tigrinum) were decapitated and pithed using procedures approved by the UCSF Committee for Animal Care. Retinas were dissected from enucleated eyes, and cells were dissociated in 0 Ca2+/papain (7 U ml−1; Worthington, Freehold, NJ, USA) saline for 25 min at room temperature (20–22 °C) and then plated onto coverslips coated with IgG/IgM (Jackson ImmunoResearch, West Grove, PA, USA) and the Sal-1 antibody (a kind gift from Dr Peter MacLeish) or 0.2 mg ml−1 Concanavalin A (Sigma, St Louis, MO, USA). All these procedures were performed in bright light. As a consequence, the photopigment in rod OSs was bleached. In the cone photoreceptors studied here, the OSs were removed during the dissociation procedure. Thus, under our experimental conditions, voltage changes in rod and cone ISs were unlikely to be influenced by photocurrents from the OSs.
Coverslips were inserted into a perfusing chamber with a volume of 90 μl (Warner Instruments, Hamden, CT, USA). The chamber was superfused via a multi-inlet manifold (MP-8, Warner Instruments) allowing complete solution exchange within 10 s. The control saline solution contained (mm): 97 NaCl, 2 KCl, 2 CaCl2, 2 MgCl2, 10 Hepes, 20 glucose, 1 pyruvic acid, 2 lactic acid, 0.3 ascorbic acid and 1 glutathione at 240 mosmol l−1. The pH was adjusted to 7.6 with NaOH.
[Ca2+] measurement and data acquisition
These methods are described fully elsewhere (Krizaj & Copenhagen, 1998). Briefly, photoreceptors were loaded with 3–5 μm fura-2 acetoxymethylester (fura-2 AM; Molecular Probes, Eugene, OR, USA) for 10 min and subsequently washed for 20 min. The fluorescence signals were acquired on an inverted microscope (Nikon Eclipse 200) using a dry × 40 objective (NA = 0.8). In all experiments, the signals were averaged over a region of interest (ROI) encompassing the perikarya (cell nucleus and the surrounding cytoplasm), the ellipsoid or the synaptic region of the IS; only the results obtained from the cell body are illustrated in this paper. Image acquisition was generally binned at 5 × 5 and was run at 0.3–1 Hz by a cooled 12 bit digital CCD camera using a Kodak KAF1400 chip (PXL, Photometrics, Tucson, AZ, USA). The camera and the shutter (Lambda 10–2, Sutter Instruments, Novato, CA, USA) were controlled by commercial software (Metafluor 4.1; Universal Imaging, West Chester, PA, USA). Ratios between the 340 and 380 nm excitation wavelengths were calculated after subtraction of the background fluorescence. Free Ca2+ levels were calibrated in vivo with 10 μm ionomycin using the standard relationship (Grzynkiewicz et al. 1985) and the KD for Ca2+ binding to fura-2 was taken to be 224 nm (Grzynkiewicz et al. 1985). The calibration was not completed for all cells, therefore data for some cells are presented as 340/380 nm ratios; the relatively low acquisition rate of the CCD camera may have resulted in an underestimation of peak [Ca2+]i in the calibrated cells. For confocal line-scan analysis, cells were loaded with 5 μm fluo-4 AM (Molecular Probes), incubated for 10 min and washed for another 10 min. Confocal images were collected in the line-scan mode using a LSM 5 Pascal confocal microscope (Zeiss, Jena, Germany) and a × 63 water-immersion objective (NA = 0.8). Fluo-4 fluorescence was excited with the 488 nm band of the Ar laser with transmission set at 1 %. The scan interval was 10–40 ms. These cells were not calibrated and the data are presented on an intensity scale in arbitrary units (a.u.).
Immunohistochemistry and confocal image acquisition
Salamander eyecups were immersion-fixed for 1.5 h in 4 % (w/v) paraformaldehyde in phosphate buffer (PB; 0.1 m; pH 7.4). The formaldehyde was obtained as a 16 % solution in sealed ampoules from Electron Microscopy Sciences (Fort Washington, PA, USA). The retinas were rinsed three times in PB and cryoprotected in 30 % sucrose overnight at 4 °C. Pieces of retina were mounted in OCT, sectioned vertically at 14 μm thickness on a cryostat, collected on Super-Frost Plus slides (Fisher, Pittsburgh, PA, USA) and stored at −20°C until use. For immunohistochemistry, retinal sections were washed in PB for 15 min then permeabilized and blocked in a solution containing 0.5 % Triton X-100 and 10 % goat serum. As secondary antibodies we utilized the Alexa 488 goat anti-rabbit IgG (H+L) conjugates (Molecular Probes, Eugene, OR, USA), diluted at 1:1000. After incubation, sections were washed in PB and mounted in Vectashield (Vector, Burlingame, CA, USA). Negative controls were performed for every set of experiments by omitting the primary antibody.
Immunofluorescence and bright field Nomarski fields of view were obtained using a confocal microscope (Zeiss LSM 5 Pascal) at 10 % power for the 488 nm argon line and 100 % power for the 543 nm He/Ne line. Acquired images were processed with Adobe Photoshop (version 7.0) software.
Caffeine, thymol, chlorocresol, ryanodine and cyclopiazonic acid (CPA) were obtained from Sigma (St Louis, MO, USA). SERCA antibodies were a generous gift from Professor Frank Wuytack (Katholieke Universiteit, Leuven, Belgium).
RESULTS
Caffeine releases more Ca2+ from intracellular stores in rods than in cones
Spatially averaged Ca2+ signals from rod and cone ISs were measured in fura-2 AM-loaded rod and cone photoreceptors. In every cell, the region of interest was drawn around the perikaryal region of the IS comprising the cell nucleus and the surrounding cytoplasm. To test whether rods and cones possess intracellular Ca2+ storage compartments, we exposed them to caffeine, a methylxanthine commonly used to stimulate Ca2+ release from RyR-gated intracellular compartments (Neering & McBurney, 1984; Sitsapesan & Williams, 1990). Caffeine, at a concentration known to evoke half-maximal activation of Ca2+ release from caffeine-sensitive stores (10 mm; Akaike & Sadoshima, 1989; Uneyama et al. 1993), has been shown previously to increase [Ca2+] in rod ISs (Krizaj et al. 1999). Our impression from these previous experiments was that caffeine was much less effective in cone ISs. Here, we systematically compared rod and cone responses to caffeine. An example of caffeine-induced changes in [Ca2+]i in rod and cone ISs is shown in Fig. 1.
Figure 1. Caffeine evokes an increase in free [Ca2+]i in the inner segment (IS) of rods but not cones.

a, simultaneous [Ca2+] measurement from salamander rod and cone ISs loaded with the Ca2+ indicator fura-2. Note that due to limited loading of the fura-2 ester into the small outer segment (OS) cytoplasmic volume, the OS Ca2+ signal is not detected at the 340/380 nm excitation used in this particular experiment. The cell body and the ellipsoid of the rod and cone ISs, respectively, are marked with white arrows. b, 10 mm caffeine evoked an increase in [Ca2+]i in the rod IS but not the cone IS. The [Ca2+]i increase was prominent in the cell body (yellow arrowheads) and modest in the ellipsoid (red arrowhead). e, caffeine washout. f, subsequent exposure to 20 mm KCl raised [Ca2+]i in both cells. The increase was again less pronounced in the ellipsoid regions of both cells (red arrowheads in g). h, [Ca2+]i returned to baseline levels 2 min following KCL washout. Scale bar = 20 μm.
A rod and cone were imaged in the same field of view using a high-resolution CCD camera. The data represent a spatial average of the fluorescence signal encompassing the perikaryal compartment (the cell body). The cells were identified by the typical shape of their cell bodies and ellipsoids (Townes-Anderson, 1985; Krizaj & Copenhagen, 1998; Krizaj et al. 1999); the rod photoreceptor consists of a somatic and an ellipsoid compartment attached to a large cylindrical OS. As seen in Fig. 1, caffeine triggered a [Ca2+]i elevation in the rod IS but not in the cone IS. The [Ca2+]i increase was most prominent at the edge of the rod soma (Fig. 1b and c), from where it spread into the interior of the IS (Fig. 1c and d). Note the comparatively smaller [Ca2+]i increase in the mitochondria-containing ellipsoid region of the rod IS (red arrowhead). To test whether both the rod and the cone possess operational voltage-gated Ca2+ influx pathways, we subsequently depolarized the cells with 20 mm KCl. Exposure to high K+ resulted in an elevation in [Ca2+]i in the rod and in the cone IS. The magnitude of evoked [Ca2+]i increase was again less in the ellipsoid compared with the rest of the IS (red arrowheads). These results indicate that Ca2+, released from caffeine-sensitive stores, differs in the extent of its contribution to steady-state [Ca2+]i in rods and cones. Furthermore, our results suggest that pronounced differences in Ca2+ homeostasis exist between the cell body and the ellipsoid regions of the IS.
