Abstract
Sound-evoked vibrations of the basilar membrane (BM) in anaesthetised guinea-pigs are shown to be affected over two distinct time scales by electrical stimulation of the medial olivocochlear efferent system: one is fast (10–100 ms), the other much slower (10–100 s). For low and moderate level tones near the BM's characteristic frequency, both fast and slow effects inhibited BM motion. However, fast inhibition was accompanied by phase leads, while slow inhibition was accompanied by phase lags. These findings are consistent with a hypothesis that both fast and slow effects decrease sound amplification in the cochlea. However, the opposing directions of the phase changes indicate that separate mechanical processes must underlie fast and slow effects. One plausible interpretation of these findings is that efferent slow effects are caused by outer-hair-cell stiffness decreases, while efferent fast effects are caused by reductions in ‘negative damping’.
The medial olivocochlear (MOC) efferent system inhibits sound-evoked responses in the auditory nerve over two time scales (Sridhar et al. 1995): fast (inhibition building up over tens of milliseconds) and slow (inhibition building up over tens of seconds). Both fast and slow effects are initiated when the efferents release acetylcholine (ACh) onto the cochlea's outer hair cells (OHCs). The OHCs act as mechanical motors in a feedback loop within the cochlea, both detecting and amplifying the sound-evoked motion of the basilar membrane (BM) (for reviews see Dallos, 1992; Patuzzi, 1996; Robles & Ruggero, 2001). Reductions in the amplification produced by the OHCs are likely to underlie both fast and slow effects of MOC efferents (see Guinan, 1996 for review).
Previous studies have shown that BM motion can be inhibited either by electrical stimulation of MOC efferents, or by direct application of ACh into the cochlea (Murugasu & Russell, 1996a,b; Dolan et al. 1997). However, these studies were not designed to compare the fast vs. slow efferent effects, and to date, no differences in BM motion that could be attributed to fast vs. slow effects have been reported.
The present study investigates the mechanical effects of MOC efferents using two paradigms that were designed to test for both fast and slow effects. The study reveals various conditions where the mechanical changes associated with the fast and slow effects occur in opposite directions, and leads us to conclude that the two effects involve fundamentally different mechanical changes.
Methods
Experiments were performed on albino and pigmented guinea-pigs (290–1000 g; n = 51), which were anaesthetised using combinations of sodium pentobarbitone (25 mg kg−1, i.p.) and Hypnorm (0.6 ml kg−1, i.m.; each millilitre of Hypnorm contains 10 mg fluanisone and 0.315 mg fentanyl citrate), or of Ketamine (50 mg kg−1, i.m.) and Xylazine (10 mg kg−1, i.m.). Artificial ventilation was used to maintain end-tidal CO2 levels of ca 4.5 %, and core temperatures were maintained around 37.6 °C. The animals were killed humanely, without recovery from the anaesthesia, at the end of the experiments. All experiments were performed in accordance with Home Office Guidelines on the Operation of the Animals (Scientific Procedures) Act, 1986.
The cochlea was exposed via a dorsolateral bulla opening. Acoustic stimuli were delivered to the ear canal via a closed sound system, and sound pressure levels (SPLs, expressed in decibels re: 20 μPa, or dB SPL) were calibrated within 2 mm of the eardrum using a probe tube microphone (Brüel & Kjær 4134, Denmark).
The physiological condition of the cochlea was monitored using compound action potential (CAP) recordings from a wire electrode placed near the round window (see Johnstone et al. 1979). CAP thresholds deteriorated by at least 10 dB from their initial values in most (but not all) experiments, but this appeared to affect only the magnitude of the efferent-evoked effects (i.e. not the patterns of the effects).
