Abstract
Hypotonicity produces a marked activation of the Na+ pump in frog sartorius muscle. The increase in net Na+ efflux under hypotonic conditions occurs despite the reductions in [Na+]i that are due to fibre swelling and Na+ loss. The pump density (ouabain binding) increases not only upon reduction of the medium osmotic pressure (π) from its normal value (π= 1) to one-half (π= 0.5), but also in muscles that are returned to π= 1 after equilibration in π= 2 medium. The equilibration in π= 2 medium does not affect pump density. Ouabain-binding increments cannot be ascribed to a rise in the Na+–K+ exchange rate of a fixed number of pumps: they also occurred in the continued presence of a saturating concentration of ouabain (50 μm). Under those conditions, the π= 1 →π= 0.5 transfer produced a 43 % increase in pump sites, while the π= 2 →π= 1 transfer induced a rise of 46 %. Actinomycin D did not alter the stimulation of Na+ extrusion elicited by hypotonicity, suggesting that de novo synthesis of pumps was not involved in the increase of the apparent number of pump sites. Disruption of microtubules by colchicine (100 μm) and intermediate filaments by acrylamide (4 mm) did not alter the hypotonic effect. Likewise, genistein (100 μm), a specific inhibitor of tyrosine kinase, did not affect significantly the hypotonic response. Microfilament-disrupting agents like cytochalasin B (5 μm) and latrunculin B (10 μm) reduced the increase in Na+ efflux induced by π= 1 →π= 0.5 transfer by about 35 % and 72 %, respectively. Latrunculin B reduced the increases in pump density generated by π= 1 →π= 0.5 and π= 2 →π= 1 transfers by about 79 % and 91 %, respectively. The results suggest that the membrane stretch due to hypotonic fibre volume increase would promote a microfilament-mediated insertion of submembranous spare Na+ pumps in the sarcolemma and, consequently, the rise in active Na+ transport.
The Na+ pump or Na+,K+-ATPase is an integral membrane protein that, in most animal cells, plays a key role in the maintenance of the transmembrane electrochemical gradients of Na+ and K+, which, in turn, are responsible for the development of the membrane potential and osmotic equilibrium.
More than 20 years ago it was found that hypotonic media produce a marked, quick and reversible increase in Na+ efflux in frog skeletal muscle. This effect is independent of external Na+, not due to membrane depolarisation and inhibitable by strophanthidin and ouabain (Venosa, 1978). Thus, it was clear that, somehow, fibre swelling stimulates the active Na+ transport. Later on, this notion was further strengthened by the findings that a concurrent rise in active K+ influx also occurs and that the increment in Na+ pump activity was accompanied by an increase in the sarcolemmal pump density (Venosa, 1991). The fact that the increase in Na+–K+ transport was percentagewise greater than the increase in pump site density, as judged from measurements of ouabain binding, suggested that hypotonicity increases the pumping activity by an increase in both the number of pump sites and the rate of Na+–K+ exchange per site.
The hypotonic effect was also found in brain synaptosomes (Mongin et al. 1992; Aksentsev et al. 1994), cardiac myocytes (Whalley et al. 1993; Sasaki et al. 1994), astrocytes (Mongin et al. 1994) and renal cells (Coutry et al. 1994; Niisato & Marunaka, 1999).
Hyposmolar syndromes, where free water clearance is compromised, like those caused by inappropriate secretion of antidiuretic hormone, congestive heart failure, hepatic cirrhosis and renal failure, are clinically important. Under these conditions, muscles, which represent about half the body mass, swell, thus promoting the stimulation of active Na+–K+ transport, which would tend to lower [K+]o. This, in turn, would reduce membrane depolarisation (caused by K+i dilution) and, consequently, the likelihood of an impairment of excitability due to Na+ channel inactivation.
Early on, it was hypothesised that fibre swelling might be translated, through membrane deformation, into the observed increase in active Na+ extrusion and K+ uptake. In addition, the swelling effect was also observed when muscles that had been equilibrated in a medium of osmotic pressure twice the normal (π= 2) were transferred to an isotonic medium (Venosa, 1991), a manoeuvre that increases the sarcolemmal tension from a subnormal value to the normal one.
In the present series of experiments it is shown that the actin filaments of the cytoskeleton play a relevant role in the hypotonic response of the active Na+–K+ transport of skeletal muscle fibres. Microtubules and intermediate filaments, on the other hand, do not seem to be involved.
A partial account of the present results has been published in abstract form (Venosa, 2001).
METHODS
Experiments were performed on isolated frog (Leptodactylus ocellatus) paired sartorius muscles. Experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (NIH publication no. 85–23, revised 1996). Before dissection, animals were chilled in an ice-water mixture to full immobility. This procedure lasted 40 min even though the animals were immobilised and did not react to stimuli after about 20 min. They were subsequently double pithed.
