Abstract
Patients with acute respiratory distress syndrome have increased lung tissue weight and therefore an increased hydrostatic pressure gradient down the lung. Also, they have a better arterial oxygenation in prone (face down) than in supine (face up) posture. We hypothesized that this effect of the direction of gravity also existed in healthy humans, when increased hydrostatic gradients were induced by hypergravity. Ten healthy subjects were studied in a human centrifuge while exposed to 1 or 5 G in anterio-posterior (supine) or posterio-anterior (prone) direction. We measured blood gases using remote-controlled sampling and gas exchange by mass spectrometry. Hypergravity led to marked impairments of arterial oxygenation in both postures and more so in supine posture. At 5 G, the arterial oxygen saturation was 84.6 ± 1.2 % (mean ±s.e.m.) in supine and 89.7 ± 1.4 % in prone posture (P < 0.001 for supine vs. prone). Ventilation and alveolar PO2 were increased at 5 G and did not differ between postures. The alveolar-to-arterial PO2 difference increased at 5 G to 8.0 ± 0.2 kPa and 6.6 ± 0.3 kPa in supine and prone postures (P = 0.003). Arterial oxygenation was less impaired in prone during hypergravity due to a better-preserved alveolo-arterial oxygen transport. We speculate that mammals have developed a cardiopulmonary structure that favours function with the gravitational vector in the posterio-anterior direction.
The lungs in patients with acute respiratory distress syndrome (ARDS) have increased interstitial and alveolar fluid content causing increased tissue weight and hydrostatic pressure gradient down the lung. In this ‘sponge model’ (Bone, 1993; Pelosi et al. 1994; Gattinoni et al. 2001) the lung tends to collapse under its own weight, creating severe ventilation/perfusion disturbances. However, such patients have improved arterial oxygen tension when lying prone (face down) compared with lying supine (face up) (Mure & Lindahl, 2001). The effect of prone positioning in such patients is remarkably quick (Fridrich et al. 1996), suggesting that it is not the underlying disease process that is reversed but rather the efficiency of oxygen transport in the lungs. Although this treatment strategy has been used for a long time, the mechanisms underlying the improvement of arterial oxygenation have not been determined (Mure & Lindahl, 2001). Performing well-controlled experiments with patients in an intensive care unit poses several ethical and practical problems. We reasoned that if a markedly increased hydrostatic pressure gradient was reversibly induced in healthy subjects, this might serve as a model in which responses to the direction of gravity in this condition could be studied.
We increased intrathoracic hydrostatic pressure gradients with resulting gas exchange impairment by exposing subjects to hypergravity (Barr, 1962; Glaister, 1967, 1970, 2001), and we hypothesized that the protective effect of prone posture seen in patients with lung insufficiency would be demonstrable also in healthy humans under conditions of hypergravity. If so, the beneficial effects of prone posture in patients with lung insufficiency would at least in part rely on basic structural properties of the lungs, rather than on reversal of a disease process. To test this hypothesis, we determined arterial oxygen partial pressure (PaO2) and alveolar-to-arterial PO2 differences ((A-a)DO2) in prone and supine subjects during transient hypergravity in a human centrifuge. Using this model, we demonstrate that this difference between postures also exists in healthy humans, when acute lung insufficiency is induced by hypergravity.
METHODS
Subjects
Six men and four women were studied. Their ages, heights and body masses ranged from 21 to 28 years, 163 to 191 cm and 55 to 90 kg, respectively. They had no history of cardiopulmonary disease and were not taking medications at the time. They were also instructed not to drink coffee or use nicotine-containing products on the day of the experiment. The subjects received written information about the procedure and informed verbal consent was obtained. All procedures used conformed with the Declaration of Helsinki, and the experimental protocol had been approved by the Ethics Committee of the Karolinska Institutet.
The human centrifuge
Subjects were placed on a padded support surface, which could be adjusted to be perpendicular to the resultant of the normal G vector and the centrifugal G vector. The subject was secured on the surface by a five-point safety belt. The head and torso of the subject were covered with a cowling to reduce air draft, noise and visual inputs. The rotational radius of the centrifuge was 7.2 m at the middle of the support surface. Slip rings at the centre of rotation allowed for audiovisual monitoring, power supply and transmission of physiological signals between the platform and a control room.