Simultaneous optical recordings of [Ca2+]i in a rod and cone IS are illustrated in Fig. 2 for a different rod-cone pair. [Ca2+]i is plotted as a function of time. Caffeine puffs were intertwined with puffs of high K+ (stars) used to load the intracellular Ca2+ stores. As illustrated in Fig. 2A and B, 10 mm caffeine evoked a transient [Ca2+]i increase in the rod but not the cone IS. [Ca2+]i in the rod did not stay elevated in the continued presence of caffeine, but returned back to the baseline with a time constant of 44 s. In a sample of 42 rods tested with fura-2, superfusion with 10 mm caffeine in control saline resulted in a 182 ± 31 nm increase in peak [Ca2+]i, whereas caffeine convincingly raised [Ca2+]i similarly in one out of 39 cone ISs tested. Caffeine-evoked responses were blocked by ryanodine, a specific antagonist of Ca2+ release from caffeine-sensitive stores in rod ISs (Krizaj et al. 1999). Ryanodine (20 μm) elevated baseline [Ca2+]i in rods by ∼30 nm, but had no effect on cone [Ca2+]i (n = 5; data not shown). These two results are consistent with the idea that ryanodine stores are much reduced or masked in cones.
Figure 2. Time course of caffeine effects on intracellular free [Ca2+] in rod and cone ISs.

A, simultaneous measurement of [Ca2+] from salamander rod and cone ISs using a CCD camera. Cells were loaded with fura-2 AM and stimulated with brief puffs of 90 mm KCl from a nearby pipette (indicated by the asterisks) followed by superfusion with 10 mm caffeine (indicated by the horizontal bar). Caffeine evoked a transient [Ca2+]i increase in the rod, but not cone, IS. B, simultaneous line-scan measurement of Ca2+ signals from salamander rod and cone ISs loaded with fluo-4 AM. Caffeine (10 mm) evoked a large fluorescence increase in the rod IS but did not change the fluorescence in the cone. C, caffeine triggered an increase in [Ca2+]i in a subset of cone ISs. Simultaneous recording of fluo-4 fluorescence from rod and cone IS. Caffeine (10 mm) triggered a relatively rapid large-amplitude increase in the rod Ca2+ signal. Concomitantly, a small Ca2+ signal with a slow rise time was observed in the cone IS. a.u. = arbitrary units.
In order to test whether caffeine evokes fast changes in [Ca2+]i that might be undetectable with the slower CCD system, we performed Ca2+ measurements at high temporal resolution using the line-scan function of the laser-scanning confocal microscope. In these experiments cells were loaded with the single-wavelength optical dye fluo-4. Caffeine evoked a [Ca2+]i increase in all rods studied (29/29). In contrast, the majority of cones (38/44) showed no response to caffeine under these conditions (Fig. 2B). We did, however, detect small [Ca2+]i elevations in response to caffeine in six cones. One illustrative example of caffeine-evoked Ca2+ increase in a cone IS is shown in Fig. 2C. The scan line was placed across the perikaryal regions of a rod and a cone IS simultaneously. Exposure to caffeine triggered a prominent [Ca2+]i elevation in the rod and a slow, small-amplitude increase in the cone. Based on our measurements using high K+ in fura-2-loaded cells, we estimate that caffeine-evoked [Ca2+]i increases in cone ISs never exceeded 50 nm. To rule out the possibility that caffeine stimulated Ca2+ influx rather than release from intracellular stores, we repeated the caffeine experiment in the absence of extracellular [Ca2+]. We found that, as shown earlier (Krizaj et al. 1999), caffeine stimulated Ca2+ release in rod ISs superfused with 0 Ca2+ and 3 mm EGTA (n = 5/11). Caffeine also evoked a small [Ca2+]i increase in one out of 12 cone ISs (data not shown). These results indicate that Ca2+ release from caffeine-sensitive stores in rod ISs has a much larger effect on global cytoplasmic [Ca2+]i than in cone ISs.
Caffeine-sensitive stores are not detectable in rod OSs
A key structural element of the rod OS are the stacks of intracellular cisternae (‘rod disks’) that fill most of the OS cytoplasm. The OS cisternae are thought to accumulate and release Ca2+ (Liebman 1974; Fain & Schroder, 1990); however, the mechanism of Ca2+ release from the cisternae has not been established (Krizaj & Copenhagen, 2002). To determine whether there was any evidence for caffeine-sensitive stores in light-adapted rods, we recorded [Ca2+]i from ROIs in rod ISs and OSs. Figure 3A shows a simultaneous optical recording from a rod IS and OS. Stimulation with caffeine caused a large elevation in the IS [Ca2+]i but had little effect on the OS [Ca2+]i. Rod OSs never showed caffeine-evoked [Ca2+]i responses (n = 8). Similarly, caffeine had no effect on [Ca2+]i in OSs detached from the rest of the cell (n = 3; data not shown). A similar absence of caffeine-mediated [Ca2+]i increase was obtained in line-scan experiments with the confocal microscope (n = 3/3 OSs/ISs). A dissociated rod with an intact OS was loaded with fluo-4 (Fig. 3B). The IS exhibited a high resting fluorescence, which increased markedly following exposure to caffeine (Fig. 3C). No fluorescence increase was observed in the OS. Note also that Ca2+, released in the IS during caffeine stimulation, did not enter the OS, presumably due to the large diffusional barrier between the two regions of the cell. Although we observed large differences in caffeine-sensitive responses between ISs and OSs, we note that these experiments were performed under circumstances when all rod pigment was bleached, and that we might have missed a potential contribution of caffeine-sensitive stores that might occur in dark-adapted rods under in vivo conditions. These experiments do demonstrate that Ca2+ released from the IS Ca2+ stores is excluded from the OS cytoplasm.
Figure 3. Simultaneous [Ca2+]i measurement from an IS and OS of a rod.

A, rod loaded with fura-2 AM. The cell was depolarized by 20 mm KCl to maximize store loading. Caffeine (10 mm) transiently elevated [Ca2+]i in the IS, but not the OS. B, confocal image of a dissociated rod photoreceptor loaded with 5 μm fluo-4. Prominent fluo-4 fluorescence is observed in the IS of the cell, whereas the OS signal is weak. C, line-scan across the IS (top) and the OS (bottom) of the cell shown in B. The abscissa represents the time axis. Exposure to 10 mm caffeine caused a large saturating increase in the fluo-4 signal in the IS but caused no fluorescence change in the OS. Note that the caffeine-evoked Ca2+ signal is confined to the IS.
Ca2+ released from caffeine-sensitive stores is rapidly extruded by PMCAs
During continuous superfusion with caffeine, rod IS [Ca2+]i fell back to the baseline level, with an average time constant of 44 ± 5 s (n = 25). We investigated the mechanism underlying the time course of this [Ca2+]i decay in the presence of caffeine. One possibility is that a negative feedback mechanism may cause RyRs to inactivate at high local [Ca2+]i (Bezprozvanny et al. 1991; Györke & Fill, 1993). This is unlikely to occur under our experimental conditions because we showed earlier that raising [Ca2+]i increases the amount of Ca2+ released from the stores (Krizaj et al. 1999). Alternatively, if significant amounts of caffeine-released Ca2+ are rapidly extruded across the plasma membrane rather than re-accumulated into the ER, caffeine-sensitive stores may gradually become depleted during prolonged exposure to caffeine, leading to a gradual decline in caffeine-evoked changes in [Ca2+]i. Thus, the amount of Ca2+ extruded from the cell should regulate both the rate of decay of caffeine-induced [Ca2+]i transients and the recovery of responsiveness to caffeine.
