Abstract
Our hypothesis was that the simultaneous activation of tongue protrudor and retractor muscles (co-activation) would constrict and stiffen the pharyngeal airway more than the independent activation of tongue protrudor muscles. Upper airway stiffness was determined by injecting known volumes of air into the sealed pharyngeal airway of the anaesthetized rat while measuring nasal pressure under control (no-stimulus) and stimulus conditions (volume paired with hypoglossal (XII) nerve stimulation). Stimulation of the whole XII nerves (co-activation) or the medial XII branches (protrudor activation) effected similar increases in total pharyngeal airway stiffness. Importantly, co-activation produced volume compression (airway narrowing) at large airway volumes (P < 0.05), but had no effect on airway dimension at low airway volumes. In comparison, protrudor activation resulted in significant volume expansion (airway dilatation) at low airway volumes and airway narrowing at high airway volumes (P < 0.05). In conclusion, both co-activation and independent protrudor muscle activation increase airway stiffness. However, their effects on airway size are complex and depend on the condition of the airway at the time of activation.
Some 30 years ago Brouillette & Thach (1979) outlined a neuromuscular mechanism for stabilizing the extrathoracic airway against negative intraluminal pressures. According to this view, phasic activities in extrathoracic airway muscles such as the genioglossus and geniohyoid increase pharyngeal airway rigidity thereby preventing inspiratory airway obstruction. Since that time, considerable attention has focused on hypoglossal (XII) nerve stimulation and the potential for tongue protrudor (genioglossus) and/or retractor (hyoglossus) muscle activation to modulate upper airway resistance and collapsibility (Miki et al. 1989; Schwartz et al. 1993; Eisele et al. 1995; Oliven et al. 1996; Fuller et al. 1999). The results of this work indicate that protrudor and retractor muscle activities can profoundly influence the collapsibility and flow characteristics of the upper airway.
The effects of tongue muscle activation on pharyngeal airway mechanics were first described in the isolated upper airway of the cat (Schwartz et al. 1993). In this preparation, the mouth and/or nostril are patent and the upper airway is exposed to a rapid reduction in hypopharyngeal pressure. Using this model in the rat, Fuller et al. (1999) showed that genioglossus activation increased the maximal pharyngeal airflow but did not change the pressure at which the airway collapsed (Pcrit). Significantly, co-activation of tongue protrudor and retractor muscles (genioglossus and hyoglossus, respectively) resulted in a more negative Pcrit (i.e. indicative of reduced airway collapsibility) and a modest increase in the maximal inspiratory flow. These results suggest that activation of the tongue protrudor muscles dilates the airway, whereas co-activation of tongue protrudor and retractor muscles stiffens the pharynx and results in only modest increases in the peak inspiratory flow.
There are two previous studies that report the effects of hypoglossal nerve stimulation on the pharyngeal airway pressure-volume relationship in the isolated and sealed upper airway (Miki et al. 1992; Hida et al. 1995). The results of this work confirm that the slope of the pressure-volume relationship (i.e. the compliance of the pharyngeal airway) decreases with stimulation. However, in both studies stimulation was applied to the whole hypoglossal nerve bilaterally, thereby co-activating both tongue protrudor and retractor muscle groups. Thus, the effects of independent activation of protrudor muscles vs. co-activation of protrudor and retractor muscles were not tested. Accordingly, the purpose of the present study was to compare the effects of co-activation and independent activation of tongue protrudor muscles on the pressure-volume relationship of the pharyngeal airway. On the basis of previous observations of tongue muscle activities and pharyngeal airflow mechanics (Fuller et al. 1998, 1999), our hypothesis was that co-activation would both constrict and stiffen the pharyngeal airway. Conversely, protrudor activation would dilate the airway without changing pharyngeal airway stiffness.
