Abstract
Single light-harvesting complexes LH-2 from Rhodopseudomonas acidophila were immobilized on various charged surfaces under physiological conditions. Polarized light experiments showed that the complexes were situated on the surface as nearly upright cylinders. Their fluorescence lifetimes and photobleaching properties were obtained by using a confocal fluorescence microscope with picosecond time resolution. Initially all molecules fluoresced with a lifetime of 1 ± 0.2 ns, similar to the bulk value. The photobleaching of one bacteriochlorophyll molecule from the 18-member assembly caused the fluorescence to switch off completely, because of trapping of the mobile excitations by energy transfer. This process was linear in light intensity. On continued irradiation the fluorescence often reappeared, but all molecules did not show the same behavior. Some LH-2 complexes displayed a variation of their quantum yields that was attributed to photoinduced confinement of the excited states and thereby a diminution of the superradiance. Others showed much shorter lifetimes caused by excitation energy traps that are only ≈3% efficient. On repeated excitation some molecules entered a noisy state where the fluorescence switched on and off with a correlation time of ≈0.1 s. About 490 molecules were examined.
The structure of the light-harvesting complex LH-2 from Rhodopseudomonas acidophila was recently determined by x-ray diffraction (1, 2). There are 18 bacteriochlorophyll (BChl) molecules arranged symmetrically in a circular ring structure supported by nine αβ-dipeptides. The principal electronic absorption of these BChls is at 850 nm (B850). The assembly contains another ring of nine BChl a molecules which absorb at 800 nm (B800). The spectroscopy of LH-2 and the energy transfer rates between B800–B800, B800–B850, and B850–B850 have been studied extensively (3–13). Energy can flow from B800 to B850 in about 650 fs at room temperature (13). In the absence of traps or other antenna complexes this excitation leads to emission at longer wavelength (>870 nm). Here we examine the photochemical properties of individual LH-2 units.
Recent exciting developments that couple various forms of optical spectroscopy with microscopy (14–20) have made possible heretofore unprecedented visualization of processes involving single biological molecules or assemblies (21–25). Time-resolved detection has also been incorporated into these approaches (15–18), thereby enabling studies of the dynamics of molecular processes at the single-assembly level. Fluorescence lifetimes and spectra obtained from the repeated excitation of a single molecule have been reported and characterized for a number of fluorescent dyes and for a few molecules of biological importance (15–18, 24–26). Such single-molecule studies can yield knowledge that is not obtainable from investigations of macroscopic systems (27, 28).
Light-harvesting complexes are protected from photooxidation by carotenoids mostly through direct quenching of the triplet BChl and, to a lesser extent, through quenching the singlet oxygen (29). These processes affect the entire light-harvesting apparatus. The exciton coupling between pigments in B850 is large enough to cause significant delocalization (30–34). The 18 B850 chromophores form an exciton band that derives from coupling between nine dimeric units (1, 2). The nine BChls in the B800 band are much further apart and exhibit more localized excitations (2, 13, 31). Because there is rapid excitation transfer or delocalization throughout the 18 cofactors, the photodamage of individual BChls can have a significant effect on the luminescence properties of the whole assembly. Our study shows that this is indeed the case.
METHODS
Sample Preparation, Immobilization, and Imaging.
Isolated LH-2 assemblies were first immobilized on mica plates with thicknesses less than 100 μm. Mica is optically transparent, slightly negatively charged, and hydrophobic. For each experiment freshly cleaved mica formed the base of a solution cell with a volume of ≈200 μl. The assemblies were also immobilized on HF-treated microscope cover glasses.
The sample was prepared from a stock solution of 0.2 μM LH-2 (Rps. acidophila strain 10050) in buffer [50 mM Tris⋅HCl, pH 7.8/0.1% lauryldimethylamine oxide (LDAO)]. This solution was diluted with buffer by a factor of 105 in four steps. Seven minutes after addition of 50 μl of the 2 × 10−12 M solution to the cell, the sample was washed five times by 150-μl aliquots of buffer and transferred to the microscope. To keep the sample immersed during the whole experiment, 150 μl of buffer was added. All manipulations were performed in the dark. These same procedures were also carried out using a buffer of 50 mM potassium phosphate, pH 7.7/0.8% n-octyl β-d-glucopyranoside (OGP).
The imaging was carried out by positioning the sample in a confocal microscope (26) with the illumination source at λ = 800 nm (850 nm in a few experiments) from a dye-laser synchronously pumped at 6.33 MHz. The diffraction-limited spot diameter was 700 nm. The scanning area is 7.2 × 7.2 μm2. Dichroic mirrors and color glass filters rejected the exciting light.
