Abstract
An important feature of ligand-gated ion channels is their exquisite ability to discriminate between ions. Still, little is known about the mechanisms underlying, or structural determinates of, this ability. We examined the structural elements underlying the ionic selectivity of ρ1 GABA receptors expressed in Xenopus oocytes and human embryonic kidney cells using site-directed mutagenesis and two-electrode voltage-clamp or patch-clamp techniques. The wild-type GABA receptor was chloride selective, with a small but significant permeability to potassium (PNa+ : PK+ : PCl-= 0 : 0.03 :1). Mutation of an alanine to glutamate at position 291 (thought to be located at the intracellular end of the second transmembrane domain), formed a channel that exhibited little discrimination among ions (0.70:0.87:1), while deletion of a neighbouring proline (290) was chloride selective, but had elevated cation permeabilities compared to the wild-type channel (0.12 : 0.14 : 1). Together, the two mutations (ΔP290/A291E) caused a reversal of selectivity (2.72 : 3.59 : 1). We also examined the effects of neutralizing and reversing the charge of the adjacent, and highly conserved, arginine. Mutation of the neighbouring arginine to glutamate (R292E) increased the cation permeability similar to the ΔP290/A291E double mutant (2.4 : 3.0 : 1), whereas neutral mutations at this position (R292M or R292C) retained chloride selectivity (0 : 0.11 : 1.0 and 0 : 0.14 : 1.0, respectively). Our experiments suggest that the effective charge near the presumed intracellular mouth of the pore is critical for ionic selectivity.
Although the members of the ligand-gated ion channel (LGIC) superfamily, which includes nicotinic acetylcholine (nACh), γ-aminobutyric acid (GABA), glycine and serotonin (5-HT3) receptors, have high sequence homology in their putative pore regions (Boulter et al. 1987; Grenningloh et al. 1987; Schofield et al. 1987; Maricq et al. 1991), there are significant differences in ion selectivity. For example, nACh α7 receptors are permeable to calcium ions and, to a lesser degree, sodium ions (Seguela et al. 1993). GABA receptors, however, exclude both sodium and calcium and permit the passage of chloride and, to a limited, extent potassium (Bormann et al. 1987). These differences in permeability are the ionic basis of chemical transmission, yet our understanding of the underlying mechanisms is limited.
The first amino acid residue implicated in LGIC selectivity forms a ring of charges at the cytoplasmic end of the second transmembrane domain (TM2) in nACh receptors. This ring is located at the −1′ position in the LGIC TM2 numbering system (Fig. 1A; Imoto et al. 1988). Comparison of amino acid sequences shows that a proline followed by alanine motif near the intracellular end of TM2 (−2′, −1′) is found in all anion channels. In cation-permeable LGICs, however, the proline is absent and a negatively charged glutamate appears in place of the neutral alanine. In addition, all of the anion channels have a hydrophilic threonine at the 13′ position, while in cation channels there is a hydrophobic valine or phenylalanine. Mutation of all three residues (−2′, −1′ and 13′) can reverse the ionic selectivity of nACh α7, glycine or 5-HT3 receptors (Galzi et al. 1992; Keramides et al. 2000; Gunthorpe & Lummis, 2001; Jensen, et al. 2002), and mutation of two of these residues in only the β subunit can reverse selectivity in GABAA receptors (Jensen et al. 2002). However, the contributions of each of these three residues in ion selectivity of GABA receptors have yet to be elucidated. In addition, it is intriguing that a positive charge is conserved at the 0′ residue, which is arginine in anion-conducting pores and lysine in cation-conducting pores. The potential role of this residue in ionic selectivity has yet to be determined.
Figure 1. Sequence alignment of second transmembrane (TM2) domains and the putative position of the four mutated residues.

A, the TM2 domain is indicated by a line, with standard TM2 numbering shown below. The cytoplasmic, internal and extracellular rings proposed to be important in charge selectivity of nicotinic receptors (Imoto et al. 1988) are marked below the sequences by an asterisk. Chloride-permeable channels are given above the dotted line, while those below are cation-permeable. Residues mutated in this study are indicated in bold. Note the conserved positive charge at the 0′ position (arginine in chloride channels and lysine in cation channels). nACh, nicotinic ACh channel. B, cartoon illustrating the proposed subunit topology. The residues mutated in this study are shown as the single-letter amino acid abbreviation.
In this study, we examined the effects of mutations at the −2′, −1′, 0′ and 13′ sites on ionic selectivity of ρ1 GABA receptors expressed in Xenopus oocytes and human embryonic kidney (HEK)293 cells using two-electrode voltage-clamp and patch-clamp techniques. Ionic permeabilities were quantified for mutant and wild-type receptors by measuring reversal potentials in varying ionic conditions. We found that the double mutation, ΔP290/A291E (−2′, −1′), reversed the charge selectivity of the GABA receptor from anionic to cationic. Surprisingly, the single mutation A291E allowed mixed cation and anion permeability. We also determined that the selectivity changes caused by these mutations did not appear to be accompanied by a significant alteration in the estimated size of the channel pore. Reversal of the charge at the 0′ position (R292E) resulted in a cation-preferring pore. Removal of the charge (R292C or R292M), or replacement with lysine (conserved in cation-selective members of this LGIC family), elevated the cation permeability, although the preference for chloride was retained. The permeability changes imparted by these mutations allowed us to locate critical elements of the GABA receptor selectivity filter at the intracellular mouth of the channel pore. We propose a mechanism in which the charge environment in this region is an important component determining LGIC selectivity.
METHODS
cDNA cloning and in vitro transcription
The cDNA encoding the human ρ1 wild-type subunit (accession number NM 002042) was cloned into pALTERMAX (Promega, Madison, WI, USA) and pGEMHE (Liman et al. 1991); the latter vector allowed high expression levels in Xenopus laevis oocytes. Seven single-point mutants (ΔP290, A291E, T305V, R292E, R292C, R292K and R292M), three double mutants (ΔP290/A291E, ΔP290/T305V, A291E/T305V) and a triple mutant (ΔP290/A291E/ T305V) were produced in ρ1 using the overlap extension method (Kammann et al. 1989). Wild-type nACh subunits α7, α3 and β4 were cloned into pGEMHE for use as controls (rat, accession numbers L31619, L31621 and U42976, respectively). The cDNA for each construct was linearized, and cRNA was synthesized using standard in vitro transcription techniques as described previously (Amin & Weiss, 1994). The yield and integrity of cRNA were verified by agarose gel electrophoresis. Unless specified, all chemicals were purchased from Sigma (St Louis, MO, USA).
Oocyte preparation and microinjection
Female Xenopus laevis (Xenopus I, Ann Arbor, MI, USA) were anaesthetized with 0.2 % MS-222, and several lobes of ovary were removed surgically. The incision was sutured, and the animal was monitored during its recovery period, after which it was returned to its tank. This procedure was approved by the UAB Institutional Animal Use and Care Committee. Ovarian lobes were placed in a calcium-free oocyte Ringer solution (OR2) that consisted of (mm): NaCl 92.5, KCl 2.5, MgCl2 1, Na2HPO4 1 and Hepes 5, plus penicillin 50 U ml−1 and streptomycin 50 μg ml−1, pH 7.5. The ovarian lobes were cut into small pieces, and then digested in 0.3 % collagenase A (Boehringer Mannheim, Indianapolis, IN, USA) in the above solution. After dispersal for about 2 h, stage VI oocytes were selected and thoroughly rinsed and maintained in OR2 with CaCl2 (1 mm) at 18 °C for several hours before cRNA injection. Typically, 60–100 nl of cRNA (25–100 ng μl−1) was injected into the oocyte with a Nanoject (Drummond Scientific, Broomall, PA, USA), and then the oocytes were incubated at 16 °C for 1–5 days. Oocytes were screened in voltage-clamp for expression of GABA receptors. Low expression levels (GABA-induced currents between 200 and 1000 nA) were used for reversal potential experiments in order to minimize the error due to series resistance (< 4 % with a 1000 nA current). Unless otherwise stated, all data were obtained from injected oocytes.