The sole Ca2+ extrusion mechanism in the plasma membrane of salamander rod and cone ISs is the PMCA (Krizaj & Copenhagen, 1998; Krizaj et al. 2002). To test whether PMCAs regulate the recovery of [Ca2+]i in the continuous presence of caffeine, we superfused the cells with 1 mm La3+, a potent blocker of PMCAs (Milanick, 1990; Krizaj & Copenhagen, 1998) and voltage-sensitive Ca2+ channels (Reichling & MacDermott, 1991). In the presence of La3+, caffeine evoked a sustained elevation of [Ca2+]i that lasted as long as the caffeine step itself and was fully reversible (n = 25; Fig. 4). These findings implicate a role for PMCA in clearing store-released Ca2+ in rod ISs.
Figure 4. Plasma membrane Ca2+ ATPases (PMCAs) shape the decay of caffeine-evoked [Ca2+]i transients.

A rod IS was superfused continually with 1 mm La3+, a blocker of PMCAs, and then stimulated with steps of 10 mm caffeine. In the presence of La3+, caffeine evoked sustained [Ca2+]i elevations. Following caffeine removal, [Ca2+]i returned to baseline in the continued presence of La3+.
Inhibition of PMCA unmasks a caffeine-sensitive store in cones
Interestingly, exposure to La3+ uncovered a response to caffeine in cones. Figure 5 illustrates a cone IS challenged with caffeine in control solution and subsequently in the presence of La3+. During exposure to La3+, caffeine evoked a pronounced [Ca2+]i elevation in this cell. In 10 La3+-treated cones, caffeine raised [Ca2+]i by 85 ± 19 nm, suggesting that both rods and cones possess caffeine-sensitive Ca2+ stores. We propose that the absence of a caffeine-evoked response in cones in control saline is not due to the absence of caffeine-sensitive intracellular stores, but that extrusion of released Ca2+ by the PMCAs is more rapid in the cones (e.g. Krizaj & Copenhagen, 1998). Consequently, the released Ca2+ is not detected by fura-2.
Figure 5. Inhibition of PMCAs unmasks a caffeine-evoked [Ca2+]i response in the cone IS.

A cone IS that was stimulated with caffeine in control saline showed no [Ca2+]i response. Subsequently, putative caffeine-sensitive stores were replenished with a step of 20 mm KCl and the solution was switched to 1 mm La3+. In the presence of La3+, caffeine evoked a sustained increase in [Ca2+]i.
RyRs are expressed in salamander photoreceptors
The Ca2+-release channels targeted by caffeine are generally thought to belong to the RyR family. To determine whether RyRs are localized to salamander photoreceptors, we immunostained salamander retinal sections with an antibody against RyRs. This pan-RyR antibody recognizes RyR isoforms expressed in the whole brain, cerebellum and skeletal and cardiac muscle (Mackrill et al. 1997; Llano et al. 2000). To examine the subcellular localization of RyRs, we also enzymatically dissociated and fixed photoreceptors from the salamander retina and immunostained them with the RyR antibody. Figure 6B and F shows that RyRs are expressed in both rod and cone ISs. Colocalization with the photoreceptor synaptic marker SV2 (Krizaj et al. 2002) showed that RyR immunostaining was especially pronounced in the synaptic terminals (Fig. 6D and H). Immunostaining was also observed in the ellipsoid region of the IS, whereas moderate labelling was observed in the perikarya of rods, single cones and double cones. These results are consistent with results obtained from our physiological experiments. Figure 6I–L shows a confocal image of a transverse section of the salamander retina immunolabelled with antibodies against RyRs and SV2. RyRs are expressed at high density in the outer nuclear and outer plexiform layers of the salamander retina (Fig. 6L). Similar to the isolated cells, the synaptic terminals and the cytoplasm surrounding perikarya and ellipsoids of ISs of rods and cones were all labelled.
Figure 6. Ryanodine receptors (RyRs) are localized to photoreceptor ISs.
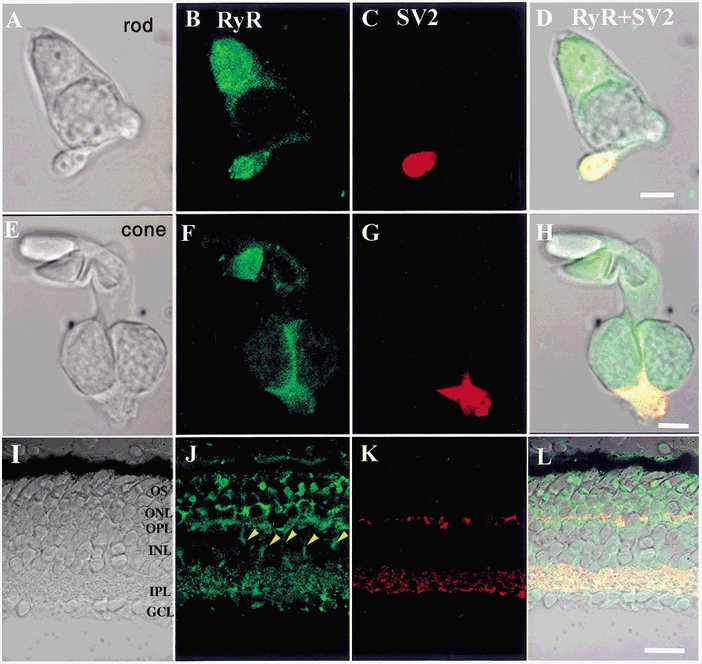
Confocal fluorescence images of retinal cells immunostained with antisera against RyRs and SV2. A and E, Nomarski images of rod and double cone ISs dissociated from the salamander retina. B and F, RyR immunofluorescence is prominent in the synaptic terminal and the ellipsoid region. A moderate RyR signal is also observed in the plasma membrane surrounding the perikaryon. C, G and K, SV2 immunolabels synaptic terminals of retinal neurons. D and H, RyR and SV2 signals colocalize in the synaptic terminals of the rod and the cone. I, Nomarski image from a salamander retinal section. J, the RyR antibody labels photoreceptor perikarya and synaptic terminals. A prominent signal is also observed in Müller cell bodies and processes (arrowheads), in ganglion cell bodies and in synaptic processes in the inner plexiform layer (IPL). Little staining is seen in photoreceptor OSs and in bipolar cell bodies. L, RyR and SV2 colocalize in synaptic processes of the outer plexiform layer (OPL) and the IPL. ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. Scale bars are 10 μm in A−H and 50 μm in I−L.
RyRs were also found in other retinal cell types. Pronounced immunolabelling was observed in Müller cells (arrowheads in Fig. 6J), consistent with a previous Ca2+ imaging report suggesting that these cells possess functional RyRs (Keirstead & Miller, 1995). The RyR antibody also labelled calbindin-immunopositive horizontal cells, which are known to possess caffeine-sensitive Ca2+ stores (Micci & Christensen, 1998; not shown). These results indicate that RyRs are selectively expressed in the retina, with a prominent localization to photoreceptor ISs.
Caffeine-evoked increases in [Ca2+]i are blocked by SERCA inhibitors
The refilling of ER stores is commonly mediated by SERCAs, a family of Ca2+ ATPases distributed widely over ER membranes (Pozzan et al. 1994; Guerini & Carafoli, 1998). SERCA pumps are selectively and reversibly blocked by CPA, a mycotoxin isolated from Aspergillus and Penicillium (Thomas & Hanley, 1994). To test whether SERCAs mediate Ca2+ sequestration into the rod ER, we exposed these cells to CPA and measured caffeine responses before, during and after that exposure. Figure 7 shows [Ca2+]i from two simultaneously recorded rods. The dark trace shows the response to a caffeine-filled pipette positioned close to the IS of this cell (rod 1). [Ca2+]i in the cell was transiently elevated by 250 ms puffs of 50 mm caffeine. As illustrated in Fig. 7, caffeine-evoked responses in rod 1 were reversibly suppressed by CPA, consistent with the hypothesis that blocking the SERCA pumps depletes the caffeine-sensitive store. We found that in most cells, CPA alone increased the basal [Ca2+], ostensibly as a result of blocked Ca2+ sequestration into the stores. This is seen clearly in a rod not exposed to caffeine (rod 2; dotted trace) that exhibited the CPA-evoked elevation in [Ca2+]i. In five cells exposed to CPA, [Ca2+]i rose by 21 ± 1 nm. The CPA-mediated [Ca2+]i increase is consistent with an inhibition of tonic Ca2+ sequestration into the ER mediated by SERCA pumps. In the presence of CPA, [Ca2+]i declined progressively towards the baseline level (Fig. 7), presumably reflecting a gradual depletion of Ca2+ stores (Thomas & Hanley, 1994; Lo & Thayer, 1995; Usachev et al. 2001). Similar results were also seen in four cells superfused with 1 μm thapsigargin, another SERCA pump inhibitor (data not shown). Preliminary experiments using antibody staining against all three SERCA isoforms in the salamander retina suggest that salamander photoreceptors express the SERCA 2b isoform (D. Krizaj & D. R. Copenhagen, unpublished results).