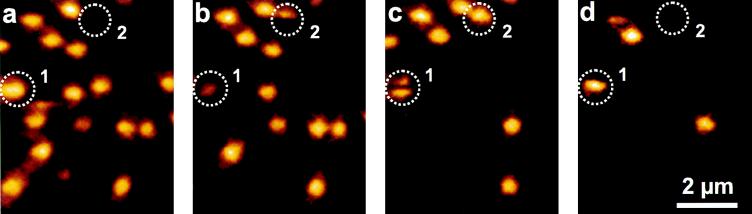
To prove that the LH-2 assemblies were immobilized on the mica, the same sample section was scanned several times. The first, fifth, ninth, and twelfth images from a typical series of scans are shown in Fig. 1. The integration time per pixel was 4 ms and the total counts per molecule in one image ranged from 1,000 to 1,400. The excitation power was 290 nW at λ = 800 nm. The assemblies remain immobilized until they are finally destroyed by photobleaching. The concentrations in this demonstration were chosen 3 times higher than those used for the single-molecule measurements. At 2 × 10−12 M LH-2 there were usually about five molecules per image. Care was taken to reduce the background signal by using thin clean mica and pure buffer and by minimizing the scattered light. At 100–200 W/cm2 the background was reduced to 20 counts per s, mainly limited by the dark counts of the detector of ≈15 counts per s. At this intensity the detected fluorescence count rate from a single molecule was 1,000–2,000 counts per s.
Figure 1.
Examples from a series of fluorescence images of single LH-2 assemblies. The image size is 102 × 128 pixels and the integration time per pixel was 4 ms. The excitation power was 290 nW at λ = 800 nm. The molecule labeled 1 is an A state in a. It goes to a B′ state in b and it recovers to an A′ state in c. During the scanning of this image, a B state was formed for ≈0.5 s before recovering to the A′ state. In d a P state is reached. The circle labeled 2 is one of the rare cases where a molecule suddenly appears. Probably it was bleached to a B state during the sample preparation.
Photobleaching and Fluorescence Lifetimes.
To record a typical photobleaching response a 25-μm2 image was scanned with low excitation power at a fast scan rate of 4 Hz per line (1 ms per pixel). The light was then blocked and the scanner was moved to a position where a molecule was now known to be located. The emission of this molecule was then recorded during constant illumination. The data for the time-resolved fluorescence decay were collected through another channel at a rate of 1 Hz with a PCA II multichannel analyzer (Nucleus, Oak Ridge, TN). These data were binned according to the different regions in the photobleaching curve, and the fluorescence lifetime for each region was obtained by standard techniques (35). After complete photobleaching of the first molecule, the same procedure was then applied to the remaining molecules in this image without ever scanning the whole image again. A sample could not be used for more than 1.5 hr, after which the fluorescence was irreversibly lost. A total of ≈490 molecules were studied. Fluorescence emission polarization experiments were carried with a variable polarizer in front of the detector or by rotating the polarization of the exciting laser beam.
RESULTS AND ANALYSIS
All except ≈10% of the 490 molecules studied gave similar fluorescent signals. These few that showed anomalously large signals were discarded and attributed to aggregates. The following results used 800 nm, but 850-nm excitation showed the same bleaching patterns.
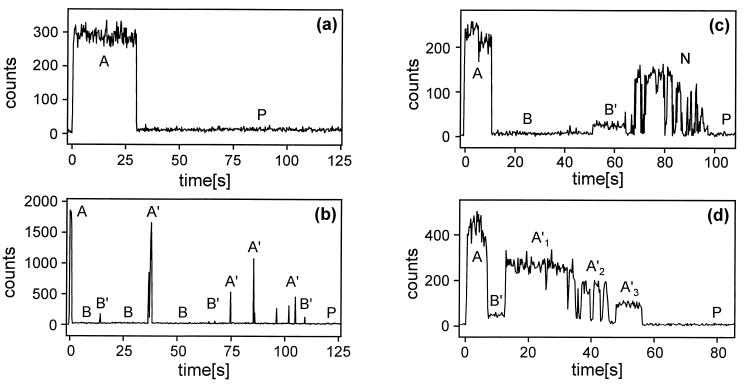
Most molecules (64%) went from an initial bright (fluorescent) state to a dark (nonfluorescent) state, from which they recovered after up to several tens of seconds. This second bright state usually showed different properties from the first one, and several other steps of dark and bright states often followed. The remaining 36% of the molecules exhibited the transition to the dark state and no further recovery. Examples for the various types of photobleaching curves are shown in Fig. 2. The different states in the bleaching curves are categorized and discussed in the following presentation of the results.
Figure 2.
Examples of bleaching curves of single LH-2 assemblies with a binning time of 0.25 s. The excitation power at λ = 800 nm was 0.5 μW, 3.7 μW, 0.3 μW, and 0.7 μW for a, b, c, and d, respectively. The state labels are discussed in the text. The corresponding lifetime measurements of the different states in d are given in Fig. 3.