Voltage-clamp experiments
The oocyte was placed in a small-volume chamber with a continuous perfusion system, as described previously (Amin & Weiss, 1994). The normal extracellular OR2 consisted of (mm): NaCl 92.5, KCl 2.5, MgCl2 1, CaCl2 1 and Hepes 5, pH 7.5. Recording microelectrodes were fabricated from thin-walled glass micropipettes (A-M Systems, Carlsborg, WA, USA) on a Sutter P87 horizontal puller (Sutter, Novato, CA, USA) and filled with 3 m KCl (resistance = 1–3 MΩ). In order to prevent errors due to junction potentials during ion-substitution experiments, the ground electrode was placed in a separate 3 m KCl well connected to the bath by an agar bridge. Solutions for ion replacement experiments had a portion of the usual NaCl replaced with an iso-osmotic amount of one of the following: sodium isethionate, caesium chloride (Gibco BRL, Gaithersburg, MD, USA), choline chloride, rubidium chloride, TEA chloride, lithium chloride or sucrose.
Reversal potential measurements
Each oocyte was allowed to equilibrate at a holding potential of −70 mV in each solution for approximately 1 min before recording sequences were begun. A group of five voltage ramps was applied from −70 to +10 mV (−40 to 0 mV ramps were used for some experiments) and then averaged. Each individual ramp lasted 1 s with 3 s intervals between ramps. The solution was then switched to a GABA-containing solution with the same ionic composition. The bath exchange time in our two-electrode voltage-clamp setup is typically < 5 s. When the GABA response reached steady state, five more ramps were taken and averaged. This was followed by a third set of ramps after complete recovery from the GABA application. For determination of the reversal potential, the initial and recovery ramps were averaged, and this average control ramp was subtracted from the average ramp during GABA application. The voltage at which this GABA minus control ramp crossed the abscissa was the reversal potential of the GABA-induced portion of the current. Reversal potentials were measured in OR2 at the beginning and end of each experiment and were typically identical (ρ1 wild-type, before, −21.2 ± 1.0 mV; after, −21.8 ± 0.6 mV; n = 9), indicating that the internal ion concentrations remained stable, even though the extracellular ion concentrations were changed many times during the course of the experiment. In the few cases where a difference was observed, those particular experiments were discarded. In all cases, GABA (Calbiochem, La Jolla, CA, USA) or carbachol were applied at the concentration required for half-maximal activation (EC50).
HEK293 expression
HEK293 cells (CRL-1573, American Type Culture Collection, Rockville, MD, USA) were grown in Dulbecco's modified Eagle's medium (DMEM)/F12 supplemented with 10 % fetal bovine serum (Atlanta Biologicals, Norcross, GA, USA), with 100 U ml−1 penicillin and 100 mg ml−1 streptomycin in a 37 °C incubator with 95 % air and 5 % CO2. At 50–90 % confluence, the cells were dispersed with 0.05 % trypsin and plated at low density onto poly-l-lysine-coated coverslips in 35 mm culture dishes. Each dish was transfected with ρ1 wild-type or mutant cDNA plus pGreenLantern (Promega, Madison, WI, USA) using Fugene (Roche Molecular Biochemicals, Indianapolis, IN, USA). A total of 2 μg of cDNA was used in each 35 mm culture dish. Dishes were then incubated for 36 h before electrophysiological recordings were made. Only cells that fluoresced and were isolated from neighbouring cells were used for experiments. Cell culture chemicals were purchased from Life Technologies (Gaithersburg, MD, USA), unless otherwise stated.
Whole-cell recording
Recording electrodes were pulled with a vertical puller (List Medical, Darmstadt, Germany) in two steps from borosilicate glass tubes (A-M Systems, Everett, WA, USA) with a 1.5 mm outer diameter and 0.8 mm inner diameter. The electrodes were filled to a level less than 10 mm from the tip with an intracellular solution designed to mimic (as closely as possible) the intracellular concentrations found in oocytes as well as the concentration gradients present in oocyte experiments, while still accommodating the osmolarity required for HEK cell stability. The intracellular solution consisted of (mm): NaCl 15, KCl 40, Hepes 10 and glucose 180, pH 7.2. After filling, electrodes had a resistance of 6–12 MΩ. Unless otherwise stated, the extracellular solution consisted of (mm): NaCl 150, KCl 2, MgCl2 2, CaCl2 2 and Hepes 4, pH 7.4. Sodium and chloride concentrations were altered by replacement with equimolar amounts of either choline chloride or sodium isethionate. The ground electrode was placed in a separate well containing a pipette solution connected to the bath by an agar bridge, and junction potentials were nulled before each experiment. Series resistance was compensated up to 80 %. Reversal potentials were measured at the beginning and end of each experiment and were typically identical (ρ1 wild-type before, −26.0 ± 1.9 mV; after, −25.7 ± 2.7 mV; n = 6), indicating that the internal ion concentrations remained stable, even though the extracellular ion concentrations were changed many times during the course of the experiment.
Drug application was controlled with a fast-switching piezo system. A control solution and the same solution plus GABA (at the EC50 value for each receptor) were connected through polyethylene tubing to a glass Θ tube (Sutter). Solutions with different ion concentrations could be selected on each side of the Θ tube by switching a six-way valve. Two solutions were perfused into the chamber continuously, one on each side of the Θ tube. The control solution was directed to the cell first, then the computer-controlled piezo translator moved the Θ tube so that the GABA-containing solution was directed at the cell. Flow of the bath solution was stopped during applications of test solutions, allowing test solutions from the Θ tube to replace the bath solution. The cell was incubated in each new test solution for at least 1 min to wash out the previous solution before measurement of the reversal potential, resulting in several complete exchanges of the chamber. Reversal potentials were determined using the same ramp protocol as in oocytes. The current was amplified by an Axopatch 200A amplifier (Axon Instruments, Union City, CA, USA), low-pass filtered at 200 Hz with an eight-pole Bessel filter (Frequency Devices, Haverhill, MA, USA), and then digitized at 2000 Hz with 16 bit resolution (ITC-16 Computer Interface; Instrutech, Port Washington, NY, USA) and analysed on a Macintosh computer (Apple Computer, Cupertino, CA, USA).
Calcium permeability determination
Calcium permeability was tested in select mutants by measuring reversal potentials before and 15 min after a 28 nl injection of 100 mm 5,5′dibromoBAPTA (RBI/Sigma, Natick, MA, USA), similar to the method used by Galzi et al. (1992). In order to minimize the effects of injections on intracellular ion concentrations, 5,5′dibromoBAPTA was dissolved in a solution containing 10 mm NaCl and 50 mm KCl. We estimate that the intracellular 5,5′dibromoBAPTA concentration would be ∼10-fold greater than the KD for calcium.
Intracellular ion activities
The oocyte internal chloride activity was calculated from our measured chloride (GABA-activated) reversal potentials using constant potassium and calcium concentrations and a relative chloride permeability of 1 in the following rearrangement of the Nernst equation,
| (1) |
where Vrev is the mean reversal potential in OR2, and F, R and T have their usual meanings. With our solutions, and an average chloride reversal potential of −20.6 mV, the calculated internal chloride activity ([Cl−]i) was estimated to be 35 mm. This value is in good agreement with the 33 mm estimate of intracellular activity from Barish (1983), whose figures for other intracellular ion activities were used ([Na+]= 6 mm and [K+]= 92 mm).
Data analysis
Concentration-response curves for GABA were constructed for each functional mutant and fitted with the Hill equation,
| (2) |
where I is peak current at agonist concentration A, Imax is the maximum current, EC50 is the concentration of agonist required for a half-maximal current, and nH is the Hill coefficient. All data are presented as the mean ±s.e.m.