The normal saline had the following composition (mm): NaCl 115, KCl 2.5, CaCl2 1.8, Na2HPO4 2.15 and NaH2PO4, 0.85 (pH 7.18). The isotonic medium used as reference was similar to normal Ringer solution except that the NaCl concentration was reduced to 53 mm and in addition had 113 mm sucrose (osmotically equivalent to 62 mm NaCl). Na+-free media had the following composition (mm): TrisCl 59, KCl 2.5, CaCl2 1.8 and sucrose 113 (pH 7.18). The relative osmotic pressure, π, was halved (π= 0.5) by suppressing sucrose, and doubled (π= 2) by making the sucrose concentration 339 mm, so that regardless of π, all experimental solutions had the same ionic composition. A Wescor 5100 osmometer was used to control the osmolarity of the media.
Na+ (22Na+) efflux was measured by exposing muscles to normal Ringer solution labelled with the isotope for ∼2.5 h. After that period they were washed in a series of tubes containing 2.6 ml of non-radioactive solutions. At the end of the run the preparations were digested in tubes containing 0.4 ml of 70 % HNO3. After digestion, distilled water was added to a total volume of 2.6 ml to make their geometry similar to that of the washout tubes. The radioactivity of all of the samples was assayed in a gamma counter. The efflux is expressed in terms of efflux rate coefficient (fractional loss per minute, min−1). 22Na+ was purchased from New England Nuclear (Boston, MA, USA).
Net non-radioactive Na+ efflux was measured by atomic absorption spectrophotometry using a washout (into Na+-free media) protocol similar to that employed for the determination of radioactive fluxes. The Na+ content of muscle fibres at any time during the washout was obtained from the Na+ content of the muscles (digested in HNO3) at the end of the run and the back-addition of the Na+ released during that period.
The density of Na+ pump sites was measured by [3H]ouabain (New England Nuclear) binding following the technique previously described (Venosa & Horowicz, 1981; Venosa, 1991). It has been shown that for a saturating concentration of ouabain (40 μm), the binding time constant is in the order of 16 min in π= 1 medium and about 10 min in π= 0.5 medium (Venosa, 1991); this means that a 40 min exposure to the glycoside at that concentration would saturate more than 90 % of the pumps. In the present experiments the exposure periods to 50 μm ouabain were at least 40 min. On the other hand, the washout of ouabain from frog muscle has two distinct components, the initial release that is relatively fast (washout of the extracellular space and non-specific binding sites), followed by a very slow one, which is monoexponential and represents the release of the glycoside from specific binding sites. In 70 determinations, the time constant of the slow component was 1073 ± 54 min with a mean correlation coefficient for a single exponential fitting of 0.990 ± 0.002. Washout periods lasted 540 min and the tonicity of the unlabelled solution used was that of the medium to which the muscle had been exposed last during the binding period. The amount of ouabain bound to specific sites (pumps) at the end of the loading period is given by the extrapolation of the slow component of the washout to time zero (see Fig. 1). The content of [3H]ouabain in the washout samples and the muscle (digested in 1 ml of Protosol from New England Nuclear) was assayed by liquid scintillation counting. In this way the muscle ouabain content at any time during the washout period could be easily obtained. Internal standards were used to account for the quenching difference between washout samples and digested muscle. Washout curves were obtained from the amount of ouabain bound to the muscle at the end of the washout and the back addition of the amounts of glycoside released in the washout tubes.
Figure 1. Estimate of the amount of ouabain bound to specific sites.
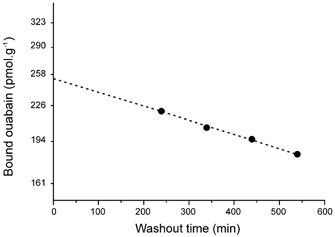
The experimental points show a semilog plot of the slow monoexponential component of the release of ouabain (labelled with [3H]ouabain) from a muscle that had been exposed to the drug for 40 min. The extrapolation to time zero represents the amount of ouabain bound to specific sites (pumps) at the end of the exposure. Most of the initial points of the washout (not shown) fall out of range and represent the release from the extracellular space and non-specific binding sites.
To monitor twitch tension, the pelvic tendon of a sartorius muscle was fastened to the lower end of a vertical holder, while the distal tendon was connected to a UFI 1030 force transducer (Morro Bay, CA, USA) whose output was recorded with an EasyGraf TA240 recorder (Gould, Valley View, OH, USA). The muscle was held at normal frog body length and stimulated supramaximally with pulses of 0.5 ms in duration delivered through a pair of platinum electrodes attached to the holder and connected to a Grass S48 stimulator (West Warwick, RI, USA).
Cytochalasin (Cyto) B (Cyto-B), latrunculin B (Lat-B) and actinomycin D were obtained from Biomol Research Laboratories (Plymouth Meeting, PA, USA), acrylamide was from Bio-Rad (Hercules, CA, USA) and colchicine and genistein from Sigma (St Louis, MO, USA).
Experiments were performed at room temperature (20–22 °C).
Student's t test was used to estimate the statistical significance of differences. Values are expressed as means ±s.e.m.