Equipment and measurement
The instrumentation for respiratory measurements included a quadrupole mass spectrometer (AMIS 2000, Innovision A/S, Odense, Denmark) and a pneumotachometer (type 3719, Hans Rudolph, Kansas City, MO, USA), coupled to a pressure transducer (Validyne, model CD12, Northridge, CA, USA) with its membrane mounted parallel to the plane of rotation of the centrifuge so that its function was not affected by hypergravity. The subjects breathed through a mouthpiece and wore a nose clip. Gases were sampled from the mouthpiece through a 10 m long capillary tube to the mass spectrometer located at the centre of the centrifuge. An accelerometer was positioned on the support surface perpendicular to the rotational radius and the support surface. Continuous signals were recorded at 200 Hz per channel in a digital data acquisition system (Biopac Inc, Goleta, CA, USA). Before and after each experiment the mass spectrometer was calibrated against gases of known composition. The flow meter was calibrated with a 3 l syringe within the experimental flow range. The response latency of the mass spectrometer was determined from a sudden, simultaneous change of gas composition and flow direction at the inlet of the sampling capillary (Bates et al. 1983). The latency between the flow meter and the mass spectrometer signals was about 3 s. The 95 % rise time for the response to a step change in gas concentration was 150 ms.
Subject instrumentation
The subjects had an ear probe for pulse oximetry (type EarSat Sensor, Datex Ohmeda Division, Helsinki, Finland). The probe was held in place by an elastic bandage and the ear lobe pre-treated with capsaicin ointment for vasodilatation to ensure a satisfactory pulse oximetry signal. ECG electrodes and the O2 saturation probe were applied to each subject. Also, a radial artery catheter was introduced under local anaesthesia. A disposable pressure transducer (pressure monitoring set with DPT-6003, PVB Medizintechnik GMBH, Kirchseeon, Germany) was connected to the arterial line and mounted beside the subject so as to be aligned with a level corresponding to 50 % of the anterio-posterior height of the reclining subject. This pressure transducer was not affected by the magnitude or direction of gravity.
Experimental procedures
The experiments were conducted in the human centrifuge at the Karolinska Institutet, Stockholm, Sweden. Before the centrifuge runs, the arterial catheter was connected to two syringe pumps (Terumo, Japan), modified to withdraw blood samples. The experiments were performed at 1 and 5 G, in prone and supine postures in a random order with one set of measurements in each condition. The subjects rested on a support surface on the platform of the centrifuge and breathed air through the mouthpiece. Approximately 6 min after the desired G level was reached, blood was sampled from the radial artery catheter for gas analysis and haematocrit determination; the first 6 ml was discarded and a 2 ml sample was obtained in the second syringe. Samples were stored on ice and were analysed in quadruplicate within 1 h (ABL 505 with OSM 3, Radiometer, Copenhagen, Denmark). Gas exchange was measured continuously by the mass spectrometer during the 30 s period of arterial sampling. High-G exposures were separated by at least 10 min at normal gravity.
Data analysis
Offline data analysis was performed with an Acknowledge 3.2 Biopac digital data handling system (Biopac Inc, Goleta, CA, USA). Offline computations included algorithms for total dry pressure correction (Scheid et al. 1971) and computation of calibrated values for all dry gas fractional concentrations. Also, concentration readings were corrected for the response latency of the mass spectrometer system (Bates et al. 1983) and gas volumes and flows were converted to BTPS (body temperature, ambient pressure, saturated with water vapour) when appropriate. We computed the (A–a)DO2 (PAO2 - PaO2) using the alveolar gas equation (West, 1995) to estimate PAO2, and using PaCO2 as an estimate of PACO2 and an assumed R value of 0.8.
Statistical analysis
Analysis of variance (Statistica 5.0, Statsoft Inc., Tulsa, OK, USA) with repeated measures design with two dependent factors (gravity (in the anterior-posterior or the posterio-anterior direction) and posture) was used in order to test for differences between changing G levels and posture. Results were considered statistically significant if P < 0.05 and all tests were two-sided. Data are presented as mean and s.e.m., if not otherwise stated.
RESULTS
All three indices of arterial oxygenation (PaO2, arterial O2 saturation (SaO2) and SaO2 obtained with pulse oximetry) showed that hypergravity-induced impairments of arterial oxygenation were significantly larger in supine posture (Tables 1 and 2, Fig. 1). This is demonstrated by the significant interaction between posture and gravity for all three variables (Table 2). SaO2 was reduced by 8.4 ± 1.8 % in prone and 13.2 ± 1.3 % (mean ±s.e.m.) in supine posture (Table 1). Pulse oximetry data were in close agreement with values obtained from arterial samples (r = 0.98, P < 0.001 at 5 G, prone, n = 10).