The permeabilities (P) of other ions relative to chloride were calculated using the Goldman-Hodgkin-Katz (GHK) equation (Goldman, 1943; Hodgkin & Katz, 1949) with our calculated internal chloride activity and the known activities for external chloride and replacement ions. Least-squares fitting was used (Igor, Wavemetrics, Lake Oswego, OR, USA) to calculate the ionic permeability coefficients from the following equation, using reversal potentials measured at various activities of extracellular chloride and sodium,
| (3) |
Permeability coefficients for sodium and potassium were fitted simultaneously (holding PCl- at 1 for normalization), with reversal potentials measured in OR2 and three different sodium concentrations and three different chloride concentrations. Data from five to seven individual cells were fitted separately. The resulting permeability values were then averaged and the standard deviation was calculated. Student's unpaired t test was applied to determine significant differences, using P < 0.05. Student's paired t test was used for determination of calcium permeability. Fits were also attempted while constraining the various permeability coefficients in turn to one or zero. An F-ratio test was used to determine if one or more permeabilities could be eliminated, using P < 0.05.
RESULTS
Sequence comparison shows similarities and differences between anion and cation channels
There is a high amino acid homology in TM2 of the LGIC subunits, especially between receptors that are permeable to ions of the same polarity (Fig. 1A). Three differences between channels permeable to cations versus anions (−2′, −1′ and 13′ in the universal LGIC TM2 numbering system) have been implicated in ion selectivity (Galzi et al. 1992; Keramides et al. 2000; Gunthorpe & Lummis, 2001; Jensen et al. 2002; Fig. 1). Mutations were produced in the ρ1 subunit to change the residues at positions −2′, −1′ and 13′ to the corresponding amino acids in the nACh α7. We examined the functional consequences of each mutation individually, as well as in all possible combinations. We also examined mutations at position 0′ which has a positive charge in all members of this LGIC family.
Mutations had significant effects on receptor expression and/or activation
All but one of the functional mutants had dose-response curves that were shifted to higher agonist concentrations (Fig. 2, Table 1). The GABA EC50 for ΔP290 receptors was 25-fold greater than for ρ1 wild-type receptors, while the T305V and A291E/T305V concentration-response curves were shifted by > 30-fold. A291E and ΔP290/A291E had smaller effects on agonist sensitivity; their EC50 values were shifted by only two- and fivefold, respectively. Mutation of R292 had only very small effects on concentration- response curves. R292K and R292E each exhibited a 20 % increase in the GABA EC50, and R292M was increased twofold. R292C exhibited a 20 % reduction in the GABA EC50. In addition to shifts in concentration-response curves, all of the mutant receptors showed a reduction in the Hill number (Table 1), as evident by the shallow slopes (Fig. 2). Concerning the changes in sensitivity, it is likely that the mutations in this study are in close proximity to elements of the channel gate (Unwin, 1993; Wilson & Karlin, 1998) or to structures that couple agonist binding to channel gating (Grosman et al. 2000). It is also possible that the mutations caused changes in subunit structure, possibly some distance from the mutated residue. Such changes could result in reduced cooperativity among subunits or altered sensitivity to agonist.
Figure 2. Dose-response relationships of mutants show effects on channel activation properties.

Current traces are shown for the wild-type and selected mutant receptors. The concentration of GABA (μm) is indicated above each trace. The ΔP290 mutant exhibited fast decay rates in the presence of GABA, returning nearly to baseline during the application. The dose-response curves of all of the mutants were shifted to higher agonist concentrations. The EC50 values and Hill coefficients for the Hill equation fits (lines) are provided in Table 1.
Table 1.
Concentration-response properties of mutant ρ1 receptors
| Mutation(s) | GABA EC50(μm) | Hill slope | n |
| ρ1 | 0.8 ± 0.0 | 3.37 ± 0.07 | 6 |
| A291E | 2.0 ± 0.1 | 1.89 ± 0.08 | 5 |
| ΔP290 | 20.3 ± 3.3 | 1.16 ± 0.16 | 6 |
| T305V | 30.0 ± 2.4 | 1.40 ± 0.01 | 5 |
| ΔP290/A291E | 4.5 ± 0.4 | 1.51 ± 0.05 | 5 |
| A291E/T305V | 39.8 ± 5.2 | 1.13 ± 0.05 | 7 |
| ΔP290/T305V | NF | NF | 30 |
| ΔP290/A291E/T305V | NF | NF | 30 |
| R292C | 0.6 ± 0.1 | 1.77 ± 0.17 | 6 |
| R292M | 1.8 ± 0.1 | 1.00 ± 0.08 | 6 |
| R292K | 5.1 ± 1.6 | 1.06 ± 0.16 | 5 |
| R292E | 1.3 ± 0.1 | 1.36 ± 0.16 | 5 |
All but one (R292C) of the mutant receptors exhibited rightward shifts in their dose-response curves, and all exhibited decreased Hill slopes. NF = non-functional.
Accompanying the dose-response shift, ΔP290 receptors exhibited dramatic kinetic differences from ρ1, as seen in the traces in Fig. 2. A characteristic feature of ρ1 is its lack of desensitization during prolonged agonist application (Amin & Weiss, 1994), but ΔP290 receptors exhibited a dramatic current decay. A reduction in current amplitude was evident even at GABA concentrations well below the EC50. At high concentrations, the current returned to baseline, or below, during agonist application. Changes in the kinetics of desensitization have been reported for point mutations at the corresponding site of glycine α1 (Breitinger et al. 2001). Two of the ρ1 constructs, the triple mutant ΔP290/A291E/T305V and the double mutant ΔP290/T305V, did not form functional channels when expressed in oocytes. Similarly, the corresponding double mutant in nACh α7 was non-functional (Galzi et al. 1992).
Ionic selectivity of mutant channels
Reversal potentials, determined by subtraction of ramps in the absence and presence of agonist, can be used to determine the underlying ionic selectivity. In Fig. 3, reversal potentials in normal OR2 are indicated by an arrow; the traces have been omitted for clarity. GABA-induced currents in wild-type receptors reverse at −20.6 ± 0.4 mV (n = 35, Fig. 3A), very close to the equilibrium potential predicted for chloride (−19.9 mV), consistent with chloride selectivity. Substitution of 50 mm choline chloride for 50 mm sodium chloride did not alter the reversal potential, indicating that the channel was impermeable to sodium. In contrast, replacement by sodium isethionate resulted in a reversal potential shift to more positive values (Vrev=−6.1 ± 0.8 mV, n = 3), consistent with chloride permeability. Replacement of 50 mm sodium chloride by an iso-osmotic amount of sucrose shifted the reversal potential to the same extent as did isethionate replacement, additional evidence of chloride permeability and sodium exclusion. Reversal potentials of T305V and the double mutant A291E/T305V showed a similar pattern as the wild-type receptor (Fig. 3B and C).
Figure 3. Voltage ramps for wild-type and mutant receptors.

Voltage ramps of GABA-induced currents from a typical experiment are shown for the wild-type and each of the mutant receptors. Reversal potentials in normal calcium-free oocyte ringer solution (OR2) are indicated by an arrow. Ramps are shown for iso-osmotic replacements of 50 mm sodium chloride by choline chloride (Chol), sodium isethionate (Ise) or sucrose (Suc). Only the GABA-induced currents are shown here as the ramp in the absence of GABA has already been subtracted from the ramp in the presence of GABA. A-C, in ρ1, T305V and A291E/T305V receptors, the OR2 reversal potential was −22 mV, and that of Chol was the same, indicating that sodium is impermeant. A similar replacement by isethionate resulted in a reversal potential shift to more positive values, which is consistent with chloride permeability. Sucrose replacement shifted reversal potential similarly to Ise replacement, additional evidence of chloride permeability and sodium exclusion. D, the deletion mutant ΔP290 had a reversal potential shift with isethionate, but no shift with choline, similar to the case for wild-type receptors. However, the OR2 reversal potential of ΔP290 was more negative than that of the wild-type receptor. E, the OR2 reversal potential of A291E was −13 mV. The choline reversal potential was shifted to −20 mV and the isethionate reversal potential was shifted in the positive direction by nearly the same magnitude (to −3 mV). The reversal potential for sucrose was the same as in OR2. This pattern suggests that the A291E mutant channel was permeable to both sodium and chloride. F, in the case of the double mutant (ΔP290/A291E), the OR2 reversal potential was −11 mV, the same as the isethionate reversal potential. Choline replacement shifted the reversal potential to −22 mV, which is the same as the sucrose reversal potential. This pattern suggests that the ΔP290/A291E mutant was selective for sodium.
