Abstract
Single skeletal muscle fibres from rat and cane toad were mechanically skinned and stimulated either electrically by initiating action potentials in the sealed transverse (t-) tubular system or by ion substitution causing depolarisation of the t-system to pre-determined levels. Depression of mitochondrial ATP-producing function with three diverse mitochondrial function antagonists (azide: 1–10 mm; oligomycin 1 μg ml−1 and carbonyl cyanide 4-trifluoromethoxyphenylhydrazone (FCCP) 1 μm), under conditions in which the cytosolic ATP was maintained high and constant, invariably reduced the excitability of rat fibres but had no obvious effect on the excitability of toad fibres, where mitochondria are less abundant and differently located. The reduction in excitability linked to mitochondria in rat fibres appears to be caused by depolarisation of the sealed t-system membrane. These observations suggest that mitochondria can regulate the functional state of mammalian muscle cells and have important implications for understanding how the balance between ATP utilisation and ATP production is regulated at the cellular level in general and in mammalian skeletal muscle fibres in particular.
It is vitally important for cells to keep the balance between ATP utilisation and ATP production and there are several well-known signalling pathways whereby the rate of ATP production is regulated by processes associated with a change in ATP demand. However, in order to ensure that the rate of ATP utilisation does not exceed the maximum capacity of ATP production it would be necessary that cells have a reverse signalling pathway whereby the ATP-generating capacity restrains the rate of ATP utilisation. This should be particularly important for cells that have a high ATP turnover. Contrary to one's intuition, the change in cytosolic [ATP] is not an appropriate signal in such a feedback mechanism because [ATP] must remain within a narrow range for normal cell function and a significant depletion of ATP has irreversible deleterious effects on cell functional integrity.
In intact cells, it is not possible to block ATP production without causing rapid, marked changes in the composition of the internal environment with respect to pH, the concentrations of Ca2+, Mg2+, inorganic phosphate and ADP and other modulators of cellular function. Therefore, changes in cellular function caused by inhibitors of various ATP-producing pathways cannot be directly linked to one particular factor. To overcome this problem we used a mechanically skinned muscle fibre preparation in which one has direct access to control the intracellular environment (Moisescu & Thieleczek, 1978; Lamb & Stephenson, 1994) while maintaining fibre structural integrity (Lamb et al. 1995) and excitability to electrical stimulation (Posterino et al. 2000). Using this skinned fibre preparation, we show that inhibiting the mitochondrial ATP-producing ability of rat fast-twitch fibres reduces fibre excitability in a dose-dependent and reversible fashion under conditions in which the composition of the cytosolic environment, including [ATP] is maintained constant. The importance of this new signalling pathway for cellular function in general, and muscle function in particular, is considerable.
Methods
Microdissection of mechanically skinned fibres
Male Long Evans hooded rats (16–18 weeks old) were killed by halothane overdose and cane toads (Bufo marinus) were stunned by a blow to the head and killed by double pithing, in accordance with the procedures approved by La Trobe University Animal Ethics Committee. The extensor digitorum longus (EDL, fast-twitch) muscle of the rat and the iliofibularis muscle of the toad were rapidly excised and immersed in paraffin oil. Mechanically skinned fibre preparations were obtained from the EDL muscle and the twitch region of the iliofibularis muscle by rolling back the sarcolemma using jewellers’ forceps (see Fig. 1A) as previously described (Fink et al. 1986; Lamb & Stephenson, 1994). The preparation was then mounted onto a force transducer (SensoNor 801, Norway), stretched to 120 % of slack length and immersed in a standard rat (or toad, as appropriate) K+-solution mimicking the cytosol.
Figure 1. Effect of mitochondrial antagonists on the excitability of mechanically skinned rat EDL fibres.