RESULTS
It is generally accepted that the extrusion of Na+ by the pump increases as [Na+]i rises. Therefore, it might be expected that under hypotonic conditions, the decrease in [Na+]i due to cytoplasmic dilution would produce a reduction in the active Na+ efflux. At variance with this notion, hypotonicity actually stimulates Na+ pumping (Venosa, 1978). To account for this behaviour of the Na+ pump, it seems appropriate first to know how the active Na+ efflux and [Na+]i change as fibres swell in muscles exposed to half the normal osmotic pressure (π= 0.5).
To estimate the changes in active Na+ efflux and [Na+]i as a function of time in π= 0.5 medium, the ouabain-sensitive net Na+ efflux from muscles pre-equilibrated in isotonic Na+-free medium (π= 1/0Na+) and then exposed to π= 0.5/0Na+ medium for 1 h was studied. Figure 2A illustrates the time course of the change in active net Na+ efflux (ΔJpump) and [Na+]i during the exposure to π= 0.5/0Na+ medium. [Na+]i was calculated at each point from the Na+ content and the corresponding intracellular water volume (Vw) estimated from ΔVw as a function of time (inset). ΔVw followed an exponential time course (τ= 32 min). This water volume change is considerably slow as compared with those seen in single fibres (Reuben et al. 1963), mainly because of diffusion delays in the extracellular space of the present multifibre preparation.
Figure 2. Effect of hypotonicity on net ouabain-blockable release of Na+ into Na+-free (Tris) media, determined by atomic absorption spectrophotometry.
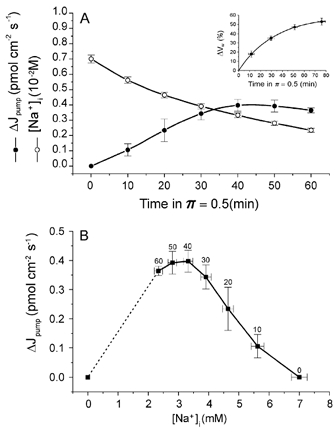
A, time course of the increase in active Na+ efflux (ΔJpump) and internal Na+ concentration ([Na+]i) upon replacement of a solution of π= 1 by one of π= 0.5 at time = 0. ΔJpump rises, in spite of the reduction in [Na+]i. Each experimental ΔJpump value represents data from four muscle pairs (one member of each pair in the presence of 50 μm ouabain) and is expressed per cm2 of superficial sarcolemma using the relationship 430 cm2 g−1 of muscle (Venosa, 1991). [Na+]i was calculated from the release of Na+ into π= 0.5 medium and the muscle Na+ content at the end of the washout period of muscles kept in ouabain-free medium. The inset shows the time course of the increment in intracellular water (ΔVW) in π= 0.5 medium (n = 8). The experimental values were obtained from wet (drained but not blotted) muscle mass determinations. In control experiments it was found that the volume of the extracellular space (Na+ space) is not significantly changed by π= 1 →π= 0.5 transfer. Values from this curve were used to calculate [Na+]i. B, ΔJpump as a function of [Na+]i. The numbers next to the experimental points indicate time (min) in π= 0.5 medium. The plot emphasises the fact that as fibres swell and [Na+]i falls, the active Na+ extrusion increases. Near swelling completion, when the hypotonic effect reaches a maximum, ΔJpump as a growing function of [Na+]i starts to become apparent.
Clearly, in π= 0.5/0Na+ medium, ΔJpump increases while [Na+]i decreases with time in π= 0.5 medium. It should be noted that [Na+]i was already low (about 7 mm) at the beginning of the run because of the pre-exposure to π= 1/0Na+ medium. This fall of [Na+]i is the result of both fibre swelling and Na+ extrusion into a Na+-free medium. The data in Fig. 2A suggest that the hypotonic effect is underlined by a process that overcomes the reduction of the Na+ pump activity that might be expected due to the steady decrease of [Na+]i. Figure 2B shows a plot of ΔJpump as a function of [Na+]i (data from Fig. 2A). The descending limb of the curve illustrates the swelling effect on ΔJpump, which, as the exposure to π= 0.5/0Na+ medium progresses and the rate of volume increase slows down, reaches a maximum. Under these conditions, as [Na+]i falls below about 3.5 mm, ΔJpump becomes apparent as a growing function of [Na+]i. It should be noted that unlike the other points, the point at the extreme left is not an experimental one but is included because at [Na+]i= 0, ΔJpump must equal zero. The dashed straight line is not meant to suggest a linear relationship between ΔJpump and [Na+]i, since it is known that in frog skeletal muscle fibres (Mullins & Frumento, 1963) as well as in guinea-pig cardiac myocytes (Gao et al. 1995) at constant volume, the active Na+ efflux appears to be a saturable function of [Na+]i3.