ΔP290 receptors showed no change in reversal potential when sodium was replaced by choline (Fig. 3D, ΔVrev= 0.4 ± 1.7 mV, n = 7), indicating that the channel was impermeable to sodium. However, the shift in isethionate was about one-third less than that observed for the wild-type channel (ΔVrev=−9.9 ± 2.3 mV, n = 7, Fig. 3D). The smaller magnitude of the reversal potential shift with isethionate replacement suggests that this mutant, while chloride permeable, is not as selective for chloride as the wild-type receptor.
A291E (Fig. 3E) results were consistent with mixed permeability to sodium and chloride. Upon replacement of 50 mm sodium chloride with either choline chloride or sodium isethionate, a comparable change in the reversal potential was observed (ΔVrev, choline =−6.1 ± 0.8 mV, n = 10; isethionate = 7.4 ± 1.3 mV, n = 9). Replacement by an iso-osmotic amount of sucrose (thus reducing the concentrations of sodium and chloride equally) resulted in no shift (ΔVrev=−0.4 ± 0.3 mV, n = 5). Evidently, A291E altered the pore such that it was permeable to sodium as well as chloride.
In contrast to the wild-type receptor, the ΔP290/A291E mutant was cation selective. The reversal potential did not change when sodium isethionate was substituted for sodium chloride (ΔVrev=−0.1 ± 0.5 mV, n = 11) and it shifted to −11.6 ± 0.9 mV (n = 11, Fig. 3F) when choline chloride was the replacement salt. The absence of a shift in the reversal potential upon chloride replacement for the ΔP290/A291E mutant was the same as that observed for the cation-selective nAChR, α3β4 (ΔVrev=−3.2 ± 3.4 mV, n = 4, not shown). Reversal potentials measured with 50 mm sodium chloride replaced by iso-osmotic amounts of sucrose (Fig. 3F) show that the reversal potential shifted to the same point as it did with the 50 mm choline chloride replacement (ΔVrev=−13.5 ± 0.7 mV, n = 4). This result was similar to the sucrose shift observed for nAChR α3β4 (ΔVrev=−16.3 ± 2.2 mV, n = 3, not shown). These experiments indicate that the double mutation near the intracellular end of TM2 resulted in a channel that was selective for sodium.
By varying the concentration of permeant ions, additional information can be gained about relative ionic permeabilities. The Nernst equation predicts that for a selective channel, the reversal potential will shift by 58 mV with a 10-fold change in permeant ion concentration. For wild-type receptors, a shift of 57.7 ± 0.5 mV was seen upon a 10-fold Cl− change, consistent with a highly selective chloride channel (Fig. 4A). In contrast, for cation-selective channels, there was no significant shift in the reversal potential upon 10-fold isethionate replacement of chloride (nACh α3β4: 0.6 ± 0.7 mV, n = 4; nACh α7: −0.3 ± 0.6 mV, n = 4, data not shown). Mutant receptors showed results that covered the range between chloride selective and sodium selective.
Figure 4. Reversal potential shifts for ionic substitution experiments.

Reversal potentials were measured in solutions with varying amounts of sodium chloride replaced by either choline chloride (filled circles) or sodium isethionate (open circles). A-C, ρ1, T305V and A291E/T305V receptors exhibited the 58 mV shift per decade change in chloride concentration expected for a chloride-selective pore. No significant change was observed when sodium was replaced. D, ΔP290 also exhibited no reversal potential shift with the replacement of sodium, but the magnitude of the chloride shift was 35 mV, less than the theoretical value for a chloride-selective pore. E, the point mutant A291E had virtually symmetrical shifts in reversal potential with sodium and chloride replacements, suggesting a comparable permeability to both chloride and sodium. F, ΔP290/A291E receptors showed no change in reversal potential with chloride substitution and a reversal potential shift of −22.7 mV per decade change in sodium concentration.
The three mutant receptors (T305V, A291E/T305V and ΔP290) exhibited no shift in reversal potential when sodium was replaced, indicating that they were sodium impermeable (Fig. 4B–D). However, only T305V had a reversal potential shift that is consistent with a high chloride selectivity (55.6 ± 1.0 mV, n = 4, Fig. 4B). ΔP290 and the double mutant A291E/T305V had reversal potential shifts smaller than expected for chloride-selective channels (ΔP290, 33.4 ± 2.9 mV, n = 10; A291E/T305V, 38.3 ± 4.7 mV, n = 4), indicating that other ions must permeate. For the point mutation A291E, reversal potential shifts with 90 mm replacements of sodium (−16.6 ± 1.0 mV, n = 7) or chloride (−17.7 ± 1.4, n = 7) were observed, indicating permeability to both sodium and chloride (Fig. 4E). However, these results suggest that chloride and sodium are not the only permeant ions. Finally, although the ΔP290/A291E double mutant was clearly sodium permeable, the shift in reversal potential for choline was −33.4 ± 2.9 mV (n = 10), indicating that sodium was not the only permeant ion (Fig. 4F). The shift in low chloride was not significant, resembling nAChR α3β4 or α7 (not shown).
Mutations that allow sodium and potassium permeability do not affect calcium permeability
Before quantifying the relative permeabilities of the wild-type and mutant receptors for the various ions, we tested for calcium permeability in the cation-permeable mutants. This was necessary since the mutations changed amino acid residues of ρ1 to the corresponding residues in the calcium-permeable nAChR α7 receptor (Seguela et al. 1993). In order to minimize interference from the endogenous calcium-activated chloride current (Barish, 1983; Miledi & Parker, 1984), we measured the reversal potentials of GABA-induced currents before and after injection of the membrane-impermeant calcium chelator 5,5′dibromoBAPTA (Galzi et al. 1992). Two calcium-permeable nAChR subtypes, α7 and α3β4, were used as positive controls, and each showed a significant shift in the reversal potential in the positive direction after BAPTA treatment (Fig. 5). However, the most cation-permeable mutants, ΔP290/A291E and A291E, exhibited negligible reversal potential shifts (ΔP290/A291E, ΔVrev= 0.98 ± 1.2 mV, n = 6; A291E, ΔVrev=−1.7 ± 1.0 mV, n = 6). Apparently, the mutations that imparted an increased permeability to sodium did not introduce a calcium permeability.
Figure 5. Cation-permeable mutant receptors showed no calcium permeability.
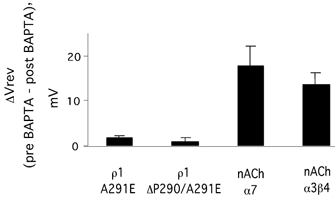
Reversal potentials were measured before (pre) and 15 min after (post) injection of 5,5′dibromoBAPTA. No change was observed for either the A291E or ΔP290/A291E receptors, although large shifts in the reversal potential were seen in the nACh receptors used as positive controls. On this basis, PCa was set to 0 for relative permeability determinations.
Relative permeabilities from GHK fits
Using the results from the experiments described in Fig. 4, along with known ionic activities, it was possible to perform least-squares fits of the GHK equation (eqn 3) to calculate the relative permeabilities of sodium, potassium and chloride for each receptor. In Fig. 6, the best fit for each receptor is shown as a continuous curve; dotted or dashed curves show fits with one or more constraints (see Table 2). For ρ1 wild-type receptors, on the basis of results shown in Fig. 4, PNa+ was 0 and PCl- was 1. The best fit was obtained with PK+= 0.03 ± 0.02, which agrees well with published values for native GABAA receptors in spinal cord neurons (Borman et al. 1987). Other possibilities were tested and then rejected on the basis of poorer fits (Table 2, Fig. 6A, dotted and dashed curves). Similarly, the best fit for T305V receptors was PK+/PCl-= 0.07 ± 0.03 (not significantly different from wild-type channels; P > 0.20). Evidently, the 13′ mutation alone had no impact on chloride permeability.