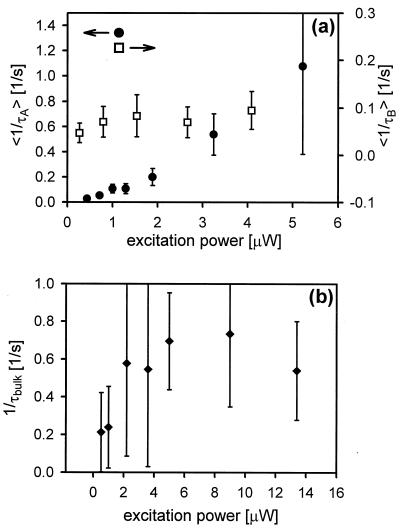
First we discuss the one-step bleaching of the initial bright state, labeled A, into a completely dark state, labeled B. A sudden switching off of the relatively constant A state fluorescence was observed for ≈90% of the molecules. This is proposed to be a result of photobleaching of one BChl molecule in the assembly. The total time in state A was measured for each of these molecules and plotted into histograms for different excitation intensities. The incident intensity was determined by the average number of counts measured in state A. The longevity of this initial state becomes shorter with increasing excitation intensity. A plot of the mean of the inverse of the lifetime as a function of the excitation power in a range from 0.5 to 5 μW suggests a linear dependency of this first bleaching time on the excitation power (Fig. 4a). For higher power levels (>5 μW) this method is difficult to apply because the survival time in the state A gets considerably shorter than 1 s. Measurements were also carried out with about 100 LH-2 assemblies in the focal spot (Fig. 4b). At low power the time constant is consistent with the statistical result from single LH-2 assemblies. However, for higher intensities (>6 μW) the bleaching rate saturates, becoming less dependent on the light intensity. This “bulk” measurement detects not only those molecules that go from an A state to the B state but also those that resume emitting photons, as described below. This result shows clearly that the interstate dynamics can be observed only through the study of single molecules.
Figure 4.
Laser power dependence of bleaching. (a) The mean of the inverse of the survival time in state A (〈1/τA〉; •) and the mean of the inverse of the time between state A and the first reemitting state (〈1/τB〉; □) are plotted against the excitation power at λ = 800 nm. The increase in 〈1/τA〉 is almost linear with the excitation power, whereas 〈1/τB〉 is almost independent of it. These results are based on the analysis of 201 and 136 single-molecule measurements for 〈1/τA〉 and 〈1/τB〉, respectively. (b) “Bulk” measurements on ≈100 molecules were fitted by a double exponential. Only the fast time constant (90%) is plotted against the excitation power. The error bars correspond to a confidence level of 95%.
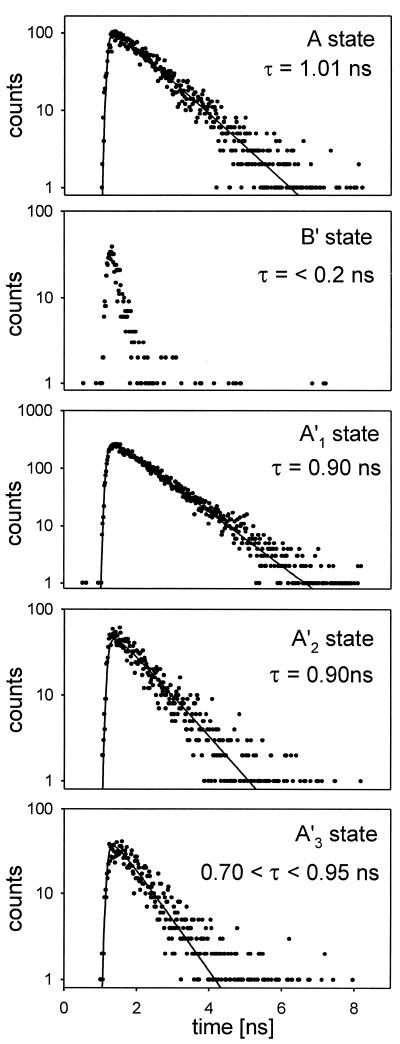
The lifetimes of the A states were all in the range 1.0 ± 0.2 ns (Fig. 3), in excellent agreement with bulk measurements of the fluorescence lifetime. The narrow distribution indicates that the sample is relatively homogeneous. After the disappearance of state A by photobleaching, the LH-2 assembly must continue to absorb photons, but its fluorescence is quenched beyond our detection limit. Traps are created that quench all fluorescence regardless of the spatial distribution of the excitations.
Figure 3.
Lifetime measurements on single LH-2 assemblies in the states corresponding to those in Fig. 2d.