METHODS
Animals and surgical procedure
All procedures adhered to the guidelines established by the Institutional Animal Care and Use Committee at the University of Arizona. Nine male Sprague-Dawley rats weighing 250–350 g were used in the experiments. All animals were anaesthetized with isoflurane in an effort to minimize spontaneous upper airway muscle activities. A subcutaneous injection of atropine sulphate (0.5 mg kg−1) was given to reduce airway secretions. No surgical procedures were performed until animals were unresponsive to a strong paw-pinch with a haemostat. Paw-pinch was also used to assess the depth of anaesthesia at regular intervals. Animals were supine with limbs secured to the operating table throughout the experiment. Prone positioning was not attempted in this protocol because of the necessity for continued access to and monitoring of the sublaryngeal catheter for purposes of clearing airway secretions before each trial. Rectal temperature was maintained at 37 °C with a thermistor connected to a servo-controlled heating pad (Haake Inc., model D1-L). At the end of the experiment animals were killed by an intravenous dose of sodium pentobarbital.
Prior to tracheotomy animals breathed humidified O2 via a nose cone connected to an isoflurane vaporizer (2–2.5 %). Following cannulation of the trachea (∼1 cm) caudal to the larynx (PE-240) the animal was mechanically ventilated (Kent Scientific) with 100 % O2 routed via an isoflurane vaporizer (1.5 %) for the duration of the experiment. Polyethylene catheters were placed in a femoral vein and artery. The femoral vein catheter (PE-50) was used to administer intravenous fluids throughout the experiment. The arterial catheter (PE-50) was connected to a pressure transducer (Coulbourn Instruments, V94–21) for blood pressure monitoring.
A second tracheotomy was made rostral to the first approximately 1 mm below the cricoid cartilage for purposes of volume injections of air. A polyethylene catheter (PE 160) connected to a 1 ml syringe was inserted into the cervical trachea and the tip advanced through the larynx (∼2 mm). A second catheter (PE 10) connected to a pressure transducer (Coulbourn Instruments, V94–21) was inserted into the nose (∼5 mm) for purposes of monitoring airway pressure (PN). The nose and mouth were sealed and the oesophagus ligated caudal to the larynx. We tested the seal of the preparation by injecting a volume of air into the isolated upper airway. The test of an acceptable seal was a plateau of pressure sustained for at least 5 s following introduction of a volume of 0.5 ml.
The vagus nerves were isolated and sectioned adjacent to the tracheotomy tube. The hypoglossal nerves were exposed with a ventral approach (Gilliam & Goldberg, 1995). Once isolated, the whole XIl nerves were placed on stainless steel bipolar hook electrodes (inter-electrode distance approximately 3 mm). The whole XII nerves then were crushed proximal to the hook electrodes to prevent retrograde current flow and to prevent spontaneous or respiratory-related tongue muscle activities. Stainless steel, fine-wire recording electrodes (0.05 mm diameter, California Fine Wire) were inserted into the bellies of the genioglossus and/or hyoglossus muscles. Electrical output from the muscles was amplified and displayed on a storage oscilloscope for purposes of monitoring of M-waves to ensure nerve stimuli were both symmetrical and supramaximal throughout the experimental protocol.
Experimental protocol
Suction was applied initially to the upper airway via the sublaryngeal catheter to clear secretions. Baseline pressure-volume measures were obtained with airway pressure at the atmospheric level, with and without nerve stimulation. Then, a volume of air (0.5 ml) was injected into the sealed airway (no-stimulus condition). Airway inflation was maintained for approximately 3 s after which the whole XII nerve (medial and lateral XII branches) was stimulated using bipolar hook electrodes (square wave pulse 0.2 ms) at 60 Hz (no-stimulus + stimulus condition). This process was repeated for 0.4, 0.3, 0.2 and 0.1 ml volumes to obtain passive and active pressure-volume curves. The procedure was repeated following sectioning of the lateral XII branches to obtain passive and active pressure-volume curves for medial XII branch stimulation.
Based on several preliminary experiments, we elected not to measure pressure changes at airway volumes below the resting airway volume. We found that the application of negative pressure via volume withdrawal resulted in blockage of the relatively narrow pressure catheters by secretions that develop in the sealed airway. Thus, when negative pressure was applied to the airway the secretions were sucked into the catheter resulting in pressure artifacts that could not be measured or interpreted with confidence. Accordingly, we decided to measure stiffness at volumes above the baseline level, where pressure artifacts were not a problem. Although this prevents us from reporting the influence of hypoglossal nerve stimulation on the pressure-volume relationship at volumes below the resting airway volume (which may indeed be clinically relevant), we were still able to obtain good stimulation-induced pressure changes over a wide range of volumes.