Figure 6. Fits of the Goldman-Hodgkin-Katz (GHK) equation to determine relative ionic permeabilities.
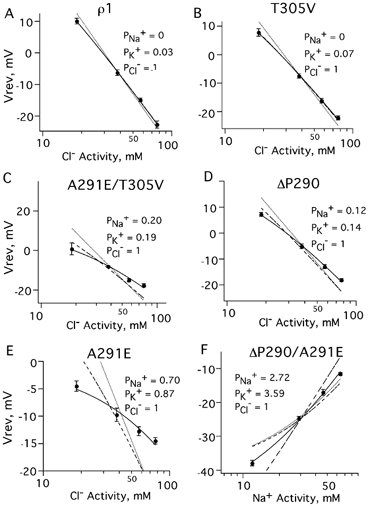
Least-squares fits using internal ion activities (Barish, 1983) and the changes in reversal potential at various chloride or sodium concentrations were used to calculate the permeability of sodium and potassium relative to chloride. An unconstrained fit including PNa+ and PK+ for each mutant is shown as a continuous line; alternate fits are shown as dotted and dashed lines (see Table 2 for permeability values and constraints). A, for ρ1 wild-type receptors, PNa+ was 0 with PCl-= 1. The best fit was obtained when PK+= 0.03 ± 0.02. Constraining PK+ to 0 gave a fit that deviated from the data at higher chloride concentrations (dotted line) while constraining PNa+ to 0 (dashed line) was the same as the unconstrained fit (continuous line). B, the best fit for T305V was similar to the wild-type channel, with relative PK+= 0.07 ± 0.03. C, since A291E/T305V was not perfectly chloride selective (Fig. 4), we fitted both PNa+ and PK+, yielding PNa+= 0.20 ± 0.10 and PK+= 0.19 ± 0.08. Excluding either sodium (dashed line) or potassium (dotted line) from the fit resulted in significant deviations from the data. D, ΔP290 was significantly more cation permeable than the wild-type receptor, with relative PNa+= 0.12 ± 0.08 and PK+= 0.14 ± 0.03. Excluding either sodium (dashed line) or potassium (dotted line) from the fit resulted in significant deviations from the data. E, for A291E, PNa+= 0.70 ± 0.11, PK+= 0.87 ± 0.10 and PCl-= 1 gave the best fit. Excluding either cation resulted in a poor fit. F, for ΔP290/A291E PCl-= 1, PK+= 3.59 ± 1.17 and PNa+= 2.72 ± 0.79 provided the best description of the data.
Table 2.
Summary of ionic permeabilities
| Symbol | PNa+ | PK+ | PCl− | Best fit | |
|---|---|---|---|---|---|
| ρ1 | —— | 0 | 0.03 | 1† | |
| …………… | 0 | 0† | 1† | ||
| –––– | 0† | 0.03 | 1† | * | |
| T305V | —— | 0 | 0.07 | 1† | |
| …………… | 0 | 0† | 1† | ||
| –––– | 0† | 0.03 | 1† | * | |
| A291E/T305V | —— | 0.20 | 0.19 | 1† | * |
| …………… | 0 | 0† | 1† | ||
| ––- | 0† | 0.10 | 1† | ||
| ΔP290 | —— | 0.12 | 0.14 | 1† | * |
| …………… | 0.05 | 0† | 1† | ||
| –––– | 0† | 0.01 | 1† | ||
| A291E | —— | 0.70 | 0.87 | 1† | * |
| …………… | 0.09 | 0† | 1† | ||
| ––––– | 0† | 0.03 | 1† | ||
| ΔP290/A291E | —— | 2.72 | 3.59 | 1† | * |
| …………… | 0.99 | 1† | 1† | ||
| –––––- | 1† | 1.05 | 1† | ||
| –– | 1.01 | 0.99 | 0† | ||
| R292E | —— | 2.4 | 3.0 | 1† | * |
| …………… | 0.92 | 1† | 1† | ||
| –––––- | 1† | 1.14 | 1† | ||
| –– | 0.87 | 1.13 | 0† | ||
| R292C | —— | 0.03 | 0.15 | 1† | |
| …………… | 0 | 0† | 1† | ||
| –––– | 0† | 0.14 | 1† | * | |
| R292M | —— | 0.06 | 0.17 | 1† | |
| …………… | 0 | 0† | 1† | ||
| –––––- | 0† | 0.11 | 1† | * | |
| R292K | —— | 0.13 | 0.30 | 1† | * |
| …………… | 0 | 0† | 1† | ||
| –––– | 0† | 014 | 1† |
Summary of the ionic permeabilities for the fits shown in Fig. 6 and Fig. 8. The line style in the symbol column refers to the fits shown in Fig. 6 and Fig. 8. Parameters constrained during fitting are indicated by
The best fit is indicated by an asterisk, and was determined by the F-ratio test (P < 0.05).
Figure 8. R292 mutant GHK fits show that a negative charge at the 0′ position resulted in a cation-preferring receptor.
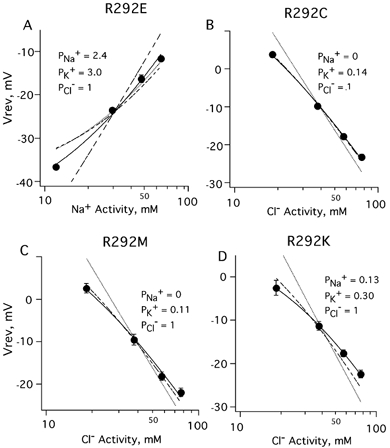
A, for the R292E mutant, the best fit of reversal potentials measured at various sodium concentrations was PNa+ : PK+ : PCl-= 2.40 ± 0.20 : 3.0 ± 0.24 : 1 (continuous curve). Constraining PCl- to 0 resulted in a poor fit (long-dashed curve), as did constraining PNa+ or PK+ to zero (short-dashed and dotted curves). B, neutralization of the 0′ positive charge by substitution with cysteine resulted in a chloride channel with elevated cation permeability. The best fit for R292C mutant channels gave PNa+= 0 and PK+= 0.14 ± 0.10 (dashed curve) and was not significantly different from a fit that included sodium permeability (continuous curve). Setting PK+ to zero gave an unsatisfactory fit (dotted curve). C, substitution by methionine also resulted in a channel with elevated cation permeability. The best fit for R292M gave PNa+= 0 and PK+= 0.11 ± 0.01 (dashed curve) and was the same as a fit that included PNa+ (continuous curve). Constraining PK+ to zero gave unsatisfactory fits (dotted curve). D, the best fit of the data upon replacement of arginine by lysine was PNa+= 0.13 ± 0.04, PK+= 0.30 ± 0.04 and PCl-= 1 (continuous curve). Setting PNa+ or PK+ to zero gave unsatisfactory fits (dashed and dotted curves).
From Fig. 4C, it appeared that the double mutant A291E/T305V was permeable to chloride, but impermeable to sodium. However, permeabilities relative to chloride of PK+= 0.20 ± 0.10 and PNa+= 0.19 ± 0.08 resulted in an excellent fit (Fig. 6C, Table 2). Setting either cation permeability coefficient to zero gave poor results. For ΔP290, an excellent fit was obtained with PK+= 0.14 ± 0.03 and PNa+= 0.12 ± 0.08 (Fig. 6D).
A291E had nearly symmetrical reversal potential shifts when chloride or sodium were replaced, indicating that both ions were permeant. The data were well fitted with PNa+= 0.70 ± 0.11 and PK+= 0.87 ± 0.10 (Fig. 6E, Table 2). Setting either cation permeability coefficient to zero resulted in large deviations from the data (Fig. 6E, Table 2). Evidently, the A291E mutation resulted in a pore that was nearly non-selective for sodium, potassium and chloride.
