Abstract
The pharyngeal muscles, such as the genioglossus (GG) muscle of the tongue, are important for effective lung ventilation since they maintain an open airspace. Rapid-eye-movement (REM) sleep, however, recruits powerful neural mechanisms that can abolish GG activity, even during strong reflex respiratory stimulation by elevated CO2. In vitro studies have demonstrated the presence of GABAA receptors on hypoglossal motoneurons, and these and other data have led to the speculation that GABAA mechanisms may contribute to the suppression of hypoglossal motor outflow to the GG muscle in REM sleep. We have developed an animal model that allows us to chronically manipulate neurotransmission at the hypoglossal motor nucleus using microdialysis across natural sleep-wake states in rats. The present study tests the hypothesis that microdialysis perfusion of the GABAA receptor antagonist bicuculline into the hypoglossal motor nucleus will prevent the suppression of GG muscle activity in REM sleep during both room-air and CO2-stimulated breathing. Ten rats were implanted with electroencephalogram and neck muscle electrodes to record sleep-wake states, and GG and diaphragm electrodes for respiratory muscle recording. Microdialysis probes were implanted into the hypoglossal motor nucleus for perfusion of artificial cerebrospinal fluid (ACSF) or 100 μm bicuculline during room-air and CO2-stimulated breathing (7 % inspired CO2). GABAA receptor antagonism at the hypoglossal motor nucleus increased respiratory-related GG activity during both room-air (P = 0.01) and CO2-stimulated breathing (P = 0.007), indicating a background inhibitory GABA tone. However, the effects of bicuculline on GG activity depended on the prevailing sleep-wake state (P < 0.005), with bicuculline increasing GG activity in non-REM (NREM) sleep and wakefulness both in room air and hypercapnia (P < 0.01), but GG activity was effectively abolished in those REM periods without phasic twitches in the GG muscle. This abolition of GG activity in REM sleep occurred regardless of ACSF or bicuculline at the hypoglossal motor nucleus, or room-air or CO2-stimulated breathing (P > 0.63). We conclude that these data in freely behaving rats confirm previous in vitro studies that GABAA receptor mechanisms are present at the hypoglossal motor nucleus and are tonically active, but the data also show that GABAA receptor antagonism at the hypoglossal motor nucleus does not increase GG muscle activity in natural REM sleep.
The genioglossus (GG) muscle of the tongue, in conjunction with other pharyngeal muscles, plays an important role in maintaining an open and less collapsible upper airway for effective breathing (Remmers et al. 1978; Fuller et al. 1999). Sleep, especially rapid-eye-movement (REM) sleep, however, causes periods of major suppression of GG activity both in humans (Sauerland & Harper, 1976) and other animals (Megirian et al. 1985; Parisi et al. 1987; Horner et al. 2002), especially during periods of REM sleep with phasic eye movements (Wiegand et al. 1991). GG activity can be abolished in periods of REM sleep even in the presence of strong excitatory influences on the hypoglossal motor nucleus, the source of motor outflow to the tongue. This is true whether hypoglossal motoneurons are excited by reflex drives such as hypercapnia (Megirian et al. 1985; Parisi et al. 1987; Horner et al. 2002) or when stimulated by local application of the excitatory neurotransmitter serotonin delivered by microdialysis (Jelev et al. 2001). These data suggest that REM sleep can recruit powerful neural mechanisms that markedly suppress motor outflow to the GG muscle. The mechanisms mediating this effect of REM sleep on GG activity, however, are unknown. Determining these mechanisms is important because suppression of GG activity in sleep can lead to airway narrowing and hypoventilation in humans and predispose to obstructive sleep apnoea (Remmers et al. 1978), a common and serious breathing disorder affecting up to 4 % of adults (Young et al. 1993).
Post-synaptic inhibitory mechanisms play a significant role in the suppression of spinal (lumbar) and cranial (massetter) motoneuron activity both in natural REM sleep (Morales et al. 1987a; Pedroarena et al. 1994) and in the REM-sleep-like state elicited by microinjection of carbachol into the pontine reticular formation of decerebrate or anaesthetised animals (Morales et al. 1987b; Lopez-Rodriguez et al. 1995). Inhibitory potentials have also been recorded from hypoglossal motoneurons in the carbachol model of REM sleep in anaesthetised cats (Yamuy et al. 1999; Fung et al. 2000), with preliminary data having also been obtained in naturally sleeping cats (Fung et al. 2001). Evidence suggests that the inhibitory potentials in the carbachol model of REM sleep are glycinergic (Yamuy et al. 1999). The presence of neural circuitry mediating the inhibition of hypoglossal motoneurons is supported by several lines of research (Berger, 2000). For example, strychnine-sensitive inhibitory post-synaptic potentials have been recorded from hypoglossal motoneurons following electrical stimulation of the lingual nerve in cats (Takata & Ogata, 1980). Moreover, several in vitro studies have documented and characterised robust glycinergic inhibitory inputs to hypoglossal motoneurons (Umemiya & Berger, 1995; Singer et al. 1998; O'Brien & Berger, 1999; Donato & Nistri, 2000). Some of these inputs arise from the medullary reticular formation and, as such, may be involved in the inhibition of hypoglossal motoneurons in REM sleep (Chase & Morales, 1994; Yamuy et al. 1999; Berger, 2000). These data, which were obtained in reduced animal preparations, may also have relevance to humans because systemic administration of strychnine to block glycine receptors caused increases in tensor palatini and GG muscle activities and reduced the severity of obstructive sleep apnoea, although data were only reported in a single patient and the relationship to NREM and REM sleep was not reported (Remmers et al. 1980). However, although these data show that glycine-mediated inhibition of hypoglossal motoneurons has been studied previously, little is known about the effects of γ-aminobutyric acid (GABA; Berger, 2000), another major inhibitory neurotransmitter in the central nervous system. Overall, given the importance of the GG muscle to the normal maintenance of upper-airway patency and the pathogenesis of obstructive sleep apnoea (Remmers et al. 1978), it is relevant to determine if GABA mechanisms modulate GG activity across sleep-wake states.
There is widespread distribution of GABA projections and receptors throughout the medulla, including respiratory structures (for reviews see McCrimmon et al. 1995; Berger, 2000). GABAA receptors have been identified on hypoglossal motoneurons, and in vitro studies show inhibitory post-synaptic potentials that are sensitive to bicuculline, a GABAA receptor antagonist (O'Brien & Berger, 1999; Donato & Nistri, 2000, 2001). In anaesthetised animals in vivo, GABAA receptor stimulation at the hypoglossal motor nucleus also markedly suppresses GG activity, with and without respiratory stimulation with CO2 (Liu et al. 2003), in a fashion that resembles the suppression of GG activity and CO2 responses observed in intact animals in REM sleep (Horner et al. 2002). In naturally sleeping cats, GABAA mechanisms play an important role in the inhibition of ascending spinal sensory pathways in REM sleep (Taepavarapruk et al. 2002). This study tests the hypothesis that in intact, freely behaving animals, GABAA receptor antagonism at the hypoglossal motor nucleus will increase GG muscle activity in natural REM sleep with and without reflex respiratory stimulation with CO2.
METHODS
All procedures conformed to the recommendations of the Canadian Council on Animal Care, and the University of Toronto Animal Care Committee approved the experimental protocols. Rats were housed individually, maintained on a 12:12 h light:dark cycle (lights on at 07.00 h), and had free access to food and water.
Anaesthesia and surgical preparation
Studies were performed on 10 male Wistar rats (Charles River; mean body weight, 275 g; range, 227–317 g). Sterile surgery was performed under anaesthesia induced with intraperitoneal ketamine (85 mg kg−1) and xylazine (15 mg kg−1), as described previously (Jelev et al. 2001; Horner et al. 2002). Rats were also injected intraperitoneally with buprenorphine (0.03 mg kg−1), atropine sulphate (1 mg kg−1) and saline (3 ml, 0.9 %). An anaesthesia mask was placed over the snout and the rats breathed spontaneously a 50:50 mixture of room air and O2. Any additional anaesthesia was given by inhalation (isoflurane, typically 0.2–2 %). Effective anaesthesia was judged by abolition of the pedal withdrawal and corneal blink reflexes.
With the rats supine, the ventral surface of the GG was exposed via a submental incision, and dissection of the overlying geniohyoid and mylohyoid muscles. Two insulated, multi-stranded stainless steel wires (AS631; Cooner Wire, Chatsworth, CA, USA) were implanted bilaterally and directly into the GG muscle and secured with sutures and tissue glue. A previous study with section of the medial branches of the hypoglossal nerves showed that GG activity was recorded with such electrodes (Morrison et al. 2002). To record diaphragm activity, two insulated, multistranded stainless steel wires (AS636: Cooner Wire) were sutured onto the costal diaphragm via an abdominal approach. To ensure adequate electrode placements during surgery, the GG and diaphragm signals were monitored on a chart recorder (Grass Model 79D polygraph, 7P511 amplifiers) and by loudspeaker (AM8 Audio Amplifier, Grass). Observation of tongue protrusion in response to electrical stimulation (1.0 V) was also used to confirm that the electrodes were in the GG muscle. The GG and diaphragm wires were tunnelled subcutaneously to a neck incision and the submental and abdominal incisions closed with absorbable sutures.