Figure 7. Ca2+ is sequestered into intracellular stores by sarcoplasmic-endoplasmic Ca2+ ATPases.

Simultaneous recordings from two rod ISs. A puffer pipette containing 50 mm caffeine was positioned close to rod 1; rod 2 was located at the opposite side of the coverslip. Brief puffs of caffeine evoked transient increases in [Ca2+]i in rod 1, but had no effect on rod 2. In both cells, cyclopiazonic acid (CPA) by itself evoked a slow elevation in [Ca2+]i by several tens of nanomoles.
The refilling of caffeine-sensitive stores depends on the magnitude of the conditioning stimulus
Previously, we have shown that the amplitude of caffeine-evoked Ca2+ transients depended on the magnitude of the steady-state [Ca2+]i resulting from Ca2+ influx via voltage-gated Ca2+ channels (Krizaj et al. 1999). We next addressed the issue of Ca2+ release from stores when plasma membrane Ca2+ channels are closed. Specifically, we examined the relationship between the filling status of Ca2+ stores and the magnitude of [Ca2+]i transients evoked by caffeine. The stores were filled to different extents by conditioning steps of 90 mm KCl (Fig. 8, asterisks). Each KCl puff transiently elevated [Ca2+]i; longer step durations raised the [Ca2+]i level higher. The range of KCl-evoked Ca2+ transients was 50–1200 nm. To test whether the refilling of stores is graded with the magnitude of [Ca2+]i increase, we waited until [Ca2+]i returned to baseline. We then measured [Ca2+]i rises in response to 10 mm caffeine. A representative experiment is illustrated in Fig. 8. The first conditioning KCl-evoked Ca2+ transient elevated [Ca2+]i by 20 nm. Subsequent exposure to caffeine raised [Ca2+]i by 25 nm (i.e. ∼50 % of baseline level). Larger [Ca2+]i transients result in the larger amplitude of subsequent caffeine-induced responses. The final conditioning transient elevated [Ca2+]i to ∼800 nm, and the caffeine-evoked response was ∼120 nm. This result suggests that the amount of Ca2+ sequestered into Ca2+ stores depends on the magnitude of the immediately prior cytoplasmic [Ca2+] transients. Similar results were observed in four cells.
Figure 8. The magnitude of caffeine-evoked Ca2+ release depends on the magnitude of the conditioning depolarization.

A rod photoreceptor IS exposed to conditioning steps of high K+ followed by superfusion with 10 mm caffeine. Each asterisk represents a single 128 ms KCl puff; bars denote superfusion. Increasing the duration of the conditioning [Ca2+]i step caused a subsequent increase in the caffeine-evoked [Ca2+]i increase. The caffeine-evoked responses saturated when the conditioning free [Ca2+]i reached ∼500 nm.
Ca2+ is released from the ER via CICR
The predominant physiological trigger for Ca2+ release from neuronal ryanodine-sensitive stores is thought to be Ca2+ itself (i.e. CICR). According to the CICR hypothesis, Ca2+ changes evoked by Ca2+ influx through voltage-gated Ca2+ channels are amplified by Ca2+ release from neuronal intracellular stores (Hua et al. 1993; Verkhratsky & Shmigol, 1996). We tested whether such amplification occurs in rods by evoking increases in [Ca2+]i with test depolarizations in the absence and presence of pharmacological agents that deplete the stores (caffeine), or block either release from the stores (ryanodine) or sequestration into the stores (CPA).
Short puffs of KCl evoked transient increases of [Ca2+]i in ISs. These rose to several hundred nanomolar in less than 1 s, the fastest interval we could record, and returned to baseline in tens of seconds (Fig. 9). Previous work showed that KCl-evoked [Ca2+]i increases disappeared in Ca2+-free saline and were reversibly reduced by 70–100 % by 1–2 μm nifedipine, a dihydropyridine antagonist of L-type Ca2+ channels (11/11 rods and 3/3 cones, data not shown; Corey et al. 1984; Krizaj & Copenhagen, 1998; Nachmann-Clewner et al. 1999). The findings show that Ca2+ influx through voltage-gated Ca2+ channels is necessary for depolarization-evoked Ca2+ transients. It is not clear, however, whether this Ca2+ influx can trigger further release of Ca2+ from intracellular stores via CICR. To examine whether Ca2+ release from caffeine-sensitive stores contributed to the magnitude of depolarization-evoked Ca2+ transients, high K+ was puffed onto cells before and during the depletion of Ca2+ stores with caffeine, CPA or ryanodine. When Ca2+ stores in rod ISs were depleted during continuous superfusion of cells with 10 mm caffeine (n = 4), 5 μm CPA (n = 4) or 20 μm ryanodine (n = 2), the magnitude of KCl-evoked Ca2+ transients was markedly reduced. Figure 9B illustrates a rod in which KCl-evoked responses were reduced by more than 50 % by caffeine. Likewise, ryanodine and CPA dramatically reduced the amplitude of KCl-evoked [Ca2+]i transients (Fig. 9B and C). Cones responded similarly. As illustrated in Fig. 9D, CPA transiently elevated baseline [Ca2+]i in cones and caused a reduction in the magnitude of KCl-evoked Ca2+ transients (n = 3). Note that caffeine itself evoked little [Ca2+]i change in the cone IS, presumably because most of the released Ca2+ was extruded by PMCAs or sequestered into the mitochondria (e.g. Fig. 5 and Fig. 11). These results provide evidence that Ca2+ release from ryanodine-sensitive Ca2+ stores in rod photoreceptors occur via CICR, and provide evidence suggestive of the involvement of Ca2+ stores in buffering and sequestration of cytoplasmic Ca2+ during depolarization-induced [Ca2+]i signalling in rod and cone ISs.
Figure 9. Ca2+-induced Ca2+ release contributes to depolarization-evoked [Ca2+]i increases in both rod and cone ISs.

A, a rod IS in which [Ca2+]i was raised periodically with 128 ms puffs of 90 mm KCl. Superfusion with 10 mm caffeine itself raised [Ca2+]i by ∼100 nm. However, the magnitude of the subsequent KCl puff was substantially reduced in the presence of caffeine. B, a rod IS in which exposure to CPA slightly elevated the [Ca2+]i baseline and significantly reduced the magnitude of KCl-evoked [Ca2+]i transients. Following recovery, caffeine itself evoked a normal [Ca2+]i response. C, a rod IS in which 20 μm ryanodine irreversibly decreased the magnitude of KCl-evoked [Ca2+]i transients. D, a cone IS in which CPA caused a transient elevation of [Ca2+]i and reversibly reduced the magnitude of KCl-evoked transients. Note that whereas KCl-evoked responses recovered following CPA washout, subsequent superfusion with 10 mm caffeine did not result in elevation of [Ca2+]i.
Figure 11. Ryanodine-sensitive Ca2+ stores communicate with the IS mitochondria in rod and cone ISs.

A, a rod IS loaded with fura-2 AM. The rod was depolarized by 20 mm KCl throughout the experiment. Caffeine (10 mm) evoked a transient [Ca2+]i increase followed by an undershoot. p-Trifluoromethoxy-phenyl hydrazone (FCCP, 1 μm) itself caused an increase in [Ca2+]i and a fivefold potentiation in the magnitude of the caffeine-evoked [Ca2+]. B, line-scan recording from a cone IS loaded with fluo-4. No response to caffeine was observed in control saline. FCCP (2 μm) by itself caused a large increase in the Ca2+ signal in the cone IS. In the presence of FCCP, caffeine evoked a significant elevation in [Ca2+]i.