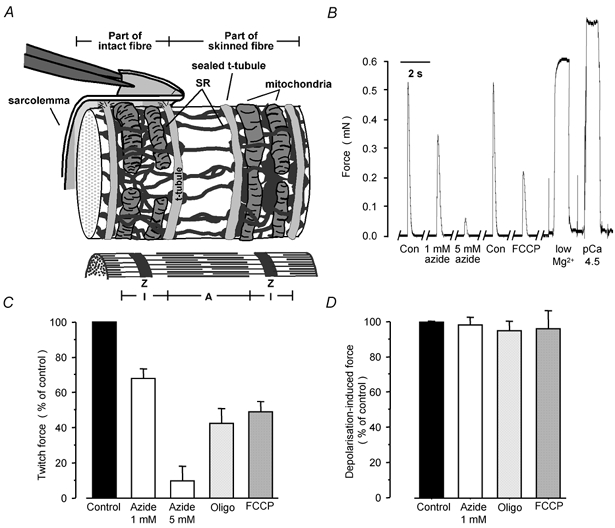
A, schematic representation of mammalian fibre ultrastructure and of the mechanical skinning procedure. Note that paired long mitochondria are transversely located at the I-band level wrapped around the contractile apparatus and in contact with SR but clearly separated from t-tubules. B, representative force responses to electrical stimulation, exposure to low-Mg2+ solution and to a maximally Ca2+-activating solution (pCa 4.5) from one preparation equilibrated in standard K-HDTA solutions with and without mitochondrial antagonists. C, summary of the twitch-force response data (n = 6–11). D, summary of the force responses when replacing the K-HDTA solution with a Na-HDTA solution (n = 3–5). Z, Z-line; A, A-band; I, I-band. Here and in subsequent figures: Con, control; Oligo, 1 μg ml−1 oligomycin; FCCP, 1 μm FCCP.
Solutions
The standard control solution (K-HDTA solution) contained (mm): K+ 127 (rat) or 117 (toad), Na+ 36, hexamethylene-diamine-tetraacetate (HDTA2−) 50, total ATP 8, creatine phosphate (CrP) 10, total Mg2+ 8.6 (1 free Mg2+), Hepes 90 (rat) or 60 (toad) (pH 7.10 ± 0.01) and total EGTA 0.05. The pCa (-log10[Ca2+]) was adjusted to 7.0 using a Ca2+-sensitive electrode (Orion Research Inc., Boston, MA, USA). In the 0 Na-HDTA solution, all Na+ was replaced by K+ and the Na-HDTA solution was identical to the K-HDTA solution, except that Na+ replaced all K+. Solutions of different [K+] were obtained by mixing K-HDTA and Na-HDTA solutions in various proportions. In Ca2+-activating solutions HDTA2− was replaced with EGTA2−/CaEGTA2− buffer. The low-Mg2+ solution was similar to the K-HDTA solution but contained only 0.8 mm total Mg2+ (15 μm free Mg2+). Rat and toad solutions had osmolalities of 290 ± 2 and 255 ± 2 mosmol kg−1, respectively. All experiments were performed at 24–25 °C. Control and test solutions containing mitochondrial function antagonists were made by dividing solutions into two and adding the antagonist to one half. Antagonist concentrations (1–10 mm azide, 1 μg ml−1 oligomycin and 1 μm FCCP (carbonyl cyanide 4-trifluoromethoxyphenylhydrazone)) were selected based on previously published work and were tested in preliminary experiments. Azide was added from an 80 mm stock in K-HDTA, 0 Na-HDTA or Na-HDTA solution as appropriate, and oligomycin and FCCP were added from concentrated stock solutions in DMSO (3 mg ml−1 and 4 mm, respectively). The [DMSO] used had no effect on force responses. All chemicals were of analytical grade. HDTA was obtained from Fluka (Buchs, Switzerland) and most other chemicals were from Sigma (St Louis, USA)
Fibre activation
Fibre excitation was achieved either by electrical field stimulation (2 ms pulses at 50 V cm−1) using two platinum wire electrodes running parallel to the skinned fibre and eliciting action potentials in the sealed t-system (Posterino et al. 2000) or by replacing the K-HDTA solution with Na-HDTA solution (ion substitution; Lamb & Stephenson, 1994). The experiments were performed such that a twitch response was evoked as soon as possible following exposure to the antagonist (within about 5 s) and subsequently every 15 s until steady-state twitch responses were obtained (usually within 90s). At the end of an experiment, the fibres were exposed to the low-Mg2+ solution to ascertain the level of Ca2+ in the sarcoplasmic reticulum (SR) and then were maximally activated in a strongly Ca2+-buffered (30 μm) solution containing 50 mm CaEGTA/EGTA. Force responses were recorded at 1 kHz using a 200 series Powerlab and the results were analysed using GraphPad Prism software (San Diego, CA, USA).