The rats were placed in a stereotaxic frame (Kopf Model 962, Tujunga, CA, USA) with blunt ear bars. To ensure consistent positioning between rats, the flat skull position was achieved with an alignment tool (Kopf model 944). Two insulated, multistranded stainless steel wires (AS636: Cooner Wire) were sutured onto the dorsal neck muscles to record the neck electromyogram (EMG). To record the electroencephalogram (EEG), two stainless steel screws (0–80) attached to insulated wire (30 gauge) were implanted in the skull (Jelev et al. 2001; Horner et al. 2002).
A small hole was drilled at the junction of the interparietal and occipital bones for placement of the microdialysis guides (CMA/11, Acton, MA, USA). The guides were lowered 13.9 ± 0.1 mm (mean ±s.e.m.) posterior to bregma (range, 13.2–14.6 mm), 0.3 ± 0.03 mm lateral to the midline (range, 0.1–0.4 mm) and aimed 3 mm above the hypoglossal motor nucleus (Jelev et al. 2001). At the end of surgery the GG, diaphragm, EEG and neck EMG electrodes were connected to pins and inserted into a miniature plug (STC-89PI-220ABS, Carleton University, Ottawa, ON, Canada). The plug and microdialysis guides were then affixed to the skull with dental acrylic and anchor screws.
After recovery from the anaesthetic, the rats were transferred to a clean cage and kept warm under a heating lamp until achieving full recovery, as judged by normal locomotor activity, grooming, drinking and eating. Rats were given soft food for the 1st day after surgery. All rats recovered fully and were housed individually. The rats recovered for an average of 6.8 ± 0.3 days (range, 5–8 days) before the experiments.
Recording procedures
For recordings, a lightweight shielded cable was connected to the plug on the rat's head. The cable was attached to a counterbalanced swivel that permitted free movement. For purposes of habituation, the rats were placed in a recording chamber (MD-1500, BAS, West Lafayette, IN, USA) the day before the experiments with fresh bedding, food and water. The recording chamber was situated inside an electrically insulated, sound-attenuated cubicle (EPC-010, BRS/LVE, Laurel, MD, USA). A video camera inside the cubicle allowed continuous remote monitoring.
A customised lid on the recording chamber contained a centre hole for the cable carrying the electrical signals from the rat. To disperse and mix the air as it flowed into the chamber, the lid also contained three air inlet ports with small computer fans (Model FP-108G/DC, 12 V, Commonwealth, driven at 6 V DC). The volume inside the chamber was approximately 20.5 l. Air or CO2 mixtures (see below) were delivered at approximately 5 l min−1 after humidification over a water reservoir. CO2 levels were measured continuously (Beckman LB-2) at a flow rate of 500 cm3 min−1, with the sampled air being returned to the chamber. A calibrated thermohygrometer probe (Model 37950–10, Cole-Parmer Instruments, Vernon Hills, IL, USA) continuously monitored temperature and relative humidity, which averaged 26.1 ± 0.1 °C (range, 23.8–28.8 °C) and 32.4 ± 0.2 % (range, 23.7–42.4 %), respectively.
The electrical signals were amplified and filtered (Super-Z head-stage amplifiers and BMA-400 amplifiers/filters, CWE, Ardmore, PA, USA). The EEG was filtered between 1 and 100 Hz, while the GG, diaphragm and neck EMGs were filtered between 100 and 1000 Hz. The ECG was removed from the diaphragm EMG using an oscilloscope and an electronic blanker (Model SB-1, CWE). The moving-time averages (time constant, 200 ms) of the GG, diaphragm and neck EMGs were also obtained (Coulbourn S76–01, Lehigh Valley, PA, USA). The EEG and EMGs were calibrated using the built-in calibrator (20 μV to 1 mV) on the head-stage amplifiers. All signals, along with the moving-time averages, were digitised and recorded visually on chart paper (Grass model 78D polygraph) and digitally on computer (Spike 2 software, 1401 interface, CED, Cambridge, UK).
Microdialysis
On the evening before the experiments the rats were gently restrained while the internal dummy cannula was removed from the guide and the microdialysis probe (CMA/11 14/01) was inserted. The probes projected 3 mm from the tip of the guide and were 240 μm in diameter with a 1 mm membrane and a 6000 Da cut-off. The ease of insertion of the probe ensured that the rat was disturbed minimally by the procedure. In each rat, a burst of GG activity was observed on chart and detected audibly on loudspeaker when the probe was initially inserted and penetrated the hypoglossal motor nucleus, and this was useful as a preliminary confirmation of probe placement (Jelev et al. 2001). The burst of GG activity observed during probe insertion was transient (lasting 4.0 ± 1.0 min) and did not occur in the neck or diaphragm signals (Jelev et al. 2001). Each probe was connected to FEP Teflon tubing (inside diameter, 0.12 mm) with this tubing connected to 1.0 ml syringes via a zero dead-space switch (Uniswitch, BAS, West Lafayette, IN, USA). The probes were continually flushed with artificial cerebrospinal fluid (ACSF) at a flow rate of 2.1 μl min−1 using a syringe pump and controller (MD-1001 and MD-1020, BAS). With the length of tubing used for the experiments, the lag time for fluid to travel from the switch to the tip of the probe was 10 min 34 s at this flow rate. The composition (mm) of the ACSF was: NaCl 125, KCl 3, KH2PO4 1, CaCl2 2, MgSO4 1, NaHCO3 25 and d-glucose 30. The ACSF was warmed to 37 °C and bubbled with CO2 to a pH of 7.36 ± 0.01. The CaCl2 was added after warming the ACSF to 37 °C (Nattie & Li, 2000). The experiments began the morning after probe insertion, and were typically performed between 07.00 and 20.00 h (i.e. the time of day during which the rats normally sleep).
Protocol
During microdialysis perfusion of ACSF (control) into the hypoglossal motor nucleus, recordings were made while the animals breathed room air and while breathing was stimulated with 7 % inspired CO2 (actual delivered CO2, 7.22 ± 0.08 %). Hypercapnia produced robust GG stimulation in NREM sleep, thereby allowing the changes from NREM to REM sleep to be observed readily (Horner et al. 2002), even during perfusion of the hypoglossal motor nucleus with ACSF when the level of GG activity was normally low (Fig. 4 and see Jelev et al. 2001; Horner et al. 2002). Stimulation with 7 % CO2 was also chosen because it is sufficient to overcome the vagal inhibition of GG activity, which is particularly strong in the intact rat (Bailey et al. 2001; Horner et al. 2002). Data were obtained for at least two full sleep cycles (i.e. periods containing wakefulness, NREM and REM sleep) with ACSF at the hypoglossal motor nucleus during room-air breathing, and for at least two full sleep cycles with CO2-stimulated breathing. The perfusion medium was then switched to bicuculline (GABAA receptor antagonist, [-]-bicuculline methiodide, FW: 509.3, Sigma) at a dose of 100 μm, pH 7.41 ± 0.06, which was similar to the pH of the ACSF controls (see above). Initial studies on anaesthetised rats showed that 100 μm bicuculline effectively blocks the inhibition of hypoglossal motor output by GABAA receptor stimulation with muscimol (Liu et al. 2003). In the presence of bicuculline, recordings were also made with the rats breathing room air and 7 % inspired CO2 (actual delivered CO2, 7.15 ± 0.06 %) again for at least two full sleep cycles for each inspired gas mixture. A minimum of 30 min was allowed to elapse after the switch between drugs before any data were analysed. At this time a clear increase in GG activity with bicuculline was observed, indicative of a drug effect (see Results). Nevertheless, the increase in GG activity after the switch to bicuculline was gradual, making determination of the latencies to a response difficult to determine with any confidence, especially because spontaneous alterations in sleep-wake states also obscured the time at which GG activity started to increase. However, in some rats serotonin was also applied to the hypoglossal motor nucleus at the end of the experiments (see below) and the increase in GG activity was more marked with this neurotransmitter and the latencies to a response are reported.
Figure 4. Group data showing GG responses to bicuculline at the hypoglossal motor nucleus.
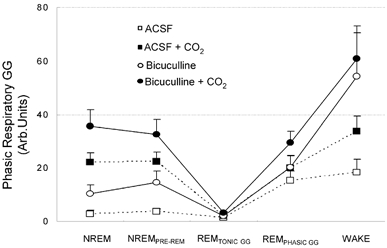
Group data showing changes in respiratory-related GG activity across all sleep-wake states during microdialysis perfusion of ACSF and bicuculline into the hypoglossal motor nucleus during both room-air and CO2-stimulated breathing. Note that GG activity was effectively abolished in periods of REMTONIC GG across all conditions. All values are means ±s.e.m.
Distribution of perfusate within the hypoglossal motor nucleus
In six separate rats, anaesthetised with ketamine and xylazine (85 and 15 mg kg−1, respectively) and maintained with isoflurane, fluorescein (Molecular Probes, Eugene, OR, USA) was delivered by microdialysis to the hypoglossal motor nucleus. We chose fluorescein as it has a molecular weight similar to bicuculline (332.3 vs. 509.3). Rats were perfused at 2.1 μl min−1 with 100 μm fluorescein dissolved in ACSF (i.e. the same dose as bicuculline) for 1 h in three rats and 6 h in three other rats.