Nevertheless, in two preliminary experiments where pressure recordings were obtained at airway volumes below the baseline level, we found that both whole and medial XII nerve stimulation resulted in a pressure drop consistent with airway dilatation. These results generally are consistent with the observations that we report in some animals at the baseline airway volume and volumes just above the baseline level (see Fig. 1 and Fig. 2).
Figure 1. Representative raw record from one animal demonstrating volume injections of 0.5, 0.4, 0.3, 0.2 and 0.1 ml and zero volume and subsequent application of a 60 Hz stimulus to the whole hypoglossal nerves.
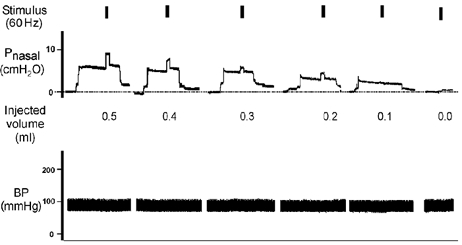
Note that prior to volume injection the airway pressure was allowed to return to atmospheric pressure. In some instances this return was immediate but in most cases several minutes elapsed before the airway pressure returned to baseline. For this reason, only that part of the pressure record that coincides with the initial volume injection and subsequent application of the stimulus are shown.
Figure 2. Representative raw record from one animal demonstrating volume injections of 0.5, 0.4, 0.3, 0.2 and 0.1 ml and zero volume and subsequent application of a 60 Hz stimulus to the medial hypoglossal branches.
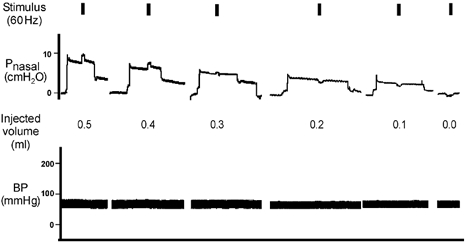
As noted in Fig. 1, airway pressure was allowed to return to atmospheric pressure prior to volume injection. Only that part of the pressure record that coincides with the initial volume injection and application of the stimulus are shown.
Data analysis and statistics
All data were acquired using Spike2 software (Cambridge Electronic Design, UK). Subsequent off-line analyses of airway pressure (PN) were performed using customized computer programs (Spike2, CED). The effects of whole XII and medial XII branch stimulation on airway compliance (whole vs. medial) were examined by multiple regression analyses with pairwise comparisons at each of the volume levels, conducted by repeated measures analysis of variance on ranks and the Student-Newman-Keuls post hoc test (P < 0.05). Differences in the mean pressure change associated with medial vs. whole nerve stimulation were determined by repeated measures analysis of variance. The significance of the pressure change with respect to the zero pressure condition was determined for each nerve at each of the volume levels by adjacent level contrasts with significance levels adjusted by the Bonferroni procedure.
RESULTS
The influence of volume injection on pharyngeal airway pressure with and without stimulation is shown for representative animals in Fig. 1 and Fig. 2. Note that pressures were atmospheric prior to volume injection, and were allowed to return to atmospheric pressure prior to subsequent injections. In these examples, volume injection from baseline always resulted in an increase in airway pressure (range 2–8 cmH2O).
Stimulation of the whole XII nerves (Fig. 1) resulted in additional increases (i.e. over and above the pressure increase associated with the initial volume injection) in upper airway pressure of between 2–5 cmH2O for volumes greater than 0.1 ml, consistent with volume compression. In this example, at the smaller injected volumes and baseline conditions, the changes in pressure associated with whole nerve stimulation were negligible.
For the medial XII branch preparation (Fig. 2), volume injection from baseline resulted in comparable increases in airway pressure as for the whole nerve preparation. However, in this example, volume injection paired with electrical stimulation produced additional increases in airway pressure only when paired with the 0.4–0.5 ml volumes. Stimulation paired with smaller volume injections i.e. < 0.4 ml, resulted in a drop in airway pressure (range 1.0–2.0 cmH2O) consistent with volume expansion.