Table 1.
Cardiopulmonary variables in resting subjects in prone and supine posture during normal and increased gravity
| Variable | Supine 1 G | Prone 1 G | Supine 5 G | Prone 5 G |
|---|---|---|---|---|
| SaO2, blood sampling (%) | 97.6 ± 0.1 | 97.5 ± 0.2 | 84.4 ± 1.3 | 89.2 ± 1.2 |
| SaO2, pulse oximetry (%) | 97.4 ± 0.2 | 97.7 ± 0.3 | 84.6 ± 1.2 | 89.7 ± 1.4 |
| Alveolar PO2 (kPa) | 13.3 ± 0.2 | 13.3 ± 0.2 | 14.3 ± 0.1 | 14.1 ± 0.1 |
| End-tidal PO2 (kPa) | 13.4 ± 0.3 | 13.2 ± 0.4 | 15.9 ± 0.4 | 15.8 ± 0.2 |
| Arterial PCO2 (kPa) | 5.38 ± 0.2 | 5.43 ± 0.1 | 4.57 ± 0.1 | 4.70 ± 0.1 |
| Haematocrit (%) | 42.3 ± 1.2 | 42.2 ± 1.3 | 44.9 ± 1.3 | 45.1 ± 1.3 |
| pH | 7.42 ± 0.005 | 7.41 ± 0.005 | 7.47 ± 0.008 | 7.46 ± 0.006 |
| Base excess (mmol I−1) | 1.4 ± 0.5 | 1.3 ± 0.5 | 1.9 ± 0.4 | 1.6 ± 0.5 |
| Ventilation (1 mir−1) BTPS | 8.8 ± 0.8 | 8.8 ± 0.8 | 16.4 ± 2.4 | 14.4 ± 1.4 |
| Respiratory rate (breaths min−1) | 14.8 ± 0.7 | 13.6 ± 0.8 | 26.6 ± 3.0 | 23.5 ± 2.2 |
| Tidal volume (1) | 0.59 ± 0.04 | 0.65 ± 0.04 | 0.60 ± 0.06 | 0.64 ± 0.05 |
| Mean arterial pressure (mmHg) | 79.5 ± 2.1 | 84.0 ± 2.3 | 96.3 ± 3.0 | 94.3 ± 2.8 |
| Heart rate (beats min−1) | 6.3 ± 2.2 | 73.5 ± 2.3 | 87.8 ± 4.4 | 96.1 ± 4.5 |
Values are means ±s.e.m.; n = 10 subjects. PO2, partial pressure of oxygen; SaO2, arterial oxygen saturation; BTPS, body temperature, ambient pressure, saturated with water vapour; G, gravity in the anterio-posterior (supine) or posterio-anterior direction (prone).
Table 2.
Analysis of variance, effects of posture and gravity.
| Variable | Prone/Supine | 1/5 G | Interaction |
|---|---|---|---|
| Arterial PO2 | — | — | 0.03 |
| SaO2 blood sampling | — | — | 0.001 |
| SaO2 pulse oximetry | — | — | <0.001 |
| Alveolar PO2 | 0.03 | <0.001 | n.s. |
| End-tidal PO2 | n.s. | <0.001 | n.s. |
| Arterial PCO2 | 0.03 | <0.001 | n.s. |
| Haematocrit | n.s. | <0.001 | n.s. |
| pHa | 0.02 | <0.001 | n.s. |
| Base excess | 0.1 | 0.03 | n.s. |
| Alveolar-to-arterial PO2 | — | — | 0.03 |
| difference Ventilation | n.s. | 0.002 | n.s. |
| Respiratory rate | n.s. | <0.001 | n.s. |
| Tidal volume | n.s. | n.s. | n.s. |
| Mean arterial pressure | n.s. | 0.003 | n.s. |
| Heart rate | 0.02 | <0.001 | n.s. |
Values are significance of effects of the two factors posture and gravity level. PO2 partial pressure of oxygen; SaO2, arterial oxygen saturation; n.s., not significant. Results are considered statistically significant if P < 0.05.
Figure 1. Hypergravity-induced arterial deoxygenation.