We investigated the properties of the first dark state B by measuring the excitation intensity dependence of the mean time between the photobleaching of the A state and its first recovery into a bright state (see Fig. 4a). The survival time of the first B state is essentially independent of the light intensity, with a mean value of 15 s and a standard deviation of 19 s. The recovery of this state is not dominantly light induced. In another experiment, the excitation light was blocked for 90 s immediately after appearance of the B state. After re-illumination, most of the molecules showed no fluorescence. However, about one-third of them did begin emitting after prolonged irradiation. This indicates that even without irradiation, the B states can undergo chemistries which lead to the permanent disappearance of the fluorescence signal. Clearly, certain steps in the recovery to a fluorescent state are light assisted.
In 64% of all cases, exemplified by the photobleaching curves of Fig. 2 b–d, emitting states reappeared before a “permanent” dark state, designated as P, was achieved. These regenerated fluorescent states are labeled A′, B′, and N (bright, dark, and noisy, respectively) representing the three major fluorescence patterns.
In these A′ states, 20–100% of the initial A state counts and a fluorescence lifetime close to that of the A state were observed. This A′ state was found for 76% of all molecules that exhibited recovery. A set of data obtained from a molecule that exhibited this behavior is shown in Fig. 2d. There are four bright states with the following relative (to A) number of counts/fluorescence lifetimes: A, 1.0/1.0 ns; A′1, 0.6/0.9 ns; A′2, 0.41/0.9 ns; and A′3, 0.21/0.8 ns. These extremely interesting states whose fluorescence yield appears to vary without much change in lifetime will be discussed more fully below. In most cases the A′ states that were formed after a B state exhibited “blinking” (e.g., A′ → B → A′ → or A′ → B′ → A′ →) with up to 10 cycles. The A′ states also evolved from B′ states.
Approximately 30% of the measurements yielded a different type of recovered state having a count rate reduced from A state emission by a factor of more than 6, and a fluorescence lifetime somewhat shorter than our resolution of 200 ps. The luminescence was not fully quenched as in B states, but the lifetime was nevertheless significantly reduced. These are evidently strongly quenched states, so they are referred to as B′. The lifetimes of the B′ states were shorter than the instrument function, but they could be estimated from the signal strengths in the time-correlated single-photon counting experiments to be in the range of 20–50 ps. Most of the B′ states were formed from a B state, but some evolved directly from A states. About half of the B′ states lead to B or P states, and the other half generated A′ states. When the illumination of a B′ molecule was interrupted for 90 s it mostly reappeared in a B′ state, indicating that light is needed to converts B′ to other states.
Some regenerated bright states exhibited a fast switching between bright and dark states. This noisy state, N, was seen in only a few cases (18%), one of which is shown in Fig. 2c. The mean fluorescence decay time of the N state was found to be similar to that of the A state. The autocorrelation function of the signal in the N state fits a single-exponential decay with correlation time of ≈0.1–0.6 s. This time constant should be viewed as the correlation time of the fluctuations between the dark and bright states.
To reveal possible protein motions that influence the fluorescence, we excited the LH-2 assemblies at a very low laser power (50 nW) to obtain trajectories up to 400 s, which were analyzed by autocorrelation. There were no decay time constants between 4 ms and 4 s. However, the signal shows a very slow variation on the time scale of tens of seconds. A control measurement ruled out that this was due to drift of our instrument. We also searched for the effects of spectral jumps in the emitted light (25, 36, 37). A narrow band-pass filter (Chroma Technology, 10-nm bandwidth, centered at λ = 885 nm) was placed in front of the detector. The trajectories obtained in this way were again analyzed with autocorrelation, but no significant decay was found.
Several polarization-sensitive measurements were performed to obtain information about the orientation of LH-2 assemblies. In a first set the emitted fluorescence was analyzed with a polarizer in front of the detector. At a fixed excitation polarization several images were recorded by alternatively flipping the emission polarizer 90° after each image. No large systematic changes in the count rates from molecules in the two orientations were observed, suggesting that the emission is approximately isotropic. This finding was confirmed by switching the polarization of the exciting laser beam and detecting the total fluorescence emission. From measurements of the count rates for 40 molecules at the two orientations, we found an average polarization ratio of 0.99. The data also showed that there was an equal chance of either polarization being the largest. The variance of this series of measurements was found to be 0.27. When 5 molecules with anomalous ratios were not included in the calculation, the variance was reduced to 0.16. When a number of molecules were viewed in an image they all exhibited similar signals, independent of the choice of polarization.
To discover whether the buffer influences the fluorescence properties of the LH-2 assemblies, we carried out some control measurements. When the Tris buffer and detergent were changed to potassium phosphate and n-octyl glucopyranoside, respectively, measurements on single LH-2 assemblies showed the same bleaching patterns as we reported above for Tris and lauryldimethylamine oxide buffer on mica. We also replaced the mica, which is slightly negatively charged, with an HF-treated thin glass plate. Although the LH-2 assemblies themselves did not stick as well on this surface, we obtained enough data to establish that the bleaching behavior was the same as with the mica surface.