Group pressure-volume and stiffness results without nerve stimulation and with stimulation applied to the whole XII nerve or medial XII branches are depicted in Fig. 3. Relative to the no-stimulus condition, whole XII nerve stimulation consistently increased nasal airway pressure (range 0.149–5.03 cmH2O) when the injected volume exceeded 0.1 ml (P < 0.05). Whole XII nerve stimulation paired with relatively small airway volumes (i.e. < 0.1 ml) resulted in both slight increases and slight decreases in airway pressure (range −0.17 to 0.82 cmH2O).
Figure 3. Average (+s.d.) pressure-volume and stiffness plots for the no-stimulus condition, whole XII nerve stimulation and medial XII branch stimulation as a function of the injected volume.
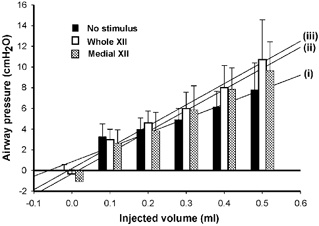
The absolute stiffness of the upper airway under each condition (represented by the slope of the pressure-volume relationship) is indicated by each of three regression lines (i, ii, iii) corresponding to: (i) no-stimulus condition (r2= 0.94), (ii) medial XII branch stimulation (r2= 0.98), and (iii) whole XII nerve stimulation (r2= 0.98). Note: there is a significant difference between the slopes of the regression lines for stimulus and no-stimulus conditions (P < 0.05).
Medial XII branch stimulation also raised airway pressures relative to the no-stimulus condition (P < 0.05) but only for injected volumes in excess of 0.3 ml, and the magnitude of the increase was less than that recorded in whole XII nerve preparations. Conversely, medial branch stimulation paired with volumes smaller than 0.3 ml typically resulted in a reduction in nasal pressure (range 0.08–1.75 cmH2O) relative to the no-stimulus condition (P < 0.05), an observation that is consistent with airway dilatation.
Total airway stiffness under each condition (represented by the slope of the pressure-volume relationship) is indicated by each of three regression lines (Fig. 3, i-iii). Accordingly, under the no-stimulus condition (i), total airway stiffness is 14.0 cmH2O ml−1 and rises to 20.5 and 20.3 cmH2O ml−1 when electrical stimulation is applied to the whole XII nerves (iii) and to the medial XII branches (ii), respectively.
Figure 4 depicts the average change (±s.d.) in airway pressure associated with co-activation or protrudor activation across the range of injected volumes. Co-activation resulted in significantly more positive changes in airway pressure relative to protrudor activation for airway volumes between 0.0 and 0.3 ml (P < 0.05). Independent protrudor activation resulted in significantly more negative airway pressures relative to the zero pressure or baseline condition for airway volumes < 0.2 ml (P < 0.05). Points falling below the zero pressure condition correspond to the airway volumes at which stimulation resulted in airway dilatation.
Figure 4. Measures of the change in airway pressure (±s.d.) associated with whole XII nerve and medial XII branch preparations in the stimulus and no-stimulus conditions across the volume range.
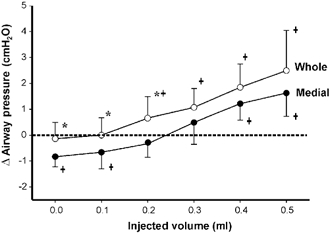
The area below zero corresponds to the airway volumes at which stimulation resulted in airway dilatation. * Significant differences between the airway pressures obtained with whole XII vs. medial XII branch stimulation (P < 0.05) at a given airway volume. †Significant differences between the zero pressure and stimulus airway conditions at a given airway volume. Note that medial branch stimulation (i.e. tongue protrudor activation) resulted in significant airway dilatation at the lowest airway volumes whereas whole nerve stimulation did not.