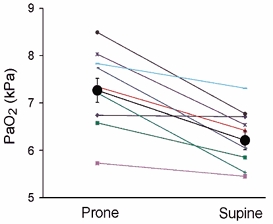
Arterial oxygen partial pressure (PaO2) after 6 min exposure to five times normal gravity in prone and supine postures (n = 10). P < 0.001 for planned comparison between prone and supine posture at this G-level. Large circles are mean values ±s.e.m. and small symbols are individual values.
Estimated mean alveolar oxygen tension (PAO2) increased with gravity in both postures: 0.8 ± 0.2 and 1.0 ± 0.2 kPa in prone and supine posture, respectively (Table 1). In both postures, there was a hypergravity-induced increase of 20 % in end-tidal PO2 and a 14 % decrease in arterial carbon dioxide tension (PaCO2; Table 1). As with arterial PO2 and saturation, there was a significant interaction between posture and gravity for (A-a)DO2 (Table 2); in prone posture (A-a)DO2 increased by 6.6 ± 0.4 kPa at 5 G compared with 1 G, and in supine posture the corresponding increase was 7.7 ± 0.3 kPa (Table 1 and Fig. 2).
Figure 2. Alveolar-to-arterial PO2 differences ((A-a)DO2) ±s.e.m. during the same conditions as in Fig. 1.
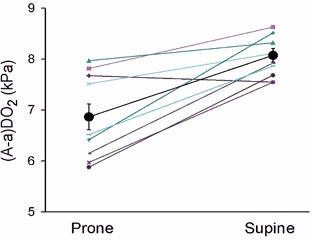
P < 0.001 for the same comparison as in Fig. 1.
There was a hypergravity-induced increase in pulmonary ventilation: +5.6 ± 1.3 l min−1 in prone and +7.6 ± 1.8 l min−1 in supine posture (Table 1). The increase in ventilation was due to a 60–80 % increase in respiratory rate but with unchanged tidal volume (Table 1).
DISCUSSION
Hypergravity induced a gas exchange impairment similar in magnitude to that seen in patients with acute lung insufficiency. Using three independent measures, we showed that arterial oxygenation was better preserved in prone than in supine posture. Conventionally the ratio PaO2/FIO2 (inspired fraction of oxygen) is used to define the degree of lung insufficiency (Bernard et al. 1994) with values below 300 mmHg per unit FIO2 defined as acute lung injury and values of 200 and below required as one of the criteria for ARDS. In the present study PaO2/FIO2 was 222 mmHg or 30 kPa per unit FIO2 in supine posture during hypergravity. The key element of ARDS is a diffuse and overwhelming inflammatory reaction of the pulmonary-capillary membrane to a variety of triggers, causing markedly increased lung fluid content, mechanical limitations to alveolar ventilation, limitations of alveolo-arterial diffusion and a disadvantageous ventilation-perfusion relationship (Curley, 1999). However, the observation that the increase in arterial oxygenation is almost instantaneous when the patient is turned into prone posture speaks against the notion that it is the underlying disease process that is reversed, but rather speaks in favour of a more uniform distribution of ventilation and perfusion (Pelosi et al. 2002).
In hypergravity, there are several possible reasons for the desaturation: for example an alveolar hypoventilation or a decreased alveolo-arterial gas transport (due to increased ventilation and perfusion inhomogeneities or decreased diffusing capacity), and therefore also different possible mechanisms responsible for a better gas exchange in prone posture. Below we will discuss the extent to which the elements typical of ARDS may have been replicated in the present model.
Alveolar hypoventilation
Smedal et al. (1963) studied arterial O2 saturation in healthy subjects exposed to up to 6 G in prone and supine posture. They observed more severe desaturation in supine posture and postulated that the difference in SaO2 between postures during hypergravity is due to alveolar hypoventilation resulting from a mechanical restriction of the thoracic cage (Smedal et al. 1963). Several independent findings of the present study when taken together speak against reduced total and alveolar ventilation as an explanation for arterial hypoxia in hypergravity. First, pulmonary ventilation was increased in hypergravity to 160 and 190 % of 1 G control in prone and supine postures, respectively. This increase of ventilation may to a large extent be wasted as dead space ventilation, as previously shown by Rosenhamer (1967) in sitting, resting subjects at 3 G. In contrast, the present findings of reduced PaCO2 in hypergravity indicate slightly increased effective alveolar ventilation. The increases of PAO2 and end-tidal PO2 also speak against alveolar hypoventilation in hypergravity. Thus, the differences in gas exchange between supine and prone postures during hypergravity cannot be explained by variations in alveolar ventilation.