In summary, the light-induced destruction of individual LH-2 assemblies involves a complex sequence of events. All the molecules showed the initial bright state A and the final dark state P, but there were many different intermediate bright and dark states that we referred to as A′, B, B′, and N. Some molecules showed only one intermediate state, whereas others had several or all of them. The periods of time spent in the A and A′ states were sometimes comparable, but they could be quite different. The recovery time of the B states exhibited a large variance and no apparent regularity. The first transition from an A to a B state initiated a progression that inevitably generated a P state in less than 3 min. If the excitation light was blocked while the system was in an A state, this state could still be found up to 30 min later. However, if the sample was left on the mica plate in the dark for times in excess of 1–1.5 hr, hardly any molecule could be detected in the focal spot. Thus there are also nonphotochemical destruction processes occurring.
DISCUSSION
When the LH-2 assembly absorbs a photon at 800 nm the B800 rings are excited, but they transfer their excitation in 0.65 ps to the B850 ring, where they have a lifetime of 1 ns. Thus the probability of photobleaching one of the nine B800 cofactor molecules is about 103 times less than that of a B850 cofactor. Therefore most of the observed dynamics occurs in the B850 ring. An excitation has equal probabilities of being located anywhere in the B850 ring after about 250 fs (13). Therefore, regardless of what mechanism transports the excitation around the ring, we will expect that repeated excitations of a single assembly eventually will sample all the B850 molecules with equal probability during our measurements.
Bleaching was not influenced by either the buffer or the type of surface on which the assemblies were immobilized, so we seek explanations for it from properties of the immobilized LH-2 on the surface immersed in buffer. Although the BChl molecules of B850 have identical chances of being excited on the time scale of our measurements, different cofactors may undergo different photochemistry. The assembly consists of nine αβ-dipeptides, but the α and β BChls are not equivalent. The α and β chains both have hydrophobic groups near the BChls, but the histidine that binds the BChl is near two phenylalanines in the β chain, whereas there are no aromatic residues nearby in the α chain. This makes the possibilities for photoreactions such as electron transfer very different for the BChls in the α and β chains. The intrinsic asymmetry introduces the possibility for at least two distinct types of photobleaching, and there could well be others if each of the BChl molecules in the assembly has a number of pathways for photobleaching.
Polarization of Excitation and Fluorescence.
The LH-2 assemblies can be viewed as cylinders with the radiative transition dipoles of each BChl a molecule arranged in a circle with its plane perpendicular to the axis of symmetry. If the cylinders were upright on the mica surface, with their circular absorption and emission oscillator plane parallel to the surface, there should be no polarization preference when the excitation beam is directed perpendicular to the surface of the mica and the absorption should be isotropic. Because of the subpicosecond energy transfer around the ring of BChls (13) the emission for this geometry should also be isotropic. The observed fluorescence signals suggest that the sample consists of approximately upright cylinders. We can estimate their orientation from the data. The polarization ratio obtained by varying the excitation polarization and detecting all the emission, is given by (1 − sin2θ cos2φ)/(1 − sin2θ sin2φ), where θ and φ give the orientation of the cylinder axis in the laboratory frame with the polar z-axis normal to the plane of the mica sheet. The mean of many measurements should yield unity, and a value of 0.99 was observed. The variance in the polarization ratio provides an estimate of θ. A numerical simulation showed that the observed variance corresponds to an angle θ of 20–30°. This assumed that the cylinder axes all have similar θ angles and that there is no rapid rotational averaging.
Trapping.
The loss of fluorescence is presumed to be the result of trapping of the excitation by photobleached regions of the assembly. Trapping of excitations in molecular aggregates (38, 39) occurs when an impurity has excited states at lower energy than the aggregate state. It may involve any of the common energy transfer mechanisms. For LH-2 the 0.9-nm separation between adjacent BChls suggests a Förster mechanism will be important in nonresonant trapping. Charge carrier generation, where an excitation forms an electron and a hole, can also occur. For low-energy excitations, electron transfer to an acceptor should also be considered. In a crystal this would be the unavoidable impurity such as a quinone generated by oxidation, but in the LH-2 it could be the peptide constituents. The bleaching then produces a BChl radical cation which has an optical absorption band near 925 nm (40, 41) and could be a trap for excitations. The calculated Förster energy transfer rate is 1012 s−1. Once created, the positive hole would migrate around the assembly. The tendency of the positive charge to delocalize is counteracted by self-trapping, which creates a distortion of the structure, again in analogy with molecular crystals (39). There is also the possibility of oxidizing an amino acid residue. Atom or ion elimination and even the disruption of the LH-2 assembly are also possible contributors to the photobleaching. The corresponding charge or atom recombination reactions could occur to regenerate fluorescent assemblies. During a typical trajectory of repeated excitations, the BChls in the single-molecule experiments are each electronically excited on average about 106 times before photobleaching. Thus the processes responsible for photobleaching are extremely slow and have extremely small efficiencies in bulk materials.