As already mentioned in the Introduction, the increased activity of the pump in π= 0.5 medium is accompanied by a significant increment in the number of pump sites; since the stimulation of the pump is also observed when swelling occurs in the subnormal fibre volume range (Venosa, 1991), it seemed appropriate to find out if under these conditions (π= 2 →π= 1 transfer), the number of pump sites increases. It was found that the magnitude of the ouabain binding in muscles that had been equilibrated in π= 2 medium is not significantly different from the value found in muscles equilibrated in π= 1 medium. On the other hand, when muscles equilibrated in π= 2 medium are exposed to π= 1 medium, the swelling of the fibres back to their normal volume is accompanied by an increase in ouabain binding to values significantly greater than those found in muscles in π= 1 medium that had not been pre-equilibrated in π= 2 medium. This is depicted in Fig. 3A. It can be seen that the increase of binding promoted by the π= 2 →π= 1 transfer is close to that generated by the π= 1 →π= 0.5 transfer.
Figure 3. Reduction of tonicity, regardless of its absolute value, increases Na+ pump density.
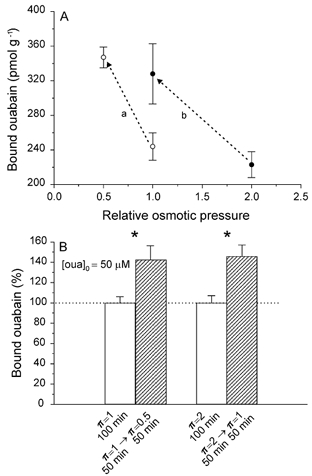
A, the number of Na+ pumps per gram of muscle (determined by [3H]ouabain binding) increases significantly when muscles pre-equilibrated in π= 1 medium are exposed to π= 0.5 (a) and in those pre-equilibrated in π= 2 medium and then transferred to π= 1 (b; n = 6 muscle pairs for each change of π). B, in the continuous (100 min) presence of a saturating concentration of ouabain ([oua]o= 50 μm), muscles that were exposed to π= 1 medium for 50 min and then transferred to π= 0.5 for another 50 min exhibited a significantly greater (43 %, P < 0.04) ouabain binding than their paired control muscles, which had been kept in π= 1 medium. Likewise, a similar increase of ouabain binding was produced by the π= 2 →π= 1 transfer (46 %, P < 0.02). These data indicate that the stimulation of the active Na+ transport promoted by fibre swelling is related to an actual increase of the pump density, although an additional effect on the Na+–K+ exchange cannot be discarded (n = 4 muscle pairs for each transfer).
The increases in ouabain binding produced by lowering the tonicity of the external medium could be due to an increase in the density of pump sites in the sarcolemma, but might also be induced by a rise in the Na+–K+ exchange rate of a fixed number of pumps (Hootman & Ernst, 1988). Previous data suggest that both mechanisms are at work in the hypotonic response (Venosa, 1991). It was reasoned that if we are dealing with a fixed number of pump sites, one would expect that in muscles equilibrated in a π= 1 medium containing a saturating concentration of ouabain and then transferred to π= 0.5 medium containing the same concentration of the glycoside, the magnitude of the ouabain binding should be similar to that of their paired preparations exposed to π= 1 and ouabain throughout the experiment. At variance with this possibility, it was found that in the continuous presence of a saturating concentration of ouabain (50 μm), muscles that had been first equilibrated in π= 1 medium and then transferred to π= 0.5 medium, exhibited a ouabain binding (i.e. a density of pump sites) significantly higher than that found in control companion muscles kept in π= 1 medium containing the same concentration of ouabain throughout. On the other hand, when a similar protocol was applied to muscles first equilibrated in π= 2 medium containing 50 μm ouabain and then transferred to π= 1 medium, keeping the concentration of ouabain constant, the binding was significantly higher than that in the companion muscle kept in π= 2 medium and the same concentration of ouabain throughout. These results, which are shown in Fig. 3B, suggest strongly that the stimulation of the active Na+ transport promoted by fibre swelling in both π= 1 →π= 0.5 and π= 2 →π= 1 transfers is mostly related to an actual increase in the number of pump sites.
The increase in pump density promoted by hypotonicity could be due to a rise of the synthesis of Na+,K+-ATPase α or α and β subunits. To evaluate this possibility, experiments were performed in which protein synthesis was blocked at the translational level by pre-equilibrating muscles in π= 1 medium plus 4 mg l−1 of actinomycin D for a period of 135 min. As can be seen in Fig. 4A, this treatment produced no effect on the hypotonic response, suggesting that de novo synthesis is unlikely to be the cause of the increase in the density of sarcolemmal pumps.
Figure 4. Stimulation of the active Na+ efflux is not the result of de novo synthesis of pumps and is independent of the integrity of either microtubules or intermediate filaments.
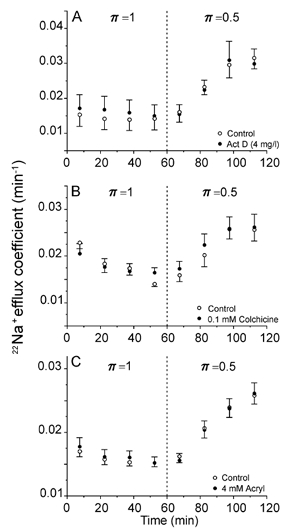
A, the exposure of the experimental muscles to 4 mg l−1 actinomycin D (Act D) to inhibit de novo synthesis of Na+ pumps began 2 h before the π= 1 →π= 0.5 transfer and continued to the end of the run. The data represent the means from four identical experiments performed in paired muscles. B, colchicine (0.1 mm), a microtubule-disrupting agent, did not alter the hypotonic response of Na+ efflux. Mean data from four identical experiments performed in paired muscles. C, acrylamide (Acryl, 4 mg l−1), a drug that disrupts intermediate filaments, failed to affect the hypotonic stimulation of Na+ efflux. Mean data from four identical experiments performed in paired muscles.