Pulmonary tissue oedema
It is tempting to assume that increased hydrostatic pressure in blood vessels of dependent lung regions during hypergravity might cause extravasation and an interstitial accumulation of fluid. Pulmonary tissue oedema would aggravate an existing gas exchange impairment by decreasing diffusing capacity. If significant amounts of fluid are lost from the circulating blood due to oedema, the haematocrit would be expected to increase. We therefore determined haematocrit values in order to assess haemoconcentration. The haematocrit was moderately higher in hypergravity but did not differ between the two postures. The observed degree of haemoconcentration corresponds to loss of approximately 0.17 l of plasma in a subject with a blood volume of 5 l. If the tissue compliance to fluid loading were the same for the lung as for other tissues such as muscles, a total of 2–3 ml would enter the lung tissue based on relative tissue mass. However, as shown by Miserocchi et al. (1993), the lung tissue is 30 times less compliant than for example intercostal muscle tissue at the same hydrostatic level. We therefore conclude that there could not be more than a modest net filtration, if any, from the blood to other tissues in hypergravity, and not to any measurable degree to the lung tissue. Accordingly, Glaister (1970, 2001) has never found convincing evidence of pulmonary tissue oedema despite many years of studies of human responses to hypergravity. Also of importance is our observation that the subjects in our study recovered from the hypergravity-induced arterial desaturation within minutes after the centrifuge was stopped, which is much faster than would be expected if it had been caused by interstitial pulmonary oedema.
Thus even though the hydrostatic pressure must have been increased in the dependent pulmonary capillaries, interstitial pulmonary oedema is not likely to have influenced our results.
Intrathoracic pressure gradients and ventilation- perfusion inhomogeneities
Another possible explanation for the better oxygenation in prone posture is a more favourable ventilation-perfusion relationship. Although this study provides no data on the topographical distributions of ventilation and perfusion, the results speak against alternative explanations such as alveolar hypoventilation and pulmonary tissue oedema. In support of this notion, evidence from studies on anaesthetized dogs in normal gravity show a more homogeneous distribution of the ventilation-to-perfusion ratio in prone than in supine posture (Beck et al. 1992). As a result, there is a more efficient gas exchange in prone posture and a higher PaO2 (Beck et al. 1992).
There is much evidence from both animal (Lai-Fook et al. 1984; Olson & Lai-Fook, 1988; Mutoh et al. 1992; Lamm et al. 1994) and human (Mayo et al. 1995) experiments that at normal gravity vertical gradients of pleural pressure and alveolar size are much smaller - if not zero - in prone compared with in supine posture. As alveolar size and alveolar expansion gradients are determined by pleural pressure, these data speak in favour of ventilation distribution having a much smaller vertical gradient in prone than in supine posture. Furthermore, Amis et al. (1984) showed, in humans, that regional ventilation increased from upper to lower parts of the lung in supine posture but was uniform in prone posture.
Studies in dogs (Reed & Wood, 1970; Beck & Rehder, 1986; Glenny et al. 1991; Beck et al. 1992) and in humans (Nyren et al. 1999) have shown a higher vascular conductance in dorso-caudal parts of the lung independent of the direction of gravity. This mechanism leads to a more homogeneous perfusion distribution in prone posture (Nyren et al. 1999), as it will counteract the effect of gravity on the perfusion distribution. There appear to be inter-species differences, however: Melsom et al. (1995), studying anaesthetized goats, found less gravity dependence of vertical perfusion distribution in prone compared with supine posture only in two out of six animals.
Corresponding data from hypergravity are sparse. Pulmonary perfusion becomes more heterogeneous with increasing G force (Hlastala et al. 1998; Glaister, 2001) and the same is true for ventilation distribution and ventilation-to-perfusion ratio in supine posture during hypergravity. To our knowledge, there are no reports of comparisons between the topographical ventilation/perfusion distributions in prone and supine posture during hypergravity.
In summary, therefore, the bulk of evidence from normal gravity points to a much more homogeneous distribution of both ventilation and perfusion in prone than in supine posture, and limited data from hypergravity support a similar conclusion. A possible interpretation of the present data is that the improved homogeneity of ventilation and perfusion in prone posture compared with supine is of little consequence for healthy humans in normal gravity but makes a difference when the function of the lung as a gas exchanger is impaired due to hypergravity or a disease process (ARDS).