Statistics
Unless otherwise stated statistical comparisons were made using one-way analysis of variance (ANOVA) and significant differences between means were located by Fisher's PLSD post hoc test. Statistical significance was accepted at P < 0.05. All values are given as means ±s.e.m.
Results
Figure 1B shows representative action potential-induced twitch-force responses elicited by electrical stimulation (see Methods) in the same mechanically skinned rat fibre under control conditions and when the mitochondrial ATP-producing function was impaired with 1 or 5 mm azide, an inhibitor of oxidative phosphorylation, and 1 μm FCCP which uncouples F1-F0 ATP synthase from electron transport. Note that the twitch response under control conditions is greater than in the intact muscle fibre because of the low [EGTA] and lack of parvalbumin in the myoplasmic environment (see Posterino et al. 2000). Oligomycin (1 μg ml−1), a third unrelated type of mitochondrial inhibitor that inhibits the F1-F0 ATP synthase was also used and the results are summarised in Fig. 1C. In all instances, the twitch response of the rat muscle fibres was rapidly (within 90 s) depressed in a dose-dependent and reversible mode. Thus, inhibition of mitochondria in rat fibres with 1 and 5 mm azide depressed twitch force by 33 ± 5 % (n = 18) and 90 ± 5 % (n = 11), respectively. There was no further decrease in the twitch response when the [azide] was increased to 10 mm. Oligomycin (1 μg ml−1, n = 6) depressed rat twitch force by 58 ± 8 % and FCCP (1 μm, n = 7) by 51 ± 6 %. After exposure to azide (Fig. 1B), the twitch response recovered almost completely within minutes, whilst recovery after exposure to FCCP and oligomycin was slower because they are lipid soluble and wash out more slowly. Importantly, when the sealed t-system was maximally depolarised by ion substitution (Fig. 1D) there was no statistically significant depression of the t-system depolarisation-induced response after exposure to 1 mm azide, 1 μg ml−1 oligomycin or 1 μm FCCP in the standard solution, indicating that the inhibition of mitochondrial ATP-generating function affects events in the excitation- contraction (E-C) coupling preceding the activation of the voltage sensors/dihydropyridine receptors (DHPRs).
Another approach demonstrating that the decrease in the twitch response was related to mitochondria was to apply the same mitochondrial inhibitors to muscle fibres in which mitochondria were differently located and occupied a smaller fractional fibre volume than in rat EDL fibres. In fast-twitch mammalian fibres most mitochondria are located transversally to the fibre axis and in close proximity to the t-tubules (Fig. 1A) while in anuran twitch fibres mitochondria are located close to the sarcolemma and in long columns between myofibrils and are also generally less abundant (Smith & Ovalle, 1973; Mobley & Eisenberg, 1975; Eisenberg & Kuda, 1976; Davey & Wong, 1980; Ogata & Yamasaki, 1987, 1993; Lännergren et al. 1999). Indeed, from the electron microscopy data obtained in this laboratory on mechanically skinned rat EDL and cane toad iliofibularis fibres in connection with a previous study (Lamb et al. 1995), we could confirm the different arrangement of mitochondria in the twitch fibres of the two species and estimate that the fractional volume occupied by mitochondria in the toad fibres was smaller than that in the rat EDL fibres (2.28 ± 1.08 % (n = 7) vs. 4.64 ± 0.87 % (n = 8), P < 0.03; Mann-Whitney one-tailed test). Figure 2B shows representative results with cane toad twitch fibres and the results are summarised in Fig. 2C and D, showing clearly that 1 mm azide, 1 μg ml−1 oligomycin and 1 μm FCCP had no effect on either the twitch response or on the t-system depolarisation-induced response by ion substitution.