DISCUSSION
We examined the influence of independent tongue protrudor muscle stimulation and co-activation of protrudor and retractor muscles on the pressure-volume relation of the sealed rodent pharynx. The major finding is that independent stimulation of tongue protrudor muscles dilated the rat pharynx at low airway volumes, but constricted the airway at higher volumes. In contrast, co-activation did not change airway volume at low volumes, and constricted the airway at higher volumes. The effects of muscle stimulation on airway stiffness also were examined. We did this by measuring pharyngeal pressure over a range of airway volumes so that the pressure- volume curve could be computed, with the slope of the curve defining airway stiffness. We found that both independent protrudor muscle stimulation and co-activation resulted in comparable increases in total airway stiffness. Thus, the influence of tongue muscle contraction on pharyngeal mechanical properties is complex, and depends importantly on airway volume.
Methodological issues
As stated in the introduction, measurement of airway stiffness necessitates the isolation and sealing of the upper airway. Although this technique has been applied previously to the canine and feline airways (Miki et al. 1989; Hida et al. 1997; Kuna & Vanoye, 1999), this is the first study of its type conducted in the rodent airway. There are several features of the present preparation that are worthy of discussion.
As anticipated, the present measures of airway stiffness obtained in the rodent airway in the stimulus and no-stimulus conditions are somewhat greater than those reported in the cat with stimulation of the pharyngeal constrictors (Kuna & Vanoye, 1999). This is expected because the rat upper airway has a smaller cross-sectional area than the cat. Thus, at a given pressure the stiffness of the feline airway should be less than that observed in the smaller rodent airway. Of particular interest, however, is the discrepancy between the present measures and those reported with whole XII stimulation in the dog (Hida et al. 1995). The measures obtained in the latter study are approximately 100 times less (average values 0.15– 0.25 cmH2O ml−1) than for the rat or cat. The magnitude of the difference between the present values and those reported in the dog may be attributed to an abundance of soft tissue surrounding the oral cavity, and to its relatively large size compared to the feline and rodent pharynx.
In the present protocol we stimulated the whole XII nerve and medial XII branches at a single stimulation frequency of 60 Hz. The decision to stimulate at this frequency was based on previously published observations in the rat isolated airway preparation (Fuller et al. 1999) which demonstrated significant increases in maximal inspiratory flow, and more negative critical collapsing pressures, for stimulation frequencies of 60 Hz or higher. Importantly, rat tongue muscles exhibit good endurance properties at this frequency, while remaining below their tetanic fusion frequency of 80–90 Hz (Gilliam & Goldberg, 1995).
A limitation of the study is the lack of evaluation of the dose-dependent effects of stimulation on airway stiffness. In this study the stimulus intensity was set at a level that would ensure that muscles were activated symmetrically and consistently throughout the protocol. Effects other than increased pharyngeal airway stiffness may emerge at more physiological stimulus intensities (Rolfe et al. 1991).
Previously published data from this laboratory show that the volume of the rodent pharyngeal airway is approximately 0.05–0.1 ml, and that total upper airway volume is approximately 0.35 ml (Brennick et al. 2001). Accordingly, in the present protocol we selected volumes between 0 and 0.5 ml to encompass a range of upper airway volumes. Airway volumes < 0.3 ml were a particular focus of the study as these were considered to represent the ‘physiological’ range of airway volumes for this species.
Selective stimulation of the tongue protrudor muscles has been shown to result in tongue protrusion and depression in intact airway preparations. In the isolated and sealed airway preparation the magnitude of tongue protrusion is attenuated, although submental bulging is clearly observed secondary to tongue depression (Fregosi & Fuller, 1997). Evidence of submental displacement with medial XII branch stimulation is of particular significance to the present findings as a potential mechanism by which dilatation of the airway may be effected.
Finally, we interpreted the increase in pressure in the sealed upper airway to indicate volume compression. In an open airway preparation, stimulation of the medial XII branches results in protrusion of the tongue beyond the teeth. However, in the closed system protrusion is prevented and under these circumstances stimulation presumably results in the tongue blade ‘filling’ much of the anterior oral cavity. Volume compression might also occur as a consequence of airway shortening secondary to laryngeal displacement (i.e. elevation). Displacement of the thyroid cartilage was observed on several occasions when XII (whole or medial branch) stimulation was paired with larger injected volumes. We attribute this displacement to the co-activation of laryngeal strap muscles, e.g. geniohyoid and thyrohyoid that are innervated by branches of the hypoglossal nerve.