The high probability of occurrence of one-step bleaching A → B can be understood as generation of a trap for electronic excitations by photobleaching. If a trap were formed, the very efficient and fast delocalization of the excitons would ensure that it could be accessed by the excitations formed in subsequent light absorption by the ring. The inverse lifetime of the A state depends linearly on the light intensity when B is the product, suggesting that the A → B bleaching involves just one trap.
Traps are also created as a result of interactions occurring among singlet and triplet excitations. The fluorescence yield is 10% (32), and the triplet yield is ≈2% to 15% (42). The assembly is excited about once per 3 μs with the source at 1 μW. Thus there is a negligible probability for two singlet excitations being present in the ring at the same time. The triplet lifetime of BChl a is known to be 10–20 μs in the absence of carotenoids (43). The triplet lifetime of BChl a is reduced to 20–30 ns in the presence of the carotenoids (29), so the probability of a double triplet excitation of BChl a being created in the ring is also negligible. However, the carotenoid triplet state has a lifetime of 3–15 μs (44) and is known to be a very efficient trap for singlet excitations in the bacterial LH-1 and LH-2 complexes (13, 45, 46). Therefore at our excitation rate 0.7% to 12% of the singlet excitations created in the experiment will be rapidly quenched by triplet carotenoid and will not contribute to the fluorescence signal. These dynamics were not seen because of the relatively low total count rates. However, the total number of counts is consistent with this analysis: at 1 μW incident power, a collection efficiency of ≈7%, and a fluorescence yield reduced to 6%, we expect a count rate of 1,250 s−1, whereas 2,000 s−1 was observed. A large proportion of the heat energy from these internal nonradiative processes is deposited into the carotenoid by repeated triplet–triplet and singlet–triplet transfer, suggesting a likely source of thermal damage of the carotenoid. The removal of the carotenoid will lengthen the LH-2 triplet lifetime and leave the assembly unprotected from oxygen (29).
The bleaching process A → B′ yields a partially quenched state of LH-2. Incomplete quenching requires a photochemical product having a low probability of trapping the excitations, which must nevertheless be able to recover to yield bright A′ type states. The B′ states may be generated at many stages in the trajectory. An analysis of the fluorescence decay signal amplitudes for B′ (see Fig. 3) suggests that the lifetime of the LH-2 excitation is reduced to about 20–50 ps. According to the energy delocalization parameters of neat LH-2 there should be about 80–200 visits to a trap during this period. Therefore there must be some barrier associated with trapping in this case.
Fluorescence Lifetimes and Quantum Yields in the A′ States.
In a given trajectory the bright A′ states have similar lifetimes, yet they can exhibit widely different signals. These intensity variations are suggested to be the result of fluorescence quantum efficiency changes. Nonradiative pathways dominate the fluorescence rate coefficient, so variations in the radiative rates are needed to alter the yield without causing large changes in the lifetime. For example, if the nonradiative yield is 90%, a fluorescence yield reduction by a factor of 2 requires a fluorescence lifetime change of only 5%. In LH-2 the inverse of fluorescence lifetime is 2–3 times larger than in BChl as a result of exciton delocalization (32). Therefore partial localization of the excitations by the destruction of the ring structure is expected to cause a diminution of the count rate by up to a factor of 3. The observed changes of the count rate in the A′ states are consistent with this interpretation, which implies that we are observing superradiant states of single molecules (the A states): the superradiance is diminished in the A′ states that have reduced yields. Superradiance arises when the BChls in the LH-2 ring all emit with a definite phase relation such as would occur if the emitting state were a superposition of the individual BChl states. The constructive interference enhances the transition moment over that expected for 18 uncoupled monomers. This type of behavior is well known in other systems (47, 48). The destruction of the superradiance leading to lower yield states with similar lifetimes would be caused by structural deformations that localize the excitations. In other instances the recovery process generated A′ states with the same count rate as the A state. These may be cases of charge recombination or other type of repair process. The polarization measurements make it unlikely that tilting of the transition dipole plane by light-induced tumbling of the whole assemblies or large structural displacements of the immobilized protein is the cause of the intensity variations in these A′ states.
SUMMARY
Single light-harvesting complexes LH-2 from Rps. acidophila were immobilized on various surfaces under physiological conditions, and about 490 of them were examined for their fluorescence lifetime and photobleaching properties by using a confocal fluorescence microscope with picosecond time resolution. The planes of the circular absorbers and the mica plate were within ≈20–30°. The photocharacteristics were independent of the immobilization procedure and the types of buffer and detergent. The photobleaching of one BChl molecule from the 18-member assembly causes the fluorescence to switch off completely because of trapping of the delocalized excitations. This process is linear in light intensity. On continued irradiation the fluorescence often reappears with a range of characteristics. Some LH-2 complexes show states having a range of radiative rates, attributed to localization of the excitations and diminution of the superradiance. Others show much shorter lifetimes caused by excitation energy traps that are only ≈3% efficient. On repeated excitation some molecules enter a noisy state where the fluorescence switches on and off with a correlation time of ≈0.1 s.