The present measurements do not permit any distinction between impairments of the ventilation-perfusion homogeneity and pure shunt as factors behind the arterial desaturation in hypergravity. Data from Rosenhamer (1967) comparing resting and exercising subjects in upright hypergravity showed an increased shunt fraction in exercise when assuming a two-compartment model with one compartment of full oxygenation and another with a pure shunt. Barr (1963) demonstrated that in sitting humans there was a more severe desaturation during hypergravity when inflating a G-suit. Both studies suggest a worsening of the ventilation-perfusion homogeneity in situations with an increased central blood volume and cardiac output in sitting subjects in hypergravity. Since a pure shunt is one extreme in the ventilation-perfusion spectrum, it cannot be excluded that the present supine and prone subjects had an element of pure shunting in hypergravity that might not have occurred in sitting posture where central blood volume and cardiac output are likely to be lower.
Albert & Hubmayr (2000) suggest an alternative mechanism in which better gas exchange in prone posture may be the result of less lung compression by the heart: in prone posture, the heart rests directly on the sternum and not - as in supine posture - on lung tissue between the heart and the dorsal thoracic wall. Hoffman Hoffman & Ritman, (1985, 1987), studying anaesthetized dogs and sloths in prone and supine posture, concluded that the intrathoracic position of the heart plays an important role in determining regional differences in alveolar expansion. The vertical gradient of regional alveolar expansion was present in supine animals but not in prone (Hoffman & Ritman, 1985, 1987). Wood and co-workers studied the topographic relationships of the heart and the lungs in dogs exposed to 1 and 6–7 G in prone and supine postures, measured from biplane roentgenograms (Rutishauser et al. 1966, 1967). Since the dorsal-ventral dimensions of the thorax of the dogs used were about 20 cm, or almost the same as for humans, the results obtained are probably of relevance also for humans. There was a severe dorsal displacement of the heart and a consequent over-distension of non-dependent alveoli during hypergravity in supine posture, whereas the position of the heart was stable in prone posture (Rutishauser et al. 1966, 1967); in supine posture the centre of the heart was located at 66 % of the lung height at 1 G and at 38 % at 6–7 G. The corresponding displacement in prone posture was from 33 to 29 %. Bearing in mind that the human heart is larger than that of the dog relative to the dorsal-ventral dimensions, cardiac displacement was probably less in our subjects. Nevertheless, a trend for cardiac displacement in supine posture must have led to a more extensive compression of lung tissue in the dorsal parts and more arterio-venous shunting than previously has been described at 1 G (Vandenberg et al. 1968; Katori et al. 1970).
Taken together, the results from Albert & Hubmayr (2000) and Wood and colleagues (Rutishauser et al. 1967) suggest that the different positions of the heart relative to the lungs in prone compared with supine posture may account both for better ventilation and better perfusion in some lung units due to the lack of lung distortion caused by the heart.
In summary, we have found that when gas exchange is compromised by hypergravity, lung function is better preserved in prone than in supine posture. This suggests that the hypergravity-induced acute lung insufficiency can be explored as a potential model to allow studies of the mechanisms behind the improved gas exchange when prone, with the advantage of using healthy subjects instead of patients but with the caveat that the model does not include other effects of the increased pulmonary fluid content of the lungs in ARDS than increased hydrostatic gradients. Thus the diffusion limitations caused by tissue and alveolar oedema are not included.
From an evolutionary standpoint it seems as if the human lung remains functionally adapted to a life on four legs.
Acknowledgments
We acknowledge the dedication of our subjects and the technical support of Barbro Bergström, Annette Ebberyd, Ola Hallert, Ingeborg Inacio, Jorges Inacio and Bertil Lindborg. This study was supported by the Swedish National Space Board, Fraenckel's Fund for Medical Research, the Laerdahl Foundation for Acute Medicine and the Swedish Research Council (Project No. 05020 and 10401).