XII nerve stimulation changes airway dimension and stiffness
Previous models of upper airway function suggested that more negative closing pressures associated with an increase in upper airway muscle activity were the result of an increase in airway size rather than wall stiffness (Horner, 1996). According to this view nerve stimulation shifts the pressure-volume curve upward without changing the slope of the relationship. However, the results of the present study demonstrate increased airway stiffness accompanied by airway dilatation at small airway volumes (< 0.3 ml) with independent protrudor activation, and by airway narrowing, at larger airway volumes (> 0.3 ml) with protrudor activation or co-activation. Hida et al. (1995) and Kuna & Vanoye (1999) also noted volume expansion consistent with airway dilatation, with stimulation of tongue or pharyngeal constrictor muscles at relatively small airway volumes in the dog and cat. Moreover, the results of regional airway compliance studies in individuals with obstructive sleep apnoea clearly demonstrate concomitant increases in stiffness and area with electrical stimulation applied to the base of tongue (Isono et al. 1999). Collectively, these findings suggest that pharyngeal muscle activation stabilizes the upper airway via dual effects on airway geometry and wall stiffness.
Protrudor activation, co-activation and the pressure-volume relation in the isolated and sealed pharyngeal airway
This is the first study of its type to examine the effects of tongue protrudor activation and co-activation of protrudor and retractor muscles on the pharyngeal airway pressure-volume relationship. Selective stimulation of the hypoglossal nerve resulted in comparable increases in airway stiffness accompanied by complex changes in airway geometry. Specifically, independent protrudor activation resulted in airway dilatation at small airway volumes but in airway compression at larger airway volumes. Co-activation had a negligible effect on airway geometry at small airway volumes but compressed the airway at larger volumes. These seemingly paradoxical findings are exemplified by the data presented in Fig. 3 and Fig. 4. As shown in Fig. 3, the slopes of the pressure-volume relationship assessed across the entire volume range are the same for the two nerve stimulation protocols. However, if the effects of stimulation are assessed in terms of the change in airway pressure at a given airway volume, it is evident that the effects differ markedly at the smaller airway volumes (i.e. < 0.3 ml). Indeed, protrudor activation at these more physiological volumes resulted in a significant lowering of airway pressure not only with respect to co-activation but also with respect to the baseline volume condition (Fig. 4).
The distinction between the effects of protrudor activation and co-activation on the pressure-volume relationship of the pharyngeal airway is an important one. While stimulation applied to the whole XII nerves or medial XII branches renders comparable changes in total pharyngeal airway stiffness and thus airway collapsibility (as defined by the slope of the pressure-volume curve), stimulation applied to the whole XII nerve or the medial XII branches does not render comparable changes in airway geometry. In conclusion, the pressure-volume relationship and airway stiffness data provide separate pieces of information regarding airway mechanics. We suggest that both measures provide valuable information regarding the complex biophysical properties of the airway and the circumstances under which electrical stimulation may best be applied to the hypoglossal nerve.
Acknowledgments
The authors wish to thank the National Institutes of Health of the United States Public Health Service for supporting the studies (grant no. HL 56876).