Acknowledgments
Thanks are due to G. J. Small for his help in the early stages of this work. This research was supported by the National Institutes of Health and the National Science Foundation, with instrumentation developed under National Institutes of Health Grant RR03148 (R.M.H.); by the Biotechnology and Biological Sciences Research Council, U.K. (R.J.C); and by the Swiss National Science Foundation and the Freiwillige Akademische Gesellschaft, Basel (M.A.B).
ABBREVIATION
- BChl
bacteriochlorophyll
Note added in Proof
After submission of this paper a fluorescence study of single, conjugated polymers was published (49) which shows some effects similar to those reported here.
References
- 1.McDermott G, Prince S M, Freer A A, Hawthornthwaite-Lawless A M, Papiz M Z, Cogdell R J, Isaacs N W. Nature (London) 1995;374:517–521. doi: 10.1016/s0969-2126(96)00050-0. [DOI] [PubMed] [Google Scholar]
- 2.Freer A A, Prince S M, Sauer K, Papiz M, Hawthornthwaite-Lawless A, McDermott G, Cogdell R J, Isaacs N W. Structure. 1996;4:449–462. doi: 10.1016/s0969-2126(96)00050-0. [DOI] [PubMed] [Google Scholar]
- 3.Shreve A P, Trautman J K, Frank H A, Owens T G, Albrecht A C. Biochim Biophys Acta. 1991;1058:280–288. doi: 10.1016/s0005-2728(05)80248-8. [DOI] [PubMed] [Google Scholar]
- 4.Hess S, Feldchtein F, Babin A, Nurgaleev I, Pullerits T, Sergeev A, Sundström V. Chem Phys Lett. 1993;216:247–257. [Google Scholar]
- 5.Trautman J K, Shreve A P, Violette C A, Frank H A, Owens T G, Albrecht A C. Proc Natl Acad Sci USA. 1990;87:215–219. doi: 10.1073/pnas.87.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monshouwer R, de Zarate I O, van Mourik F, van Grondelle R. Chem Phys Lett. 1995;246:341–346. [Google Scholar]
- 7.Joo T, Jia Y, Yu J, Jonas D M, Fleming G R. J Phys Chem. 1996;100:2399–2409. [Google Scholar]
- 8.Chachisvilis M, Pullerits T, Jones M R, Hunter C N, Sundström V. Chem Phys Lett. 1994;224:345–354. [Google Scholar]
- 9.Kramer H J M, van Grondelle R, Hunter C N, Westerhuis W H J, Amesz J. Biochim Biophys Acta. 1984;765:156–165. [Google Scholar]
- 10.De Caro C, Visschers R W, van Grondelle R, Völker S J. Chem Phys Lett. 1994;98:10584–10590. [Google Scholar]
- 11.Bergström H, Sundström V, van Grondelle R, Gillbro T, Cogdell R J. Biochim Biophys Acta. 1988;936:90–98. [Google Scholar]
- 12.Reddy N R S, Small G J, Seibert M, Picorel R. Chem Phys Lett. 1991;181:391–399. [Google Scholar]
- 13.Jimenez R, Dikshit S N, Bradforth S E, Fleming G R. J Phys Chem. 1996;100:6825–6834. [Google Scholar]
- 14.Betzig E, Chichester R. Science. 1993;262:1422–1425. doi: 10.1126/science.262.5138.1422. [DOI] [PubMed] [Google Scholar]
- 15.Ambrose W P, Goodwin P M, Martin J C, Keller R A. Science. 1994;265:364–367. doi: 10.1126/science.265.5170.364. [DOI] [PubMed] [Google Scholar]
- 16.Bian R X, Dunn R C, Xie X S, Leung P T. Phys Rev Lett. 1995;75:4772–4775. doi: 10.1103/PhysRevLett.75.4772. [DOI] [PubMed] [Google Scholar]
- 17.Trautman J K, Macklin J J, Brus L E, Betzig E. Nature (London) 1994;369:40–42. [Google Scholar]
- 18.Trautman J K, Macklin J J. Chem Phys. 1996;205:221–229. [Google Scholar]
- 19.Eigen M, Rigler R. Proc Natl Acad Sci USA. 1994;91:5740–5747. doi: 10.1073/pnas.91.13.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Funatsu T, Harada M, Tokunaga M, Saito K, Yanagida T. Nature (London) 1995;374:555–559. doi: 10.1038/374555a0. [DOI] [PubMed] [Google Scholar]
- 21.Dunn R C, Holtom G R, Mets L, Xie X S. J Phys Chem. 1994;98:3094–3098. [Google Scholar]