BM vibrations were monitored in the basal turn of the cochlea using a laser interferometer (Cooper, 1999). The BM was exposed either by shaving a small hole into scala tympani of the basal turn, or by tearing a small hole through the round window membrane. Gold-coated polystyrene microbeads (PolySciences Inc., Germany; 15–25 μm diameter) were used to enhance the reflectivity of the BM. A small glass coverslip was placed over the interface between the cochlear fluids and the air to ensure the validity of the interferometric measurements (cf. Cooper & Rhode, 1992)
MOC efferents were stimulated electrically via a bipolar electrode at the floor of the fourth ventricle (see Guinan & Stankovic, 1996). Monophasic, 300 μs-wide current pulses were presented at 200–300 pulses s−1 in bursts of either 100 ms duration at 330 ms intervals (Fig. 1B), or 300 ms duration at 1.5 s intervals (Fig. 1C). Pulse amplitudes were adjusted at the beginning of each experiment, and were limited by the twitch thresholds for various muscles (for details see Murugasu & Russell, 1996a).
Figure 1. Experimental paradigms.
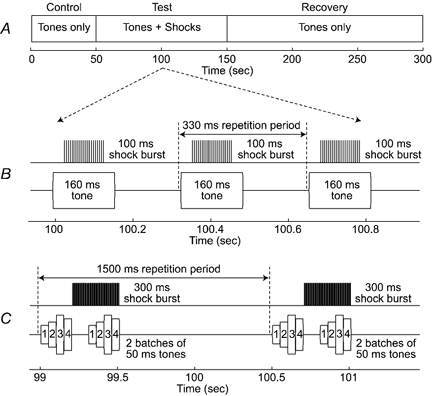
A, each ‘run’ of an experiment was divided into three parts: (1) a control period, where tones were presented without efferent stimulation; (2) a test period, where tones were paired with electrical stimulation of MOC efferents; and (3) a recovery period, where tones were again presented without efferent stimulation. The detailed timing of the two experimental paradigms is shown in panels B and C. B, in the single-tone paradigm, responses were averaged across multiple presentations of a 160 ms-long tone pip, and efferent stimulation was delivered in 100 ms-long shockbursts. C, in the multiple-tone paradigm, responses were averaged across multiple presentations of an acoustic stimulus consisting of two batches of four 50 ms-long tone pips, and efferent stimulation was delivered in 300 ms-long shockbursts. Individual shock bursts were timed to produce minimal effects near the start of each acoustic stimulus, and to produce maximal effects near the end of each stimulus.
Two paradigms were used to study the effects of MOC stimulation on BM motion: one involved repeated presentations of a single tone burst (cf. Fig. 1B), and the other involved repeated presentations of multiple tone bursts (cf. Fig. 1C). Both paradigms conformed to the overall timing pattern shown in Fig. 1A. The acoustic stimuli were presented repeatedly over several hundreds of seconds. After a control period of ca 50 s, each stimulus was paired with a short burst of efferent stimulation in a test period lasting ca 100 s. The pairing of efferent shock bursts and acoustic stimuli was then terminated, and recovery from the preceding efferent stimulation was monitored for at least 100 s.
The two stimulation paradigms differed in their fine temporal detail, as shown in Fig. 1B and C. In the single-tone paradigm (Fig. 1B), the repeated acoustic stimulus was a single tone (160 ms duration, 1 ms rise/fall times), presented once every 330 ms. In the multiple-tone paradigm (Fig. 1C), the repeated acoustic stimulus was a string of eight short tones, presented once every 1.5 s. The eight tones were arranged in two batches of four tones each (each tone was 50 ms long, with 1 ms rise/fall times; there were no delays between tones within each batch, but the two batches were separated by 110 ms). The tones within each batch could differ in frequency and/or in intensity, but all batches within a single run of the paradigm were identical.