Figure 2. Effect of mitochondrial antagonists on the excitability of mechanically skinned cane toad muscle fibres.

A, representative force responses from one preparation equilibrated in standard K-HDTA solutions with and without mitochondrial antagonists. Summary of twitch data (B) (n = 6–7) and of force responses when replacing the K-HDTA solution with a Na-HDTA solution (C) (n = 7, 5 and 2 for azide, oligomycin and FCCP, respectively).
Together, the above observations suggest that mitochondrial inhibition elicits a signal in mitochondria leading to decreased excitability of the sealed t-system. The signal is expected to be weaker in toad than in rat fibres due to the reduced presence of mitochondria and their different location away from the t-tubules, thus explaining the lack of effect of mitochondrial inhibition on action potential-induced twitch responses in toad fibres.
Several lines of evidence indicate that this mitochondrially linked twitch depression is caused by t-system depolarisation. Thus, within a matter of seconds after the control myoplasmic solution was substituted with test solutions containing mitochondrial antagonists, there were either spontaneous twitch-like responses (often larger than the control twitch responses) or slower and smaller transient force responses, which decreased in size over several seconds and then normally disappeared. The responses are similar to responses when the control myoplasmic solution is substituted with lower [K+]-containing solutions causing partial depolarisation of the t-system. Secondly, an initial twitch potentiation over a period of several seconds was observed in 50, 33 and 29 % of fibres following exposure to 1 mm azide, oligomycin and FCCP, respectively. Thirdly, in the presence of azide, the force-frequency curves obtained with mechanically skinned rat fibres were reduced in size to 50.9 ± 17.2 % (n = 4, 1 mm azide) and 9.7 ± 2.6 % (n = 3, 2 mm azide) of controls and shifted to higher frequencies of stimulation (log ratio of frequencies to generate 50 % maximum tetanic force in azide and in control solutions: 0.60 ± 0.20 (n = 4 for 1 mm azide) and 0.87 ± 0.27 (n = 3 for 2 mm azide)), and in one instance also displayed a negative slope at higher frequencies, as it would be expected if the t-system were partially depolarised. Fourthly, as shown in Fig. 3A, the effect of the inhibitors on the twitch response was stronger when the sealed t-system of rat fibres was initially partially depolarised for several minutes by incubation in solutions with lower [K+] than in the standard control solution. This would be expected if the mitochondrial function inhibitors caused a certain amount of depolarisation. In order to show this, one can use the fibre excitability curve shown in Fig. 3C (continuous line) for the action potential-induced twitch forces when the preparations were equilibrated in solutions of different [K+]. The estimated membrane potential (Vm) across the t-system is shown on the lower abscissa assuming that [K+] in the sealed t-system was 3 mM and that the ratio between Na+- and K+-permeability (PNa/PK) was 0.01 (Hodgkin & Horowicz, 1959). From the excitability curve one can see that for a certain depolarisation, there would be a larger percentage drop in the twitch response if the fibre was partially depolarised. Figure 3C also shows the (inactivation) curve (dotted line) obtained for the t-system depolarisation-induced force responses by Na+ substitution as [K+] and Vm were altered. Note that this curve is displaced to markedly more positive Vm values compared with the twitch curve because the size of the ion substitution-induced response depends on the level of voltage sensor/DHPR inactivation while the size of the action potential-induced response depends on the level of Na+ channel inactivation (Ruff, 1999). The different positions on the x-axis of the two curves in Fig. 3C also fully explain why the action potential-induced twitch responses were more sensitive to the mitochondrial antagonists than the depolarisation-induced force responses by ion substitution in rat fibres (Fig. 1C and D).
Figure 3. Effects of t-system depolarisation on force responses in rat fibres.