For ΔP290/A291E, the cation-preferring mutant, the best fit was obtained with PK+= 3.59 ± 1.07, PNa+= 2.72 ± 0.79 and PCl-= 1 (Fig. 6, Table 2). This double mutation resulted in a channel with equal sodium and potassium permeability and little chloride permeability, a reversal of ionic selectivity from the wild-type receptor.
Permeability characteristics confirmed with known intracellular composition
Since the results shown in Fig. 6 were based on estimated intracellular ion concentrations, we repeated select reversal potential measurements in whole-cell patch-clamp mode in transiently transfected HEK cells, where the intracellular ion concentrations are controlled. In general, the data from HEK cells were similar to the results from oocytes. The wild-type receptor formed a chloride-selective pore with potassium permeability of 0.05 ± 0.04 (Table 3) and no change in reversal potential when 130 mm sodium was replaced by choline (sodium Vrev=−26.0 ± 1.9 mV, n = 7; choline Vrev=−25.7 ± 2.7 mV, n = 6, P > 0.2, not shown). ΔP290 was chloride selective, with small cation permeabilities (0.09 ± 0.04, 0.20 ± 0.07, 1), not significantly different from those derived from oocyte experiments (0.12 ± 0.08, 0.14 ± 0.03, 1). A291E expressed in HEK cells formed channels that were nearly non-selective (0.77 ± 0.11, 0.96 ± 0.12, 1), with permeability values similar to those from oocyte experiments (Table 3). The double mutant, ΔP290/A291E, was cation selective. The best fit showed that sodium permeability and potassium permeability were about threefold greater than chloride permeability (3.24 ± 0.60, 2.98 ± 0.69, 1). Neither the wild-type channels nor the mutants we tested showed any significant change in permeability profiles that depended on whether receptors were expressed in Xenopus oocytes or in the mammalian HEK293 cell line.
Table 3.
Relative permeabilities measured in oocytes and human embryonic kidney (HEK)293 cells
| Oocyte | HEK293 | |
|---|---|---|
| Wild-type | 0 ± 0.01: 0.03 ±0.02: 1 | 0 ± 0.05: 0.05 ± 0.05: 1 |
| ΔP290 | 0.12 ±0.08: 0.14 ± 0.03: 1 | 0.09 ± 0.04: 0.20 ± 0.07: 1 |
| A291E | 0.70 ±0.11: 0.87 ± 0.10: 1 | 0.77 ± 0.11: 0.96 ± 0.12: 1 |
| ΔP290/A291E | 2.72 ± 0.50: 3.59 ±0.60: 1 | 3.24 ± 0.60: 2.98 ± 0.69: 1 |
Values are the means ± standard deviations of relative permeabilities; PNa+ : PK+ : Pcl−. There is no significant difference between permeability values from different expression systems (P > 0.20).
Size selectivity in cation-permeable mutants
We have estimated the ρ1 wild-type pore to be 6 Å in diameter by fitting permeabilities as a function of anion size (Wotring et al. 1999). It is possible that the mutations described in this study changed the effective size of the pore, and this in turn resulted in altered selectivity. To test this possibility for the cation-permeable mutants, we measured reversal potentials in solutions containing cations of various sizes (results shown in Fig. 7). In this experiment, a change in the reversal potential (plotted as the ordinate) would indicate that the test cation permeability differs from the sodium permeability. The reversal potentials of the A291E mutant did not shift significantly for lithium, potassium, rubidium or caesium, but there were shifts in choline and TEA (Fig. 7, open circles). Using fitted values from Table 2 (0.70, 0.87, 1), the predicted reversal potential for replacement of 50 mm sodium by a totally impermeant cation would be −10 mV (shown in Fig. 7 as a dotted open circle), which is in excellent agreement with the observed TEA shift of −9.3 ± 0.9 mV. Evidently, cations smaller than 5 Å are permeant, while cations larger than 7 Å are excluded.
Figure 7. Permeability changes in A291E and ΔP290/A291E are not associated with changes in the size of the pore.

In order to estimate the size of the channel pore of the cation-permeable mutants, reversal potentials were measured in solutions containing cations of different sizes. For both A291E (open circles) and ΔP290/A291E (filled circles) receptors, the TEA reversal potential was the same as the predicted value for an impermeant ion (shown as dotted circles). Both mutant receptors seem to have a size cut-off between 5 and 7 Å, within the range of the 6 Å wild-type anionic cut-off (Wotring et al. 1999).
For the ΔP290/A291E mutant receptor, positive reversal potential shifts were seen for lithium, potassium, rubidium, caesium and hydroxylammonium, indicating that these cations were somewhat more permeant than sodium (Fig. 7, filled circles). In TEA, however, the reversal potential shifted to −24.3 ± 0.5 mV (n = 5) from −10.1 ± 0.5 mV (n = 25) in OR2, the same as when sodium chloride was replaced by iso-osmotic sucrose (−23.5 ± 0.9 mV, n = 5). This result is in good agreement with the predicted reversal potential shift for an impermeant cation in a chloride-impermeable channel (TEA ΔVrev=−13.1 ± 0.8 mV, n = 25; predicted Vrev is shown in Fig. 7 as a dotted closed circle). Similar to A291E, an ionic radius between 5 and 6 Å seemed to be a threshold for cation permeability of the ΔP290/A291E mutant. Evidently, neither of these mutations significantly altered the apparent size of the pore.
Mutations at the 0′ positive charge and ion selectivity
As evident in Fig. 1, all members of this LGIC family have a positive charge at the 0′ position. In anion-conducting pores, however, the 0′ residue is arginine, while in cation-conducting pores (excluding the 5-HT3 receptor which has arginine), it is lysine. In order to establish the role of the highly conserved positive charge and to directly test the idea that charged residues at the intracellular mouth of the pore are involved in selectivity, we constructed several mutations at this position. R292M and R292C eliminated the positive charge, R292E reversed its polarity, and R292K conserved the positive charge. Elimination of the positive charge (R292M and R292C) slightly elevated the cation permeability compared to wild-type. The best GHK fits for R292C and R292M were PNa+ : PK+ : PCl-= 0 : 0.14 ± 0.01 : 1, and 0 : 0.11 ± 0.01 : 1, respectively. The R292K mutant was somewhat more cation-permeable with PNa+ : PK+ : PCl-= 0.13 ± 0.04 : 0.30 ± 0.04 : 1. Replacement of arginine with glutamate produced a channel that favoured passage of cations (PNa+ : PK+ : PCl-= 2.4 ± 0.20 : 3.0 ± 0.24 : 1), very similar to the double mutant ΔP290/A291E.
DISCUSSION
Summary and comparison with other studies
Three mutations at the −2′, −1′ and 13′ positions in TM2 have been shown previously to reverse the ion selectivity of nACh α7, Gly α1, GABAA and 5-HT3 receptors, suggesting that these sites represent a conserved structural feature in this family of receptors (Galzi et al. 1992; Corringer et al. 1999; Keramides et al. 2000; Gunthorpe & Lummis, 2001; Jensen, et al. 2002). We have shown that for the homomeric ρ1 GABA receptor, mutation of only two amino acids (at positions −2′ and −1′) was sufficient to reverse pore selectivity from anionic to cationic. Similarly, the homologous pair of mutations in glycine α1 had a reduced permeability to chloride (PCl-/PNa+= 0.13) (Keramides et al. 2002). Recently, homologous mutations of the GABAAβ subunit also resulted in a cation-preferring channel (Jensen et al. 2002). At present we do not know if the ρ1 receptor exhibits a similar stoichiometric requirement for reversal of selectivity. We also show that the −2′/-1′ pair of mutations is not unique in its effects on selectivity, since mutation of the neighbouring 0′ arginine to glutamate resulted in a cation-preferring pore. While we cannot rule out the effects of distant structural changes caused by mutagenesis, there appears to be some flexibility or redundancy in the placement of charged residues that can result in selectivity changes. Evidently, key elements of the ρ1 selectivity filter are near the intracellular end of TM2. We found that mutation of the 13′ residue alone had almost no effect on permeability, but when combined with A291E, some cation permeability was seen, but to a much smaller extent than the A291E mutation alone. The basis for the difference between the ρ1 GABA receptor and the other members of this superfamily is unclear. Wang et al. (1999) have shown that a mutation of an asparagine adjacent to the TM2 extracellular ring (Imoto et al. 1988) of the Drosophila GABA-gated chloride channel (Rdl) increased cation permeability. They concluded that this increased permeability was the result of a structural change extending beyond the substituted residue.