In the test period of each paradigm, the pairing of the efferent shocks and the acoustic stimuli was arranged so that the fast and slow effects of the efferent activity could be distinguished clearly. To facilitate the distinction, the onset of each shock burst was delayed from the onset of each acoustic stimulus (by ca 30 ms in the single-tone paradigm, and ca 210 ms in the multiple-tone paradigm), and the BM responses were analysed in two separate time windows. As illustrated in Fig. 2, one analysis window (○) covered the early part of each acoustic stimulus, ending just before the onset of the efferent shock burst. Any changes to the BM responses in this ‘early’ window had to be attributed to the slow effects of previous efferent stimulation, because the early window was preceded by at least 200 ms of efferent ‘silence’ (during which time the fast effects of any preceding stimulation disappeared). A second analysis window (× in Fig. 2) coincided with a time towards the end of each acoustic stimulus, when the fast effects of each shock burst peaked (cf. Fig. 2B and C). Response changes during this ‘late’ window could result from either fast or slow efferent effects (cf. Fig. 2C), but comparisons between the responses in the early and late windows allowed the two effects to be distinguished: slow effects were estimated by comparing the responses in the early (○) windows with responses in the control period of each run (cf. Fig. 2C, E and F), while fast effects were estimated by comparing the responses in each late (×) window with those in the corresponding early (○) window (cf. Figs. 2B, C, E and F). Amplitude changes were expressed in decibels, and phase changes in degrees (i.e. δAslow effect(dB) = 20log10(A○/A○,control); δAfasteffect(dB) = 20log10(A×/A○); δΦslow effect=Φ○ – Φ○,control; and δΦfast effect=Φ× – Φ○; where A is amplitude, Φ is phase, ○ and × denote the early and late analysis windows, and ‘○, control’ denotes the early window during the control period).
Figure 2. Fast and slow effects of efferent stimulation on BM motion during one run of the single-tone paradigm.
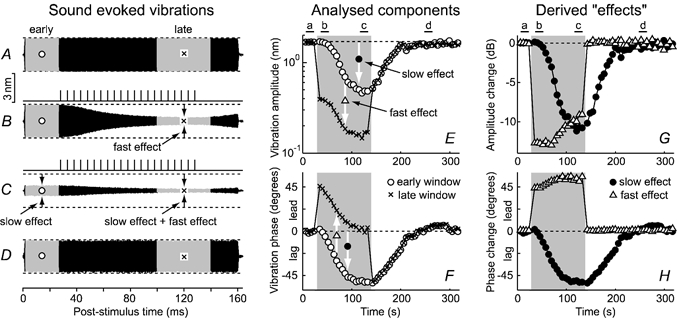
A-D, BM responses to CF tones (19 kHz, 35 dB SPL) at the times indicated above E and G. Shading in A-D marks the early (○) and late (×) analysis windows. Pulse trains above B and C illustrate the fine timing patterns of the efferent stimulation. Responses have been high-pass filtered (fc = 15 kHz) to clarify illustration of the fast and slow effects. E and F, variations in BM response amplitudes (E) and phases (F) across the period of the run. Shading indicates the overall period of efferent stimulation (the test period; cf. Fig. 1A). Slow effects of the efferent stimulation are manifest as changes in the responses in the early analysis windows (○) across time (i.e. as differences from the control, or ‘baseline’ responses). Fast effects are manifest as differences between the responses in the early (○) and late (×) analysis windows during the test period. G and H, derived amplitude and phase changes attributed to the fast (▵) and slow (•) effects. Experiment GP16, run 2.
Simultaneous BM and CAP recordings were made in most, but not all experiments. Since CAPs are triggered by tone onsets, and in the single-tone paradigm these onsets occur at least 200 ms after any efferent shocks, most of our CAP recordings only provide information about slow effects.
Both BM and CAP responses were averaged across multiple presentations of a stimulus (usually 16 successive presentations) to improve their signal-to-noise ratios. The averaged responses were then inspected for efferent-evoked changes (in either amplitude or phase) using Fourier analysis.
Results
Results are based on BM measurements in 24 guinea-pigs. The data were sufficient to separate fast and slow effects in 18 of these experiments. In five BM experiments, sound-evoked vibrations of the middle-ear ossicles were also measured, under conditions identical to those used for the BM recordings. The middle-ear vibrations showed none of the effects that were seen on the BM (thus ruling out the possibility of artefacts caused by middle-ear muscle contractions, etc.).