references
- Albert RK, Hubmayr RD. The prone position eliminates compression of the lungs by the heart. Am J Respir Crit Care Med. 2000;161:1660–1665. doi: 10.1164/ajrccm.161.5.9901037. [DOI] [PubMed] [Google Scholar]
- Amis TC, Jones HA, Hughes JM. Effect of posture on inter-regional distribution of pulmonary ventilation in man. Respir Physiol. 1984;56:145–167. doi: 10.1016/0034-5687(84)90100-2. [DOI] [PubMed] [Google Scholar]
- Barr P. Hypoxemia in man induced by prolonged acceleration. Acta Physiol Scand. 1962;54:128–137. doi: 10.1111/j.1748-1716.1962.tb02337.x. [DOI] [PubMed] [Google Scholar]
- Barr P. Pulmonary gas exchange in man as affected by prolonged gravitational stress. Acta Anaesthesiol Scand Suppl 207. 1963;58:1–46. doi: 10.1111/j.1748-1716.1963.tb00082.x. [DOI] [PubMed] [Google Scholar]
- Bates JH, Prisk GK, Tanner TE, McKinnon AE. Correcting for the dynamic response of a respiratory mass spectrometer. J Appl Physiol. 1983;55:1015–1022. doi: 10.1152/jappl.1983.55.3.1015. [DOI] [PubMed] [Google Scholar]
- Beck KC, Rehder K. Differences in regional vascular conductances in isolated dog lungs. J Appl Physiol. 1986;61:530–538. doi: 10.1152/jappl.1986.61.2.530. [DOI] [PubMed] [Google Scholar]
- Beck KC, Vettermann J, Rehder K. Gas exchange in dogs in the prone and supine positions. J Appl Physiol. 1992;72:2292–2297. doi: 10.1152/jappl.1992.72.6.2292. [DOI] [PubMed] [Google Scholar]
- Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- Bone RC. The ARDS lung. New insights from computed tomography. JAMA. 1993;269:2134–2135. doi: 10.1001/jama.269.16.2134. [DOI] [PubMed] [Google Scholar]
- Curley MA. Prone positioning of patients with acute respiratory distress syndrome: a systematic review. Am J Crit Care. 1999;8:397–405. [PubMed] [Google Scholar]
- Fridrich P, Krafft P, Hochleuthner H, Mauritz W. The effects of long-term prone positioning in patients with trauma-induced adult respiratory distress syndrome. Anesth Analg. 1996;83:1206–1211. doi: 10.1097/00000539-199612000-00013. [DOI] [PubMed] [Google Scholar]
- Gattinoni L, Caironi P, Pelosi P, Goodman LR. What has computed tomography taught us about the acute respiratory distress syndrome? Am J Respir Crit Care Med. 2001;164:1701–1711. doi: 10.1164/ajrccm.164.9.2103121. [DOI] [PubMed] [Google Scholar]
- Glaister DH. The Effects of Gravity and Acceleration on the Lung. Slough UK: Blackwell Science Inc; 1970. [Google Scholar]
- Glaister DH. Effects of acceleration on the lung. In: Prisk GK, Paiva M, West JB, editors. Gravity and the Lung; Lessons from Microgravity. New York: Blackwell Science Inc; 2001. pp. 39–74. [Google Scholar]
- Glenny RW, Lamm WJ, Albert RK, Robertson HT. Gravity is a minor determinant of pulmonary blood flow distribution. J Appl Physiol. 1991;71:620–629. doi: 10.1152/jappl.1991.71.2.620. [DOI] [PubMed] [Google Scholar]
- Hlastala MP, Chornuk MA, Self DA, Kallas HJ, Burns JW, Bernard S, Polissar NL, Glenny RW. Pulmonary blood flow redistribution by increased gravitational force. J Appl Physiol. 1998;84:1278–1288. doi: 10.1152/jappl.1998.84.4.1278. [DOI] [PubMed] [Google Scholar]
- Hoffman EA, Ritman EL. Effect of body orientation on regional lung expansion in dog and sloth. J Appl Physiol. 1985;59:481–491. doi: 10.1152/jappl.1985.59.2.481. [DOI] [PubMed] [Google Scholar]
- Hoffman EA, Ritman EL. Heart-lung interaction: effect on regional lung air content and total heart volume. Ann Biomed Eng. 1987;15:241–257. doi: 10.1007/BF02584282. [DOI] [PubMed] [Google Scholar]
- Katori R, Amorim D, Theye RA, Wood EH. Influence of body position on regional pulmonary arterial-venous shunting in intact dogs. J Appl Physiol. 1970;29:288–296. doi: 10.1152/jappl.1970.29.3.288. [DOI] [PubMed] [Google Scholar]