Stretching of the sarcolemma could, through a deformation of the cytoskeleton, be the trigger for the hypotonic response. To test this possibility, the relevance of three components of the cytoskeleton, namely microtubules, intermediate filaments and microfilaments, was studied. Colchicine (0.1 mm), a destabiliser of the microtubules, did not alter the effect of hypotonicity on the active Na+ transport (Fig. 4B). Similarly, the exposure of muscles to 4 mm acrylamide, a disrupting agent of intermediate filaments did not alter the hypotonic response of the Na+ pump (Fig. 4C).
To disrupt microfilaments, two agents were used: Cyto-B (5 μm) and Lat-B (10 μm). The effect of Cyto-B on the stimulation of active Na+ efflux by π= 0.5 medium is illustrated in Fig. 5A. It can be seen that in muscles exposed to this drug, the response to hypotonicity was appreciably smaller than that of the controls, the difference being about 35 % towards the end of the period in π= 0.5 medium.
Figure 5. Microfilament disruption reduces the hypotonic stimulation of the active Na+ efflux.
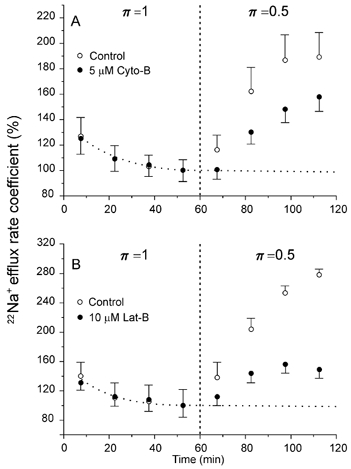
A, cytochalasin B (Cyto-B, 5 μm), a depolymeriser of microfilaments, reduced by ∼35 % the rise of the Na+ efflux induced by π = 0.5. The dotted curve represents a baseline obtained by fitting a single exponential to the points in π= 1 medium. Mean data from nine identical experiments performed in paired homologous muscles. B, latrunculin B (Lat-B, 10 μm), a microfilament depolymerising agent that is 1–2 orders of magnitude more potent than cytochalasins, reduced the hypotonic response of Na+ efflux by ∼72 %. Dotted curve as in A (n = 4 muscle pairs).
The depolymerising capacity of Lat-B is 1–2 orders of magnitude greater than that of cytochalasins (Spector et al. 1983). Figure 5B shows the effect of 10 μm Lat-B. It can be seen that the inhibition produced by this fungal toxin on the hypotonic response of the Na+ efflux was about twice (∼72 %) that of Cyto-B. It should be noticed that colchicine, acrylamide, Cyto-B and Lat-B did not affect Na+ efflux per se in π= 1 medium. On the other hand, in control experiments, half concentrations of Cyto-B (2.5 μm) and Lat-B (5 μm) produced effects similar to those reported here. Furthermore, Fig. 6 shows that the effect of Lat-B is not due to a non-specific disruption of fibre structure: prolonged exposure (∼2 h) to this agent did not affect muscle twitch substantially. In two other experiments (not shown), the exposure to the toxin lasted 80 and 95 min, respectively. They also showed no major effect of Lat-B on contractility. The mean twitch tension at 80 min from the beginning of the exposure to 10 μm Lat-B was 88.5 ± 2.5 % (n = 3) of that just before the application of the toxin. A decline in developed tension of this sort is not unusual in sartorii twitching under control conditions. Therefore, it may be concluded that at the concentration used here, Lat-B neither impaired the generation of action potentials nor destabilised the actin filaments of the contractile apparatus.
Figure 6. Lat-B (10 μm) and twitch tension.
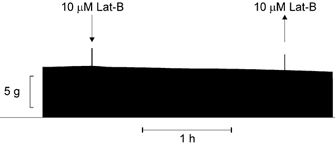
Twitch tension from a sartorius muscle. The record shows that the inhibitory effect of Lat-B on the hypotonic response of the Na+ pump is not due to a non-specific disruption of muscle structural integrity, since a prolonged exposure (117 min) to this toxin does not generate any substantial change in twitch tension, which means that this toxin produces no significant electrical and/or mechanical disarray of the fibres. The two tension spikes are artefacts produced by the solution changes: application (downward arrow) and withdrawal (upward arrow) of the toxin. Frequency of stimulation: 0.1 Hz.