Data analysis
Analysis of the EMG and EEG signals. The EMGs were analysed every 5 s from the respective moving-time average signal (above electrical zero) and were quantified in arbitrary units. Electrical zero was the voltage recorded with the amplifier inputs grounded. GG was quantified as mean tonic activity (i.e. basal activity in expiration), peak activity and phasic respiratory activity (i.e. peak inspiratory activity – tonic activity). Mean neck muscle activity, diaphragm amplitudes (i.e. phasic respiratory diaphragm activity) and respiratory rates were also calculated for each 5 s period. Diaphragm minute activity was calculated as the product of diaphragm amplitude and respiratory rate. The EEG was sampled by computer at 500 Hz then analysed on overlapping segments of 1024 samples, windowed using a raised cosine (Hamming) function and subjected to a fast Fourier transform to yield the power spectrum. The window was advanced in steps of 512 samples, and the mean power spectrum of the EEG signal over each 5 s epoch was calculated. The power contained within six frequency bands was recorded as absolute power and as a percentage of the total power of the signal. The band limits were δ2 (0.5–2 Hz), δ1 (2–4 Hz), Θ (4–7.5 Hz), α (7.5–13.5 Hz), β1 (13.5–20 Hz) and β2 (20–30 Hz). Overall, the analysis provided breath-by-breath measurements of GG and diaphragm activities, and mean neck and EEG frequencies calculated and averaged in consecutive 5 s bins for the periods of sleep-wake states that were analysed in each rat (see below).
Measurements across sleep-wake states. The moving-time averages of the GG, neck and diaphragm EMGs were analysed in established periods of wakefulness, NREM and REM sleep identified by standard EEG and EMG criteria (Jelev et al. 2001). Measurements were made during 30 s periods of quiet wakefulness (in the absence of behaviours such as eating, drinking or grooming) and were compared to preceding periods of NREM and REM sleep that occurred closest in time to each other. Accordingly, the periods of NREM that were analysed occurred immediately before the REM periods and were therefore designated NREMPRE-REM. However, neuronal systems that contribute to the generation and maintenance of REM sleep can increase their discharge during NREM periods prior to the onset of EEG-defined REM sleep (Trulson & Jacobs, 1979; Aston-Jones & Bloom, 1981; el Mansari et al. 1989). This effect may therefore have minimised the chance of detecting a potential contribution of GABAA mechanisms to the suppression of GG activity in REM sleep if such mechanisms were recruited prior to the onset of EEG-defined REM sleep. Accordingly, measurements of GG activity were also performed in additional NREM periods that preceded, and were separated in time (36.3 ± 41.0 s) from, the subsequent NREMPRE-REM and REM periods. Furthermore, since previous studies in rats (Jelev et al. 2001) and cats (Richard & Harper, 1991) have also shown that GG activity in REM sleep is characterised by periods both with and without phasic bursts of GG activity (i.e. periods of REMPHASIC GG and REMTONIC GG, respectively), these periods were also analysed and the proportion of the whole REM sleep time spent in these tonic and phasic periods was also calculated. Periods of REMPHASIC GG were identified from the moving-time average signal when bursts of GG activity occupied > 25 % of the analysed period and achieved a level > 25 % of GG activity in REM sleep. To prevent potential bias in the selection of periods of tonic and phasic GG activity, analyses were also performed on GG activity throughout the whole of the REM periods. Finally, to determine the time course of the GG responses to the onset of REM sleep, the average GG activity in each 5 s epoch for 1 min periods immediately prior to and following the onset of EEG-defined REM sleep were also calculated.
Each rat served as its own control, with all interventions performed in one experiment, thus allowing for consistent effects of experimental condition (e.g. ACSF or bicuculline, or inspired CO2 level) to be observed across sleep-wake states within and between rats. Two to six periods of each sleep-wake state were analysed for each experimental condition in each rat, and then for each animal a grand mean was calculated for each variable, for each sleep-wake state, at each CO2 level and for each drug delivered to the hypoglossal motor nucleus.
Histology and GG electrodes
At the end of the experiments the rats were re-anaesthetised with ketamine and xylazine (85 and 15 mg kg−1, respectively, as described above), and documentation of tongue protrusion was tested again in response to electrical stimulation of the GG wires. In all rats the electrodes were in place at the beginning and the end of the study.
To mark the lesion site left by the microdialysis probe, potassium permanganate (1 %) was microinjected for 10 min at 2.1 μl min−1 via a microdialysis probe with the membrane cut at the tip (Sun et al. 2000). The rats were then overdosed with urethane (0.5 g) and perfused intracardially with 40 ml of 0.9 % saline followed by 20 ml of 10 % formalin. The brains were then removed and fixed in 10 % formalin. The medullary regions were blocked, transferred to 30 % sucrose and cut in 50 μm coronal sections with a cryostat (Leica, CM 1850, Nussloch, Germany). Each section with the lesion site was mounted and stained with Neutral Red. Microdialysis sites were localised from the stained sections and marked on standard brain maps (Paxinos & Watson, 1998). For those rats used for fluorescence studies, alternate sections were placed on separate glass slides for fluorescence microscopy or conventional Neutral Red staining. The distribution of fluorescein was also marked on standard brain maps (Paxinos & Watson, 1998).
Statistical analysis
For all comparisons, differences were considered significant if the null hypothesis was rejected at P < 0.05 using a two-tailed test. Data was analysed using either analysis of variance with repeated measures (RM-ANOVA) or paired t tests. Student-Newman-Keuls test was used for post hoc comparisons. Analyses were performed using Sigmastat (SPSS, Chicago, IL, USA). All data are expressed as means ±s.e.m.
RESULTS
Histology and distribution of perfusate in the hypoglossal motor nucleus
Figure 1 shows the distribution of individual microdialysis sites from all rats. Probes were located in the hypoglossal motor nucleus in seven rats and in the midline immediately adjacent to both nuclei in three rats. Figure 2 shows examples in two rats of the spread within the dorsal medulla of fluorescein dialysed for 1 h or 6 h. For all rats, the fluorescence was concentrated around the site of the lesion left by the microdialysis probe and was largely centred within the hypoglossal motor nucleus. Examples of the lesion sites left by the microdialysis probes are shown in the histological sections at the top of the figure.
Figure 1. Location of the microdialysis probes.
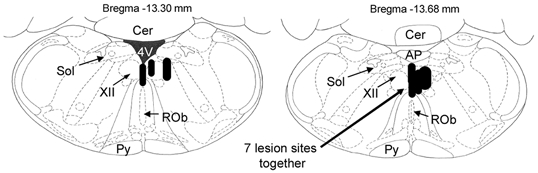
Distribution of individual microdialysis sites from all rats. Probes were located in the hypoglossal motor nucleus in seven rats and in the midline immediately adjacent to both nuclei in three rats. The size of the bar represents the apparent size of the lesion from the histological sections. Abbreviations: Cer, cerebellum; 4V, fourth ventricle; Sol, nucleus of the tractus solitarius; XII, hypoglossal motor nucleus; ROb, raphe obscurus; Py, pyramidal tract; AP, area postrema.
Figure 2. Distribution of fluorescein dye dialysed into the hypoglossal motor nucleus.
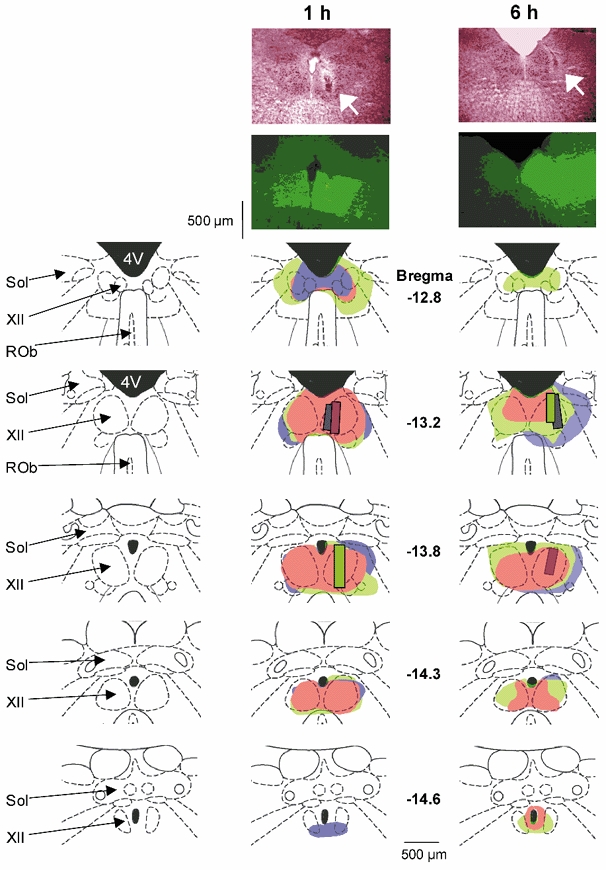
The top two photographs show examples in two rats of the spread of fluorescein dialysed for 1 h and 6 h into the hypoglossal motor nucleus. Neutral Red-stained sections containing the lesion sites left by the microdialysis probe are shown. The arrow indicates the sites of microdialysis. The distribution of fluorescence around the probe site is also shown from the adjacent histological section. The distribution of fluorescence in the hypoglossal motor nuclei for each individual rat is shown in the lower panels. Each rat is shown in a different colour and the bars indicate the sites of microdialysis. Note that the spread of fluorescence is largely within the medullary regions containing the hypoglossal motor nuclei. The traces on the left show the anatomical structures for orientation. Abbreviations as for Fig. 1.