Caffeine-insensitive stores are a component of intracellular Ca2+ stores in photoreceptors
We estimated the amount of Ca2+ stored in non-caffeine-sensitive intracellular compartments. In order to empty the caffeine-sensitive stores, we first superfused cells with 10 mm caffeine in the absence of external Ca2+. To visualize the amount of Ca2+ stored in other intracellular compartments, we then exposed cells to 10 μm ionomycin. Ionomycin, which is commonly used to calibrate [Ca2+]i, is an ionophore that selectively shuttles Ca2+ across lipid bilayers. In addition to equilibrating Ca2+ across the plasma membrane, ionomycin molecules insert themselves into the membranes of intracellular compartments, causing an equilibration of their Ca2+ with the cytosol. In the rod shown in Fig. 10, in 0 Ca2+/EGTA saline, exposure to caffeine raised [Ca2+]i by ∼290 nm; the cell was superfused with caffeine for 2.2 min so as to completely deplete the caffeine-sensitive store. Subsequent exposure to ionomycin raised [Ca2+]i by 800 nm, which was followed by a [Ca2+]i decline to the very low level imposed by the 0 Ca2+ saline. In 69 rods, ionomycin raised [Ca2+]i by 354 ± 45 nm. Prolonged exposure to 10 mm caffeine in the absence of extracellular Ca2+ depletes the caffeine stores completely. We found that subsequent applications of caffeine evoke no further response (data not shown). In the absence of external Ca2+, caffeine increased [Ca2+]i by 61 ± 26 nm in 5/11 cells. In the other six rods, no effect of caffeine on [Ca2+]i was observed, possibly due to the long exposure times in 0 Ca2+. The ionomycin experiments demonstrate that rod photoreceptors possess both caffeine-sensitive Ca2+ stores and one or more separate, caffeine-insensitive, Ca2+ storage compartments. Furthermore, these results suggest that the amount of releasable Ca2+ can vary substantially due to the previous history of cytoplasmic exposure to Ca2+; at low [Ca2+]i, the amount of released Ca2+ is much less than that released in normal saline or high K+ due to the gradual depletion of intracellular stores.
Figure 10. Photoreceptors possess multiple types of internal Ca2+ stores.

Ryanodine stores were depleted via a prolonged exposure to caffeine in 0 Ca2+ supplemented with 3 mm EGTA. Subsequent exposure to ionomycin caused an additional elevation of Ca2+ by ∼800 nm, consistent with the presence of caffeine-insensitive stores in the IS.
We also tested for the presence of caffeine-insensitive stores in cones. Caffeine slightly elevated [Ca2+]i in only one cone out of 12, superfused with 0 Ca2+ saline. Ionomycin by itself, however, increased [Ca2+]i in 31/31 cones superfused with 0 Ca2+ saline (163 ± 44 nm), suggesting that these cells also store Ca2+ in intracellular compartments.
Mitochondria buffer Ca2+ released by the ER
We demonstrated that Ca2+ release from ryanodine stores is much more prominent in rods than in cones (Fig. 1 and Fig. 2). We also showed that both cell types possess caffeine-insensitive Ca2+ stores. To determine whether Ca2+ sequestration into caffeine-insensitive stores plays a role in influencing Ca2+ release from the ryanodine store, we examined the role of mitochondria in modulating Ca2+ release from ryanodine stores. Mitochondria are known to contribute significantly to Ca2+ sequestration from the cell cytoplasm (Duchen, 1999; Rizzuto et al. 2000). Since mitochondria are often located near the mouths of Ca2+-release channels across the ER membrane (Landolfi et al. 1998), they may rapidly sequester released Ca2+ before it is captured by cytosolic Ca2+ dyes. We hypothesized that mitochondria may be especially effective in capturing Ca2+ released from caffeine-sensitive stores in cone ISs. To fill the putative mitochondrial Ca2+ stores, [Ca2+]i was elevated with high K+ and release from caffeine-sensitive stores was evoked in the absence or presence of blockers of oxidative phosphorylation. The protonophore p-trifluoromethoxy-phenyl hydrazone (FCCP) abolishes the membrane potential at the inner membrane of the mitochondria and thus blocks Ca2+ uptake into the mitochondrial matrix (Gunter et al. 1994; Babcock et al. 1997). Figure 11A illustrates a caffeine-induced [Ca2+]i signal from a rod IS. The test response evoked a transient increase in [Ca2+]i. When exposed to 1 μm FCCP alone, basal [Ca2+]i levels increased by 300 nm, presumably because Ca2+ was released from mitochondrial stores. Subsequent application of caffeine in the presence of FCCP revealed a significant potentiation of the magnitude of the caffeine-evoked [Ca2+]i transient. Similar results were observed in 9/9 rods. FCCP-evoked [Ca2+]i increases were also observed in cone ISs (16/16 cells). In addition, a significant response to caffeine in cone ISs was unmasked in the presence of FCCP (n = 10/13 cones). Figure 11B shows a confocal line-scan recording from a cone IS superfused with control saline. Caffeine by itself caused little change in the intensity of fluo-4 fluorescence. FCCP by itself triggered a large increase in fluorescence, presumably reflecting an increase in cytoplasmic [Ca2+] following inhibition of mitochondrial Ca2+ uptake (Babcock et al. 1997; Duchen, 1999). When the cell was exposed to caffeine in the presence of FCCP, a significant elevation of [Ca2+]i was observed (Fig. 11B).
These results indicate that mitochondria contribute significantly to Ca2+ homeostasis by buffering Ca2+ released from the ER as well as Ca2+ entering the cell through the plasma membrane.
DISCUSSION
The aim of this study was to explore the role of ryanodine-sensitive stores in the regulation of intracellular Ca2+ in rod and cone photoreceptors. We sought to understand how these stores were filled and emptied and how Ca2+ extrusion and sequestration processes regulated Ca2+ release from the stores. We found that although RyRs are expressed in both classes of cell, Ca2+ released from stores in rod ISs contributes more prominently to global Ca2+ homeostasis than that from cone ISs. We provide evidence for CICR in both rod and cone ISs and show that mitochondrial uptake and extrusion via PMCAs play important roles in removing Ca2+ released from intracellular stores
Photoreceptors possess caffeine-sensitive Ca2+ stores
We found that RyRs play an important role in Ca2+ homeostasis within ISs of rod photoreceptors, but contribute much less to changes in the cytoplasmic free [Ca2+]i of cone ISs. Several lines of evidence reveal the localization and functional roles of caffeine- and ryanodine-sensitive Ca2+ stores in photoreceptor ISs. RyR-selective antibodies labelled the ISs of rods and cones. Caffeine, theophylline, 4-chlorocresol and thymol, compounds that cause Ca2+ release from caffeine-sensitive stores (Akaike & Sadoshima, 1989; Cseresnyes et al. 1997), all produced an increase in [Ca2+]i (Krizaj et al. 1999). The effect of caffeine on [Ca2+]i was blocked irreversibly by ryanodine and reversibly by CPA, which inhibit release and refilling of Ca2+ stores, respectively (Fig. 7; Thomas & Hanley, 1994).