In view of these results, it was further speculated that if the apparent upregulation of the Na+ pump sites produced by hypotonicity (Venosa, 1991) was accomplished by a microfilament-dependent insertion of spare pumps into the sarcolemma, then, in the presence of Lat-B, the increase of ouabain binding brought about by hypotonic media should be reduced. Thus, experiments were performed similar to those shown in Fig. 3B except that all solutions contained 10 μm Lat-B. Figure 7 illustrates the inhibitory effect of Lat-B on the increase in ouabain binding that takes place upon fibre swelling in both π= 1 →π= 0.5 and π= 2 →π= 1 transfers. As in the case of Na+ efflux, Lat-B per se did not affect ouabain binding. By comparison with data in Fig. 3B, Lat-B reduced the increase in ouabain binding by ∼79 % (π= 1 →π= 0.5 transfer) and ∼91 % (π= 2 →π= 1 transfer).
Figure 7. The hypotonic increase of ouabain binding depends on the integrity of microfilaments.
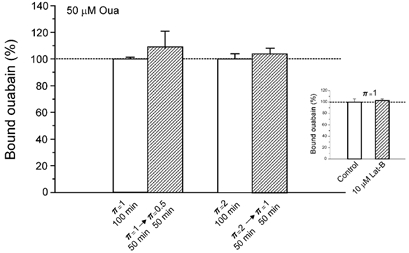
Experimental protocol similar to that used in the experiment depicted in Fig. 3B, except that it was performed in the presence of 10 μm Lat-B. The increases in ouabain binding promoted by reducing the tonicity from π= 1 to π= 0.5 (n = 8 paired muscles) and from π= 2 to π= 1 (n = 8 paired muscles) were virtually absent in the presence of this microfilament-disrupting agent (compare with data in Fig. 3A and B). The inset shows that Lat-B does not affect per se the ouabain binding (n = 8 paired muscles).
In cardiac myocytes, hypotonicity elicits a fast activation of tyrosine kinase (Sadoshima et al. 1996) that appears to be associated with Na+ pump stimulation (Bewick et al. 1999). To test the role of this kinase in the hypotonic response of skeletal muscle, sartorii were pre-equilibrated (1 h) in π= 1 medium containing genistein (100 μm), to inhibit tyrosine kinase, and then transferred to π= 0.5 medium in the continued presence of genistein. After 1 h in π= 0.5/genistein medium, the Na+ efflux rate coefficient was 0.0204 ± 0.0009 min−1, while in homologous control muscles it was 0.0230 ± 0.0015 min−1 (P > 0.18; n = 4 muscle pairs), suggesting that the hypotonic response of skeletal muscle fibres, unlike that of cardiac myocytes, is either slightly, or not tyrosine-kinase dependent.
DISCUSSION
When the activation of the Na+ pump by hypotonicity was first shown in frog skeletal muscle (Venosa, 1978), it was clear that the effect is [Na+]o-independent and, therefore, not due to a net Na+ influx, which would raise [Na+]i in the neighbourhood of the pumps and, thus, stimulate the active Na+ extrusion. In the present study it is further shown that in the absence of external Na+, the net ouabain-sensitive Na+ efflux increases as a function of time during the exposure to π= 0.5, despite the continuous fall of [Na+]i produced by both Na+ loss and dilution due to fibre swelling. This means that hypotonicity raises the pumping capacity of the fibres as they swell and, as shown in Fig. 2, with a time course similar to that of the cell volume increase. Clearly, the hypotonic stimulation of the Na+ pump must overcome the opposite effect of the [Na+]i fall.
Fibre swelling, whether it occurs in the π= 1 →π= 0.5 or the π= 2 →π= 1 transfer, stimulates the Na+ pump and elicits an increase in the sarcolemmal density of pump sites, which might be the result of an increase in the de novo synthesis of pumps. However, the lack of effect of the translational blockage of protein synthesis by actinomycin D on the hypotonic response indicates that this mechanism is probably not involved.
It is known that integral membrane proteins, including Na+,K+-ATPase, are connected to the membrane cytoskeleton. The linkage would be mediated by ankyrins, a family of proteins that bind to the α subunit of the Na+,K+-ATPase and to a submembranous spectrin-actin network (Devarajan et al. 1994; Zhang et al. 1998). Based on these structural relationships, it was thought that the upregulation of pumps in swelling fibres could be related to the stretch and deformation of the sarcolemma and its associated cytoskeleton. Therefore, it seemed appropriate to explore the participation of three well-characterised components of the cytoskeleton, namely microtubules, intermediate filaments and microfilaments. The hyposmotic response of the Na+ pump was unaffected by prolonged exposure of preparations to either colchicine or acrylamide, indicating that neither microtubules nor intermediate filaments play a role in this phenomenon. On the contrary, the depolymerisation of microfilaments by Cyto-B, and particularly by Lat-B, produced a marked inhibition of both the rise of active Na+ efflux and the increase in the number of pumps generated by hypotonicity.