Efferent-evoked changes in BM motion occurred on both fast (τ≈ 30–60 ms) and slow (τ≈ 10–50 s) time scales, as illustrated in Fig. 2. The slow effects can be seen by comparing responses across the early (○) windows of the control, test and recovery periods in Fig. 2A–F, and are shown explicitly by the • symbols in Fig. 2G–H. The slow effects continued for at least 100 s beyond the end of the test period, and were often terminated by a short ‘rebound’ period (see below; cf. Fig. 3C). The fast effects were manifest as differences between the sound-evoked responses in the early (i.e. pre-shockburst) and late (end-of-shockburst) analysis windows of each stimulus. These can be seen as differences between the ○ and × analysis windows in Fig. 2B and C and the ○ and × symbols in Fig. 2E and F, and are shown explicitly by the ▵ symbols in Fig. 2G and H (note that fast effects were only seen during the test period, i.e. in the shaded regions of Fig. 2E and H).
Figure 3. Fast and slow effects vary with tone frequency and intensity, and with the time course of efferent stimulation.
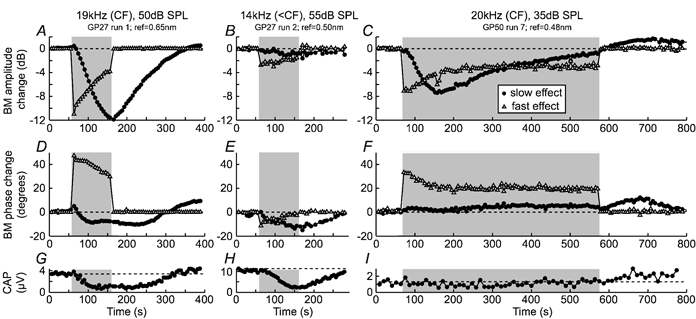
BM vibration amplitude (A-C) and phase (D-F) changes attributed to fast (▵) and slow (•) effects are shown for three runs of the single-tone paradigm (cf. Fig. 1B). Stimulus details and reference vibration levels for 0 dB amplitude changes are indicated at the top. Shading indicates the overall period of efferent stimulation (the test period; cf. Fig. 1 A). G-I, amplitudes of simultaneously recorded CAPs, illustrating the slow effects of the efferent stimulation on the auditory nerve.
Both fast and slow effects varied with the frequency and intensity of the acoustic stimulation. Both effects were largest for low-to-moderate level tones near the BM's most-sensitive or ‘characteristic’ frequency (CF), and both effects were negligible for tones more than an octave below CF.
For near-CF tones, both fast and slow effects inhibited BM motion (e.g. Figs 2G, 3A and C). The amounts of fast and slow inhibition near the CF were usually similar, and ranged up to 13 dB each. However, the amount of fast inhibition always decreased over the first 100 s of efferent stimulation, while the amount of slow inhibition always increased over the same time period (cf. Figs 2G, 3A and C). In one run where efferent stimulation was continued for 500 s, the slow inhibition peaked at 8 dB (after ca 100 s) before decreasing to less than 1 dB (while the fast inhibition remained constant; cf. Fig. 3C). The slow inhibition was often followed by a ‘rebound’ period of BM hypersensitivity, lasting 100–200 s (cf. Fig. 3C).
The phase changes that accompanied the fast and slow inhibition of near-CF responses differed markedly: slow inhibition was associated with phase lags of between 10 and 50 deg (e.g. Figs 2H, 3D and E), while fast inhibition was associated with phase leads of between 20 and 70 deg (e.g. Figs 2H, 3D and F, 4G and H). Oppositely directed fast and slow phase changes were observed for most tones: the only exceptions were for low-to-moderate level tones around 1/4–1/2 octave below CF, where both fast and slow effects involved phase lags of up to 20 deg (e.g. Fig. 3E).
Figure 4. Frequency and intensity dependencies of fast and slow effects during a single run of the multiple-tone paradigm.