The caffeine-evoked Ca2+ increases observed in this study are likely to occur from release by the networks of ER cisternae that ramify within ISs and synaptic terminals (Sjöstrand & Nilsson, 1965; Ripps et al. 1976; Holtzman & Mercurio, 1980; Mercurio & Holtzmann, 1982; Ungar et al. 1984; Townes-Anderson, 1995). Ungar et al. (1984) showed that the ER in the IS accumulates large amounts of Ca2+ deposits. We now show that this Ca2+ can be released by caffeine and that the Ca2+, sequestered within the ER is in dynamic equilibrium with the cytoplasmic [Ca2+]i. Our results suggest that the stores in photoreceptor ISs are already partially filled at very low levels of [Ca2+]i. As evidence of this we find that CPA, a specific inhibitor of Ca2+ sequestration via SERCA pumps, itself increased [Ca2+]i in rod and cone ISs (Fig. 7 and Fig. 10). We interpret the CPA-mediated [Ca2+]i increase as suggestive of the inhibition of tonic Ca2+ sequestration into the ER. A continuous leak and sequestration of Ca2+ into the ER is therefore likely to occur even in hyperpolarized rods at low [Ca2+]i. It is possible that IS Ca2+ stores in light-adapted cells are replenished by Ca2+ influx through store-operated Ca2+ channels, as described in hippocampal and dorsal root ganglion neurones (Garaschuk et al. 1997; Usachev & Thayer, 1999). This possibility is supported by our observation that the magnitude of caffeine-evoked Ca2+ transients was always much larger in control Ca2+-containing saline than in the absence of extracellular Ca2+. In any case, our results indicate strongly that the IS ER acts as a continuous source and sink for cytoplasmic Ca2+ at both high and low steady-state [Ca2+]i (e.g. Friel & Tsien, 1992; Thomas & Hanley, 1994; Solovyova et al. 2002).
Ca2+ homeostasis in rods and cones is regulated by the interaction between ryanodine-sensitive stores and PMCAs
Rod and cone ISs respond differently to caffeine. Typically, under conditions in which robust caffeine-evoked [Ca2+]i increases were seen in rods, cone ISs were unresponsive to caffeine. Basal [Ca2+]i in cone ISs was almost half that measured in the rod, consistent with the notion that PMCA-mediated extrusion is more efficacious in cones and that Ca2+ stores are not as full in cones compared to rods. Indeed, we have shown previously that plasma membrane Ca2+ extrusion in cones is more efficient compared to Ca2+ extrusion from rod ISs (Krizaj & Copenhagen, 1998). Our current results suggest that the Ca2+ released from IS Ca2+ stores is intercepted by PMCAs and by the mitochondria before diffusing into the cytoplasm, and that this mechanism is likely to be much more effective in cone than in rod ISs. The ER in cone ISs may exist as subsurface cisternae that create a restricted cytosolic space within tens of nanometres of the plasma membrane and in which Ca2+ may accumulate without diffusing into the bulk cytosol (Berger, 1967; Baumann & Walz, 2001; Delmas & Brown, 2002).
PMCAs also shaped caffeine-evoked responses in rod ISs. In control saline, [Ca2+]i typically returned to baseline in the continued presence of caffeine. However, we found that exposure to extracellular La3+ caused caffeine-evoked [Ca2+]i responses to be much more sustained. La3+ reversibly blocks Ca2+ extrusion from ISs by its action on PMCAs, which are the exclusive mechanism for Ca2+ extrusion from salamander photoreceptor ISs (Krizaj & Copenhagen, 1998). We thus propose that the transient nature of caffeine-evoked responses results from of Ca2+ extrusion and a gradual depletion of Ca2+ stores. In the absence of PMCA-mediated Ca2+ extrusion, release of Ca2+ via RyRs was well matched by Ca2+ sequestration via SERCAs, as evidenced by [Ca2+]i recovery following the removal of caffeine (Fig. 5). This suggests that SERCAs also play important roles in IS Ca2+ homeostasis and that the steady-state [Ca2+]i in ISs reflects a dynamic, fine-tuned interplay between both classes of Ca2+ transporter (e.g. Brini et al. 2000).
The role of mitochondria
Although mitochondria are concentrated in the ellipsoid, they are found across all regions of the IS, including the synaptic terminal, and are often seen in intimate contact with ER cisternae (Sjöstrand & Nilsson, 1965; Holtzman & Mercurio, 1980; Mercurio & Holtzman, 1982; Brandstätter et al. 1999). In this paper we show that mitochondria in the photoreceptor IS accumulate significant amounts of Ca2+ and buffer the Ca2+ released from ryanodine stores. We were able to release mitochondrial Ca2+ with the protonophore FCCP, which abolishes the driving force for Ca2+ accumulation into the mitochondrial matrix (Gunter et al. 1994). In the presence of FCCP, the magnitude of caffeine-evoked Ca2+ release was potentiated, suggesting that IS mitochondria act as a large-capacity Ca2+ buffer that accumulates Ca2+ during rapid Ca2+ release from the internal stores. We conclude that the mitochondria are likely to constitute a significant component of the caffeine-insensitive intracellular Ca2+ pool in photoreceptors. For example, Ca2+ sequestration by mitochondria may be responsible for the low baseline [Ca2+]i levels in the ellipsoid regions of rod and cone ISs as well as the small [Ca2+] amplitude responses during depolarization and stimulation with caffeine (e.g. Fig. 1).
Functional considerations for RyR signalling in photoreceptors
The physiological impact of Ca2+ stores in the IS will be determined by the spatial distribution and functional properties of signalling microdomains formed by Ca2+-release channels and pumps and the three-dimensional layout of the ER and mitochondria within the synaptic terminal, cell body and the ellipsoid. Our results show that Ca2+ release can significantly amplify the Ca2+ signal triggered by influx through voltage-gated Ca2+ channels, suggesting a role in Ca2+ homeostasis in depolarized cells. It remains to be determined to what extent CICR influences light-evoked responses in these subcompartments. For example, during the light response, IS ryanodine stores may function as a sink to sequester excess cytoplasmic Ca2+, whereas CICR might help improve the kinetics of the light response during light decrements at background illumination. An added benefit of Ca2+ sequestration might be to prevent potential neurotoxic effects of elevated Ca2+ (Nicotera & Orrenius, 1998; Mattson et al. 2000). Both ER and mitochondria have been implicated in Ca2+-mediated apoptosis in many cell types, including photoreceptors (Edward et al. 1991; Duchen, 1999; Linden et al. 1999; He et al. 2000; Yang et al. 2001).
Taken together, our data indicate that Ca2+ signalling in photoreceptors is complex, involving communication between several different compartments. We show links among influx through L-type voltage-gated Ca2+ channels, sequestration and release by the ER and by the mitochondria, and activation of the plasma membrane Ca2+ pump by Ca2+ released from the caffeine-sensitive compartment. Our results suggest that releasable Ca2+ stores provide a mechanism by which diverse signalling pathways and the functional history of the rod photoreceptor are integrated to modulate cell metabolism and synaptic transmission. Our results thus contribute to the understanding of Ca2+ homeostatic mechanisms within photoreceptor ISs operating under light and dark conditions. Caffeine itself has been shown to shorten reaction times in a variety of visual stimulation protocols (e.g. Lorist et al. 1994). It remains to be seen whether the effects of caffeine manifest themselves via its action of Ca2+ stores at the very first synapse of the visual system.
Acknowledgments
We thank Dr René Rentería for insightful comments on the manuscript. This project was supported by research grants from NIH (EY 01869 and NS16033 to D.R.C. and EY 13870 to D.K.) and a NEI core grant to U.C.S.F. Additional support was provided by Research to Prevent Blindness foundation (D.R.C., Senior Investigator Award), the Wheeler Center for the Neurobiology of Addiction (D.K. and D.R.C.), Academic Senate Individual Investigator Grant (D.K.), Steele Award from That Man May See (D.R.C.) and Sandler Award in Basic Science (D.R.C.).