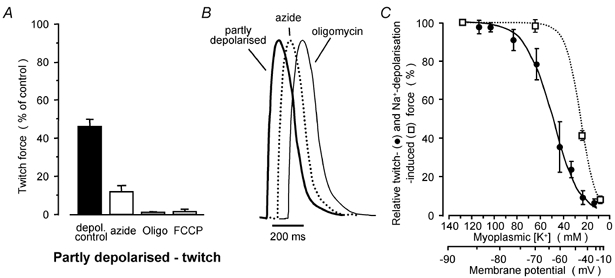
A, summary of twitch-force responses of partly depolarised rat fibres incubated in the presence of 43 mm K+ (Vm about −60 mV) (n = 7, 6, 4 and 3 for controls, 1 mm azide, 1 μg ml−1 oligomycin and 1 μm FCCP, respectively). B, representative twitch responses at high temporal resolution in the same fibre when partially depolarised in the 43 mm K+ solution and when equilibrated in standard myoplasmic solution with 1 mm azide or 1 μg ml−1 oligomycin. Responses were normalised to the same peak height and overlaid. C, action potential-induced twitch-force responses (continuous line) and force responses induced by t-system depolarisation with the Na-HDTA solution (dotted line) in rat fibres equilibrated in solutions of different myoplasmic [K+]. The lower x-axis is the estimated t-system membrane potential (see text). The test twitch responses were bracketed by twitch responses in the standard K-HDTA solution. Data points were best fitted by the Bolzmann-equation (Frelative = 1/(1 + exp(F50 – [K+])/slope)), with half-maximal responses (F50) at 50 and 27 mm K+ and slopes of 12 and 9 mm for the twitch and ion substitution curves, respectively.
Representative twitch responses obtained in the same fibre when equilibrated with 1 mm azide, 1 μg ml−1 oligomycin under standard conditions, or partially depolarised under control conditions (43 mm myoplasmic K+) to cause a similar level of twitch response depression (Fig. 1C) are overlaid in Fig. 3B with high temporal resolution after the peak force responses were normalised. The lack of any significant difference in the time course of the three twitch responses in Fig. 3B provides further support for the conclusion that mitochondrial antagonists cause partial depolarisation of the t-system, and also indicates that steps in the E-C coupling (Stephenson et al. 1998) following triggering of action potentials are not affected by inhibition of mitochondria.
The extent of t-system depolarisation by mitochondrial signalling can be deduced from the (continuous line) curve relating action potential-induced twitches to Vm in Fig. 3C. From this curve, one can estimate that 1 mm azide caused Vm depolarisation of 20 and 16 mV in the fully and partly polarised fibres, and that 5 and 10 mm azide depressed Vm by 45 mV whilst 1 μg ml−1 oligomycin and 1 μm FCCP caused Vm depolarisation of about 30–35 mV both in the 43 mm K+ and in the standard solution.
Important information concerning the mechanism responsible for the observed depolarisation of the t-system in the presence of mitochondrial antagonists can be obtained from the rate of decline of the twitch responses after rapidly blocking the Na+-K+-ATPase by removing Na+ from the myoplasmic environment (within 2–3 s; see the description of the last trace in Fig. 4 legend). When azide was present in solutions, the twitch response was smaller to start with and, importantly, declined much faster upon the removal of Na+ from the myoplasmic environment than when azide was absent (Fig. 4). This indicates that Vm depolarised considerably faster upon turning off the Na+-K+-ATPase in the presence of azide. Thus, the average rate of twitch force decline from 70 to 20 % of maximum twitch response was 4.4 ± 0.4 (n = 11) times faster in the presence of 1 mm azide than in the control. According to Fig. 3C, this corresponds to a Vm depolarisation from −67 to −58 mV. Considering that the [Na+] and [K+] in the sealed t-system must have changed by similar amounts in the presence and absence of azide when Vm changed from −67 to −58 mV, the 4.4-fold faster depolarisation between these Vm values, indicates that the rate of Na+ loss from the sealed t-tubules, and consequently the rate of K+ entry into the sealed t-system, must have increased by a similar factor. Assuming that PNa/PK is 0.01 under standard conditions (see above), the increase in the rate of Na+ loss from the t-tubules in the presence of 1 mm azide implies an increase in PNa by a factor close to 4.4, increasing PNa/PK to about 0.04, which fully accounts for the 20 mV depolarisation observed in the presence of 1 mm azide in the standard K-HDTA solution (see text and Fig. 1C and Fig. 3C).