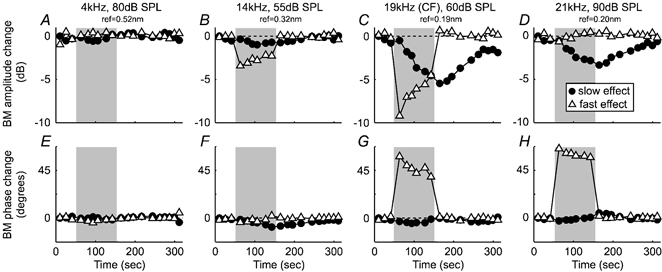
The relative strengths of the fast (▵) and slow (•) amplitude changes (A-D) and phase changes (E-H) vary systematically with frequency. The tones were presented in a pseudo-randomly interleaved manner (19, 14, 21 and 4 kHz data correspond to tones 1–4 in Fig. 1C). Shading indicates the overall period of efferent stimulation (the test period; cf. Fig. 1A). Stimulus details and reference vibration levels are indicated at the top. Experiment GP27, run 4.
The frequency and intensity dependence of fast and slow effects differed in at least two ways. Firstly, while slow effects always involved phase lags, fast effects ‘flipped’ from involving phase lags at low-to-moderate levels, 1/4–1/2 octave below CF, to involving phase leads at higher levels and/or frequencies. A second difference occurred for high-level tones ca 1–3 kHz above the CF. Fast effects often facilitated the BM responses to such tones (typically by ca 1–2 dB), but slow facilitation was never seen. In three of the 12 runs where we have data on this issue, between 1 and 3 dB of slow inhibition was observed at the same time as 1–2 dB of fast facilitation; in two other runs, up to 3 dB of slow inhibition was seen without either fast inhibition or fast facilitation (although fast phase leads were seen; cf. Fig. 4D and H); and in the remaining seven runs, no slow effects were seen (despite fast phase leads).
The slow effects seen during this study varied considerably both within and across experiments. This made the frequency and intensity dependence of the slow effect very difficult to assess in detail. However, some of the variability seemed to be linked to the efferent stimulation history of each experiment, and this could be overcome using the multiple-tone paradigm of Fig. 1C. This paradigm makes it possible to compare effects across different frequencies and intensities of stimulation, while maintaining the efferent stimulation history almost constant. Example data from one run of the paradigm are shown in Fig 4: in this case, the same sets of efferent shock bursts produced effects which were negligible well below CF (Fig. 4A and E), predominantly ‘fast’ just below CF (Fig. 4B and F), both ‘fast’ and ‘slow’ at CF (Fig. 4C and G), and predominantly ‘slow’ above CF (Fig. 4D and H). Three other runs of the multiple-tone paradigm (each in a different experiment) gave similar results.
Discussion
This report provides the first detailed examination of the time course of MOC efferent effects on cochlear mechanics. The results provide direct evidence that both fast and slow effects of MOC stimulation have mechanical underpinnings. For low-to-moderate level, near-CF stimuli in particular, the data are consistent with a hypothesis that the major source of the efferent-evoked inhibition seen in the auditory nerve (in this and previous studies) results from mechanical inhibition on the BM. However, a more detailed study (including extensive use of well-below CF tones, cf. Stankovic & Guinan, 1999) is needed to test this hypothesis fully. The data of this report are also consistent with a hypothesis that the gain of the cochlear amplifier (i.e. the amount of amplification that a sound undergoes within a healthy cochlea) decreases during both fast and slow effects of efferent stimulation.
The most intriguing finding of the present report is that the mechanical phase changes evoked by the fast and slow effects usually occur in opposite directions. Our findings on efferent-evoked phase changes differ from those reported previously by Murugasu & Russell (1996a, b), who showed both efferent- and ACh-evoked BM phase changes to be either absent or ‘variable’. However, Murugasu and Russell's studies were not designed to distinguish fast and slow effects, and it is possible that oppositely directed fast and slow phase changes could have cancelled each other in their data.
The oppositely directed phase changes found in the present report imply that fundamentally different mechanical changes underlie the efferent system's fast and slow effects. Given that both effects almost certainly originate in the OHCs (for reasoning see Sridhar et al. 1995; Guinan, 1996), this means that OHCs must affect BM motion in at least two ways. Perhaps slow inhibition is caused by OHC-induced decreases in the stiffness of the cochlear partition (e.g. as suggested by Dallos et al. 1997), while fast inhibition is caused by decreases in the ‘negative damping’ that OHC motility normally provides (for reviews see Dallos, 1992; Guinan, 1996; Patuzzi, 1996). This scenario is consistent with the direction of the phase changes observed here (in cochlear models, decreased stiffnesses generally produce phase lags, while increased damping can produce phase lags below CF, and phase leads above CF; see Kolston, 2000 for examples).