REFERENCES
- Akaike N, Sadoshima J. Caffeine affects four different ionic currents in the bull-frog sympathetic neurone. J Physiol. 1989;412:221–244. doi: 10.1113/jphysiol.1989.sp017612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock DF, Herrington J, Goodwin PC, Park YB, Hille B. Mitochondrial participation in the intracellular Ca2+ network. J Cell Biol. 1997;136:833–844. doi: 10.1083/jcb.136.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann O, Walz B. Endoplasmic reticulum of animal cells and its organization into structural and functional domains. Int Rev Cytol. 2001;205:149–214. doi: 10.1016/s0074-7696(01)05004-5. [DOI] [PubMed] [Google Scholar]
- Berger ER. Subsurface membranes in paired cone photoreceptor inner segments of adult and neonatal Lebistes retinae. J Ultrastruct Res. 1967;17:220–232. doi: 10.1016/s0022-5320(67)80044-3. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Brandstätter JH, Fletcher EL, Garner CC, Gundelfinger ED, Wässle H. Differential expression of the presynaptic cytomatrix protein bassoon among ribbon synapses in the mammalian retina. Eur J Neurosci. 1999;11:3683–3693. doi: 10.1046/j.1460-9568.1999.00793.x. [DOI] [PubMed] [Google Scholar]
- Brini M, Bano D, Manni S, Rizzuto R, Carafoli E. Effects of PMCA and SERCA pump overexpression on the kinetics of cell Ca(2+) signalling. EMBO J. 2000;19:4926–4935. doi: 10.1093/emboj/19.18.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey DP, Dubinsky JM, Schwartz EA. The calcium current in inner segments of rods from the salamander (Ambystoma tigrinum) retina. J Physiol. 1984;354:557–575. doi: 10.1113/jphysiol.1984.sp015393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cseresnyes Z, Bustamante AI, Klein MG, Schneider MF. Release-activated Ca2+ transport in neurons of frog sympathetic ganglia. Neuron. 1997;19:403–419. doi: 10.1016/s0896-6273(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Delmas P, Brown DA. Junctional signaling microdomains: bridging the gap between the neuronal cell surface and Ca2+ stores. Neuron. 2002;36:787–790. doi: 10.1016/s0896-6273(02)01097-8. [DOI] [PubMed] [Google Scholar]
- Duchen MR. Mitochondria and calcium: from cell signalling to cell death. J Physiol. 1999;529:57–68. doi: 10.1111/j.1469-7793.2000.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edward DP, Lam TT, Shahinfar S, Li J, Tso MO. Amelioration of light-induced retinal degeneration by a calcium overload blocker. Arch Ophthalmol. 1991;109:554–562. doi: 10.1001/archopht.1991.01080040122042. [DOI] [PubMed] [Google Scholar]
- Emptage NJ, Reid CA, Fine A. Calcium stores in hippocampal synaptic boutons mediate short-term plasticity, store-operated Ca2+ entry, and spontaneous transmitter release. Neuron. 2001;29:197–208. doi: 10.1016/s0896-6273(01)00190-8. [DOI] [PubMed] [Google Scholar]
- Fain GL, Matthews HR, Cornwall MC, Koutalos Y. Adaptation in vertebrate photoreceptors. Physiol Rev. 2001;81:117–149. doi: 10.1152/physrev.2001.81.1.117. [DOI] [PubMed] [Google Scholar]
- Fain GL, Schroder WH. Light-induced calcium release and re-uptake in toad rods. J Neurosci. 1990;10:2238–2249. doi: 10.1523/JNEUROSCI.10-07-02238.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel DD, Tsien RW. A caffeine- and ryanodine-sensitive Ca2+ store in bullfrog sympathetic neurones modulates effects of Ca2+ entry on [Ca2+]i. J Physiol. 1992;450:217–246. doi: 10.1113/jphysiol.1992.sp019125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaschuk O, Yaari Y, Konnerth A. Release and sequestration of calcium by ryanodine-sensitive stores in rat hippocampal neurones. J Physiol. 1997;502:13–30. doi: 10.1111/j.1469-7793.1997.013bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Guerini D, Carafoli E. The calcium pumps. In: Carafoli E, Klee C, editors. Calcium as a Cellular Regulator. New York: Oxford University Press; 1998. pp. 249–278. [Google Scholar]
- Gunter TE, Gunter KK, Sheu SS, Gavin CE. Mitochondrial calcium transport: physiological and pathological relevance. Am J Physiol. 1994;267:C313–339. doi: 10.1152/ajpcell.1994.267.2.C313. [DOI] [PubMed] [Google Scholar]
- Györke S, Fill M. Ryanodine receptor adaptation: control mechanism of Ca(2+)-induced Ca2+ release in heart. Science. 1993;260:807–809. doi: 10.1126/science.8387229. [DOI] [PubMed] [Google Scholar]
- He L, Poblenz AT, Medrano CJ, Fox DA. Lead and calcium produce rod photoreceptor cell apoptosis by opening the mitochondrial permeability transition pore. J Biol Chem. 2000;275:12175–12184. doi: 10.1074/jbc.275.16.12175. [DOI] [PubMed] [Google Scholar]
- Hernandez-Cruz A, Diaz-Muñoz M, Gomez-Chavarin M, Cañedo-Merino R, Protti DA, Escobar AL, Sierralta J, Suarez-Isla BA. Properties of the ryanodine-sensitive release channels that underlie caffeine-induced Ca2+ mobilization from intracellular stores in mammalian sympathetic neurons. Eur J Neurosci. 1995;7:1684–1699. doi: 10.1111/j.1460-9568.1995.tb00690.x. [DOI] [PubMed] [Google Scholar]
- Holtzman E, Mercurio AM. Membrane circulation in neurons and photoreceptors: some unresolved issues. Int Rev Cytol. 1980;67:1–67. doi: 10.1016/s0074-7696(08)62426-2. [DOI] [PubMed] [Google Scholar]
- Hong K, Nishiyama M, Henley J, Tessier-Lavigne M, Poo M-M. Calcium signaling in the guidance of nerve growth by netrin-1. Nature. 2000;403:93–98. doi: 10.1038/47507. [DOI] [PubMed] [Google Scholar]
- Hua S, Nohmi M, Kuba K. Characteristics of Ca2+ release induced by Ca2+ influx in cultured bullfrog sympathetic neurones. J Physiol. 1993;464:245–272. doi: 10.1113/jphysiol.1993.sp019633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirstead SAA, Miller RF. Calcium waves in dissociated retinal glial (Muller) cells are evoked by release of calcium from intracellular stores. Glia. 1995;14:14–22. doi: 10.1002/glia.440140104. [DOI] [PubMed] [Google Scholar]
- Korenbrot JI. Ca2+ flux in retinal rod and cone outer segments: differences in Ca2+ selectivity of the cGMP-gated ion channels and Ca2+ clearance rates. Cell Calcium. 1995;18:285–300. doi: 10.1016/0143-4160(95)90025-x. [DOI] [PubMed] [Google Scholar]
- Krizaj D, Bao JX, Schmitz Y, Witkovsky P, Copenhagen DR. Caffeine-sensitive calcium stores regulate synaptic transmission from retinal rod photoreceptors. J Neurosci. 1999;19:7249–7261. doi: 10.1523/JNEUROSCI.19-17-07249.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizaj D, Copenhagen DR. Compartmentalization of calcium extrusion mechanisms in the outer and inner segments of photoreceptors. Neuron. 1998;21:249–256. doi: 10.1016/s0896-6273(00)80531-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizaj D, Copenhagen DR. Calcium regulation in photoreceptors. Front Biosci. 2002;7:2023–2044. doi: 10.2741/a896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizaj D, De Marco S, Johnson J, Strehler EE, Copenhagen DR. Call-specific expression of plasma membrane calcium ATPase isoforms in retinal neurons. J Comp Neurol. 2002;451:1–21. doi: 10.1002/cne.10281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolfi B, Curci S, Debellis L, Pozzan T, Hofer AM. Ca2+ homeostasis in the agonist-sensitive internal store: functional interactions between mitochondria and the ER measured in situ in intact cells. J Cell Biol. 1998;142:1235–1243. doi: 10.1083/jcb.142.5.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman PA. Light-dependent Ca++ content of rod outer segment disc membranes. Invest Ophthalmol. 1974;13:700–701. [PubMed] [Google Scholar]
- Linden R, Rehen SK, Chiarini LB. Apoptosis in developing retinal tissue. Prog Retin Eye Res. 1999;18:133–165. doi: 10.1016/s1350-9462(98)00020-2. [DOI] [PubMed] [Google Scholar]