Figure 4. Change in rat fibre excitability after rapidly stopping the Na+-K+-ATPase by Na+ removal from the myoplasmic environment.
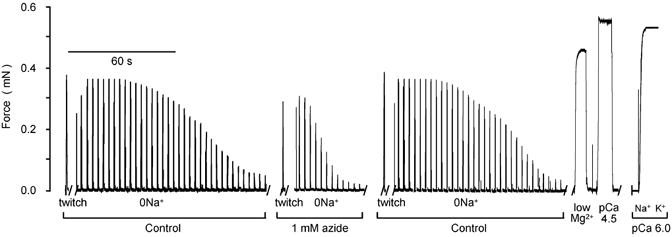
Twitch-force responses from a representative fibre elicited every 3 s in a 0 Na-solution in the absence and the presence of 1 mm azide. The last trace is the normalised response of the change in the level of activation of the contractile apparatus when changing from a Na-EGTA (pCa 6.0) to a K-EGTA (pCa 6.0) solution. This change in level of activation is due to the lower Ca2+ sensitivity in the presence of Na+ (Fink et al. 1986) and approximates the time taken (2–3 s) to remove Na+ from the myoplasmic environment.
Discussion
Results obtained in this study with rat EDL and toad iliofibularis twitch fibres, which differ with respect to location and fractional volume of mitochondria, and three molecularly diverse antagonists of mitochondrial function (each of which may also affect other different cell structures: see e.g. Cho et al. 1997; Collins & Larson, 2002) suggest that mitochondria play a more active role than previously thought in modulating muscle function. The observations cannot be explained by: (i) local depletion of cytosolic ATP and consequently by cytosolic ATP-dependent processes (ATPases, ionic channels) because myoplasmic ATP was high, was freely exchangeable with an almost infinite ATP pool in the bathing solution and was also buffered with CrP in the presence of endogenous creatine phosphokinase (Walliman et al. 1977) or (ii) differences in the ionic composition between test and control solutions (see Methods).
Evidence is also provided that this loss of fibre excitability is caused by depolarisation of the t-system due to an increase in PNa via a signalling pathway originating in mitochondria. Channels that are relatively permeant to Na+ and may become activated via this mitochondrial pathway include persistent Na+ channels (Gage et al. 1989), voltage-dependent anion channels channels that have been located in the t-system (Junankar et al. 1995) and non-selective cationic channels (Sipido & Marban, 1991).
Since there is no tight physical coupling between mitochondria and the t-system to produce a non-accessible ‘fuzzy space’ (Eisenberg & Kuda, 1976; Ogata & Yamasaki, 1993), the signal originating in the mitochondria when its ATP production-function is impaired must be converted into a chemical messenger to bridge the gap between mitochondria and t-system membranes. There is clear evidence that chemical messengers modulating cellular activity are produced in the mitochondria (Duchen, 2000), as shown during glucose-stimulated insulin secretion in the pancreatic β-cells (Maechler & Wollheim, 2000). Signalling pathways originating in the mitochondria that would appear compatible with the described observations are those involving a decrease in GTP level or production of reactive oxygen species (ROS). However, preliminary results with high [GTP] in solutions do not support a GTP-dependent pathway and the depression of twitch responses with exogenously produced ROS (Posterino et al. 2003) does not show reversibility as observed in this study.
In the intact mammalian skeletal muscle, metabolic stress would be expected to induce depolarisation of the t-system, which, in turn, would result in reduced force response as observed with rat diaphragm muscle preparations (Mainwood et al. 1982), thus reducing the rate of ATP utilisation and protecting the muscle cell from irreversible damage.