Rosado & Sage (2000), using confocal microscopy, have shown in human platelets that Cyto-D induced a reorganisation of membrane skeleton actin filaments into dense foci. It seems reasonable to assume that in skeletal muscle, a similar reorganisation could be responsible for the inhibitory effect of Cyto-B and Lat-B on the hypotonic stimulation of active Na+ efflux. Thus, it might be imagined that spare pumps during the hypotonic response would be pulled, perhaps due to membrane stretch, towards the sarcolemma by the membrane skeleton microfilaments, a process that would be abolished by the formation of dense foci. Somehow at variance with the present results, it has been reported that unpolymerised actin filaments stimulate the activity of rat kidney Na+,K+-ATPase (Cantiello, 1995).
While in cardiac myocytes, inhibition of tyrosine kinase blocks the hypotonic response of the pump (Bewick et al. 1999), a prolonged exposure of sartorius muscles to genistein, a specific inhibitor of tyrosine kinase, failed to significantly affect the response. To this author, an explanation for the apparent discrepancy, which certainly deserves to be further investigated, is not readily available.
The present results are consistent with the idea that membrane stretching in either the π= 1 →π= 0.5 or the π= 2 →π= 1 transfer would elicit a microfilament-mediated insertion of submembranous spare pumps into the sarcolemma. A mechanism akin to this hypothesis has been proposed in which the membrane surface area would be modulated by its tension so that the stretch produced by swelling would promote an addition of plasma membrane, provided by a cytosolic pool through an exocytotic process (for a review see Morris & Homann, 2001).
Interestingly, while in the π= 1 →π= 0.5 transfer, the effect on Na+ efflux is sustained, in the π= 2 →π= 1 transfer it is transient (Venosa, 1991), as if the swelling activation of the pump has two components: one associated with the increase of membrane tension (T) as a function of time (dT/dt > 0) and the other with the constant degree of tension reached at equilibrium (dT/dt = 0). Thus, in the π= 2 →π= 1 protocol we have at the beginning in π= 1 medium a dT/dt > 0, and later on, when dT/dt = 0, the membrane tension is that which is observed under normal physiological conditions. In muscles pre-equilibrated in π= 2 medium and then transferred to π= 1 medium containing ouabain, the magnitude of ouabain binding exceeds significantly that found in muscles in π= 1 medium that had not been pre-exposed to π= 2 (see Fig. 3A), the difference corresponds to the previously described transient increase in active Na+ efflux observed in this transfer (Venosa, 1991). Ouabain binding in frog muscle is practically irreversible (Venosa & Horowicz, 1981), so that the extra glycoside bound during the transient upregulation of pumps will remain attached to them for many hours.
In the case of the π= 1 →π= 0.5 transfer, not only the increase of T with time (dT/dt > 0), but also the value of T at equilibrium in π= 0.5 medium (dT/dt = 0), which is greater than that in π= 1 medium, will contribute to raise the number of sarcolemmal pumps. In fact, the increase in pump density produced by hypotonicity was first demonstrated by exposing muscles to [3H]ouabain after prolonged equilibration in π= 0.5 medium (Venosa, 1991). Therefore, it seems as if dT/dt > 0 would always increase the number of pump sites, while constant T would do so if it is greater than T at π= 1.
In summary, in frog skeletal muscle, the stimulation of the active Na+ transport elicited by hypotonicity seems to be due mainly to an increase in the density of Na+ pumps, apparently produced by a microfilament-mediated membrane insertion of submembranous spare pumps. Although at present details of the role of microfilaments in the hypotonic response are lacking, it probably involves a series of reactions, for instance the secretion-like coupling model type proposed for the store-operated Ca2+ entry (Patterson et al. 1999; see Rosado & Sage, 2000 for a review), at the end of which these components of the cytoskeleton would be instrumental in the increase of sarcolemmal pump density produced by hypotonicity.
Acknowledgments
This work was supported by CONICET (The National Research Council of Argentina). R.A.V. is an established investigator of CONICET.