The −1′ alanine aligns with the previously identified intermediate ring of the nACh receptor. Several studies have proposed that this particular region forms the narrowest part of the pore (Imoto et al. 1988, 1991; Cohen et al. 1992a,b; Villarroel & Sakmann, 1992; Wang & Imoto, 1992; Corringer et al. 1999). Mutation of the nearby 2′ threonine in nACh α1 altered the single-channel conductance in proportion to the size of the substituted residue, suggesting a constriction in this region that may be acting like a molecular sieve (Villarroel & Sakmann, 1992). Substitutions of the glutamate at the intermediate ring of the nACh receptor decreased the permeability of organic cations in a manner that depended upon the number of subunits carrying the charge. The authors proposed a model in which repulsion helps sustain the proper pore size (Wang & Imoto, 1992). It has also been suggested that this narrow region is essentially formed by a single turn of an α-helix and the pore opens up into a wider vestibule on either side (Cohen et al. 1992a). In this regard, the electrostatic potential at the mouth of the pore (entirely due to the intermediate ring) can be sensed by ions approaching the narrow selectivity filter from either side of the membrane (Wilson & Karlin, 1998). While such details must await the structural resolution of the pore, the notion that the domain at the intracellular end of TM2 forms a narrow part of the pore and plays a key role in ion selectivity seems established.
Replacing the alanine at position 291 with glutamate resulted in a pore that was permeable to both monovalent cations and anions with a diameter less than 6 Å. Calcium, as well as ions with a diameter of >7 Å, were impermeant. This result is similar to that of Keramides et al. (2002) for the corresponding glycine α1 point mutant with PCl-/PNa+= 0.34. A variety of non-selective, cation-permeable pores have been characterized, but none that demonstrates a comparable permeability to both cations and anions. For example, the gap junction channel is one of the least discriminatory channels documented, but still shows a 5-fold preference for cations over anions (Veenstra et al. 1994; Trexler et al. 1996).
Since the mutations changed the ρ1 amino acid sequence, at least partially, to that of the calcium-permeable nAChR α7 receptor, we considered the possibility that the mutant receptors might be calcium-permeable. In fact, the A291E and ΔP290/A291E mutants, although cation-permeable, exhibited no calcium permeability. In previous studies examining homologous mutations in other LGIC subunits, calcium permeability has proven difficult to associate with specific mutations. For example, using homologous mutations in the glycine α1 subunit, a −2′/-1′ double mutant exhibited calcium permeability, while a −2′/-1′/13′ triple mutant and a −1′ point mutant were impermeable to calcium (Keramides et al. 2000, 2002). In contrast, mutation of the −1′ residue of the nAChR α7 was sufficient to eliminate its calcium permeability (Galzi et al. 1992). Although some combinations of −2′/-1′/13′ mutants have been shown to alter calcium permeability in other superfamily members, significant calcium permeability was not observed in any of the ρ1 mutants we tested. It has been shown that mutations at the 16′ and 17′ residues of nAChR α7 TM2 alter permeability to calcium without affecting monovalent cations (Bertrand et al. 1993), and our data is consistent with their conclusion that permeabilities to divalent and monovalent cations appear to be determined by distinct domains.
Models of ion permeation in chloride channels
Although the mechanism of ion selectivity in these LGICs has not yet been elucidated, a hypothesis has been proposed to explain selectivity in the related nACh receptor. Wang & Imoto (1992) noted that mutations at −5′, −1′ and 20′ positions of M2 (the intracellular, intermediate and extracellular rings, respectively) altered ion selectivity. They suggested that these three rings of negative charges governed cation selectivity in nicotinic receptors. However, this reasoning cannot be used to explain anion selectivity in GABA and glycine receptors, since anion channel subunits do not contain three corresponding rings of positive charge.
There are, however, models proposed to explain the mechanism of chloride selectivity in other types of anionic pores. Franciolini & Nonner (1987) proposed that in a voltage-dependent chloride channel, anion permeation requires the presence of a cation in the pore to screen a negatively charged site. This creates a positively charged site that acts as the selectivity filter. This model explained their observation that current was always carried by anions but was detected only in the presence of cations. Another model, based on the cystic fibrosis transmembrane conductance regulator, suggests that chloride selectivity can be explained by an energetically favourable interaction between chloride ions and a polarizable, as opposed to charged, pore (Smith et al. 1999). This model minimizes the role of any specific amino acid residues and focuses instead on a more general interaction between the ion and pore wall. Another recent model put forward by Dutzler et al. (2002) is based on the first crystal structure of the ClC channel. These authors propose that the selectivity filter is formed by partial positive charges that stabilize the presence of an anion in the pore. Coordination of an ion by several partial charges results in the ideal situation for ion throughput; the ion is stabilized to some degree, but not so stable that conductance is limited. Partial charges are contributed by the inherent dipole of α-helices along with side-chain hydroxyls and backbone nitrogens. The extracellular and intracellular vestibules are also somewhat positively charged, thus tending to concentrate anions, or even to help pull them through the pore. At present we do not know if any of these chloride channel models provide a good description of anion selectivity and permeation in GABA receptors. However, our results are consistent with a simple electrostatic model in which charge near the intracellular end of M2 is a critical element of the selectivity filter. While a validation of this model must await a structural comparison of cationic and anionic members of this LGIC superfamily, it seems an inescapable conclusion that this domain plays a pivotal role in ionic selectivity.
Acknowledgments
This work was supported by National Institutes of Health grants NS35291 (DSW) and MH11793 (VEW).