Finally, results reported in this study offer a direct explanation for the many observations of plasma membrane depolarisation by up to 40 mV during metabolic stress in various tissues such as cardiac muscle (Hasin & Barry, 1984), neurons (Buckler & Vaughan-Jones, 1998; Nowicky & Duchen, 1998), glial cells (Brismar & Collins, 1993) and endothelium (Park et al. 2002).
Acknowledgments
This work was supported by grants from Team Denmark, the Danish Sports Research Council and the National Health and Medical Research Council of Australia.
REFERENCES
- Brismar T, Collins VP. Effect of external cation concentration and metabolic inhibitors on membrane potential of human glial cells. J Physiol. 1993;460:365–383. doi: 10.1113/jphysiol.1993.sp019476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ, Vaughan-Jones RD. Effects of mitochondrial uncouplers on intracellular calcium, pH and membrane potential in rat carotid body type I cells. J Physiol. 1998;513:819–833. doi: 10.1111/j.1469-7793.1998.819ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JH, Balasubramanyam M, Chernaya G, Gardner JP, Aviv A, Reeves JP. Oligomycin inhibits store-operated channels by a mechanism independent of its effects on mitochondrial ATP. Biochem J. 1997;324:971–980. doi: 10.1042/bj3240971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins A, Larson M. Differential sensitivity of inward rectifier K+ channels to metabolic inhibitors. J Biol Chem. 2002;39:35815–35818. doi: 10.1074/jbc.M206032200. [DOI] [PubMed] [Google Scholar]
- Davey DF, Wong SY. Morphometric analysis of rat extensor digitorum longus and soleus muscles. Aust J Exp Biol Med Sci. 1980;58:213–230. doi: 10.1038/icb.1980.22. [DOI] [PubMed] [Google Scholar]
- Duchen MR. Mitochondria and calcium: from cell signalling to cell death. J Physiol. 2000;529:57–68. doi: 10.1111/j.1469-7793.2000.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg BR, Kuda AM. Discrimination between fiber populations in mammalian skeletal muscle by using ultrastructural parameters. J Ultrastruct Res. 1976;54:76–88. doi: 10.1016/s0022-5320(76)80010-x. [DOI] [PubMed] [Google Scholar]
- Fink RH, Stephenson DG, Williams DA. Potassium and ionic strength effects on the isometric force of skinned twitch muscle fibres of the rat and toad. J Physiol. 1986;370:317–337. doi: 10.1113/jphysiol.1986.sp015937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage PW, Lamb GD, Wakefield BT. Transient and persistent sodium currents in normal and denervated mammalian skeletal muscle. J Physiol. 1989;418:427–439. doi: 10.1113/jphysiol.1989.sp017850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin Y, Barry WH. Myocardial metabolic inhibition and membrane potential, contraction, and potassium uptake. Am J Physiol. 1984;247:H322–329. doi: 10.1152/ajpheart.1984.247.2.H322. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, Horowicz P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junankar PR, Dulhunty AF, Curtis SM, Pace SM, Thinnes FP. Porin-type 1 proteins in sarcoplasmic reticulum and plasmalemma of striated muscle fibres. J Muscle Res Cell Motil. 1995;16:595–610. doi: 10.1007/BF00130241. [DOI] [PubMed] [Google Scholar]
- Lamb GD, Junankar PR, Stephenson DG. Raised intracellular [Ca2+] abolishes excitation-contraction coupling in skeletal muscle fibres of rat and toad. J Physiol. 1995;489:349–62. doi: 10.1113/jphysiol.1995.sp021056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD, Stephenson DG. Effects of intracellular pH and [Mg2+] on excitation-contraction coupling in skeletal muscle fibres of the rat. J Physiol. 1994;478:331–339. doi: 10.1113/jphysiol.1994.sp020253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren J, Bruton JD, Westerblad H. Vacuole formation in fatigued single muscle fibres from frog and mouse. J Muscle Res Cell Motil. 1999;20:19–32. doi: 10.1023/a:1005412216794. [DOI] [PubMed] [Google Scholar]