Bicuculline at the hypoglossal motor nucleus
Example of response.Figure 3 shows an example of the responses of the GG muscle to perfusion of bicuculline into the hypoglossal motor nucleus in a naturally sleeping rat, both with and without reflex respiratory stimulation with increased inspired CO2. Note that in NREM sleep, bicuculline produced clear increases in GG activity compared to ACSF controls, both with room-air (C vs. A) and CO2-stimulated breathing (D vs. B). However, regardless of the presence of bicuculline at the hypoglossal motor nucleus or respiratory stimulation with CO2, major suppression of GG activity occurred in periods of REMTONIC GG. These records also show the periods of phasic bursts of GG activity (i.e. REMPHASIC GG), which are normally associated with REM sleep (Richard & Harper, 1991; Jelev et al. 2001). This example shows that these transient GG bursts were increased compared to ACSF, in the presence of combined bicuculline and hypercapnia (D vs. B).
Figure 3. Genioglossus (GG) responses to bicuculline at the hypoglossal motor nucleus in natural sleep.
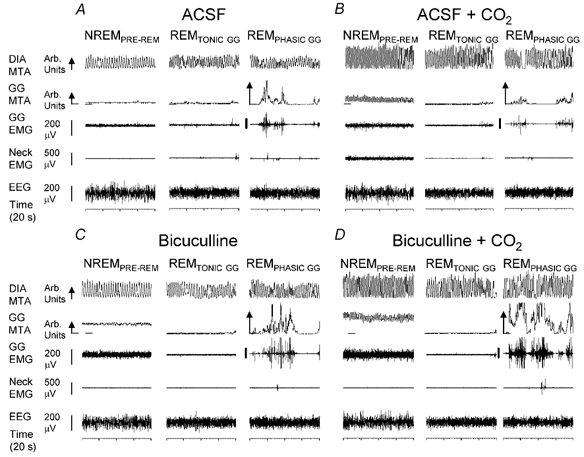
Sleep patterns and respiratory muscle activities with microdialysis perfusion of artificial cerebrospinal fluid (ACSF) and bicuculline into the hypoglossal motor nucleus during room-air and CO2-stimulated breathing. In non-rapid-eye-movement (NREM) sleep bicuculline produced clear increases in GG activity compared to ACSF both in room air (C vs. A) and with CO2 (D vs. B). However, major suppression of GG activity occurred in periods of rapid eye movement (REM) sleep without phasic GG twitches (i.e. REMTONIC GG) across all conditions. The GG and diaphragm (DIA) signals are displayed as their moving-time averages (MTA) in arbitrary (Arb.) units. The integrator baseline (i.e. electrical zero) is shown for the GG MTA. The arrow on the DIA and GG calibration bars denotes the direction of inspiration. REMPHASIC GG, REM associated with phasic GG muscle twitches; NREMPRE-REM, periods of NREM that were analysed occurring immediately prior to REM; EEG, electroencephalogram; EMG, electromyogram.
Group data
Effects of bicuculline and sleep-wake states on GG activity.Figure 4 shows that administration of bicuculline at the hypoglossal motor nucleus led to significant increases in respiratory-related GG activity during both room-air and CO2-stimulated breathing. Specifically, in room air there was a significant increase in GG activity after bicuculline compared to ACSF (F1,9= 10.76, P = 0.01, two-way RM-ANOVA) but GG activity still varied across sleep-wake states (F4,36= 8.43, P < 0.001). Post hoc analyses showed that GG activity was greater in wakefulness than in any stage of NREM and REM sleep (all P < 0.02, Student-Newman-Keuls). Similar effects were observed for the influence of bicuculline and sleep-wake states on tonic GG activity (both P < 0.03, two-way RM-ANOVA).
In hypercapnia, bicuculline also significantly increased respiratory-related GG activity compared to ACSF (F1,9= 12.27, P = 0.007, two-way RM-ANOVA), but again GG activity varied across sleep-wake states (F4,36= 22.86, P < 0.001) with activity being greater in wakefulness than in any stage of NREM and REM sleep (all P < 0.001, Student-Newman-Keuls). Tonic GG activity also varied across sleep-wake states (F4,36= 13.49, P < 0.001, two-way RM-ANOVA) but, unlike the effects in room air, tonic GG activity was not increased in hypercapnia by bicuculline (F1,9= 3.13, P = 0.111).
GG activity from NREM to REM sleep and the effects of bicuculline.Figure 4 shows that with either room air or CO2-stimulated breathing, respiratory-related GG activity was minimal in REMTONIC GG, regardless of whether ACSF or bicuculline was present at the hypoglossal motor nucleus. Statistical analyses confirmed that the stimulating effect of bicuculline on respiratory-related GG activity did depend on sleep state both in room air and hypercapnia (both F2,18 > 7.28, both P < 0.005, two-way RM-ANOVA). Post hoc analyses showed that although bicuculline increased GG activity in NREM and NREMPRE-REM sleep in either room air or hypercapnia (all P < 0.009, Student-Newman-Keuls), there was no effect of bicuculline on GG activity in REMTONIC GG compared to ACSF (P > 0.63, Student-Newman-Keuls). As such, in the presence of bicuculline there remained significant suppression of GG activity from NREM to REM sleep both in room air and hypercapnia (all P < 0.001 Student-Newman-Keuls).
Although there was no effect of bicuculline on GG activity in REM sleep during room-air breathing, REMPHASIC GG was increased by bicuculline, but only in hypercapnia (P = 0.004, Student-Newman-Keuls, see Fig. 3 and Fig. 4). There was no difference in GG activity between NREM and NREMPRE-REM sleep in either room air or hypercapnia (all P > 0.08, Student-Newman-Keuls).
Effects of CO2 on GG activity. There was a significant stimulating effect of CO2 on respiratory-related GG activity with both ACSF and bicuculline at the hypoglossal motor nucleus (both F1, 9 > 11.37, both P < 0.009, two-way RM-ANOVA). Further analyses showed that the percent change in GG activity with CO2 stimulation was not different for ACSF or bicuculline (F1,9= 2.24, P = 0.169, two-way RM-ANOVA). Nevertheless, for both ACSF and bicuculline, the effects of CO2 on GG activity depended on sleep state (both F3,27 > 5.89, P < 0.004, two-way RM-ANOVA). Post hoc analyses showed that CO2 caused significant increases in respiratory-related GG activity in both NREM and NREMPRE-REM sleep with either ACSF or bicuculline at the hypoglossal motor nucleus (all P < 0.004, Student-Newman-Keuls), but there was no independent stimulating effect of CO2 in any period of REM sleep (all P > 0.05, Student-Newman-Keuls).
Tonic and phasic GG activity in REM sleep.Figure 5 shows the percentage of the entire REM episode that was associated with periods of tonic and phasic GG activity for both ACSF and bicuculline at the hypoglossal motor nucleus. Analysis showed that the effects of ACSF or bicuculline on the amount of REMTONIC GG or REMPHASIC GG depended on the level of respiratory stimulation with CO2 (F1,9= 5.73, P = 0.04, two-way RM-ANOVA). Post hoc analyses confirmed that during room-air breathing, the amount of REM sleep accompanied by phasic bursts of GG activity was decreased by bicuculline compared to ACSF (P = 0.03, Student-Newman-Keuls), although all other effects were of marginal statistical significance (range, P = 0.164–0.064). For the neck EMG, CO2 stimulation decreased the percentage of the REM episode that was accompanied by phasic twitches in neck muscle activity (F1,9= 49.23, P < 0.01, two-way RM-ANOVA) although there was no effect of bicuculline per se on neck muscle activity (F1,9= 2.23, P = 0.164, two-way RM-ANOVA).
Figure 5. The percentage of REM sleep associated with periods of tonic and phasic GG activity.
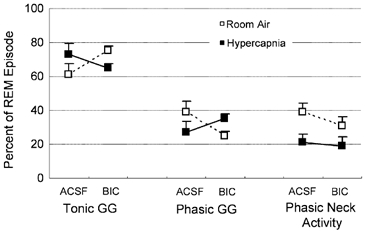
Group data showing the changes in tonic and phasic GG activities in REM sleep with bicuculline (BIC) and ACSF at the hypoglossal motor nucleus compared to neck muscle activity during room-air and CO2-stimulated breathing. The percentage of REM sleep accompanied by phasic bursts of GG activity was decreased by bicuculline compared to ACSF. No other changes were statistically significant. See text for further details. Abbreviations are as for Fig. 3.
GG activity in entire REM periods
The data in Fig. 4 show that there was major suppression of GG activity from NREM and NREMPRE-REM sleep to REM sleep periods without phasic bursts of GG activity (i.e. REMTONIC GG). Importantly, GG activity was minimal during these periods regardless of the presence of ACSF or bicuculline at the hypoglossal motor nucleus, or room air and CO2-stimulated breathing. However, to avoid any potential bias in analysing only selected periods of tonic and phasic GG activity in REM sleep, further analysis was performed on GG activity measured throughout the entire REM episodes (i.e. inclusive of all tonic and phasic GG events as they occurred). These data are shown in Fig. 6. Analysis showed that although there was a tendency for GG activity to increase in overall REM sleep in the presence of combined hypercapnia and bicuculline at the hypoglossal motor nucleus (Fig. 6), there was no statistically significant effect of bicuculline on GG activity in total REM sleep (F1,9= 4.00, P = 0.08, two-way RM-ANOVA).
Figure 6. GG activity analysed across the entire REM period.