references
- Amin J, Weiss DS. Homomeric ρ1 GABA channels: activation properties and domains. Receptors Channels. 1994;2:227–236. [PubMed] [Google Scholar]
- Barish ME. A transient calcium-dependent chloride current in the immature Xenopus oocyte. J Physiol. 1983;342:309–325. doi: 10.1113/jphysiol.1983.sp014852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand D, Galzi J-L, Devillers-Thiery A, Bertrand S, Changeux JP. Mutations at two distinct sites within the channel domain M2 alter calcium permeability of neuronal α7 nicotinic receptor. Proc Natl Acad Sci U S A. 1993;90:6971–6975. doi: 10.1073/pnas.90.15.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann J, Hamill OP, Sakmann B. Mechanism of ion permeation through channels gated by glycine and γ-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter J, Connolly E, Deneris E, Goldman D, Heinemann S, Patrick J. Functional expression of two neuronal nicotinic acetylcholine receptors from cDNA clones identifies a gene family. Proc Natl Acad Sci U S A. 1987;84:7763–7767. doi: 10.1073/pnas.84.21.7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitinger H-G, Villmann C, Becker K, Becker C-M. Opposing effects of molecular volume and charge at the hypereckplexia site α1(P250) govern glycine receptor activation and desensitization. J Biol Chem. 2001;276:29657–29663. doi: 10.1074/jbc.M100446200. [DOI] [PubMed] [Google Scholar]
- Cohen BN, Labarca C, Davidson N, Lester HA. Tris+/Na+ permeability ratios of nicotinic acetylcholine receptors are reduced by mutations near the intracellular end of the M2 region. J Gen Physiol. 1992a;99:545–572. doi: 10.1085/jgp.99.4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen BN, Labarca C, Davidson N, Lester HA. Mutations in M2 alter the selectivity of the mouse nicotinic acetylcholine receptor for organic and alkali metal cations. J Gen Physiol. 1992b;100:373–400. doi: 10.1085/jgp.100.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corringer P-J, Bertrand S, Galzi J-L, Devillers-Thiery A, Changeux J-P, Bertrand D. Mutational analysis of the charge selectivity filter of the α7 nicotinic acetylcholine receptor. Neuron. 1999;22:831–843. doi: 10.1016/s0896-6273(00)80741-2. [DOI] [PubMed] [Google Scholar]
- Dutzler R, Campbell EB, Cadene M, Chalt BT, MacKinnon R. X-ray structure of a ClC chloride channel at 3. 0 Å reveals the molecular basis of anion selectivity. Nature. 2002;415:287–294. doi: 10.1038/415287a. [DOI] [PubMed] [Google Scholar]
- Franciolini F, Nonner W. Anion and cation permeability of a chloride channel in rat hippocampal neurons. J Gen Physiol. 1987;90:453–478. doi: 10.1085/jgp.90.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galzi J-L, Devillers-Thiery A, Hussy N, Bertrand S, Changeux J-P, Bertrand D. Mutations in the channel domain of a neuronal nicotinic receptor convert ion selectivity from cationic to anionic. Nature. 1992;359:500–505. doi: 10.1038/359500a0. [DOI] [PubMed] [Google Scholar]
- Gerzanich V, Wang F, Kuryatov A, Lindstrom J. α5 subunit alters desensitization, pharmacology, Ca++ permeability, and Ca++ modulation of human neuronal α3 nicotinic receptors. J Pharmacol Exp Ther. 1998;286:311–320. [PubMed] [Google Scholar]
- Goldman DE. Potential, impedance and rectification in membranes. J Gen Physiol. 1943;27:37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenningloh G, Rienitz A, Schmitt B, Methfessel C, Zensen M, Beyreuther K, Gundelfinger ED, Betz H. The strychnine-binding subunit of the glycine receptor shows homology with nicotinic acetylcholine receptors. Nature. 1987;328:215–220. doi: 10.1038/328215a0. [DOI] [PubMed] [Google Scholar]
- Grosman C, Salamone F, Sine S, Auerbach A. The extracellular linker of muscle acetylcholine receptor channels is a gating control element. J Gen Physiol. 2000;116:327–339. doi: 10.1085/jgp.116.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunthorpe MJ, Lummis SCR. Conversion of the ion selectivity of the 5-HT3A receptor from cationic to anionic reveals a conserved feature of the ligand-gated ion channel superfamily. J Biol Chem. 2001;276:10977–10983. [PubMed] [Google Scholar]
- Hodgkin AL, Katz B. The effect of sodium ions on the electrical activity of the giant axon of the squid. J Physiol. 1949;108:37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imoto K, Busch C, Sakmann B, Mishina M, Konno T, Nakai J, Bujo H, Mori Y, Fukuda K, Numa S. Rings of negatively charged amino acids determine the acetylcholine receptor channel conductance. Nature. 1988;335:645–648. doi: 10.1038/335645a0. [DOI] [PubMed] [Google Scholar]
- Imoto K, Konno T, Nakai J, Wang F, Mishina M, Numa S. A ring of uncharged polar amino acids as a component of channel constriction in the nicotinic acetylcholine receptor. FEBS Lett. 1991;289:193–200. doi: 10.1016/0014-5793(91)81068-j. [DOI] [PubMed] [Google Scholar]
- Jensen M, Timmermann D, Johansen T, Schousboe A, Varming T, Ahring P. The β subunit determines the ion selectivity of the GABAA receptor. J Biol Chem. 2002;277:41438–41447. doi: 10.1074/jbc.M205645200. [DOI] [PubMed] [Google Scholar]
- Kammann M, Laufs J, Schell J, Gronenborn B. Rapid insertional mutagenesis of DNA by polymerase chain reaction (PCR) Nucleic Acids Res. 1989;17:5404. doi: 10.1093/nar/17.13.5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keramides A, Moorhouse AJ, French CR, Schofield PR, Barry PH. M2 pore mutations convert the glycine receptor channel from being anion- to cation-selective. Biophys J. 2000;78:247–259. doi: 10.1016/S0006-3495(00)76287-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keramides A, Moorhouse AJ, Pierce KD, Schofield PR, Barry PH. Cation-selective mutations in the M2 domain of the inhibitory glycine receptor channel reveal determinants of ion-charge selectivity. J Gen Physiol. 2002;119:393–410. doi: 10.1085/jgp.20028552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liman ER, Hess P, Weaver F, Koren G. Voltage-sensing residues in the S4 region of a mammalian K+ channel. Nature. 1991;353:752–756. doi: 10.1038/353752a0. [DOI] [PubMed] [Google Scholar]
- Maricq A, Peterson A, Brake A, Myers R, Julius D. Primary structure and functional expression of the 5HT3 receptor, a serotonin-gated ion channel. Science. 1991;25:432–436. doi: 10.1126/science.1718042. [DOI] [PubMed] [Google Scholar]
- Miledi R, Parker I. Chloride current induced by injection of calcium into Xenopus oocytes. J Physiol. 1984;357:173–183. doi: 10.1113/jphysiol.1984.sp015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield PR, Darlison MG, Fujita N, Burt DR, Stephenson FA, Rodriguez H, Rhee LM, Ramachandran J, Reale V, Glencorse T, Seeburg PH, Barnard EA. Sequence and functional expression of the GABAA receptor shows a ligand-gated receptor super-family. Nature. 1987;328:221–227. doi: 10.1038/328221a0. [DOI] [PubMed] [Google Scholar]
- Seguela P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain α7: A nicotine cation channel highly permeable to calcium. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Steinle ED, Meyerhoff ME, Dawson DC. Cystic fibrosis transmembrane conductance regulator: physical basis for lyotropic anion selectivity patterns. J Gen Physiol. 1999;114:799–817. doi: 10.1085/jgp.114.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trexler EB, Bennett MVL, Bargiello TA, Verselis VK. Voltage gating and permeation in a gap junction hemichannel. Proc Natl Acad Sci U S A. 1996;93:5836–5841. doi: 10.1073/pnas.93.12.5836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin N. Nicotinic acetylcholine receptors at 9 Å resolution. J Mol Biol. 1993;229:1101–1124. doi: 10.1006/jmbi.1993.1107. [DOI] [PubMed] [Google Scholar]
- Veenstra RD, Wang H-Z, Beyer EC, Brink PR. Selective dye and ionic permeability of gap junction channels formed by connexion45. Circ Res. 1994;75:483–490. doi: 10.1161/01.res.75.3.483. [DOI] [PubMed] [Google Scholar]
- Villarroel A, Sakmann B. Threonine in the selectivity filter of the acetylcholine receptor channel. Biophys J. 1992;62:196–208. doi: 10.1016/S0006-3495(92)81805-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Imoto K. Pore size and negative charge as structural determinants of permeability in the Torpedo nicotinic acetylcholine receptor channel. Proc R Soc Lond B Biol Sci. 1992;250:11–17. doi: 10.1098/rspb.1992.0124. [DOI] [PubMed] [Google Scholar]
- Wang CT, Zhang HG, Rocheleau TA, Ffrench-Constant, Jackson MB. Cation permeability and cation-anion interactions in a mutant GABA-gated chloride channel from Drosophila. Biophys J. 1999;77:691–700. doi: 10.1016/S0006-3495(99)76924-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson GG, Karlin A. The location of the gate in the acetylcholine receptor channel. Neuron. 1998;20:1269–1281. doi: 10.1016/s0896-6273(00)80506-1. [DOI] [PubMed] [Google Scholar]
- Wotring VE, Chang Y, Weiss DS. Permeability and single channel conductance of human homomeric ρ1 GABAC receptors. J Physiol. 1999;521:327–336. doi: 10.1111/j.1469-7793.1999.00327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