- Maechler P, Wollheim CB. Mitochondrial signals in glucose-stimulated insulin secretion in the beta cell. J Physiol. 2000;529:49–56. doi: 10.1111/j.1469-7793.2000.00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainwood GW, Alward M, Eislet B. The effects of metabolic inhibitors on the contraction of creatine-depleted muscle. Can J Physiol Pharmacol. 1982;60:114–119. doi: 10.1139/y82-019. [DOI] [PubMed] [Google Scholar]
- Mobley BA, Eisenberg BR. Sizes of components in frog skeletal muscle measured by methods of stereology. J Gen Physiol. 1975;66:31–45. doi: 10.1085/jgp.66.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisescu DG, Thieleczek R. Calcium and strontium concentration changes within skinned muscle preparations following a change in the external bathing solution. J Physiol. 1978;275:241–262. doi: 10.1113/jphysiol.1978.sp012188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicky AV, Duchen MR. Changes in [Ca2+]i and membrane currents during impaired mitochondrial metabolism in dissociated rat hippocampal neurons. J Physiol. 1998;507:131–145. doi: 10.1111/j.1469-7793.1998.131bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata T, Yamasaki Y. High-resolution scanning electron-microscopic studies on the three-dimensional structure of mitochondria and sarcoplasmic reticulum in the different twitch muscle fibers of the frog. Cell Tissue Res. 1987;250:489–497. doi: 10.1007/BF00218939. [DOI] [PubMed] [Google Scholar]
- Ogata T, Yamasaki Y. Ultra-high resolution scanning electron microscopic studies on the sarcoplasmic reticulum and mitochondria in various muscles: a review. Scanning Microsc. 1993;7:145–156. [PubMed] [Google Scholar]
- Park KS, Jo I, Pak K, Bae SW, Rhim H, Suh SH, Park J, Zhu H, So I, Kim KW. FCCP depolarizes plasma membrane potential by activating proton and Na+ currents in bovine aortic endothelial cells. Pflugers Arch. 2002;443:344–352. doi: 10.1007/s004240100703. [DOI] [PubMed] [Google Scholar]
- Posterino GS, Cellini MA, Lamb GD. Effect of oxidation and cytosolic redox conditions on excitation-contraction coupling in rat skeletal muscle. J Physiol. 2003;547:807–823. doi: 10.1113/jphysiol.2002.035204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posterino GS, Lamb GD, Stephenson DG. Twitch and tetanic force responses and longitudinal propagation of action potentials in skinned skeletal muscle fibres of the rat. J Physiol. 2000;527:131–137. doi: 10.1111/j.1469-7793.2000.t01-2-00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff RL. Effects of temperature on slow and fast inactivation of rat skeletal muscle Na+ channels. Am J Physiol. 1999;277:C937–947. doi: 10.1152/ajpcell.1999.277.5.C937. [DOI] [PubMed] [Google Scholar]
- Sipido KR, Marban E. L-type calcium channels, potassium channels, and novel nonspecific cation channels in a clonal muscle cell line derived from embryonic rat ventricle. Circ Res. 1991;69:1487–1499. doi: 10.1161/01.res.69.6.1487. [DOI] [PubMed] [Google Scholar]
- Smith RS, Ovalle WK., Jr Varieties of fast and slow extrafusal muscle fibres in amphibian hind limb muscles. J Anat. 1973;116:1–24. [PMC free article] [PubMed] [Google Scholar]
- Stephenson DG, Lamb GD, Stephenson GM. Events of the excitation-contraction-relaxation (E-C-R) cycle in fast- and slow-twitch mammalian muscle fibres relevant to muscle fatigue. Acta Physiol Scand. 1998;162:229–245. doi: 10.1046/j.1365-201X.1998.0304f.x. [DOI] [PubMed] [Google Scholar]
- Wallimann T, Turner DC, Eppenberger HM. Localization of creatine kinase isoenzymes in myofibrils. I Chicken skeletal muscle. J Cell Biol. 1977;75:297–317. doi: 10.1083/jcb.75.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]