The suggestion that OHCs affect BM motion in more than one way leads to the question of how MOC efferents produce their effects at a cellular level. This is a well-studied question, with evidence of at least two mechanisms. The first involves efferent-evoked OHC conductance increases. These are largely mediated by calcium-activated potassium channels (Housley & Ashmore, 1991; Evans, 1996), and occur during both fast and slow efferent effects (Sridhar et al. 1995, 1997; Yoshida et al. 2001). In terms of their ability to affect cochlear mechanics, increased OHC conductances both hyperpolarise the cells and shunt the potentials that drive OHC motility, potentially leading to decreases in the negative damping that this motility could provide in the cochlea (for reviews see Dallos, 1992; Guinan, 1996; Patuzzi, 1996). The second form of change is a decrease in the OHCs’ axial stiffnesses. This occurs much more slowly than efferent fast effects, because it relies on various second messengers and protein phosphorylation, etc. (Dallos et al. 1996, 1997). The OHCs’ axial stiffnesses contribute only marginally towards the overall stiffness of the cochlear partition (cf. Hallworth, 1995), but changes in OHC stiffness could still produce large changes in BM motion at frequencies close to CF (cf. Allen, 1990). Since only conductance changes are present during fast effects, but both conductance and stiffness changes are present during slow effects, the stiffness change must dominate during slow effects in order to produce the oppositely directed phase changes found for fast vs. slow effects.
The finding that the fast and slow effects of efferent stimulation have different underlying mechanisms may be related to the function of these effects in hearing. The slow effect may be important in preventing cochlear damage due to loud sounds (Reiter & Liberman, 1995), while the fast effect is more likely to be involved in producing perceptual changes (e.g. by reducing masking: for review see Guinan, 1996). Since these functions may be needed in different circumstances, and on different time scales, it is perhaps not surprising that different mechanisms have evolved to provide them.
Acknowledgments
This work was supported by the Royal Society, the Wellcome Trust and NIDCD RO1 DC00235.
References
- Allen JB. Modeling the noise damaged cochlea. In: Dallos P, Geisler CD, Mathews JW, Ruggero MA, Steele CR, editors. The Mechanics and Biophysics of Hearing. Vol. 87. Berlin: Blackwell Science Inc; 1990. pp. 324–332. [Google Scholar]
- Cooper NP. An improved heterodyne laser interferometer for use in studies of cochlear mechanics. J Neurosci Meth. 1999;88:93–102. doi: 10.1016/s0165-0270(99)00017-5. [DOI] [PubMed] [Google Scholar]
- Cooper NP, Rhode WS. Basilar membrane mechanics in the hook region of cat and guinea-pig cochleae: sharp tuning and nonlinearity in the absence of baseline position shifts. Hear Res. 1992;63:163–190. doi: 10.1016/0378-5955(92)90083-y. [DOI] [PubMed] [Google Scholar]
- Dallos P. The active cochlea. J Neurosci. 1992;12:4575–4585. doi: 10.1523/JNEUROSCI.12-12-04575.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P, He DZ, Lin X, Evans BN. Efferent control of cochlear mechanics: outer hair cells. In: Lewis ER, Long GR, Lyon RF, Narins PM, Hecht-Poinar E, editors. Diversity in Auditory Mechanics. Singapore: Blackwell Science Inc; 1996. pp. 501–508. [Google Scholar]
- Dallos P, He DZ, Lin X, Sziklai I, Mehta S, Evans BN. Acetylcholine, outer hair cell electromotility, and the cochlear amplifier. J Neurosci. 1997;17:2212–2226. doi: 10.1523/JNEUROSCI.17-06-02212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan DF, Guo MH, Nuttall AL. Frequency-dependent enhancement of basilar membrane velocity during olivocochlear bundle stimulation. J Acoust Soc Am. 1997;102:3587–3596. doi: 10.1121/1.421008. [DOI] [PubMed] [Google Scholar]