references
- Aksensev SL, Mongin AA, Orlov SN, Rakovich AA, Kaler GV, Konev SV. Osmotic regulation of sodium pump in rat brain synaptosomes: the role of cytoplasmic sodium. Brain Res. 1994;644:1–6. doi: 10.1016/0006-8993(94)90338-7. [DOI] [PubMed] [Google Scholar]
- Bewick NL, Fernández C, Pitt AD, Rasmussen HH, Whalley DW. Mechanisms of Na+–K+ pump regulation in cardiac myocytes during hyposmolar swelling. Am J Physiol. 1999;276:C1091–1099. doi: 10.1152/ajpcell.1999.276.5.C1091. [DOI] [PubMed] [Google Scholar]
- Cantiello HF. Actin filaments stimulate the Na+–K+-ATPase. Am J Physiol. 1995;269:F637–643. doi: 10.1152/ajprenal.1995.269.5.F637. [DOI] [PubMed] [Google Scholar]
- Coutry N, Farman N, Bonvalet JP, Blot-Chabaud M. Role of cell volume variations in Na+–K+-ATPase recruitment and/or activation in cortical collecting duct. Am J Physiol. 1994;C266:1342–1349. doi: 10.1152/ajpcell.1994.266.5.C1342. [DOI] [PubMed] [Google Scholar]
- Devarajan P, Scaramuzzino DA, Morrow JS. Ankyrin binds to two distinct cytoplasmic domains of Na,K-ATPase α subunit. Proc Natl Acad Sci U S A. 1994;91:2965–2969. doi: 10.1073/pnas.91.8.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Mathias RT, Cohen IS, Balbo GJ. Two functionally different Na/K pumps in cardiac ventricular myocytes. J Gen Physiol. 1995;106:995–1030. doi: 10.1085/jgp.106.5.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hootman SR, Ernst AE. Estimation of Na,K-pump numbers and turnover in intact cells with [3H]ouabain. Methods Enzymol. 1988;156:213–229. doi: 10.1016/0076-6879(88)56023-8. [DOI] [PubMed] [Google Scholar]
- Mongin AA, Aksensev SL, Orlov SN, Slepko NG, Kozlova MV, Maximov GV, Konev SV. Swelling-induced K+ influx in cultured primary astrocytes. Brain Res. 1994;655:110–114. doi: 10.1016/0006-8993(94)91603-9. [DOI] [PubMed] [Google Scholar]
- Mongin AA, Aksensev SL, Rakovhich AA, Okun IM, Konev SV, Orlov SN. Osmotic regulation of the sodium pump in rat brain synaptosomes. Biophysics. 1992;37:847–852. [PubMed] [Google Scholar]
- Morris CE, Homann U. Cell surface area regulation and membrane tension. J Membr Biol. 2001;179:79–102. doi: 10.1007/s002320010040. [DOI] [PubMed] [Google Scholar]
- Mullins LJ, Frumento AS. The concentration dependence of sodium efflux from muscle. J Gen Physiol. 1963;46:629–654. doi: 10.1085/jgp.46.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niisato N, Marunaka Y. Activation of the Na+–K+ pump by hypoosmolality through tyrosine kinase-dependent Cl− conductance in Xenopus renal epithelial A6 cells. J Physiol. 1999;518:417–432. doi: 10.1111/j.1469-7793.1999.0417p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson AB, Van Rosuum DB, Gill DL. Store-operated Ca2+ entry: evidence for a secretion-like coupling model. Cell. 1999;98:487–499. doi: 10.1016/s0092-8674(00)81977-7. [DOI] [PubMed] [Google Scholar]
- Reuben JP, López E, Brandt PW, Grundfest H. Muscle: volume changes in isolated muscle fibers. Science. 1963;142:246–248. doi: 10.1126/science.142.3589.246. [DOI] [PubMed] [Google Scholar]
- Rosado JA, Sage OS. The actin cytoskeleton in store-mediated calcium entry. J Physiol. 2000;526:221–229. doi: 10.1111/j.1469-7793.2000.t01-2-00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoshima J, Qiu Z, Morgan JP, Izumo S. Tyrosine kinase activation is an immediate and essential step in hypotonic cell swelling-induced ERK activation and c-fos gene expression in cardiac myocytes. EMBO J. 1996;15:5535–5546. [PMC free article] [PubMed] [Google Scholar]
- Sasaki N, Mitsuiye T, Wang Z, Noma A. Increase of the delayed rectifier K and Na+–K+ pump currents by hypotonic solutions in guinea pig cardiac myocytes. Circ Res. 1994;75:887–895. doi: 10.1161/01.res.75.5.887. [DOI] [PubMed] [Google Scholar]
- Spector I, Shochet NR, Kashman Y, Groweiss A. Latrunculin: novel marine toxins that disrupt microfilament organization in culture cells. Science. 1983;219:493–495. doi: 10.1126/science.6681676. [DOI] [PubMed] [Google Scholar]
- Venosa RA. Stimulation of the Na+ pump by hypotonic solutions in skeletal muscle. Biochim Biophys Acta. 1978;510:378–383. doi: 10.1016/0005-2736(78)90038-x. [DOI] [PubMed] [Google Scholar]
- Venosa RA. Hypo-osmotic stimulation of active Na+ transport in frog muscle: apparent upregulation of Na+ pumps. J Membr Biol. 1991;120:97–104. doi: 10.1007/BF01872392. [DOI] [PubMed] [Google Scholar]
- Venosa RA. Estimulación hypotónica de (EH) del transporte activo de Na+ en músculo esquelético: participación del citoesqueleto. Medicina. 2001;61:707. (abstract) [Google Scholar]
- Venosa RA, Horowicz P. Density and apparent location of sodium pump in frog sartorius muscle. J Membr Biol. 1981;59:225–232. doi: 10.1007/BF01875427. [DOI] [PubMed] [Google Scholar]
- Whalley DW, Hool LC, Ten Eick RE, Rasmussen HH. Effect of osmotic swelling and shrinkage on Na+–K+ pump activity in mammalian cardiac myocytes. Am J Physiol. 1993;265:1201–1210. doi: 10.1152/ajpcell.1993.265.5.C1201. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Dvarajan P, Dorfman AI, Morrow JS. Structure of ankyrin binding domain of α-Na,K-ATPase. J Biol Chem. 1998;273:18681–18684. doi: 10.1074/jbc.273.30.18681. [DOI] [PubMed] [Google Scholar]