references
- Brennick MJ, Trouard TP, Gmitro AF, Fregosi RF. MRI study of pharyngeal airway changes during stimulation of the hypoglossal nerve branches in rats. J Appl Physiol. 2001;90:1373–1384. doi: 10.1152/jappl.2001.90.4.1373. [DOI] [PubMed] [Google Scholar]
- Brouillette RT, Thach BT. A neuromuscular mechanism maintaining extrathoracic airway patency. J Appl Physiol. 1979;46:772–779. doi: 10.1152/jappl.1979.46.4.772. [DOI] [PubMed] [Google Scholar]
- Eisele DW, Schwartz AR, Hari A, Thut DC, Smith PL. The effects of selective nerve stimulation on upper airway flow mechanics. Arch Otolaryngol Head Neck Surg. 1995;121:1361–1364. doi: 10.1001/archotol.1995.01890120021004. [DOI] [PubMed] [Google Scholar]
- Fregosi RF, Fuller DD. Respiratory-related control of extrinsic tongue muscle activity. Respir Physiol. 1997;110:295–306. doi: 10.1016/s0034-5687(97)00095-9. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Mateika JH, Fregosi RF. Co-activation of tongue protrudor and retractor muscles during chemoreceptor stimulation in the rat. J Physiol. 1998;507:265–276. doi: 10.1111/j.1469-7793.1998.265bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Williams JS, Janssen PL, Fregosi RF. Effect of co-activation of tongue protrudor and retractor muscle on tongue movements and pharyngeal airflow mechanics in the rat. J Physiol. 1999;519:601–613. doi: 10.1111/j.1469-7793.1999.0601m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliam EE, Goldberg SJ. Contractile properties of tongue muscles: Effects of hypoglossal nerve and extracellular motoneuron stimulation in rat. J Neurophysiol. 1995;74:547–555. doi: 10.1152/jn.1995.74.2.547. [DOI] [PubMed] [Google Scholar]
- Hida W, Hajime K, Shinichi O, Kikuchi Y, Midorikawa J, Chung Y, Takishima T, Shirato K. Hypoglossal nerve stimulation affects the pressure-volume behavior of the upper airway. Am J Respir Crit Care Med. 1995;151:455–460. doi: 10.1164/ajrccm.151.2.7842206. [DOI] [PubMed] [Google Scholar]
- Horner RL. Motor control of the pharyngeal musculature and implications for the pathogenesis of obstructive sleep apnea. Sleep. 1996;19:827–853. doi: 10.1093/sleep/19.10.827. [DOI] [PubMed] [Google Scholar]
- Isono S, Tanaka A, Nishino T. Effects of tongue electrical stimulation on pharyngeal mechanics in anesthetized patients with obstructive sleep apnoea. Eur Respir J. 1999;14:1258–1265. doi: 10.1183/09031936.99.14612589. [DOI] [PubMed] [Google Scholar]
- Kuna ST, Vanoye CR. Mechanical effects of pharyngeal constrictor activation on pharyngeal airway function. J Appl Physiol. 1999;86:411–417. doi: 10.1152/jappl.1999.86.1.411. [DOI] [PubMed] [Google Scholar]
- Miki H, Hida W, Kikuchi Y, Chonan T, Satoh M, Iwase N, Takishima T. Effects of pharyngeal lubrication on the opening of obstructed upper airway. J Appl Physiol. 1992;72:2311–2316. doi: 10.1152/jappl.1992.72.6.2311. [DOI] [PubMed] [Google Scholar]
- Miki H, Hida W, Shindoh C, Kikuchi Y, Chonan T, Taguchi O, Inoue H, Takishima T. Effects of electrical stimulation of the genioglossus on upper airway resistance in anesthetized dogs. Am Rev Respir Dis. 1989;140:1279–1284. doi: 10.1164/ajrccm/140.5.1279. [DOI] [PubMed] [Google Scholar]
- Oliven A, Odeh M, Schnall RP. Improved upper airway patency elicited by electrical stimulation of the hypoglossus nerves. Respiration. 1996;63:213–216. doi: 10.1159/000196547. [DOI] [PubMed] [Google Scholar]
- Rolfe I, Olson LG, Saunders NA. Pressure-volume properties of the upper airway in man. Respir Physiol. 1991;86:15–23. doi: 10.1016/0034-5687(91)90036-i. [DOI] [PubMed] [Google Scholar]
- Schwartz AR, Thut DC, Russ B, Seelagy M, Yuan X, Brower RG, Permutt S, Wise RA, Smith PL. Effect of electrical stimulation of the hypoglossal nerve on airflow mechanics in the isolated upper airway. Am Rev Respir Dis. 1993;147:1144–1150. doi: 10.1164/ajrccm/147.5.1144. [DOI] [PubMed] [Google Scholar]