- Evans MG. Acetylcholine activates two currents in guinea-pig outer hair cells. J Physiol. 1996;491:563–578. doi: 10.1113/jphysiol.1996.sp021240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinan JJ., Jr . Physiology of olivocochlear efferents. In: Dallos P, Popper A, Fay R, editors. The Cochlea. Vol. 8. New York: Blackwell Science Inc; 1996. pp. 435–502. [Google Scholar]
- Guinan JJ, Jr, Stankovic KM. Medial efferent inhibition produces the largest equivalent attenuations at moderate to high sound levels in cat auditory-nerve fibers. J Acoust Soc Am. 1996;100:1680–1690. doi: 10.1121/1.416066. [DOI] [PubMed] [Google Scholar]
- Hallworth R. Passive compliance and active force generation in the guinea pig outer hair cell. J Neurophysiol. 1995;74:2319–2328. doi: 10.1152/jn.1995.74.6.2319. [DOI] [PubMed] [Google Scholar]
- Housley GD, Ashmore JF. Direct measurement of the action of acetylcholine on isolated outer hair cells of the guinea pig cochlea. Proc R Soc Lond B Biol Sci. 1991;244:161–167. doi: 10.1098/rspb.1991.0065. [DOI] [PubMed] [Google Scholar]
- Johnstone JR, Alder VA, Johnstone BM, Robertson D, Yates GK. CAP threshold and single unit thresholds. J Acoust Soc Am. 1979;65:254–257. doi: 10.1121/1.382244. [DOI] [PubMed] [Google Scholar]
- Kolston PJ. The importance of phase data and model dimensionality to cochlear mechanics. Hear Res. 2000;145:25–36. doi: 10.1016/s0378-5955(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Murugasu E, Russell IJ. The effect of efferent stimulation on basilar membrane displacement in the basal turn of the guinea pig cochlea. J Neurosci. 1996a;16:325–332. doi: 10.1523/JNEUROSCI.16-01-00325.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugasu E, Russell IJ. The role of calcium on the effects of intracochlear acetylcholine perfusion on basilar membrane displacement in the basal turn of the guinea pig cochlea. Auditory Neuroscience. 1996b;2:363–376. doi: 10.1523/JNEUROSCI.16-01-00325.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patuzzi RB. Cochlear micromechanics and macromechanics. In: Dallos P, Popper A, Fay R, editors. The Cochlea. Vol. 8. New York: Blackwell Science Inc; 1996. pp. 186–257. [Google Scholar]
- Reiter ER, Liberman MC. Efferent-mediated protection from acoustic overexposure: relation to slow effects of olivocochlear stimulation. J Neurophysiol. 1995;73:506–514. doi: 10.1152/jn.1995.73.2.506. [DOI] [PubMed] [Google Scholar]
- Robles L, Ruggero MA. Mechanics of the mammalian cochlea. Physiol Rev. 2001;81:1305–1352. doi: 10.1152/physrev.2001.81.3.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar TS, Brown MC, Sewell WF. Unique postsynaptic signaling at the hair cell efferent synapse permits calcium to evoke changes on two time scales. J Neurosci. 1997;17:428–437. doi: 10.1523/JNEUROSCI.17-01-00428.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhar TS, Liberman MC, Brown MC, Sewell WF. A novel cholinergic “slow effect” of efferent stimulation on cochlear potentials in the guinea pig. J Neurosci. 1995;15:3667–3678. doi: 10.1523/JNEUROSCI.15-05-03667.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankovic KM, Guinan JJ., Jr Medial efferent effects on auditory-nerve responses to tail-frequency tones. I. Rate reduction. J Acoust Soc Am. 1999;106:857–869. doi: 10.1121/1.427102. [DOI] [PubMed] [Google Scholar]
- Yoshida N, Liberman MC, Brown MC, Sewell WF. Fast, but not slow, effects of olivocochlear activation are resistant to apamin. J Neurophysiol. 2001;85:84–88. doi: 10.1152/jn.2001.85.1.84. [DOI] [PubMed] [Google Scholar]