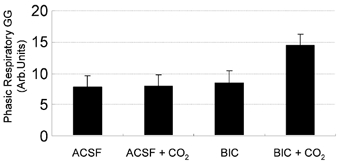
Group data showing GG activity measured throughout the entire REM episodes. Although there was a tendency for GG activity to increase in overall REM sleep in the presence of combined CO2 stimulation and bicuculline (BIC) at the hypoglossal motor nucleus there was no statistically significant effect of bicuculline on GG activity in total REM sleep.
GG activity at transition from NREM to REM sleep
The previous analysis was performed on selected periods of stable NREM and REM sleep data separated in time. To allow the characterisation of GG suppression at the onset of REM sleep, further analyses were performed for 1 min periods immediately preceding and following the onset of REM sleep as identified by EEG criteria. Figure 7 shows an example of the change in GG activity from NREM to REM sleep with bicuculline at the hypoglossal motor nucleus. Note the major suppression of GG activity at the onset of REM sleep, despite the GABAA receptor antagonism. Periods of phasic bursts of GG activity occur later in the REM episode.
Figure 7. GG activity upon transition from NREM to REM sleep with bicuculline at the hypoglossal motor nucleus.
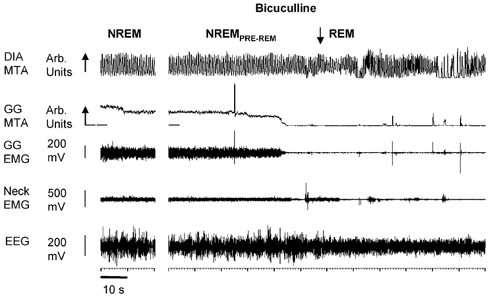
Example showing the abolition of GG activity at the onset of REM sleep despite the presence of bicuculline at the hypoglossal motor nucleus. Abbreviations are as for Fig. 3.
Overall group data.Figure 8 shows group data for the change in GG activity from NREM to REM sleep with both ACSF and bicuculline at the hypoglossal motor nucleus, during both room-air and CO2-stimulated breathing. In confirmation of the previous analyses, bicuculline increased respiratory-related GG activity in both room air and hypercapnia for NREM and NREMPRE-REM sleep (all F1,9 > 7.24, all P < 0.03, two-way RM-ANOVA). In contrast, in REM sleep bicuculline did not increase GG activity during room-air breathing compared to ACSF (F1,9= 0.16, P = 0.698). There was, however, a significant stimulating effect of bicuculline on GG activity in REM sleep in the presence of hypercapnia (F1,9= 16.49, P = 0.003); this is in keeping with previous observations (Figs 3 (D vs. B), 4 and 6).
Figure 8. Group data showing changes in GG activity from NREM to REM sleep.
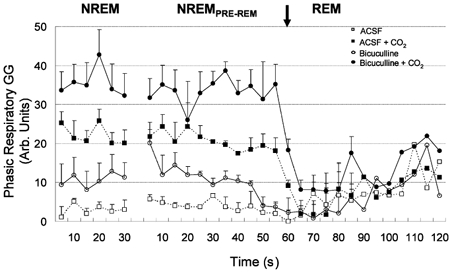
Group data showing changes in respiratory-related GG activity from NREM to REM sleep during microdialysis perfusion of ACSF and bicuculline into the hypoglossal motor nucleus during both room-air and CO2-stimulated breathing. GG activity in REM sleep was similar across all conditions. See text for further details.
GG activity over time in NREM sleep. GG activity was stable across time in NREM sleep so that there was no statistical effect of time on GG activity either in room air or hypercapnia, irrespective of whether ACSF or bicuculline were present at the hypoglossal motor nucleus (both F1,9 > 0.71, both P > 0.623, two-way RM-ANOVA). In NREMPRE-REM sleep, however, GG activity showed graded suppression prior to the onset of EEG-defined REM sleep during both room air and CO2-stimulated breathing (both F11,99 > 3.20, P < 0.001, two-way RM-ANOVA). However, this change in GG activity over time did not depend on the presence of ACSF or bicuculline at the hypoglossal motor nucleus during room air breathing (F11,99= 1.80, P = 0.064), but did in hypercapnia (F11,99= 2.08, P = 0.028). These data are consistent with the observation that the decline in GG activity in NREMPRE-REM sleep with bicuculline was less than with ACSF during hypercapnia (Fig. 8).
GG activity over time in REM sleep. In both room air and hypercapnia there was a significant effect of time on GG activity (both F11,99 > 2.36, P < 0.02, two-way RM-ANOVA) consistent with REMTONIC GG being prominent at the onset of REM sleep, and REMPHASIC GG occurring later into the REM episode (e.g. see Fig. 7). However, the change in GG activity over time was not different between ACSF and bicuculline at the hypoglossal motor nucleus in either room air or hypercapnia (both F11,99 < 1.44, P > 0.160).
Specificity of bicuculline effects on GG activity
Figure 9 shows the grouped mean data for the effects of bicuculline vs. ACSF at the hypoglossal motor nucleus on respiratory and sleep-related parameters during both room-air and CO2-stimulated breathing. In hypercapnia there was no effect of bicuculline on respiratory rate, respiratory-related diaphragm activity, the ratio of high to low frequencies in the EEG (i.e. %β2/%δ1) and neck muscle activity (all F1,9 < 3.32, P > 0.102, two-way RM-ANOVA). Likewise, in room air there was no effect of bicuculline on the ratio of high to low frequencies in the EEG or neck muscle activity (both F1,9 < 3.22, P > 0.110). In room air, however, bicuculline caused a consistent decrease in respiratory rate (Fig. 9A, F1,9= 14.10, P = 0.004) and an increase in respiratory-related diaphragm activity (Fig. 9B, F1,9= 18.98, P = 0.002) that led to overall increases in diaphragm minute activity (mean increase = 10.5 %, F1,9= 10.76, P = 0.009).
Figure 9. Group data showing specificity of responses to bicuculline at the hypoglossal motor nucleus.
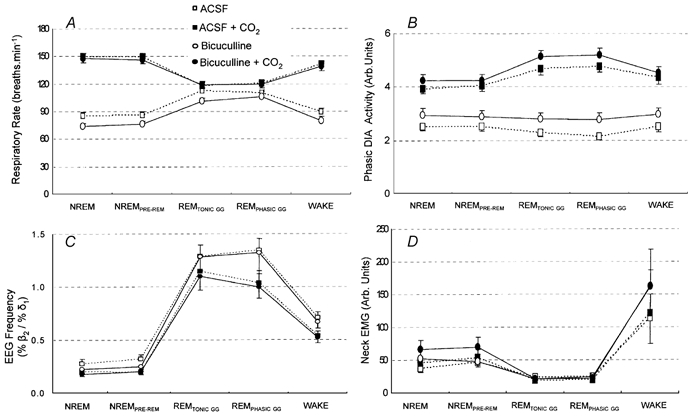
Responses of respiratory rate (A), phasic diaphragm (DIA) activity (B), the ratio of high to low frequencies in the EEG (C) and neck EMG (D) are shown across all sleep-wake states for microdialysis perfusion of ACSF and bicuculline into the hypoglossal motor nucleus during both room-air and CO2-stimulated breathing. There are no effects of bicuculline on any parameter in hypercapnia but bicuculline decreased respiratory rate and increased diaphragm activity in room air. See text for further details.
The independent effects of sleep-wake states on respiratory variables (e.g. increased respiratory rates in REM) and sleep data (e.g. changes in EEG frequencies and neck EMG), and their response to CO2, were as described previously (Jelev et al. 2001; Horner et al. 2002).
Stimulation of the hypoglossal motor nucleus with serotonin
The consistent increase in GG activity in NREM sleep and wakefulness with bicuculline was initial evidence for an intact hypoglossal motor nucleus that was able to respond to manipulation of neurotransmission. In three rats, further confirmation of a functional hypoglossal motor nucleus was performed at the end of the experiments by perfusing the probes with 10 mm serotonin (creatinine sulphate complex, FW: 387.4, Sigma), a dose that increases GG activity in sleeping rats (Jelev et al. 2001). Figure 10 shows an example of the increase in GG activity following application of serotonin to the hypoglossal motor nucleus. Similar responses were observed in the other two rats. The increase in GG activity with serotonin was marked and easily distinguishable, thereby allowing determination of latencies. The latency to an increase in GG activity following the switch to serotonin was 16.7 ± 4.0 min, consistent with a previous study (Jelev et al. 2001).
Figure 10. GG activation by 5-hydroxytryptamine (5-HT) at the hypoglossal motor nucleus.
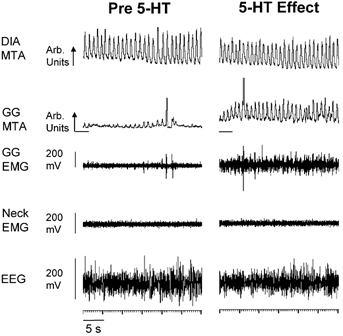
Example showing an increase in GG activity with microdialysis perfusion of serotonin (5-HT) into the hypoglossal motor nucleus. These traces from one rat were both obtained in NREM sleep. Abbreviations are as for Fig. 3.