- Llano I, Gonzalez J, Caputo C, Lai FA, Blayney LM, Tan YP, Marty A. Presynaptic calcium stores underlie large-amplitude miniature IPSCs and spontaneous calcium transients. Nat Neurosci. 2000;3:1256–1265. doi: 10.1038/81781. [DOI] [PubMed] [Google Scholar]
- Lo TM, Thayer SA. Pharmacologic characterization of refilling inositol 1,4,5-trisphosphate-sensitive Ca2+ stores in NG 108–15 cells. Brain Res. 1995;704:10–18. doi: 10.1016/0006-8993(95)01099-8. [DOI] [PubMed] [Google Scholar]
- Lorist MM, Snel J, Kok A. Influence of caffeine on information processing stages in well rested and fatigued subjects. Psychopharmacology. 1994;113:411–421. doi: 10.1007/BF02245217. [DOI] [PubMed] [Google Scholar]
- Mackrill JJ, Challiss RA, O'Connell DA, Lai FA, Nahorski SR. Differential expression and regulation of ryanodine receptor and myo-inositol 1,4,5-trisphosphate receptor Ca2+ release channels in mammalian tissues and cell lines. Biochem J. 1997;327:251–258. doi: 10.1042/bj3270251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson PS, Kim YK, Valdivia H, Knudson CM, Takekura H, Franzini-Armstrong C, Coronado R, Campbell K. The brain ryanodine receptor: a caffeine-sensitive calcium release channel. Neuron. 1991;7:17–25. doi: 10.1016/0896-6273(91)90070-g. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Laferla FM, Chan SL, Leissring MA, Shepard PN, Geiger JD. Calcium signaling in the ER: its role in neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2000;23:222–229. doi: 10.1016/s0166-2236(00)01548-4. [DOI] [PubMed] [Google Scholar]
- Mercurio AM, Holtzman E. Smooth endoplasmic reticulum and other agranular reticulum in frog retinal photoreceptors. J Neurocytol. 1982;11:263–293. doi: 10.1007/BF01258247. [DOI] [PubMed] [Google Scholar]
- Micci MA, Christensen BN. Na+/Ca2+ exchange in catfish retina horizontal cells: regulation of intracellular Ca2+ store function. Am J Physiol. 1998;274:C1625–1633. doi: 10.1152/ajpcell.1998.274.6.C1625. [DOI] [PubMed] [Google Scholar]
- Milanick MA. Proton fluxes associated with the Ca pump in human red blood cells. Am J Physiol. 1990;258:C552–562. doi: 10.1152/ajpcell.1990.258.3.C552. [DOI] [PubMed] [Google Scholar]
- Nachman-Clewner M, St Jules R, Townes-Anderson E. L-type calcium channels in the photoreceptor ribbon synapse: localization and role in plasticity. J Comp Neurol. 1999;415:1–16. [PubMed] [Google Scholar]
- Narita K, Akita T, Hachisuka J, Huang S, Ochi K, Kuba KJ. Functional coupling of Ca(2+) channels to ryanodine receptors at presynaptic terminals. Amplification of exocytosis and plasticity. J Gen Physiol. 2000;115:519–532. doi: 10.1085/jgp.115.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neering IR, McBurney RN. Role for microsomal Ca storage in mammalian neurones? Nature. 1984;309:158–160. doi: 10.1038/309158a0. [DOI] [PubMed] [Google Scholar]
- Ohyama T, Picones A, Korenbrot JI. Voltage-dependence of ion permeation in cyclic GMP-gated ion channels is optimized for cell function in rod and cone photoreceptors. J Gen Physiol. 2002;119:341–354. doi: 10.1085/jgp.20028565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzan T, Rizzuto R, Volpe P, Meldolesi J. Molecular and cellular physiology of intracellular calcium stores. Physiol Rev. 1994;74:595–636. doi: 10.1152/physrev.1994.74.3.595. [DOI] [PubMed] [Google Scholar]
- Reichling DB, MacDermott AB. Lanthanum actions on excitatory amino acid-gated currents and voltage-gated calcium currents in rat dorsal horn neurons. J Physiol. 1991;441:199–218. doi: 10.1113/jphysiol.1991.sp018746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripps H, Shakib M, MacDonald ED. Peroxidase uptake by photoreceptor terminals of the skate retina. J Cell Biol. 1976;70:86–96. doi: 10.1083/jcb.70.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Bernardi P, Pozzan T. Mitochondria as all-round players of the calcium game. J Physiol. 2000;529:37–47. doi: 10.1111/j.1469-7793.2000.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini BL, Maravall M, Svoboda K. Ca2+ signaling in dendritic spines. Curr Opinion Neurobiol. 2001;11:349–356. doi: 10.1016/s0959-4388(00)00218-x. [DOI] [PubMed] [Google Scholar]
- Sampath AP, Matthews HR, Cornwall MC, Bandarchi J, Fain GL. Light-dependent changes in outer segment free-Ca2+ concentration in salamander cone photoreceptors. J Gen Physiol. 1999;113:267–277. doi: 10.1085/jgp.113.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnapf JL, Copenhagen DR. Differences in the kinetics of rod and cone synaptic transmission. Nature. 1982;296:862–864. doi: 10.1038/296862a0. [DOI] [PubMed] [Google Scholar]
- Sitsapesan R, Williams AJ. Mechanisms of caffeine activation of single calcium-release channels of sheep cardiac sarcoplasmic reticulum. J Physiol. 1990;423:425–439. doi: 10.1113/jphysiol.1990.sp018031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The structure of the rabbit retina as revealed by electron microscopy. In: Prince JS, editor; Sjöstrand FS, Nilsson SE, editors. The Rabbit in Eye Research. IL, USA: Thomas, Springfield; 1965. pp. 1–65. [Google Scholar]
- Smith AB, Cunnane TC. Ryanodine-sensitive calcium stores involved in neurotransmitter release from sympathetic nerve terminals of the guinea-pig. J Physiol. 1996;497:657–664. doi: 10.1113/jphysiol.1996.sp021797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solovyova N, Veselovsky N, Toescu EC, Verkhratsky A. Ca(2+) dynamics in the lumen of the endoplasmic reticulum in sensory neurons: direct visualization of Ca(2+)-induced Ca(2+) release triggered by physiological Ca(2+) entry. EMBO J. 2002;21:622–630. doi: 10.1093/emboj/21.4.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D, Hanley MR. Pharmacological tools for perturbing intracellular calcium storage. Methods Cell Biol. 1994;40:65–89. doi: 10.1016/s0091-679x(08)61110-3. [DOI] [PubMed] [Google Scholar]
- Townes-Anderson E. Intersegmental fusion in vertebrate rod photoreceptors. Rod cell structure revisited. Invest Ophthal Vis Sci. 1985;36:1918–1933. [PubMed] [Google Scholar]
- Uneyama H, Munakata M, Akaike N. Caffeine response in pyramidal neurons freshly dissociated from rat hippocampus. Brain Res. 1993;604:24–31. doi: 10.1016/0006-8993(93)90348-q. [DOI] [PubMed] [Google Scholar]
- Ungar F, Piscopo I, Letizia J, Holtzman E. Uptake of calcium by the endoplasmic reticulum of the frog photoreceptor. J Cell Biol. 1984;98:1645–1655. doi: 10.1083/jcb.98.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usachev YM, Thayer SA. Ca2+ influx in resting rat sensory neurones that regulates and is regulated by ryanodine-sensitive Ca2+ stores. J Physiol. 1999;519:115–130. doi: 10.1111/j.1469-7793.1999.0115o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usachev YM, Toutenhoofd SL, Goellner GM, Strehler EE, Thayer SA. Differentiation induces up-regulation of plasma membrane Ca2+-ATPase and concomitant increase in Ca2+ efflux in human neuroblastoma cell line IMR-32. J Neurochem. 2001;76:1756–1765. doi: 10.1046/j.1471-4159.2001.00169.x. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Shmigol A. Calcium-induced calcium release in neurones. Cell Calcium. 1996;19:1–14. doi: 10.1016/s0143-4160(96)90009-3. [DOI] [PubMed] [Google Scholar]
- Yang JH, Gross RL, Basinger SF, Wu SM. Apoptotic cell death of cultured salamander photoreceptors induced by cccp: CsA-insensitive mitochondrial permeability transition. J Cell Sci. 2001;114:1655–1664. doi: 10.1242/jcs.114.9.1655. [DOI] [PubMed] [Google Scholar]
- Yoshizaki K, Hoshino T, Sato M, Koyano H, Nohmi M, Hua SY, Kuba K. Ca2+-induced Ca2+ release and its activation in response to a single action potential in rabbit otic ganglion cells. J Physiol. 1995;486:177–187. doi: 10.1113/jphysiol.1995.sp020801. [DOI] [PMC free article] [PubMed] [Google Scholar]