- Lai-Fook SJ, Beck KC, Southorn PA. Pleural liquid pressure measured by micropipettes in rabbits. J Appl Physiol. 1984;56:1633–1639. doi: 10.1152/jappl.1984.56.6.1633. [DOI] [PubMed] [Google Scholar]
- Lamm WJ, Graham MM, Albert RK. Mechanism by which the prone position improves oxygenation in acute lung injury. Am J Respir Crit Care Med. 1994;150:184–193. doi: 10.1164/ajrccm.150.1.8025748. [DOI] [PubMed] [Google Scholar]
- Mayo JR, MacKay AL, Whittall KP, Baile EM, Paré PD. Measurement of lung water content and pleural pressure gradient with magnetic resonance imaging. J Thorac Imaging. 1995;10:73–81. doi: 10.1097/00005382-199501010-00007. [DOI] [PubMed] [Google Scholar]
- Melsom MN, Flatebo T, Kramer-Johansen J, Aulie A, Sjaastad OV, Iversen PO, Nicolaysen G. Both gravity and non-gravity dependent factors determine regional blood flow within the goat lung. Acta Physiol Scand. 1995;153:343–353. doi: 10.1111/j.1748-1716.1995.tb09872.x. [DOI] [PubMed] [Google Scholar]
- Miserocchi G, Negrini D, Del Fabbro M, Venturoli D. Pulmonary interstitial pressure in intact in situ lung: transition to interstitial edema. J Appl Physiol. 1993;74:1171–1177. doi: 10.1152/jappl.1993.74.3.1171. [DOI] [PubMed] [Google Scholar]
- Mure M, Lindahl SG. Prone position improves gas exchange - but how? Acta Anaesthesiol Scand. 2001;45:150–159. [PubMed] [Google Scholar]
- Mutoh T, Guest RJ, Lamm WJ, Albert RK, Lindahl SG. Prone position alters the effect of volume overload on regional pleural pressures and improves hypoxemia in pigs in vivo. Am Rev Respir Dis. 1992;146:300–306. doi: 10.1164/ajrccm/146.2.300. [DOI] [PubMed] [Google Scholar]
- Nyren S, Mure M, Jacobsson H, Larsson SA, Lindahl SG. Pulmonary perfusion is more uniform in the prone than in the supine position: scintigraphy in healthy humans. J Appl Physiol. 1999;86:1135–1141. doi: 10.1152/jappl.1999.86.4.1135. [DOI] [PubMed] [Google Scholar]
- Olson LE, Lai-Fook SJ. Pleural liquid pressure measured with rib capsules in anesthetized ponies. J Appl Physiol. 1988;64:102–107. doi: 10.1152/jappl.1988.64.1.102. [DOI] [PubMed] [Google Scholar]
- Pelosi P, Brazzi L, Gattinoni L. Prone position in acute respiratory distress syndrome. Eur Respir J. 2002;20:1017–1028. doi: 10.1183/09031936.02.00401702. [DOI] [PubMed] [Google Scholar]
- Pelosi P, D'Andrea L, Vitale G, Pesenti A, Gattinoni L. Vertical gradient of regional lung inflation in adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994;149:8–13. doi: 10.1164/ajrccm.149.1.8111603. [DOI] [PubMed] [Google Scholar]
- Reed JH, Wood EH. Effect of body position on vertical distribution of pulmonary blood flow. J Appl Physiol. 1970;28:303–311. doi: 10.1152/jappl.1970.28.3.303. [DOI] [PubMed] [Google Scholar]
- Rosenhamer G. Acta Physiol Scand Suppl. Vol. 68. 1967. Influence of increased gravitational stress on the adaptation of cardiovascular and pulmonary function to exercise. [PubMed] [Google Scholar]
- Rutishauser WJ, Banchero N, Tsakiris AG, Wood EH. Effect of gravitational and inertial forces on pleural and esophagal pressures. J Appl Physiol. 1967;22:1041–1052. doi: 10.1152/jappl.1967.22.6.1041. [DOI] [PubMed] [Google Scholar]
- Scheid P, Slama H, Piiper J. Electronic compensation of the effects of water vapor in respiratory mass spectrometry. J Appl Physiol. 1971;30:258–260. doi: 10.1152/jappl.1971.30.2.258. [DOI] [PubMed] [Google Scholar]
- Smedal HA, Holden GR, Smith JR. Cardiovascular responses to transversely applied accelerations. Aerosp Med. 1963;34:749–752. [PubMed] [Google Scholar]
- Vandenberg RA, Nolan AC, Reed JH, Wood EH. Regional pulmonary arterial-venous shunting caused by gravitational and inertial forces. J Appl Physiol. 1968;25:516–527. doi: 10.1152/jappl.1968.25.5.516. [DOI] [PubMed] [Google Scholar]
- West JB. Respiratory Physiology - The Essentials. Baltimore: Blackwell Science Inc; 1995. [Google Scholar]