DISCUSSION
Differential GG responses to bicuculline at the hypoglossal motor nucleus
The results of the present study show that delivery of the GABAA receptor antagonist bicuculline to the hypoglossal motor nucleus by in vivo microdialysis in freely behaving rats caused consistent increases in GG activity in wakefulness and NREM sleep but not in REM sleep. The increase in GG activity after bicuculline in NREM sleep is consistent with in vitro studies showing inhibitory GABAA receptors on hypoglossal motoneurons (O'Brien & Berger, 1999; Donato & Nistri, 2000, 2001) and in vivo studies in anaesthetised animals showing that such receptors are tonically active (Liu et al. 2003). The greater increase in GG activity in response to bicuculline in wakefulness is probably due to removal of the same inhibitory GABAA tone against a background of increased endogenous excitatory neurotransmitters associated with the waking state (e.g. serotonin, thyrotropin-releasing hormone, substance P and noradrenaline; for review see Kubin et al. 1998). However, no previous studies have addressed whether such inhibitory GABAA mechanisms at the hypoglossal motor nucleus are recruited in specific behaviours (e.g. to explain the major suppression of GG activity in periods of natural REM sleep; Sauerland & Harper, 1976; Megirian et al. 1985; Parisi et al. 1987; Wiegand et al. 1991; Horner et al. 2002). If so, antagonism of such GABAA mechanisms may be expected to lead to increased GG activity in REM sleep compared to ACSF controls. The results of the present study show, however, that despite the increase in GG activity in wakefulness and NREM sleep with bicuculline, GG activity was minimal upon transition into REM sleep and remained effectively abolished in those REM sleep periods without phasic twitches in the GG muscle. Therefore, this study shows that the prevailing sleep-wake states exert significant modulating effects on the GG responses to GABAA receptor antagonism at the hypoglossal motor nucleus. These results highlight the significant differences in the neurobiology of hypoglossal motor control between NREM and REM sleep, and emphasise the importance of studies in intact freely behaving animals in extrapolating results from reduced preparations to specific behaviours such as natural sleep-wake states.
Control of GG activity in REM sleep
Several in vitro studies have characterised the inhibitory control of hypoglossal motoneurons (Umemiya & Berger, 1995; Singer et al. 1998; O'Brien & Berger, 1999; Donato & Nistri, 2000), with these circuits thought to be involved in the documented inhibition of hypoglossal motoneurons in the carbachol model of REM sleep (Yamuy et al. 1999; Fung et al. 2000) and potentially natural REM sleep (Fung et al. 2001). Evidence suggests that glycine mediates a significant component of this inhibition in REM sleep (Yamuy et al. 1999), as it does for lumbar and massetter motoneurons (Morales et al. 1987a,b; Pedroarena et al. 1994; Lopez-Rodriguez et al. 1995). In humans, administration of strychnine to a patient with obstructive sleep apnoea also led to increased GG activity (i.e. in support of glycinergic inhibition), although sleep states were not reported in that study and further patients were not presented (Remmers et al. 1980). Although disfacilitation via withdrawal of excitatory neurotransmitters, such as serotonin, may also be involved concurrently in suppressing hypoglossal motoneurons in REM sleep (Kubin et al. 1993; Woch et al. 1996), the relative inadequacy of pharmacological attempts to increase GG activity in REM sleep by modulating serotonin levels has been disappointing both in humans and animal models (for review see Horner, 2001 and references therein), even with direct application of serotonin to the hypoglossal motor nucleus (Jelev et al. 2001). These data suggest that post-synaptic inhibition plays a more dominant role in suppression of GG activity in REM sleep than does disfacilitation. This effect may become especially apparent as serotonin levels are withdrawn prior to and during REM sleep (Trulson & Trulson, 1982; Jacobs & Azmitia, 1992; Woch et al. 1996), an effect that would be expected to increase the efficacy of any ongoing inhibitory glycinergic inputs to the hypoglossal motor nucleus (Umemiya & Berger, 1995).
However, the potential role of GABA in this process of REM-sleep-related inhibition of hypoglossal motor activity had not been addressed prior to this study, despite evidence for robust GABAA inhibition of hypoglossal motoneurons in vitro (O'Brien & Berger, 1999; Donato & Nistri, 2000, 2001) and in vivo (Liu et al. 2003). The results of the present study in freely behaving rats show that GABAA receptor antagonism at the hypoglossal motor nucleus does not increase GG muscle activity in natural REM sleep during normal breathing as well as reflex respiratory stimulation, despite evidence for tonically active GABAA receptors at the hypoglossal motor nucleus that caused the observed increase in GG activity after bicuculline in states outside of REM sleep. It remains to be tested whether recruitment of glycinergic inhibition explains the major suppression of GG activity that persists in REM sleep despite the GABAA receptor antagonism. However, the results of the present study do suggest that therapeutic strategies aimed at increasing GG activity via modulation of GABAA mechanisms at the hypoglossal motor nucleus may be less effective in reducing the severity of obstructive sleep apnoea in REM sleep compared with NREM sleep. In the present study, the absence of an effect of GABAA antagonism on GG activity specifically in REM sleep was confirmed by three complimentary analyses: (a) analysis of selected periods of REM sleep distinguishing between epochs with tonic and phasic bursts of GG activity, (b) analysis of the whole REM period to avoid potential bias in the selection of the tonic and phasic GG activities, and (c) analysis of the change in GG activity during transition from NREM to REM sleep. Of relevance, a similar inability of GABAA receptor antagonism to reverse the suppression of hypoglossal nerve activity has also been observed in the carbachol model of REM sleep in decerebrate cats (Kubin et al. 1993).
It is possible, however, that mechanisms unrelated to sleep neural processes could also influence GG activity in REM sleep. For example, central respiratory drive and respiratory rate normally increase in REM sleep (Fig. 9 and Orem et al. 2000) and this could lead to reduced GG activity via decreased arterial partial pressure of CO2 (Pa,CO2). However, the minimal GG activity in REM sleep during room air was similar to during hypercapnia, specifically at the onset of REM sleep and in REM periods without the phasic bursts of GG activity. In addition, since respiratory rates decline rather than increase in REM sleep in hypercapnia (Fig. 9A and Horner et al. 2002), a REM-related decrease in Pa,CO2 is unlikely to explain the periods of major suppression of GG activity.
Effects of GABAA receptor antagonism on tonic vs. respiratory-related GG activity
GABAA antagonism at the hypoglossal motor nucleus increased both tonic and respiratory-related GG activity in room air, but increased only respiratory-related GG activity in hypercapnia. This preferential increase in respiratory-related GG activity with GABAA receptor antagonism has also been observed in anaesthetised rats where the respiratory drive to GG muscle was increased by vagotomy (Liu et al. 2003). While there are clear inhibitory GABAergic influences on hypoglossal motoneurons (O'Brien & Berger, 1999; Berger, 2000; Donato & Nistri, 2000, 2001) it is noteworthy that inspiratory hypoglossal motoneurons are not inhibited during expiration (Withington-Wray et al. 1988; Woch & Kubin, 1995; Peever et al. 2001), unlike phrenic (Lipski et al. 1985) and inspiratory intercostal motoneurons (Kirkwood et al. 1993). Rather, inspiratory hypoglossal motoneurons receive synaptic inhibition towards the end of inspiration (Withington-Wray et al. 1988) and at the transition to expiration (Woch & Kubin, 1995). Therefore, if such inspiratory-related inhibition were mediated, at least in part, by GABAA mechanisms, then bicuculline at the hypoglossal motor nucleus may be expected to increase inspiratory GG activity at the expense of tonic activity, particularly during periods of heightened respiratory drive, such as during hypercapnia.
Tonic vs. phasic twitches in GG activity in REM sleep
Periods of transient suppression of GG activity and ventilation occur in phasic REM sleep in humans (Wiegand et al. 1991). In rats, periods of complete cessation of GG activity occur for the majority of the REM period, although characteristic twitches in GG activity also occurred (e.g. see Fig. 3 and Fig. 7, and Jelev et al. 2001). These bursts of GG activity are sporadic and not always associated with bursts of neck EMG activity, and since rapid eye movements were not measured in this study, due to the number of wires that we could reasonably implant, it is unknown if these GG twitches were associated with REM sleep with or without eye movements. Nevertheless, the REM sleep state is not solely associated with motor inhibition, and it is therefore possible that the transient bursts of GG activity may be typical of the transient membrane depolarisations that characterise REM sleep in the hypoglossal (Richard & Harper, 1991) as well as other motoneuron pools (Glenn et al. 1978; Chase & Morales, 1983; Orem, 1994).
Bicuculline at the hypoglossal motor nucleus increased GG activity in wakefulness and NREM sleep, but no such increase was observed when REM sleep was analysed as a whole. Further analysis of the REM sleep periods showed that the only significant effect of bicuculline observed in REM sleep was the increase in GG activity associated with phasic GG twitches, but this occurred only in the presence of CO2. It is unlikely that this effect was caused by increased amounts of phasic REM sleep per se, because the proportion of phasic neck muscle activity in REM sleep was decreased by hypercapnia (Fig. 5). The increased GG activity is therefore likely to be a bicuculline effect that was revealed only at times of heightened respiratory drive to the GG muscle by the mechanisms discussed in the previous section, in conjunction with periods of transient excitatory motor drives characteristic of normal REM sleep (Glenn et al. 1978; Chase & Morales, 1983; Orem, 1994).