- 22.Nie S, Chiu D T, Zare R N. Science. 1995;266:1018–1021. doi: 10.1126/science.7973650. [DOI] [PubMed] [Google Scholar]
- 23.Haab B B, Mathies R A. Anal Chem. 1995;67:3253–3260. doi: 10.1021/ac00114a023. [DOI] [PubMed] [Google Scholar]
- 24.Ha T, Enderle T, Ogletree D F, Chemla D S, Selvin P R, Weiss S. Proc Natl Acad Sci USA. 1996;93:6264–6268. doi: 10.1073/pnas.93.13.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ha T, Enderle T, Chemla D S, Selvin P R, Weiss S. Phys Rev Lett. 1996;77:3979–3982. doi: 10.1103/PhysRevLett.77.3979. [DOI] [PubMed] [Google Scholar]
- 26.Jia Y, Sytnik A, Li L, Vladimirov S, Cooperman B S, Hochstrasser R M. Proc Natl Acad Sci USA. 1997;94:7932–7936. doi: 10.1073/pnas.94.15.7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, Wolynes P. Phys Rev Lett. 1995;74:4317–4320. doi: 10.1103/PhysRevLett.74.4317. [DOI] [PubMed] [Google Scholar]
- 28.Bopp M A, Meixner A J, Tarrach G, Zschokke-Gränacher I, Novotny L. Chem Phys Lett. 1996;263:721–726. [Google Scholar]
- 29.Cogdell R J, Frank H A. Biochim Biophys Acta. 1987;895:63–79. doi: 10.1016/s0304-4173(87)80008-3. [DOI] [PubMed] [Google Scholar]
- 30.Cogdell R J, Scheer H. Photochem Photobiol. 1985;42:669–678. [Google Scholar]
- 31.Sauer K, Cogdell R J, Prince S M, Freer A, Isaacs N W, Scheer H. Photochem Photobiol. 1996;64:564–576. [Google Scholar]
- 32.Monshouwer, R., Abrahamsson, M., van Mourik, F. & van Grondelle, R. (1997) J. Phys. Chem., in press.
- 33.Alden R G, Johnson E, Nagarajan V, Parson W W, Law C J, Cogdell R G. J Phys Chem. 1997;101:4667–4680. [Google Scholar]
- 34.Meier, T., Chernyak, V. & Mukamel, S. (1997) J. Phys. Chem., in press.
- 35.Holtom G. Proc SPIE Int Soc Opt Eng. 1990;1204:2–12. doi: 10.1117/12.17758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moerner W E. Acc Chem Res. 1996;29:563–571. [Google Scholar]
- 37.Lu H P, Xie X S. Nature (London) 1997;385:143–146. [Google Scholar]
- 38.Agranowich V M, Galanin M D. In: Modern Problems in Condensed Matter Science. Agranowich V M, Maradudin A A, editors. Vol. 3. Amsterdam: North–Holland; 1982. [Google Scholar]
- 39.Silinsh E, Capek V. Organic Molecular Crystals: Interaction, Localization and Transport Phenomena. New York: Am. Inst. Phys.; 1994. [Google Scholar]
- 40.Fajer J, Borg D C, Forman A, Felton R H, Dolphin D, Vegh L. Proc Natl Acad Sci USA. 1974;71:994–998. doi: 10.1073/pnas.71.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Picorel R, Lefebvre S, Gingras G. Eur J Biochem. 1994;142:305–311. doi: 10.1111/j.1432-1033.1984.tb08286.x. [DOI] [PubMed] [Google Scholar]
- 42.Cogdell R J, Hipkins M F, MacDonald W, Truscott T G. Biochim Biophys Acta. 1981;634:191–202. doi: 10.1016/0005-2728(81)90138-9. [DOI] [PubMed] [Google Scholar]
- 43.Davidson E, Cogdell R J. Biochim Biophys Acta. 1981;635:295–303. doi: 10.1016/0005-2728(81)90028-1. [DOI] [PubMed] [Google Scholar]
- 44.Angerhofer A, Bornhäuser F, Gall A, Cogdell R J. Chem Phys. 1995;194:259–274. [Google Scholar]
- 45.Monger T G, Parson W W. Biochim Biophys Acta. 1977;460:393–407. doi: 10.1016/0005-2728(77)90080-9. [DOI] [PubMed] [Google Scholar]
- 46.Bradforth S E, Jimenez R, van Mourik F, van Grondelle R, Fleming G R. J Phys Chem. 1995;99:16179–16191. [Google Scholar]
- 47.Kim Y R, Lee M, Thorne J R G, Hochstrasser R M, Zeigler J M. Chem Phys Lett. 1988;145:75–80. [Google Scholar]
- 48.Fidder H, Knoester J, Wiersma D A. J Chem Phys. 1993;98:6564–6566. [Google Scholar]
- 49.Vanden Bout D A, Yip W-T, Hu D, Fu D-K, Swager T A, Barbara P F. Science. 1997;277:1074–1077. [Google Scholar]