Specificity of responses
A specific effect of bicuculline at the hypoglossal motor nucleus is strongly indicated if there are no changes in control measurements compared to ACSF. Such control measurements included the neck EMG (control postural muscle), diaphragm (control respiratory muscle) and the EEG (marker of arousal). In hypercapnia, none of these control measurements were altered by bicuculline, indicating a specific effect at the hypoglossal motor nucleus. Likewise, in room air neither the neck EMG nor EEG frequencies were altered by bicuculline. In room air, however, bicuculline caused a consistent decrease in respiratory rate and increase in diaphragm activity that led to overall increases in diaphragm minute activity by approximately 10 %. Similar effects on respiratory activity with bicuculline at the hypoglossal motor nucleus were observed in anaesthetised, tracheotomised rats (Liu et al. 2003), thereby ruling out that the effects on diaphragm activity were indirect and mediated by changes in upper-airway patency via increased GG activity. However, the increase in diaphragm activity may suggest spread of bicuculline beyond the hypoglossal motor nucleus. GABAB receptors have been implicated in the reticular formation surrounding the hypoglossal motor nucleus (Okabe et al. 1994), although there is little information for GABAA receptors (Tebecis & Ishikawa, 1973). Nevertheless, as shown in Fig. 2, bicuculline may have diffused into the nucleus of the tractus solitarius, where it could have affected respiratory rhythm via modulation of afferent inputs or dorsal respiratory group neurons (Kubin et al. 1993; McCrimmon et al. 1995; Paton & Richter, 1995; Shao & Feldman, 1997; Berger, 2000). There is also the possibility that the perfusate could have spread to the surface of the medulla and hence into the fourth ventricle (Fig. 2). Although to our knowledge, the effects on hypoglossal nerve activity of the application of GABA or bicuculline directly into the fourth ventricle have not been reported, GABA in the fourth ventricle has been shown to cause respiratory depression, whereas bicuculline increased minute ventilation via increased tidal volume but without a change in respiratory rate (Yamada et al. 1982). However, these potential effects produced by spread of the perfusate outside of the hypoglossal motor nucleus do not detract from the main results of the present study, since any increase in overall diaphragm activity would be expected to produce a degree of relative hypocapnia, and as such would not fully explain the increase in GG activity produced by bicuculline in NREM sleep and wakefulness but not REM sleep.
The delivery of agents to the hypoglossal motor nuclei was by unilateral microdialysis; this may have influenced our results since the nuclei are distributed bilaterally. However, most probe placements were at, or close to, the midline (Fig. 1). Given the close proximity of both motor nuclei and the extensive bilateral distribution of dendrites of adult hypoglossal motoneurons (Paxinos, 1995), it is unlikely that our interventions only affected one nucleus, although this was not specifically tested with bilateral interventions. Nevertheless, GG activity was measured with electrodes placed either side of the tongue (i.e. recording activity across the whole muscle innervated by both hypoglossal nerves), and the fluorescence was typically distributed bilaterally (Fig. 2). Indeed, the dialysed fluorescence was typically distributed to dorsal regions of the medulla encompassing the hypoglossal motor nuclei.
Although pharyngeal muscle recordings were made only from the GG in this study, it is likely that tongue retractors were also activated in hypercapnia (Fuller et al. 1998) and would be influenced by bicuculline. However, this latter influence may be to a lesser degree than for GG motoneurons, since the probe sites were located in rostral regions of the motor nucleus (Fig. 1 and Paxinos & Watson, 1998). Nevertheless, activity in both the tongue retractor and protruder muscles is beneficial for the maintenance of airway patency (Fuller et al. 1999). Accordingly, the mechanisms at the hypoglossal motor nucleus modulating pharyngeal muscle activity across sleep-wake states, especially REM sleep, will be significant in understanding the neural mechanisms maintaining upper-airway patency. Such studies will also be important in guiding therapeutic strategies for obstructive sleep apnoea to determine if the pharyngeal muscles can be selectively activated in sleep, especially REM sleep.
Acknowledgments
This work was supported by funds from the Canadian Institutes of Health Research (CIHR, Grant MT-15563). J.L.M. is a recipient of Post-Doctoral Fellowships from the Department of Medicine at the University of Toronto and Merck Frosst. R.L.H. is a recipient of a CIHR Scholarship. Funds from the Premier's Research Excellence Award from the Ministry of Science, Energy and Technology, Ontario Government to R.L.H. also helped fund this research. The authors thank Dr James Duffin, Department of Physiology, for use of the fluorescence microscope.
references
- Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey EF, Jones CL, Reeder JC, Fuller DD, Fregosi RF. Effect of pulmonary stretch receptor feedback and CO2 on upper airway and respiratory pump muscle activity in the rat. J Physiol. 2001;532:525–534. doi: 10.1111/j.1469-7793.2001.0525f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger AJ. Determinants of respiratory motoneuron output. Respir Physiol. 2000;122:259–269. doi: 10.1016/s0034-5687(00)00164-x. [DOI] [PubMed] [Google Scholar]
- Chase MH, Morales FR. Subthreshold excitatory activity and motoneuron discharge during REM periods of active sleep. Science. 1983;221:1195–1198. doi: 10.1126/science.6310749. [DOI] [PubMed] [Google Scholar]
- Chase MH, Morales FR. The control of motoneurons during sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Philadelphia: Blackwell Science Inc; 1994. pp. 163–175. [Google Scholar]
- Donato R, Nistri A. Relative contribution by GABA or glycine to Cl−-mediated synaptic transmission on rat hypoglossal motoneurons in vitro. J Neurophysiol. 2000;84:2715–2724. doi: 10.1152/jn.2000.84.6.2715. [DOI] [PubMed] [Google Scholar]
- Donato R, Nistri A. Differential short-term changes in GABAergic or glycinergic synaptic efficacy on rat hypoglossal motoneurons. J Neurophysiol. 2001;86:565–574. doi: 10.1152/jn.2001.86.2.565. [DOI] [PubMed] [Google Scholar]
- el Mansari M, Sakai K, Jouvet M. Unitary characteristics of presumptive cholinergic tegmental neurons during the sleep-waking cycle in freely moving cats. Exp Brain Res. 1989;76:519–529. doi: 10.1007/BF00248908. [DOI] [PubMed] [Google Scholar]
- Fuller D, Mateika JH, Fregosi RF. Co-activation of tongue protrudor and retractor muscles during chemoreceptor stimulation in the rat. J Physiol. 1998;507:265–276. doi: 10.1111/j.1469-7793.1998.265bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Williams JS, Janssen PL, Fregosi RF. Effect of co-activation of tongue protruder and retractor muscles on tongue movements and pharyngeal airflow mechanics in the rat. J Physiol. 1999;519:601–613. doi: 10.1111/j.1469-7793.1999.0601m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung SJ, Yamuy J, Xi MC, Engelhardt JK, Morales FR, Chase MH. Changes in electrophysiological properties of cat hypoglossal motoneurons during carbachol-induced motor inhibition. Brain Res. 2000;885:262–272. doi: 10.1016/s0006-8993(00)02955-3. [DOI] [PubMed] [Google Scholar]
- Fung SJ, Yamuy J, Xi MC, Pandi-Perumal SR, Morales FR, Chase MH. Cat hypoglossal motoneurons are postsynaptically inhibited during naturally occurring active sleep. Soc Neurosci Abstr. 2001;27:572. [Google Scholar]
- Glenn LL, Foutz AS, Dement WC. Membrane potential of spinal motoneurons during natural sleep in cats. Sleep. 1978;1:199–204. [PubMed] [Google Scholar]
- Horner RL. The neuropharmacology of upper airway motor control in the awake and asleep states: implications for obstructive sleep apnoea. Respir Res. 2001;2:286–294. doi: 10.1186/rr71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner RL, Liu X, Gill H, Nolan P, Liu H, Sood S. Effects of sleep-wake state on the genioglossus vs. diaphragm muscle response to CO2 in rats. J Appl Physiol. 2002;92:878–887. doi: 10.1152/japplphysiol.00855.2001. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Jelev A, Sood S, Liu H, Nolan P, Horner RL. Microdialysis perfusion of 5-HT into hypoglossal motor nucleus differentially modulates genioglossus activity across natural sleep-wake states in rats. J Physiol. 2001;532:467–481. doi: 10.1111/j.1469-7793.2001.0467f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood PA, Schmid K, Sears TA. Functional identities of thoracic respiratory interneurones in the cat. J Physiol. 1993;461:667–687. doi: 10.1113/jphysiol.1993.sp019535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubin L, Davies RO, Pack L. Control of upper airway motoneurons during REM sleep. News Physiol Sci. 1998;13:637–656. doi: 10.1152/physiologyonline.1998.13.2.91. [DOI] [PubMed] [Google Scholar]
- Kubin L, Kimura H, Tojima H, Davies RO, Pack AI. Suppression of hypoglossal motoneurons during the carbachol-induced atonia of REM sleep is not caused by fast synaptic inhibition. Brain Res. 1993;611:300–312. doi: 10.1016/0006-8993(93)90517-q. [DOI] [PubMed] [Google Scholar]
- Lipski J, Fyffe RE, Jodkowski J. Recurrent inhibition of cat phrenic motoneurons. J Neurosci. 1985;5:1545–1555. doi: 10.1523/JNEUROSCI.05-06-01545.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Sood S, Liu H, Nolan P, Morrison JL, Horner RL. Suppression of genioglossus muscle tone and activity during reflex hypercapnic stimulation by GABA-A mechanisms at the hypoglossal motor nucleus in-vivo. Neuroscience. 2003;116:249–259. doi: 10.1016/s0306-4522(02)00564-x. [DOI] [PubMed] [Google Scholar]
- Lopez-Rodriguez F, Kohlmeier KA, Yamuy J, Morales FR, Chase MH. Muscle atonia can be induced by carbachol injections into the nucleus pontis oralis in cats anesthetized with alpha-chloralose. Brain Res. 1995;699:201–207. doi: 10.1016/0006-8993(95)00899-2. [DOI] [PubMed] [Google Scholar]
- McCrimmon DR, Mitchell GS, Dekin MS. Glutamate, GABA, and serotonin in ventilatory control. In: Dempsey JA, Pack AI, editors. Regulation of Breathing. New York: Blackwell Science Inc; 1995. pp. 151–218. [Google Scholar]
- Megirian D, Hinrichsen CF, Sherrey JH. Respiratory roles of genioglossus, sternothyroid, and sternohyoid muscles during sleep. Exp Neurol. 1985;90:118–128. doi: 10.1016/0014-4886(85)90045-7. [DOI] [PubMed] [Google Scholar]
- Morales FR, Boxer P, Chase MH. Behavioral state-specific inhibitory postsynaptic potentials impinge on cat lumbar motoneurons during active sleep. Exp Neurol. 1987a;98:418–435. doi: 10.1016/0014-4886(87)90252-4. [DOI] [PubMed] [Google Scholar]
- Morales FR, Engelhardt JK, Soja PJ, Pereda AE, Chase MH. Motoneuron properties during motor inhibition produced by microinjection of carbachol into the pontine reticular formation of the decerebrate cat. J Neurophysiol. 1987b;57:1118–1129. doi: 10.1152/jn.1987.57.4.1118. [DOI] [PubMed] [Google Scholar]
- Morrison JL, Sood S, Liu X, Liu H, Park E, Nolan P, Horner RL. Glycine at the hypoglossal motor nucleus: genioglossus activity, CO2 responses and the additive effects of GABA. J Appl Physiol. 2002;93:1786–1796. doi: 10.1152/japplphysiol.00464.2002. [DOI] [PubMed] [Google Scholar]
- Nattie E, Li A. Muscimol dialysis in the retrotrapezoid nucleus region inhibits breathing in the awake rat. J Appl Physiol. 2000;89:153–162. doi: 10.1152/jappl.2000.89.1.153. [DOI] [PubMed] [Google Scholar]
- O'Brien JA, Berger AJ. Cotransmission of GABA and glycine to brain stem motoneurons. J Neurophysiol. 1999;82:1638–1641. doi: 10.1152/jn.1999.82.3.1638. [DOI] [PubMed] [Google Scholar]
- Okabe S, Woch G, Kubin L. Role of GABAB receptors in the control of hypoglossal motoneurons in vivo. Neuroreport. 1994;5:2573–2576. doi: 10.1097/00001756-199412000-00042. [DOI] [PubMed] [Google Scholar]
- Orem J. Respiratory neurons and sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Philadelphia: Blackwell Science Inc; 1994. pp. 177–193. [Google Scholar]
- Orem J, Lovering AT, Dunin-Barkowski W, Vidruk EH. Endogenous excitatory drive to the respiratory system in rapid eye movement sleep in cats. J Physiol. 2000;527:365–376. doi: 10.1111/j.1469-7793.2000.00365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi RA, Neubauer JA, Frank MM, Edelman NH, Santiago TV. Correlation between genioglossal and diaphragmatic responses to hypercapnia during sleep. Am Rev Respir Dis. 1987;135:378–382. doi: 10.1164/arrd.1987.135.2.378. [DOI] [PubMed] [Google Scholar]
- Paton JF, Richter DW. Role of fast inhibitory synaptic mechanisms in respiratory rhythm generation in the maturing mouse. J Physiol. 1995;484:505–521. doi: 10.1113/jphysiol.1995.sp020682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G. The Rat Nervous System. San Diego: Blackwell Science Inc; 1995. [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Blackwell Science Inc; 1998. [Google Scholar]
- Pedroarena C, Castillo P, Chase MH, Morales FR. The control of jaw-opener motoneurons during active sleep. Brain Res. 1994;653:31–38. doi: 10.1016/0006-8993(94)90368-9. [DOI] [PubMed] [Google Scholar]
- Peever JH, Mateika JH, Duffin J. Respiratory control of hypoglossal motoneurones in the rat. Pflugers Arch. 2001;442:78–86. doi: 10.1007/s004240000502. [DOI] [PubMed] [Google Scholar]
- Remmers JE, Anch AM, Degroot WJ, Baker JP, Sauerland EK. Oropharyngeal muscle tone in obstructive sleep apnea before and after strychnine. Sleep. 1980;3:447–453. doi: 10.1093/sleep/3.3-4.447. [DOI] [PubMed] [Google Scholar]
- Remmers JE, Degroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol. 1978;44:931–938. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- Richard CA, Harper RM. Respiratory-related activity in hypoglossal neurons across sleep-waking states in cats. Brain Res. 1991;542:167–170. doi: 10.1016/0006-8993(91)91014-r. [DOI] [PubMed] [Google Scholar]
- Sauerland EK, Harper RM. The human tongue during sleep: electromyographic activity of the genioglossus muscle. Exp Neurol. 1976;51:160–170. doi: 10.1016/0014-4886(76)90061-3. [DOI] [PubMed] [Google Scholar]
- Shao XM, Feldman JL. Respiratory rhythm generation and synaptic inhibition of expiratory neurons in pre-Bötzinger complex: differential roles of glycinergic and GABAergic neural transmission. J Neurophysiol. 1997;77:1853–1860. doi: 10.1152/jn.1997.77.4.1853. [DOI] [PubMed] [Google Scholar]
- Singer JH, Talley EM, Bayliss DA, Berger AJ. Development of glycinergic synaptic transmission to rat brain stem motoneurons. J Neurophysiol. 1998;80:2608–2620. doi: 10.1152/jn.1998.80.5.2608. [DOI] [PubMed] [Google Scholar]
- Sun M-H, Hildenbrandt L, Curran AK, Darnall R, Chen G, Filiano J. Potassium permanganate can mark the site of microdialysis in brain sections. J Histotechnol. 2000;23:151–154. [Google Scholar]
- Taepavarapruk N, McErlane SA, Soja PJ. State-related inhibition by GABA and glycine of transmission in Clarke's column. J Neurosci. 2002;22:5777–5788. doi: 10.1523/JNEUROSCI.22-13-05777.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata M, Ogata K. Two components of inhibitory postsynaptic potentials evoked in hypoglossal motoneurons by lingual nerve stimulation. Exp Neurol. 1980;69:299–310. doi: 10.1016/0014-4886(80)90213-7. [DOI] [PubMed] [Google Scholar]
- Tebecis AK, Ishikawa T. Glycine and GABA as inhibitory transmitters in the medullary reticular formation. Studies involving intra- and extracellular recording. Pflugers Arch. 1973;338:273–278. doi: 10.1007/BF00587392. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Jacobs BL. Raphe unit activity in freely moving cats: correlation with level of behavioral arousal. Brain Res. 1979;163:135–150. doi: 10.1016/0006-8993(79)90157-4. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Trulson VM. Activity of nucleus raphe pallidus neurons across the sleep-waking cycle in freely moving cats. Brain Res. 1982;237:232–237. doi: 10.1016/0006-8993(82)90572-8. [DOI] [PubMed] [Google Scholar]
- Umemiya M, Berger AJ. Presynaptic inhibition by serotonin of glycinergic inhibitory synaptic currents in the rat brain stem. J Neurophysiol. 1995;73:1192–1201. doi: 10.1152/jn.1995.73.3.1192. [DOI] [PubMed] [Google Scholar]
- Wiegand L, Zwillich CW, Wiegand D, White DP. Changes in upper airway muscle activation and ventilation during phasic REM sleep in normal men. J Appl Physiol. 1991;71:488–497. doi: 10.1152/jappl.1991.71.2.488. [DOI] [PubMed] [Google Scholar]
- Withington-Wray DJ, Mifflin SW, Spyer KM. Neuroscience. Vol. 25. 1988. Intracellular analysis of respiratory-modulated hypoglossal motoneurons in the cat; pp. 1041–1051. [DOI] [PubMed] [Google Scholar]
- Woch G, Davies RO, Pack AI, Kubin L. Behaviour of raphe cells projecting to the dorsomedial medulla during carbachol-induced atonia in the cat. J Physiol. 1996;490:745–758. doi: 10.1113/jphysiol.1996.sp021182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woch G, Kubin L. Non-reciprocal control of rhythmic activity in respiratory-modulated XII motoneurons. Neuroreport. 1995;6:2085–2088. doi: 10.1097/00001756-199510010-00031. [DOI] [PubMed] [Google Scholar]
- Yamada KA, Norman WP, Hamosh P, Gillis RA. Medullary ventral surface GABA receptors affect respiratory and cardiovascular function. Brain Res. 1982;248:71–78. doi: 10.1016/0006-8993(82)91148-9. [DOI] [PubMed] [Google Scholar]
- Yamuy J, Fung SJ, Xi M, Morales FR, Chase MH. Hypoglossal motoneurons are postsynaptically inhibited during carbachol-induced rapid eye movement sleep. Neuroscience. 1999;94:11–15. doi: 10.1016/s0306-4522(99)00355-3. [DOI] [PubMed] [Google Scholar]
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]


